Abstract
Background
A human chorionic gonadotropin (hCG) cut-off of ≤300 IU/l for starting actinomycin D (ActD) in post-molar gestational trophoblastic neoplasia (GTN) patients developing methotrexate resistance (MTX-R) reduced the number of women needing toxic multi-agent chemotherapy (etoposide, MTX and ActD alternating weekly with cyclophosphamide and vincristine; EMA/CO) without affecting survival. Here we assess whether an increased hCG cut-off of ≤1000 IU/l spares more women EMA/CO.
Patients and methods
All post-molar GTN patients treated with first-line methotrexate and folinic acid (MTX/FA) were identified in a national cohort between 2009 and 2016. Data collected included age, FIGO score, the hCG levels at MTX-R, and treatment outcomes.
Results
In total, 609 GTN patients commenced treatment with MTX/FA achieving a complete response in 57% (348/609). Resistance developed in 25.1% (153/609) at an hCG ≤ 1000 IU/l and switching to ActD achieved remission in 92.8% without any major toxicity with the remaining 7.2% remitting on EMA/CO. Comparative analysis of patients switching at an hCG <100 versus 100-300 versus 300-1000 IU/l revealed a significant fall in the cure rate with second-line ActD from 97% (93/96) to 87% (34/39) to 78% (14/18), respectively, P = 0.009. However, by increasing the hCG cut-off from ≤300 to ≤1000 IU/l, 14 patients were spared EMA/CO chemotherapy. Moreover, in the present series, all post-molar GTN remain in remission.
Conclusion
This study demonstrates that increasing the hCG cut-off from ≤300 to ≤1000 IU/l for choosing patients for ActD following MTX-R spares more women with GTN from the greater toxicity of EMA/CO without compromising 100% survival outcomes.
Key words: gestational trophoblastic neoplasia, molar pregnancy, hCG
Highlights
-
•
An hCG cut-off of ≤1000 IU/l for ActD over EMA/CO treatment in MTX-R GTN spares women toxicity without affecting survival.
-
•
On developing MTX-R, as the hCG cut-off for selecting ActD versus EMA/CO rises, the complete response rate for ActD falls.
-
•
Half of FIGO-7 patients were cured on single-agent treatment (MTX/FA or sequential ActD), warranting further investigation.
Introduction
The commonest forms of gestational trophoblastic disease are complete hydatidiform moles (CHM) and partial hydatidiform moles (PHM) that affect 1-3 in 1000 pregnancies.1 These usually present in the first trimester of pregnancy with vaginal bleeding prompting an early pelvic ultrasound and recognition of an abnormal gestation. The diagnosis is made by histopathological examination of the products from uterine evacuation. Both CHM and PHM are best regarded as pre-malignant conditions because about 16% and 1%, respectively, will develop into gestational trophoblastic neoplasia (GTN), usually in the form of invasive mole or choriocarcinoma.1 Rarer forms of GTN such as placental site trophoblastic tumour (PSTT) and epithelioid trophoblastic tumours (ETT) arise after any type of pregnancy and comprise 0.23% of all gestational trophoblastic disease (GTD).2, 3, 4 These cancers are biologically distinct, managed differently from post-molar GTN, and are not further discussed here.
In the UK, all women following uterine evacuation of a CHM or PHM are registered for centralised histopathology review and human chorionic gonadotrophin (hCG) monitoring.1 Malignant change to GTN is diagnosed if the hCG plateaus over three values or rises over two consecutive values as previously described.1,5 Patients from the UK are then urgently reviewed at one of the two GTD national centres – the majority at Charing Cross Hospital in London and those living in North England or Wales seen at Weston Park Hospital in Sheffield. This allows for clinical assessment and determination of the FIGO score (Table 1) which is used to decide the type of treatment required.6 Most post-molar GTN patients will have a FIGO score of 0-6 and therefore be at low risk (LR) of developing disease resistant to single-agent chemotherapy with either methotrexate (MTX) or actinomycin D (ActD).1,7 These treatments carry few short-term and virtually no long-term toxicities.8 In the UK, all such LR patients commence intramuscular MTX 50 mg on days 1, 3, 5, and 7 alternating daily with oral folinic acid (FA) 15 mg on days 2, 4, 6, and 8 repeated every 2 weeks (MTX/FA) until the hCG has been normal for 6 weeks.1 Moreover, rarely high-risk patients with a FIGO score of >6 refuse multi-agent therapy and so start single-agent treatment.9 However, methotrexate resistance (MTX-R), as evidenced by a plateaued or rising hCG, and more occasionally, toxicity can occur, necessitating a change in treatment.1,10 Previously, this meant using combination agent chemotherapy such as etoposide, MTX, and ActD, alternating weekly with cyclophosphamide and vincristine (EMA/CO).11,12 This treatment is considerably more toxic both in the short-term (nausea/vomiting, alopecia, significant myelosuppression, neuropathy, lethargy) and in later-life (earlier menopause, increased risk of some cancers if treatment prolonged).8
Table 1.
FIGO Scoring in GTN
| 0 | 1 | 2 | 4 | |
|---|---|---|---|---|
| Age (years) | <40 | >40 | — | — |
| Antecedent pregnancy | Mole | Abortion | Term | — |
| Interval from index pregnancy (months) | <4 | 4-6 | 7-12 | >12 |
| Pre-treatment hCG (IU/l) | <103 | 103-104 | >104-105 | >105 |
| Largest tumour size (cm) | — | 3-4 | ≥5 | — |
| Site of metastases | Lung | Spleen/kidney | GI tract | Brain/liver |
| Number of metastases | — | 1-4 | 5-8 | >8 |
| Previous chemotherapy | — | — | Single drug | >1 drug |
GI, gastrointestinal; GTN, gestational trophoblastic neoplasia; hCG, human chorionic gonadotrophin.
To spare women the toxicity of EMA/CO, at the Charing Cross Hospital GTD Centre, we adopted an hCG cut-off to determine whether to switch from MTX to the other single agent, ActD (1.25 mg/m2 intravenously every 2 weeks, which is also relatively non-toxic), or to employ EMA/CO.13 We initially chose an hCG cut-off of 100 IU/l whereby patients developing MTX resistance (MTX-R) at or below this value received ActD and those above had EMA/CO. This spared some patients unnecessary EMA/CO as 87% of the ActD treated women were cured and the remainder were all salvaged with EMA/CO providing 100% overall survival.13 We then increased the hCG cut-off to ≤300 IU/l and produced similar results, sparing more women the toxicity of EMA/CO.9 Since 2009 we have raised the hCG cut-off to ≤1000 IU/l. Here, we report for the first time the effects of this increase on single-agent ActD-induced cure rates, the reduction in EMA/CO usage, and on overall survival of women starting MTX/FA for post-molar GTN.
Patients and methods
Patient database and selection
The Charing Cross Hospital GTD database was screened for the years 2009-2016 to identify all cases of post-molar GTN who received MTX/FA as initial therapy together with those individuals who developed MTX-R and switched to ActD or EMA/CO. Patients were excluded if they came from overseas, had a non-molar histological diagnosis such as choriocarcinoma or PSTT/ETT, or their follow-up was <12 months following completion of therapy. The following variables were analysed: age, FIGO score, number of cycles of MTX/FA before switching to ActD, reason for the switch (MTX-R or toxicity), hCG level at change of treatment, number of cycles of ActD to achieve cure, toxicity, outcome of treatment, necessity of third-line chemotherapy regimens or surgical treatments following ActD, the development of subsequent relapse, and overall survival. The ability of ActD to induce remission following MTX-R was also analysed according to the following hCG levels post-MTX-R: <100, 100-300, and 301-1000 IU/l.
Follow-up and treatment protocols
All patients following a CHM/PHM had central pathology review and were registered for hCG monitoring. The hCG monitoring following uterine evacuation of CHM and PHM was carried out as previously described using the Charing Cross radio-immunoassay (RIA) and measured in serum every 2 weeks until normal.1 The diagnosis of post-molar GTN was nearly always established on the basis of a rising or plateaued hCG, with a few cases due to a serum hCG >20 000 IU/l more than 4 weeks post-evacuation or heavy bleeding requiring transfusion.14 All were staged using the FIGO criteria (Table 1).6
Patients with LR disease (as per FIGO score 0-6) were initiated on MTX/FA. High-risk patients (FIGO score >6) commenced on EMA-CO were excluded from this study, but if they started MTX/FA because of patient choice, they were included. Treatment regimens were as previously described1,14 and as outlined in the introduction. Serum hCG was monitored twice-weekly during treatment. Patients failing EMA/CO were considered for hysterectomy/resection of resistant disease sites and/or given paclitaxel plus etoposide alternating every 2 weeks with paclitaxel and cisplatin (TE/TP) as described.15 Resistance to treatment was defined as a rising hCG on ≥2 or plateaued hCG on ≥3 occasions and toxicity was assessed according to the standard common terminology criteria for adverse events (CTCAE) grading system.
Statistical analysis
Median and standard deviation of the continuous variables were calculated; in case of nominal variables, frequency and percentages were calculated. Contrast between groups was done using U Mann–Whitney non-parametric testing. For comparison between groups when variables were nominals, Pearson's chi-square test was used, and P < 0.05 was considered a statistically significant value. Analyses were carried out using JMP-SAS 9 software (https://www.jmp.com/en_gb/home.html).
All patient data were rendered non-clinically identifiable using a coding system. The study was considered a service evaluation and development by Imperial College NHS Healthcare Trust with all data de-identified, so formal ethics approval was not required.
Results
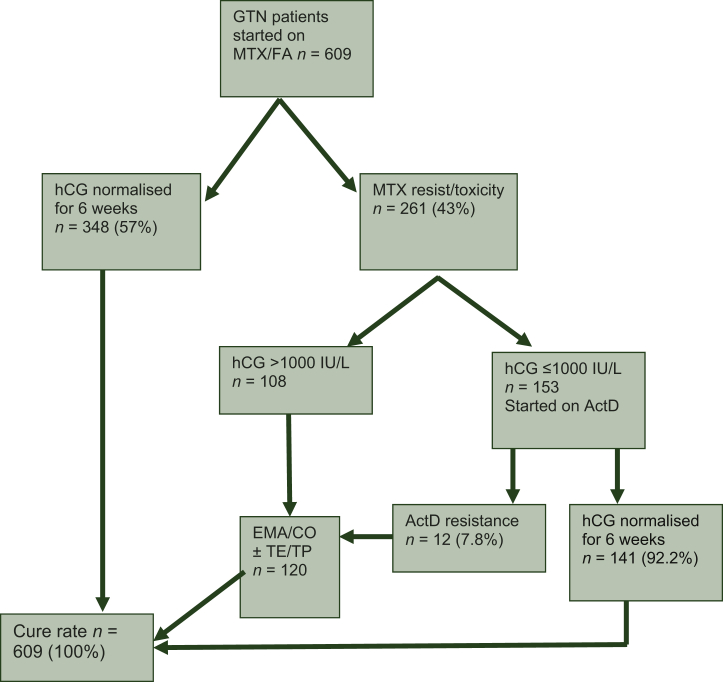
The Charing Cross Hospital GTD database screen revealed 8105 patients registered with a diagnosis of molar pregnancy between 2009 and 2016. GTN subsequently developed in 787 women but 178 were excluded using the criteria outlined above. Thus, 609 women commenced single-agent MTX/FA for confirmed post-molar GTN. At the start of treatment, the median age was 32 years (range 14-56 years) and the median hCG was 12140 IU/l (range 8-410, 804 IU/l). The treatment outcomes for these women are displayed in Figure 1. Overall, 348 (57%) women were cured on MTX/FA alone, whilst 261 needed to change treatment either because of MTX-R (90%) or toxicity (10%). Of these, 153 went on to receive ActD as their hCG was ≤1000 IU/l and 108 had an hCG >1000 IU/l and were therefore switched to EMA/CO (Figure 1).
Figure 1.
Flow chart of outcomes in post-molar GTN patients at CXH between 2009 and 2016.
ActD, actinomycin D; CXH, Charing Cross Hospital; EMA/CO, etoposide, MTX and ActD alternating weekly with cyclophosphamide and vincristine; GTN, gestational trophoblastic neoplasia; hCG, human chorionic gonadotrophin; MTX/FA, methotrexate and folonic acid; TE/TP, paclitaxel/cisplatin alternating with paclitaxel/etoposide.
The outcomes for patients according to their initial FIGO score are given in Table 2. Most patients (542; 89%) had a FIGO score of 0-4 whilst 62 (10%) were 5-6 and 5 patients were actually high risk with a score of 7 but nevertheless started on MTX/FA. Table 2 shows that there was no obvious relationship between FIGO score and the chance of cure with second-line ActD therapy with an overall success rate of 92.2%. The remaining 7.8% (12/153) who failed ActD because of resistance were successfully salvaged by EMA/CO (10 patients) or additional TE/TP (2 patients) (Figure 1 and Table 2).
Table 2.
The successful treatment rates for single-agent MTX/FA chemotherapy and second- and third-line therapies grouped by FIGO score
| FIGO score | 1st line MTX/FA | Success rate % (n) |
2nd line ActD | Success rate % (n) |
2nd line EMA/ CO | Success rate % (n) |
3rd-line (EMA/CO or TE/TP) | Success rate % (n) |
|---|---|---|---|---|---|---|---|---|
| 0 | 57 | 74 (42) | 13 | 92 (12) | 2 | 100 (2) | 1 | 100 (1) |
| 1 | 91 | 78 (71) | 16 | 100 (16) | 4 | 100 (4) | ||
| 2 | 128 | 64 (82) | 34 | 94 (32) | 12 | 92 (11) | 3 | 100 (3) |
| 3 | 145 | 52(75) | 40 | 90 (36) | 30 | 90 (27) | 7 | 100 (7) |
| 4 | 120 | 44 (53) | 34 | 91 (31) | 33 | 100 (33) | 3 | 100 (3) |
| 5 | 32 | 44 (14) | 11 | 82 (9) | 7 | 86 (6) | 3 | 100 (3) |
| 6 | 31 | 32 (10) | 4 | 100 (4) | 17 | 94 (16) | 1 | 100 (1) |
| 7 | 5 | 20 (1) | 1 | 100 (1) | 3 | 100 (3) | ||
| Total | 609 | 57.2 (348) | 153 | 92.2 (141) | 108 | 94.4 (102) | 18 | 100 (18) |
ActD, actinomycin D; EMA/CO, etoposide; MTX and ActD alternating weekly with cyclophosphamide and vincristine; MTX/FA, methotrexate and folonic acid; TE/TP, paclitaxel/cisplatin alternating with paclitaxel/etoposide.
Table 3 shows the five patients who had FIGO score 7 and the reasons why they were started on MTX/FA rather than the expected EMA/CO therapy. Interestingly, two of these five patients were cured on single-agent chemotherapy, one with MTX/FA alone and the second following a switch to ActD due to MTX-R. The remaining three were switched to EMA-CO, either due to rescoring from FIGO 6 to 7 following imaging review (two patients) or developing MTX-R with plateaued hCG levels >1000 IU/l (one patient). All five patients were cured with no relapse.
Table 3.
Breakdown of patients with FIGO score 7 and treatment outcomes
| Age (years) | Initial hCG value IU/l | Number of metastases | Size of uterine mass | Cycles of MTX | Outcome | Notes |
|---|---|---|---|---|---|---|
| 45 | 101 563 | 0 | >5 cm | 8 | Cured on MTX alone | Advised high risk but patient chose MTX/FA |
| 31 | 30 782 | >8 | 3-5 cm | 7 | Switched to ActD and cured | Advised high risk but patient wanted MTX/FA and upon MTX-R insisted on ActD |
| 18 | 158 000 | 1 | >5 cm | 1 | Switch to EMA/CO | Initial score 6 as CXR report normal but MDT noted lung metastasis so upgraded to FIGO 7, switched to EMA/CO |
| 46 | 184 951 | 0 | >5 cm | 1 | Switch to EMA/CO | Initial score 6 in error but post-MDT review scored 7 switched to EMA/CO |
| 28 | 142 552 | 1 | >5 cm | 7 | Switch to EMA/CO | CXR reported as suspicious so FIGO 7, later CT chest normal so downgraded to FIGO 6 and continued MTX/FA |
ActD, actinomycin D; CT, computer tomography scan; CXR, chest X-Ray; EMA/CO, etoposide; hCG, human chorionic gonadotrophin; MDT, multi-disciplinary team meeting; MTX, methotrexate; MTX/FA, methotrexate and folonic acid; MTX-R, methotrexate resistance.
We next determined the effect of increasing hCG cut-offs on the efficacy of ActD to induce remission following MTX-R and to prevent the need for subsequent EMA/CO. We therefore analysed our data according to whether MTX-R occurred at an hCG cut-off of <100, 100-300, or 301-1000 IU/l (Tables 4A-C, respectively). Tables 4A-C demonstrate that as the hCG cut-off rises from <100 to 100-300 to 301-1000 IU/l, remission rates to ActD significantly fall from 97% (93/96) to 87% (34/39) to 78% (14/18), respectively (P = 0.009). This reduction in ActD efficacy does not obviously reflect an increase in the FIGO scores in these segmented groups. Importantly, by increasing the cut-off of hCG from ≤300 to ≤1000 IU/l we succeeded in preventing 14 women from unnecessarily receiving the more toxic EMA/CO therapy (Table 4C).
Table 4.
Distribution of patients switched to second-line actinomycin D (ActD), sub-grouped according to human chorionic gonadotrophin (hCG) level. (A) <100 IU/l, (B) 100-300 IU/l, (C) 301-1000 IU/l
| FIGO score | n | Success % (n) | No. of cycles |
Relapse | |
|---|---|---|---|---|---|
| Min | Max | ||||
| A. Switch to second-line ActD, hCG <100 IU/l | |||||
| 0 | 10 | 100 (10) | 1 | 5 | |
| 1 | 12 | 100 (12) | 3 | 9 | |
| 2 | 21 | 95 (20) | 2 | 7 | 1 |
| 3 | 23 | 91 (21) | 3 | 7 | |
| 4 | 21 | 100 (21) | 3 | 6 | 2 |
| 5 | 7 | 100 (7) | 4 | 6 | |
| 6 | 2 | 100 (2) | 4 | 5 | |
| 96 | 97 (93) | 1 | 9 | 0.3% (3) | |
| B. Switch to second-line ActD, hCG 100-300 IU/l | |||||
| 0 | 3 | 66 (2) | 3 | 6 | 0 |
| 1 | 3 | 100 (3) | 5 | 7 | 0 |
| 2 | 10 | 80 (8) | 4 | 9 | 0 |
| 3 | 12 | 83 (10) | 2 | 7 | 0 |
| 4 | 7 | 100 (7) | 3 | 9 | 1 |
| 5 | 2 | 100 (2) | 6 | 6 | 0 |
| 6 | 2 | 100 (2) | 8 | 8 | 1 |
| 39 | 87 (34) | 2 | 9 | 0.5% (2) | |
| C. Switch to second-line ActD, hCG 301-1000 IU/l | |||||
| 0 | 0 | ||||
| 1 | 1 | 100 (1) | 8 | 1 | |
| 2 | 3 | 67 (2) | 3 | 7 | |
| 3 | 5 | 80 (4) | 3 | 6 | 1 |
| 4 | 6 | 83 (5) | 2 | 9 | |
| 5 | 2 | 50 (1) | 2 | 7 | |
| 6 | 0 | ||||
| 7 | 1 | 100 (1) | 9 | ||
| 18 | 78 (14) | 2 | 9 | 11% (2) | |
After achieving a complete response with second-line ActD, 4.5% (7/153) of women subsequently relapsed with a rising hCG but all were successfully salvaged with further chemotherapy (five with EMA/CO, two with TE/TP) and/or surgery (one hysteroscopic nodule resection, four hysterectomies).
Importantly, all 609 women who started on MTX/FA for post-molar GTN remain in remission with a minimum follow-up of 48 months.
Discussion
Chemotherapy for LR-GTN produces cure rates of around 100% using single-agent MTX or ActD and, if resistance develops, followed by the much more toxic EMA/CO. Consequently, any approaches that reduce the use of EMA/CO whilst maintaining these excellent outcomes would be highly desirable. Accordingly, over the past 20 years, we have been evaluating the sequential use of single-agent therapy to reduce the number of patients receiving EMA/CO. We initially used an hCG cut-off of ≤100 IU/l at the point of MTX-R for selecting ActD and as this was successful, then showed that ≤300 IU/l also preserved the 100% survival rate and spared more women from EMA/CO.9,13
Here, for the first time, we demonstrate that an hCG cut-off of ≤1000 IU/l is safe and saves more women from EMA/CO toxicity. Similar to our previous reports,9,13 about 60% of LR-GTN patients achieved successful remission with MTX/FA alone. Indeed, a systematic review from multiple centres including our own data also reveals that around two-thirds of LR-GTN patients are cured with first-line MTX/FA.16 Importantly, 92.2% of patients with MTX-R who were switched to ActD entered remission with this second single agent. Superficially, this looked very similar to our previous remission rates for ActD of 87% and 95% when we had employed an hCG cut-off of ≤100 and ≤300 IU/l, respectively.9,13 However, with rising hCG cut-off points, one might have anticipated a drop in these remission rates. This is because of the correlation of higher hCG levels with larger volumes of disease and higher FIGO risk scores, and hence a greater possibility of developing resistance to ActD.
We therefore investigated this more carefully by undertaking a comparative analysis of subgroups of MTX-R patients switching to ActD at an hCG of <100 versus 100-300 versus 301-1000 IU/l. This did indeed reveal a significant fall in the cure rate with ActD as the hCG cut-off increased (Table 4, P = 0.009). However, by raising the hCG cut-off from ≤300 to ≤1000 IU/l, 14 more patients were spared EMA/CO treatment. Importantly, all patients with failing ActD treatment were able to enter remission with EMA/CO. Moreover, overall relapse rates were low at 3% (18/609), consistent with our previous data9 and all were successfully salvaged with subsequent treatment preserving our 100% cure rate.
We previously reported no success with MTX-FA in three high-risk patients with a FIGO score of 7 although all were cured with multi-agent treatment.9 Interestingly, in the present study, five women with a FIGO score of 7 and GTN were treated with MTX-FA, largely due to patient choice. Whilst one of these was rescored as 6 with further imaging, it is quite striking that in 50% of the four genuine FIGO-7 patients, sustained remission was achieved with MTX/FA either alone or with sequential ActD (Table 3). Thus, whilst the case numbers here are small, the possibility that women scoring FIGO 7 could benefit from MTX/FA with or without sequential use of ActD to avoid the toxicity of EMA/CO urgently warrants further investigation.
The next question must be whether more patients could be safely spared EMA/CO toxicity through further raising the hCG cut-off point or by using other relatively non-toxic, single-agent treatments. The former is attractive and some argue that all LR-GTN patients developing MTX-R should receive ActD regardless of the hCG level.17 We have adopted a more cautious approach and are currently testing a ≤3000 IU/l cut-off recognising that we may reach a point of failure given our present findings. The use of other single agents has recently been piloted using carboplatin following MTX-R but currently results have demonstrated mixed success.18,19 In addition, checkpoint immunotherapy with pembrolizumab has shown promise in saving the lives of women with GTN in whom all existing treatments failed20 and avelumab has now been tested in both MTX/FA-resistant and EMA-CO-resistant patients with early signs of activity.21 However, little is known about the long-term side-effects of these exciting and expensive new therapies.
Limitations of our study include its retrospective rather than prospective nature but it has the advantage that the study cohort is large and population-based rather than hospital acquired and so is less subject to case ascertainment bias. However, we recognise that the numbers of cases within subgroups are still small and so this could be associated with errors in our analysis. In particular, the numbers of cases with an hCG between 300 and 1000 IU/l were much smaller than the number of cases with a lower hCG cut-off at the time of MTX-R. Thus, whilst the fall in cure rate with ActD as the hCG cut-off rises might seem logical, and was statistically significant, we cannot exclude the possibility of a type 1 error. It is important to note that, due to variation between hCG immunoassay methods, the hCG cut-offs evaluated in this study are specific to the hCG method in use at our centre (RIA).22 Nevertheless, our own unpublished data using a commercially available assay suggests that these cut-offs may be more broadly applicable at least for one other hCG assay.
In conclusion, we have demonstrated that increasing the hCG cut-off point from ≤300 to ≤1000 IU/l to select patients for ActD following MTX-R spares more women with post-molar GTN the greater toxicity of EMA/CO without compromising 100% survival outcomes. The possibility that patients with FIGO score 7 could also be spared this toxicity merits further investigation.
Acknowledgments
Funding
None declared.
Disclosure
The authors have declared no conflicts of interest.
References
- 1.Seckl M.J., Sebire N.J., Berkowitz R.S. Gestational trophoblastic disease. Lancet. 2010;376:717–729. doi: 10.1016/S0140-6736(10)60280-2. [DOI] [PubMed] [Google Scholar]
- 2.Schmid P., Nagai Y., Agarwal R. Prognostic markers and long-term outcome of placental-site trophoblastic tumours: a retrospective observational study. Lancet. 2009;374:48–55. doi: 10.1016/S0140-6736(09)60618-8. [DOI] [PubMed] [Google Scholar]
- 3.Frijstein M.M., Lok C.A.R., van Trommel N.E. Management and prognostic factors of epithelioid trophoblastic tumors: results from the International Society for the Study of Trophoblastic Diseases Database. Gynecol Oncol. 2019;152:361–367. doi: 10.1016/j.ygyno.2018.11.015. [DOI] [PubMed] [Google Scholar]
- 4.Froeling F.E.M., Ramaswami R., Papanastasopoulos P. Intensified therapies improve survival and identification of novel prognostic factors for placental-site and epithelioid trophoblastic tumours. Br J Cancer. 2019;120:587–594. doi: 10.1038/s41416-019-0402-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ngan H.Y.S., Seckl M.J., Berkowitz R.S. Update on the diagnosis and management of gestational trophoblastic disease. Int J Gynaecol Obstet. 2018;143(Suppl 2):79–85. doi: 10.1002/ijgo.12615. [DOI] [PubMed] [Google Scholar]
- 6.Kohorn E.I. The new FIGO 2000 staging and risk factor scoring system for gestational trophoblastic disease: description and critical assessment. Int J Gynecol Cancer. 2001;11:73–77. doi: 10.1046/j.1525-1438.2001.011001073.x. [DOI] [PubMed] [Google Scholar]
- 7.Maesta I., Growdon W.B., Goldstein D.P. Prognostic factors associated with time to hCG remission in patients with low-risk postmolar gestational trophoblastic neoplasia. Gynecol Oncol. 2013;130:312–316. doi: 10.1016/j.ygyno.2013.05.017. [DOI] [PubMed] [Google Scholar]
- 8.Savage P., Cooke R., O'Nions J. Effects of single-agent and combination chemotherapy for gestational trophoblastic tumors on risks of second malignancy and early menopause. J Clin Oncol. 2015;33:472–478. doi: 10.1200/JCO.2014.57.5332. [DOI] [PubMed] [Google Scholar]
- 9.Sita-Lumsden A., Short D., Lindsay I. Treatment outcomes for 618 women with gestational trophoblastic tumours following a molar pregnancy at the Charing Cross Hospital, 2000-2009. Br J Cancer. 2012;107:1810–1814. doi: 10.1038/bjc.2012.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goldstein D.P., Berkowitz R.S., Horowitz N.S. Optimal management of low-risk gestational trophoblastic neoplasia. Expert Rev Anticancer Ther. 2015;15:1293–1304. doi: 10.1586/14737140.2015.1088786. [DOI] [PubMed] [Google Scholar]
- 11.Newlands E.S., Bagshawe K.D., Begent R.H., Rustin G.J., Holden L. Results with the EMA/CO (etoposide, methotrexate, actinomycin D, cyclophosphamide, vincristine) regimen in high risk gestational trophoblastic tumours, 1979-1989. Br J Obstet Gynaecol. 1991;98:550–557. doi: 10.1111/j.1471-0528.1991.tb10369.x. [DOI] [PubMed] [Google Scholar]
- 12.Bower M., Newlands E.S., Holden L. EMA/CO for high-risk gestational trophoblastic tumours: results from a cohort of 272 patients. J Clin Oncol. 1997;15:2636–2643. doi: 10.1200/JCO.1997.15.7.2636. [DOI] [PubMed] [Google Scholar]
- 13.McNeish I.A., Strickland S., Holden L. Low risk persistent gestational trophoblastic disease: outcome following initial treatment with low-dose methotrexate and folinic acid, 1992-2000. J Clin Oncol. 2002;20:1838–1844. doi: 10.1200/JCO.2002.07.166. [DOI] [PubMed] [Google Scholar]
- 14.Seckl M.J., Sebire N.J., Fisher R.A. Gestational trophoblastic disease: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24(Suppl 6):vi39–vi50. doi: 10.1093/annonc/mdt345. [DOI] [PubMed] [Google Scholar]
- 15.Wang J., Short D., Sebire N.J. Salvage chemotherapy of relapsed or high-risk gestational trophoblastic neoplasia (GTN) with paclitaxel/cisplatin alternating with paclitaxel/etoposide (TP/TE) Ann Oncol. 2008;19:1578–1583. doi: 10.1093/annonc/mdn181. [DOI] [PubMed] [Google Scholar]
- 16.Lawrie T.A., Alazzam M., Tidy J. First-line chemotherapy in low-risk gestational trophoblastic neoplasia. Cochrane Database Syst Rev. 2016:CD007102. doi: 10.1002/14651858.CD007102.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maesta I., Nitecki R., Desmarais C.C.F. Effectiveness and toxicity of second-line actinomycin D in patients with methotrexate-resistant postmolar low-risk gestational trophoblastic neoplasia. Gynecol Oncol. 2020;157:372–378. doi: 10.1016/j.ygyno.2020.02.001. [DOI] [PubMed] [Google Scholar]
- 18.Mora P.A.R., Sun S.Y., Velarde G.C. Can carboplatin or etoposide replace actinomycin-d for second-line treatment of methotrexate resistant low-risk gestational trophoblastic neoplasia? Gynecol Oncol. 2019;153:277–285. doi: 10.1016/j.ygyno.2019.03.005. [DOI] [PubMed] [Google Scholar]
- 19.Winter M.C., Tidy J.A., Hills A. Risk adapted single-agent dactinomycin or carboplatin for second-line treatment of methotrexate resistant low-risk gestational trophoblastic neoplasia. Gynecol Oncol. 2016;143:565–570. doi: 10.1016/j.ygyno.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 20.Ghorani E., Kaur B., Fisher R.A. Pembrolizumab is effective for drug-resistant gestational trophoblastic neoplasia. Lancet. 2017;390:2343–2345. doi: 10.1016/S0140-6736(17)32894-5. [DOI] [PubMed] [Google Scholar]
- 21.You B., Bolze P.-A., Lotz J.-P. Avelumab in patients with gestational trophoblastic tumors with resistance to single-agent chemotherapy: cohort a of the TROPHIMMUN phase II trial. J Clin Oncol. 2020;38:3129–3137. doi: 10.1200/JCO.20.00803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harvey R.A., Mitchell H.D.C., Stenman U.-H. Differences in total human chorionic gonadotropin immunoassay analytical specificity and ability to measure human chorionic gonadotropin in gestational trophoblastic disease and germ cell tumors. J Reprod Med. 2010;55:285–295. [PubMed] [Google Scholar]