Summary
Follicular T helper (TFH) cells are specialized T cells that support B cells, which are essential for humoral immunity. TFH cells express the transcription factor B‐cell lymphoma 6 (Bcl‐6), chemokine (C‐X‐C motif) receptor (CXCR) 5, the surface receptors programmed cell death protein 1 (PD‐1) and inducible T‐cell costimulator (ICOS), the cytokine IL‐21 and other molecules. The activation, proliferation and differentiation of TFH cells are closely related to dynamic changes in cellular metabolism. In this review, we summarize the progress made in understanding the development and functional differentiation of TFH cells. Specifically, we focus on the regulatory mechanisms of TFH cell functional differentiation, including regulatory signalling pathways and the metabolic regulatory mechanisms of TFH cells. In addition, TFH cells are closely related to immune‐associated diseases, including infections, autoimmune diseases and cancers.
Keywords: follicular T helper cells, metabolic reprogramming, signalling, infectious diseases, cancer, inflammation
The progress about the development and functional differentiation of TFH cells. The regulatory mechanisms of TFH cell functional differentiation, including regulatory signalling pathways and the metabolic regulatory mechanisms of TFH cells. TFH cells are closely related to immune‐associated diseases, including infections, autoimmune diseases, and cancers.
![]()
Abbreviations
- AITD
autoimmune thyroid disease
- AMP
adenosine 5’‐monophosphate
- AMPK
activated protein kinase
- APCs
antigen‐presenting cells
- ApoAI
apolipoprotein AI
- AS
atherosclerosis
- BC
breast cancer
- Bcl‐6
B cell lymphoma 6
- BTLA
B‐ and T‐lymphocyte attenuator
- CIA
collagen‐induced arthritis
- CRC
colorectal cancer
- CXCR 5
chemokine (C‐X‐C motif) receptor 5
- DAMPs
damage‐associated molecular patterns
- DCs
dendritic cells
- FDCs
follicular dendritic cells
- Foxo
forkhead box O
- GATA‐3
GATA‐binding protein 3
- GCs
germinal centres
- GD
glucose deprivation
- HIV
human immunodeficiency virus
- IBD
inflammatory bowel disease
- ICOS
inducible T cell co‐stimulator
- IFN‐γ
interferon‐γ
- IRF4
interferon regulatory factor 4
- JDM
juvenile dermatomyositis
- LCMV
lymphocytic choriomeningitis virus
- LZ
light zone
- MS
multiple sclerosis
- PAMPs
pathogen‐associated molecular patterns
- PD‐1
programmed cell death protein 1
- PKC
protein kinase C
- PPs
peyer’s patches
- pSS
sjogren’s syndrome
- RA
rheumatoid arthritis
- RORγt
retinoid‐related orphan nuclear receptor γt
- S1PR1
sphingosine‐1‐phosphate receptor
- SAP
SLAM‐associated protein
- SLE
systemic lupus erythaematosus
- STATs
signal transducers and transcriptional activators
- T1D
type 1 diabetes
- TCR
T cell receptor
- TFH
follicular T helper
- TH1
type 1 T helper cells
- TNF‐α
tumour necrosis factor‐α
- Tox
thymocyte selection‐associated high mobility group box protein
- WT
wild‐type
INTRODUCTION
Effector CD4+ T helper cells (TH) include multiple cell subsets, such as type 1 T helper (TH1) cells, TH2 cells, TH9 cells and TH17 cells, regulatory T (Treg) cells and follicular T helper (TFH) cells. 1 , 2 , 3 , 4 (Figure 1). Th cells are divided into different subgroups according to the different effector cytokines secreted and the specific transcription factors expressed, which play decisive roles in each subgroup. 3 , 4 , 5 In response to stimulation by antigens and cytokines, naïve T cells differentiate into TH1, TH9, TH17 and other cell subsets (Figure 1). The main effector factors secreted by TH1 cells are interferon‐γ (IFN‐γ) and tumour necrosis factor‐α (TNF‐α), and TH1 differentiation is mainly regulated by the transcription factor T‐Box transcription factor (T‐bet); TH2 cells are regulated by the transcription factor GATA‐binding protein 3 (GATA‐3) and produce the cytokines interleukin (IL)‐4 and IL‐13; TH17 cells express the transcription factor retinoid‐related orphan nuclear receptor γt (RORγt) and produce the cytokine IL‐17; and TH9 cells express the transcription factors PU.1 and interferon regulatory factor (IRF) 4 and produce the cytokine IL‐9. 3 , 4 , 5 , 6 , 7 Treg cells express the specific transcription factor Foxp3, produce the cytokine TGF‐β and play immunosuppressive and immunomodulatory roles. 8 , 9 The differentiation of T‐cell subsets is determined by the cytokines expressed during T‐cell activation. 10 For example, IL‐12 induces T‐bet in TH1 cells, and IL‐6 or IL‐23 can induce RORγt activation during TH17 cell differentiation. 8 , 11 , 12 , 13
Figure 1.
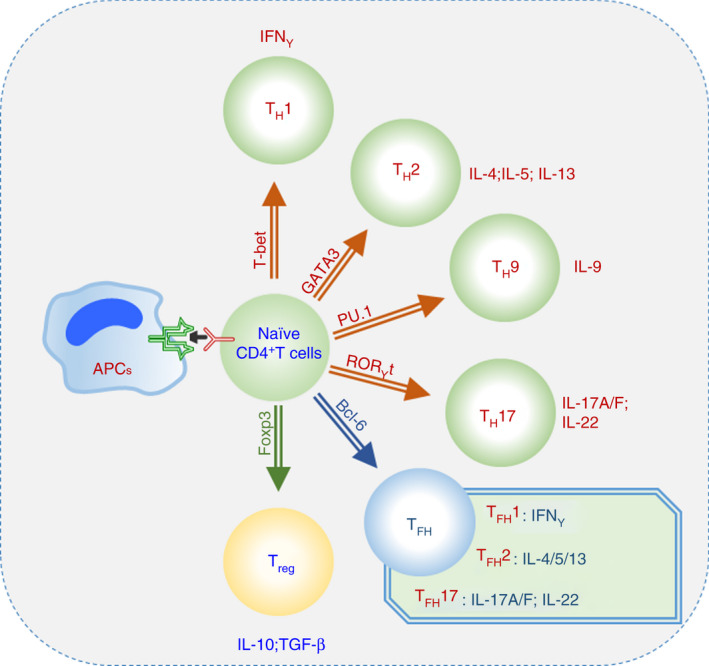
Different T helper cell subsets. Effector CD4+ T‐cell lineage differentiation and the expression of transcription factors.
TFH cells develop differently from other subsets of effector CD4+ T helper cells, express the main regulatory transcription factor B‐cell lymphoma 6 (Bcl‐6) and produce the cytokine IL‐21. 1 , 2 , 3 , 4 TFH cells are a newly discovered subset of T cells that play an important role in regulating B‐cell‐mediated humoral immunity (Figures 1 and 2). TFH cells were originally characterized as unique activated CD4+ T cells in the germinal centres (GCs) of the human tonsil that express chemokine (C‐X‐C motif) receptor (CXCR) 5 and promote the differentiation and function of B cells. 14 Subsequent gene expression and phenotypic analysis showed the anatomical localization and upregulated expression of cell surface proteins, including inducible T‐cell costimulatory molecule (ICOS). 15 , 16 , programmed cell death (PD)‐1. 17 , B‐ and T‐lymphocyte attenuator (BTLA) and CD40L. 18 , 19 , which indicate that TFH cells are different from other T helper cell populations. TFH cells express the master transcription factor Bcl‐6 and the cytoplasmic adapter and signalling molecule SLAM‐associated protein (SAP), which are required for TFH cell differentiation, and secrete the cytokine IL‐21. 18 , 20
Figure 2.
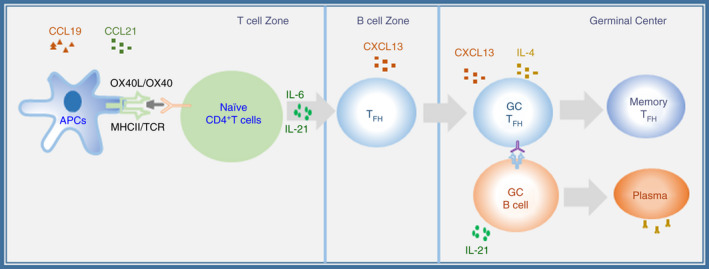
Overview of TFH cell differentiation and localization. The process of TFH cell differentiation highlights the chemokines needed for CD4+ T‐cell trafficking into the T‐cell and B‐cell zones. The expression of chemokine receptors on the surface of T cells plays a decisive role in the migration of TFH cells. CXCL13 is the ligand of CXCR5 and is secreted by B‐cell follicles and germinal centres. However, CCL19 and CCL21, the ligands of CCR7, are highly present in the T‐cell region.
TFH cells, as a specific subset of CD4+ T cells, can be classified by chemokine receptor expression. CXCR3 and CCR6 are characteristic receptors used to define subgroups of TFH cells. 21 , 22 , 23 CXCR3 is a chemokine receptor that is highly expressed on the surface of TH1 cells, while CCR6 is expressed in TH17 cell subsets. 24 , 25 TFH cells can also be further divided into subgroups according to the chemokines expressed on the cell surface. Studies have defined CXCR3+ CCR6− TFH cells as TFH1 cells, which express the transcription factor T‐bet and secrete the cytokine IFN‐γ; CXCR3− CCR6− cells are termed TFH2 cells, express the transcription factor GATA3 and secrete the cytokines IL‐4, IL‐5 and IL‐13; and CXCR3− CCR6+ TFH cells are termed TFH17 cells, express the transcription factor RORγt and secrete the cytokines IL‐17A, IL‐17F and IL‐22. 21 , 22 , 23 , 24 , 25
DEVELOPMENT AND FUNCTIONAL DIFFERENTIATION OF TFH CELLS
TFH cells are differentiated through three processes (Figures 2 and 3). Because of their tissue‐specific anatomical location, TFH cells possess unique functions that differ from those of other TH subsets. The three‐stage differentiation process is divided according to TFH cell migration and localization. 18 , 26 , 27 , 28
Figure 3.
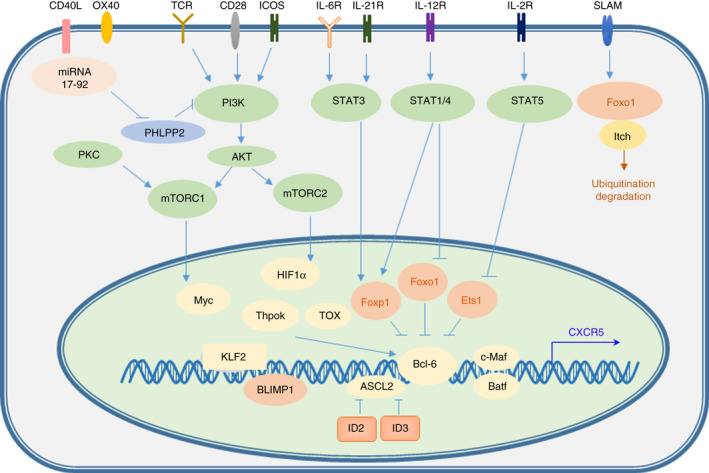
Transcriptional regulation of TFH cell differentiation. IL‐6R/IL‐21R‐STAT3 signalling functions in the differentiation of TFH cells. Bcl‐6, c‐Maf, Batf and other transcription factors play regulatory roles in TFH cell differentiation.
The first stage of TFH cell differentiation is the migration of TFH progenitor cells to B‐cell follicles. 29 Naïve T cells receive antigen signals presented by dendritic cells (DCs) and activate T‐cell receptor (TCR) signalling. 28 Stimulatory cytokines, such as IL‐6, induce the expression of the master transcription factor Bcl‐6 and the critical chemokine receptor CXCR5 in TFH progenitor cells. 29 , 30 The first stage has been extensively studied and is vital in TFH differentiation.
Currently, research on TFH cells is mainly performed in mice. In humans, most studies on TFH cells are focused on tonsillar and circulating TFH cells in peripheral blood. 29 The costimulatory receptor and cytokine signals transduced during the differentiation of TFH cells are almost identical in mice and humans. During the first differentiation stage, naïve T‐cell interactions with DCs are mainly regulated by IL‐6, ICOS, IL‐2 and the TCR signalling pathways. 31 , 32 ICOS plays an important role in regulating the differentiation and migration of TFH cells. 15 , 16 ICOSL signalling is necessary for antigen‐specific B cells in most cases, except when in the presence of a large number of antigens or during the migration of a large number of antigen‐specific B cells. 15 , 16 B cells act as not only antigen‐presenting cells (APCs) but also the ICOS ligand (ICOSL) in TFH cell differentiation. 33 During acute infection and immune responses, B cells are the main APCs. Antigen presentation is important for antigen‐specific CD4+ T‐cell‐required antigen recognition during each cell division. 33 During the migration of activated CD4+ T cells from the T‐cell zone to the B‐cell zone, a series of changes take place in their surface molecules. CXCL13 is the ligand of CXCR5 and is highly expressed in the follicles and germinal centres of B cells. However, CCL19 and CCL21, the ligands of CCR7, are highly expressed in the T‐cell region. 18 , 33 , 34 , 35 , 36 CD4+ T cells that are sensitized by DCs upregulate CXCR5 and downregulate CCR7 to induce B‐cell migration to the T‐B cell junction. 17 , 37 ICOS and CXCR5 promote PI3 K signalling, PD‐1 inhibits PI3 K activity, and PI3 K signalling determines whether TFH cell precursors enter the follicle. The structural expression of bystander B‐cell ICOSL and PD‐1 mediates TFH ICOS signalling. ICOS signalling promotes PI3 K activation, T‐cell pseudopodia formation and T‐cell migration. The interaction of CXCL13‐CXCR5 also promotes PI3 K signalling, which stimulates precursor TFH cells to enter the follicular region. 18 miR19‐72 promotes the differentiation of TFH cells. miR19‐72 downregulates the phosphatases PHLPP2 and PTEN, which inhibit the PI3 K‐ICOS signalling pathways. 38 IL‐2 inhibits the differentiation of TFH cells. Early TFH differentiation is regulated by a variety of signalling pathways, such as the IL‐6, ICOS, IL‐2 and TCR pathways, mainly by DCs, and by the regulated expression of CXCR5 and Bcl‐6. 39 IL‐6 promotes CD4+ T‐cell and B‐cell activation during Plasmodium infection. IL‐6 promotes CD4+ T‐cell activation and B‐cell responses during the blood stage of Plasmodium infection, which contributes to the production of parasite‐specific antibodies. The IL‐21 and IL‐21 receptors show increased expression and activity levels during the immunopathogenesis of multiple sclerosis. Thus, several related regulators play critical regulatory roles in the early stage of TFH cell differentiation and function.
The second stage of TFH cell differentiation occurs during the interaction between TFH cells and antigen‐specific B cells at the junction of the T‐B cell border and in the follicles and interfollicular region. The chemokine environment of the T‐cell region and B‐cell region affects the migration of T cells. 14 , 18 , 36 , 40
The third stage of TFH differentiation takes place in GCs. GCs are specialized structures found in the B‐cell follicles of secondary lymphoid tissues, where B cells produce high‐affinity antibodies and differentiate into memory B cells and long‐lived plasma cells. 41 , 42 GCs are special structures composed of GC TFH cells, GC B cells, follicular dendritic cells (FDCs), macrophages and stroma. 43 The B7 receptor PD‐1 is highly expressed on TFH cells and is one of the main markers of TFH cells located in GCs. TFH cell precursors initially express PD‐1 during cell activation because of their interaction with antigen‐presenting DCs. The expression of PD‐1 is enhanced when precursor TFH cells interact with B cells at the junction of the T‐B border. The steady interaction between T and B cells leads to the formation of GCs, and the expression of PD‐1 on TFH cells peaks in GCs. 41 , 42 , 43 Notably, the role of PD‐1 in the localization of TFH cells varies with the location and maturation stage of T cells. The expression levels of the chemokine receptors CXCR5 and CXCR4 are high, and the expression levels of PSGL1 and sphingosine‐1‐phosphate receptor (S1PR1) are low in GC TFH cells during this stage. 44 , 45 The expression of G protein‐coupled receptor 2 (EBI2), which is induced by the Epstein–Barr virus, requires special attention because EBI2 ligands are present in B‐cell follicles but not in GCs. The decreased expression of EBI2 in GC B cells and GC TFH cells is important for their correct localization in GCs. 46 , 47 In addition, adhesion molecules play important roles in GC TFH cells, regulating the interaction and localization with GC B cells. The signalling lymphocyte activation molecule (SLAM) family receptors SLAMF6, also known as Ly108 and NTB‐A; CD84 and SLAM, are self‐ligands that are differentially expressed in GC TFH cells and GC B cells. 48 The expression of SLAM‐associated protein (SAP) is necessary for the development of GC TFH cells and the production of most memory B cells and memory plasma cells. The absence of SAP weakens the adhesion of TFH cells to GC B cells, making TFH cells unable to remain in GCs and impairing the maturation and function of B cells. The importance of SAP is largely due to its ability to block the powerful inhibitory signals of SLAMF6. After the competitive binding of SAP and the phosphatase SHP‐1 to SLAMF6 and SAP, respectively, SLAMF6 transmits a positive TFH cell differentiation signal, which supports the adhesion and function of TFH cells. In contrast, when SLAMF6 binds to SHP‐1, a negative regulatory TFH cell differentiation signal is transmitted that inhibits the adhesion of TFH cells and B cells. 48 , 49 , 50
However, TFH cell localization to GCs is not the end‐point of TFH cell differentiation. TFH cells migrate from one GC to another or migrate into the blood or lymph for circulation. 51 , 52 , 53 On the other hand, the position of B cells in the GCs is fixed; that is, they cannot be removed. After moving out of the GC, TFH cells become memory TFH cells, and the expression of Bcl‐6 is downregulated. After TFH cells leave the GC, the activity and polarization of TFH cells decrease, IL‐7Rα is upregulated, and these cells differentiate into resting memory TFH cells. 51 , 52 , 53 , 54 , 55 Memory TFH cells have a central memory phenotype, are located mainly in the spleen, lymph nodes and bone marrow and can be recycled in the blood. Approximately 20% of human central memory CD4+ T cells are CXCR5+, indicating that memory TFH cells are the main components of the human memory T‐cell population. Memory TFH cells are less predominant than reactivated TFH cells and GC TFH cells. 18 , 51 , 52 , 53 , 54 , 55 In humans, memory TFH cells are phenotypically heterogeneous, at least in the blood, and in this population, a considerable number are resting memory TFH cells that express low levels of PD‐1. These PD‐1low memory TFH cells are the most polarized and powerful memory TFH cells. The expression of Bcl‐6 in TFH cells is unstable and requires continuous enhancement. 18 , 51 , 52 , 53 , 54 , 55 Therefore, when GC TFH cells leave GCs, the expression of Bcl‐6 is decreased, and as the cells transform into resting memory TFH cells, the expression of Bcl‐6 decreases further (Figure 2).
REGULATION OF TFH CELL FUNCTION AND DIFFERENTIATION
Regulatory roles of cytokines in TFH cell differentiation
Cytokines play important roles in lymphocyte differentiation (Figure 3). IL‐6 and IL‐21 play roles in TFH cell differentiation and act directly on B cells. IL‐6 transiently induces the expression of Bcl‐6 in the early stage of TFH cell differentiation, and it is also an inducer of IL‐21 expression in inactivated mouse CD4+ T cells. IL‐6 is the most important cytokine involved in the early differentiation of TFH cells. 32 IL‐6 is produced by DCs, macrophages, B cells and other types of cells in response to a series of external and internal pathogen‐associated molecular patterns (PAMPs) and damage‐associated molecular patterns (DAMPs). 18 In the absence of IL‐6, the early differentiation of mouse TFH cells is inhibited, but differentiation is increased in response to supplementation with IL‐21 and IL‐27. Many kinds of DCs and monocytes regulate the differentiation of TFH cells. 32 , 56 , 57 , 58
IL‐2 negatively regulates TFH cell differentiation. 59 Furthermore, IL‐2‐STAT5 signalling inhibits the expression of the transcription factor Bcl‐6 in TFH cells. STAT5 promotes the expression of BLIMP1, which inhibits the expression of Bcl‐6. The expression of PD‐1 in GC TFH cells is high, and PD‐1 signalling inhibits the production of IL‐2, which suggests that IL‐2 inhibits the expression of PD‐1. 39 However, during naïve T‐cell differentiation, IL‐2 signalling is enhanced to different degrees. 59 Therefore, the regulatory effects of IL‐2 signalling on TFH cell differentiation are different and depend on the intensity of IL‐2 signalling.
IL‐12 is an important cytokine that induces naïve CD4+ T cells to differentiate into TH1 cells and promotes the expression of the specific transcription factor T‐bet. IL‐12 but not IFN‐γ is induced during Salmonella infection and represses TFH cell differentiation. 60 TFH1 cells, a subgroup of TFH cells, produce both the TFH‐associated cytokine IL‐21 and the TH1 cytokine interferon‐γ. IL‐12 promotes TFH1 differentiation and function by increasing T‐bet and Bcl‐6 expression. 61
Type I IFN‐α/β participates in the incomplete differentiation of TFH cells and induces the expression of Bcl‐6, CXCR5 and PD‐1 through STAT1 signalling without producing IL‐21. 62 , 63 , 64 Type II IFN‐γ plays an active role in the differentiation of TFH cells. As shown in the Sanroque lupus model, the accumulation of TFH is the result of excessive IFN‐γ. IFN‐γ blockade reduces the number of TFH cells in lupus tissue, indicating that IFN‐γ plays an important and active role in the production of TFH cells. In terms of mechanism, excessive IFN‐γ signalling leads to increased expression of Bcl‐6. In contrast, in the late stage of TH1 cell differentiation, Bcl‐6 binds to the IFN‐γ gene locus and inhibits excessive Ifn mRNA expression. 62 Thus, Bcl‐6 and IFN‐γ regulate each other through a negative feedback mechanism.
Therefore, several cytokines show distinct regulatory roles in determining TFH cell lineage differentiation. IL‐6, IL‐21, IL‐27 and IL‐12 have positive regulatory effects on TFH cell differentiation, and IFN‐γ, IL‐2 and IL‐10 play inhibitory roles in TFH cell differentiation.
Transcriptional regulation of TFH cell function and differentiation
Multiple transcription factors synergistically promote the development and differentiation of T cells (Figure 3). T cells have different functional subsets, and their transcription factors are expressed in different forms and levels at different stages of development and differentiation. During TFH differentiation, different transcription factors promote or inhibit the differentiation of TFH cells.
Some transcription factors play positive roles in TFH differentiation. 65 Signal transducers and transcriptional activators (STATs) are also closely related to the differentiation of TFH cells. STAT3 plays an important role in the differentiation of TFH cells in the mouse CD4+ T‐cell population, and STAT1 and STAT4 are also very important in the differentiation of TFH cells. 66 , 67 The expression of STAT3 and IL‐21 in mouse CD4+ T cells is very important. 65 Mouse CXCR5 and IL‐6 drive STAT3 and STAT1 to interact with Maf and Batf, bind to the promoter and initiate the expression of CXCR5. In contrast to their effects in mice, STAT3 and STAT4 are equally important for the differentiation of TFH cells in humans. 65 , 68 STAT5 inhibits the differentiation of TFH cells, and the opposite effects of STAT3 and STAT5 on the differentiation of TFH cells are similar to the effects of STAT3 and STAT5 on the differentiation of TH17 cells. 69 , 70
The promoter of Bcl‐6 harbours binding sites for the transcription factors STAT and Forkhead box O (Foxo). 65 , 71 Under low IL‐2 conditions, STAT3 binds to the promoter of Bcl‐6, and when IL‐2 increases, STAT5 binds to the promoter of Bcl‐6. Interestingly, STAT5 replaces the STAT3 complex and excessively recruits the inhibitory complex, which explains why IL‐2 inhibits the differentiation of TFH cells. 71 Maf is highly expressed in TFH cells and is related to the expression of CXCR5, IL‐21 and IL‐4. c‐Maf is a basic leucine zipper transcription factor that binds to the IL‐4 promoter and plays an important role in the differentiation of TH2 cells. 15 , 72 The loss of c‐Maf leads to a reduction in IL‐17 production. ICOS‐mediated c‐Maf expression is necessary for IL‐21 production and TFH cell expansion. 72
IRF4 and basic leucine zipper transcription factor (Batf) also play important roles in regulating the differentiation of TFH cells. 73 , 74 Batf is a positive regulator of Bcl‐6. In the absence of Batf, the coexpression of Bcl‐6 and Maf is required for the expression of CXCR5 in vivo. 73 Although Batf is only moderately increased in TFH cells, Batf −/− mice cannot produce TFH cells. Batf directly binds to the promoters of Bcl‐6 and Maf. The E protein Ascl2 also promotes the differentiation of TFH cells. Ascl2 is not the only regulatory factor of CXCR5. There is redundancy among several E proteins that are expressed in T cells. Multiple E proteins promote the expression of CXCR5 by binding to enhanced subregions. 74 The function of E proteins is tightly regulated by DNA‐binding inhibitor 2 (ID2) and ID3 (Figure 3).
T helper cell‐inducing POZ/Kruppel‐like factor (Thpok) and thymocyte selection‐associated high mobility group box protein (Tox) have been reported to regulate other transcription factors that are crucial to TFH cell differentiation, such as Bcl‐6 and c‐Maf. Thpok and Tox are critical for the development of T cells. 75 , 76 It has been shown that these factors bind to the promoters or other transcriptional regulatory regions of TFH differentiation‐related transcription factors and regulators (such as CXCR5 and ICOS) to promote their expression. There may be other upstream transcription factors that promote TFH cell development.
Negative regulatory transcription factors in TFH cell differentiation have also been reported in other studies (Figure 3). Foxp1 and Foxo1 are expressed in resting immature CD4+ T cells and are necessary for immature CD4+ T cells to maintain resting and homing states. 77 Downregulating the expression of Foxo1 and Foxp1 can positively regulate the differentiation of TFH. For example, itchy E3 ubiquitin protein ligase (Itch) can bind to Foxo1 and accelerate Foxo1 degradation. ICOS and PI3 K‐Akt signalling promote the differentiation of TFH cells, while Akt signalling leads to a decrease in Foxo1 transcriptional activity. 78 Inhibiting Foxo1 and Foxp1 enhances the expression level of ICOS. 79 E26 transformation‐specific sequence‐1 (Ets1) downregulates the expression of GATA3 and IL‐4. The specific loss of Ets1 in mouse CD4+ T cells leads to systemic lupus erythematosus (SLE) and the terminal differentiation of TFH2 cells. 80
Taken together, these data suggest that Bcl‐6 is the master transcription factor in TFH cell differentiation; STAT3, STAT4 and STAT1 promote the expression of Bcl‐6 and TFH differentiation; and transcription factors such as IRF4, MAF, BATF, Ascl2 and ThpoK also play positive roles in TFH cell differentiation and function. Transcription factors such as Foxo1, Foxo3a and BLIMP1 negatively regulate the differentiation and function of TFH cells.
METABOLIC REGULATION OF TFH CELL FUNCTION AND DIFFERENTIATION
Metabolic regulation plays an important role in the differentiation and function of TFH cells (Figure 4). Some studies of glucose metabolism in TFH cells suggest that glycolysis, which is mediated by mTOR‐ and HIF1α‐related signalling, is positively regulated during the differentiation process. However, some studies suggest that TFH cells have less robust metabolic functions than other Th cell subsets, such as TH1 cells, and oxidative phosphorylation but not glycolysis plays a major role in differentiation.
Figure 4.
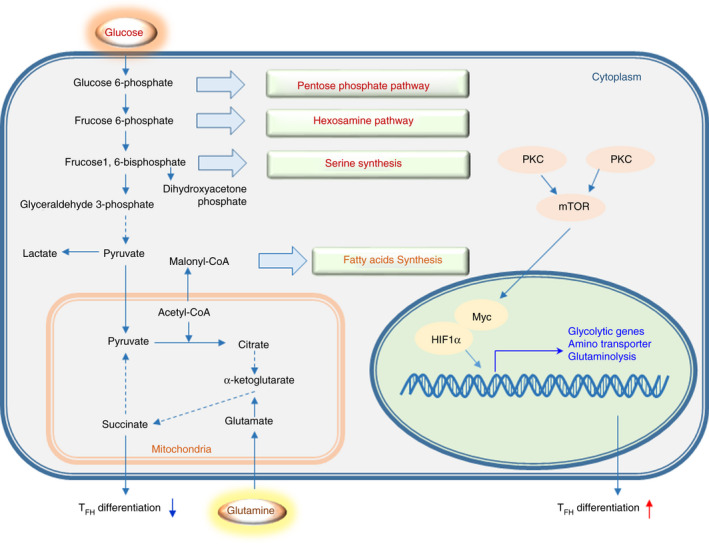
Regulation of metabolism in TFH cell differentiation. Basic processes and correlations of glucose, lipid and amino acid metabolism. mTOR signalling and transcription factors such as Myc and HIF1α promote TFH cell differentiation related to improvements in metabolism. The differentiation of TFH cells is inhibited by the glycolysis inhibitors 2‐DG and succinate, a substrate of oxidative phosphorylation.
mTORC1 and mTORC2 are essential for the differentiation of TFH cells and other TH cell subsets. 81 , 82 Specifically, mTORC1 promotes TH1 and TH17 cell differentiation, whereas mTORC2 favours TH2 cell differentiation by orchestrating metabolic reprogramming and lineage‐specific gene transcription. It has been reported that mTORC1 and mTORC2 are critical for TFH differentiation based on studies of the phenotype acquired in Raptor‐ and Rictor‐knockout mice. 82 The loss of Rictor or Raptor results in a decrease in the TFH cell population and attenuates the humoral immune response. 82 Aberrant activation of mTORC1 can lead to autoimmune diseases. The mTORC1‐4E‐BP‐eIF4E axis can promote Bcl‐6 protein synthesis. 83 In addition, some studies suggest that mTORC1 inhibits the differentiation of TFH cells through the IL‐2‐mTORC1 axis. 84 The regulatory effects of mTORC1 on TFH cell differentiation are contradictory, and the precise regulatory mechanisms need to be further studied. It was recently reported that mTORC2 plays a positive regulatory role in the differentiation and function of TFH cells. Additionally, mTORC1 inhibits the differentiation and expansion of Treg cells. However, some studies have reported that mTORC1 is essential for the differentiation and function of Treg cells by activating the STAT3‐TCF‐1‐Bcl‐6 axis. 5 mTORC1 participates in the regulation of multiple signal pathways, and its regulatory functions on the differentiation and function of TFH cells and Treg cells might differ due to different experimental conditions and external stimuli.
mTOR signalling‐related glucose metabolism is critical for TFH cell differentiation. A deficiency in PTEN, a negative regulator of mTOR, promotes TFH cell differentiation and GC formation. 82 , 85 mTORC1 and mTORC2 signalling links ICOS to anabolic metabolism and TFH cell‐associated transcriptional programmes in Peyer's patches (PPs). 82 This finding suggests that mTOR activation and glycolysis are required for TFH cell differentiation. K/BxN mice are models of spontaneous rheumatoid arthritis and autoimmune sero‐positive arthritis. The production of high levels of anti‐GPI IgG requires CD4+ follicles to facilitate the expansion of TFH cells. The metabolic activity of CD4+ T cells and B cells in K/BxN mice was higher than that in KRN control mice. Prophylactic inhibition of glycolysis with 2‐deoxy‐D‐glucose (2‐DG) significantly reduced joint inflammation, the activation of adaptive and innate immune cells, and the production of pathogenic autoantibodies. The self‐reactive TFH cells of K/BxN mice exhibit a high level of glycolysis, and their function can be inhibited by downregulating glucose metabolism. The high expression of hexokinase HK2 in joint‐infiltrating lymphocytes in patients with rheumatoid arthritis (RA) indicates that the metabolism of these cells is highly glycolytic. 86 These results suggest that glycolysis and/or mTOR signalling is critical for the differentiation of TFH cells.
Roquin inhibits the PI3 K‐mTOR signalling pathway, spontaneous activation of T cells and abnormal differentiation of TFH and TH17 cells. 87 The deletion of Roquin leads to an increase in the ratio of TFR cells to TFH cells, and the inhibition of PI3 K‐mTOR signalling restores the increase in TFR and TFH cells caused by Roquin deletion. 88 The PI3 K‐mTOR signalling pathway has been suggested to play an important positive regulatory role in glycolysis; therefore, it is suggested that glycolysis signalling mediated by the PI3 K‐mTOR pathway promotes the differentiation of TFH and TFR cells. 87 TFR cells, similar to Treg cells, play immunosuppressive roles. TFR cells express CXCR5, Bcl‐6 and other characteristic factors that function in the germinal centre. 88 mTORC1 promotes the differentiation of TFR cells, suggesting that mTORC1 positively regulates the expression of CXCR5, ICOS and Bcl‐6 in T cells. However, other studies present opposite findings. For example, it was found that TFH cells exhibit reduced glycolysis and mitochondrial respiration, accompanied by reduced mTOR activity compared with those of TH1 cells in acute viral infection. 84 It has been shown that overactivated PI3Kδ affects humoral immunity in APDS mouse model (Pik3cd E1020 K/+). 89 In Pik3cd E1020 K/+ mice, TFH cells and GC B cells are particularly abundant, but the efficiency of the B‐cell response to the immune‐based conversion of antigen‐specific B cells was decreased. TFH cells in Pik3cd E1020 K/+ mice are typically limited to the light zone (LZ), which specifically targets high‐affinity B cells and induces survival and proliferation. 89 APDS patients present with structural disorders in germinal centres, and the increased invasion of TFH cells impairs humoural immune response.
Protein kinase C (PKC) plays important roles in cell metabolism and signal transduction. The function of mitochondria in B cells is downregulated in Pkc −/− mice, and the antigen presentation ability of B cells is inhibited. It was also found that the reaction of GCs in these mice can be decreased, and the production of plasma cells and immunoglobulin is decreased. The mitochondrial regulation mediated by PKC is partly regulated through the mTOR signalling pathway. Thus, mitochondria are considered to be particularly important for the GC response, which requires the participation of TFH cells. 90 These findings suggest that PKC also regulates the function of TFH cells by regulating mitochondrial functions.
Bcl‐6 is an important transcription factor in the differentiation of TFH cells. 91 The expression of Bcl‐6 is significantly upregulated in activated CD4+ T cells subjected to glucose deprivation (GD) or 2‐DG. Adenosine 5’‐monophosphate (AMP)‐activated protein kinase (AMPK), a metabolic sensor, is activated when glycolysis is decreased. The AMPK antagonist compound C inhibits the expression of Bcl‐6 induced by glucose deprivation. 91 A study showed that AMPK plays a critical role in the differentiation of TFH cells. Mature TFH cells have a lower metabolic state than TH1 cells. 91 These data show that a decrease in cell metabolism is an inducer of TFH differentiation, not merely the result of TFH differentiation.
Resting T cells mainly rely on oxidative phosphorylation in mitochondria to produce ATP. Under antigen stimulation, the downstream AKT signalling pathway is mediated by the TCR pathway and costimulatory receptor CD28, and the AKT signalling pathway has been extensively studied with respect to the positive regulation of glycolysis. Under the metabolic conditions generated by glycolysis, naïve CD4+ T cells differentiate into TH1, TH17 and other cell subsets. DUSP6, a MAPK phosphatase, connects TCR signalling to activation‐induced metabolism that favours glycolysis and attenuates TFH cell differentiation. 92 Bcl‐6 expression is highly upregulated in activated CD4+ T cells following glucose deprivation, and this pathway is insensitive to inhibition by IL‐2. In a manner similar to glucose deprivation, the glucose analogue 2‐DG inhibits glycolysis, and 2‐DG induces Bcl‐6 expression in activated CD4+ T cells. 91 Although these studies revealed that Bcl‐6 is induced when glycolysis is inhibited, the inhibition of glycolysis is also detrimental to T‐cell activation. Therefore, during robust T‐cell proliferation, glucose uptake and glycolysis are required, and Bcl‐6 is activated by low‐energy signals transmitted by AMPK during high T‐cell proliferation and activity. 86 , 91 , 93 These results suggest that the mTOR pathway positively regulates TFH cell differentiation, but the mechanism by which the transcription programme is regulated by mTOR has not been clarified.
Ovalbumin (OVA) sensitization and influenza virus infection are considered to be models for the study of TFH cells. TFH cells are induced to differentiate during OVA sensitization and viral infection. Succinate is a substrate in oxidative phosphorylation. The injection of succinate into wild‐type (WT) mice sensitized by OVA inhibited TFH cell differentiation and the GC response. In addition, 2‐DG is an inhibitor of glycolysis. Under OVA sensitization and viral infection, the injection of 2‐DG into the body inhibited the differentiation and function of TFH cells. 94 Therefore, the inhibition of glycolysis or the enhancement of oxidative phosphorylation inhibits the differentiation of TFH cells in vivo, suggesting that glycolysis is the main metabolic mechanism regulating TFH cell differentiation.
TFH CELLS IN IMMUNE‐ASSOCIATED DISEASES
TFH cells affect humoural immunity by regulating B‐cell development. In recent years, with continuous research on TFH cell differentiation and functions, it has been revealed that TFH cells are closely related to many immune‐associated diseases, including infectious diseases, autoimmune diseases and cancers (Figure 5 and Table 1).
Figure 5.
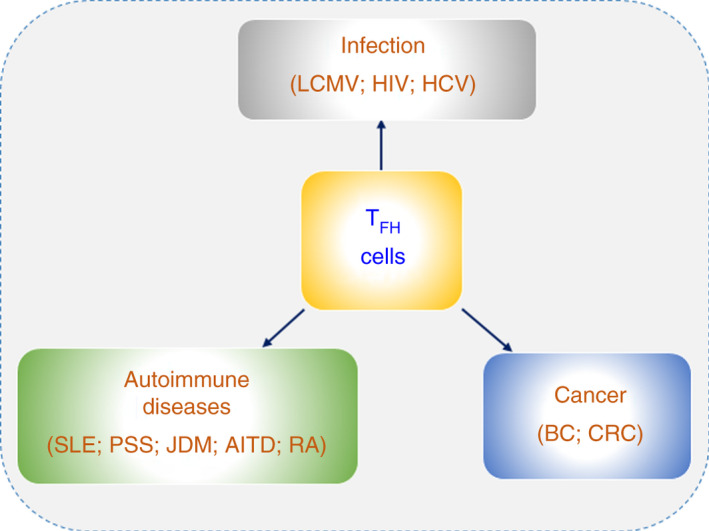
TFH cell‐associated immune‐mediated diseases. TFH cells play regulatory roles in immune‐associated diseases, including infections, autoimmune diseases and cancers.
Table 1.
Immunoregulation of TFH cells in diseases.
| Disease | Prognosis effects | Immune functions | ||
|---|---|---|---|---|
| Infection | LCMV | Mouse | Promote viral clearance | IL−6 and Bcl−6 upregulation. 96 |
| Production of late‐stage antibodies. 97 | ||||
| HCV | Human | Anti‐infection | IL−21 and ICOS upregulation. 99 | |
| HIV | Human | Anti‐infection | Plasma cell and autoantibody production decreases. 100 , 102 , 103 | |
| GAS | Human | Promote bacterial clearance | Granzyme B expression. 128 | |
| Autoimmune diseases | SLE | Mouse; human | Hyperactive | Autoantibody production. 51 , 80 , 106 , 107 |
| pSS | Human | Hyperactive | Autoantibody production. 108 , 109 | |
| JDM | Human | Hyperactive | Autoantibody production. 52 | |
| AITD | Human | Hyperactive | Autoantibody production; IL−21 and Bcl−6 upregulation. 110 ; CXCR5 and CXCL13 expression. 111 | |
| RA | Human | Hyperactive | Autoantibody production; IL−21 upregulation. 112 ; | |
| Mouse | Autoantibody production; CXCR5 expression. 113 | |||
| Cancers | BC | Human | Positive correlation with good prognosis | 8‐gene TFH cell signature. 123 ; CXCL13 expression. 124 |
| CRC | Mouse; human | Positive correlation with good prognosis | CXCL13 and IL−21 expression. 125 | |
| Other cancers | Mouse | Downregulate antitumour immunity | IL−4 expression. 126 | |
| Upregulate antitumour immunity | IL−21 expression. 127 | |||
Immunoregulatory roles of TFH cells in infection
TFH cells help B cells differentiate into plasma cells to produce antibodies, which are crucial for eliminating viruses and bacteria. In the context of viral and bacterial infection, some investigations have been performed on lymphocytic choriomeningitis virus (LCMV) infection, hepatitis C virus (HCV) infection, human immunodeficiency virus (HIV) infection and group A Streptococcus (GAS) bacterial infection.
In mice infected with LCMV Clone 13, a persistent variant of LCMV, persistent viral infection progressively drives CD4+ T‐cell development to disfavour TH1 cells and favour TFH cells. 95 In other words, LCMV infection promotes TFH cell differentiation. In addition, in persistent LCMV infection in vivo, TFH cells are crucial for viral clearance. 96 Cytokine production and other changes were analysed in mice infected with LCMV Clone 13. IL‐6 signalling in TFH cells promoted the clearance of LCMV Clone 13 and enhanced the function of TFH cells by upregulating Bcl‐6. A similar study also demonstrated that TFH cells were essential in helping B cells produce late‐stage antibodies to neutralize LCMV in mice. 97
TFH cells are also essential in controlling HCV infection in a manner similar to the control of LCMV. During HCV infection, it has been observed that TFH cells also significantly increase the percentage of cells coexpressing CXCR5 and PD‐1 in both acute infection and chronic infection. 98 , 99 In patients with acute HCV infection, TFH cells express specific markers and secrete IL‐21 in response to HCV. Virus‐specific TFH cells can be detected in blood samples, and ICOS expression is significantly increased in CXCR3‐expressing HCV‐specific TFH cells. 99 In the livers of patients with chronic HCV infection, virus‐specific TFH cells are enriched compared with other types of cells. 99
HIV causes defects in the human immune system, and an increasing number of people die from HIV infection. Research has shown that TFH cells are severely dysregulated in HIV‐infected patients. 100 , 101 CXCR5+PD‐1+Bcl‐6+ cells harbouring HIV DNA are significantly increased in infected patients. Deregulation of TFH‐mediated B cells contributes to diminished B‐cell responses during HIV infection, which suggests that TFH cells have a significant impact on the control of HIV infection. 100 GC TFH cells have a higher probability of harbouring HIV infection than other cells in vitro, as indicated by the higher frequency of infected cells. 102 , 103
GAS bacterial infection is the main cause of recurrent tonsillitis (RT) in childhood. 104 Aberrant pyrogenic exotoxin A‐activated GC TFH cells express granzyme B to kill B cells in RT patients. 63
Immunoregulatory roles of TFH cells in autoimmunity
Autoimmunity is closely associated with excessive autoantibody production. The differentiation and function of TFH cells are closely related to autoimmune diseases. 105 Overactive TFH cells were found in subsets of patients with systemic lupus erythematosus (SLE), Sjogren's syndrome (pSS), juvenile dermatomyositis (JDM), autoimmune thyroid disease (AITD) and rheumatoid arthritis (RA) (Figure 5 and Table 1).
SLE is an autoimmune disease that is currently well understood in the field of TFH cell‐related diseases. The number of circulating TFH cells is increased in mice and patients with the autoimmune disease SLE. 51 , 106 , 107 TFH cells are closely associated with SLE, suggesting that disordered homeostasis of the T‐cell–B‐cell equilibrium is a major cause of SLE. 106 , 107 In Ets‐deficient mice, TFH2 (Bcl6+GATA3high) cells are the main TFH cell subset, and these mice are highly likely to develop SLE. 80 Similarly, there are more TFH2 cells in SLE patients than in healthy individuals. 80 It has been found that TFH cells play an important role in the severity of pSS. 108 , 109 The proportion of peripheral TFH cells is increased in patients. The percentage of TFH cells is positively correlated with the numbers of both activated T cells and Th1 cells. 108 In JDM patients, the TFH cell subset proportions are severely unbalanced, with a profound skewing of the TFH cell subset towards TH2 and TH17 cells. 52 Studies have shown that TFH cells play important roles in AITD. 110 , 111 In patients with AITD, the frequency of circling TFH cells is increased, and the mRNA expression of IL‐21 and Bcl‐6 is also enhanced. 110 In addition, the chemokine receptor CXCR5 and its ligand CXCL13 are essential in regulating T‐cell and B‐cell compartmentalization in the thyroid tissue of patients with AITD. 111 TFH cell and serum IL‐21 levels are significantly increased in the RA patient group compared with those of the corresponding healthy control group. Moreover, B‐cell proliferation and antibody secretion are increased in RA patients. Research has also shown that IL‐21 promotes B‐cell activity. Thus, IL‐21 may be related to the pathogenesis of rheumatoid arthritis. 112 In a collagen‐induced arthritis (CIA) mouse model, CXCR5‐deficient mice showed strong resistance to CIA, suggesting that CXCR5 is an indispensable factor for inducing autoimmune diseases. 113
TFH cells contribute to metabolic‐associated autoimmune disorders, such as diabetes and atherosclerosis. Type 1 diabetes (T1D) is called insulin‐dependent diabetes mellitus (IDDM) and is associated with metabolic disorders. TFR cells negatively regulate the function of TFH cells during inflammation. It has been reported that TFR cell deficiency is involved in the pathogenesis of T1D. Thus, it was hypothesized that TFH cells participate in the onset of diabetes. 114 Type 2 diabetes (T2D) mellitus is a chronic metabolic disease that is strongly associated with obesity. In mucosal biopsy samples, B cells and plasma blasts from T2D patients exhibited a slight but significant increase in the frequency of cells with surface IgG expression compared with those from healthy individuals. To a large extent, the pathogenesis of T2D is related to obesity, but some patients with T2D have a body mass index (BMI) that is inconsistent with obesity. It was found that non‐obese T2D patients exhibited significantly higher levels of faecal IgG and higher frequencies of CD4+CXCR5+ T (TFH) cells than BMI‐matched healthy controls. 115 Atherosclerosis (AS) is associated with lipid metabolism disorders and is the main cause of coronary heart disease, cerebral infarction and peripheral vascular disease. Treg cells function in antiatherosclerosis. Treg cells lose Foxp3 expression during inflammation and are then converted into other CD4+ T‐cell subsets. During atherosclerosis development, Treg cells can switch their phenotype and become pro‐atherogenic TFH cells and apolipoprotein AI (ApoAI), this conversion can be achieved by reducing the expression of IL‐2Rα and p‐STAT5 levels. 116 There is cross‐regulation between atherosclerosis and other autoimmune diseases, such as SLE. Dyslipidaemia in atherosclerotic diseases significantly increases the severity of SLE, with enhanced production of autoantibodies, such as IgG2c, and the TFH cell response via IL‐27. 117 STAT4 suppresses Foxp3 expression, regulates TFH cell functions and promotes atherogenesis in insulin‐resistant low‐density lipoprotein receptor‐deficient (Ldlr −/−) mice. 118 Pre‐B‐cell leukaemia transcription factor 1 isoform d (Pbx1d) can regulate TFH cell expansion and Treg cell homeostasis as a lupus susceptibility gene. Cholesterol favours TFH cell differentiation or maintenance, and the interaction between Pbx1d and dyslipidaemia alters TFH cell and Treg cell fates and functions. 119
Furthermore, different subsets of TFH cells (TFH2 or TFH13 cells) are specifically involved in autoimmune diseases. Dysfunction or imbalance in CD4+ T cells leads to the pathogenesis of autoimmune diseases. For example, TH1 cells play critical roles in insulin‐dependent T1D, inflammatory bowel disease (IBD) and RA; TH17 cells participate in the pathogenesis of autoimmune encephalomyelitis (EAE); and TH22 cells are critical in psoriasis, obsessive–compulsive spondylitis and multiple sclerosis (MS). TFH cells are critical for antibody production and humoural immunity, and their aberrant activity is closely related to the pathogenesis of autoimmune diseases. Among the TFH cell subsets, TFH2 and TFH13 cells are recognized as crucial participants in the onset and exacerbation of autoimmune diseases. Asthma causes airflow obstruction and is induced by inflammatory disorders. Circulating TFH2 cells are polarized, and the ratio of TFH2 cells to TFH1 cells is increased in the peripheral blood of patients with asthma. 120 TFH2 cells are polarized in both allergic rhinitis (AR) and AR +asthma cases. In one study, it was found that peripheral blood lymphocytes of patients with AR or AR +asthma diseases were preferentially polarized to the TFH2 phenotype. TFH2 cells can produce IgE‐related cytokines, such as IL‐4, IL‐5 and IL‐13, similar to TH2 cells, leading to allergic inflammation. 121 It was reported that the expression of CD23 was enhanced on CD19+CD20+CD27+IgD− switching memory B cells and positively correlated with antigen‐specific IgE levels and the TFH2 cell ratio in AR patients. TFH2 cells have the capacity to promote CD23 expression on switching memory B cells by secreting the cytokine IL‐4. 122
Immunomodulatory roles of TFH cells in cancer
An increasing number of studies have shown that TFH cells have an indispensable role in a variety of cancers, including breast cancer (BC) and colorectal cancer (CRC) (Figure 5 and Table 1).
It has been observed in breast cancer patients an 8‐gene TFH cell signature in tumour tissue who has a significant positive correlation with organized antitumour immunity, long‐term patient survival and preoperative response to chemotherapy. 123 In addition, researchers have also shown that TFH cells promote local memory B‐cell differentiation, thus enhancing antitumour immunity. 124 In human CRC, TFH cells also have a positive effect on the survival of cancer patients. A study showed that the density of TFH cells increased, whereas the levels of most T cells decreased during tumour progression. CXCL13 and IL‐21 expression are also associated with cancer progression. 125
However, studies have shown that TFH cells expressing IL‐4 are associated with downregulated antitumour immunity in other cancers. 126 CNS2‐deleted mice were injected subcutaneously with various tumour cells and exhibited stronger antitumour immunity than control WT mice. In addition to IL‐4, IL‐21 is another major cytokine that is expressed by TFH cells. However, in contrast to the effect of IL‐4, IL‐21 enhances antitumour immunity in mouse models. 127 When PD‐1 or CTLA‐4 blockade was combined with IL‐21, the mice showed improved treatment outcomes. 127
CONCLUDING REMARKS
TFH cells have been extensively investigated in mouse and human studies. These studies have demonstrated that TFH cells contribute to both humoural immunity and immune‐related diseases. However, despite in‐depth study, the regulatory factors and mechanisms of TFH cell differentiation, especially in the pathogenesis of various important immune‐related diseases, remain unclear and need further study. Further study of TFH cell subgroups, including the TFH1, TFH2 and TFR cell subgroups, and other subpopulations may be helpful to further clarify the roles of these cells in immune‐related diseases, which will provide the basis for the diagnosis and treatment of diseases in the future.
AUTHOR CONTRIBUTIONS
L.D., Y.H. and Y.C. consulted the references; Y.X.W., A.J., Y.F.W., Q.Y. and W.L. participated in discussions, L.D., Y.B. and G.L. contributed to writing the manuscript and participated in discussions.
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
ACKNOWLEDGEMENTS
The authors’ research is supported by grants from the National Natural Science Foundation for Key Programs of China (31730024, G.L.), National Natural Science Foundation for General Programs of China (31671524, G.L.) and Beijing Municipal Natural Science Foundation of China (5202013, GL).
Lin Dong, Ying He, Yejin Cao, and Yuexin Wang contributed equally to this work as cofirst authors.
Contributor Information
Yujing Bi, Email: liugw@bnu.edu.cn, Email: byj7801@sina.com.
Guangwei Liu, Email: liugw@bnu.edu.cn.
DATA AVAILABILITY STATEMENT
Not applicable.
REFERENCES
- 1. King C, Tangye SG, Mackay CR. T follicular helper (TFH) cells in normal and dysregulated immune responses. Annu Rev Immunol. 2008; 26:741–66. [DOI] [PubMed] [Google Scholar]
- 2. Liu G, Burns S, Huang G, Boyd K, Proia RL, Flavell RA, et al. The receptor S1P1 overrides regulatory T cell‐mediated immune suppression through Akt‐mTOR. Nat Immunol. 2009; 10:769–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Groom JR. Regulators of T‐cell fate: Integration of cell migration, differentiation and function. Immunol Rev. 2019; 289:101–14. [DOI] [PubMed] [Google Scholar]
- 4. Masopust D, Schenkel JM. The integration of T cell migration, differentiation and function. Nat Rev Immunol. 2013; 13:309–20. [DOI] [PubMed] [Google Scholar]
- 5. Wang Y, Bi Y, Chen X, Li C, Li Y, Zhang Z, et al. Histone Deacetylase SIRT1 Negatively Regulates the Differentiation of Interleukin‐9‐Producing CD4(+) T Cells. Immunity 2016; 44:1337–49. [DOI] [PubMed] [Google Scholar]
- 6. Zhu J, Paul WE. Peripheral CD4+ T‐cell differentiation regulated by networks of cytokines and transcription factors. Immunol Rev. 2010; 238:247–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Li Y, Yu Q, Zhang Z, Wang J, Li S, Zhang J, et al. TH9 cell differentiation, transcriptional control and function in inflammation, autoimmune diseases and cancer. Oncotarget. 2016; 7:71001–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Liu G, Bi Y, Xue L, Zhang Y, Yang H, Chen X, et al. Dendritic cell SIRT1‐HIF1alpha axis programs the differentiation of CD4+ T cells through IL‐12 and TGF‐beta1. Proc Natl Acad Sci USA. 2015; 112:E957–E965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wang X, Bi Y, Xue L, Liao J, Chen X, Lu Y, et al. The calcineurin‐NFAT axis controls allograft immunity in myeloid‐derived suppressor cells through reprogramming T cell differentiation. Mol Cell Biol. 2015; 35:598–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhang Y, Bi Y, Yang H, Chen X, Liu H, Lu Y, et al. mTOR limits the recruitment of CD11b+Gr1+Ly6Chigh myeloid‐derived suppressor cells in protecting against murine immunological hepatic injury. J Leukoc Biol. 2014; 95:961–70. [DOI] [PubMed] [Google Scholar]
- 11. Li C, Bi Y, Li Y, Yang H, Yu Q, Wang J, et al. Dendritic cell MST1 inhibits Th17 differentiation. Nat Commun. 2017; 8:14275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Liu G, Yang K, Burns S, Shrestha S, Chi H. The S1P(1)‐mTOR axis directs the reciprocal differentiation of T(H)1 and T(reg) cells. Nat Immunol. 2010; 11:1047–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Liu G, Bi Y, Wang R, Yang H, Zhang Y, Wang X, et al. Targeting S1P1 receptor protects against murine immunological hepatic injury through myeloid‐derived suppressor cells. J Immunol. 2014; 192:3068–79. [DOI] [PubMed] [Google Scholar]
- 14. Ma CS, Deenick EK, Batten M, Tangye SG. The origins, function, and regulation of T follicular helper cells. J Exp Med. 2012; 209:1241–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bauquet AT, Jin H, Paterson AM, Mitsdoerffer M, Ho IC, Sharpe AH, et al. The costimulatory molecule ICOS regulates the expression of c‐Maf and IL‐21 in the development of follicular T helper cells and TH‐17 cells. Nat Immunol. 2009; 10:167–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Choi YS, Kageyama R, Eto D, Escobar TC, Johnston RJ, Monticelli L, et al. ICOS receptor instructs T follicular helper cell versus effector cell differentiation via induction of the transcriptional repressor Bcl6. Immunity 2011; 34:932–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shi J, Hou S, Fang Q, Liu X, Liu X, Qi H. PD‐1 Controls Follicular T Helper Cell Positioning and Function. Immunity 2018; 49(264–74):e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Crotty S. T follicular helper cell differentiation, function, and roles in disease. Immunity 2014; 41:529–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kurata I, Matsumoto I, Ohyama A, Osada A, Ebe H, Kawaguchi H, et al. Potential involvement of OX40 in the regulation of autoantibody sialylation in arthritis. Ann Rheum Dis. 2019; 78:1488–96. [DOI] [PubMed] [Google Scholar]
- 20. Choi YS, Yang JA, Yusuf I, Johnston RJ, Greenbaum J, Peters B, et al. Bcl6 expressing follicular helper CD4 T cells are fate committed early and have the capacity to form memory. J Immunol. 2013; 190:4014–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ueno H. Human Circulating T Follicular Helper Cell Subsets in Health and Disease. J Clin Immunol. 2016; 36(Suppl 1):34–9. [DOI] [PubMed] [Google Scholar]
- 22. Schmitt N, Ueno H. Human T follicular helper cells: development and subsets. Adv Exp Med Biol. 2013; 785:87–94. [DOI] [PubMed] [Google Scholar]
- 23. Schmitt N, Bentebibel SE, Ueno H. Phenotype and functions of memory Tfh cells in human blood. Trends Immunol. 2014; 35:436–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Acosta‐Rodriguez EV, Rivino L, Geginat J, Jarrossay D, Gattorno M, Lanzavecchia A, et al. Surface phenotype and antigenic specificity of human interleukin 17‐producing T helper memory cells. Nat Immunol. 2007; 8:639–46. [DOI] [PubMed] [Google Scholar]
- 25. Sallusto F, Lenig D, Mackay CR, Lanzavecchia A. Flexible programs of chemokine receptor expression on human polarized T helper 1 and 2 lymphocytes. J Exp Med. 1998; 187:875–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nance JP, Belanger S, Johnston RJ, Takemori T, Crotty S. Cutting edge: T follicular helper cell differentiation is defective in the absence of Bcl6 BTB repressor domain function. J Immunol. 2015; 194:5599–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Linterman MA, Vinuesa CG. Signals that influence T follicular helper cell differentiation and function. Semin Immunopathol. 2010; 32:183–96. [DOI] [PubMed] [Google Scholar]
- 28. Zhang Y, Garcia‐Ibanez L, Toellner KM. Regulation of germinal center B‐cell differentiation. Immunol Rev. 2016; 270:8–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rautajoki KJ, Kylaniemi MK, Raghav SK, Rao K, Lahesmaa R. An insight into molecular mechanisms of human T helper cell differentiation. Ann Med. 2008; 40:322–35. [DOI] [PubMed] [Google Scholar]
- 30. Schmitt N, Ueno H. Regulation of human helper T cell subset differentiation by cytokines. Curr Opin Immunol. 2015; 34:130–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Goenka R, Barnett LG, Silver JS, O'Neill PJ, Hunter CA, Cancro MP, et al. Cutting edge: dendritic cell‐restricted antigen presentation initiates the follicular helper T cell program but cannot complete ultimate effector differentiation. J Immunol. 2011; 187:1091–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Eto D, Lao C, DiToro D, Barnett B, Escobar TC, Kageyama R, et al. IL‐21 and IL‐6 are critical for different aspects of B cell immunity and redundantly induce optimal follicular helper CD4 T cell (Tfh) differentiation. PLoS One 2011; 6:e17739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Krishnaswamy JK, Alsen S, Yrlid U, Eisenbarth SC, Williams A. Determination of T Follicular Helper Cell Fate by Dendritic Cells. Front Immunol. 2018; 9:2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fazilleau N, McHeyzer‐Williams LJ, Rosen H, McHeyzer‐Williams MG. The function of follicular helper T cells is regulated by the strength of T cell antigen receptor binding. Nat Immunol. 2009; 10:375–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Luthje K, Kallies A, Shimohakamada Y, Belz GT, Light A, Tarlinton DM, et al. The development and fate of follicular helper T cells defined by an IL‐21 reporter mouse. Nat Immunol. 2012; 13:491–8. [DOI] [PubMed] [Google Scholar]
- 36. Weinstein JS, Herman EI, Lainez B, Licona‐Limon P, Esplugues E, Flavell R, et al. TFH cells progressively differentiate to regulate the germinal center response. Nat Immunol. 2016; 17:1197–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Good‐Jacobson KL, Szumilas CG, Chen L, Sharpe AH, Tomayko MM, Shlomchik MJ. PD‐1 regulates germinal center B cell survival and the formation and affinity of long‐lived plasma cells. Nat Immunol. 2010; 11:535–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Baumjohann D, Kageyama R, Clingan JM, Morar MM, Patel S, de Kouchkovsky D, et al. The microRNA cluster miR‐17 approximately 92 promotes TFH cell differentiation and represses subset‐inappropriate gene expression. Nat Immunol. 2013; 14:840–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ballesteros‐Tato A, Leon B, Graf BA, Moquin A, Adams PS, Lund FE, et al. Interleukin‐2 inhibits germinal center formation by limiting T follicular helper cell differentiation. Immunity 2012; 36:847–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Haynes NM, Allen CD, Lesley R, Ansel KM, Killeen N, Cyster JG. Role of CXCR5 and CCR7 in follicular Th cell positioning and appearance of a programmed cell death gene‐1high germinal center‐associated subpopulation. J Immunol. 2007; 179:5099–108. [DOI] [PubMed] [Google Scholar]
- 41. Crotty S. T Follicular Helper Cell Biology: A Decade of Discovery and Diseases. Immunity 2019; 50:1132–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Shulman Z, Gitlin AD, Targ S, Jankovic M, Pasqual G, Nussenzweig MC, et al. T follicular helper cell dynamics in germinal centers. Science 2013; 341:673–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Papa I, Vinuesa CG. Synaptic Interactions in Germinal Centers. Front Immunol. 2018; 9:1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Poholek AC, Hansen K, Hernandez SG, Eto D, Chandele A, Weinstein JS, et al. In vivo regulation of Bcl6 and T follicular helper cell development. J Immunol. 2010; 185:313–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lee JY, Skon CN, Lee YJ, Oh S, Taylor JJ, Malhotra D, et al. The transcription factor KLF2 restrains CD4(+) T follicular helper cell differentiation. Immunity 2015; 42:252–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Li J, Lu E, Yi T, Cyster JG. EBI2 augments Tfh cell fate by promoting interaction with IL‐2‐quenching dendritic cells. Nature 2016; 533:110–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lu E, Cyster JG. G‐protein coupled receptors and ligands that organize humoral immune responses. Immunol Rev. 2019; 289:158–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Keszei M, Detre C, Castro W, Magelky E, O'Keeffe M, Kis‐Toth K, et al. Expansion of an osteopontin‐expressing T follicular helper cell subset correlates with autoimmunity in B6.Sle1b mice and is suppressed by the H1‐isoform of the Slamf6 receptor. FASEB J. 2013; 27:3123–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wang N, Halibozek PJ, Yigit B, Zhao H, O'Keeffe MS, Sage P, et al. Negative Regulation of Humoral Immunity Due to Interplay between the SLAMF1, SLAMF5, and SLAMF6 Receptors. Front Immunol. 2015; 6:158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hu J, Havenar‐Daughton C, Crotty S. Modulation of SAP dependent T: B cell interactions as a strategy to improve vaccination. Curr Opin Virol. 2013; 3:363–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. He J, Tsai LM, Leong YA, Hu X, Ma CS, Chevalier N, et al. Circulating precursor CCR7(lo)PD‐1(hi) CXCR5(+) CD4(+) T cells indicate Tfh cell activity and promote antibody responses upon antigen reexposure. Immunity 2013; 39:770–81. [DOI] [PubMed] [Google Scholar]
- 52. Morita R, Schmitt N, Bentebibel SE, Ranganathan R, Bourdery L, Zurawski G, et al. Human blood CXCR5(+)CD4(+) T cells are counterparts of T follicular cells and contain specific subsets that differentially support antibody secretion. Immunity 2011; 34:108–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ricard L, Jachiet V, Malard F, Ye Y, Stocker N, Riviere S, et al. Circulating follicular helper T cells are increased in systemic sclerosis and promote plasmablast differentiation through the IL‐21 pathway which can be inhibited by ruxolitinib. Ann Rheum Dis. 2019; 78:539–50. [DOI] [PubMed] [Google Scholar]
- 54. Chevalier N, Jarrossay D, Ho E, Avery DT, Ma CS, Yu D, et al. CXCR5 expressing human central memory CD4 T cells and their relevance for humoral immune responses. J Immunol. 2011; 186:5556–68. [DOI] [PubMed] [Google Scholar]
- 55. Ise W, Inoue T, McLachlan JB, Kometani K, Kubo M, Okada T, et al. Memory B cells contribute to rapid Bcl6 expression by memory follicular helper T cells. Proc Natl Acad Sci USA. 2014; 111:11792–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Batten M, Ramamoorthi N, Kljavin NM, Ma CS, Cox JH, Dengler HS, et al. IL‐27 supports germinal center function by enhancing IL‐21 production and the function of T follicular helper cells. J Exp Med. 2010; 207:2895–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Coquet JM, Schuijs MJ, Smyth MJ, Deswarte K, Beyaert R, Braun H, et al. Interleukin‐21‐Producing CD4(+) T Cells Promote Type 2 Immunity to House Dust Mites. Immunity 2015; 43:318–30. [DOI] [PubMed] [Google Scholar]
- 58. Harker JA, Dolgoter A, Zuniga EI. Cell‐intrinsic IL‐27 and gp130 cytokine receptor signaling regulates virus‐specific CD4(+) T cell responses and viral control during chronic infection. Immunity 2013; 39:548–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. DiToro D, Winstead CJ, Pham D, Witte S, Andargachew R, Singer JR, et al. Differential IL‐2 expression defines developmental fates of follicular versus nonfollicular helper T cells. Science 2018; 361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Elsner RA, Shlomchik MJ. IL‐12 Blocks Tfh Cell Differentiation during Salmonella Infection, thereby Contributing to Germinal Center Suppression. Cell Rep. 29(9):2796–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Powell MD, Read KA, Sreekumar BK, Jones DM, Oestreich KJ. IL‐12 signaling drives the differentiation and function of a TH1‐derived TFH1‐like cell population. Sci Rep. 2019; 9:13991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Lee SK, Silva DG, Martin JL, Pratama A, Hu X, Chang PP, et al. Interferon‐gamma excess leads to pathogenic accumulation of follicular helper T cells and germinal centers. Immunity 2012; 37:880–92. [DOI] [PubMed] [Google Scholar]
- 63. Fang D, Cui K, Mao K, Hu G, Li R, Zheng M, et al. Transient T‐bet expression functionally specifies a distinct T follicular helper subset. J Exp Med. 2018; 215:2705–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Jogdand GM, Mohanty S, Devadas S. Regulators of Tfh Cell Differentiation. Front Immunol. 2016; 7:520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Liu X, Nurieva RI, Dong C. Transcriptional regulation of follicular T‐helper (Tfh) cells. Immunol Rev. 2013; 252:139–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Ray JP, Marshall HD, Laidlaw BJ, Staron MM, Kaech SM, Craft J. Transcription factor STAT3 and type I interferons are corepressive insulators for differentiation of follicular helper and T helper 1 cells. Immunity 2014; 40:367–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Qiu H, Wu H, Chan V, Lau CS, Lu Q. Transcriptional and epigenetic regulation of follicular T‐helper cells and their role in autoimmunity. Autoimmunity 2017; 50:71–81. [DOI] [PubMed] [Google Scholar]
- 68. Schmitt N, Liu Y, Bentebibel SE, Munagala I, Bourdery L, Venuprasad K, et al. The cytokine TGF‐beta co‐opts signaling via STAT3‐STAT4 to promote the differentiation of human TFH cells. Nat Immunol. 2014; 15:856–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Sahoo A, Wali S, Nurieva R. T helper 2 and T follicular helper cells: Regulation and function of interleukin‐4. Cytokine Growth Factor Rev. 2016; 30:29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Johnston RJ, Choi YS, Diamond JA, Yang JA, Crotty S. STAT5 is a potent negative regulator of TFH cell differentiation. J Exp Med. 2012; 209:243–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Qi H, Liu D, Ma W, Wang Y, Yan H. Bcl‐6 controlled TFH polarization and memory: the known unknowns. Curr Opin Immunol. 2014; 28:34–41. [DOI] [PubMed] [Google Scholar]
- 72. Andris F, Denanglaire S, Anciaux M, Hercor M, Hussein H, Leo O. The Transcription Factor c‐Maf Promotes the Differentiation of Follicular Helper T Cells. Front Immunol. 2017; 8:480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Betz BC, Jordan‐Williams KL, Wang C, Kang SG, Liao J, Logan MR, et al. Batf coordinates multiple aspects of B and T cell function required for normal antibody responses. J Exp Med. 2010; 207:933–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Sahoo A, Alekseev A, Tanaka K, Obertas L, Lerman B, Haymaker C, et al. Batf is important for IL‐4 expression in T follicular helper cells. Nat Commun. 2015; 6:7997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Vacchio MS, Ciucci T, Gao Y, Watanabe M, Balmaceno‐Criss M, McGinty MT, et al. A Thpok‐Directed Transcriptional Circuitry Promotes Bcl6 and Maf Expression to Orchestrate T Follicular Helper Differentiation. Immunity 2019; 51(3):465–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Xu W, Zhao X, Wang X, Feng H, Gou M, Jin W, et al. The Transcription Factor Tox2 Drives T Follicular Helper Cell Development via Regulating Chromatin Accessibility. Immunity 2019; 51(5):826–39. [DOI] [PubMed] [Google Scholar]
- 77. Miyazaki M, Miyazaki K, Chen S, Chandra V, Wagatsuma K, Agata Y, et al. The E‐Id protein axis modulates the activities of the PI3K‐AKT‐mTORC1‐Hif1a and c‐myc/p19Arf pathways to suppress innate variant TFH cell development, thymocyte expansion, and lymphomagenesis. Genes Dev. 2015; 29:409–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Xiao N, Eto D, Elly C, Peng G, Crotty S, Liu YC. The E3 ubiquitin ligase Itch is required for the differentiation of follicular helper T cells. Nat Immunol. 2014; 15:657–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Stone EL, Pepper M, Katayama CD, Kerdiles YM, Lai CY, Emslie E, et al. ICOS coreceptor signaling inactivates the transcription factor FOXO1 to promote Tfh cell differentiation. Immunity 2015; 42:239–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Kim CJ, Lee CG, Jung JY, Ghosh A, Hasan SN, Hwang SM, et al. The Transcription Factor Ets1 Suppresses T Follicular Helper Type 2 Cell Differentiation to Halt the Onset of Systemic Lupus Erythematosus. Immunity 2018; 49(6):1034–48. [DOI] [PubMed] [Google Scholar]
- 81. Yang J, Lin X, Pan Y, Wang J, Chen P, Huang H, et al. Critical roles of mTOR Complex 1 and 2 for T follicular helper cell differentiation and germinal center responses. Elife. 2016; 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Zeng H, Cohen S, Guy C, Shrestha S, Neale G, Brown SA, et al. mTORC1 and mTORC2 Kinase Signaling and Glucose Metabolism Drive Follicular Helper T Cell Differentiation. Immunity 2016; 45:540–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Yi W, Gupta S, Ricker E, Manni M, Jessberger R, Chinenov Y, et al. The mTORC1‐4E‐BP‐eIF4E axis controls de novo Bcl6 protein synthesis in T cells and systemic autoimmunity. Nat Commun. 2017; 8:254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Ray JP, Staron MM, Shyer JA, Ho PC, Marshall HD, Gray SM, et al. The Interleukin‐2‐mTORc1 Kinase Axis Defines the Signaling, Differentiation, and Metabolism of T Helper 1 and Follicular B Helper T Cells. Immunity 2015; 43:690–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Shrestha S, Yang K, Guy C, Vogel P, Neale G, Chi H. Treg cells require the phosphatase PTEN to restrain TH1 and TFH cell responses. Nat Immunol. 2015; 16:178–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Abboud G, Choi SC, Kanda N, Zeumer‐Spataro L, Roopenian DC, Morel L. Inhibition of Glycolysis Reduces Disease Severity in an Autoimmune Model of Rheumatoid Arthritis. Front Immunol. 2018; 9:1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Essig K, Hu D, Guimaraes JC, Alterauge D, Edelmann S, Raj T, et al. Roquin Suppresses the PI3K‐mTOR Signaling Pathway to Inhibit T Helper Cell Differentiation and Conversion of Treg to Tfr Cells. Immunity 2017; 47(6):1067–82. [DOI] [PubMed] [Google Scholar]
- 88. Xu L, Huang Q, Wang H, Hao Y, Bai Q, Hu J, et al. The Kinase mTORC1 Promotes the Generation and Suppressive Function of Follicular Regulatory T Cells. Immunity 2017; 47(3):538–51. [DOI] [PubMed] [Google Scholar]
- 89. Preite S, Cannons JL, Radtke AJ, Vujkovic‐Cvijin I, Gomez‐Rodriguez J, Volpi S, et al. Hyperactivated PI3Kdelta promotes self and commensal reactivity at the expense of optimal humoral immunity. Nat Immunol. 2018; 19:986–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Tsui C, Martinez‐Martin N, Gaya M, Maldonado P, Llorian M, Legrave NM, et al. Protein Kinase C‐beta Dictates B Cell Fate by Regulating Mitochondrial Remodeling, Metabolic Reprogramming, and Heme Biosynthesis. Immunity 2018; 48: 1144–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Xie MM, Amet T, Liu H, Yu Q, Dent AL. AMP kinase promotes Bcl6 expression in both mouse and human T cells. Mol Immunol. 2017; 81:67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Hsu WC, Chen MY, Hsu SC, Huang LR, Kao CY, Cheng WH, et al. DUSP6 mediates T cell receptor‐engaged glycolysis and restrains TFH cell differentiation. Proc Natl Acad Sci USA. 2018; 115:E8027–E8036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Liu RT, Zhang M, Yang CL, Zhang P, Zhang N, Du T, et al. Enhanced glycolysis contributes to the pathogenesis of experimental autoimmune neuritis. J Neuroinflammation. 2018; 15:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Dong L, He Y, Zhou S, Cao Y, Li Y, Bi Y, et al. HIF1alpha‐Dependent Metabolic Signals Control the Differentiation of Follicular Helper T Cells. Cells 2019; 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Fahey LM, Wilson EB, Elsaesser H, Fistonich CD, McGavern DB, Brooks DG. Viral persistence redirects CD4 T cell differentiation toward T follicular helper cells. J Exp Med. 2011; 208:987–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Harker JA, Lewis GM, Mack L, Zuniga EI. Late interleukin‐6 escalates T follicular helper cell responses and controls a chronic viral infection. Science 2011; 334:825–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Greczmiel U, Krautler NJ, Pedrioli A, Bartsch I, Agnellini P, Bedenikovic G, et al. Sustained T follicular helper cell response is essential for control of chronic viral infection. Sci Immunol. 2017; 2(18):eaam8686. [DOI] [PubMed] [Google Scholar]
- 98. Vella LA, Herati RS, Wherry EJ. CD4(+) T Cell Differentiation in Chronic Viral Infections: The Tfh Perspective. Trends Mol Med. 2017; 23:1072–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Raziorrouh B, Sacher K, Tawar RG, Emmerich F, Neumann‐Haefelin C, Baumert TF, et al. Virus‐Specific CD4+ T Cells Have Functional and Phenotypic Characteristics of Follicular T‐Helper Cells in Patients With Acute and Chronic HCV Infections. Gastroenterology 2016; 150(3):696–706. [DOI] [PubMed] [Google Scholar]
- 100. Cubas RA, Mudd JC, Savoye AL, Perreau M, van Grevenynghe J, Metcalf T, et al. Inadequate T follicular cell help impairs B cell immunity during HIV infection. Nat Med. 2013; 19:494–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Perreau M, Savoye AL, De Crignis E, Corpataux JM, Cubas R, Haddad EK, et al. Follicular helper T cells serve as the major CD4 T cell compartment for HIV‐1 infection, replication, and production. J Exp Med. 2013; 210:143–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Kohler SL, Pham MN, Folkvord JM, Arends T, Miller SM, Miles B, et al. Germinal Center T Follicular Helper Cells Are Highly Permissive to HIV‐1 and Alter Their Phenotype during Virus Replication. J Immunol. 2016; 196:2711–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Pallikkuth S, Sharkey M, Babic DZ, Gupta S, Stone GW, Fischl MA, et al. Peripheral T Follicular Helper Cells Are the Major HIV Reservoir within Central Memory CD4 T Cells in Peripheral Blood from Chronically HIV‐Infected Individuals on Combination Antiretroviral Therapy. J Virol. 2015; 90:2718–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Roberts AL, Connolly KL, Kirse DJ, Evans AK, Poehling KA, Peters TR, et al. Detection of group A Streptococcus in tonsils from pediatric patients reveals high rate of asymptomatic streptococcal carriage. BMC Pediatr. 2012; 12:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Linterman MA, Rigby RJ, Wong RK, Yu D, Brink R, Cannons JL, et al. Follicular helper T cells are required for systemic autoimmunity. J Exp Med. 2009; 206:561–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Simpson N, Gatenby PA, Wilson A, Malik S, Fulcher DA, Tangye SG, et al. Expansion of circulating T cells resembling follicular helper T cells is a fixed phenotype that identifies a subset of severe systemic lupus erythematosus. Arthritis Rheum. 2010; 62:234–44. [DOI] [PubMed] [Google Scholar]
- 107. Choi JY, Ho JH, Pasoto SG, Bunin V, Kim ST, Carrasco S, et al. Circulating follicular helper‐like T cells in systemic lupus erythematosus: association with disease activity. Arthritis Rheumatol. 2015; 67:988–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Szabo K, Papp G, Barath S, Gyimesi E, Szanto A, Zeher M. Follicular helper T cells may play an important role in the severity of primary Sjogren's syndrome. Clin Immunol. 2013; 147:95–104. [DOI] [PubMed] [Google Scholar]
- 109. Li XY, Wu ZB, Ding J, Zheng ZH, Li XY, Chen LN, et al. Role of the frequency of blood CD4(+) CXCR5(+) CCR6(+) T cells in autoimmunity in patients with Sjogren's syndrome. Biochem Biophys Res Commun. 2012; 422:238–44. [DOI] [PubMed] [Google Scholar]
- 110. Zhu C, Ma J, Liu Y, Tong J, Tian J, Chen J, et al. Increased frequency of follicular helper T cells in patients with autoimmune thyroid disease. J Clin Endocrinol Metab. 2012; 97:943–50. [DOI] [PubMed] [Google Scholar]
- 111. Aust G, Sittig D, Becherer L, Anderegg U, Schutz A, Lamesch P, et al. The role of CXCR5 and its ligand CXCL13 in the compartmentalization of lymphocytes in thyroids affected by autoimmune thyroid diseases. Eur J Endocrinol. 2004;250:225–34. [DOI] [PubMed] [Google Scholar]
- 112. Liu R, Wu Q, Su D, Che N, Chen H, Geng L, et al. A regulatory effect of IL‐21 on T follicular helper‐like cell and B cell in rheumatoid arthritis. Arthritis Res Ther. 2012; 14:R255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Moschovakis GL, Bubke A, Friedrichsen M, Falk CS, Feederle R, Forster R. T cell specific Cxcr5 deficiency prevents rheumatoid arthritis. Sci Rep. 2017; 7:8933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Xu X, Shi Y, Cai Y, Zhang Q, Yang F, Chen H, et al. Inhibition of increased circulating Tfh cell by anti‐CD20 monoclonal antibody in patients with type 1 diabetes. PLoS One 2013; 8:e79858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Zhou J, Wang Y, He Y, Gao Y, Wan R, Cai M, et al. Non‐obese type 2 diabetes patients present intestinal B cell dysregulations associated with hyperactive intestinal Tfh cells. Mol Immunol. 2018; 97:27–32. [DOI] [PubMed] [Google Scholar]
- 116. Gaddis DE, Padgett LE, Wu R, McSkimming C, Romines V, Taylor AM, et al. Apolipoprotein AI prevents regulatory to follicular helper T cell switching during atherosclerosis. Nat Commun. 2018; 9:1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Ryu H, Lim H, Choi G, Park YJ, Cho M, Na H, et al. Atherogenic dyslipidemia promotes autoimmune follicular helper T cell responses via IL‐27. Nat Immunol. 2018; 19:583–93. [DOI] [PubMed] [Google Scholar]
- 118. Taghavie‐Moghadam PL, Waseem TC, Hattler J, Glenn LM, Dobrian AD, Kaplan MH, et al. STAT4 Regulates the CD8(+) Regulatory T Cell/T Follicular Helper Cell Axis and Promotes Atherogenesis in Insulin‐Resistant Ldlr(‐/‐) Mice. J Immunol. 2017;199:3453–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Li W, Elshikha AS, Cornaby C, Teng X, Abboud G, Brown J, et al. T cells expressing the lupus susceptibility allele Pbx1d enhance autoimmunity and atherosclerosis in dyslipidemic mice. JCI. Insight 2020;5:e13827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Gong F, Qian C, Zhu H, Zhu J, Pan Y, Dong Q, et al. Circulating follicular T‐helper cell subset distribution in patients with asthma. Allergy Asthma Proc. 2016;37:154–61. [DOI] [PubMed] [Google Scholar]
- 121. Kamekura R, Shigehara K, Miyajima S, Jitsukawa S, Kawata K, Yamashita K, et al. Alteration of circulating type 2 follicular helper T cells and regulatory B cells underlies the comorbid association of allergic rhinitis with bronchial asthma. Clin Immunol. 2015; 158:204–11. [DOI] [PubMed] [Google Scholar]
- 122. Yao Y, Wang N, Chen CL, Pan L, Wang ZC, Yunis J, et al. CD23 expression on switched memory B cells bridges T‐B cell interaction in allergic rhinitis. Allergy 2020; 75(10):2599–612. [DOI] [PubMed] [Google Scholar]
- 123. Gu‐Trantien C, Loi S, Garaud S, Equeter C, Libin M, de Wind A, et al. CD4(+) follicular helper T cell infiltration predicts breast cancer survival. J Clin Invest. 2013; 123:2873–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Gu‐Trantien C, Migliori E, Buisseret L, de Wind A, Brohee S, Garaud S, et al. CXCL13‐producing TFH cells link immune suppression and adaptive memory in human breast cancer. JCI Insight. 2017; 2:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Bindea G, Mlecnik B, Tosolini M, Kirilovsky A, Waldner M, Obenauf AC, et al. Spatiotemporal dynamics of intratumoral immune cells reveal the immune landscape in human cancer. Immunity 2013; 39:782–95. [DOI] [PubMed] [Google Scholar]
- 126. Shirota H, Klinman DM, Ito SE, Ito H, Kubo M, Ishioka C. IL4 from T Follicular Helper Cells Downregulates Antitumor Immunity. Cancer Immunol Res. 2017; 5:61–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Lewis KE, Selby MJ, Masters G, Valle J, Dito G, Curtis WR, et al. Interleukin‐21 combined with PD‐1 or CTLA‐4 blockade enhances antitumor immunity in mouse tumor models. Oncoimmunology. 2017; 7:e1377873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Dan JM, Havenar‐Daughton C, Kendric K, Al‐kolla R, Kaushik K, Rosales SL, et al. Recurrent group A Streptococcus tonsillitis is an immunosusceptibility disease involving antibody deficiency and aberrant TFH cells. Science Translational Medicine. 2019; 11(478):eaau3776. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.


