Abstract
Introduction:
Clinical presentation of oromandibular dystonia (OMD) is variable that can be further complicated by the presence of temporomandibular disorder (TMD) symptoms. We sought to evaluate variations in the clinical presentation of OMD patients, particularly TMD related characteristics, in two clinic settings.
Methods:
In a cross-sectional study design, a web-based data collection survey was provided to eligible patients with OMD from Movement Disorder (MD) and Orofacial Pain (OFP) clinics. The survey questionnaire was designed to collect information on demographic characteristics, clinical presentation particularly related to TMD, quality of life and treatment outcomes. Validated questionnaires were used when available such as the TMD Pain Screener, EQ-5D-5L, Jaw Functional Limitation Scale, and Global Rating of Change Scale.
Results:
Of 53 eligible patients, 31 responded to the survey for a 58% response rate. Forty-eight percent of patients in the MD clinic and 60% of patients in the OFP clinic reported jaw pain along with involuntary movements. Of those, 90% from the MD group and 83% from the OFP group screened positive with the TMD Pain Screener at the onset of symptoms based on recall. Positive TMD Pain Screener response was observed in about 40% of patients in both clinics within 30 days of questionnaire response. No statistically significant differences were observed between two groups for any measured variables.
Conclusion:
Patients with OMD have features of TMD, irrespective of the clinical setting in which they seek and receive care. OMD patients from both clinics were similar in terms of clinical presentation, quality of life and treatment outcomes.
INTRODUCTION
Oromandibular dystonia (OMD) involves sustained or repetitive involuntary movements of the masticatory, facial, and lingual muscles. Different population studies of OMD have shown prevalence of 2.1 to 6.9 per 100,000 persons (1–3). Clinical characteristics of OMD are a reflection of different muscles undergoing dystonic spasm causing involuntary movements like opening, closing, side or protrusive jaw movements, facial grimacing, tongue movements, lip movements like pursing, sucking or smacking, and retraction of the corners of the mouth (4). Data regarding the occurrence of temporomandibular disorder (TMD) or jaw pain in OMD patients is variable in the literature (5–7). TMD was often considered in the differential diagnosis of OMD, particularly to jaw opening dystonia due to similarity with temporomandibular joint subluxation (4). Costa and colleagues found that patients with oromandibular dystonia frequently report some form of TMD (8). In a previous study, we evaluated the clinical characteristics of OMD patients in our Orofacial Pain (OFP) clinic and found that all patients had an associated diagnosis of TMD (9). Given the increasing reports of occurrence of TMD in OMD patients and heterogeneity in clinical characteristics of OMD, we intend to study the variation in clinical presentation of OMD in a Movement Disorder (MD) clinic and an OFP clinic.
Involuntary movements in OMD typically appear and worsen with jaw functions that impart negative effects on quality of life (10, 11). Despite the significant disease burden, there is a paucity of literature on health status and quality of life of OMD patients (12–14). Since OMD is not curable, treatment is often focused on reducing dystonic movements and improving one’s functions. Commonly reported treatment options include botulinum toxin injections, oral medications, oral appliances, physical therapy, behavioral therapy and occasionally deep brain stimulation (15, 16). Therefore, we wanted to explore clinical characteristics, evaluate quality of life and assess treatment outcomes in OMD patients who presented to MD and OFP clinics.
METHODS
This is a cross-sectional survey study in which 43 questions about OMD and TMD were asked of eligible participants receiving care within two different services, a MD clinic where care is provided by physicians and an OFP clinic where care is provided by dentists with advanced training in OFP. Both clinics are part of the University of Minnesota system and located on the East Bank of the Twin Cities campus. The University of Minnesota Institutional Review Board approved the study protocol, and all the subjects provided their informed consent. The research was conducted in accordance with the rules of Declaration of Helsinki of 1975, as revised in 2013.
Patient selection
After the approval of institutional review boards, patients with OMD were identified using ICD-9 (333.82 Orofacial dyskinesia) and ICD-10 (G24.4 Idiopathic orofacial dystonia) diagnostic codes from electronic health record systems of the MD clinic and OFP clinic when they were seen between October 2012 and February 2019. Exclusion criteria were patients <18 years old, pregnant women, prisoners, non-English speakers, patients with dementia, OMD diagnosis secondary to Parkinson’s disease, Tardive dyskinesia, or other movement disorders like chorea, hemifacial spasm, cervical dystonia, tremor, isolated blepharospasm, and functional movement disorder. We identified 42 patients from the MD clinic and 11 patients from the OFP clinic who met eligibility criteria (Figure 1).
Figure 1:
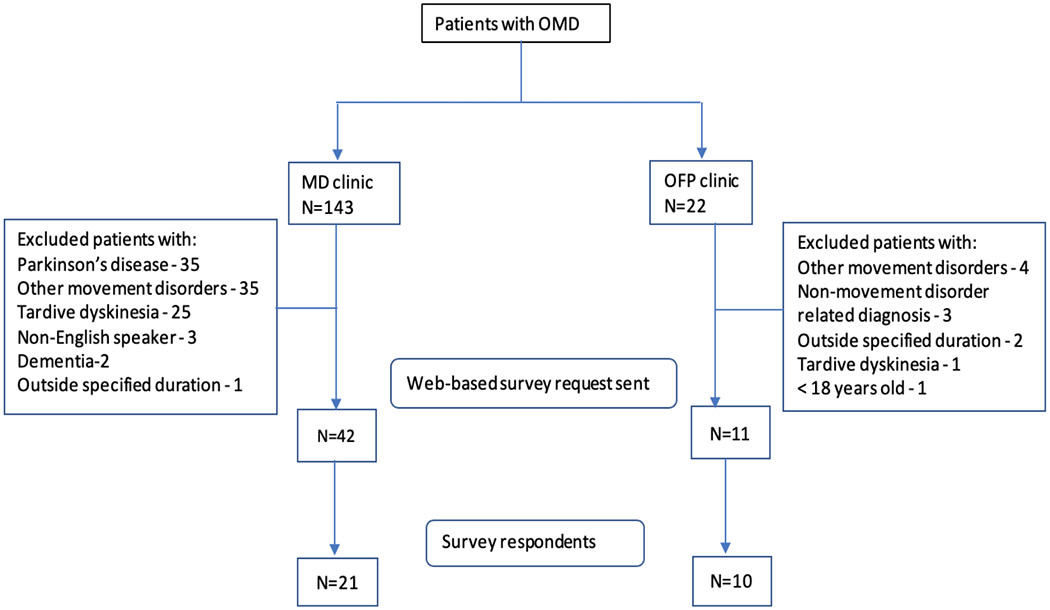
Flow chart of study inclusion
Recruitment strategy
Eligible patients were contacted through a recruitment letter for voluntary participation in the survey for data collection. Recruitment letters included information about the research, reasons for conducting the study, benefits of taking part in the survey and our contact details for further questions. The letters had a URL link and QR code for participants to access the web-based questionnaire. The survey was designed and administered through QualtricsXM. The survey reminders were sent three additional times at 4-week intervals. Upon completion of the survey, participants were sent a thank you letter along with a $25 gift card to compensate for their time.
Data collection instruments
A 43-item questionnaire was designed to collect information on demographic features (age, gender, ethnicity, education), clinical features of involuntary movements (onset, trigger, kind of involuntary movements, its effect on function, sensory tricks, associated muscular tension, and jaw pain with relation to involuntary movements), clinical care received (number of providers seen, kind of treatments received, most effective treatment, reason for discontinuing treatment), and TMD related characteristics. The questionnaire was divided into categories like symptoms pre-treatment, clinical care received, symptoms post-treatment, general health status and demographics and were based on existing validated questionnaires when available.
The specific instruments used were:
TMD pain screener (17), which is a 6-item instrument for identifying patients with painful TMD. Scores range from 0-7 with a score of 3 or more indicating a positive screen. The TMD Pain screener was displayed twice to participants: first to participants when they were experiencing jaw pain along with involuntary movements and second when all the participants in both groups were requested to fill out the TMD Pain screener for the symptoms in the last 30 days irrespective of the onset of jaw pain with involuntary movements.
Characteristic Pain Intensity from Graded chronic pain scale (18), and a 30-day version from Diagnostic Criteria for TMD (DC/TMD) (19) was used for assessment of average pain severity that ranges from 0 (no pain) to 10 (pain as bad as could be). Scores range from 0 to 100 points.
Jaw Functional Limitation Scale (JFLS) involving an 8-item instrument (20) (short version) was used to assess functional limitation of the masticatory system. For each item, the participants rate their limitation on a 0 – 10 point scale, where “0” is no limitation and “10” is severe limitation. A “not applicable” option was available to participants to indicate that the item was irrelevant for their situation, which was considered as missing. The global score was computed from the mean of the available items with no more than 2 items missing.
EQ-5D-5L (21) is a measure of health status developed by the EuroQol group that provides a generic measure of clinical and economic burden of disease. It includes 5 dimensions (mobility, self-care, usual activities, pain/discomfort, and anxiety/depression) with 5 levels in each dimension (no problems, slight problems, moderate problems, severe problems, and extreme problems). EQ-5D-5L states were converted to a summary number or index value using the United States value sets (22). The index value represents how good or bad a health state is according to preferences of the general population in that country (in our case, USA) and it can range from −0.573 (worse than dead) to 1 (perfectly healthy).
Global Rating of Change Scale (23) involves a single question that measures self-perceived change in health status. It is an 11-point scale ranging from −5 (very much worse), through 0 (unchanged), to +5 (completely recovered).
Data management and analysis
The survey data was analyzed using JASP statistical software. Descriptive statistics were presented as mean and standard deviation for continuous data and counts and proportions for categorical data. Mann-Whitney’s U test was used to compare means due to non-normal distribution of data and small sample sizes. Chi-square test was used to compare proportions of two groups.
RESULTS
Demographic features and general health status
Ten of eleven (91%) patients in the OFP clinic and 21 of 42 (50%) patients in the MD clinic responded to our survey with no missing values to any questions for all completed questionnaires. All the participants identified themselves as Caucasians with a predominance of females in both groups (3:1 female: male). The EQ-5D-5L scale showed no significant difference in summary index value of participants from both groups (p-value 0.374). Both groups had comparable baseline characteristics and general health status (Table I).
Table I.
Demographic characteristics
| Characteristic | N | MD group (N=21) |
OFP group (N=10) |
p-value |
|---|---|---|---|---|
| Age in years (mean, range) * | 31 | 59 (31-82) | 64 (55-84) | 0.498 |
| Sex – Female (n, %) ^ | 31 | 17 (81%) | 6 (60%) | 0.213 |
| Race – Caucasian (n, %) ^ | 31 | 21 (100%) | 10 (100%) | 1 |
| Level of education (n, %) ^ | 31 | 0.775 | ||
| High school graduate | 3 (14%) | 3 (30%) | ||
| Some college/Associate’s degree | 8 (38%) | 3 (30%) | ||
| Bachelor’s degree | 7 (33%) | 3 (30%) | ||
| Graduate degree | 3 (14%) | 1 (10%) | ||
| EQ-5D-5L Index Value (mean, SD) * | 31 | 0.789 (0.214) | 0.855 (0.131) | 0.374 |
Mann-Whitney U test;
Chi-square test
Symptoms before receiving treatment
Survey participants in the MD group had noted involuntary movements for a mean of 15 years compared to 8 years in the OFP group (p-value 0.330). A portion of the participants in both groups (43% in the MD and 30% in the OFP group) were not aware of particular precipitating factors for their involuntary movements (Figure 2). Jaw closing and jaw opening movements were more commonly reported in the MD group, while jaw closing, and tongue movements were often reported in the OFP group. On an ordinal scale, involuntary movements bothered patients “a lot” in 60% of the OFP group compared to 43% of the MD group. (p-value 0.313). Sixty-seven percent of participants in the MD group had sensory tricks compared to 50% of participants in the OFP group (p-value 0.373) (Table II).
Figure 2:
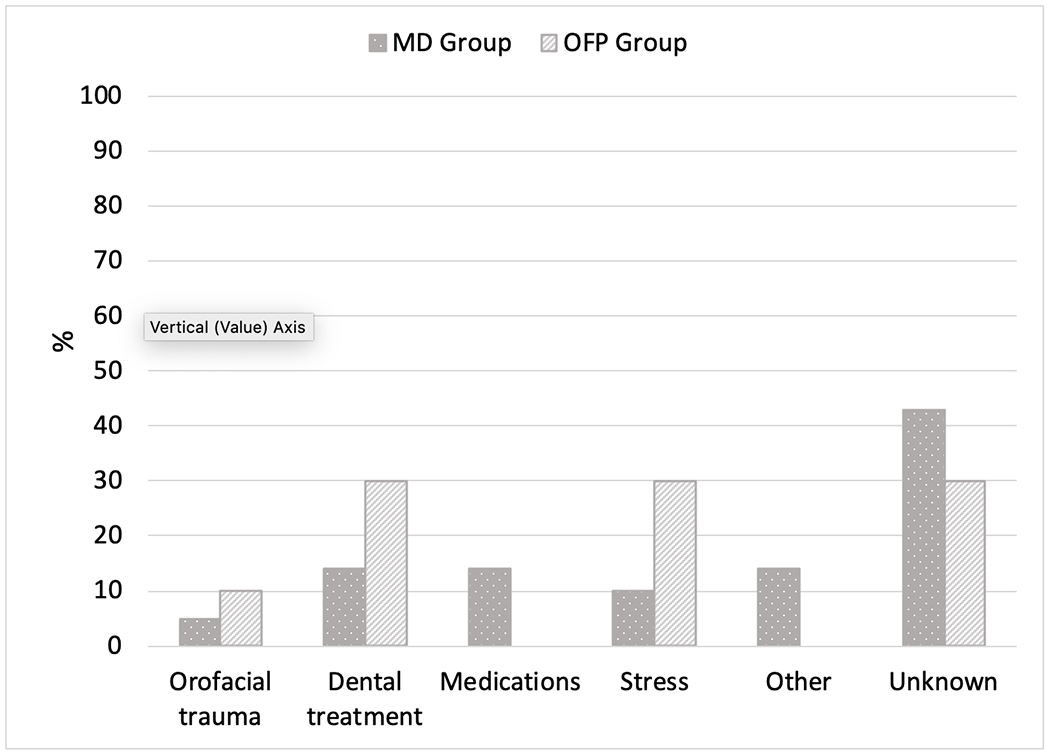
Precipitating factors for involuntary movements
Table II. Symptom description before receiving treatment.
(including OMD features and TMD Screener at the onset of involuntary movements)
| Item | N | MD Group (N=21) |
OFP group (N=10) |
P-value |
|---|---|---|---|---|
| Duration of involuntary movements noted * | ||||
| Months | 31 | 176 | 97 | 0.330 |
| Bothered by involuntary movements ^ | ||||
| A lot | 31 | 9 (43%) | 6 (60%) | 0.313 |
| A moderate amount | 8 (38%) | 4 (40%) | ||
| A little | 4 (19%) | 0 | ||
| Not at all | 0 | 0 | ||
| Presence of sensory tricks ^ | ||||
| Yes | 31 | 14 (67%) | 5 (50%) | 0.373 |
| Involuntary movements affected ability to perform daily functions ^ | ||||
| Yes | 31 | 15 (71%) | 8 (80%) | 0.610 |
| Muscle tension associated with involuntary movements ^ | ||||
| Yes | 31 | 15 (71%) | 9 (90%) | 0.248 |
| Jaw pain associated with involuntary movements ^ | ||||
| Yes | 31 | 10 (48%) | 6 (60%) | 0.519 |
Mann-Whitney U test;
Chi-square test
Muscle tension associated with involuntary movements was reported by 71% of participants in the MD group compared to 90% participants in OFP group (p-value 0.248). However, jaw pain associated with involuntary movements was reported by 48% (10 out of 21) of participants in the MD group compared to 60% (6 out of 10) in the OFP group (p-value 0.519) (Table II). These 16 participants who experienced pain with involuntary movements were further asked the six-item TMD screening instrument. In the MD group, 9 of 10 screened positive for TMD as opposed to 5 of 6 in the OFP group (p-value 0.696). Involuntary movements bothered participants more than jaw pain in 7 out of 10 from the MD group and 3 out of 6 in the OFP group. Further, involuntary movements were the chief reason for seeking healthcare in 8 of 10 participants in the MD group and 3 of 6 participants in the OFP group (Table III).
Table III.
Characteristics of patients who reported jaw pain with involuntary movements
| Item | N | MD group (N=10) |
OFP group (N=6) |
P-value |
|---|---|---|---|---|
| TMD Screener before receiving treatment ^ | ||||
| Positive (≥3) | 16 | 9 (90%) | 5 (83%) | 0.696 |
| Negative (<3) | 1(10%) | 1(17%) | ||
| Association between your pain and involuntary movements ^ | ||||
| Pain preceded your involuntary movements | 16 | 1 (10%) | 0 | 0.362 |
| Involuntary movements preceded pain | 3 (30%) | 3 (50%) | ||
| Both started around the same time | 6 (60%) | 2 (33%) | ||
| There was no association between pain and involuntary movements | 0 | 1 (17%) | ||
| What bothered you the most ^ | ||||
| Involuntary movements | 16 | 7 (70%) | 3 (50%) | 0.424 |
| Jaw pain | 3 (30%) | 3 (50%) | ||
| Reasons for seeking health care for the first time ^ | ||||
| Involuntary movements | 16 | 8 (80%) | 3 (50%) | 0.210 |
| Jaw pain | 2 (20%) | 3 (50%) | ||
Chi-square test
Clinical care
The average number of providers seen by participants in the MD group was 8 (range 1-25) compared to 5 (range 3-7) in the OFP group (p-value 0.231). Botulinum toxin injection was the most commonly received treatment in both groups followed by medications in the MD group and mouth guards in the OFP group. Botulinum toxin injections were the most effective treatment per participant self-report (MD: 48%, OFP: 40%, p-value 0.350). Up to 76% of participants in both groups were still receiving treatment under the care of a health provider. Three patients from the MD group and 2 from the OFP group discontinued care due to ineffectiveness of treatment (Table IV). On the Global Rating of Change Scale, 81% from the MD group and 70% from the OFP group reported some level of improvement in involuntary movements (p-value 0.087). Fifty-seven percent from the MD group and 80% from the OFP group reported some level of improvement in their jaw pain (p-value 0.338) (Figures 3 & 4).
Table IV.
Clinical care
| Item | N | MD group (N=21) |
OFP group (N=10) |
p-value |
|---|---|---|---|---|
| Mean number of providers seen in total * | ||||
| 31 | 8 | 5 | 0.231 | |
| Treatments that provided you the most relief with the involuntary movements ^ | ||||
| Medication(s) | 31 | 5 (24%) | 0 | 0.350 |
| Botulinum toxin injection(s) | 10 (48%) | 4 (40%) | ||
| Physical therapy | 0 | 1 (10%) | ||
| Chiropractic care | 0 | 0 | ||
| Self-care or homecare recommendations or behavior modification instructions | 0 | 0 | ||
| Mouth guard/oral appliance | 1 (5%) | 1 (10%) | ||
| Surgery | 2 (10%) | 2 (20%) | ||
| Talk therapy or counseling | 0 | 0 | ||
| Acupuncture | 0 | 0 | ||
| Other | 3 (14%) | 2 (20%) | ||
| Participants receiving treatment under the care of a health provider ^ | ||||
| Yes | 31 | 16 (76%) | 7 (70%) | 0.713 |
| Reasons for discontinuing treatment ^ | ||||
| Complete relief of symptoms | 8 | 1 (20%) | 0 | 0.688 |
| Treatment not effective | 3 (60%) | 2 (67%) | ||
| Side effects from treatment | 0 | 0 | ||
| Financially unaffordable | 0 | 0 | ||
| Other | 1 (20%) | 1 (33%) | ||
Mann-Whitney U test;
Chi-square test
Figure 3:
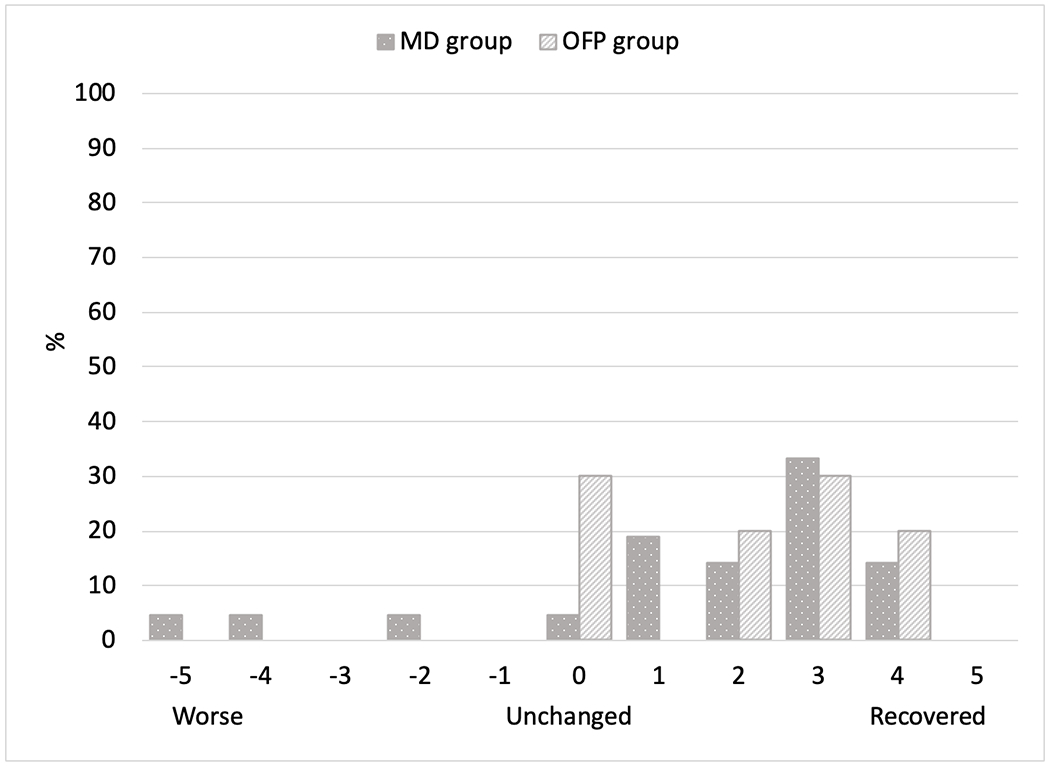
Global Rating of Change Scale for involuntary movements
Figure 4:
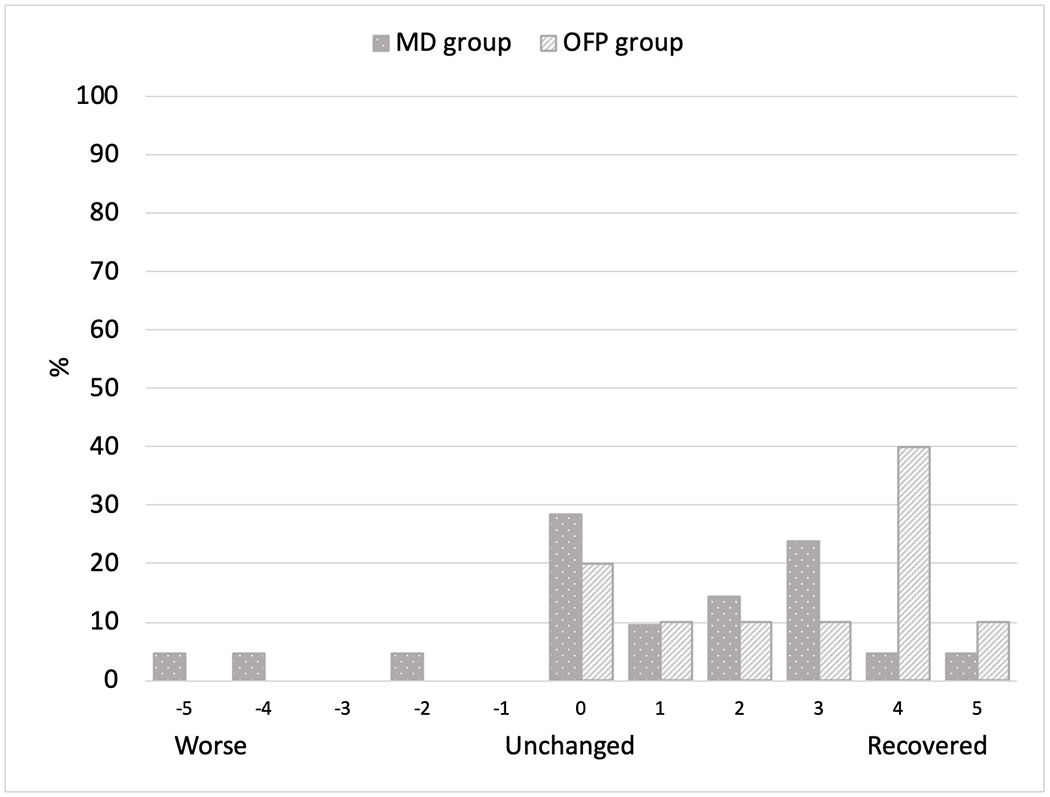
Global Rating of Change Scale for jaw pain
TMD symptoms after receiving treatment (within the last 30 days of responding to the survey)
All of the participants in both groups were requested to fill out the six-item TMD Pain Screener for the symptoms in the last 30 days irrespective of the onset of involuntary movements or pain. Nine participants in the MD group (43%) and 4 participants in the OFP group (40%) screened positive for TMD (p-value 0.880). There was no difference in the average pain rating for jaw or temple pain for participants in both groups (p-value 0.592). The number of days participants noticed jaw or temple pain in the last 30 days averaged 13 days in the MD group compared to 2 days in the OFP group (p-value 0.185). Global score of JFLS was 1.8 in the MD group and 1.6 in the OFP group; the difference was not statistically significant (Table V).
Table V:
Symptoms after receiving treatment within the last 30 days of survey response
| Items | N | MD group (N=21) |
OFP group (N=10) |
p-value |
|---|---|---|---|---|
| TMD screener after receiving treatment for all participants ^ | ||||
| Positive (≥3) | 31 | 9 (43%) | 4 (40%) | 0.880 |
| Negative (<3) | 31 | 12 (57%) | 6 (60%) | |
| Average rating of jaw or temple pain * | ||||
| Mean (SD) | 31 | 25 (27) | 20 (22) | 0.845 |
| Mean number of days with jaw or temple pain * | ||||
| Mean (SD) | 31 | 12.5 days (13.9) | 1.5 days (1.8) | 0.185 |
| Global score of Jaw Functional Limitation Scale * | ||||
| N | 27 | 19 | 8 | 0.811 |
| Mean (SD) | 1.839 (1.61) | 1.594 (1.599) | ||
Mann-Whitney U test;
Chi-square test
DISCUSSION
To our knowledge, this is the first study comparing clinical characteristics and treatment outcomes of OMD patients in two different clinic settings. In this study, we developed a sampling strategy to obtain patients that are most likely to be similar in characteristics by choosing to recruit from referral-based academic settings, one medical (MD clinic) and the other dental (OFP clinic), that are co-located in a major metropolitan area two and a half blocks from each other. Given the similarities in demographic and clinical features, we feel the sampling strategy met this goal.
Both groups had small sample sizes with comparable baseline and clinical characteristics. Demographic features like age and female prevalence noted in our study were similar to other published studies (1, 7). It was interesting to note that all the participants who responded to our survey were Caucasians. It is unclear from the literature whether OMD is more prevalent in Caucasians (24), this is due to the predominant local population with access to healthcare, or it is just a non-response bias.
Most of the studies measuring Quality of Life (QoL) in dystonia patients are based on cervical dystonia with very few studies on OMD patients (12, 14, 25–27). These OMD studies have variably used Glasgow Benefit Inventory (GBI), oromandibular dystonia questionnaire-25 (OMDQ-25) or communication-related quality of life (CR-QoL) to measure quality of life after botulinum toxin treatment. We used generic health related Quality of Life measure [EuroQol (EQ)-5D-5L] as it also takes non-motor components (pain, anxiety, depression) of OMD into consideration. Further, it represents OMD participants’ health at the time of completion of the survey, making it less susceptible to recall bias. Based on EQ-5D-5L index value, OMD patients from both clinics did not reveal any significant difference in quality of life. The pooled mean EQ-5D-5L of 0.810 (SD 0.19) suggests that these patients have lower health quality than population norms of the United States (28). This amount of burden experienced by patients with OMD is comparable to the disease burden of people with osteoporosis or dry eye disease, while less burdensome than heart failure, stroke, poorly controlled asthma, and pulmonary embolism (29–34).
TMD-Pain Screener has 99% sensitivity and 97% specificity in detecting painful TMD versus healthy controls (17). TMD Pain Screener has been used by various studies to report prevalence of painful TMD which varies from 16-22% (35–37). Our study showed that 48% of OMD patients from the MD clinic and 60% from the OFP clinic reported jaw pain along with involuntary movements. In these patients with jaw pain, about 90% had a positive TMD Pain Screener. Our findings parallel with Costa et al. (8) in which painful TMD was reported in 85% of OMD patients. This suggests the higher prevalence of TMD in OMD patients when compared to the prevalence in non-OMD patients. Further, our previous work on this topic revealed that myofascial pain of masticatory muscles, a form of TMD, was noted in all the OMD patients (9). It suggests that the presence of TMD related characteristics is simply not a referral bias and the positive TMD Pain Screener could be due to involvement of the masticatory system with dystonic muscle contractions. Further, we tried to assess the temporal relationship of involuntary movements and jaw pain. Of the 16 participants in both groups who reported pain, 50% reported onset of pain and involuntary movements around the same time, while 37% noted that involuntary movements preceded the pain. It is important to note that this study is unable to determine a cause or effect relationship between of involuntary movements and jaw pain in OMD patients; therefore, this relationship remains unclear.
Botulinum toxin was observed to be the treatment of choice in OMD patients and reported to be most beneficial by both groups of patients, which is consistent with published evidence (12, 38). Clinical care in both clinics differed in terms of the second-line treatment provided to OMD patients, i.e. oral medications (e.g. baclofen, deutetrabenazine, clonazepam) in the MD clinic vs. oral appliance use in the OFP clinic. Twenty-four percent (5/21) of participants from the MD group said medications provided the most relief, which is similar to that reported in literature (39). Several case reports have been published in reducing dystonic movements with oral appliances (40–42) and 1 participant from each group (i.e. a total of 2 patients) reported oral appliance was the most effective treatment for involuntary movements. It remains questionable if medications or oral appliance is the choice of treatment in patients when Botox is contraindicated or not found to be effective.
On the Global Rating of Change Scale, OMD patients in the MD clinic noticed more improvement in their involuntary movements than jaw pain. Even after treatment, the TMD Pain Screener was positive in about 40% patients in both clinics in the last 30 days questionnaire responses. Although the average jaw or temple pain was about the same in OMD patients from both clinics on graded chronic pain scale, the number of days with pain was greater in the MD group compared to the OFP group. This suggests that treatment of TMD symptoms in addition to involuntary movements could lead to better patient outcomes.
The JFLS is a predictor of persistent TMD pain (43) and was found to be significantly higher in TMD cases than pain-free controls (44). Even though norms have not yet been established for interpretation of the JFLS, the global score in both groups is comparable to the TMD cases in the OPPERA case-control study. In another study by Bakke et al., OMD participants revealed problems chewing, talking, and singing, showing significant correlation of JFLS with impact of OMD as measured by Visual Analogue Scale (45). These findings suggest that instead of considering TMD as a differential diagnosis of OMD (46, 47), we may need to think of TMD as a co-existing feature along with involuntary movements in patients with OMD.
Our study also highlights the feasibility of online platforms to conduct surveys. Our survey response rate was 58%, higher than the 20% reported for an incentivized, lengthy, and web-based population survey (48). This could be attributed to the additional reminders, monetary incentive upon completion of the survey, personally addressed recruitment letters, and easy access to this survey on handheld electronic devices after scanning the QR code or typing the short URL code into a browser (49).
Our study findings need to be interpreted considering some of the limitations. Probably the most important limitation is smaller sample size that limits our ability to infer comparisons between two groups. This was attempted to be addressed with wider timeframe for subject inclusion, repeated survey reminders and incentives for participation. Another limitation is assessing clinical outcomes based upon patient’s responses rather than objective scales. However, given the nature of problems being studied in clinic, objective evaluation is limited. Further, not all the patients in our study are actively following in the clinic. To overcome these, we have used a unique approach of electronic survey format where visual aid of scales would mimic those that would be given in clinic if patient was present. In addition, wherever possible, validated questionnaire was used on the subject matter of interest. Finally, electronic survey format may have selectively eliminated the subjects who are not technically savvy and hesitant to participate. In spite of these limitations, the standardization of the questions, use of validated questionnaires wherever possible and similar clinical settings for both the groups provided snapshot of real-world experience on this interesting topic.
CONCLUSION
Within the consideration of limitations, our study demonstrates that OMD patients presenting in two clinical settings do not seem to be different from each other in their clinical characteristics, quality of life and treatment outcomes by patient report. OMD patients from both clinics screened positive for TMD and reported higher scores on JFLS, suggesting TMD symptoms could be a coexisting feature in patients with OMD.
ACKNOWLEDGMENT AND FUNDING
This research was supported by the National Institutes of Health’s National Center for Advancing Translational Sciences, grant UL1TR002494. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health’s National Center for Advancing Translational Sciences.
List of Abbreviations
- OMD
Oromandibular dystonia
- TMD
Temporomandibular disorder
- MD
Movement disorder
- OFP
Orofacial pain
- DC/TMD
Diagnostic Criteria for Temporomandibular disorders
- JFLS
Jaw Functional Limitation Scale
- QoL
Quality of Life
- EQ-5D-5L
EuroQol 5-Dimensions 5-Levels
- GBI
Glasgow Benefit Inventory
- OMDQ-25
Oromandibular dystonia questionnaire-25
- CR-QoL
Communication-Related Quality of Life
- JASP
Jeffrey’s Amazing Statistics Program
Footnotes
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
DATA AVAILABILITY STATEMENT
The data presented in this study is contained within the article
Bibliography
- 1.Nutt JG, Muenter MD, Aronson A, Kurland LT, Melton LJ 3rd. Epidemiology of focal and generalized dystonia in Rochester, Minnesota. Mov Disord. 1988;3(3):188–94. [DOI] [PubMed] [Google Scholar]
- 2.Joensen P High prevalence of primary focal dystonia in the Faroe Islands. Acta Neurol Scand. 2016. January;133(1):55–60. [DOI] [PubMed] [Google Scholar]
- 3.Asgeirsson H, Jakobsson F, Hjaltason H, Jonsdottir H, Sveinbjornsdottir S. Prevalence study of primary dystonia in Iceland. Mov Disord. 2006. March;21(3):293–8. [DOI] [PubMed] [Google Scholar]
- 4.Balasubramaniam R, Ram S. Orofacial movement disorders. Oral Maxillofac Surg Clin North Am. 2008. May;20(2):273–85, vii. [DOI] [PubMed] [Google Scholar]
- 5.Blanchet PJ, Rompre PH, Lavigne GJ, Lamarche C. Oral dyskinesia: a clinical overview. Int J Prosthodont. 2005. Jan-Feb;18(1):10–9. [PubMed] [Google Scholar]
- 6.Raoofi S, Khorshidi H, Najafi M. Etiology, Diagnosis and Management of Oromandibular Dystonia: an Update for Stomatologists. J Dent (Shiraz). 2017. June;18(2):73–81. [PMC free article] [PubMed] [Google Scholar]
- 7.Slaim L, Cohen M, Klap P, Vidailhet M, Perrin A, Brasnu D, et al. Oromandibular Dystonia: Demographics and Clinical Data from 240 Patients. J Mov Disord. 2018. May;11(2):78–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Costa AL, Campos LS, Franca MC Jr., D’Abreu A. Temporomandibular disorders in patients with craniocervical dystonia. Arq Neuropsiquiatr. 2011. December;69(6):896–9. [DOI] [PubMed] [Google Scholar]
- 9.Sude A, Nixodrf DR. Prevalence and Clinical Characteristics of Oromandibular Dystonia Patients in Orofacial Pain Clinic: A Retrospective Study. Oral Surg Oral Med Oral Pathol Oral Radiol. 2020. [DOI] [PubMed] [Google Scholar]
- 10.Sankhla C, Lai EC, Jankovic J. Peripherally induced oromandibular dystonia. J Neurol Neurosurg Psychiatry. 1998. November;65(5):722–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Page AD, Siegel L. Perspectives on the Psychosocial Management of Oromandibular Dystonia. Semin Speech Lang. 2017. July;38(3):173–83. [DOI] [PubMed] [Google Scholar]
- 12.Bhattacharyya N, Tarsy D. Impact on quality of life of botulinum toxin treatments for spasmodic dysphonia and oromandibular dystonia. Arch Otolaryngol Head Neck Surg. 2001. April;127(4):389–92. [DOI] [PubMed] [Google Scholar]
- 13.Charous SJ, Comella CL, Fan W. Jaw-opening dystonia: Quality of life after botulinum toxin injections. Ear Nose Throat J. 2011. February;90(2):E9. [DOI] [PubMed] [Google Scholar]
- 14.Merz RI, Deakin J, Hawthorne MR. Oromandibular dystonia questionnaire (OMDQ-25): a valid and reliable instrument for measuring health-related quality of life. Clin Otolaryngol. 2010. October;35(5):390–6. [DOI] [PubMed] [Google Scholar]
- 15.Jinnah HA, Factor SA. Diagnosis and treatment of dystonia. Neurol Clin. 2015. February;33(1):77–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Batla A, Stamelou M, Bhatia KP. Treatment of focal dystonia. Curr Treat Options Neurol. 2012. June;14(3):213–29. [DOI] [PubMed] [Google Scholar]
- 17.Gonzalez YM, Schiffman E, Gordon SM, Seago B, Truelove EL, Slade G, et al. Development of a brief and effective temporomandibular disorder pain screening questionnaire: reliability and validity. J Am Dent Assoc. 2011. October;142(10):1183–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Von Korff M, Ormel J, Keefe FJ, Dworkin SF. Grading the severity of chronic pain. Pain. 1992. August;50(2):133–49. [DOI] [PubMed] [Google Scholar]
- 19.Dworkin SF, Sherman J, Mancl L, Ohrbach R, LeResche L, Truelove E. Reliability, validity, and clinical utility of the research diagnostic criteria for Temporomandibular Disorders Axis II Scales: depression, non-specific physical symptoms, and graded chronic pain. J Orofac Pain. 2002;16(3):207–20. [PubMed] [Google Scholar]
- 20.Ohrbach R, Larsson P, List T. The jaw functional limitation scale: development, reliability, and validity of 8-item and 20-item versions. J Orofac Pain. 2008. Summer;22(3):219–30. [PubMed] [Google Scholar]
- 21.EuroQol Research Foundation. EQ-5D-5L User Guide. [Internet] 2019; Available from: https://euroqol.org/publications/user-guides.
- 22.Pickard AS, Law EH, Jiang R, Pullenayegum E, Shaw JW, Xie F, et al. United States Valuation of EQ-5D-5L Health States Using an International Protocol. Value Health. 2019. August;22(8):931–41. [DOI] [PubMed] [Google Scholar]
- 23.Kamper SJ, Maher CG, Mackay G. Global rating of change scales: a review of strengths and weaknesses and considerations for design. J Man Manip Ther. 2009;17(3):163–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gonzalez-Alegre P, Schneider RL, Hoffman H. Clinical, Etiological, and Therapeutic Features of Jaw-opening and Jaw-closing Oromandibular Dystonias: A Decade of Experience at a Single Treatment Center. Tremor Other Hyperkinet Mov (N Y). 2014;4:231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weiss D, Hieber L, Sturm J, Bortlein A, Mayr I, Appy M, et al. Botulinumtoxin Improves both Generic and Disease-Specific Quality of Life in Cervical Dystonia. Front Neurol. 2017;8:561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Page AD, Siegel L, Jog M. Self-Rated Communication-Related Quality of Life of Individuals With Oromandibular Dystonia Receiving Botulinum Toxin Injections. Am J Speech Lang Pathol. 2017. June 22;26(2S):674–81. [DOI] [PubMed] [Google Scholar]
- 27.Girach A, Vinagre Aragon A, Zis P. Quality of life in idiopathic dystonia: a systematic review. J Neurol. 2019. December;266(12):2897–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Janssen B, Szende A. Population Norms for the EQ-5D. In: Szende A, Janssen B, Cabases J, editors. Self-Reported Population Health: An International Perspective based on EQ-5D. Dordrecht (NL)2014. p. 19–30. [Google Scholar]
- 29.Gold T, Williams SA, Weiss RJ, Wang Y, Watkins C, Carroll J, et al. Impact of fractures on quality of life in patients with osteoporosis: a US cross-sectional survey. J Drug Assess. 2019;8(1):175–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dana R, Meunier J, Markowitz JT, Joseph C, Siffel C. Patient-Reported Burden of Dry Eye Disease in the United States: Results of an Online Cross-Sectional Survey. Am J Ophthalmol. 2020. April 8. [DOI] [PubMed] [Google Scholar]
- 31.Boczor S, Daubmann A, Eisele M, Blozik E, Scherer M. Quality of life assessment in patients with heart failure: validity of the German version of the generic EQ-5D-5L. BMC Public Health. 2019. November 6;19(1):1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Golicki D, Niewada M, Karlinska A, Buczek J, Kobayashi A, Janssen MF, et al. Comparing responsiveness of the EQ-5D-5L, EQ-5D-3L and EQ VAS in stroke patients. Qual Life Res. 2015. June;24(6):1555–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee LK, Ramakrishnan K, Safioti G, Ariely R, Schatz M. Asthma control is associated with economic outcomes, work productivity and health-related quality of life in patients with asthma. BMJ Open Respir Res. 2020. March;7(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chuang LH, Gumbs P, van Hout B, Agnelli G, Kroep S, Monreal M, et al. Health-related quality of life and mortality in patients with pulmonary embolism: a prospective cohort study in seven European countries. Qual Life Res. 2019. August;28(8):2111–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ortiz-Culca F, Cisneros-Del Aguila M, Vasquez-Segura M, Gonzales-Vilchez R. Implementation of TMD pain screening questionnaire in peruvian dental students. Acta Odontol Latinoam. 2019. August 1;32(2):65–70. [PubMed] [Google Scholar]
- 36.Jivnani HM, Tripathi S, Shanker R, Singh BP, Agrawal KK, Singhal R. A Study to Determine the Prevalence of Temporomandibular Disorders in a Young Adult Population and its Association with Psychological and Functional Occlusal Parameters. J Prosthodont. 2019. January;28(1):e445–e9. [DOI] [PubMed] [Google Scholar]
- 37.Chuinsiri N, Jitprasertwong P. Prevalence of self-reported pain-related temporomandibular disorders and association with psychological distress in a dental clinic setting. J Int Med Res. 2020. September;48(9):300060520951744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sinclair CF, Gurey LE, Blitzer A. Oromandibular dystonia: long-term management with botulinum toxin. Laryngoscope. 2013. December;123(12):3078–83. [DOI] [PubMed] [Google Scholar]
- 39.Teemul TA, Patel R, Kanatas A, Carter LM. Management of oromandibular dystonia with botulinum A toxin: a series of cases. Br J Oral Maxillofac Surg. 2016. December;54(10):1080–4. [DOI] [PubMed] [Google Scholar]
- 40.Schneider R, Hoffman HT. Oromandibular dystonia: a clinical report. J Prosthet Dent. 2011. December;106(6):355–8. [DOI] [PubMed] [Google Scholar]
- 41.Khan J, Anwer HM, Eliav E, Heir G. Oromandibular dystonia: differential diagnosis and management. J Am Dent Assoc. 2015. September;146(9):690–3. [DOI] [PubMed] [Google Scholar]
- 42.Watt E, Sangani I, Crawford F, Gillgrass T. The role of a dentist in managing patients with dystonia. Dent Update. 2013. December;40(10):846–8. [DOI] [PubMed] [Google Scholar]
- 43.Kapos FP, Look JO, Zhang L, Hodges JS, Schiffman EL. Predictors of Long-Term Temporomandibular Disorder Pain Intensity: An 8-Year Cohort Study. J Oral Facial Pain Headache. 2018. Spring;32(2):113–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ohrbach R, Fillingim RB, Mulkey F, Gonzalez Y, Gordon S, Gremillion H, et al. Clinical findings and pain symptoms as potential risk factors for chronic TMD: descriptive data and empirically identified domains from the OPPERA case-control study. J Pain. 2011. November;12(11 Suppl):T27–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bakke M, Baram S, Dalager T, Biernat HB, Moller E. Oromandibular dystonia, mental distress and oro-facial dysfunction-A follow-up 8-10 years after start of treatment with botulinum toxin. J Oral Rehabil. 2019. May;46(5):441–9. [DOI] [PubMed] [Google Scholar]
- 46.Jankovic J. Etiology and differential diagnosis of blepharospasm and oromandibular dystonia. Adv Neurol. 1988;49:103–16. [PubMed] [Google Scholar]
- 47.Evatt ML, Freeman A, Factor S. Adult-onset dystonia. Handb Clin Neurol. 2011;100:481–511. [DOI] [PubMed] [Google Scholar]
- 48.Guo Y, Kopec JA, Cibere J, Li LC, Goldsmith CH. Population Survey Features and Response Rates: A Randomized Experiment. Am J Public Health. 2016. August;106(8):1422–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Phillips AW, Reddy S, Durning SJ. Improving response rates and evaluating nonresponse bias in surveys: AMEE Guide No. 102. Med Teach. 2016;38(3):217–28. [DOI] [PubMed] [Google Scholar]


