Abstract
Dipeptidyl peptidase IV (DPP-IV) inhibitors improve glycemic control by prolonging the action of glucagon-like peptide-1 (GLP-1). In contrast to GLP-1 analogues, DPP-IV inhibitors are weight-neutral. DPP-IV cleavage of PYY and NPY gives rise to PYY3-36 and NPY3-36 which exert potent anorectic action by stimulating Y2 receptor (Y2R) function. This invites the possibility that DPP-IV inhibitors could be weight-neutral by preventing conversion of PYY/NPY to Y2R-selective peptide agonists. We therefore investigated whether co-administration of an Y2R-selective agonist could unmask potential weight lowering effects of the DDP-IV inhibitor linagliptin. Male diet-induced obese (DIO) mice received once daily subcutaneous treatment with linagliptin (3 mg/kg), a Y2R-selective PYY3-36 analogue (3 or 30 nmol/kg) or combination therapy for 14 days. While linagliptin promoted marginal weight loss without influencing food intake, the PYY3-36 analogue induced significant weight loss and transient suppression of food intake. Both compounds significantly improved oral glucose tolerance. Because combination treatment did not further improve weight loss and glucose tolerance in DIO mice, this suggests that potential negative modulatory effects of DPP-IV inhibitors on endogenous Y2R peptide agonist activity is likely insufficient to influence weight homeostasis. Weight-neutrality of DPP-IV inhibitors may therefore not be explained by counter-regulatory effects on PYY/NPY responses.
Subject terms: Pharmacology, Endocrine system and metabolic diseases
Introduction
Defective pancreatic β-cell function is a key pathological hallmark in type 2 diabetes (T2D). Because progressive loss of β-cell insulin secretory capacity is associated with worsening hyperglycemia, targeting intrinsic β-cell activity is considered essential in T2D management. A canonical role of the gut-derived incretin hormone glucagon-like peptide-1 (GLP-1) is potentiation of glucose-stimulated insulin secretion by activation of GLP-1 receptors in islet β-cells. The incretin effect is estimated to account for approximately 50–70% of total insulin secreted following oral glucose administration1. Diminished incretin effect is an early characteristic of T2D2. In support of the important role of GLP-1 in glucose homeostasis, supraphysiological doses of native GLP-1 can normalize glucose levels and improve insulin sensitivity in T2D patients3–5. In addition to glucoregulatory effects, GLP-1 have pleiotropic CNS effects and promotes weight loss by suppressing appetite function 6. Active GLP-1 (GLP-17–36) is susceptible to rapid inactivation by the ubiquitous enzyme dipeptidyl peptidase IV (DPP-IV)7. Consequently, GLP-1 is effectively degraded upon secretion from enteroendocrine cells and when released into the circulation, resulting in GLP-17–36 having a circulatory half-life of approximately 1 min7,8.
While the extremely short half-life prevents the therapeutic use of GLP-17–36, incretin-based therapies have established a foothold in T2D management through the introduction of oral DPP-IV inhibitors and injectable long-acting GLP-1 receptor agonists 9. Several DPP-IV inhibitors (‘gliptins’) have been approved for T2D management10. The classical therapeutic mechanism for DPP-IV inhibitors is prevention of GLP-1 degradation which promotes two to threefold increases in post-prandial plasma GLP-17–36 levels, leading to improvement of glucose homeostasis due to combined insulinotropic effect and suppression of glucagon secretion11. Local inhibitory effects on intestinal GLP-1 degradation may potentially contribute to the glycemic effects of DPP-IV inhibitors12. Another incretin, glucose-dependent insulinotropic polypeptide (GIP), is also a substrate for DPP-IV. While the insulinotropic effect of GLP-1 in T2D patients is relatively more preserved compared to GIP4,13,14, several lines of evidence suggests that prevention of GIP degradation may potentially contribute to the antihyperglycemic effects of DPP-IV inhibitors. For example, combined GLP-1 and GIP receptor deficiency is necessary to fully eliminate the glucose-lowering action of DPP-IV inhibition in mice15, β-cell responsiveness to both GLP-17–36 and GIP is increased in T2D patients with improved glycemic control16, and DPP-IV inhibition can partially restore the insulinotropic effect of GIP in T2D patients17.
Unlike long-acting GLP-1 receptor agonists, DPP-IV inhibitors do not promote weight loss18. Weight neutrality of DPP-IV inhibitors has been explained by increases in circulating GLP-1 concentrations being insufficient to facilitate centrally mediated satiety responses. However, other regulatory peptides involved in energy and weight homeostasis qualify to be DPP-IV substrates19. NPY-family peptides such as peptide YY (PYY, i.e. PYY1–36) and neuropeptide Y (NPY, i.e. NPY1–36) are of particular interest because DPP-IV catalytic rates for these peptides are higher than those for GLP-17–36 and GIP20. While NPY is expressed at all levels of the gut-brain axis, PYY is almost exclusively expressed in enteroendocrine cells and co-secreted with GLP-1 in response to nutrient ingestion21,22. In contrast to loss of insulinotropic GLP-1 and GIP-1 activity, DPP-IV induced degradation of PYY and NPY changes the receptor affinity and biological action of these peptides. Full-length NPY and PYY bind to Y receptors with little subtype selectivity. While Y1 receptors (Y1R) play a dominant role in PYY1–36 and NPY1–36 induced feeding upon central administration23–25, cleavage by DPP-IV gives rise to NPY3–36 and PYY3–36 which are high-affinity agonists for the Y2 receptor (Y2R) that mediate appetite suppression and weight loss26,27. It has therefore been proposed that DPP-IV inhibitors are weight-neutral as the appetite-suppressive effects of enhanced GLP-1 activity may potentially be counterbalanced by accumulation of intact PYY/NPY resulting in attenuation of anorectic Y2R signaling11.
To test this hypothesis, the present study aimed to determine whether combined pharmacological DPP-IV inhibition and Y2R stimulation would unmask potential weight loss promoting effects of the DPP-IV inhibitor. To this end, we administered linagliptin (Tradjenta) together with a Y2R-selective PYY3–36 analogue in diet-induced obese (DIO) mice and characterized effects on food intake, body weight and glucose homeostasis.
Materials and methods
Animals
The Danish Animal Experiments Inspectorate approved all experiments which were conducted using internationally accepted principles for the use of laboratory animals (license #2013-15-2934-00784). The study was carried out in compliance with the ARRIVE guidelines. 99 C57BL/6J mice were from Janvier Labs (Le Genest Saint Isle, France) and housed in a controlled environment (12 h light/dark cycle, lights on at 3 AM, 21 ± 2 °C, humidity 50 ± 10%). Each animal was identified by an implantable subcutaneous microchip (PetID Microchip, E-vet, Haderslev, Denmark). Mice arrived at 5 weeks of age and were made diet-induced obese (DIO) by offering ad libitum access to tap water and high-fat diet (5.15 kcal/g; 60 kcal-% fat, 20 kcal-% carbohydrate, 20% kcal-% protein; D12492, Ssniff, Soest, Germany) for 26–27 weeks before study start. The mice were kept on the diet throughout the study.
Drug treatment
DIO mice were randomized into individual treatment groups (n = 9–10 per group) based on body weight measured 3 days prior to initiation of drug treatment. Vehicle was phosphate-buffered saline added 5% mannitol. In study 1, linagliptin monotreatment (3 mg/kg/day, s.c.) was characterized over a dosing period of 14 days. The applied linagliptin dose and treatment time is within the ranges reported to obtain robust DPP-IV inhibition and significant glycemic effects in DIO rodent models28–30. In study 2, linagliptin (3 mg/kg/day, s.c.) was administered alone or in combination with an Y2R-selective PYY3–36 analogue (compound 38; 3 or 30 nmol/kg/day, s.c.)31 for 14 days followed by 6 days of wash-out (no treatment). All drugs were administered once daily at 1 h before lights off. The first dose was given on day 0. Body weight and food intake was monitored daily during the treatment period.
Oral glucose tolerance test
In study 2, an oral glucose tolerance test (OGTT) was performed one week prior to treatment start (day -7) and on treatment day 12. Animals were fasted for 4 h prior to OGTT. At t = 0 an oral glucose load (2 g/kg glucose; 200 mg/mL, Fresenius Kabi, Sweden) was administered via a gastrically placed tube. Blood samples for measuring blood glucose were collected from the tail vein at t = 0, 15, 30, 60, 120, 180, 240 min. A pre-OGTT blood sample was collected at − 60 min (OGTT on day − 7) and − 240 min (OGTT on treatment day 12). Glucose area under the curve (AUC) calculations were determined as total AUC from the sampling period of 0 to 240 min.
Blood biochemistry
Terminal cardiac blood samples were collected in EDTA-coated tubes with (for GLP-1 and GIP analysis) or without (for DPP-IV enzyme analysis) 10 µl DPP-IV inhibitor/ml blood (Millipore, Copenhagen, Denmark) prior to sampling. Plasma was separated by centrifugation (3000 × g for 10 min at 4 °C) and immediately frozen on dry ice and stored at − 80 °C until further analysis. DPP-IV enzyme catalytic activity was determined as reported previously 32. Active GLP-1 and GIP levels were assessed by ELISA (#K150JWC, MesoScale Discovery, Rockville, MD; #27702, IBL, Fujioka, Japan) according to the manufacturer’s instructions. Plasma levels of insulin were measured by ELISA (#K152BZC, MesoScale Discovery, Rockville, MD), according to the manufacturer’s instructions.
Linagliptin exposure
Linagliptin concentrations in heparinized plasma samples were determined by LC–MS/MS analysis as described previously33. Linagliptin exposure was determined in plasma samples before (pre-dosing, i.e. approximately 24 after the previous dose, study 1) and after administration of the last dose (4 h post-dosing) on treatment day 14 (study 1 and 2).
In vitro assessment of linagliptin effect on NPY and PYY degradation
Human plasma was used as source of endogenous DPP-IV. Healthy adult individuals were enrolled after they provided written informed consent. The local Ethics Committee at the Hannover Medical School approved the protocol. All methods were carried out by observing the applicable legal provisions, including data protection regulations, as well as the principles of medical and professional ethics as laid down in the Declaration of Helsinki issued by the World Medical Association and the ICH/EU recommendations Note for Guidance on Good Clinical Practice (GCP recommendations). Blood samples were collected from the cubital vein into blood collection tubes (EDTA-plasma). Immediately after withdrawal, plasma was separated from cells by a two-step centrifugation procedure (10 min at 2000 × g followed by 15 min at 2500 × g, both centrifugation steps at room temperature). Plasma aliquots were transferred into 2 ml vials and stored − 80 °C. 30 pmol of human PYY1–36 or NPY1–36 was incubated in 85 µl PBS, and reaction was started by adding 5 µl plasma with or without 1 µM linagliptin and incubated for 8 h (NPY) or 18 h (PYY) at 37 °C. Parent NPY, PYY and corresponding fragments were determined by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF–MS) as described previously34.
In vitro assessment of PYY3–36 analogue effect on NPY and PYY degradation
NPY and PYY were incubated in human EDTA-plasma in presence or absence of linagliptin and/or Y2R-selective PYY3–36 analogue and subsequently subjected to MALDI-TOF–MS analysis to determine the rate of N-terminal cleavage by DPP-4. PYY (30 pmol) and NPY (30 pmol) were solubilised in PBS and added to 5 µl human plasma with or without linagliptin (1 µM) and/or Y2R-selective PYY3–36 analogue at different concentrations (30–30,000 fmol). The plasma was then incubated for 18 h at 37 °C. Subsequently the reaction was stopped, prepared via solid-phase extraction and subjected to mass spectrometry. After measurement, signal intensities were exported and conversion rates for NPY and PYY were expressed as product/(product + substrate). Statistical significance against controls was tested using Welch’s t-test. Each incubation was carried out in duplicate and measured in duplicate at two different concentrations (n = 8). Data were pooled from three individual experiments.
Statistics
Data were subjected to relevant statistical analyses using GraphPad v8.4 software (GraphPad, La Jolla, CA). Results are presented as mean ± S.E.M. (standard error of the mean). Statistical evaluation of the data was carried out using an unpaired t-test (plasma analytes in study 1, in vitro data), paired t-test (plasma linagliptin), two-way analysis of variance (ANOVA) with Dunnett’s post-hoc test (body weight, food intake, OGTT), or Kruskal–Wallis test (plasma analytes in study 2). A p-value less than 0.05 was considered statistically significant.
Results
Linagliptin promotes marginal weight loss while significantly increasing circulating active GLP-1 and GIP levels
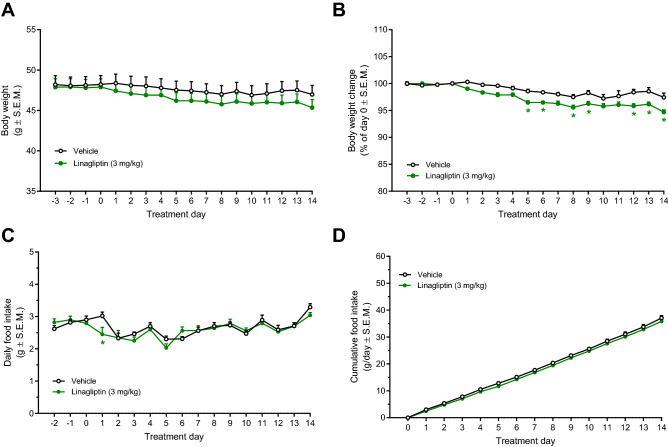
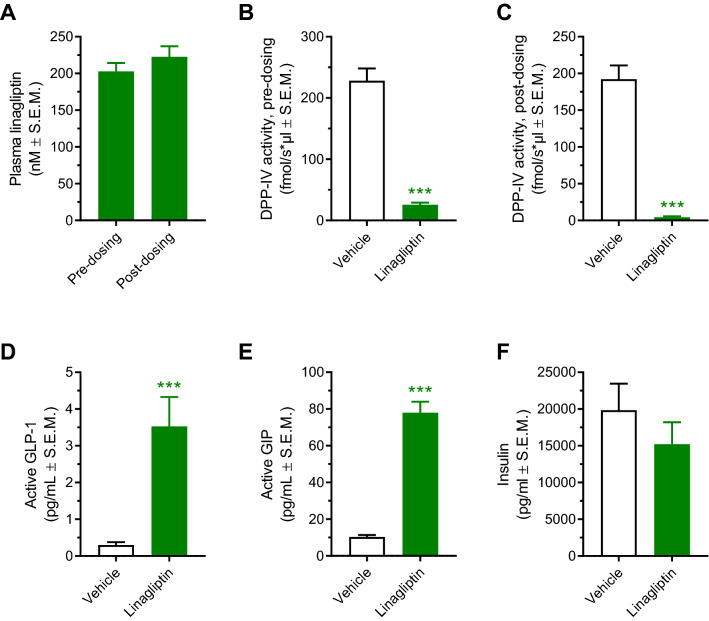
In order to obtain maximal DPP-IV inhibition, DIO mice were administered linagliptin using a dose of 3 mg/kg/day (s.c.). Linagliptin monotreatment was initially characterized for effects on key metabolic parameters in DIO mice. Compared to vehicle controls, linagliptin promoted a marginal weight loss (2.8%, p < 0.05) following 14 days of treatment (Fig. 1A,B). Food intake was essentially unaffected by linagliptin treatment (Fig. 1C,D). Linagliptin treatment once daily resulted in high linagliptin exposure (~ 200 nM), as measured in plasma samples before (approximately 24hrs after the previous dose) and 4 h after administration of the last dose on treatment day 14 (Fig. 2A). At both sampling times, almost complete inhibition of plasma DPP-IV catalytic activity was observed (Fig. 2B,C). Correspondingly, linagliptin treatment significantly elevated plasma levels of active GLP-1 (12-fold increase) and GIP (8-fold increase), as measured 4 h post-dosing (Fig. 2D,E). Terminal plasma insulin levels were unaffected by linagliptin treatment (Fig. 2F). Except for significantly reduced resistin and TNF-α concentrations, other plasma markers, including total PYY and pancreatic polypeptide, were unaffected by linagliptin treatment (Supplementary Fig. 1).
Figure 1.
Linagliptin promotes marginal weight loss without influencing high-fat diet intake in DIO mice. *p < 0.05 vs. vehicle controls (two-way ANOVA with Dunnett’s post-hoc test).
Figure 2.
Once daily linagliptin administration for 14 days results in sustained systemic linagliptin exposure, almost complete inhibition of plasma DPP-IV activity and significantly elevated plasma concentrations of active GLP-1 and GIP in DIO mice. (A) Plasma linagliptin concentrations before (pre-dosing, i.e. approximately 24 h after the previous dose) and 4 h after the last dosing (post-dosing) on treatment day 14, paired t-test (p > 0.05). (B) Plasma DPP-IV activity before the last dose of linagliptin (pre-dosing, i.e. approximately 24 h after the previous dose). (C) Plasma DPP-IV activity determined 4 h after the last dosing (post-dosing). (D) Plasma levels of active GLP-1. (E) Plasma levels of active GIP. (F) Plasma levels of insulin. Plasma GLP-1, GIP and insulin levels were measured determined 4 h after the last dosing. ***p < 0.001 vs. vehicle control (unpaired t-test).
Linagliptin inhibits conversion of NPY and PYY in vitro
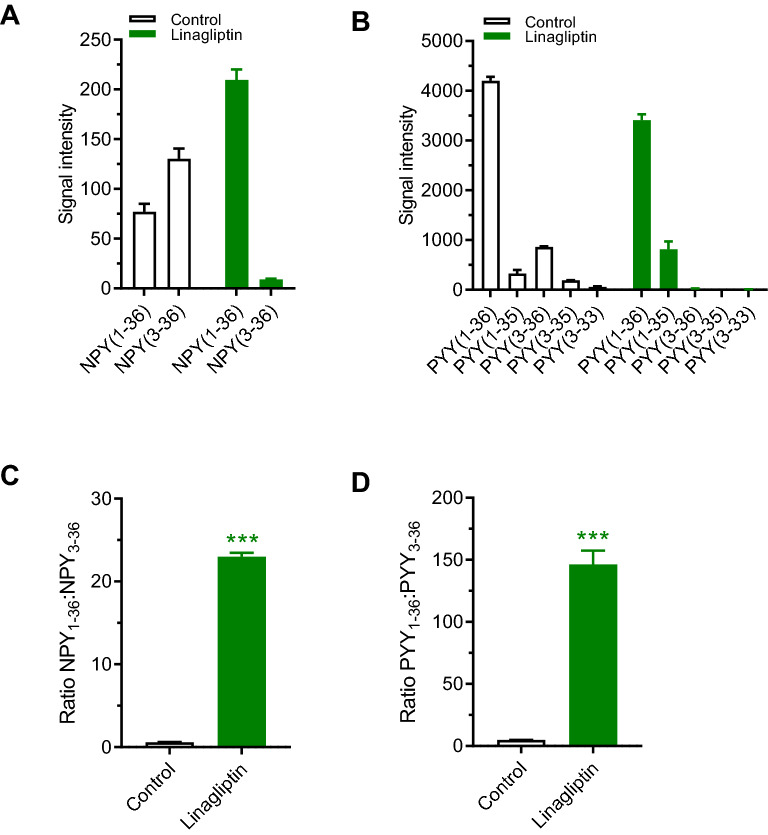
Synthetic NPY1–36 and PYY1–36 was evaluated as DPP-IV substrates in human plasma with or without addition of linagliptin. Degradation of NPY and PYY was relatively slow. After 8 h approximately two-thirds of NPY was processed by DPP-IV. In contrast, 30%-50% of PYY was processed after 18 h of incubation. In control samples, NPY3–36 was the principal NPY fragment (Fig. 3A). Only small quantities of C-terminal processed NPY entities were detectable (data not shown). In contrast, PYY was subjected to both N- and C-terminal cleavage yielding several peptide fragments, with PYY3–36 being the predominant fragment (Fig. 3B). N-terminal processing of PYY and NPY was significantly prevented by linagliptin as demonstrated by accumulation of NPY1–36, PYY1–36 and PYY1-35 (Fig. 3A,B). Compared to control, linagliptin shifted ratios of NPY1–36:NPY3–36 from 0.6 to 23 (Fig. 3C) and PYY1–36:PYY3–36 from 5 to 146 (Fig. 3D).
Figure 3.
Linagliptin prevents conversion of full-length NPY and PYY to NPY3–36 and PYY3–36 in human plasma samples. Synthetic NPY1–36 and PYY1–36 was evaluated as DPP-IV substrates with or without addition of linagliptin [1 µM, incubation times: 8 h (NPY), 18 h (PYY)]. (A) Concentrations of full-length NPY1–36 and NPY3–36. (B) Concentrations of full-length PYY1–36 and PYY3–36. (C) NPY1–36:NPY3–36 ratio, (D) PYY1–36:PYY3–36 ratio. ***p < 0.001 vs. vehicle control.
Linagliptin does not augment Y2R agonist-induced food intake inhibition and weight loss
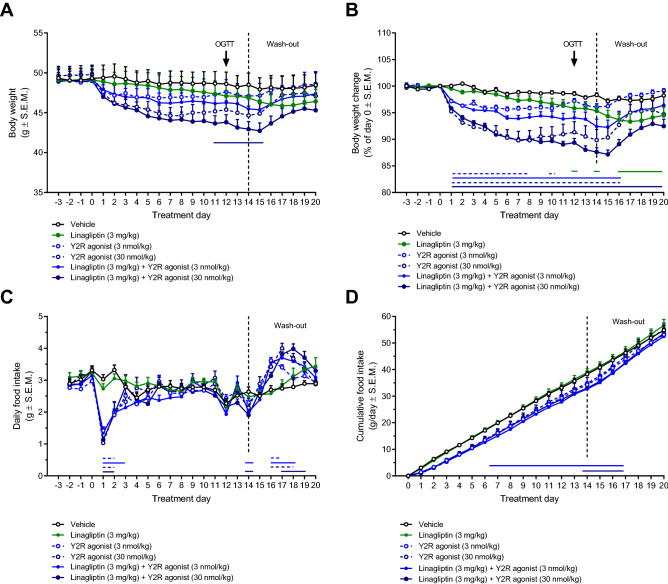
To investigate potential implications of linagliptin-induced bidirectional regulation of GLP-1 and neuropeptide Y-family peptides, we characterized the effect of a Y2R-selective agonist in DIO mice with or without co-administration of linagliptin. Weight curves are depicted in Fig. 4A,B. Compared to baseline, linagliptin monotreatment (3 mg/kg, s.c.) promoted a marginal vehicle-subtracted weight loss (vehicle-subtracted weight loss, − 3.0 ± 1.1%, p = 0.019 vs. vehicle) on the last day of dosing (day 14). Whereas the lowest dose of the PYY analogue (3.0 nmol/kg) showed no statistically significant effect on body weight (vehicle-subtracted weight loss on day 14, − 2.2 ± 0.6%, p = 0.06 vs. vehicle), the highest dose (30 nmol/kg) promoted a robust weight drop (vehicle-subtracted weight loss on day 14, − 8.5 ± 2.0%, p = 0.001, vs. vehicle). Linagliptin + Y2R agonist (3 nmol/kg) co-treatment promoted a slightly greater weight loss than corresponding Y2R agonist monotreatment (vehicle-subtracted weight loss on day 14, − 5.9 ± 1.3%; p = 0.001 vs. vehicle; p = 0.007 vs. PYY analogue 3 nmol/kg), but not when compared to linagliptin monotreatment (p = 0.07). Linagliptin + Y2R agonist (30 nmol/kg) promoted no further weight loss compared to Y2R agonist monotreatment (vehicle-subtracted weight loss on day 14, − 10.7 ± 0.8%, p = 0.001 vs. vehicle; p < 0.001 vs. linagliptin; p = 0.41 vs. PYY analogue 30 nmol/kg). The body weight lowering effect of Y2R agonist mono- and combination treatments gradually wore off after the last day of administration. Weight loss following linagliptin dosing appeared maximal during the wash-out phase (vehicle-subtracted weight loss on day 16–20, 3.5–4.0%; p < 0.05 vs. vehicle). Linagliptin did not influence food intake in DIO mice. In comparison, Y2R agonist treatment suppressed food intake during the first days of dosing (Fig. 4C,D). Compensatory overeating was observed following treatment cessation (Fig. 4C,D).
Figure 4.
Linagliptin does not augment Y2R receptor agonist-induced food intake inhibition and weight loss in DIO mice. Linagliptin (3 mg/kg, SC) and long-acting Y2 receptor agonist (PYY analogue, 3 or 30 nmol/kg, SC) was administered once daily for 14 days followed by 6 days of wash-out (no treatment) before study termination. (A) Absolute body weight. (B) Body weight change (relative to day 0). (C) Daily food intake. (D) Cumulative food intake. Horizontal lines denote significant change in body weight compared to vehicle control (p < 0.05, two-way ANOVA with Dunnett’s post-hoc test). Compared to Y2R receptor agonist monotreatment, combined linagliptin and Y2R receptor agonist administration did not promote further changes in body weight and daily food intake (p > 0.05, two-way ANOVA with Dunnett’s post-hoc test). Compensatory overeating was observed following treatment cessation.
Equivalent improvement in oral glucose tolerance following linagliptin monotreatment and combined linagliptin-Y2R agonist administration
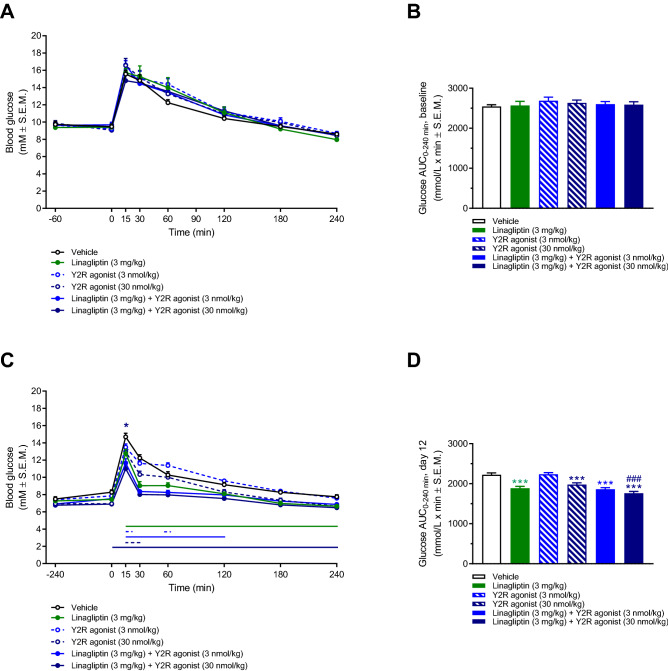
Oral glucose tolerance was determined one week prior to treatment start (day − 7) and on treatment day 12. On day − 7, fasted blood glucose levels (t = 0) were similar in all DIO mouse groups (group mean 9.1–9.7 mM, p > 0.05) as well as glucose excursion curves and AUC-glucose values (Fig. 5A,B). Compared to day -7, fasted blood glucose levels were significantly lower in all treatment groups (p < 0.05, paired t-test), including vehicle controls, on treatment day 12 (group mean 6.9–8.2 mM, Fig. 5C). Compared to vehicle dosing, linagliptin significantly improved glucose tolerance. Y2R agonist treatment also improved glucose tolerance, albeit with less robust effect on glucose excursions. Improvements in glucose tolerance following combined linagliptin and Y2R agonist administration were superior to Y2R agonist, but not linagliptin, monotreatment (Fig. 5C,D).
Figure 5.
No synergistic effects of linagliptin and Y2 receptor agonist treatment on oral glucose tolerance in DIO mice. An oral glucose tolerance test (OGTT) was performed one week before treatment start (day − 7, A,B) and on treatment day 12 (C,D). Horizontal lines denote significant change in blood glucose concentrations compared to vehicle control (p < 0.05, two-way ANOVA with Dunnett’s post-hoc test). *p < 0.05, linagliptin (3 mg/kg) + Y2R agonist (30 nmol/kg) vs. linagliptin (3 mg/kg), two-way ANOVA with Dunnett’s post-hoc test.
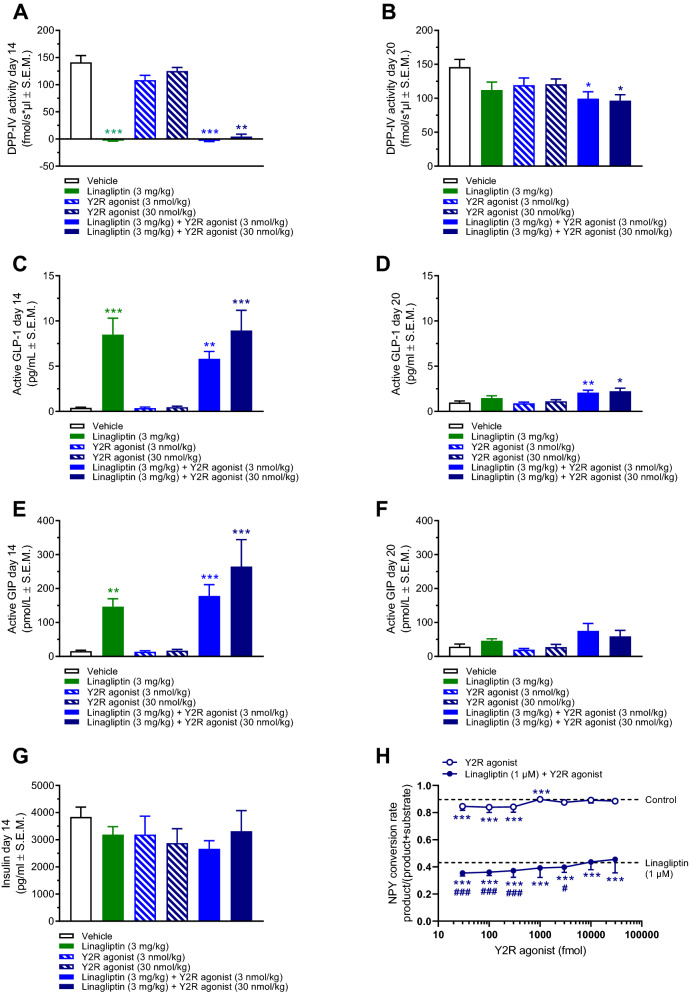
Linagliptin-induced DPP-IV inhibition is unaffected by co-administration of a Y2R-selective agonist
The selected dose of linagliptin (3 mg/kg) completely suppressed plasma DPP-IV activity as measured 4 h post-dosing on the last dosing day (day 14, Fig. 6A). All linagliptin-treated groups showed equally elevated plasma levels of active GLP-1 and GIP on treatment day 14 (Fig. 6C,E). Residual DPP-IV inhibitory activity was detected 6 days after the last linagliptin dose (day 20), being most consistent for linagliptin-Y2R agonist co-treatment groups (Fig. 6B). In the treatment combination groups, this was also reflected by slight, however significantly, increased active GLP-1, but not GIP, levels on day 20 (Fig. 6D,F). The Y2R agonist had no effect on DPP-IV activity as well as active GLP-1 and GIP levels. Treatments did not influence plasma insulin levels (Fig. 6G). Because linagliptin and Y2R agonist co-administration demonstrated stronger inhibitory effects on DPP-IV activity as compared to linagliptin administration alone, we investigated whether the Y2R agonist influenced DPP-IV activity in human EDTA-plasma. In the low fmol range (30–300 fmol), the Y2R agonist slightly reduced NPY conversion rate. Also, linagliptin and the Y2R agonist showed additive inhibitory effects on NPY conversion rate (Fig. 6H). PYY conversion rate was also slightly reduced by the Y2R agonist alone (30–1000 fmol), but unaffected by combined Y2R agonist and linagliptin application (data not shown). At higher Y2R agonist amounts (≥ 1000 fmol), the inhibitory effect of NPY/PYY conversion inhibition was diminished or absent, which was ascribed to reduced solubility of the Y2R agonist.
Figure 6.
Linagliptin-induced DPP-IV inhibition is unaffected by Y2R agonist co-administration. Linagliptin (3 mg/kg, SC) and long-acting Y2 receptor agonist (PYY analogue, 3 or 3 nmol/kg, SC) was administered once daily for 14 days followed by 6 days of wash-out (no treatment) before study termination. (A) Plasma DPP-IV activity measured on treatment day 14 (4 h post-dosing). (B) Terminal plasma DPP-IV activity measured on study day 20. (C) Plasma active GLP-1 levels measured on treatment day 14 (4 h post-dosing). (D) Terminal plasma active GLP-1 levels measured on study day 20. (E) Plasma active GIP levels measured on treatment day 14 (4 h post-dosing). (F) Terminal plasma active GIP levels measured on study day 20. *p < 0.05, **p < 0.01, ***p < 0.001 vs. vehicle control (Kruskal–Wallis test). (G) Plasma insulin levels measured on treatment day 14 (4 h post-dosing). (H) NPY conversion rate in human EDTA-plasma added linagliptin (1 µM), Y2 receptor agonist (30–30,000 fmol) or linagliptin (1 µM) + Y2R agonist (30–30,000 fmol). ***p < 0.001 vs. control, #p < 0.05, ###p < 0.001 vs. linagliptin alone (Welch’s t-test).
Discussion
The present study characterized the metabolic effects of linagliptin with or without co-administration of a Y2R-selective PYY3–36 analogue in DIO mice. The linagliptin dose (3 mg/kg, s.c.) promoted complete plasma DPP-IV inhibition (> 98%) maintained throughout the dosing interval. The subcutaneous dosing regimen results in more pronounced DPP-IV inhibition as compared to oral dosing in DIO mice (~ 80% inhibition)28. In the clinic, ≥ 80% DPP-IV inhibition by linagliptin administration is sufficient to improve glucose tolerance35. Sustained increases in circulating incretin levels were observed in DIO mice treated with linagliptin. Although both linagliptin and the Y2R-selective PYY3–36 analogue improved glucose tolerance and Y2R receptor agonist treatment promoted significant weight loss, no synergistic metabolic effects were observed following combined treatment. Therefore, our study in DIO mice indirectly suggests that weight-neutrality of DPP-IV inhibitors is not explained by reduced endogenous Y2R agonist action, and lends further support to the notion that increasing circulating GLP-1 levels by DPP-IV inhibition is insufficient to promote satiety responses.
DPP-IV inhibition is a therapeutic option to extend circulating half-life of GLP-1 and GIP and improve glycemic control in patients with T2D. DPP-IV inhibitors are generally weight-neutral18. Our preclinical study corroborates end extends previous studies on linagliptin administration in DIO mice28,30,36, by demonstrating that linagliptin induces marginal weight loss when applying a dosing regimen that promotes sustained linagliptin exposure, nearly complete inhibition of plasma DPP-IV activity and ≥ 10-fold increases in plasma GLP-1 and GIP levels. The mild weight loss attained by linagliptin administration in DIO mice may potentially associate to beneficial effects on white adipose tissue mass, liver fat and thermogenesis28,30.
Weight-neutrality of DPP-IV inhibitors contrasts the weight loss efficacy of long-acting GLP-1 analogues which are DPP-IV resistant and have slow elimination kinetics37,38. It has therefore been speculated that DPP-IV inhibitors are weight-neutral by regulating the biological activity of other peptide hormones involved in energy homeostasis11. NPY-family peptides may be potential candidates for promoting metabolic counter-regulatory responses to DPP-IV inhibitor treatment. DPP-IV shows greater affinity for PYY and NPY at physiological concentrations as compared to GLP-1 and GIP20. Studies on DPP-IV substrates other than GLP-1 and GIP are limited. Because reliable techniques are lacking to distinguish between intact and cleaved PYY and NPY immunoreactive forms there is little information on the distribution of circulating PYY and NPY cleavage products following DPP-IV inhibitor treatment. We therefore used MALDI-TOF–MS to determine PYY/NPY isoforms in human plasma following incubation with synthetic human full-length NPY and PYY. Linagliptin prevented N-terminal cleavage of PYY and NPY resulting in substantial lowering of PYY3–36 and NPY3–36 concentrations. Consistent with previous studies39,40, PYY3–36 and NPY3–36 appeared to be predominant PYY/NPY fragments generated by DPP-IV catalytic activity in human plasma. Correspondingly, PYY3–36 and NPY3–36 represent the predominant circulating NPY/PYY isoforms19,41,42. PYY3–36 is a potent anorectic peptide which activates anorexigenic neurocircuits by stimulating vagal and hypothalamic arcuate Y2R signaling43–45. The functional relevance of DPP-IV mediated PYY cleavage is further emphasized by the absence of anorectic effect of exogenous PYY1–36 in rats carrying an inactivating DPP-IV point mutation46. Sitagliptin has been demonstrated to increase plasma PYY1–36:PYY3–36 ratios in T2D patients, indicating that PYY is a physiological substrate for DPP-IV47. NPY is a neuronal-derived peptide that regulates many aspects of energy balance, including feeding behavior end energy expenditure48. NPY1–36 is an orexigenic neuropeptide which acts by stimulating hypothalamic Y1R signaling25. The anorexigenic actions of PYY3–36 are thought partially mediated by inhibiting arcuate NPY neuronal activity49. The N-terminal tyrosine residue of NPY/PYY is critical for efficient Y1R binding 50. As for PYY3–36, NPY3–36 has markedly reduced Y1R affinity but retained anorexigenic Y2R agonist activity51. In contrast to NPY, C-terminally truncated PYY variants were detected in human plasma which supports the notion that PYY is a substrate for several endopeptidases 52,53. C-terminal cleavage renders the peptide Y2R inactive54. While our in vitro study demonstrates that linagliptin-induced inhibition of DPP-IV activity prevents conversion of synthetic full-length NPY/PYY to NPY3–36/PYY3–36, further studies are required to confirm if DPP-IV inhibitors can also modulate endogenous circulating levels of NPY3–36/PYY3–36.
It is currently unknown if modulation of PYY and NPY biological activity may contribute to shape the therapeutic effects of DPP-IV inhibitors. Because PYY3–36 and NPY3–36 signaling converge at the level of Y2R function, it may be hypothesized that DPP-IV inhibitors are weight-neutral because lowering of circulating PYY3–36 and NPY3–36 levels shifts PYY/NPY activity towards Y1R signaling. As a consequence, food intake inhibitory responses subsequent to DPP-IV induced increases in active GLP-1 levels could be counterbalanced by lowered Y2R anorectic activity relative to Y1R orexigenic activity. We therefore sought to determine if pharmacological stimulation of Y2R activity could unmask potential contributory weight-lowering effects of linagliptin in DIO mice. The Y2R-selective agonist (compound 38) was selected based on a patent, demonstrating that compound 38 was one of the most efficacious PYY3–36 analogues on food intake inhibition in db/db mice31. In the current study, food intake suppression was only observed during the first days of Y2R agonist administration while body weight continued to drop over the 14-day treatment period. Sustained weight loss by Y2R agonist treatment may be potentially explained by contributory effects from increased lipolysis and energy expenditure55,56. Linagliptin co-administration did not enhance the anorectic response or weight loss attained by Y2R agonist treatment. Following cessation of drug treatment, DIO mice showed compensatory overeating and gradually resumed baseline body weight. The rate of weight regain was different in linagliptin and Y2R agonist treated mice which is likely explained by different pharmacokinetics. Co-administration of linagliptin and the Y2R agonist resulted in slightly stronger inhibitory effects on DPP-IV activity as compared to linagliptin administration alone. This effect likely explains the slightly higher plasma active GLP-1 levels observed in DIO mice receiving combination treatment. Additive DPP-IV inhibitory effects of linagliptin and the PYY3–36 analogue were confirmed in vitro. Because NPY was added in 100-fold excess in comparison to the PYY3–36 analogue, substrate inhibition seems less plausible. Therefore, future studies must aim to address the underlying mechanism for DPP-IV inhibition conferred by the PYY3–36 analogue.
Our findings contrast mounting evidence from pharmacological studies suggesting additive/synergistic effects of GLP-17–36 and PYY3–36. In infusion studies, the combination of PYY3–36 and GLP-1 confers stronger suppression of food intake and hunger sensation compared to monotreatment in healthy and obese subjects57–59. Correspondingly, preclinical studies have demonstrated synergistic anorectic effects and robust weight loss following administration of a GLP-1 analogue in combination with native PYY3–36 or PYY3–36 analogue treatment60–63. Compared to the marked metabolic effects of pharmacological administration of GLP-1 and PYY3–36, weight-neutral metabolic effects of DPP-IV inhibitors may therefore result from the modest changes in circulating levels of peptide hormones involved in appetite regulation and weight homeostasis. Both linagliptin and the Y2R agonist significantly improved oral glucose tolerance in DIO mice. As for body weight, combined linagliptin and Y2R agonist treatment showed no additive glycemic effects. In agreement, PYY3–36 lacks insulinotropic properties and does not enhance insulin responses to GLP-17–36 administration64. Therefore, Y2R agonists are thought to improve glucose tolerance by increasing peripheral insulin sensitivity as result of weight loss65.
Conclusion
We report that linagliptin does not enhance anorectic and weight loss responses to Y2R agonist treatment in DIO mice. DPP-IV inhibitors may reduce circulating levels of PYY3–36 and NPY3–36, however, any negative modulatory effect on Y2R activity may likely not contribute to shape the therapeutic profile of DPP-IV inhibitors. Our study lends support to the notion that weight neutrality of DPP-IV inhibitors is determined by active GLP-1 levels being insufficiently increased to elicit satiety responses.
Supplementary Information
Authors contributions
Conceived and designed the experiments: R.V.G., N.V., T.K.; performed the experiments: R.V.G., T.B.-P., H.T., L.K.L.; analyzed and interpreted the data: H.H.H., R.V.G., T.B.-P., P.H., H.T., L.K.L., T.K.; wrote the manuscript: H.H.H., P.H., J.J., T.K.
Competing interests
HHH, RVG, and LKL are employed by Gubra; NV and JJ are owners of Gubra; TB-P, PH and TK are employed by Boehringer Ingelheim Pharma; HT is employed by PXBioVisioN. This study was supported by Boehringer Ingelheim Pharma.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-87539-7.
References
- 1.Nauck MA, et al. Incretin effects of increasing glucose loads in man calculated from venous insulin and C-peptide responses. J. Clin. Endocrinol. Metab. 1986;63:492–498. doi: 10.1210/jcem-63-2-492. [DOI] [PubMed] [Google Scholar]
- 2.Holst, J. J., Knop, F. K., Vilsbøll, T., Krarup, T. & Madsbad, S. Loss of incretin effect is a specific, important, and early characteristic of type 2 diabetes. Diabetes Care34 (2011). [DOI] [PMC free article] [PubMed]
- 3.Zander M, Madsbad S, Madsen JL, Holst JJ. Effect of 6-week course of glucagon-like peptide 1 on glycaemic control, insulin sensitivity, and β-cell function in type 2 diabetes: A parallel-group study. Lancet. 2002;359:824–830. doi: 10.1016/S0140-6736(02)07952-7. [DOI] [PubMed] [Google Scholar]
- 4.Nauck MA, et al. Preserved incretin activity of glucagon-like peptide 1 [7-36 amide] but not of synthetic human gastric inhibitory polypeptide in patients with type-2 diabetes mellitus. J. Clin. Invest. 1993;91:301–307. doi: 10.1172/JCI116186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rachman J, Barrow BA, Levy JC, Turner RC. Near-normalisation of diurnal glucose concentrations by continuous administration of glucagon-like peptide-1 (GLP-1) in subjects with NIDDM. Diabetologia. 1997;40:205–211. doi: 10.1007/s001250050664. [DOI] [PubMed] [Google Scholar]
- 6.Drucker DJ. Mechanisms of action and therapeutic application of glucagon-like peptide-1. Cell Metab. 2018;27:740–756. doi: 10.1016/j.cmet.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 7.Deacon CF, et al. Both subcutaneously and intravenously administered glucagon-like peptide I are rapidly degraded from the NH2-terminus in type II diabetic patients and in healthy subjects. Diabetes. 1995;44:1126–1131. doi: 10.2337/diab.44.9.1126. [DOI] [PubMed] [Google Scholar]
- 8.Holst JJ, Deacon CF. Glucagon-like peptide-1 mediates the therapeutic actions of DPP-IV inhibitors. Diabetologia. 2005;48:612–615. doi: 10.1007/s00125-005-1705-7. [DOI] [PubMed] [Google Scholar]
- 9.Tran S, Retnakaran R, Zinman B, Kramer CK. Efficacy of glucagon-like peptide-1 receptor agonists compared to dipeptidyl peptidase-4 inhibitors for the management of type 2 diabetes: A meta-analysis of randomized clinical trials. Diabetes Obes. Metab. 2018;20:68–76. doi: 10.1111/dom.13137. [DOI] [PubMed] [Google Scholar]
- 10.Gallwitz, B. Clinical use of DPP-4 inhibitors. Front. Endocrinol. 10 (2019). [DOI] [PMC free article] [PubMed]
- 11.Deacon, C. F. Physiology and pharmacology of DPP-4 in glucose homeostasis and the treatment of type 2 diabetes. Front. Endocrinol. (Lausanne).10 (2019). [DOI] [PMC free article] [PubMed]
- 12.Omar B, Ahrén B. Pleiotropic mechanisms for the glucose-lowering action of DPP-4 inhibitors. Diabetes. 2014;63:2196–2202. doi: 10.2337/db14-0052. [DOI] [PubMed] [Google Scholar]
- 13.Vilsbøll T, Krarup T, Madsbad S, Holst J. Defective amplification of the late phase insulin response to glucose by gip in obese type II diabetic patients. Diabetologia. 2002;45:1111–1119. doi: 10.1007/s00125-002-0878-6. [DOI] [PubMed] [Google Scholar]
- 14.Vilsbøll T, et al. The pathophysiology of diabetes involves a defective amplification of the late-phase insulin response to glucose by glucose-dependent insulinotropic polypeptide—Regardless of etiology and phenotype. J. Clin. Endocrinol. Metab. 2003;88:4897–4903. doi: 10.1210/jc.2003-030738. [DOI] [PubMed] [Google Scholar]
- 15.Hansotia T, et al. Double incretin receptor knockout (DIRKO) mice reveal an essential role for the enteroinsular axis in transducing the glucoregulatory actions of DPP-IV inhibitors. Diabetes. 2004;53:1326–1335. doi: 10.2337/diabetes.53.5.1326. [DOI] [PubMed] [Google Scholar]
- 16.Højberg PV, et al. Four weeks of near-normalisation of blood glucose improves the insulin response to glucagon-like peptide-1 and glucose-dependent insulinotropic polypeptide in patients with type 2 diabetes. Diabetologia. 2009;52:199–207. doi: 10.1007/s00125-008-1195-5. [DOI] [PubMed] [Google Scholar]
- 17.Aaboe, K. et al. Restoration of the insulinotropic effect of glucose-dependent insulinotropic polypeptide contributes to the antidiabetic effect of dipeptidyl peptidase-4 inhibitors. Diabetes Obes. Metab.17, 74–81 (2015). [DOI] [PubMed]
- 18.Sesti G, et al. Ten years of experience with DPP-4 inhibitors for the treatment of type 2 diabetes mellitus. Acta Diabetol. 2019;56:605–617. doi: 10.1007/s00592-018-1271-3. [DOI] [PubMed] [Google Scholar]
- 19.Mulvihill EE, Drucker DJ. Pharmacology, physiology, and mechanisms of action of dipeptidyl peptidase-4 inhibitors. Endocr. Rev. 2014;35:992–1019. doi: 10.1210/er.2014-1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mentlein R. Dipeptidyl-peptidase IV (CD26)-role in the inactivation of regulatory peptides. Regul. Pept. 1999;85:9–24. doi: 10.1016/S0167-0115(99)00089-0. [DOI] [PubMed] [Google Scholar]
- 21.Ekblad E, Sundler F. Distribution of pancreatic polypeptide and peptide YY. Peptides. 2002;23:251–261. doi: 10.1016/S0196-9781(01)00601-5. [DOI] [PubMed] [Google Scholar]
- 22.Holzer P, Reichmann F, Farzi A. Neuropeptide Y, peptide YY and pancreatic polypeptide in the gut-brain axis. Neuropeptides. 2012;46:261–274. doi: 10.1016/j.npep.2012.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kanatani A, et al. Role of the Y1 receptor in the regulation of neuropeptide Y-mediated feeding: Comparison of wild-type, Y1 receptor-deficient, and Y5 receptor-deficient mice. Endocrinology. 2000;141:1011–1016. doi: 10.1210/endo.141.3.7387. [DOI] [PubMed] [Google Scholar]
- 24.Kaga T, et al. Modest overexpression of neuropeptide Y in the brain leads to obesity after high-sucrose feeding. Diabetes. 2001;50:1206–1210. doi: 10.2337/diabetes.50.5.1206. [DOI] [PubMed] [Google Scholar]
- 25.Clark JT, Sahu A, Kalra PS, Balasubramaniam A, Kalra SP. Neuropeptide Y (NPY)-induced feeding behavior in female rats: comparison with human NPY ([Met17]NPY), NPY analog ([norLeu4]NPY) and peptide YY. Regul. Pept. 1987;17:31–39. doi: 10.1016/0167-0115(87)90030-9. [DOI] [PubMed] [Google Scholar]
- 26.Nguyen AD, Herzog H, Sainsbury A. Neuropeptide y and peptide YY: Important regulators of energy metabolism. Curr. Opin. Endocrinol. Diabetes Obes. 2011;18:56–60. doi: 10.1097/MED.0b013e3283422f0a. [DOI] [PubMed] [Google Scholar]
- 27.Shi, Y. C. et al. NPY neuron-specific Y2 receptors regulate adipose tissue and trabecular bone but not cortical bone homeostasis in mice. PLoS One5 (2010). [DOI] [PMC free article] [PubMed]
- 28.Kern, M. et al. Linagliptin improves insulin sensitivity and hepatic steatosis in diet-induced obesity. PLoS One7 (2012). [DOI] [PMC free article] [PubMed]
- 29.Hansen HH, et al. The DPP-IV inhibitor linagliptin and GLP-1 induce synergistic effects on body weight loss and appetite suppression in the diet-induced obese rat. Eur. J. Pharmacol. 2014;741:254–263. doi: 10.1016/j.ejphar.2014.08.010. [DOI] [PubMed] [Google Scholar]
- 30.de Oliveira Correia, B. R. et al. High dose of linagliptin induces thermogenic beige adipocytes in the subcutaneous white adipose tissue in diet-induced obese C57BL/6 mice. Endocrine65, 252–262 (2019). [DOI] [PubMed]
- 31.Østergaard, S., Jessen, C., Wulff, B. S. & Sanfridson, A. Selective PYY compounds and uses thereof. 1–123 (2016).
- 32.Schürmann C, et al. The dipeptidyl peptidase-4 inhibitor linagliptin attenuates inflammation and accelerates epithelialization in wounds of diabetic ob/ob mice. J. Pharmacol. Exp. Ther. 2012;342:71–80. doi: 10.1124/jpet.111.191098. [DOI] [PubMed] [Google Scholar]
- 33.Fuchs H, Runge F, Held HD. Excretion of the dipeptidyl peptidase-4 inhibitor linagliptin in rats is primarily by biliary excretion and P-gp-mediated efflux. Eur. J. Pharm. Sci. 2012;45:533–538. doi: 10.1016/j.ejps.2011.11.018. [DOI] [PubMed] [Google Scholar]
- 34.Tammen H, et al. Mass spectrometric phenotyping of Val34Leu polymorphism of blood coagulation factor XIII by differential peptide display. Clin. Chem. 2004;50:545–551. doi: 10.1373/clinchem.2003.028209. [DOI] [PubMed] [Google Scholar]
- 35.Heise, T. et al. Pharmacokinetics, pharmacodynamics and tolerability of multiple oral doses of linagliptin, a dipeptidyl peptidase-4 inhibitor in male type 2 diabetes patients. Diabetes Obes. Metab.11, 786–794 (2009). [DOI] [PubMed]
- 36.Manrique, C. et al. Dipeptidyl peptidase-4 inhibition with linagliptin prevents western diet-induced vascular abnormalities in female mice. Cardiovasc. Diabetol.15 (2016). [DOI] [PMC free article] [PubMed]
- 37.Nauck, M. A., Quast, D. R., Wefers, J. & Meier, J. J. GLP-1 receptor agonists in the treatment of type 2 diabetes—State-of-the-art. Mol. Metab. 101102 10.1016/j.molmet.2020.101102 (2020). [DOI] [PMC free article] [PubMed]
- 38.Gilbert, M. P. & Pratley, R. E. GLP-1 analogs and DPP-4 inhibitors in type 2 diabetes therapy: Review of head-to-head clinical trials. Front. Endocrinol.11 (2020). [DOI] [PMC free article] [PubMed]
- 39.Abid, K. et al. Kinetic study of neuropeptide Y (NPY) proteolysis in blood and identification of NPY3–35. A new peptide generated by plasma kallikrein. J. Biol. Chem.284, 24715–24724 (2009). [DOI] [PMC free article] [PubMed]
- 40.Frerker N, et al. Neuropeptide Y (NPY) cleaving enzymes: Structural and functional homologues of dipeptidyl peptidase 4. Peptides. 2007;28:257–268. doi: 10.1016/j.peptides.2006.09.027. [DOI] [PubMed] [Google Scholar]
- 41.Grandt D, et al. Two molecular forms of Peptide YY (PYY) are abundant in human blood: Characterization of a radioimmunoassay recognizing PYY 1–36 and PYY 3–36. Regul. Pept. 1994;51:151–159. doi: 10.1016/0167-0115(94)90204-6. [DOI] [PubMed] [Google Scholar]
- 42.Mentlein R, Dahms P, Grandt D, Krüger R. Proteolytic processing of neuropeptide Y and peptide YY by dipeptidyl peptidase IV. Regul. Pept. 1993;49:133–144. doi: 10.1016/0167-0115(93)90435-B. [DOI] [PubMed] [Google Scholar]
- 43.Batterham RL, et al. Gut hormone PYY3-36 physiologically inhibits food intake. Nature. 2002;418:650–654. doi: 10.1038/nature00887. [DOI] [PubMed] [Google Scholar]
- 44.Ueno H, Yamaguchi H, Mizuta M, Nakazato M. The role of PYY in feeding regulation. Regul. Pept. 2008;145:12–16. doi: 10.1016/j.regpep.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 45.Koda S, et al. The role of the vagal nerve in peripheral PYY 3–36-induced feeding reduction in rats. Endocrinology. 2005;146:2369–2375. doi: 10.1210/en.2004-1266. [DOI] [PubMed] [Google Scholar]
- 46.Unniappan S, et al. Effects of dipeptidyl peptidase IV on the satiety actions of peptide YY. Diabetologia. 2006;49:1915–1923. doi: 10.1007/s00125-006-0310-8. [DOI] [PubMed] [Google Scholar]
- 47.Aaboe, K. et al. Twelve weeks treatment with the DPP-4 inhibitor, sitagliptin, prevents degradation of peptide YY and improves glucose and non-glucose induced insulin secretion in patients with type 2 diabetes mellitus. Diabetes Obes. Metab.12, 323–333 (2010). [DOI] [PubMed]
- 48.Mercer RE, Chee MJS, Colmers WF. The role of NPY in hypothalamic mediated food intake. Front. Neuroendocrinol. 2011;32:398–415. doi: 10.1016/j.yfrne.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 49.Acuna-Goycolea C, Van Den Pol AN. Peptide YY3-36 inhibits both anorexigenic proopiomelanocortin and orexigenic neuropeptide Y neurons: Implications for hypothalamic regulation of energy homeostasis. J. Neurosci. 2005;25:10510–10519. doi: 10.1523/JNEUROSCI.2552-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Michel, M. C. et al. XVI. International Union of Pharmacology recommendations for the nomenclature of neuropeptide Y, peptide YY, and pancreatic polypeptide receptors. Pharmacol. Rev.50, 143–150 (1998). [PubMed]
- 51.Pedrazzini T, Pralong F, Grouzmann E. Neuropeptide Y: The universal soldier. Cell. Mol. Life Sci. 2003;60:350–377. doi: 10.1007/s000180300029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Santos Medeiros, M. Dos & Turner, A. J. Processing and metabolism of peptide-yy: Pivotal roles of dipeptidylpeptidase-IV, aminopeptidase-p, and endopeptidase-24.11. Endocrinology134, 2088–2094 (1994). [DOI] [PubMed]
- 53.Dos Santos Medeiros, M. & Turner, A. J. Metabolism and functions of neuropeptide Y. Neurochem. Res.21, 1125–1132 (1996). [DOI] [PubMed]
- 54.Beck-Sickinger AG, Jung G. Structure–activity relationships of neuropeptide Y analogues with respect to Y1 and Y2 receptors. Biopolymers. 1995;37:123–142. doi: 10.1002/bip.360370207. [DOI] [PubMed] [Google Scholar]
- 55.Sloth, B., Holst, J. J., Flint, A., Gregersen, N. T. & Astrup, A. Effects of PYY1–36 and PYY3–36 on appetite, energy intake, energy expenditure, glucose and fat metabolism in obese and lean subjects. Am. J. Physiol. - Endocrinol. Metab.292 (2007). [DOI] [PubMed]
- 56.Boey D, et al. PYY transgenic mice are protected against diet-induced and genetic obesity. Neuropeptides. 2008;42:19–30. doi: 10.1016/j.npep.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 57.Neary NM, et al. Peptide YY3-36 and glucagon-like peptide-17-36 inhibit food intake additively. Endocrinology. 2005;146:5120–5127. doi: 10.1210/en.2005-0237. [DOI] [PubMed] [Google Scholar]
- 58.Schmidt, J. B. et al. Effects of PYY3–36 and GLP-1 on energy intake, energy expenditure, and appetite in overweight men. Am. J. Physiol. Endocrinol. Metab.306 (2014). [DOI] [PubMed]
- 59.De Silva A, et al. The gut hormones PYY 3–36 and GLP-1 7–36 amide reduce food intake and modulate brain activity in appetite centers in humans. Cell Metab. 2011;14:700–706. doi: 10.1016/j.cmet.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dalbøge LS, et al. A Hamster model of diet-induced obesity for preclinical evaluation of anti-obesity, anti-diabetic and lipid modulating agents. PLoS ONE. 2015;10:e0135634. doi: 10.1371/journal.pone.0135634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kjaergaard M, et al. PYY(3–36) and exendin-4 reduce food intake and activate neuronal circuits in a synergistic manner in mice. Neuropeptides. 2019;73:89–95. doi: 10.1016/j.npep.2018.11.004. [DOI] [PubMed] [Google Scholar]
- 62.Rangwala SM, et al. A long-acting PYY 3–36 analog mediates robust anorectic efficacy with minimal emesis in nonhuman primates. Cell Metab. 2019;29:837–843.e5. doi: 10.1016/j.cmet.2019.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Talsania T, Anini Y, Siu S, Drucker DJ, Brubaker PL. Peripheral exendin-4 and peptide YY3-36 synergistically reduce food intake through different mechanisms in mice. Endocrinology. 2005;146:3748–3756. doi: 10.1210/en.2005-0473. [DOI] [PubMed] [Google Scholar]
- 64.Tan TM, et al. Combination of peptide YY3-36 with GLP-17-36 amide causes an increase in first-phase insulin secretion after IV glucose. J. Clin. Endocrinol. Metab. 2014;99:E2317–E2324. doi: 10.1210/jc.2014-2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Khoo B, Tan TMM. Combination gut hormones: Prospects and questions for the future of obesity and diabetes therapy. J. Endocrinol. 2020;246:R65–R74. doi: 10.1530/JOE-20-0119. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.