Introduction
The safety and efficacy of tofacitinib in Crohn’s disease (CD) has been studied in two phase II trials in patients with moderate-to-severe CD with no new safety signals observed, but no significant difference from placebo in the primary efficacy endpoint of clinical response.1–3 However, post-hoc analyses and smaller studies have observed clinical and biological response to tofacitinib in patients with CD.2, 4, 5 There is a paucity of real-world effectiveness and safety data for tofacitinib in non-FDA label usage in CD and Inflammatory Bowel Disease-Unclassified (IBD-U) patients.
Methods
Data was collected in the Tofacitinib Real-world Outcomes in Patients with ulceratIve colitis and Crohn’s disease (TROPIC) consortium (supplementary methods).6 The primary outcome was clinical response (>50% reduction in symptoms) at weeks 8 and/or 16 determined by Physician Global Assessment (PGA). Secondary outcomes included corticosteroid-free response/remission, clinical remission, and endoscopic remission (ulcer resolution).
Results
Seventy-six patients with CD and IBD-U were included with a median follow-up time of 7.6 months (IQR 3.3-12.1). Most patients (98.7%) had previously been treated with a biological therapy prior to initiating tofacitinib, with 48.7% having failed at least two prior classes of biological therapy. The dosing of tofacitinib was 10mg BID in the majority of patients during induction (75.0%) and maintenance (65.3%). The treatment outcomes stratified by CD or IBD-U are shown in figure 1A. Among 73 patients with recorded week 8/16 data, 46.6% were in clinical response, 39.7% in corticosteroid-free clinical response, 15.1% in clinical remission in and 13.7%. in corticosteroid-free clinical remission. Clinical response at last documented assessment (N = 75) was reported in 42.7% (20% in clinical remission), while 57.3% either had no response or lost response. Univariate comparison of baseline characteristics based on clinical response at 8/16 weeks found significant differences in male gender (p=0.002), BMI (p=0.04) and baseline hemoglobin (p=0.01) but not for disease location (ileal vs. ileal/ileocolonic, p = 0. 16) (Supplementary Table 1). In a multivariate analysis, male gender was associated with increased odds of clinical response (aOR 5.4; CI, 1.9 - 15.5, p = 0.002) and corticosteroid-free clinical response (aOR 4.2; CI,1.4-12.5, p = 0.009). Endoscopic remission among those with baseline ulcers was seen after initiation of tofacitinib in 44% (Figure 1A). The treatment outcomes were not statistically different based on the number of failed biologics prior to the initiation of tofacitinib (Figure 1B). Tofacitinib was discontinued in 54.5% of the patients (42/76), most frequently due to no response (30.3%) or loss of response (15.8%).
Figure 1:
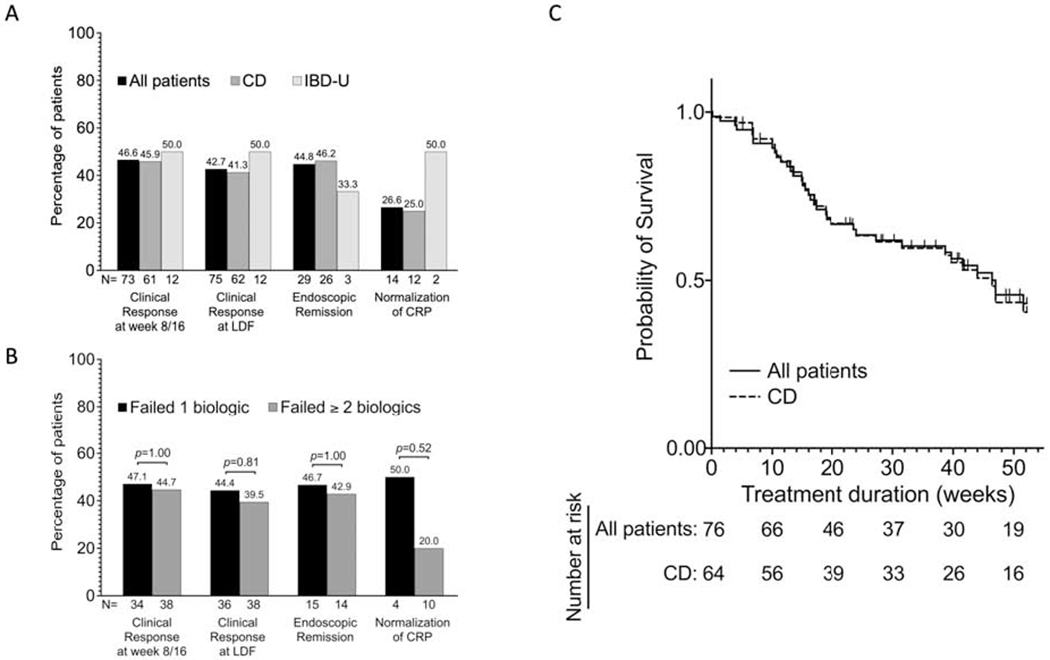
Real-world effectiveness of tofacitinib in moderate to severe Crohn’s disease and IBD-U- clinical, endoscopic and laboratory data.
A. Bars showing the percentages of all patients, Crohn’s Disease and IBD-U patients who had clinical response at week 8 and/or week 16, clinical response at last day of follow up (LDF), endoscopic remission (among those with abnormal endoscopy at baseline) and normalization of CRP (among those with elevated CRP at baseline) while on tofacitinib. N represents the total number of patients in each subgroup.
B. Association between number of prior failed biologics and the different outcomes shown in figure 1A. Bars represents proportion of patients in each outcome subgroup who failed one biologic and those who failed two or more biologics. Proportions were compared using Fisher exact test, and P-values are shown. N represents the total number of patients in each subgroup.
C. Kaplan-Meier survivor curve of tofacitinib persistence among all patients (64 CD and 12 IBD-U) and CD patients only during the first year of treatment. Failure event was defined as withdraw due to no response, loss of response or adverse events. All patients still on tofacitinib as at week 52 of treatment were censored. The median times on tofacitinib for all patients and CD patients only were 47.0 weeks and 46.4 weeks respectively.
CD, Crohn’s disease; CRP, C-reactive protein; IBD-U, Inflammatory bowel disease-unclassified; LDF, Last day of follow up.
AEs occurred in 17.1% (13/76) of our patients, with an incidence rate of 20.4 (16.5-26.9) per 100 patient years follow-up (Supplementary Table 2). Serious AEs occurred in 6 patients (7.9%) with a median age of 30 years and a median time to AE of 93 days [IQR, 19 – 329]. One patient developed HZ and there was one case of VTE (Supplementary Table 2). Surgical outcomes in patients undergoing surgery following tofacitinib therapy is included in supplementary table 2.
Discussion
Clinical response during induction in our cohort (46.6%) was lower than the rate of clinical response in prior clinical induction trials with 10mg BID of Tofacitinib in CD (69.8%). A possible explanation is the difference in baseline CD severity between the two cohorts. Our patient population had failed at least one (98.7%) or two (48.7%) classes of biologic therapy prior to initiating tofacitinib, whereas about 25% of the clinical trial cohort had not trialed a prior biologic, and no patients had received two prior biologic classes of therapy. Furthermore, 36.7% of placebo-treated patients in the clinical trial achieved clinical remission at 8 weeks, an unusually high placebo response rate.
In this cohort, male sex was associated with 4 to 5-fold increased odds of clinical response and corticosteroid-free response. Prior data in RA suggests that men may have overall increased response to biologic therapy.7 In IBD, the age at onset of IBD has been shown to vary with sex but to our knowledge this is the first report of sex-based difference in response to therapy in IBD8. Further prospective studies are needed to assess sex as a predictor of response to tofacitinib.
Limitations of the study include the retrospective design with the use of the PGA for clinical response assessment, and the lack of standardized endoscopic scoring criteria.
In summary, we report that tofacitinib is effective in achieving clinical response in a subset of real-world patients with CD and IBD-U refractory to prior biologic therapy. Additionally, we report no new significant safety signals with the use of tofacitinib in patients with refractory CD and IBD-U.
Supplementary Material
Acknowledgments
Grant support:
PD is supported by a Junior Faculty Development Award from the American College of Gastroenterology. M.A.C. is supported by DK109384, a Crohn’s and Colitis Foundation Daniel H Present Senior Research Award (Ref. 370763) and philanthropic support from the Givin’ it all for Guts Foundation (https://givinitallforguts.org) and the Lawrence C. Pakula MD IBD Research Innovation and Education Fund. Additional grant support for the REDCap database was provided by the Clinical and Translational Science Award (UL1 TR000448) and Siteman Cancer Center Support Grant (P30-CA091842). The content is solely the responsibility of the authors and does not necessarily represent the official views of the Center for Translational Medicine, the National Center for Advancing Translational Sciences, or the National Institutes of Health. RCU is supported by a Career Development Award from the Crohn’s and Colitis Foundation and an NIH K23 Career Development Award (K23KD111995-01A1). RPH is supported by a Career Development Award from the Crohn’s and Colitis Foundation
Disclosures:
Marc Fenster, Quazim A. Alayo, Aava Khatiwada, Wenfei Wang, Anish Patel, Gaurav Syal, and George P. Christophi disclose no relevant conflicts of interest.
Parakkal Deepak: PD has served as a consultant or advisory board member for Janssen and Pfizer, speaker’s bureau for AbbVie; research grants from Takeda.
Alexandra Gutierrez: AG has served as a speaker for Janssen.
Matthew A. Ciorba: MAC has done consultancy for Takeda and has been on the speaker bureau for AbbVie, Pfizer, Takeda, and UCB.
Jean-Frederic Colombel: JFC has served as an advisory board member or consultant for AbbVie, Amgen, Boehringer-Ingelheim, Arena Pharmaceuticals, Celgene Corporation, Celltrion, Enterome, Eli Lilly, Ferring Pharmaceuticals, Genentech, Janssen and Janssen, Medimmune, Merck & Co., Nextbiotix, Novartis Pharmaceuticals Corporation, Otsuka Pharmaceutical Development & Commercialization, Inc., Pfizer, Protagonist, Second Genome, Gilead, Seres Therapeutics, Shire, Takeda, Theradiag. Speaker for AbbVie, Ferring, Takeda, Celgene Corporation; stock options for Intestinal Biotech Development, Genefit; and research grants from AbbVie, Takeda, Janssen and Janssen.
David T. Rubin: DTR is a consultant for Abbvie, Abgenomics, Allergan, Inc., Arena Pharmaceuticals, Biomica, Bristol-Myers Squibb, Dizal Pharmaceuticals, Ferring Pharmaceuticals, Inc., Genentech/Roche, Janssen Pharmaceuticals, Lilly, Mahana Therapeutics, Medtronic, Merck & Co., Inc, Napo Pharmaceuticals, Pfizer, Prometheus Laboratories, Shire, Takeda, and Target PharmaSolutions, Inc.
Christina Ha: CH has served as a consultant or on advisory boards of AbbVie, Genentech, Janssen, Pfizer, Samsung Bioepis, and Takeda.
Ryan C. Ungaro: RCU has served as an advisory board member or consultant for Eli Lilly, Janssen, Pfizer, and Takeda; research grants from Abbvie, Boehringer Ingelheim, and Pfizer.
Robert P. Hirten: RPH has served as a speaker, a consultant, or an advisory board member for Takeda and Janssen and has received research support from Intralytix, Inc.
Joel Pekow: JP has received grants from Takeda and Abbvie, and serves as a consultant for Verastem. He was on the advisory board for Pfizer and Janssen.
Benjamin L. Cohen: BLC has served as a speaker, a consultant or, an advisory board member for Abbvie, Alfasigma, Allergan, Celltrion, Ferring, Grifols, Janssen, and Sublimity Therapeutics
Andres Yarur: AY has received consulting fees from Takeda Pharmaceuticals and Prometheus Laboratories and has served on the speakers bureau for AbbVie, Takeda Pharmaceuticals, and Prometheus Laboratories.
Abbreviations:
- AEs
Adverse events
- CI
Confidence interval
- CD
Crohn’s disease
- CRP
C-reactive protein
- DVT
Deep vein thrombosis
- FDA
Food and Drug Administration
- GI
Gastrointestinal
- HZ
Herpes Zoster
- IBD
Inflammatory bowel disease
- IL
Interleukins
- IQR
Interquartile range
- IRs
Incidence rates
- JAK
Janus Kinase
- MACE
Major adverse coronary events
- OLE
Open label extension
- OR
Odds ratio
- PE
Pulmonary embolism
- PGA
Physician Global Assessment
- PYF
Patient-Years Follow-up
- RA
Rheumatoid arteritis
- STROBE
Strengthening the Reporting of Observational Studies in Epidemiology
- TNF
Tumor Necrosis Factor
- TROPIC
Tofacitinib Real-world Outcomes in Patients with ulceratIve colitis and Crohn’s disease
- UC
Ulcerative colitis
- VTE
Venous thromboembolism
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Sandborn WJ, Ghosh S, Panes J, et al. A phase 2 study of tofacitinib, an oral Janus kinase inhibitor, in patients with Crohn’s disease. Clin Gastroenterol Hepatol 2014;12:1485–93 e2. [DOI] [PubMed] [Google Scholar]
- 2.Panes J, Sandborn WJ, Schreiber S, et al. Tofacitinib for induction and maintenance therapy of Crohn’s disease: results of two phase IIb randomised placebo-controlled trials. Gut 2017;66:1049–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Panes J, D’Haens GR, Higgins PDR, et al. Long-term safety and tolerability of oral tofacitinib in patients with Crohn’s disease: results from a phase 2, open-label, 48-week extension study. Aliment Pharmacol Ther 2019;49:265–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Theodorou E, Cao P, Karagiannis F, et al. P135 Biological evidence of tofacitinib efficacy in crohn’s disease. Gastroenterology 2018;154:S70–S71. [Google Scholar]
- 5.Sands BE, Panés J, Higgins PDR, et al. 14 Post-hoc analysis of tofacitinib Crohn’s disease phase 2 induction efficacy in subgroups with baseline endoscopic or biomarker evidence of inflammation. Gastroenterology 2018;154:S81. [Google Scholar]
- 6.Deepak P, Alayo QA, Khatiwada A, et al. Safety of Tofacitinib in a Real-World Cohort of Patients With Ulcerative Colitis. Clin Gastroenterol Hepatol 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hojgaard P, Ballegaard C, Cordtz R, et al. Gender differences in biologic treatment outcomes-a study of 1750 patients with psoriatic arthritis using Danish Health Care Registers. Rheumatology (Oxford) 2018;57:1651–1660. [DOI] [PubMed] [Google Scholar]
- 8.Shah SC, Khalili H, Gower-Rousseau C, et al. Sex-Based Differences in Incidence of Inflammatory Bowel Diseases-Pooled Analysis of Population-Based Studies From Western Countries. Gastroenterology 2018;155:1079–1089.e3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


