Abstract
Objective:
National guidelines advise against breastfeeding for women who use non-prescribed substances in the third trimester. This reduces the number of women who are supported in breastfeeding initiation despite limited evidence on the prognostic value of third trimester substance use. We sought to examine the degree to which prenatal non-prescribed substance use is associated with non-prescribed use postpartum.
Methods:
Retrospective cohort study of pregnant women with opioid use disorder (OUD) on methadone or buprenorphine between 2006-2015. Non-prescribed use was defined by a positive urine drug testing (UDT). Sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were calculated comparing three prenatal periods with postpartum UDT results. Generalized estimating equations were used to examine the extent to which prenatal non-prescribed use was associated with postpartum use.
Results:
Included were 545 deliveries by 503 women. Mean age was 28.3 years, 88% were white/non-Hispanic, 93% had public insurance, and 43% received adequate prenatal care. The predictive value of UDT’s 90 to 31 days prior to delivery, 30 to 0 days prior to delivery, and at delivery showed low sensitivity (44, 26, 27% respectively) and PPV (36, 36, 56% respectively), but higher NPV (80, 85, and 78% respectively), p-values all <0.05. In the final adjusted model, only non-prescribed use at delivery was significantly associated with postpartum non-prescribed use.
Conclusion:
Non-prescribed use at delivery was most strongly associated with postpartum use compared with earlier time periods currently prioritized in guidelines. In women with OUD prenatal UDT results alone are insufficient to guide breastfeeding decisions.
Keywords: Toxicology, Breastfeeding, Guidelines, Pregnancy, opioid-exposed dyad
Introduction
There has been more than a fourfold increase in the number of deliveries impacted by maternal opioid use disorder (OUD), estimated to now affect 6.5 per 1000 live births in the United States.1 Correspondingly, the rates of neonatal opioid withdrawal syndrome (NOWS) have also increased five-fold from 1999 to 2013.2,3 Improving outcomes for mother-infant dyads impacted by perinatal opioid use has been identified as a key focus to improve maternal-child health in the United States.4 There is strong evidence that breastfeeding has positive effects for maternal and neonatal health, but there are specific advantages for the opioid-exposed mother-infant dyads, including reducing the severity of NOWS and decreasing the need for pharmacologic treatment for withdrawal.5–12 Despite this, breastfeeding rates among women with OUD vary widely, ranging from 17-81% across different sites in North America and in Europe.12–14 Beyond individual preferences towards breastfeeding, a number of hospital and policy-level factors may contribute to low breastfeeding rates among women with OUD including hospital and practice guidelines.15,16
Current recommendations from the Academy of Breastfeeding Medicine (ABM), American College of Obstetrics and Gynecology, and American Academy of Pediatrics all support breastfeeding in women with OUD who are consistently engaging in prenatal care, stable in recovery, receiving substance use disorder (SUD) treatment, are not actively using non-prescribed substances, and have no other contraindications to breastfeeding.17–19 The World Health Organization recommends that “mothers with SUD should be encouraged to breastfeed unless the risks clearly outweigh the benefits” and that “breastfeeding women using alcohol or drugs should be advised and supported to cease alcohol or drug use; however, substance use is not necessarily a contraindication to breastfeeding” but describes the strength of the recommendation as conditional and the quality of the evidence to support this recommendation as low.20 These recommendations were developed in response to documented adverse events associated with maternal substance use while breastfeeding, such as infant toxicity.21 However, the impact of non-prescribed substance use prior to delivery on breastfeeding safety is understudied.
Urine drug testing (UDT) is frequently used in drug treatment and office-based addiction programs to measure adherence with medication treatment and assess for non-prescribed use, despite known limitations, including risk of adulterated samples and frequent lack of confirmatory testing.22 The results of these tests are used in a variety of ways to guide post-partum decision making. For example, they are commonly requested by child protective services as a marker for stability of recovery and risk of recurrence of use.22,23 They are also routinely used to guide breastfeeding recommendations prior to delivery, as a proxy for maternal stability. The ABM guideline recommends the use of prenatal maternal UDT to guide breastfeeding initiation, supporting clinical consideration of risk factors (not defined in statement). They stipulate using UDT to assess non-prescribed use between 90 to 30 days before delivery prior to making a breastfeeding recommendation and recommend against breastfeeding with any non-prescribed use within 30 days of delivery.17–19 There remains great heterogeneity however in how hospital practices interpret and implement these guidelines, with a range of 1 to 12 weeks of prenatal drug testing results absent of non-prescribed substances required to support breastfeeding, and limited evidence to support these practices.20,24
Given the benefits to breastfeeding among opioid-exposed mother-infant dyads and the variability in use of prenatal UDT to guide breastfeeding recommendations, we aimed to assess the correlation between non-prescribed use on prenatal and postpartum UDT findings as a surrogate marker for return to substance use22,25–27 among women with OUD engaged in a treatment program. We hypothesized that non-prescribed prenatal substance measured by UDT would have a low sensitivity and positive predictive value of ongoing postpartum drug use, and that UDT at time of delivery would be most strongly predictive of postpartum use compared with UDT in the third trimester.
Methods
We studied a retrospective cohort of women that delivered at Boston Medical Center (BMC), an urban, safety-net hospital in Boston, Massachusetts. Pregnant women at BMC are cared for in a multidisciplinary program that provides addiction treatment, mental health services, and obstetrical care called Project RESPECT (Recovery, Empowerment, Social Services, Prenatal Care, Education, Community, and Treatment). Since 1999, BMC has been designated as a Baby-Friendly institution,28 with more than 95% of non-opioid exposed dyads initiating breastfeeding compared to only 30% of opioid exposed dyads prior to 2015.29,30 After the adoption of a more liberal breastfeeding policy at BMC in 2015, which used a 30 day substance free cut off prior to delivery rather than 90 day, breastfeeding initiation increased to 50%.14 We studied women who delivered between January 1, 2006 and December 31, 2015. The study period was based on when initial tracking of opioid-exposed dyads in Project RESPECT began through to the most recent available data. Data were collected through medical chart review and extraction from the Boston University Clinical Data Warehouse. The institutional Review Board at the Boston University Medical Campus reviewed and approved this study.
We included women engaged in Project RESPECT with a DSM-IV or V diagnosis of OUD. We only included women who were receiving medication for opioid use disorder (MOUD) at time of delivery, defined as receiving either methadone or buprenorphine, as naltrexone was not recommended for treatment of OUD in pregnancy during our study period. We restricted our sample to this population, as prenatal care and addiction treatment program involvement are criteria in the ABM breastfeeding guidelines.17 To be included in analyses, women had to have completed at least one third trimester urine toxicology test and one postpartum visit with a urine toxicology test, defined as a standard urine panel (testing for barbiturates, benzodiazepines, cocaine, opiates, or oxycodone) and an expanded opioid panel (to identify methadone and buprenorphine). The standard urine panel at BMC does not test for tetrahydrocannabinol (THC). Additionally, testing for fentanyl was not standard of care until the latter half of study period, but was included when available. Intermittently-observed UDT were routinely obtained at all prenatal visits. For women who had multiple deliveries in the study period, each unique delivery was included as toxicology testing in the prenatal and postpartum period were felt to be unique to each pregnancy episode. Finally, women were excluded from analyses if they had other contraindications to breastfeeding, including HIV, Hepatitis C with cracked and bleeding nipples, or receiving other medications which are contraindicated (e.g. chemotherapy).
The main outcome variable was any non-prescribed substance in the first six months following delivery in postpartum women with OUD. We defined non-prescribed use as a UDT positive for any amphetamines, barbiturates, benzodiazepines, cocaine methadone, and opioids (including buprenorphine, fentanyl, heroin, oxycodone) as a marker for substance use. For each positive toxicology test, we performed manual chart review to confirm that a woman was not receiving a prescription for that substance or a known cross reactant at the time of the urine test. For short-term prescriptions, a woman was considered to be taking said medication starting at the time it was prescribed and ending based on the number of pills prescribed. For recurring prescriptions, prescriptions in the same year as a pregnancy were considered to be active medications throughout the pregnancy and postpartum period.
The primary explanatory variable was non-prescribed substance use prenatally using the same definition described above. We examined three distinct time periods in the third trimester: between 90 to 31 days before delivery, between 30 days before and up to delivery hospitalization, and at the delivery hospitalization. We chose these time periods as they are used in the 2015 ABM breastfeeding guidelines. We defined a test at delivery hospitalization as one within four days before or after the delivery to account for prolonged labor or inductions and testing that was obtained in the hospital before maternal discharge. Additionally, we looked at UDT results by trimester. As with our outcome, when more than one UDT was performed within a given period, we defined the presence of any non-prescribed finding as evidence of non-prescribed use for that given time-period.
We also extracted data from medical charts on maternal demographics including age, race/ethnicity, insurance type, timing of MOUD initiation (including buprenorphine, and methadone), history of any medical or psychiatric diagnoses, psychiatric medications, hepatitis C infection, Kessner prenatal care adequacy index,31 and number of pregnancies within study period.
Data were analyzed in SAS version 9.4 and R version 3.4.3. Findings were confirmed by two independent analyses. Descriptive statistics were used to summarize the proportion and type of non-prescribed use during the time periods outlined above. We examined the association between participant characteristics and non-prescribed use postpartum using Student’s t-test for continuous variables and Pearson’s Chi-squared test for categorical variables. We constructed 2×2 tables to calculate sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) of non-prescribed use prenatally at: 90-31 days prior to delivery, 30 days prior to delivery, at the time of delivery hospitalization, and by trimester. Chi-square tests were used to assess statistical significance of independence. Generalized estimating equations (GEE) were used to account for women who had more than one delivery in our study period using the GENMOD procedure. We first conducted simple regression analyses characterizing the strength of the association between prenatal urine toxicological results at each of the time periods described above and postpartum toxicology results. In our multivariable model we included three mutually exclusive time periods (90-31 days before delivery, within 30 days before delivery, and delivery) as covariates.32 The final model was also adjusted based on factors hypothesized a priori to be associated with non-prescribed substance use including maternal demographics, type of MOUD, presence of medical or psychiatric diagnoses, and adequacy of prenatal care. A p-value of <0.05 was considered statistically significant for all testing.
We performed a sensitivity analysis including a sample of 100 individuals who had been excluded from the analysis due to a missing postpartum toxicology test. We conservatively assumed that all had UDTs with non-prescribed findings postpartum to account for women who may have been lost to follow up due to return to active drug use.
Results
Figure 1 shows a schematic of the study sample. From a possible 1034 deliveries by women treated in an integrated prenatal care and addiction treatment program, 847 had OUD and were receiving MOUD at delivery and 545 deliveries by 503 unique women met inclusion criteria. Table 1 summarizes the baseline demographics. The mean age was 28.3 years, most of the participants were White/Non-Hispanic, less than half had adequate prenatal care based on the Kessner Index, and sample was split roughly equally between buprenorphine and methadone treatment at delivery. Compared to deliveries excluded from our cohort, those meeting inclusion criteria were more likely to be white/non-Hispanic, be on buprenorphine compared with methadone, have adequate prenatal care, not have Hepatitis C, and have more post-partum visits (Supplementary Table 1). Being on psychiatric medications and having inadequate prenatal care based on Kessner Index were associated with non-prescribed use within 6 months following delivery (Table 1).
Figure 1.
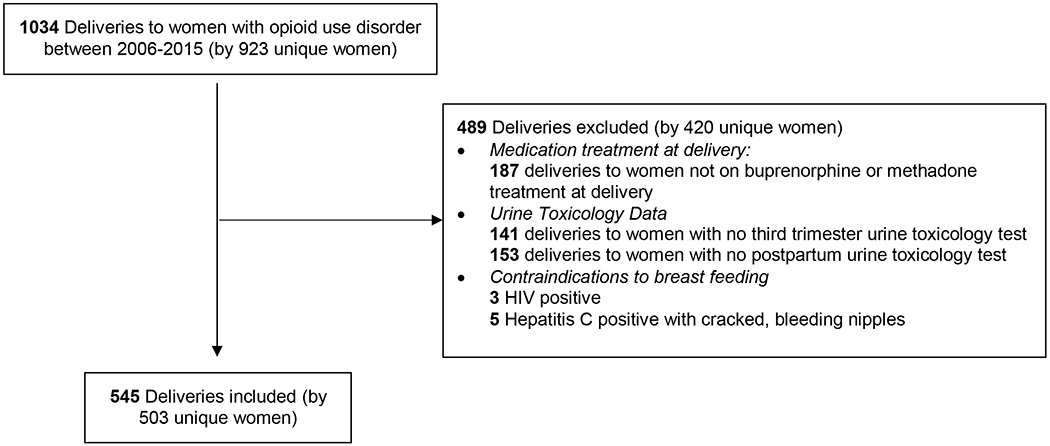
Study Flow Diagram
Table 1.
Demographic Characteristics of Deliveries to Women with Opioid Use Disorder by Postpartum Toxicology Test Results
| Demographic characteristics | Overall (N=503) | No postpartum non-prescribed substance use (N=378) | Any postpartum non-prescribed substance use (N=125) | P-value |
|---|---|---|---|---|
| Age Mean SD | 28.3 ± 5.0 | 28.2 ± 4.9 | 28.4 ± 5.0 | P = 0.76 |
| Race N (%White non-Hispanic) | 443 (88.1) | 338 (89.4) | 105 (84.0) | P = 0.11 |
| Insurance N (%) | P = 0.81 | |||
| Public payer | 467 (92.8) | 350 (92.6) | 117 (93.6) | |
| Private | 35 (7.0) | 27 (7.1) | 8 (6.4) | |
| Uninsured/Other | 1 (0.2) | 1 (0.3) | 0 (0) | |
| MOUD at first visit N, (%) | P = 0.62 | |||
| None | 10 (2.1) | 7 (1.9) | 3 (2.4) | |
| Buprenorphine | 262 (56.0) | 192 (50.8) | 70 (56.0) | |
| Methadone | 196 (42.8) | 151 (40.0) | 45 (36.0) | |
| Missing | 35 | 28 (7.4) | 7 (5.6) | |
| MOUD at delivery, N (%) | P = 0.20 | |||
| Buprenorphine | 269 (53.5) | 196 (51.9) | 73 (58.4) | |
| Methadone | 234 (46.5) | 182 (48.2) | 52 (41.6) | |
| Medical Problems, N (% any yes) | 86 (17.2) | 70 (18.6) | 16 (12.9) | P = 0.14 |
| Psychiatric Diagnosis, N (% any yes) | 343 (69.0) | 251 (67.3) | 92 (74.2) | P = 0.15 |
| On any psych medications, N (% any yes) | 241 (47.9) | 165 (43.7) | 76 (60.8) | P <0.001 |
| Hep C, N (% positive) | 300 (59.8) | 217 (57.6) | 83 (66.4) | P = 0.08 |
| Distance of residence from hospital by address at delivery (km), Mean SD |
17.7±18.5 | 17.4±17.5 | 18.8±21.3 | P = 0.44 |
| Kessner prenatal care adequacy index, N (%) | P = 0.003 | |||
| Adequate | 216 (43.0) | 179 (47.4) | 37 (29.8) | |
| Intermediate | 184 (36.7) | 129 (34.1) | 55 (44.4) | |
| Inadequate | 102 (20.3) | 70 (18.5) | 32 (25.8) | |
| Number of postpartum visits, Mean SD | 2.3±1.5 | 2.3±1.4 | 2.4±1.8 | P = 0.31 |
N; Number; UDT, urine drug tests; SD, standard deviation; MOUD, medication for opioid use disorder; Hep C, hepatitis c; km, kilometer
Women included in the cohort received 4,004 toxicology tests during the study period. Table 2 summarizes the proportion of deliveries with non-prescribed use throughout pregnancy and in the first six months postpartum. The rate of non-prescribed use on UDTs decreased as women progressed in their pregnancy, with the lowest rate at time of delivery. Non-prescribed opioids were the most common cause of non-prescribed substance use during pregnancy and postpartum. At delivery 12.5% of the study sample had evidence of non-prescribed substance use, 7.8% being opiates (Table 2).
Table 2:
Timing and Type of Any Non-Prescribed Substance Use on Urine Drug Tests During Pregnancy and Postpartum (Yes/No)
| Time Period N (%) | Amphetamine | Barbiturate | Benzodiazepine | Buprenorphine | Cocaine | Methadone | Opiate | Oxycodone | Any Non-Prescribed Substance | Total # w/ ≥1 Test/Period |
|---|---|---|---|---|---|---|---|---|---|---|
| 1st Tri | 5 (1.7) | 2 (0.7) | 24 (8.2) | 14 (4.8) | 46 (15.6) | 8 (2.7) | 103 (35.0) | 20 (6.8) | 133 (45.2) | 294 |
| 2nd Tri | 11(2.4) | 5 (1.1) | 46 (10.2) | 21 (4.6) | 59 (13.0) | 5 (1.1) | 132 (29.1) | 32 (7.1) | 193 (42.6) | 453 |
| 3rd Tri | 7 (1.3) | 7 (1.3) | 41 (7.5) | 10 (1.8) | 63 (11.6) | 9 (1.7) | 122 (22.4) | 30 (5.5) | 193 (35.4) | 545 |
| 90-31d before delivery | 5 (1.0) | 6 (1.2) | 32 (6.2) | 8 (1.5) | 55 (10.6) | 3 (0.6) | 99 (19.0) | 22 (4.2) | 157 (30.2) | 520 |
| 30d before delivery | 3 (0.6) | 3 (0.6) | 21 (4.0) | 5 (1.0) | 22 (4.2) | 6 (1.1) | 48 (9.2) | 11 (2.1) | 92 (17.6) | 524 |
| Delivery | 3 (0.6) | 3 (0.6) | 12 (2.5) | 1 (0.2) | 16 (3.3) | 1 (0.2) | 38 (7.8) | 2 (0.4) | 61 (12.5) | 487 |
| PP | 15 (2.8) | 9 (1.7) | 34 (6.2) | 2 (0.4) | 28 (5.1) | 5 (0.9) | 74 (13.6) | 24 (4.4) | 135 (24.8) | 545 |
Table 3 shows the sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of non-prescribed use on prenatal urine drug tests as a predictor of non-prescribed post-partum use, with p-values all less than 0.05. At the three main time periods tested: 90-31 days prior to delivery, 30 days to delivery hospitalization, and at delivery, the sensitivity was less than 50% and the specificity was 74.4%, 85.2%, and 92.5% respectively. The NPV was similar at all time points, at 80.2%, 85.2%, and 78.2% at 90-31d, <30d, and delivery respectively. Finally, the PPV (36.3%, 35.9%, and 55.74% at 90-31d, <30d, and delivery respectively) was lower than the NPV at all time periods. Results by first, second, and third trimester are shown in Supplementary Table 2.
Table 3:
Any Prenatal Non-Prescribed Substance Use as a Predictor of Non-Prescribed Postpartum Use using Urine Drug Tests
| 90-30d before delivery | 30d before delivery | At delivery | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Post-Partum | ≥ 1 Positive UDT 90-31d before delivery | Negative UDT 90-31d before delivery | Total | ≥ 1 Positive UDT 30d before delivery | Negative UDT 30d before delivery | Total | ≥ 1 Positive UDT at delivery | Negative UDT at delivery | Total |
| ≥ 1 Positive UDT Post- Partum | 57 | 72 | 129 | 33 | 93 | 126 | 34 | 93 | 127 |
| Negative UDT Post- Partum | 100 | 291 | 391 | 59 | 339 | 398 | 27 | 333 | 360 |
| Total | 157 | 363 | 520 | 92 | 432 | 524 | 61 | 426 | 487 |
| Sensitivity | 44.2% | 26.2% | 26.8% | ||||||
| Specificity | 74.4% | 78.5% | 92.5% | ||||||
| Pos Predictive Value | 36.3% | 35.9% | 55.74% | ||||||
| Neg Predictive Value | 80.2% | 85.2% | 78.2% | ||||||
| Chi-Squared Test | P=0.033 | P=0.006 | P<0.001 | ||||||
D, days; UDT, urine drug test
In the unadjusted model, non-prescribed use at delivery was associated with the highest odds of non-prescribed use postpartum (odds ratio (OR) 4.51 [95% confidence interval (CI) 2.57-7.91]) compared to other testing interval periods prior to delivery (Supplementary Table 3). In our final multivariable model adjusting for all covariates, the aOR at delivery was 3.72 (95% CI 1.84-7.51), compared to 1.40 (95% CI 0.73, 2.72) within 30 days of delivery and 1.68 (95% CI 0.98, 2.90) within 90-31 days of delivery, shown in Table 4. Only delivery hospitalization non-prescribed use was significantly associated with non-prescribed postpartum use in the final model. Additionally, receiving methadone at delivery compared with buprenorphine was associated with postpartum use (aOR 1.96, 95% CI 1.20, 3.22), whereas Hepatitis C was negatively associated with postpartum use (aOR 0.97, 95% CI 0.94, 0.99).
Table 4:
Multivariable Model using Generalized Estimating Equations (GEE)* Looking at Factors Predictive of Any Non-Prescribed Substance Use Post-Partum
| Parameter | Estimate | Standard Error | aOR** | 95% CI | |
|---|---|---|---|---|---|
| Positive Delivery UDT | 1.31 | 0.36 | 3.72 | 1.84 | 7.51 |
| Positive 30d to Delivery hospitalization UDT | 0.34 | 0.34 | 1.40 | 0.73 | 2.72 |
| Positive 90-31d before Delivery | 0.52 | 0.28 | 1.68 | 0.98 | 2.90 |
| White non-Hispanic v. non-white race/ethnicity | −0.47 | 0.34 | 0.62 | 0.32 | 1.20 |
| Public insurance v. Private/other | 0.11 | 0.49 | 1.11 | 0.43 | 2.89 |
| Methadone v. Buprenorphine treatment at delivery | 0.67 | 0.25 | 1.96 | 1.20 | 3.22 |
| Any psychiatric diagnosis | 0.27 | 0.27 | 1.30 | 0.77 | 2.21 |
| Hepatitis C | −0.038 | 0.014 | 0.97 | 0.94 | 0.99 |
| Adequate v. Intermediate/ Inadequate Prenatal Care | 0.46 | 0.26 | 1.59 | 0.95 | 2.64 |
OR, odds ratio; CI, confidence interval; UDT, urine drug test; d, days
GEE was used to account for clustering of deliveries by the same women across the study period. A binary outcome was used (0=no non-prescribed substances on toxicology testing within six months of delivery, 1=evidence of non-prescribed substances on toxicology testing within six months of delivery)
Model was adjusted for all covariates listed
Given demographic differences in the excluded sample comparted to the included sample (Supplementary Table 1), we conducted a sensitivity analysis (Supplementary Table 4). In this analysis, the NPV decreased at each time interval throughout the peripartum period with the 91-30 days prior to delivery yielding the highest NPV. The PPV increased with the greatest increase at delivery. There were however no significant differences in the direction of the adjusted odds ratios in our multivariable model (Supplementary Table 5).
Discussion
We analyzed over 4000 urine drug tests from pregnant women with OUD at a single academic center engaged in prenatal care and MOUD treatment. We found that non-prescribed substance use, as measured by UDT, declined throughout pregnancy, with the lowest rate at delivery, but then increased in the postpartum period. We identified that the positive predictive value and sensitivity of prenatal UDT results to predict postpartum use was significantly lower than the negative predictive value. This finding suggests that while having no non-prescribed use prenatally was correlated with continued abstinence, the corollary was less strong. For women who had a positive test within the third trimester, our multivariable model found that non-prescribed substance use at delivery, but not between 90 and 31 or between 30 days and delivery hospitalization was statistically associated with ongoing non-prescribed use postpartum.
Upon initial review of these findings, we grappled with the significance of finding a poorer correlation between prenatal and postpartum UDTs in our 2×2 tables with a strongly positive association between prenatal and postpartum UDTs in the simple and multivariable regression models. We propose two key interpretations of these results. First, the unadjusted correlation results highlight how up to two thirds of women who had evidence of non-prescribed substance use in the third trimester did not have evidence of continued use postpartum. Importantly, these women would have been discouraged from breastfeeding when applying the ABM guidelines. This suggests a possible missed opportunity to promote breastfeeding among these opioid-exposed dyads. Next, the factors that contribute to postpartum substance use are multifactorial as identified by variables in our analysis like the presence of mood disorders, type of MOUD treatment, adequacy of prenatal care, and unmeasured covariates including the degree of community supports, degree of self-efficacy, cravings, recovery capital, loss of child custody, and length of abstinence.33
That said, it is not surprising that one of the strongest predictors of future use is the history of recent use. A strength of our multivariable model is that we were able to compare the degree to which non-prescribed use at three mutually exclusive time periods were associated with substance use postpartum, and we identified that only the UDTs at delivery had a statistically significant association. These findings are discordant with current breastfeeding guidelines, which recommends using the third trimester or non-prescribed use within 30 days of delivery be used to determine breastfeeding recommendations.
While there have been no randomized trials that have assessed the impact of encouraging, not encouraging, discouraging, or recommending intermittent use of breastmilk on key maternal or infant outcomes among women with opioid use disorder to date, given the benefits of breastfeeding the risks of discouraging breastfeeding should also be weighed.34 Using a shared decision-making process with provider and patient UDT at the time of delivery could be used to guide specific harms and benefits discussions for women who are interested in breastfeeding. In addition to the benefits for the infant experiencing opioid withdrawal symptoms, breastfeeding may also help women to decrease stress and support and maintain their recovery, thereby reducing their risk of postpartum depression.35 Additional support through enhanced lactation and home visiting programs to monitor ongoing sobriety could allow for a greater number of opioid-exposed dyads to initiate and successfully breastfeed.
Infant substance exposure in maternal milk, which can be estimated by the relative infant dose (RID), is dependent on drug pharmacology, maternal exposure and metabolism, and infant gastric absorption, metabolism, and gestational age.36 While the RID of some substances are well understood, for example regardless of maternal dose only a small amount of methadone or buprenorphine is transferred to breastmilk, little is known about the RID of most illicit substances. The literature which does exist suggests that the RID is greatest during active use and decreases as it is maternally metabolized and eliminated.36 For example, cocaine is be metabolized and eliminated 24-60hrs after exposure.36,37 Therefore, for women who are able to discontinue substance use by delivery or during the delivery hospitalization, monitoring UDTs for clearance of substances may be useful to guide breastfeeding recommendations as most substances are eliminated in hours to days rather than days to weeks from the maternal system.
It is also important to acknowledge that in our sample, about one fifth of women who had negative toxicology tests during the third trimester and thus would be recommended to breastfeed, had evidence of non-prescribed use postpartum. While we are unable to determine if these women initiated or continued breastfeeding at time of these toxicology tests, there are clear harms to breastfeeding by women with active substance use. These include altered ability to respond to infant feeding cues secondary to maternal somnolence, lack of adequate sleep-wake cycling, or brain changes that result in normal infant behaviors seen as highly stressful rather than rewarding.38,39 Finally, for women who intend to breastfeed, but have difficulty with successfully providing breastmilk to their infants due to factors such as inability to latch or delayed milk production, it is also possible that strongly promoting breastfeeding among women with OUD could create increased stress and hinder postpartum stability.35 In this context, patient centered recommendations are particularly important, including ongoing screening and assessment of parental substance use and safe infant sleep following delivery hospitalization.
Our study has several limitations. First, just under 50% of the eligible population were excluded based on study inclusion criteria risking selection bias, yet our findings remained consistent in our sensitivity analysis. Next, by giving equal weight to someone with ten positive UDTs as someone who had one positive test and nine other tests showing no non-prescribed use, we may have overemphasized the significance of a single positive test. However, we purposefully chose this design given this is how current breastfeeding guidelines interpret toxicology findings. Next, by including any test within six months postpartum for our primary outcome, we may have misclassified individuals with a negative test immediately postpartum as no non-prescribed use who were actually later lost to follow up due to relapse. Our study focused on opioid-exposed mother-infant dyads therefore our findings may not be generalizable to women with other SUDs. Additionally, our study used urine toxicological testing as a surrogate marker for non-prescribed substance use. UDT are imperfect makers of substance use, dependent on collection time relative to the substance exposure, properties of a given test,40 and patient collection. However, this reflects the standard practice of the BMC program, and while susceptible to error, UDT is often used as metric of non-prescribed use in substance use literature and clinical practice.22,25–27 Lastly, given the retrospective, observational design, residual confounding is possible. Despite these limitations our study includes data collected over a ten-year period, is of a large cohort of women, and captures the real-world clinical use of urine toxicology testing.
Conclusions
In a clinical sample of women engaged in prenatal care receiving treatment for opioid use disorder, we found a limited clinical correlation between prenatal non-prescribed substance use and postpartum use as measured by UDTs. Our findings suggest that prenatal UDT results alone are insufficient to support or recommend against breastfeeding. We found that non-prescribed use at delivery had the strongest association with ongoing non-prescribed use postpartum compared to the third trimester. UDT at the time of delivery could be used to guide a shared-decision making process for breastfeeding and the need for close follow up and support for mother-infant dyads after delivery. Prospective research and randomized controlled trials are needed to assess the value of prenatal urine toxicology testing to guide breastfeeding recommendations. Additionally, qualitative studies are needed to explore maternal views on breastfeeding regarding barriers, facilitators, and effects on maternal wellbeing.
Supplementary Material
Acknowledgements
Preliminary data was presented at The Association for Multidisciplinary Education and Research in Substance use and Addiction Conference (November 2017, Washington DC, USA)
Source of Funding: Dr. Harris was supported by the Research in Addiction Medicine Fellowship NIDA (R25DA033211). Dr. Joseph was supported by the Boston University Medical Student Summer Research Program. Dr. Wachman was supported by NICHD (R01 HD096798). Dr. Schiff was supported by NIDA (K12 DA043490 and K23DA048169).
Footnotes
Conflicts of Interest: None declared
References
- 1.Haight SC, Ko JY, Tong VT, Bohm MK, Callaghan WM. Opioid use disorder documented at delivery hospitalization—United States, 1999–2014. Morbidity and Mortality Weekly Report. 2018;67(31):845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patrick SW, Schiff DM, Committee On Substance USE, Prevention. A Public Health Response to Opioid Use in Pregnancy. Pediatrics. 2017;139(3). [DOI] [PubMed] [Google Scholar]
- 3.Ko JY, Patrick SW, Tong VT, Patel R, Lind JN, Barfield WD. Incidence of Neonatal Abstinence Syndrome - 28 States, 1999–2013. MMWR Morb Mortal Wkly Rep 2016;65(31):799–802. [DOI] [PubMed] [Google Scholar]
- 4.NICHD. National Institue of Child Health and Human Development Research Priorities. National Institute of Health.https://www.nichd.nih.gov/grants-contracts/research-areas/priorities. Published 2017. Updated 12/302018. Accessed September 20, 2019. [Google Scholar]
- 5.O’Connor AB, Collett A, Alto WA, O’Brien LM. Breastfeeding rates and the relationship between breastfeeding and neonatal abstinence syndrome in women maintained on buprenorphine during pregnancy. Journal of midwifery & women’s health. 2013;58(4):383–388. [DOI] [PubMed] [Google Scholar]
- 6.Grossman MR, Berkwitt AK, Osborn RR, et al. An initiative to improve the quality of care of infants with neonatal abstinence syndrome. Pediatrics. 2017;139(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Welle‐Strand GK, Skurtveit S, Jansson LM, Bakstad B, Bjarkø L, Ravndal E. Breastfeeding reduces the need for withdrawal treatment in opioid‐exposed infants. Acta paediatrica. 2013;102(11):1060–1066. [DOI] [PubMed] [Google Scholar]
- 8.Abrahams RR, Kelly SA, Payne S, Thiessen PN, Mackintosh J, Janssen PA. Rooming-in compared with standard care for newborns of mothers using methadone or heroin. Canadian Family Physician. 2007;53(10):1722–1730. [PMC free article] [PubMed] [Google Scholar]
- 9.Smirk CL, Bowman E, Doyle LW, Kamlin O. Home‐based detoxification for neonatal abstinence syndrome reduces length of hospital admission without prolonging treatment. Acta Paediatrica. 2014;103(6):601–604. [DOI] [PubMed] [Google Scholar]
- 10.Abdel-Latif ME, Pinner J, Clews S, Cooke F, Lui K, Oei J. Effects of breast milk on the severity and outcome of neonatal abstinence syndrome among infants of drug-dependent mothers. Pediatrics. 2006;117(6):e1163–e1169. [DOI] [PubMed] [Google Scholar]
- 11.Wachman EM, Schiff DM, Silverstein M. Neonatal abstinence syndrome: advances in diagnosis and treatment. Jama. 2018;319(13):1362–1374. [DOI] [PubMed] [Google Scholar]
- 12.Tsai LC, Doan TJ. Breastfeeding among Mothers on Opioid Maintenance Treatment: A Literature Review. J Hum Lact. 2016;32(3):521–529. [DOI] [PubMed] [Google Scholar]
- 13.Wachman EM, Byun J, Philipp BL. Breastfeeding rates among mothers of infants with neonatal abstinence syndrome. Breastfeed Med. 2010;5(4):159–164. [DOI] [PubMed] [Google Scholar]
- 14.Wachman EM, Saia K, Humphreys R, Minear S, Combs G, Philipp BL. Revision of Breastfeeding Guidelines in the Setting of Maternal Opioid Use Disorder: One Institution’s Experience. J Hum Lact 2016;32(2):382–387. [DOI] [PubMed] [Google Scholar]
- 15.Schiff DM, Wachman EM, Philipp B, et al. Examination of hospital, maternal, and infant characteristics associated with breastfeeding initiation and continuation among opioid-exposed mother-infant dyads. Breastfeeding Medicine 2018;13(4):266–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bogen DL, Whalen BL, Kair LR, Vining M, King BA. Wide variation found in care of opioid-exposed newborns. Academic pediatrics. 2017;17(4):374–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reece-Stremtan S, Marinelli KA. ABM clinical protocol #21: guidelines for breastfeeding and substance use or substance use disorder, revised 2015. Breastfeed Med. 2015;10(3):135–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.ASAM. Committee Opinion No. 711: Opioid Use and Opioid Use Disorder in Pregnancy. Obstetrics & Gynecology 2017;130(2):e81–e94. [DOI] [PubMed] [Google Scholar]
- 19.Section on B. Breastfeeding and the use of human milk. Pediatrics. 2012;129(3):e827–841. [DOI] [PubMed] [Google Scholar]
- 20.Whittaker A Guidelines for the Identification and Management of Substance Use and Substance Use Disorders in Pregnancy By World Health Organization Geneva, Switzerland: WHO Press, 2014. ISBN: 9789241548731, 224 pp. Available free online http://www.who.int/substance_abuse/publications/pregnancy_guidelines/en. Drug Alcohol Rev. 2015;34(3):340-341. [PubMed] [Google Scholar]
- 21.Schultz ML, Kostic M, Kharasch S. A Case of Toxic Breast-feeding? Pediatr Emerg Care 2019;35(1):e9–e10. [DOI] [PubMed] [Google Scholar]
- 22.Jarvis M, Williams J, Hurford M, et al. Appropriate Use of Drug Testing in Clinical Addiction Medicine. Journal of Addiction Medicine. 2017;11(3):163–173. [DOI] [PubMed] [Google Scholar]
- 23.Abuse NCoS, Welfare C, America USo. Drug Testing in Child Welfare: Practice and Policy Considerations. 2010. [Google Scholar]
- 24.Breastfeeding Guidelines for Women with a Substance Use Disorder. Illinois Perinatal Quality Collaborative https://med.dartmouth-hitchcock.org/documents/NNEPQIN-Breastfeeding-Guidelines.pdf.Accessed October 10, 2019.
- 25.Perrone J, De Roos F, Jayaraman S, Hollander JE. Drug screening versus history in detection of substance use in ED psychiatric patients. Am J Emerg Med. 2001;19(1):49–51. [DOI] [PubMed] [Google Scholar]
- 26.Dupouy J, Mémier V, Catala H, Lavit M, Oustric S, Lapeyre-Mestre M. Does urine drug abuse screening help for managing patients? A systematic review. Drug Alcohol Depend. 2014;136:11–20. [DOI] [PubMed] [Google Scholar]
- 27.Baheiraei A, Banihosseini SZ, Heshmat R, Mota A, Mohsenifar A. Association of self-reported passive smoking in pregnant women with cotinine level of maternal urine and umbilical cord blood at delivery. Paediatr Perinat Epidemiol. 2012;26(1):70–76. [DOI] [PubMed] [Google Scholar]
- 28.USA BF. Baby‐Friendly USA: the gold standard of care. [Google Scholar]
- 29.Arnaudo CL, Andraka-Christou B, Allgood K. Psychiatric Co-Morbidities in Pregnant Women with Opioid Use Disorders: Prevalence, Impact, and Implications for Treatment. Current addiction reports 2017;4(1):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schiff DM, Wachman EM, Philipp B, et al. Examination of Hospital, Maternal, and Infant Characteristics Associated with Breastfeeding Initiation and Continuation Among Opioid-Exposed Mother-Infant Dyads. Breastfeed Med 2018;13(4):266–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kessner DM. Infant death: an analysis by maternal risk and health care. Vol 1: Institute of Medicine; 1973. [Google Scholar]
- 32.Rodriguez RN. An Overview of ODS Statistical Graphics in SAS 9.3. Citeseer;2011. [Google Scholar]
- 33.Clark RE, Baxter JD, Aweh G, O’Connell E, Fisher WH, Barton BA. Risk factors for relapse and higher costs among Medicaid members with opioid dependence or abuse: opioid agonists, comorbidities, and treatment history. Journal of substance abuse treatment. 2015;57:75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.WHO Guidelines Approved by the Guidelines Review Committee. In: Guidelines for the Identification and Management of Substance Use and Substance Use Disorders in Pregnancy. Geneva: World Health Organization Copyright (c) World Health Organization; 2014.; 2014. [PubMed] [Google Scholar]
- 35.Chapman SLC, Wu L-T. Postpartum substance use and depressive symptoms: a review. Women & health 2013;53(5):479–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.D’Apolito K Breastfeeding and Substance Abuse. Clinical Obstetrics and Gynecology 2013;56(1):202–211. [DOI] [PubMed] [Google Scholar]
- 37.Cressman AM, Koren G, Pupco A, Kim E, Ito S, Bozzo P. Maternal cocaine use during breastfeeding. Canadian family physician Medecin de famille canadien 2012;58(11):1218–1219. [PMC free article] [PubMed] [Google Scholar]
- 38.Fitzsimons HE, Tuten M, Vaidya V, Jones HE. Mood disorders affect drug treatment success of drug-dependent pregnant women. J Subst Abuse Treat. 2007;32(1):19–25. [DOI] [PubMed] [Google Scholar]
- 39.Rutherford H, Williams S, Moy S, Mayes L, Johns J. Disruption of maternal parenting circuitry by addictive process: rewiring of reward and stress systems. Frontiers in psychiatry 2011;2:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moeller KE, Kissack JC, Atayee RS, Lee KC. Clinical Interpretation of Urine Drug Tests: What Clinicians Need to Know About Urine Drug Screens. Mayo Clin Proc. 2017;92(5):774–796. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


