Summary
The role of antigen‐presenting cells in the skin immune system, in particular Langerhans cells and dendritic cells, has not been well defined. We recently published a study in ‘Immunology’ where we reported that the loss of langerin‐positive cells in the skin accelerated wound repair in the Lang‐DTR mouse. The study published here by Li, et al. reports delayed wound closure following depletion of CD11c‐positive cells in the CD11c‐DTR mouse. In this commentary, we attribute the differences between these results to several factors that differ between the studies including the depletion of different cell populations; differences in the age and the sex of mice; differences in antibiotic use between the studies; and differences in the location of the biopsies that were taken. Here, we describe the impact of these differences on wound healing and conclude that further standardization of the wound model, and further characterization of the specific cells that are depleted in these mice, is necessary to better understand how antigen‐presenting cells contribute to wound healing.
Keywords: antigen‐presenting cells, wound healing, depletion models, mouse
The aim of this commentary is to highlight both the importance of antigen presenting cells in wound healing, and the impact of a range of factors on results when using different antigen presenting cell depletion models.
Abbreviations
- DCs
Dendritic cells
- DT
Diphtheria toxin
- LCs
Langerhans cells
- MHCII
Major histocompatibility complex class II
- MSB
Martius Scarlet Blue
- PBS
Phosphate‐buffered saline
Introduction
Given the tremendous strain that chronic wounds place on healthcare systems, considerable efforts are underway to investigate the regulation of wound healing. In particular, the immune system has been found to play a central role in healing due to its impact on all the phases of wound repair. 1 The innate immune system is capable of rapid activation in response to a breach to the integument and is crucial for the recruitment of cells of the adaptive immune response. However, the role of antigen‐presenting cells in the skin immune cell system, in particular Langerhans cells (LCs) and dendritic cells (DCs), has not been well defined.
In our recently published study in Immunology, we reported that loss of langerin(+) cells in the skin accelerated wound repair in Lang‐DTR mice. 2 The more rapid healing was characterized by increased re‐epithelialization and granulation tissue formation. The study published here by Li et al. investigated the role of CD11c+ cells in wound healing using the CD11c‐depletable CD11c‐DTR mouse. In contrast to our observations in the Lang‐DTR mouse, the authors showed a significant delay in wound closure in the absence of CD11c(+) cells using that model. The aim of this commentary is to highlight the importance of antigen‐presenting cells in wound healing and the differences in experimental approaches between the two studies, and the impact of those differences on the results of those studies.
Methods
Mice and wounding
Female transgenic Lang‐DTR 3 mice were used for this skin wound model between 8 and 14 weeks of age. Mice were kept in a specific pathogen‐free environment at the Hercus Taieri Research Unit of the University of Otago under Animal Ethical Approval 20/13.
The transgenic mice were separated into control and experimental groups and given intraperitoneal injections of phosphate‐buffered saline (PBS) or diphtheria toxin (DT; 100 μl of 1 μg diluted in PBS), 4 respectively. These injections were administered at 4 and 1 day prior to wounding to deplete mice of langerin(+) cells in the experimental groups.
Full‐thickness wounds (4 mm thick) were created on each shaved hind limb of anaesthetized mice (ketamine 75 mg/kg; Domitor 1 mg/kg) using a sterile biopsy punch (Shoof International®). Prior to wounding, pain relief was administered to the site by subcutaneous injections of bupivacaine (2 mg/kg). A subcutaneous injection of Amphoprim (10·2 mg/kg) was administered in the scruff of the neck to prevent infection. Once the procedure was completed, Antisedan (5 mg/kg) was given by subcutaneous injection to the inguinal region to reverse the effects of the anaesthesia.
Photographs of each wound were taken using a single‐lens reflex camera (Nikon®), and a ruler for scale, immediately post‐wounding and at day 1. Wound tissue was harvested after euthanasia of the mice by CO2 asphyxiation at day 1 – post‐wounding. Wound biopsies were excised (1 cm × 1·5 cm) and fixed in 0·5% zinc salt fixative and transferred into 70% ethanol for 4 h. The tissues were dehydrated with xylene and graded ethanol water baths to displace the water. The tissues were then embedded in paraffin. Serial 4‐μm sections were cut, and one section from the centre of each wound was used for Martius Scarlet Blue (MSB) staining, following a standard protocol. 5 All embedding, section cutting and MSB staining were performed at the Histology Services Unit, Department of Pathology, Dunedin School of Medicine.
Immunofluorescence and histochemical analyses
Antigen‐presenting cells were stained using a FITC‐conjugated anti‐MHCII monoclonal antibody (Cat. No. ab93560; clone M5/114·15·2; 1:400 dilution; Abcam) and supernatant from cells expressing anti‐langerin (Cell line 99·1; clone 929 F3; undiluted) monoclonal antibody in conjunction with an Alexa Fluor® 594 goat anti‐rat IgG2a.
Slides were incubated at 37°C in oven overnight before staining to improve adhesion of the tissues onto the slide. Sections were de‐paraffinized and taken to water, and antigen retrieval was achieved by incubating the sections in 37°C Tris‐buffered saline, pH 7·4 (TBS) for 20 min for the MHCII staining and then at RT for a further 20 min, followed by three 5‐min washes in TBS containing 10% Tween‐20 (TBST). Non‐specific antigen‐binding sites were blocked by incubation with 10% normal sheep serum in TBS containing Fc block (rat anti‐mouse CD16/CD32 (1:700; BD Biosciences) and Rat IgG (1:500, Jackson ImmunoResearch) for 1 h RT. The sections were then incubated with primary antibodies diluted in antibody diluent (1% BSA, 10% Triton‐X‐100 in TBS) overnight at 4°C, followed by three 5‐min washes in TBST. Secondary antibody was added, if necessary, to the tissues for an hour at RT. At around 30 min into the incubation, the nuclear stain DAPI (1:50) was added to counterstain the nuclei. Sections were washed three times for 5 min in TBST, mounted with SlowFade™ Diamond anti‐fade reagent (Invitrogen) and stored overnight at 4°C. Images of the entire tissue section were taken using a fluorescent microscope (Olympus BX51 TRF®) under the 40× objective lens. Photographs were merged and converted into panoramas using Fiji (https://imagej.net/Fiji).
Morphometric analyses
The area of the wound was measured using Fiji by setting the scale, then using the free‐hand polygon tool to trace the neo‐epidermal area, length and width along with granulation tissue area and selecting the ‘measure’ key from the ‘analyse’ menu. All analyses were conducted on sections from the middle of the wound.
The numbers of Langerin(+) and MHCII(+) cells within the epidermis (avoiding glands and hair follicles), dermis and hypodermis were quantified in the immunofluorescent histochemistry sections at day 1 wounding. The skin within each section was divided into different zones: wound bed (between the cut edges of the PC), adjacent region (adjacent to the wound) and periphery (peripheral to the wound edge) with the latter two zones measuring 700 μm in width.
Statistical analyses
All data are presented as the mean ± standard error of the mean, and statistical analyses were conducted in GraphPad Prism 8 and performed using one‐ or two‐way ANOVA with multiple comparisons determined using the Bonferroni or Sidak's multiple comparisons tests.
Results and Discussion
There are some similarities and a number of notable differences in both the models and the approaches taken by Li, et al., and us, are summarized in Table 1, and which we will discuss here.
Table 1.
Summary of the differences between the Rajesh, et al. and Li, et al. studies
| Rajesh et al. | Li et al. | |
|---|---|---|
| Mouse |
C57BL/6 background Females aged 8–12 weeks |
C57BL/6 background Females aged 6–9 weeks and males aged 8–18 weeks |
| Model used | Lang‐DTR | CD11c‐DTR |
| Number and timing of DT administration | 2 (−4 and −1 days before wounding) | 2 (−3 and −1 days before wounding) |
| Depleted cells | Langerhans cells and langerin(+) dermal dendritic cells | Dendritic cells |
|
Wound Location of the biopsy |
4‐µm full‐thickness excision Lateral–caudal dorsum |
4‐µm full‐thickness excision Medial–caudal dorsum |
| Antibiotic used | Amphoprim | None |
|
Wound closure analysis method Primary technique used to detect immune cells |
Two‐dimensional imaging Immunofluorescence staining |
Three‐dimensional imaging Flow cytometry |
The closure of the wounds in the untreated mice appears somewhat similar for both studies, as might be expected. The common time‐point reporting closure for both studies is day 5. Li, et al. carried out three‐dimensional imaging of the wound in the untreated (no diphtheria toxin; DT) CD11c‐DTR mice to provide a measure of ‘wound openness,’ with a mean of approximately 36% at day 5. In our report, we used two‐dimensional imaging of the wound and reported a mean ‘% original wound size’ of 32% in the untreated Lang‐DTR mice at day 5. These data indicate that healing in these transgenic mice is similar in the absence of DT depletion; however, a direct comparison should be carried out experimentally to robustly confirm this.
Administration of DT in the Lang‐DTR and CD11c‐DTR models results in the depletion of different and only partially overlapping populations of cells, likely contributing to the varied responses observed in wound repair in these models. In our study, administration of DT at −4 and −1 days prior to wounding in the Lang‐DTR model resulted in the total depletion of epidermal LCs and a reduction in dermal langerin(+) cells at day 1 post‐wounding. 2
Unlike the Lang‐DTR mice, repeated DT injections in the CD11c‐DTR model result in severe toxicity and death of the mice, limiting the number of injections that can be administered. This leads to only partial depletion of CD11c(+) cells 6 and restricts the time interval when the impact of depletion can be measured. 7 In the report by Li, et al., two injections were administered, which resulted in partial depletion of CD11c(+) cells.
Other studies using the CD11c‐DTR mouse model have reported loss of several other cell populations in addition to CD11c(+) cells following the administration of DT, 8 , 9 , 10 , 11 including plasmacytoid DCs, activated T cells, natural killer cells and certain populations of macrophages in the skin. Broader depletion of cells in the CD11c‐DTR model, for example the loss of macrophages prior to wound healing, has been known to impair healing processes 12 and thus might be contributing to the observed delay in healing.
CD11c‐DTR mice have been reported to display neutrophilia in the blood following injection of 8 ng/g body weight of DT (noting that Li, et al. administered two doses of 100 ng DT 48 h prior to wounding and on the day of wounding). 11 Following DT administration, polymorphonuclear cells were increased by twofold at 24 h, fivefold at 72 h and twofold at 7 days. 11 In contrast, the expansion of neutrophils has not been reported in the Lang‐DTR mouse following DT administration. Depletion of neutrophils in vivo by injecting anti‐mouse neutrophil serum results in the acceleration of wound closure, 13 which suggests by implication that an increase in neutrophils may delay healing. This should be tested directly, and any functional implications of the neutrophilia in the CD11c‐DTR model should be taken into consideration when interpreting results.
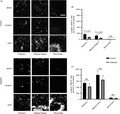
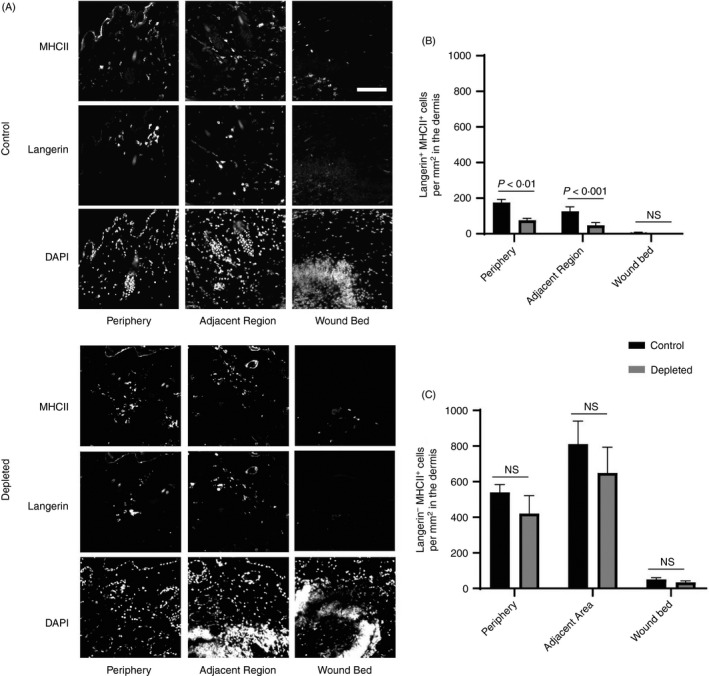
In order to look more broadly at the impact of DT injection on the numbers of langerin(−) mononuclear phagocytes in the Lang‐DTR mouse, we enumerated dermally located major histocompatibility complex class II (MHCII)‐positive cells that were negative for langerin in 8‐ to 14‐week‐old mice following DT administration (Figure 1A). MHCII‐positive cells in mouse dermis are reported to consist predominantly of dermal macrophages and dermal dendritic cells, 14 including migrating LCs and langerin(+) dermal DCs. Langerin(−) and MHCII(+) cells were enumerated in the dermis of the wound bed, adjacent area and periphery at day 1 post‐wounding (Figure1B), using the same protocol that we reported previously. 2 There was a slight, but not statistically significant, drop in the number of MHCII(+), langerin(−) cells in both the periphery and adjacent to the wound bed, indicating that there no significant depletion of these cells occurred (Figure 1B). The number of MHCII(+), langerin(−) cells were very few in the wound bed in either the DT‐treated or control groups, and there was no significant difference between the two groups. In contrast, the MHCII(+), langerin(+) cells were lost from the epidermis in the depleted group (data not shown), were significantly reduced in the periphery (ANOVA, P < 0·01) and the adjacent region (ANOVA, P < 0·001) of the wound 1 day following DT administration (Figure 1C) and were absent in the wound bed. These trends echoed our previous report, with some inter‐experimental variations in the absolute numbers that were observed. We do note that there was a difference in the ages of the mice between the two studies (8–12 weeks in our previously published study, 10–14 weeks in this study) that may have contributed to this variability. These findings indicate that the accelerated healing observed in this model 2 occurs as a consequence of depletion of langerin(+) cells, which includes LCs and a proportion of the langerin+ dermal DCs. Importantly, we show here that langerin(−), MHCII(+) cells are not significantly altered following the administration of DT in this model and therefore do not contribute to the accelerated healing that we observe in this model.
Figure 1.
Effect of DT treatment on the langerin(−) MHCII(+) and langerin(+) MHCII(+) cells in the dermis of Lang‐DTR mice. (A) Representative images of mouse tissues treated with PBS (control) or diphtheria toxin (depleted) at day 1‐ post‐wounding stained with antibodies against MHCII, langerin and nuclear stain DAPI (blue). Scale bar represents 50 µm. The number of (B) langerin(+) MHCII(+) double‐positive cells and (C) langerin(−) MHCII(+) cells per mm2 of the wound in the periphery, adjacent region and wound bed were quantified at day 1 – post‐wounding in both the control and depleted mice. Data presented as mean ± SEM from n = 8 on mice aged 10–14 weeks. Statistical differences between the groups were determined by two‐way ANOVA followed by Bonferroni multiple comparisons test
While differences in the cell populations depleted in the Lang‐DTR and CD11c‐DTR models provide an explanation for the incongruent healing responses observed, there were methodological differences between our paper and the work of Li, et al. that could contribute to the differences in the findings between these studies (Table 1). We might expect these factors to also be reflected in differences between the untreated mice between the two studies; however, apart from the day 5 data mentioned earlier, there are no other data presented that are common to both studies that can be directly compared to help establish if this is the case. Further experimentation is required to establish which parameters might impact only following cell depletion in these models.
In our previous study, we used only female mice that were aged between 8 and 12 weeks, 2 while Li et al. used female mice aged 6–9 weeks but also some males that ranged from 8 to 16 weeks. 15 Male skin is 40% stronger due to their thicker dermis, while female skin exhibits a thicker epidermis, hair shaft and hypodermis. These differences in the structure of the skin between male and female mice, along with the influence of sex hormones, 16 may contribute to different rates of healing observed in the depleted mice in these studies.
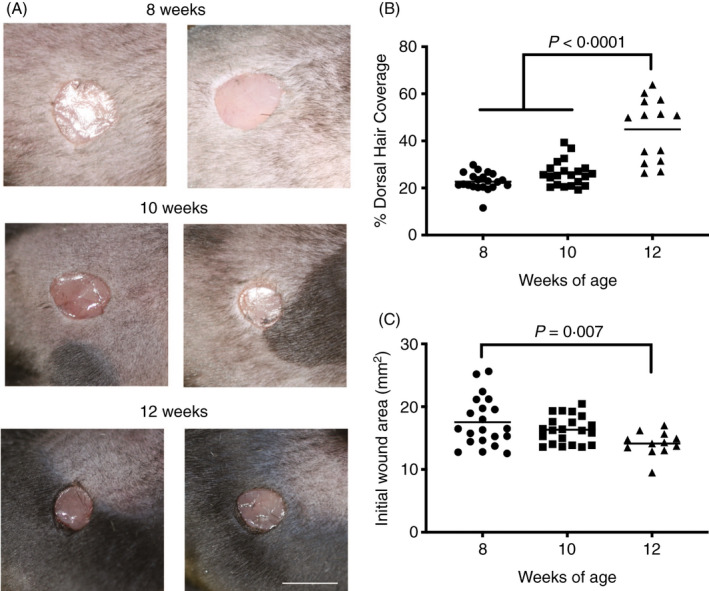
Hair cycling changes as mice age, which impacts on wound repair. While in the hair follicle growing phase (anagen phase), mouse skin exhibits accelerated wound re‐epithelialization relative to skin in other stages. 17 It has been shown increased numbers of hair follicles facilitate the migration of epithelial stem cells to aid faster re‐epithelialization. 18 We have explored the impact of anagen in the Lang‐DTR mouse model. We found that the percentage of mice exhibiting anagen phase (demonstrated by hair growth) increased as the age increased (Figure 2A,D). The mean dorsal hair coverage was nearly doubled (Figure 2A,B), which likely contributed to the creation of a smaller wound in the 12‐week‐old mice, when compared to their younger counterparts (Figure 2A,C). A limitation of both studies is the use of a broad range of ages of mice, hence potential variability in mice in the anagen phase. Moving forward, it will be important to use tighter age ranges and to compare mice at different ages, to differentiate between the wound healing effects of cell depletion and hair cycling.
Figure 2.
Relationship between the age of the mice and dorsal hair coverage and initial wound sizes. (A) Representing macroscopic images dorsal hair coverage and initial size upon wounding in Lang‐DTR mice at ages 8, 10 and 12 weeks. Scale bar represents 4 mm. (B) The percentage of dorsal hair coverage (dark hair growth), and (C) initial area of the wound, with each data point representing the mean of two wounds per animal, was determined in mice aged 8 (n = 21), 10 (n = 21) and 12 (n = 14) weeks of age. Mean ± SEM is shown. Statistical analysis was carried out using a one‐way ANOVA with Sidak’s multiple comparisons test
The location of the punch biopsies on the dorsum of the mice varied between our study and that of Li, et al. (Table 1). The rate of healing processes such as wound contraction, re‐epithelialization, total tissue repair and thus wound closure varies depending on the location. 19 Wound contraction was reported to be higher in medial–caudal wounds when compared to lateral–caudal wounds in a porcine model. 8 Although both depletion studies described here standardized the wound site for all mice, the impact of cell depletion on rate of healing may differ between anatomical locations, thus making comparisons between studies difficult.
Another major difference between the studies described here related to the use of antibiotics (Table 1). In the Lang‐DTR model, both depleted and non‐depleted groups of mice were comparably treated with an antibiotic at the time of wounding. 2 Li et al. did not use antibiotics, 10 posing a higher risk of wound infection, and when combined with the loss of a broad range of immune cells, could contribute to delayed healing or non‐closure of wounds. 20 Indeed, previous studies have shown that antibiotic use can accelerate cutaneous healing, reducing the wound area and inflammatory infiltration, increasing fibroblast proliferation and synthesis of extracellular matrix, formation of epithelial tissue and wound closure. 21
The methods of immune cell analysis differed between the studies utilizing the Lang‐DTR and CD11c‐DTR models (Table 1). Li et al. utilized flow cytometry, which is a useful means of identifying populations of cell types with high sensitivity and specificity; however, it also has limitations. Calculations of cell populations as a percentage can mask changes to immune cell populations post‐depletion, while absolute numbers relative to tissue weight can better represent those changes. 22 Immunofluorescence staining of wound sections, by contrast, provides spatial information on the location of cells relative to each other and to the architecture of the tissue, which is particularly relevant when studying changes within the wound during healing. Evidence shows that these two analyses are comparable 23 and there are benefits to using both techniques together. While these approaches provide considerable insights, they could be used in conjunction with novel technologies such as single cell sequencing to gain better understanding of the functions of LCs and DCs during skin repair.
In conclusion, these studies utilizing depletion models have provided valuable information regarding the disparate roles of skin antigen‐presenting cells in the murine wound microenvironment. The Lang‐DTR and CD11c‐DTR mouse models, and the wounding and analytical methods used in these studies have their own share of advantages and limitations. The depletion of particular cell types is not always specific, and the depletion may only be partial. Depletion in the Lang‐DTA mouse model is exclusive to the epidermal LCs and is therefore more selective than the Lang‐DTR mouse so is beneficial for the study of this cell type. 24 The use of different mouse models to study the depletion of different cell types not surprisingly may show variable effects on the rate of healing, and we must display caution while comparing studies. There is utility in standardizing experimental approaches in these models so as to improve reproducibility and comparability. Both the studies discussed here provide valuable information regarding the regulatory roles of antigen‐presenting cells in healing. Addressing the points raised here in future studies will allow for an improved understanding of the contribution of innate immune cells to the regulation of healing.
Disclosure
The authors have no conflicting interests.
Acknowledgements
This work was supported by grants from the Otago Medical Research Foundation and the University of Otago. Ms Aarthi Rajesh is the recipient of a postgraduate scholarship from the University of Otago.
Joint Senior authors: Wise and Hibma
This Perspective is a response to the ‘Letter to the editor’ https://onlinelibrary.wiley.com/doi/abs/10.1111/imm.13312 by Li et al. Both articles discuss this Original Article (https://onlinelibrary.wiley.com/doi/abs/10. 1111/imm.13202) by Rajesh et al.
Data availability statement
The data that support the findings of this study are available on request from the corresponding author.
References
- 1. MacLeod AS, Mansbridge JN. The innate immune system in acute and chronic wounds. Adv Wound Care. 2016;5:65–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rajesh A, Stuart G, Real N, Ahn J, Tschirley A, Wise L, et al. Depletion of langerin(+) cells enhances cutaneous wound healing. Immunology. 2020;160:366–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kissenpfennig A, Henri S, Dubois B, Laplace‐Builhe C, Perrin P, Romani N, et al. Dynamics and function of Langerhans cells in vivo: dermal dendritic cells colonize lymph node areas distinct from slower migrating Langerhans cells. Immunity 2005;22:643–54. [DOI] [PubMed] [Google Scholar]
- 4. Pappenheimer AM, Harper AA, Moynihan M, Brockes JP. Diphtheria toxin and related proteins: effect of route of injection on toxicity and the determination of cytotoxicity for various cultured cells. J Infect Dis. 1982;145:94–102. [DOI] [PubMed] [Google Scholar]
- 5. Lendrum AC, Fraser DS, Slidders W, Henderson R. Studies on the character and staining of fibrin. J Clin Pathol. 1962;15:401–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bennett CL, Clausen BE. DC ablation in mice: promises, pitfalls, and challenges. Trends Immunol. 2007;28:525–31. [DOI] [PubMed] [Google Scholar]
- 7. Zaft T, Sapoznikov A, Krauthgamer R, Littman DR, Jung S. CD11chigh dendritic cell ablation impairs lymphopenia‐driven proliferation of naive and memory CD8+ T cells. J Immunol. 2005;175:6428–35. [DOI] [PubMed] [Google Scholar]
- 8. Hildner K, Edelson BT, Purtha WE, Diamond M, Matsushita H, Kohyama M, et al. Batf3 deficiency reveals a critical role for CD8alpha+ dendritic cells in cytotoxic T cell immunity. Science. 2008;322:1097–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jung S, Unutmaz D, Wong P, Sano G, De los Santos K, Sparwasser T, et al. In vivo depletion of CD11c+ dendritic cells abrogates priming of CD8+ T cells by exogenous cell‐associated antigens. Immunity. 2002;17:211–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Li Z, Gothard E, Coles MC, Ambler CA. Quantitative methods for measuring repair rates and innate‐immune cell responses in wounded mouse skin. Front Immunol. 2018;9:347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tittel AP, Heuser C, Ohliger C, Llanto C, Yona S, Hammerling GJ, et al. Functionally relevant neutrophilia in CD11c diphtheria toxin receptor transgenic mice. Nat Methods. 2012;9:385–90. [DOI] [PubMed] [Google Scholar]
- 12. Rodero MP, Khosrotehrani K. Skin wound healing modulation by macrophages. Int J Clin Exp Pathol. 2010;3:643–53. [PMC free article] [PubMed] [Google Scholar]
- 13. Dovi JV, He LK, DiPietro LA. Accelerated wound closure in neutrophil‐depleted mice. J Leukoc Biol. 2003;73:448–55. [DOI] [PubMed] [Google Scholar]
- 14. Dupasquier M, Stoitzner P, van Oudenaren A, Romani N, Leenen PJ. Macrophages and dendritic cells constitute a major subpopulation of cells in the mouse dermis. J Invest Dermatol. 2004;123:876–9. [DOI] [PubMed] [Google Scholar]
- 15. Li Z, Hodgkinson T, Gothard EJ, Boroumand S, Lamb R, Cummins I, et al. Epidermal Notch1 recruits RORgamma(+) group 3 innate lymphoid cells to orchestrate normal skin repair. Nat Commun. 2016;7:11394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Azzi L, El‐Alfy M, Martel C, Labrie F. Gender differences in mouse skin morphology and specific effects of sex steroids and dehydroepiandrosterone. J Invest Dermatol. 2005;124:22–7. [DOI] [PubMed] [Google Scholar]
- 17. Ansell DM, Kloepper JE, Thomason HA, Paus R, Hardman MJ. Exploring the "hair growth‐wound healing connection": anagen phase promotes wound re‐epithelialization. J Invest Dermatol. 2011;131:518–28. [DOI] [PubMed] [Google Scholar]
- 18. Ito M, Liu Y, Yang Z, Nguyen J, Liang F, Morris RJ, et al. Stem cells in the hair follicle bulge contribute to wound repair but not to homeostasis of the epidermis. Nat Med. 2005;11:1351–4. [DOI] [PubMed] [Google Scholar]
- 19. Koschwanez HE, Broadbent E. The use of wound healing assessment methods in psychological studies: a review and recommendations. Br J Health Psychol. 2011;16:1–32. [DOI] [PubMed] [Google Scholar]
- 20. Edwards R, Harding KG. Bacteria and wound healing. Curr Opin Infect Dis. 2004;17:91–6. [DOI] [PubMed] [Google Scholar]
- 21. Altoe LS, Alves RS, Sarandy MM, Morais‐Santos M, Novaes RD, Goncalves RV. Does antibiotic use accelerate or retard cutaneous repair? A systematic review in animal models. PLoS One. 2019;14:e0223511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bennett JR, Lateef Z, Fleming SB, Mercer AA, Wise LM. Orf virus IL‐10 reduces monocyte, dendritic cell and mast cell recruitment to inflamed skin. Virus Res. 2016;213:230–7. [DOI] [PubMed] [Google Scholar]
- 23. Jung KM, Bae IH, Kim BH, Kim WK, Chung JH, Park YH, et al. Comparison of flow cytometry and immunohistochemistry in non‐radioisotopic murine lymph node assay using bromodeoxyuridine. Toxicol Lett. 2010;192:229–37. [DOI] [PubMed] [Google Scholar]
- 24. Kaplan DH, Jenison MC, Saeland S, Shlomchik WD, Shlomchik MJ. Epidermal langerhans cell‐deficient mice develop enhanced contact hypersensitivity. Immunity 2005;23:611–20. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author.


