Abstract
Introduction:
Lung-MAP S1400K was designed to evaluate the response to telisotuzumab vedotin, an antibody-drug conjugate targeting c-MET, in patients with c-MET positive squamous cell carcinoma (SCC).
Methods:
Patients with previously treated SCC with c-MET positive tumors (H score ≥150, Ventana SP44 assay) were enrolled into 2 cohorts: cohort 1 (immune checkpoint inhibitor naïve) and cohort 2 (immune checkpoint inhibitor refractory). Telisotuzumab vedotin 2.7 mg/kg was administered intravenously every 3 weeks until disease progression or unacceptable toxicity. Response assessments were performed every 6 weeks. The primary endpoint was response by RECIST 1.1. Secondary endpoints included progression-free survival (PFS), overall survival (OS), response within cohort, duration of response, and toxicities. Interim analysis was planned after 20 evaluable patients, with ≥3 responses needed to continue enrollment.
Results:
Forty-nine patients (14% of screened patients) were assigned to S1400K, 28 patients enrolled (15 in cohort 1 and 13 in cohort 2) and 23 were eligible. S1400K closed on 12/21/2018 due to lack of efficacy. Two responses (response rate of 9%; 95% CI: 0–20%) were reported in cohort 1 (1 complete and 1 unconfirmed partial response), while 10 patients had stable disease with disease control rate of 52%. The median OS and PFS were 5.6 and 2.4 months. There were 3 grade 5 events (2 pneumonitis, in cohort 2, and 1 bronchopulmonary hemorrhage, in cohort 1).
Conclusion:
Telisotuzumab vedotin failed to meet the pre-specified response needed to justify continuing enrollment to S1400K. Pneumonitis was an unanticipated toxicity observed in patients with SCC.
Keywords: Squamous cell lung cancer, c-MET, antibody-drug conjugate
INTRODUCTION:
The original Lung Master Protocol (Lung-MAP), led by the SWOG Cancer Research Network, was developed as a cooperative effort by the National Cancer Institute (NCI) and the National Clinical Trials Network (NCTN) groups to efficiently evaluate biomarker-driven therapies in previously-treated squamous cell lung cancer (SCC) using an umbrella master protocol design.1,2 The overarching goal was to establish a single infrastructure for genomic screening of previously treated SCC patients to expeditiously evaluate targeted therapies in biomarker-matched subpopulations (biomarker-driven sub-studies), as well as therapies with broad activity for patients not eligible for the biomarker-driven sub-studies (non-match sub-studies) and streamline the approval process of efficacious regimens.
The MET proto-oncogene, located on chromosome 7q21-q31, encodes a cell surface receptor tyrosine kinase called c-MET, that is activated by its ligand hepatocyte growth factor (HGF), also known as scatter factor.3,4 Binding of HGF to MET leads to homodimerization and activation of kinase activity.5 HGF/MET pathway activation plays a crucial role in normal development through mediating mesenchymal-epithelial interactions in epithelial cells and the liver, and also plays a role in skeletal muscle development and guiding motor neuron development.6 MET pathway activation in malignancies can occur through activating mutations or MET amplification.7,8 Using the commercially available Ventana Benchmark anti-total c-MET SP44 rabbit monoclonal primary antibody, and a threshold of at least 2+ staining in 50% of cells, approximately 25–29% of non-small cell lung cancer (NSCLC) tumors are noted to be positive.9,10 MET amplifications occur in 3% of squamous cell carcinomas, while MET mutations are noted in 2% of squamous cell carcinomas in The Cancer Genome Atlas.11,12
Several therapeutic strategies have been employed to target MET in NSCLC, using monoclonal antibodies directed against either HGF or MET or tyrosine kinase inhibitors, but none have shown efficacy in unselected patient populations or in patients with MET expressing tumors, as measured by immunohistochemistry (IHC).13–15 Telisotuzumab vedotin (formerly known as ABBV-399) is a first-in-class antibody-drug conjugate (ADC) comprised of ABT-700, an anti-c-MET monoclonal antibody, that is linked to monomethyl auristatin E (MMAE), a potent microtubule inhibitor.16 ABT-700 inhibits HGF-dependent and HGF-independent c-MET activation.17 It inhibits c-MET receptor dimerization and has shown anti-tumor activity in MET amplified NSCLC and gastric cancer.17,18 Telisotuzumab vedotin is cytotoxic to cancer cells with c-MET over-expression and demonstrated antitumor activity in human tumor xenografts. In a first in human phase 1 dose escalation and expansion clinical trial of telisotuzumab vedotin in solid tumors, the maximum tolerated dose was not formally identified, though the recommended phase 2 dose was defined is 2.7 milligrams/kilogram on the basis of overall safety and tolerability.19 In the 16 patients with c-MET positive NSCLC, the overall response rate was 18.8% with 3/16 patients achieving a partial response (PR), all of whom had squamous histology. The most frequent grade 3 or higher adverse events related to telisotuzumab vedotin were fatigue, anemia, neutropenia, and hypoalbuminemia, each observed in 4.2% of patients.19
SCC is a disease where there is a dire need for effective targeted therapies, and given the 29% frequency of c-MET overexpression in squamous lung cancer, telisotuzumab vedotin addresses an unmet need.9 Here we report the results of SWOG S1400K, a phase 2 study evaluating telisotuzumab vedotin in patients with previously treated metastatic c-MET positive squamous cell carcinoma of the lung.
PATIENTS AND METHODS:
Eligible patients identified through the screening platform were required to have c-MET IHC positive SCC as defined by an H-score of ≥ 150 membrane staining using the Ventana SP44 assay. Tissue specimens collected for the S1400 biomarker profiling were used, which were shipped by sites to Foundation Medicine, Inc. (FMI). Two 4–5 micron unstained, charged and unbaked formalin fixed and paraffin embedded (FFPE) slides per patient were prepared by FMI and shipped to ARUP Laboratories for staining within 2 calendar days, which were then scored by Flagship Biosciences. Flagship then sent the c-MET IHC report back to FMI, where the results were compiled and sent to SWOG Statistical Center. Patients could be enrolled prior to disease progression (defined as prescreening) or at the time of disease progression (defined as screening). Sub-study specific eligibility criteria included presence of measurable disease, ECOG performance status of 0 or 1, adequate organ function, peripheral neuropathy ≤ grade 2, edema ≤ grade 2, albumin ≥3, hepatic involvement by tumor <50% (due to potential for hepatotoxicity from telisotuzumab vedotin), and patients with treated and stable brain metastases were allowed. Patients were enrolled into 2 cohorts: Cohort 1 (immune checkpoint inhibitor (ICI) naïve) and cohort 2 (ICI refractory), which included patients with a history of prior ICI therapy at any point in the course of their treatment.
All patients had tissue that underwent next generation sequencing (NGS) testing by Foundation Medicine, Inc (FMI). Mutational analysis was performed on archival formalin-fixed paraffin-embedded (FFPE) tumor specimens in a Clinical Laboratory Improvement Amendments (CLIA)-certified and College of American Pathologists (CAP)-accredited laboratory (Foundation Medicine, Cambridge, MA).
Telisotuzumab vedotin was administered intravenously at a dose of 2.7 mg/kg over 30 minutes on day 1 of 21 day cycles. Disease response assessment occurred every two cycles (every 6 weeks), and treatment was continued until disease progression or untoward toxicity. Dose reductions and adjustments had to be discussed with the study chair and were followed as specified in the protocol (appendix).
Statistical Considerations
The primary objective was to evaluate the RECIST 1.1 response rate (RR; confirmed and unconfirmed, complete and partial) in patients with SCC treated with Telisotuzumab vedotin.20 The accrual goal was 40 response-evaluable patients, although accrual was stratified into two cohorts: 1) patients naïve to ICI and 2) patients previously-treated with ICI therapy. The sample size was based on a design with 91% power to rule out a RR of 15% at the 1-sided 5% level, if the true rate were 35%. The observation of 10/40 (25% RR) would be considered evidence to rule out RR of 15% and evidence to pursue an independent randomized phase III trial. The design included an interim analysis at 20 patients evaluable for response. If 2 or fewer responses were observed, accrual to the study would be terminated with the conclusion of insufficient evidence to continue. A key secondary objective was an assessment of investigator-assessed progression-free survival (IA-PFS). If the study continued to full accrual and the RR rate was less than 25% but the median IA-PFS was at least 4.5 months, this could be considered sufficient evidence to continue to the follow-on Phase III. With 40 patients, this design had 90% power to rule out a median IA-PFS of 3 months or less, if the true mPFS were 6 months, at the 0.05 1-sided level. The observation of a median IA-PFS of at least 4.5 months would be considered evidence to rule out a median IA-PFS of 3 months or less. Other objectives included an assessment of duration of response (DoR) among responders and evaluation of the frequency and severity of toxicities.
Binary proportions and associated 95% confidence intervals were estimated. Survival distributions were estimated using the method of Kaplan-Meier and the Brookmeyer-Crowley method was used to estimate confidence intervals around median times.
RESULTS:
Demographics:
S1400K was activated on February 5, 2018 with the first patient enrolled on March 16, 2018. In total, c-MET testing was performed on samples from 364 patients, 68 of these sample were unevaluable (18.7% tissue fail rate), and 102 samples tested positive (34.5% of evaluable samples) and 194 tested negative. Of the 102 positive samples, 49 patients (32 screened at progression and 17 pre-screened) were assigned to S1400K. S1400K was temporarily closed for safety concerns and for preplanned interim analysis on October 18, 2018, and finally closed for futility on December 21, 2018. Of these 49 patients (Figure 1), 28 patients were enrolled;(15 patients to cohort 1 (immunotherapy naïve), and 13 patients to cohort 2 (immunotherapy exposed). Of these 28 patients registered, 5 patients were not included in any analyses due to the following reasons: 2 patients were ineligible due to baseline scans performed outside of protocol specific window, 1 patient was ineligible due to extent of liver lesions and never started protocol treatment, 2 patients did not receive protocol treatment (1 of these patients died).
Figure 1:
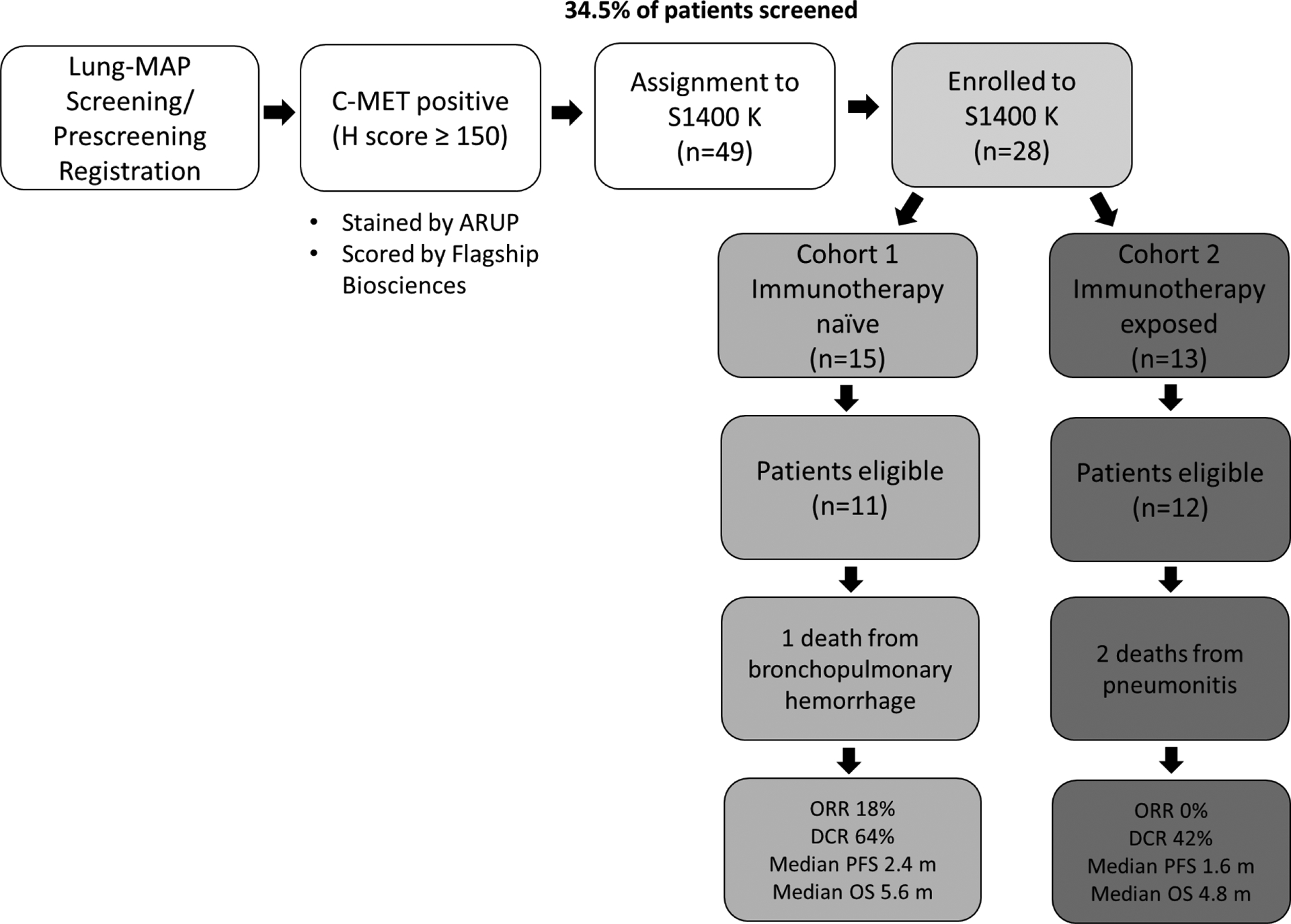
Flow diagram showing patients screened and enrolled to S1400K
Of the 21 patients who did not register: 7 patients were not eligible, 4 patients had symptomatic deterioration, 1 patient passed away, 3 were not enrolled due to investigator decision, 3 patients refused, 2 patients were not eligible for the screening study and 1 Canadian patient did not have study drug available.
Table 1 shows the demographics of the 23 eligible and analyzable patients of whom 11 were enrolled to Cohort 1 and 12 patients to Cohort 2. The median age of patients enrolled to the study was 65 years (range 58–82); 13/23 (57%) were males and 21/23 (91%) were white. Eighteen out of 23 patients (78%) had ECOG performance status of 1 and 17/23 (74%) had less than 5% weight loss in the past 6 months. Seven patients were current smokers (30%), 15 former smokers (65%), while 1 patient was a never smoker (4%). Nine of 23 patients (39%) had received 2 or more prior lines of treatment for stage 4 disease.
Table 1.
Patient Demographics
| N = 23 | |
|---|---|
| Age median(range) | 65.3 (57.7–81.6) |
| Male gender | 13 (57%) |
| Race/Ethnicity | |
| White | 21 (91%) |
| Asian | 2 (9%) |
| Hispanic | 0 (0%) |
| Prior anti-PD-(L)1 Therapy | 12 (52%) |
| Number of lines of prior therapy for Stage IV SCC | |
| 0 | 7 (30%) |
| 1 | 7 (30%) |
| 2 | 5 (22%) |
| 3 | 3 (13%) |
| 4 | 1 (4%) |
| ECOG Performance Status | |
| 0 | 5 (22%) |
| 1 | 18 (78%) |
| Weight Loss in the last 6 months | |
| < 5% | 17 (74%) |
| 5 – < 10% | 3 (13%) |
| 10 – < 20% | 3 (13%) |
| Smoking Status | |
| Current | 7 (30%) |
| Former | 15 (65%) |
| Never smoker | 1 (4%) |
| Brain metastases at baseline | 2 (9%) |
Table 2 describes the H-score values for the MET IHC assay among the 23 patients enrolled. The median score was 180 (range 150–260). This table also describes the gene alterations detected on the FMI NGS screening as required by the Lung-MAP protocol. Exon 14 c-MET mutation was noted in one patient sample.
Table 2.
Study Biomarker and Gene Alterations Detected on FMI NGS Screening
| H-score (S1400K biomarker) | N = 23 | |
|---|---|---|
| Median | 180 | |
| Range | 150–260 | |
| Interquartile Range | 160–230 | |
| Frequency of detection of other Concomitant Gene Alterations (N=22)* | N | % |
| Short Variants | ||
| TP53 | 21 | 95.5% |
| MLL2, NF1 | 4 | 18.2% |
| CDKN2A, PARP4, RB1 | 3 | 13.6% |
| AKT1, KEAP1, KRAS, NFE2L2, | 2 | 9.1% |
| MET EXON 14 | 1 | 4.5% |
| ALK, APC, ARID1A, CDH1, | 1 | 4.5% |
| Copy Number Alterations | ||
| CDKN2A, CDKN2B | 5 | 22.7% |
| FGFR1 | 4 | 18.2% |
| MDM2 | 2 | 9.1% |
| AKT1, BAP1, CCND1, CDK4, | 1 | 4.5% |
| Rearrangements | ||
| CDKN2A, MLH1, RAF1 | 1 | 4.5% |
One patient had unsuccessful NGS testing, but was still found to be c-MET positive, thus this patient has no additional biomarker data. Variants with same frequency are grouped together for ease of reporting (e.g. “MLL2, NF1” indicates that 4 patients were found to have the MLL2 alteration and 4 patients had the NF1 alteration).
Treatment Summary:
Patients received a median of 3 cycles of telisotuzumab vedotin with a range of 1–21 cycles and interquartile range of 2–5 cycles. One patient remains on treatment with 22 patients off treatment for the following reasons:, 18 (82%) due to progressive disease, 3 (14%) due to side effects of the drug and 1 due to death.
Adverse events:
There were 3 deaths on study possibly attributed to treatment (Table 3): 1 patient in cohort 1 died from a bronchopulmonary hemorrhage and 2 patients in cohort 2 died from pneumonitis. The patient in Cohort 1 had previous hemoptysis and a central tumor, and also had grade 3 anorexia. Both patients in Cohort 2 who died from pneumonitis experienced Grade 3 and 4 events prior to death. One patient had had grade 4 neutropenia, grade 4 respiratory failure and several preceding grade 3 events reported including febrile neutropenia, lymphopenia, urinary tract infection, fatigue, nausea, vomiting, dehydration, increased bilirubin, increased AST, hypokalemia, hypocalcemia, and hypophosphatemia. The second patient had grade 3 fatigue, grade 4 cardiac arrest, with no prior cardiac history, and a history of pneumonitis that had resolved prior to initiating telisotuzumab vedotin. The pneumonitis developed after 3 doses of telisotuzumab vedotin. No additional patients experienced Grade 4 events. Four (17%) additional patients experienced Grade 3 events attributed to treatment, which included anemia (4%), hyponatremia (4%), hypophosphatemia (4%) and peripheral sensory neuropathy (4%).
Table 3.
Grade 3 or higher Adverse Events Attributable to Treatment
| Adverse Event | Grade | ||
|---|---|---|---|
| 3 | 4 | 5 | |
| Anemia | 1(4%) | ||
| Anorexia | 1(4%) | ||
| AST increased | 1(4%) | ||
| Blood bilirubin increased | 1(4%) | ||
| Bronchopulmonary hemorrhage | 1(4%) | ||
| Cardiac arrest | 1(4%) | ||
| Dehydration | 1(4%) | ||
| Fatigue | 2(9%) | ||
| Febrile neutropenia | 1(4%) | ||
| Hypocalcemia | 1(4%) | ||
| Hypokalemia | 1(4%) | ||
| Hyponatremia | 1(4%) | ||
| Hypophosphatemia | 2(9%) | ||
| Lymphocyte count decreased | 1(4%) | ||
| Nausea | 1(4%) | ||
| Neutrophil count decreased | 1(4%) | ||
| Peripheral sensory neuropathy | 1(4%) | ||
| Pneumonitis | 2(9%) | ||
| Respiratory failure | 1(4%) | ||
| Urinary tract infection | 1(4%) | ||
| Vomiting | 1(4%) | ||
| Maximum Grade of any adverse event | 4(17%) | 0 | 3(13%) |
Response to treatment:
Two patients in cohort 1 responded (Figure 2), 1 patient had a complete response while the 2nd had unconfirmed partial response for a response rate of 9% (95% CI: 0–20%). The second patient had disease progression at the next assessment. The patient with complete response remains on treatment at 12.7+ months. The patient with unconfirmed partial response had a duration of response of 2.3 months. Ten patients had stable disease as their best response, and the disease control rate was 52% (95% CI: 32–73%). . MET amplifications were not observed. The 2 responders did not have met Exon 14 mutation on molecular testing of the tumor. The patient with MET exon 14 had progressive disease at first disease assessment.
Figure 2.
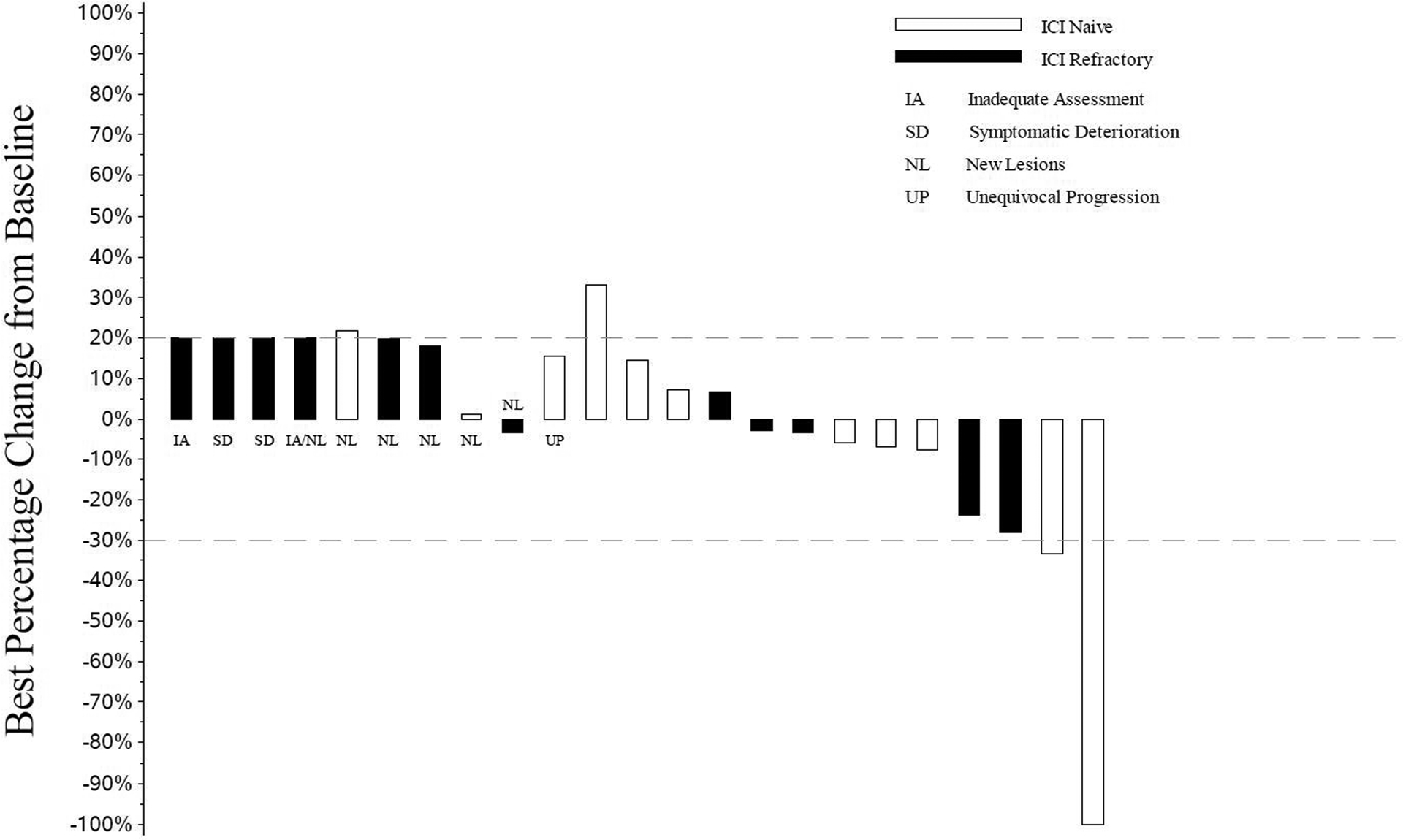
Waterfall Plot with Annotations for Individual Alterations
All patients who received at least one dose of telisotuzumab vedotin are represented in this plot.
- IA: Measurements for patients who did not have any follow-up tumor disease assessments are are labeled with “IA” and displayed as a 20% increase, the threshold for progressive disease.
- SD: Measurements for patients who had symptomatic deterioration at first disease assessment are labeled as “SD” and displayed as a 20% increase, the threshold for progressive disease.
- NL: Measurements for patients who had progressive disease at their first assessment based on new lesions are labeled by “NL”. Their best percent decrease in tumor burden is displayed.
- UP: Measurements for patients who had unequivocal progression in non-target lesions at their first follow-up assessment are labeled as “UP”. Their best percent decrease in tumor burden is displayed.
- Unlabeled bars: To the right of the labeled bars are bars representing the change in measurements for patients not coded as SD, NL, or UP with at least one follow-up disease assessment.
Bars extending above the dashed line at +20% or labeled as IA, SD, NL, or UP are coded as progressive disease. Unlabeled bars between the dashed lines (+20% to −30%) are coded as best response of stable disease and unlabeled bars extending below the −30% line are coded as best response of partial or complete response.
Figure 3 depicts the Kaplan-Meier curves for OS and PFS. The median overall survival for all patients was 5.6 months (95% CI 3.9–9.5 months) and median IA-PFS was 2.4 months (95% CI 1.4–3.0 months). There was no correlation between H score (per 10 unit increase) and PFS (HR 1.04, 95% CI:0.92–1.18, p=0.53) or OS (HR 0.96, 95% CI : 0.84–1.09, p=0.51).
Figure 3.
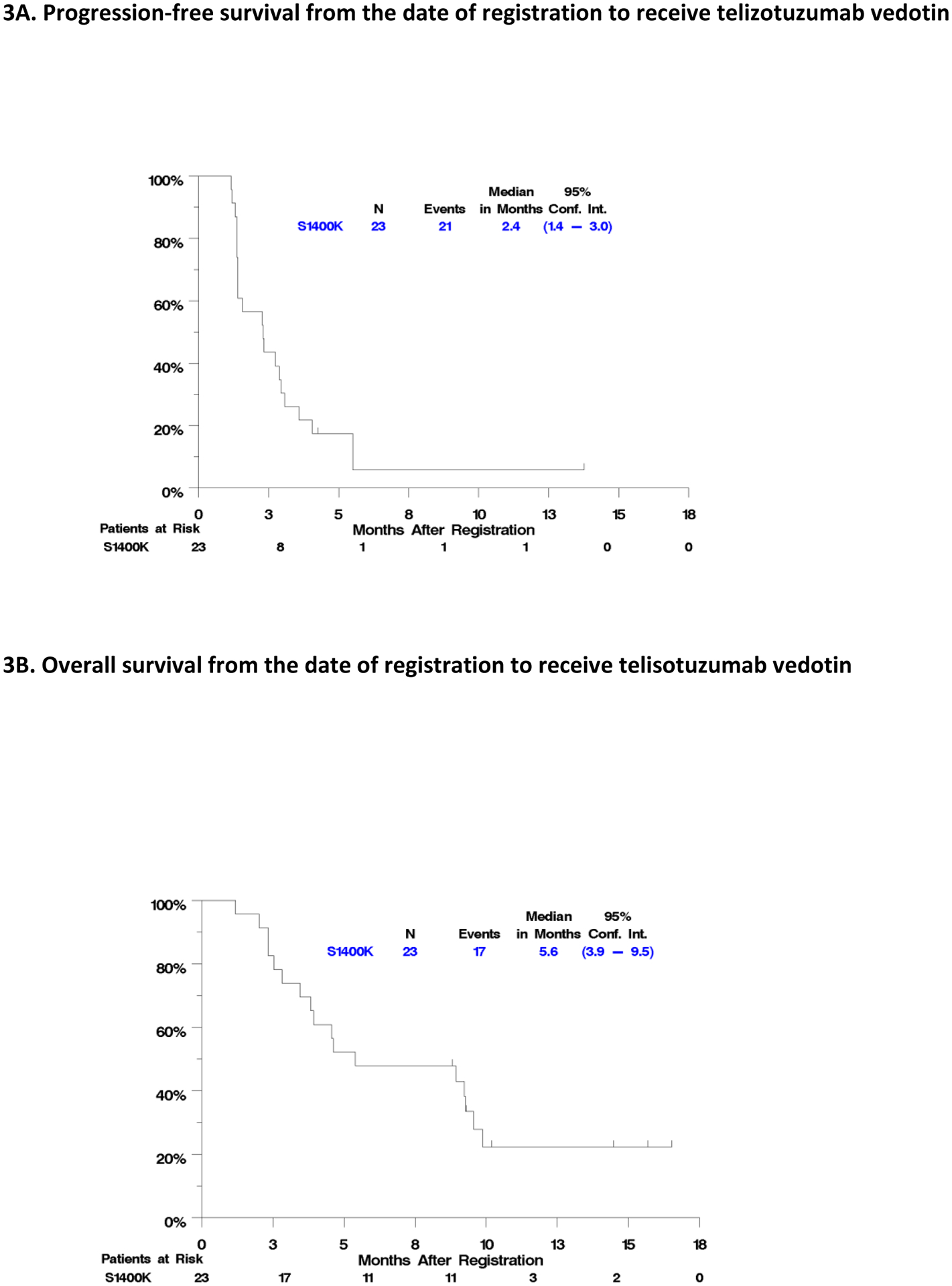
PFS and OS for Eligible Patients
DISCUSSION:
c-MET overexpression has been reported in NSCLC, with varying prevalence depending on method of evaluation and scoring. For example, MET positivity was has been reported in 25% – 29% of patients with SCC.9,10,21 In LUNG MAP, increased c-MET expression occurred in 34.5% of those screened, consistent with prior observations. Though MET protein overexpression is common in NSCLC, strategies thus far to select patients based off this for treatment with anti-MET monoclonal antibodies and small molecule inhibitors of MET have failed to show clinical benefit in large randomized studies, possibly related to the failure of the companion diagnostic.22 Preliminary evidence from a phase I study demonstrating antitumor activity in a subset of lung cancer patients with squamous cell histology and an acceptable safety profile warranted further evaluation in this patient population. However, our study was closed due to futility based on interim analysis results. The response rate seen was lower than that described in the phase 1 clinical trial of telisotuzumab vedotin in c-MET positive NSCLC, where 3/16 c-MET positive patients with NSCLC treated with telisotuzumab vedotin at a dose of 2.4–3.0 mg per kg had a partial response; all patients with response had squamous histology. Despite not meeting threshold for continuation to stage II, telisotuzumab vedotin did show clinical activity with a disease control rate of 52%, which is a meaningful benefit in patients with refractory SCC, where there is an unmet need for improved therapeutic options.
Pneumonitis was an unanticipated toxicity observed in our study, and was not an expected off-target effect, as c-Met is expressed in epithelial cells of the organs including liver, pancreas, kidney muscle and prostate, but is not expressed in the lung tissue.23 Pneumonitis has been reported with additional antibody drug conjugates, including brentuximab vedotin, which shares the same cytotoxic drug MMAE to telisotuzumab vedotin.24,25
There were 2 deaths from pneumonitis in our study. The first patient received 2 doses of telisotuzumab vedotin and developed pneumonitis 27 days after starting treatment, while the second patient received 3 doses of telisotuzumab vedotin and developed pneumonitis 42 days after starting treatment. Both patients had previously been exposed to immunotherapy, and 1 of them had a previous history of pneumonitis from immunotherapy requiring treatment with steroids 2–3 months prior to initiating study treatment, and one of them had prior thoracic radiation. While the relationship of these events to telisotuzumab vedotin was not clear, the study was placed on temporary closure for safety concerns and the protocol was amended to exclude patients with immune mediated pneumonitis or interstitial lung disease from prior immune checkpoint inhibitor and additional guidance on pneumonitis management was included. The baseline and on-study CT scans for these two patients were reviewed by the SWOG PI, co-PI and the radiology committee, who agreed that there was no evidence of pneumonitis on imaging at baseline, but emergent pneumonitis occurred after exposure to telisotuzumab vedotin. A detailed investigation into 217 patients who had previously received telisotuzumab vedotin on any study showed that only 1 immunotherapy naïve patient had grade 2 pneumonitis.
Despite closing for futility, S1400K provides valuable lessons. First, we demonstrated feasibility of adding additional biomarker tests to the existing Foundation One platform in a nimble manner, without added delays in patient assignment to this substudy. Second, we showed in a large cohort that the frequency of c-MET positivity was consistent with known c-MET expression in NSCLC, and corroborated results from the phase 1 study of telisotuzumab vedotin, in which responses were associated with qualitative c-MET positivity as determined by H score greater than 150, rather than quantitative higher H-scores being associated with depth of response. However, several MET-IHC antibodies are available and whether SP-44 assay used in the present study is the most optimal remains to be discussed, as well as the applied cut-off value for “positive” versus “negative” tumors. Interobeserver variability in MET-IHC assessment was recently published.26 While IHC was chosen as the primary biomarker assay in this study, it was recently demonstrated that Met-IHC is a poor screen for Met-amplification or -mutations, which eventually could be more useful biomarkers in retrospect.27 Third, we demonstrated commitment to patient’s safety with immediate notification to regulatory bodies as determined by SWOG protocol, notification of investigators and treating sites of pneumonitis risk, with amendment of the protocol eligibility and guidance on management of pneumonitis, which has also impacted inclusion criteria for other protocols using this agent. Finally, despite not meeting criteria to progress to phase 2, telisotuzumab vedotin did show meaningful clinical activity in the study, and development of this agent is currently being pursued by AbbVie as part of an ongoing phase 2 clinical trial (NCT03539536) in patients with platinum and immunotherapy exposed refractory metastatic NSCLC. This study has an estimated sample size enrollment of up to 310 participants, and includes telisotuzumab vedotin monotherapy cohorts of patients with non-squamous EGFR wild-type, EGFR mutant and squamous NSCLC.
CONCLUSION:
Telisotuzumab vedotin failed to meet the pre-specified response needed to justify continuing enrollment to S1400K. Pneumonitis was an unanticipated toxicity observed in patients with SCC.
Supplementary Material
CLINICAL PRACTICE POINTS:
Lung Master Protocol (Lung‐MAP) was developed as a public‐private partnership between the National Cancer Institute (NCI), Food and Drug Administration (FDA), and industry. Led by the SWOG cancer research network with the National Clinical Trials Network (NCTN) groups, the S1400 Lung‐MAP trial was opened in 2014 to evaluate biomarker‐driven therapies in previously treated squamous cell lung cancer (SCC) using an umbrella design. Lung-MAP Sub-study S1400K is an example of how a master protocol trial design allowed for rapid accrual to evaluate response to telisotuzumab vedotin, an antibody drug conjugate targeting c‐MET, in patients with c‐MET positive squamous cell carcinoma. Interim analysis was planned after 20 evaluable patients, with ≥3 responses needed to continue enrollment. This study was closed on 12/21/2018 due to lack of efficacy. Telisotuzumab vedotin failed to meet the pre-specified response needed to justify continuing enrollment to S1400K. Pneumonitis was an unanticipated toxicity observed in patients with SCC.
Funding:
Lung-MAP trial supported in part by NIH/NCI grants CA180888, CA180819, CA180820, CA180821, CA180868, CA189997, CA189858, CA189873, CA180828, CA189830, CA189971, CA189952, CA189804, CA239767, CA189808, CA189958 and by AbbVie through the Foundation for the National Institutes of Health, in partnership with Friends of Cancer Research.
Footnotes
Conflicts of Interest
Dr. Waqar reports grants from 1 UM1 CA186704–01 outside of this submitted work, and research support to Washington University School of Medicine from AbbVie outside of this submitted work. Dr. Arnold reports grants from AbbVie for other clinical trials, outside the submitted work. Dr. Hirsch has received research grants from AbbVie for laboratory studies at University of Colorado (through University of Colorado), laboratory support (through University of Colorado) from Merck, Biodesix, Amgen, Rain Therapeutics and Mersana and has also participated in scientific advisory boards for AbbVie, BMS, Merck, Genentech, Lilly, Novartis, AstraZeneca. Dr. Mack reports personal fees from AstraZeneca and Amgen outside the submitted work. Dr. Schwartz has served as a DSMB member for independent review of response assessment imaging studies blinded to treatment and outcomes for Merck, Boehringer Ingelheim, Hoffman La Roche and Novartis outside this submitted work. Dr. Gandara has served on the advisory board for AbbVie, outside of this submitted work. Dr. Ramalingam reports personal fees for Advisory board from AbbVie, Amgen, BMS, Genentech, Lilly, Takeda and Loxo and grants from AstraZeneca, Merck, and Tesaro outside the submitted work. Dr. Kelly reports research grants and personal fees for Advisory Board from AbbVie outside this submitted work, personal fees from AstraZeneca for participation in Advisory Board and DMC meeting, research grant (drug only) and personal fees for Advisory Board from Bristol Meyers Squibb, research funding (drug only) and personal fees for DMC meeting from Genentech, personal fees for Advisory Board from Pfizer outside the submitted work. Dr. Herbst reports personal fees from Abbvie Pharmaceuticals, ARMO Biosciences, Biodesix, Bristol-Myers Squibb, EMD Serrano, Genmab, Halozyme, Heat Biologics, Loxo Oncology, Nektar, Novartis, Pfizer, Sanofi, Seattle Genetics, Shire PLC, Spectrum Pharmaceuticals, Symphogen, Tocagen, Tesaro, IMAB Biopharma, Immunocore, Midas Health Analytics, Mirati Tnerapeutics, and Takeda; and grants and personal fees from AstraZeneca, Merck and Company, Eli Lilly and Company, Genentech/Roche; personal fees for Scientific Advisory Board from Infinity Pharmaceuticals, , Neon Therapeutics, and NextCure; and personal fees for Board Membership (non-executive/ independent) from Junshi Pharmaceuticals outside the submitted work. Dr Papadimitrakopoulou is currently an employee of Pfizer, Inc. and has received personal fees for Advisory Board from Abbvie, and personal fees from Eli Lilly, Novartis, Merck, Nektar Therapeutics, Janssen, Araxes, Arrys Therapeutics, Clovis Oncology, Exelixis, Gritstone, Ideaya, Leeds Biolabs, Loxo Oncology, Takeda, Tesaro, TRM Oncology outside this submitted work, and grants from Eli Lilly, Novartis, Merck, Nektar Therapeutics, Janssen Checkmate and Incyte outside the submitted work.
No Conflicts of Interest
Dr. Redman, Dr. Stinchcombe, Dr. Leighl, Dr. Tanna, Dr. Raddin, Dr. Minichiello, and Dr. Bradley report no relevant conflicts of interest.
REFERENCES:
- 1.Herbst RS, Gandara DR, Hirsch FR, et al. Lung Master Protocol (Lung-MAP)-A Biomarker-Driven Protocol for Accelerating Development of Therapies for Squamous Cell Lung Cancer: SWOG S1400. Clin Cancer Res. 2015;21(7):1514–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Malik SM, Pazdur R, Abrams JS, et al. Consensus report of a joint NCI thoracic malignancies steering committee: FDA workshop on strategies for integrating biomarkers into clinical development of new therapies for lung cancer leading to the inception of “master protocols” in lung cancer. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2014;9(10):1443–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ma PC, Maulik G, Christensen J, Salgia R. c-Met: structure, functions and potential for therapeutic inhibition. Cancer Metastasis Rev. 2003;22(4):309–325. [DOI] [PubMed] [Google Scholar]
- 4.Park M, Dean M, Kaul K, Braun MJ, Gonda MA, Vande Woude G. Sequence of MET protooncogene cDNA has features characteristic of the tyrosine kinase family of growth-factor receptors. Proc Natl Acad Sci U S A. 1987;84(18):6379–6383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sadiq AA, Salgia R. MET as a possible target for non-small-cell lung cancer. J Clin Oncol. 2013;31(8):1089–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Birchmeier C, Gherardi E. Developmental roles of HGF/SF and its receptor, the c-Met tyrosine kinase. Trends Cell Biol. 1998;8(10):404–410. [DOI] [PubMed] [Google Scholar]
- 7.Gherardi E, Birchmeier W, Birchmeier C, Vande Woude G. Targeting MET in cancer: rationale and progress. Nat Rev Cancer. 2012;12(2):89–103. [DOI] [PubMed] [Google Scholar]
- 8.Waqar SN, Morgensztern D, Sehn J. MET Mutation Associated with Responsiveness to Crizotinib. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2015;10(5):e29–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Edelman M, Spigel D, O’Byrne K, Mok T, Mocci S, Yu W, Paton V, Paz-Ares L The prevalence of MET expression by immunohistochemistry (IHC) in the MetLung (OAM4971g) trial: a randomized, placebo-controlled, phase III study with erlotinib + onartuzumab (MetMab) vs erlotinib + placebo in patients with previously treated non-small cell lung cancer Journal of Thoracic Oncology. 2013;8(11):S2–S1348, [Abstract MO1312.1307]. [Google Scholar]
- 10.Dziadziuszko R, Wynes MW, Singh S, et al. Correlation between MET gene copy number by silver in situ hybridization and protein expression by immunohistochemistry in non-small cell lung cancer. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2012;7(2):340–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gao J, Aksoy BA, Dogrusoz U, et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013;6(269):pl1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schildhaus HU, Schultheis AM, Ruschoff J, et al. MET amplification status in therapy-naive adeno- and squamous cell carcinomas of the lung. Clin Cancer Res. 2015;21(4):907–915. [DOI] [PubMed] [Google Scholar]
- 13.Mok TS, Geater SL, Su WC, et al. A Randomized Phase 2 Study Comparing the Combination of Ficlatuzumab and Gefitinib with Gefitinib Alone in Asian Patients with Advanced Stage Pulmonary Adenocarcinoma. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2016;11(10):1736–1744. [DOI] [PubMed] [Google Scholar]
- 14.Spigel DR, Ervin TJ, Ramlau RA, et al. Randomized phase II trial of Onartuzumab in combination with erlotinib in patients with advanced non-small-cell lung cancer. J Clin Oncol. 2013;31(32):4105–4114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scagliotti G, von Pawel J, Novello S, et al. Phase III Multinational, Randomized, Double-Blind, Placebo-Controlled Study of Tivantinib (ARQ 197) Plus Erlotinib Versus Erlotinib Alone in Previously Treated Patients With Locally Advanced or Metastatic Nonsquamous Non-Small-Cell Lung Cancer. J Clin Oncol. 2015;33(24):2667–2674. [DOI] [PubMed] [Google Scholar]
- 16.Wang J, Anderson MG, Oleksijew A, et al. ABBV-399, a c-Met Antibody Drug Conjugate that Targets Both MET Amplified and c-Met Overexpressing Tumors, Irrespective of MET Pathway Dependence. Clin Cancer Res. 2016. [DOI] [PubMed] [Google Scholar]
- 17.Strickler JH, LoRusso P, Yen C-J, et al. Phase 1, open-label, dose-escalation, and expansion study of ABT-700, an anti-C-met antibody, in patients (pts) with advanced solid tumors. ASCO Meeting Abstracts. 2014;32(15_suppl):2507. [Google Scholar]
- 18.Kang Y-K, LoRusso P, Salgia R, et al. Phase I study of ABT-700, an anti-c-Met antibody, in patients (pts) with advanced gastric or esophageal cancer (GEC). ASCO Meeting Abstracts. 2015;33(3_suppl):167. [Google Scholar]
- 19.Strickler JH, Weekes CD, Nemunaitis J, et al. First-in-Human Phase I, Dose-Escalation and -Expansion Study of Telisotuzumab Vedotin, an Antibody-Drug Conjugate Targeting c-Met, in Patients With Advanced Solid Tumors. J Clin Oncol. 2018;36(33):3298–3306. [DOI] [PubMed] [Google Scholar]
- 20.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–247. [DOI] [PubMed] [Google Scholar]
- 21.Watermann I, Schmitt B, Stellmacher F, et al. Improved diagnostics targeting c-MET in non-small cell lung cancer: expression, amplification and activation? Diagnostic pathology. 2015;10:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hirsch FR, Bunn PA Jr, Herbst RS “Companion diagnostics”: has their time come and gone? Clin Cancer Res. 2014;20(17):4422–4424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Organ SL, Tsao MS. An overview of the c-MET signaling pathway. Ther Adv Med Oncol. 2011;3(1 Suppl):S7–S19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sabet Y, Ramirez S, Rosell Cespedes E, et al. Severe Acute Pulmonary Toxicity Associated with Brentuximab in a Patient with Refractory Hodgkin’s Lymphoma. Case Rep Pulmonol. 2016;2016:2359437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Egloff H, Kidwell KM, Schott A. Ado-Trastuzumab Emtansine-Induced Pulmonary Toxicity: A Single-Institution Retrospective Review. Case Rep Oncol. 2018;11(2):527–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boyle TA, Khalil FK, Mino-Kenudson M, et al. Round Robin Evaluation of MET Protein Expression in Lung Adenocarcinomas Improves Interobserver Concordance. Appl Immunohistochem Mol Morphol. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guo R, Berry LD, Aisner DL, et al. MET IHC Is a Poor Screen for MET Amplification or MET Exon 14 Mutations in Lung Adenocarcinomas: Data from a Tri-Institutional Cohort of the Lung Cancer Mutation Consortium. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2019;14(9):1666–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


