Abstract
We present our institutional experience with 46 patients diagnosed with micropapillary bladder carcinoma compared to conventional urothelial carcinoma, alongside data from Surveillance, Epidemiology, and End Results (SEER)-Medicare. We identified comparable pathologic response to neoadjuvant chemotherapy (NAC) across histologic subtypes, while micropapillary bladder carcinoma was not independently associated with worse outcomes, despite presenting with more aggressive features. The role of NAC should be further evaluated with additional studies in this setting.
Background:
Micropapillary urothelial carcinoma (MPC) is a rare urothelial carcinoma variant with conflicting data guiding clinical practice. In this study, we explored oncologic outcomes in relation to neoadjuvant chemotherapy (NAC) in a retrospective cohort of patients with MPC, alongside data from Surveillance, Epidemiology, and End Results (SEER)-Medicare.
Patients and Methods:
We retrospectively identified patients with MPC or conventional urothelial carcinoma (CUC) without any variant histology undergoing radical cystectomy (RC) in our institution (2003-2018). SEER-Medicare was also queried to identify patients diagnosed with MPC (2004-2015). Clinicopathologic data and treatment modalities were extracted. Overall survival (OS) was estimated with the Kaplan-Meier method. Mann-Whitney-Wilcoxon and chi-square tests were used for comparative analysis and Cox regression for identifying clinical covariates associated with OS.
Results:
Our institutional database yielded 46 patients with MPC and 457 with CUC. In SEER-Medicare, 183 patients with MPC were identified, and 63 (34%) underwent RC. In the institutional cohort, patients with MPC had significantly higher incidence of cN+ (17% vs. 8%), pN+ stage (30% vs. 17%), carcinoma-in-situ (43% vs. 25%), and lymphovascular invasion (30% vs. 16%) at RC versus those with CUC (all P < .05). Pathologic complete response (ypT0N0) to NAC was 33% for MPC and 35% for CUC (P = .899). Median OS was lower for institutional MPC versus CUC in univariate analysis (43.6 vs. 105.3 months, P = .006); however, MPC was not independently associated with OS in the multivariate model. Median OS was 25 months in the SEER MPC cohort for patients undergoing RC, while NAC was not associated with improved OS in that group.
Conclusion:
Pathologic response to NAC was not significantly different between MPC and CUC, while MPC histology was not an independent predictor of OS. Further studies are needed to better understand biological mechanisms behind its aggressive features as well as the role of NAC in this histology variant.
Keywords: Cystectomy, Urinary bladder neoplasms
Introduction
Micropapillary urothelial carcinoma (MPC) was originally described in 1994, in a case series of 18 patients, as a variant of urothelial origin with papillary processes and cell clusters bearing resemblance to papillary serous ovarian carcinoma.1 The incidence of MPC varies in the literature, ranging 0.6% to 6% of urothelial carcinoma (UC) cases.2 MPC is almost invariably encountered alongside conventional urothelial carcinoma (CUC)2 cells and has demonstrated high-grade histologic features3 and advanced clinical stage at diagnosis.4 Increased rates of upstaging at radical cystectomy (RC), extravesical and nodal spread at RC,5-7 as well as high rates of lymphovascular invasion (LVI)8 and associated carcinoma-in-situ (CIS),1,9 have consistently been reported in the literature. In general, recurrence and survival outcomes in MPC appear worse compared to CUC, although the numbers are small.6,7,10
Management of MPC still remains controversial.11 Data on neoadjuvant chemotherapy (NAC) are conflicting, with a number of centers reporting inferior response to NAC for MPC compared with CUC,12,13 and others finding comparable pathologic response.14 As a result, there is no consensus among clinicians regarding the use of NAC in MPC, both broadly and within our own institution. If MPC is not adequately responsive to NAC, then its delivery may delay time to definitive surgery and possibly compromise survival outcomes in a number of patients.
Considering the conflicting reports on response to NAC in MPC, we studied our institutional experience with MPC treated with RC, with the aim to investigate patterns of disease in terms of clinical and pathologic behavior, treatment response, and clinical outcomes, such as recurrence and survival, compared to CUC. We hypothesized that MPC may not be as responsive to NAC and may be associated with worse outcomes compared to CUC. To further extend beyond our institutional experience, we present our findings alongside data from Surveillance, Epidemiology, and End Results (SEER)-Medicare for patients diagnosed with MPC.
Patients and Methods
Patient Selection and Study Design (Institutional Cohort)
After obtaining institutional review board approval, we retrospectively assessed our institutional RC database with the aim to identify two distinct patient populations: patients with MPC and CUC. The following inclusion criteria were used: (1) adult patients (≥ 18 years old), (2) diagnosed with UC on pathology review by bladder cancer pathology experts, (3) with histologic confirmation of micropapillary component (any percentage) at transurethral resection of bladder tumor (TURBT) (MPC group) or presence of conventional (nonvariant) UC only, both in the TURBT and RC specimen (CUC group). Demographic information, baseline comorbidities, clinical stage, CIS, and LVI at TURBT, pathologic stage, CIS, LVI, and surgical margin status at RC, percentage of micropapillary component, and survival outcomes were extracted. If the percentage of MPC was ≥ 50% of the TURBT specimen, the amount of the variant was considered predominant. We also captured the NAC regimens, number of cycles, dates of infusion, and pathologic response to NAC. MPC and CUC were compared for certain prespecified outcomes of interest.
Identification of MPC Cases in SEER-Medicare
The SEER-Medicare database was searched for patients diagnosed with MPC between 2004 and 2015, using International Classification of Disease topography codes for bladder cancer (C67.0-C67.9), as well as the MPC morphologic code 8131, defined by the 2016 World Health Organization classification.15 The SEER database utilizes a combination of clinical and pathologic information from imaging, biopsies, TURBT, RC, and autopsies to estimate tumor, node, metastasis classification system (TNM) stage. For that reason, we used the general terms T, N, or M for a composite stage, without the defining clinical (c) or pathologic (p) terms (eg, cT, pT) because the origin of the stage-defining specimen (TURBT vs. RC) was not always identifiable. Pathologic response to NAC in SEER-Medicare cannot be captured in the current form because the composite stage reported in the SEER database refers to the highest stage (noted at TURBT and/or RC).
Outcomes of Interest
Primary outcomes of interest explored in the institutional data set were: (1) comparison of clinicopathologic characteristics between MPC and CUC; (2) pathologic overall (< ypT2N0) and complete (ypT0N0) response to NAC, stratified by histology (MPC vs. CUC); (3a) overall survival (OS) from time of diagnosis to death from any cause, stratified by (i) histology (MPC vs. CUC) or (ii) NAC receipt (the latter explored separately in the MPC and CUC cohort); (3b) recurrence-free survival (RFS), measured from the time of RC to first radiographic evidence of localized recurrence/distant metastasis or death from any cause, stratified by histology (MPC vs. CUC). Secondary outcomes of interest were: (1) clinicopathologic characteristics of MPC in SEER-Medicare database; and (2) OS stratified by NAC receipt in SEER-Medicare database.
Statistical Analysis
Statistical analysis was performed by SPSS Statistics for Windows 23.0 (IBM, Armonk, NY). For univariate comparison of clinicopathologic features between MPC and CUC histologic groups, Mann-Whitney-Wilcoxon test was used for continuous variables with nonnormal distribution and chi-square test (Pearson chi-square, Fischer exact test) for categorical variables. Logistic regression adjusting for clinical features (age, sex assigned at birth, Eastern Cooperative Oncology Group (ECOG) performance status, estimated glomerular filtration rate [GFR], clinical stage, histology) was used to estimate likelihood of NAC receipt and pathologic response to NAC. GFR was calculated with the MDRD equation. Kaplan-Meier method was used for estimation of OS and RFS (as defined above). Mantel-Cox log-rank test was used for univariate comparison of OS and RFS between groups. Multivariate Cox proportional hazards model was utilized to explore the association of NAC and micropapillary histology with OS, adjusting for other covariates of clinical interest associated with OS, including age at diagnosis, sex assigned at birth, ECOG performance status, GFR, pT, pN stage, positive surgical margins, and LVI at RC. An alpha error of 5% (2 tailed) was set as the cutoff of statistical significance for all statistical tests utilized.
Results
Clinicopathologic Characteristics and Comparison of MPC and CUC Institutional Cohorts
Out of a total of 789 consecutive patients undergoing RC, 46 (6%) had MPC at TURBT and 457 (58%) had pure CUC. Table 1 summarizes the clinicopathologic data of both groups. At TURBT, micropapillary component was identified amid a UC background, comprising 1% to 70% of the specimen. In certain cases, the term “micropapillary predominant” or “micropapillary UC” was used, without an exact percentage approximation. In these cases, the MPC percentage was considered to be ≥ 50%. In a similar fashion, terms such as “focal micropapillary component” and “focal micropapillary differentiation” were considered to be < 50%. Overall, 32 (70%) of 46 patients had nonpredominant MPC and 8 (17%) predominant, while it was not feasible to assess in 6 (13%).
Table 1.
Univariate Group Comparison of Baseline Clinicopathologic Characteristics Between Patients With MPC or CUC Who Underwent RC (Mann-Whitney U, Chi-Square Test) in Institutional Data Set
| Characteristic | MPC (N = 46) |
CUC (N = 457) |
P |
|---|---|---|---|
| Sex assigned at birth | |||
| Male | 38 (83) | 366 (80) | .682 |
| Female | 8 (17) | 91 (20) | |
| Age (y), median (IQR) | 68 (61-72) | 67 (59-75) | .441 |
| Smoking | .564 | ||
| Never | 13 (28) | 131 (29) | |
| Past | 20 (43) | 227 (50) | |
| Active | 13 (28) | 99 (22) | |
| ECOG PS | .127 | ||
| 0-1 | 43 (93) | 446 (98) | |
| ≥2 | 3 (7) | 11 (2) | |
| Clinical T stage | .245 | ||
| <cT2 | 9 (20) | 130 (28) | |
| cT2 | 18 (39) | 188 (41) | |
| cT3/4 | 19 (41) | 139 (30) | |
| CIS (TURBT) | 18 (39) | 136 (30) | .196 |
| LVI (TURBT) | 7 (15) | 48 (11) | .334 |
| Clinical N stage | .009 | ||
| cN0 | 38 (83) | 422 (92) | |
| cN1 | 8 (17) | 35 (8) | |
| Pathologic T stage (RC) | .954 | ||
| pT0 | 14 (30) | 112 (25) | |
| pTa/Tis | 10 (22) | 96 (21) | |
| pT1 | 4 (9) | 54 (12) | |
| pT2 | 5 (11) | 66 (14) | |
| pT3/T4 | 13 (28) | 129 (28) | |
| Pathologic N stage (RC) | .029 | ||
| pTanyN0 | 32 (70) | 378 (83) | |
| pTanyN1-3 | 14 (30) | 79 (17) | |
| Other findings (RC) | |||
| Positive margins | 4 (9) | 28 (6) | .521 |
| CIS | 20 (43) | 113 (25) | .006 |
| LVI | 14 (30) | 73 (16) | .013 |
| NAC | 27 (59) | 188 (41) | .022 |
| <ypT2N0 | 15/27 (56) | 116/188 (62) | .540 |
| ypT0N0 | 9/27 (33) | 65/188 (35) | .899 |
Data are presented as n (%) unless otherwise indicated.
Abbreviations: c = clinical; CIS = carcinoma-in-situ; CUC = conventional urothelial carcinoma; ECOG PS = Eastern Cooperative Oncology Group performance status; IQR = interquartile range; LVI = lymphovascular invasion; MPC = micropapillary carcinoma; N = nodal stage; NAC = neoadjuvant chemotherapy; p = pathologic; RC = radical cystectomy; T = tumor stage; TURBT = transurethral resection of bladder tumor; yp = pathologic stage after NAC receipt.
We performed a comparison of clinicopathologic data at the time of TURBT and RC, between MPC and CUC (Table 1). Both groups had a male predominant population, with a comparable age distribution, smoking exposure, ECOG performance status, and clinical and pathologic tumor staging breakdown. However, MPC showed a higher proportion of radiographically enlarged lymph nodes (cN positive) during initial diagnostic imaging (17% vs. 8%, P = .009), which was concordant with the significantly higher rates of nodal involvement at RC (30% vs. 17%, P = .029). The incidence of CIS (43% vs. 25%, P = .006) and LVI positivity (30% vs. 16%, P = .013) at the time of RC was also significantly higher in the MPC group.
NAC Receipt and Response Across Histologies (Institutional Cohort)
Characteristics of Treatment Groups and Factors Associated with NAC Receipt.
NAC was administered to 27 patients (59%) with MPC and 188 patients (41%) with CUC (P = .022) (Table 1). All patients with MPC received a cisplatin-based regimen. In the CUC group, cisplatin-based NAC was provided to 173 (92%) and non-cisplatin in 8 (4%), while no data for the exact NAC regimen were available for 7 patients (4%). Table 2 compares the baseline clinicopathologic features of patients receiving NAC versus those treated with up-front RC across the two histologies evaluated (MPC/CUC). In the MPC cohort, patients who received NAC had a significantly higher proportion of advanced clinical stage compared to the RC only group and a similar trend was noted for adequate renal function (GFR ≥ 60 mL/min), although not statistically significant. In the CUC cohort, NAC group was characterized by significantly higher rates of patients who were women, had advanced clinical T stage, suspicious lymph nodes at imaging, and GFR ≥ 60 mL/min. Additionally, patients who received NAC had lower median age (P = .001). In multivariate logistic regression for NAC receipt in the combined cohort of MPC and CUC, MPC histology was independently associated with higher odds of NAC receipt, alongside GFR ≥ 60 mL/min and cT3/4 stage. In converse, age ≥ 70 years was significantly associated with lower odds of NAC receipt (Table 3).
Table 2.
Comparison of Clinicopathologic Characteristics (Mann-Whitney U, Chi-Square) Between Patients Treated With Up-front RC or NAC Before RC, Across Histologic Types in Institutional Data Set
| MPC (N = 46) | CUC (N = 457) x | |||||
|---|---|---|---|---|---|---|
| Characteristic | RC Only (N = 19) |
NAC + RC (N = 27) |
P | RC Only (N = 269) |
NAC + RC (N = 188) |
P |
| Sex assigned at birth | .246 | .041 | ||||
| Male | 14 (74) | 24 (89) | 224 (83) | 142 (76) | ||
| Female | 5 (26) | 3 (11) | 45 (17) | 46 (24) | ||
| Age (y), median (IQR) | 68 (65-74) | 65 (58-72) | .116 | 69 (61-77) | 65 (58-72) | .001 |
| Smoking | .545 | .875 | ||||
| Never | 5 (26) | 8 (30) | 75 (28) | 56 (30) | ||
| Past | 7 (37) | 13 (48) | 134 (50) | 93 (49) | ||
| Active | 7 (37) | 6 (22) | 60 (22) | 39 (21) | ||
| ECOG PS | .372 | |||||
| 0-1 | 18 (95) | 25 (93) | 264 (98) | 182 (97) | ||
| ≥2 | 1 (5) | 2 (7) | 5 (2) | 6 (3) | ||
| GFR | .079 | <.001 | ||||
| ≥60 mL/min | 11 (58) | 21 (78) | 183 (68) | 142 (76) | ||
| <60 mL/min | 8 (42) | 4 (15) | 81 (30) | 21 (11) | ||
| NA | 0 | 2 (7) | 5 (2) | 25 (13) | ||
| Clinical T stage | <.001 | <.001 | ||||
| <cT2 | 9 (47) | 0 | 128 (48) | 2 (1) | ||
| cT2 | 7 (37) | 11 (41) | 96 (36) | 92 (49) | ||
| cT3/4 | 3 (16) | 16 (59) | 45 (17) | 94 (50) | ||
| Clinical N stage | .115 | <.001 | ||||
| cN0 | 18 (95) | 20 (74) | 265 (99) | 157 (84) | ||
| cN1 | 1 (5) | 7 (26) | 4 (1) | 31 (16) | ||
| Pathologic T stage | .404 | .031 | ||||
| <pT2 | 11 (58) | 17 (63) | 141 (52) | 121 (64) | ||
| pT2 | 1 (5) | 4 (15) | 41 (15) | 25 (13) | ||
| pT3/4 | 7 (37) | 6 (22) | 87 (32) | 42 (22) | ||
| Pathologic N stage | .149 | .530 | ||||
| pN0 | 11 (58) | 21 (78) | 220 (82) | 158 (84) | ||
| pN1-3 | 8 (42) | 6 (22) | 49 (18) | 30 (16) | ||
Data are presented as n (%) unless otherwise indicated.
Abbreviations: c = clinical; CUC = conventional urothelial carcinoma; ECOG PS = Eastern Cooperative Oncology Group performance status; GFR = glomerular filtration rate; IQR = interquartile range; MPC = micropapillary carcinoma; N = nodal stage; NA = not available; NAC = neoadjuvant chemotherapy; P = pathologic; RC = radical cystectomy; T = tumor stage.
Table 3.
Results of Univariate and Multivariate Logistic Regression for Odds of NAC Receipt and Pathologic Overall Response (< ypT2N0) in Combined Institutional Cohort of Patients With MPC and CUC
| MPC and CUC (N = 503) | NAC Receipt | |||
|---|---|---|---|---|
| Univariate | Multivariate | |||
| Variable | OR (95% CI) | P | OR (95% CI) | P |
| Age ≥ 70 y | 0.51 (0.35-0.75) | .001 | 0.53 (0.33-0.85) | .009 |
| Female (assigned at birth) | 1.41 (0.90-2.18) | .131 | 1.67 (0.98-2.85) | .059 |
| ECOG PS > 1 | 1.82 (0.62-5.32) | .276 | 0.96 (0.23-3.96) | .955 |
| cT3/4 | 5.24 (3.48-7.89) | <.001 | 6.92 (4.29-11.16) | <.001 |
| GFR ≥ 60 mL/min | 2.99 (1.83-4.88) | <.001 | 4.07 (2.26-7.32) | <.001 |
| MPC component (any) | 1.18 (1.05-1.32) | .007 | 1.22 (1.01-1.46) | .036 |
| Patients Receiving NAC (N = 215) |
Pathologic Overall Response (< ypT2N0) | |||
| Variable | OR (95% CI) | P | OR (95% CI) | P |
| Age ≥ 70 y | 1.45 (0.77-2.74) | .257 | 1.54 (0.71-3.35) | .275 |
| cT3/4 | 0.58 (0.33-1.00) | .050 | 0.47 (0.25-0.87) | .016 |
| GFR ≥ 60 mL/min | 1.09 (0.46-2.57) | .851 | 1.36 (0.53-3.54) | .524 |
| MPC component (any) | 0.94 (0.77-1.15) | .541 | 1.00 (0.80-1.24) | .962 |
Abbreviations: CI = confidence interval; cT = clinical tumor stage; CUC = conventional urothelial carcinoma; ECOG PS = Eastern Cooperative Oncology Group performance status; GFR = glomerular filtration rate; MPC = micropapillary carcinoma; NAC = neoadjuvant chemotherapy; OR = odds ratio; yp = pathologic stage after NAC receipt.
Evaluation of Pathologic Response to NAC Across Histologies.
Pathologic overall (< ypT2N0) and complete (ypT0N0) response to NAC was noted in 15 (56%) of 27 and 9 (33%) of 27 patients with MPC respectively; 6 (67%) of 9 with ypT0N0 had MPC < 50%. Micropapillary predominance was not associated with significant differences in NAC receipt, as 5 (63%) of 8 patients with predominant MPC received NAC compared to 21 (66%) of 32 with nonpredominant MPC (P = .108). Pathologic overall response (< ypT2N0) to NAC was noted in 3 of 5 patients with predominant MPC and 12 of 21 with nonpredominant (60% vs. 57%, P = 1.000), with ypT0N0 rates of 3 (60%) of 5 and 6 (29%) of 21, respectively (P = .302). Of the remaining 6 patients with undetermined predominance, only one received NAC. In the CUC group, pathologic overall and complete response to NAC was achieved in 116 patients (62%) and 65 patients (35%), and it was comparable to MPC (Table 1). In multivariate logistic regression for pathologic response in the entire combined cohort of patients with MPC and CUC undergoing NAC, advanced clinical stage (cT3/4) was independently associated with lower odds of achieving < ypT2N0 (Table 3).
Survival Outcomes in Institutional Data Set
Survival Outcomes Stratified by Histology.
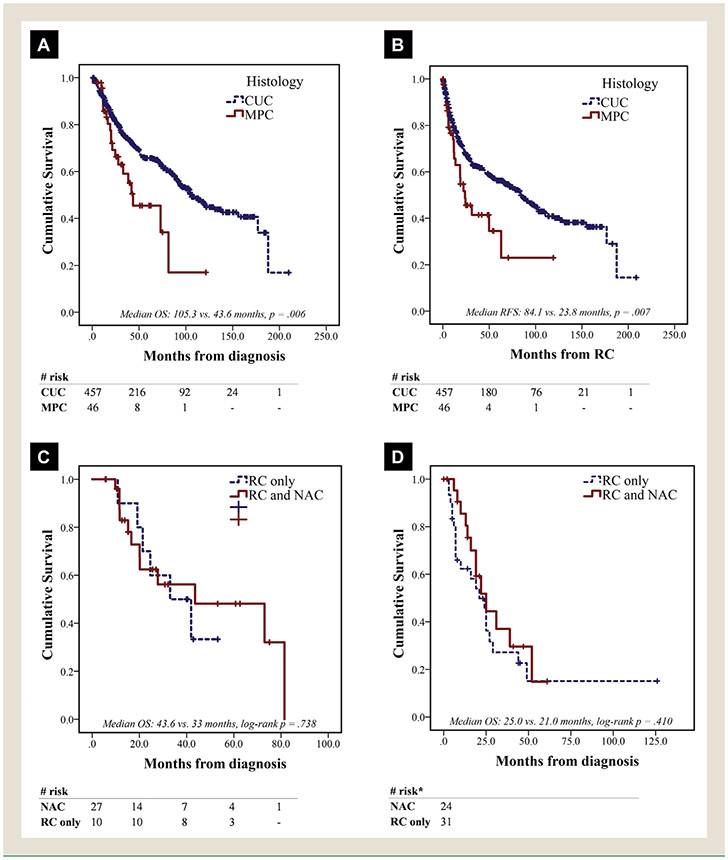
Median follow-up in the entire cohort (MPC and CUC combined) was 76.5 months (95% confidence interval [CI], 68.0-85.1), but significantly higher in patients with CUC compared to those with MPC (80.4 vs. 40.4 months, P < .001); At the end of the follow-up period, 276 and 26 patients were censored in the CUC and MPC cohorts respectively. Patients with CUC had significantly longer median OS compared to CUC (105.3 vs. 43.6 months, P = .006) and RFS (84.1 vs. 23.8 months, P = .007) (Figure 1A and B). In multivariate Cox regression for OS in the entire cohort (MPC and CUC), the presence of micropapillary histology was not independently associated with OS (hazard ratio, 1.11; 95% CI, 0.99-1.25), whereas age ≥ 70 years, presence of extravesical disease at RC (pT3/4), nodal involvement (pN+) and LVI at RC demonstrated a significant association with OS (Table 4).
Figure 1.
Survival by Histology and Receipt of NAC. (A) OS in Institutional Cohort, Stratified by Histology. (B) RFS in Institutional Cohort, Stratified by Histology. (C) OS in Institutional Cohort of Patients With cT2-4 MPC, Stratified by NAC Receipt. (D) OS in SEER-Medicare Cohort of Patients With cT2-4 MPC, Stratified by NAC Receipt. *Exact Counts Masked in Compliance With Centers for Medicare and Medicaid Services/Surveillance, Epidemiology, and End Results (CMS/SEER) Publication Policy of No Counts With < 11 Cases
Abbreviations: CUC = conventional urothelial carcinoma; MPC = micropapillary urothelial carcinoma; NAC = neoadjuvant chemotherapy; OS = overall survival; RC = radical cystectomy; RFS = recurrence-free survival.
Table 4.
Results of Univariate and Multivariate Cox Regression for Overall Survival in Institutional Cohort
| Univariate Cox | Multivariate Cox | |||
|---|---|---|---|---|
| Variable | HR (95% CI) | P | HR (95% CI) | P |
| MPC cT2-4 (N = 37) | ||||
| NAC | 0.84 (0.30-2.34) | .739 | 2.38 (0.48-11.7) | .288 |
| Age ≥ 70 y | 0.76 (0.26-2.19) | .615 | 2.47 (0.49-12.5) | .274 |
| Female (assigned at birth) | 0.87 (0.25-3.06) | .830 | 0.53 (0.11-2.5) | .426 |
| GFR ≥ 60 mL/min | 0.98 (0.95-1.02) | .327 | 1.30 (0.33-5.19) | .710 |
| pT3/4 | 3.27 (1.16-9.19) | .025 | 5.37 (1.15-25.01) | .032 |
| pN+ | 1.63 (0.61-4.39) | .332 | 2.41 (0.56-10.31) | .236 |
| CUC cT2-4 (N = 327) | ||||
| NAC | 0.70 (0.49-0.99) | .043 | 0.91 (0.61-1.35) | .626 |
| Age≥ 70 | 1.52 (1.06-2.19) | .023 | 1.30 (0.87-1.93) | .199 |
| Female (assigned at birth) | 1.76 (1.20-2.57) | .004 | 1.78 (1.18-2.69) | .006 |
| GFR ≥ 60 mL/min | 0.59 (0.40-0.88) | .009 | 0.78 (0.51-1.21) | .273 |
| pT3/4 | 2.61 (1.84-3.70) | <.001 | 1.85 (1.22-2.82) | .004 |
| pN+ | 2.62 (1.81-3.80) | <.001 | 2.21 (1.45-3.38) | .001 |
| Total patients (MPC and CUC, n = 503) | ||||
| Any MPC component | 1.18 (1.05-1.32) | .007 | 1.11 (0.99-1.25) | .088 |
| NAC | 0.88 (0.66-1.18) | .380 | 1.07 (0.78-1.47) | .685 |
| Age ≥ 70 | 1.42 (1.07-1.90) | .016 | 1.38 (1.01-1.89) | .043 |
| Female (assigned at birth) | 1.31 (0.94-1.83) | .109 | 1.25 (0.87-1.79) | .230 |
| ECOG PS > 1 | 2.31 (1.13-4.69) | .021 | 1.62 (0.70-3.72) | .259 |
| GFR ≥ 60 mL/min | 0.64 (0.47-0.87) | .004 | 0.77 (0.55-1.09) | .136 |
| pT3/4 (RC) | 2.56 (1.93-3.39) | <.001 | 1.67 (1.18-2.37) | .004 |
| pN1-3 | 2.47 (1.82-3.37) | <.001 | 1.83 (1.27-2.65) | .001 |
| Positive margins | 2.13 (1.33-3.42) | .002 | 0.88 (0.51-1.53) | .656 |
| LVI (RC) | 2.74 (2.00-3.75) | <.001 | 1.88 (1.28-2.78) | .001 |
Abbreviations: CI = confidence interval; CUC = conventional urothelial carcinoma; GFR = glomerular filtration rate; HR = hazard ratio; LVI = lymphovascular invasion; MPC = micropapillary carcinoma; NAC = neoadjuvant chemotherapy; pN = pathologic nodal stage; pT = pathologic tumor stage; RC = radical cystectomy.
Survival Outcomes Stratified by NAC Receipt in Patients with cT2-4 Stage Disease Across Histologies.
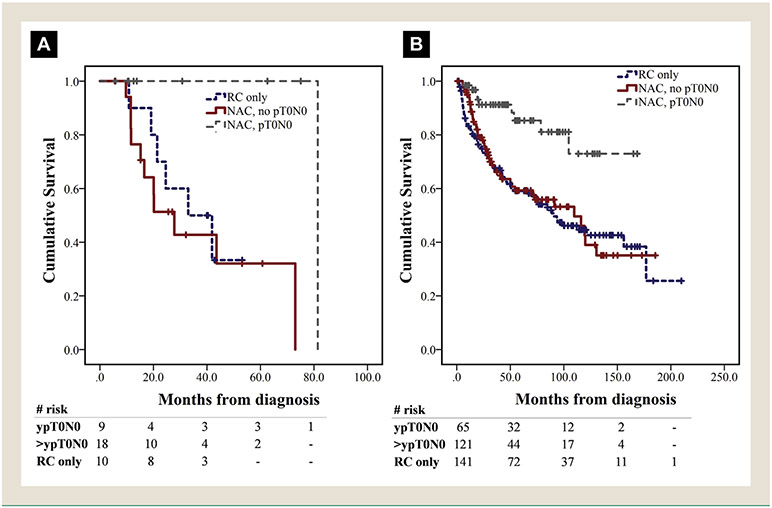
Median OS was longer in patients with cT2-4 MPC (n = 37) treated with NAC and RC versus RC alone, but the result was not statistically significant (43.6 vs. 33 months, P = .738) (Figure 1C). Of those, patients with micropapillary predominant disease (n = 6) at TURBT also had inferior median OS compared to those with nonpredominant (n = 26), although the result did not reach statistical significance (11.6 vs. 43.6 months, P = .322) in this small patient subset. Histology predominance data was not available for 5 of 37 patients. Eight of 9 patients with ypT0N0 were still alive at the time of the last follow-up, with median OS of 81.4 months, while 1 patient died from non—bladder cancer—related causes (Figure 2A). In multivariate Cox regression, NAC was not associated with improved OS in the MPC cohort, when adjusted for age, gender, renal function and pathologic stage at RC (Table 4).
Figure 2.
Survival by Therapy Cohort. (A) OS in Institutional MPC Cohort, Stratified by NAC Receipt and Pathologic Response, for Patients With cT2-4 Stage Disease. (B) OS in Institutional CUC Cohort, Stratified by NAC Receipt and Pathologic Response, for Patients With cT2-4 Stage Disease
Abbreviations: CUC = conventional urothelial carcinoma; MPC = micropapillary urothelial carcinoma; NAC = neoadjuvant chemotherapy; NAC = neoadjuvant chemotherapy; NR = not reached; OS = overall survival; RC = radical cystectomy.
In the CUC cohort, patients with cT2-4 stage (n = 327) treated with NAC plus RC had significantly longer median OS compared to those undergoing RC only (120 vs. 90.3 months, P = .042). However, in multivariate Cox regression for OS, NAC was not independently associated with OS when adjusted for other clinically relevant variables (Table 4). Patients who achieved ypT0N0 had the highest OS benefit both in the CUC and the MPC cohort (Figure 2A and B).
MPC Data From SEER-Medicare
Out of 90,899 cases of bladder cancer screened in SEER-Medicare (2004-2015), 183 cases (0.2%) of MPC were identified. Composite stage breakdown was Ta/Tis in 12 (7%), T1 in 59 (32%), T2 in 70 (38%), T3 in 25 (14%) and T4 in 17 (9%) patients; nodal disease (N1-3) was identified in 38 (21%) and distant metastasis in 19 (10%). RC was the treatment of choice for 63 patients. NAC was administered to 27 (43%) of 63, with 18 (67%) of 27 receiving a cisplatin-based regimen. Median follow-up time was 47 months (95% CI, 33.7-60.3) in the RC cohort and median OS was 25 months (95% CI, 19.1-30.9). No significant difference in OS was noted between patients with composite T2-4 stage who received NAC (25 months; 95% CI, 15.2-34.8) versus those treated with RC alone (21 months; 95% CI, 13.2-28.8, P = .410) (Figure 1D). In multivariate Cox-regression for patients treated with RC, advanced composite T and N stage were independently associated with shorter OS, while cisplatin-based NAC also showed a nonsignificant trend towards longer OS (hazard ratio, 0.45; 95% CI, 0.15-1.30, P = .140) (Table 5).
Table 5.
Results of Univariate and Multivariate Cox Regression for Overall Survival in SEER-Medicare Cohort of 63 Patients With Micropapillary Urothelial Carcinoma Treated With Radical Cystectomy
| Univariate Cox | Multivariate Cox | ||||
|---|---|---|---|---|---|
| Characteristic | Value | HR (95% CI) | P | HR (95% CI) | P |
| Age category | |||||
| 66-70 y | 17 (27) | Ref | Ref | ||
| 71-75 y | 22 (35) | 1.73 (0.76-3.96) | .196 | 1.56 (0.60-4.02) | .362 |
| 76-80 y | 13 (21) | 1.82 (0.67-4.92) | .238 | 1.43 (0.46-4.45) | .542 |
| 81+ y | 11 (17) | 1.96 (0.68-5.61) | .213 | 1.31 (0.36-4.72) | .683 |
| Sex assigned at birth | |||||
| Male | 52 (83) | Ref | Ref | ||
| Female | 11 (17) | 2.12 (0.89-5.04) | .091 | 1.58 (0.62-4.02) | .337 |
| T stage | |||||
| <T2 | <20%a | Ref | Ref | ||
| T2 | >30%a | 2.29 (0.62-8.44) | .214 | 2.76 (0.71-10.76) | .145 |
| T3/4 | 34 (54) | 7.10 (1.87-27.02) | .004 | 7.12 (1.71-29.72) | .007 |
| N stage | |||||
| N0 | 38 (60) | Ref | Ref | ||
| N+ | 25 (40) | 2.58 (1.31-5.06) | .006 | 3.02 (1.36-6.68) | .006 |
| NAC | |||||
| No NAC | 36 (57) | Ref | Ref | ||
| Non—cisplatin-based NAC | <20%a | 1.16 (0.49-2.71) | .738 | 0.98 (0.39-2.46) | .961 |
| Cisplatin-based NAC | >25%a | 0.60 (0.26-1.41) | .259 | 0.45 (0.15-1.30) | .140 |
Data are presented as n (%) unless otherwise indicated.
Abbreviations: CI = confidence interval; HR = hazard ratio; NAC = neoadjuvant chemotherapy; N = composite nodal stage; T = composite tumor stage.
Exact counts masked in compliance with Centers for Medicare and Medicaid Services/Surveillance, Epidemiology, and End Results (CMS/SEER) publication policy of no counts with fewer than 11 cases.
Discussion
In this study, we retrospectively assessed the oncologic outcomes and response to NAC of patients with bladder cancer and MPC, treated with RC in our institution, compared to a cohort of patients with CUC. We also investigated prognosis in relation to NAC utilization in a cohort of patients with MPC provided from SEER-Medicare database. In that regard, we demonstrated that MPC had an overall (< ypT2N0) and complete (ypT0N0) pathologic response to NAC that was comparable to CUC in our institution. This resulted in a longer but not statistically significant median OS in the NAC subgroup, with complete responders (ypT0N0) deriving the greatest survival benefit (Figure 2A). To add to that, data collected from SEER-Medicare MPC cohort also failed to demonstrate a significant association between OS and NAC receipt. Of note, cisplatin-based treatment had a hazard ratio of 0.45 (95% CI, 0.15-1.30) in the SEER cohort, a finding that dictates further investigation in larger patient samples. In addition, unadjusted comparison of median OS and RFS between MPC and CUC institutional cohorts showed significantly inferior outcomes for patients with MPC. However, presence of MPC was not independently associated with OS in multivariate analysis. Overall, these findings indicate that although MPC presents with more aggressive pathologic characteristics, micropapillary histology alone may not confer worse prognosis in comparison to CUC, after accounting for these adverse features, while cisplatin-based NAC may have a beneficial role in this variant.
In general, there is a lack of universal agreement on the role and efficacy of NAC in MPC (Table 6). A consensus statement provided by the European Society of Medical Oncology and European Association of Urology showed that 63% of providers favored offering NAC to patients with muscle invasive MPC.18 At the same time, varying pathologic complete response rates have been reported in the literature, with several small series reporting ypT0 rates of 0% to 13% for MPC.12,13,16,17 In contrast, Meeks et al14 reported a 28% ypT0 and 55% < ypT2 rate in patients with MPC who received NAC before cystectomy. OS was generally not improved with NAC in that study, unless ypT0 was achieved. Likewise, data from a systematic review19 report a pathologic complete response rate of 11% to 55% across studies, without improvement in OS and RFS. Similarly, in our cohort, 8 of 9 patients with ypT0N0 after NAC were still alive at the time of the last follow-up, achieving prolonged median OS, although NAC was not significantly associated with OS benefit for the entire MPC cohort. In converse, patients who did not achieve ypT0N0, showed a nonsignificant trend for worse median OS, compared to those proceeding directly to RC (Figure 2A). Also, we failed to detect an association between MPC predominance and response to NAC. The above observations may suggest that although there is a sizeable subset of micropapillary cases well-responsive to NAC, nonresponsive patients may lose significant time to definitive surgery and this could affect OS. This underlines the unmet need for optimized patient selection, potentially via predictive biomarker identification, that would guide the decision to administer NAC. In a clinical setting, it is recommended that these unique cases should be carefully evaluated by a multidisciplinary team of specialists, including real-time radiology and pathology review, in order to optimize treatment decisions.20
Table 6.
Summary of Studies Reporting Response to NAC in Patients With MPC Undergoing RC
| Study | No. MPC | NAC | < ypT2 | ypT0 | Median OS (Months) |
OS Benefit (NAC) |
|---|---|---|---|---|---|---|
| Present series | 46 | 27 (59) | 15 (56) | 9 (33) | 44 (NAC+)/33 (NAC−) | NS |
| Ghoneim12 | 37 | 7 (19) | 1 (14) | 0 (0) | 40 (all MPC cases) | NA |
| Sui13 | 380 | 32/96 (33)a | NA | 4 (13) | 45 (all MPC cases) | NS |
| Meeks14 | 44 | 29 (66) | 16 (55) | 8 (28) | 40 (all MPC cases) | NS |
| Mendiratta16 | 83 | 16 (19) | 4/12 (33)b | 1/12 (8) | 47 (all MPC cases) | NA |
| Vetterlein17 | 154 | 35 (23) | NA | 4 (11) | 52 (NAC+)/29 (NAC−) | NS |
Data are presented as n (%).
Abbreviations: MPC = micropapillary urothelial carcinoma; NA = not available; NAC = neoadjuvant chemotherapy; NS = not statistically significant; OS = overall survival; RC = radical cystectomy; yp = pathologic stage after NAC receipt.
A total of 96 of 380 patients with RC were included in analysis of response to NAC.
Pathologic data were available for 12 of 16 patients who underwent NAC + RC. Four patients had “downstaging” that was not explicitly described as < ypT2.
In terms of clinicopathologic characteristics, and consistent with the literature, we found high frequency of adverse pathology for MPC, including nodal spread,2,5 associated CIS,1,9 and LVI8 at RC (Table 1). Alvarado-Cabrero et al3 reported that patients with micropapillary histology had frequent nodal disease and LVI and demonstrated a significantly lower 3-year cancer-specific survival (40% vs. 55%), when compared with CUC. The authors also concluded that the presence of > 50% micropapillary component was associated with higher mortality risk. Similarly, a retrospective study, which included MPC and CUC cohorts, showed that MPC had higher propensity towards nodal disease than CUC at RC (46% vs. 22%).4 However, no significant differences in OS and RFS were identified and the amount of MPC present in the histology specimen was not associated with survival. Meanwhile, Wang et al6 demonstrated that patients with MPC were more likely to have pT3/4 stage (66% vs. 35%), positive lymph nodes (50% vs. 10%) and LVI (73% vs. 24%) compared to CUC. Ten-year cancer-specific survival was significantly shorter in the MPC group (31% vs. 53%) but the difference was not significant after stage-adjustment between the groups. The percentage of MPC component in that study did not seem to impact cancer-specific survival. Another study with CUC as a comparison group, showed inferior OS in patients with micropapillary histology, while the presence of MPC along with TNM stage were considered as significant predictors of survival in multivariate analysis.7 On the other hand, a recent systematic review and meta-analysis in patients with MPC showed that micropapillary histology was not associated with OS and RFS when adjusted for other pathologic factors at RC.19 Overall, literature data seems to concur that MPC is associated with high incidence of advanced stage at diagnosis and aggressive pathologic features at RC, as well as inferior OS and RFS compared to CUC. However, most literature data sets also indicate lack of significant MPC association with survival outcomes, when other clinicopathologic factors are included in the multivariate analyses, which is consistent with our findings.
This study has the inherent caveats of being a retrospective cohort, including small sample size for the MPC subgroup in a single center, potential selection and confounding biases (including confounding by indication in the NAC vs. no NAC groups), variable treatments and monitoring schedules, as well as loss to follow-up in a number of patients, which could have affected the reported outcomes to a certain extent. Those caveats could probably explain why we did not see a significant benefit of NAC in the CUC group in the multivariate analysis, despite achieving a ypT0N0 rate comparable to the literature and the known OS benefit derived from NAC in nonvariant UC.21 In terms of histology, micropapillary percentage in the pathologic specimen was inconsistently reported, while the majority of MPC cases were nonpredominant and this could potentially have affected the relatively high rates of pathologic response, although we failed to detect such an association. In addition, interpretation of a histologic component as micropapillary may differ across specialists; thus, availability or observer bias is possible.22 However, the cases were reviewed by a few very highly trained genitourinary expert pathologists in a single department at our tertiary center. We did not evaluate specific molecular biomarkers in this study. Lastly, associations and identification of potential prognostic factors can be deduced but direct causality cannot be inferred in the retrospective setting. Data extracted from SEER database were also lacking granularity in terms of clinical versus pathologic stage and response to NAC, as the database was built to collect only the highest stage encountered at every patient. As a result, pathologic complete response (ypT0N0) could not be assessed in that cohort. In addition, a relatively small number of patients was yielded from this database. This could be explained by the fact that the SEER-Medicare population was limited to those with full coverage so that healthcare utilization could be fully characterized. Roughly 40% of the Medicare population has managed care which excludes them from analysis. Furthermore, SEER only covers 34% of the US population, whereas the NCDB captures 70% of incident cancer cases in the US. Thus, it is not unexpected that there would be fewer cases in the SEER-Medicare linked files. To ensure data accuracy, we also crossed checked our case count in other published SEER populations.
Conclusion
We demonstrated that the presence of micropapillary component at TURBT correlates with aggressive clinicopathologic behavior, yet micropapillary histology was not independently associated with shorter OS, when adjusted for other adverse prognostic factors. In addition, we could not identify significant OS benefit with NAC before RC in the MPC setting, despite achieving pathologic overall and complete response rates comparable to CUC. These results highlight the fact that a subset of patients with MPC may benefit from NAC, creating a need for elucidating the underlying biology of MPC, with the aim to identify patient populations most suitable for this approach.
Clinical Practice Points.
MPC is a rare histology with limited data guiding NAC utilization.
We explored clinicopathologic characteristics and outcomes of MPC compared to CUC, related to NAC receipt, with data derived from an institutional cohort and SEER-Medicare.
Patients with MPC treated with NAC demonstrated comparable pathologic complete response rate to CUC in our institutional cohort, although NAC was not significantly associated with improved OS in the institutional and the SEER-MPC cohorts.
MPC showed a predilection for LVI and nodal infiltration and shorter OS compared to CUC, without independently predicting OS when adjusted for these aggressive pathologic features.
NAC could be a viable option in patients with MPC, although studies with larger sample sizes and biomarker assessment are needed in this setting.
Acknowledgments
A.R.K. is supported by the National Cancer Institute (training grant T32CA009515). We would also like to extend our gratitude to Seattle Translational Tumor Research for making this work possible.
Footnotes
Disclosure
S.P.P. reports honoraria, travel, accommodations, and expenses from Prime Education. J.L.G. reports research grant funding from Ferring Pharmaceuticals. D.W.L. reports DSMB for the POTOMAC study with AstraZeneca; consulting or advisory role for Astellas Pharma, Clovis Oncology, and Dendreon; and research funding form GenomeDx, Genomic Health, and MDxHealth. R.S. reports patents, royalties, other intellectual property from Global Cancer Technology. A.C.H. reports honoraria from Hotspot Therapeutics; research funding from eFFECTOR Inc; patents, royalties, or other intellectual property regarding mTOR modulators and uses thereof (patent 9629843; and “use of translational profiling to identify target molecules for therapeutic treatment,” publication 20140288097. J.K.L. reports research funding from Immunomedics. M.T.S. reports research grant funding to University of Washington from Janssen, AstraZeneca, Zenith, Pfizer, Immunomedics, Madison Vaccines, and Hoffman-La Roche. H.H.C. reports research funding from Astellas Medivation, Clovis Oncology, Color Foundation, INOVIO Pharmaceuticals, Janssen, and Sanofi; and royalties from UpToDate. E.Y.Y. reports consulting for Amgen, AstraZeneca, Bayer, Churchill, Dendreon, EMD Serono, Incyte, Janssen, Merck, Pharmacyclics, QED, Seattle Genetics, and Tolmar; institutional research support from Bayer, Daiichi-Sankyo, Dendreon, Merck, Taiho, and Seattle Genetics; and personal fees from Clovis (last 3 years). L.D.T. reports stock and other ownership interests in Lightspeed Micro research funding and Ventana Medical Systems; and patents, royalties, other intellectual property for using a lens on an open-top lightsheet microscope. R.B.M. reports research funding from AstraZeneca, ESSA, Ferring, and Janssen Oncology. P.G. reports consulting for AstraZeneca, Bayer, Bristol-Myers Squibb, Clovis Oncology, Driver, EMD Serono, Exelixis, Foundation Medicine, GlaxoSmithKline, Genentech, Genzyme, Heron Therapeutics, Janssen, Merck, Mirati Therapeutics, Pfizer, Roche, Seattle Genetics, and QED Therapeutics; participation in educational program for Bristol-Myers Squibb; institutional research funding from AstraZeneca, Bayer, Genentech, Merck, Mirati Therapeutics, Oncogenex, Pfizer, Clovis Oncology, Bavarian Nordic, Immunomedics, Debiopharm, Bristol-Myers Squibb, QED Therapeutics, GlaxoSmithKline, and Kure It Cancer Research, all unrelated to this study, within the last 3 years. J.L.W. reports royalties from UpToDate; clinical trials for Movember Foundation, Merck, Nucleix, and Altor Biosciences; and consulting for Sanofi Genzyme. The other authors have stated that they have no conflict of interest.
References
- 1.Amin MB, Ro JY, el-Sharkawy T, et al. Micropapillary variant of transitional cell carcinoma of the urinary bladder. Histologic pattern resembling ovarian papillary serous carcinoma. Am J Surg Pathol 1994; 18:1224–32. [DOI] [PubMed] [Google Scholar]
- 2.Comperat E, Roupret M, Yaxley J, et al. Micropapillary urothelial carcinoma of the urinary bladder: a clinicopathological analysis of 72 cases. Pathology 2010; 42:650–4. [DOI] [PubMed] [Google Scholar]
- 3.Alvarado-Cabrero I, Sierra-Santiesteban FI, Mantilla-Morales A, Hernandez-Hernandez DM. Micropapillary carcinoma of the urothelial tract. A clinicopathologic study of 38 cases. Ann Diagn Pathol 2005; 9:1–5. [DOI] [PubMed] [Google Scholar]
- 4.Fairey AS, Daneshmand S, Wang L, et al. Impact of micropapillary urothelial carcinoma variant histology on survival after radical cystectomy. Urol Oncol 2014; 32:110–6. [DOI] [PubMed] [Google Scholar]
- 5.Kamat AM, Dinney CP, Gee JR, et al. Micropapillary bladder cancer: a review of the University of Texas MD Anderson Cancer Center experience with 100 consecutive patients. Cancer 2007; 110:62–7. [DOI] [PubMed] [Google Scholar]
- 6.Wang JK, Boorjian SA, Cheville JC, et al. Outcomes following radical cystectomy for micropapillary bladder cancer versus pure urothelial carcinoma: a matched cohort analysis. World J Urol 2012; 30:801–6. [DOI] [PubMed] [Google Scholar]
- 7.Lopez-Beltran A, Montironi R, Blanca A, Cheng L. Invasive micropapillary urothelial carcinoma of the bladder. Hum Pathol 2010; 41:1159–64. [DOI] [PubMed] [Google Scholar]
- 8.McQuitty E, Ro JY, Truong LD, Shen SS, Zhai Q, Ayala AG. Lymphovascular invasion in micropapillary urothelial carcinoma: a study of 22 cases. Arch Pathol Lab Med 2012; 136:635–9. [DOI] [PubMed] [Google Scholar]
- 9.Holmang S, Thomsen J, Johansson SL. Micropapillary carcinoma of the renal pelvis and ureter. J Urol 2006; 175:463–6. [DOI] [PubMed] [Google Scholar]
- 10.Mitra AP, Fairey AS, Skinner EC, et al. Implications of micropapillary urothelial carcinoma variant on prognosis following radical cystectomy: a multi-institutional investigation. Urol Oncol 2019; 37:48–56. [DOI] [PubMed] [Google Scholar]
- 11.Willis DL, Flaig TW, Hansel DE, et al. Micropapillary bladder cancer: current treatment patterns and review of the literature. Urol Oncol 2014; 32:826–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ghoneim IA, Miocinovic R, Stephenson AJ, et al. Neoadjuvant systemic therapy or early cystectomy? Single-center analysis of outcomes after therapy for patients with clinically localized micropapillary urothelial carcinoma of the bladder. Urology 2011; 77:867–70. [DOI] [PubMed] [Google Scholar]
- 13.Sui W, Matulay JT, James MB, et al. Micropapillary bladder cancer: insights from the National Cancer Database. Bladder Cancer 2016; 2:415–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meeks JJ, Taylor JM, Matsushita K, et al. Pathological response to neoadjuvant chemotherapy for muscle-invasive micropapillary bladder cancer. BJU Int 2013; 111:E325–30. [DOI] [PubMed] [Google Scholar]
- 15.Humphrey PA, Moch H, Cubilla AL, Ulbright TM, Reuter VE. The 2016 WHO Classification of Tumours of the Urinary System and Male Genital Organs—Part B: Prostate and Bladder Tumours. Eur Urol 2016; 70:106–19. [DOI] [PubMed] [Google Scholar]
- 16.Mendiratta P, Barata PC, Koshkin VS, et al. Clinicopathologic factors, treatment patterns, and outcomes in micropapillary urothelial carcinoma (UC). J Clin Oncol 2018; 36:439. [Google Scholar]
- 17.Vetterlein MW, Wankowicz SAM, Seisen T, et al. Neoadjuvant chemotherapy prior to radical cystectomy for muscle-invasive bladder cancer with variant histology. Cancer 2017; 123:4346–55. [DOI] [PubMed] [Google Scholar]
- 18.Witjes JA, Babjuk M, Bellmunt J, et al. EAU-ESMO consensus statements on the management of advanced and variant bladder cancer—an international collaborative multistakeholder effort: under the auspices of the EAU-ESMO guidelines committees. Eur Urol 2020; 77:223–50. [DOI] [PubMed] [Google Scholar]
- 19.Abufaraj M, Foerster B, Schernhammer E, et al. Micropapillary urothelial carcinoma of the bladder: a systematic review and meta-analysis of disease characteristics and treatment outcomes. Eur Urol 2019; 75:649–58. [DOI] [PubMed] [Google Scholar]
- 20.Diamantopoulos LN, Winters BR, Grivas P, et al. Bladder cancer multidisciplinary clinic (BCMC) model influences disease assessment and impacts treatment recommendations. Bladder Cancer 2019; 5:289–98. [Google Scholar]
- 21.Yin M, Joshi M, Meijer RP, et al. Neoadjuvant chemotherapy for muscle-invasive bladder cancer: a systematic review and two-step meta-analysis. Oncologist 2016; 21:708–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sangoi AR, Beck AH, Amin MB, et al. Interobserver reproducibility in the diagnosis of invasive micropapillary carcinoma of the urinary tract among urologic pathologists. Am J Surg Pathol 2010; 34:1367–76. [DOI] [PubMed] [Google Scholar]