Graphical abstract
Keywords: Cryptosporidium, Disseminated, CD40L deficiency
Abstract
The protozoan Cryptosporidium affects the digestive tract of humans and animals. Cryptosporidiosis leads to diarrhoea mimicking a cholera-like course with dehydration and may even result in death in immunodeficient patients, as patients with hyper-IgM syndrome. We describe a rare case of disseminated Cryptosporidium infection in a seven- year-old boy with CD40 L deficiency. During the pre-graft phase, the patient presented an intestinal cryptosporidiosis which became complicated few days later during the aplasia period with a cholangitis and pulmonary cryptosporidiosis. Cryptosporidium hominis was identified. After treatment with nitazoxanide and azithromycine the patient was doing well.
Cryptosporidiosis is an infection caused by the protozoan Cryptosporidium that affects the digestive tract of humans and animals. Nearly 20 species and genotypes have been reported in humans, of which C. hominis and C. parvum represent the vast majority of cases around the world [1]. These obligate pathogens infects the small intestinal epithelium, causing acute gastroenteritis, abdominal pain, and persistent diarrhea [2]. Transmission primarily occurs via the fecal–oral route, by ingesting viable oocysts, emitted in the faeces of animal or human origin, and which contaminate food or water [3]. Although healthy individuals may acquire infection, immunosuppressed patients such as those infected with the human immunodeficiency virus, bone marrow or solid organ transplantation recipients or primary immunodeficient patients, are at increased risk of developing more severe long-lasting and disseminated disease [4,5].
The hyper-immunoglobulin M (HIGM) syndrome is a heterogeneous group of primary immune deficiency disorders characterised by the failure of immunoglobulin class switching in B-cells resulting in considerable deficiency of immunoglobulins (Ig) G, IgA, or IgE and a normal or elevated serum IgM level. X-linked HIGM is caused by defects in the CD40 ligand (CD40 L), a molecule predominantly expressed by activated CD4+ T-lymphocytes. Loss of interaction between CD40 L and its ligand CD40 (constitutively expressed by B-lymphocytes and monocytes) results in the impairment of terminal B-lymphocyte differentiation [6]. This leads to increased susceptibility to bacterial and intracellular pathogens. More particularly, it has been reported that male infants with primary or acquired immunodeficiency (including HIGM) are at a significant risk of infection with Cryptosporidium spp., i.e. cryptosporidiosis [7,8].
The gold standard techniques for the diagnosis of cryptosporidiosis is based on the detection of the Cryptosporidium oocysts in stool samples. The use of molecular methods has led to significant improvements in diagnosis with better detection sensitivity and characterisation at the level of species, genotypes, or subtypes of Cryptosporidium spp. [9]. Dissemination from the intestinal gut is rare and sparsly described in the literature. But Cryptosporidium spp. is able to spread from the digestive tract to the bile ducts, where it is responsible for cholangitis, and the lungs. In this report, we describe a disseminated Cryptosporidium infection in an infant with CD40 L deficiency (Fig. 1).
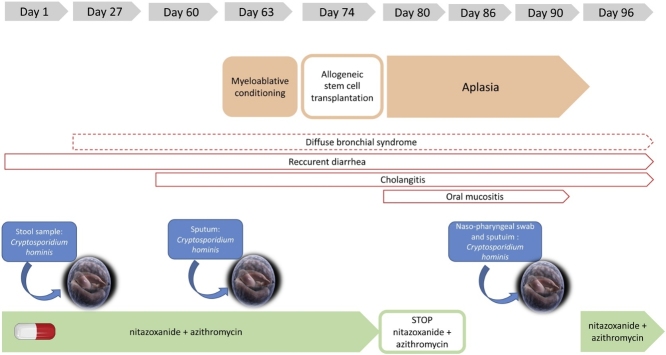
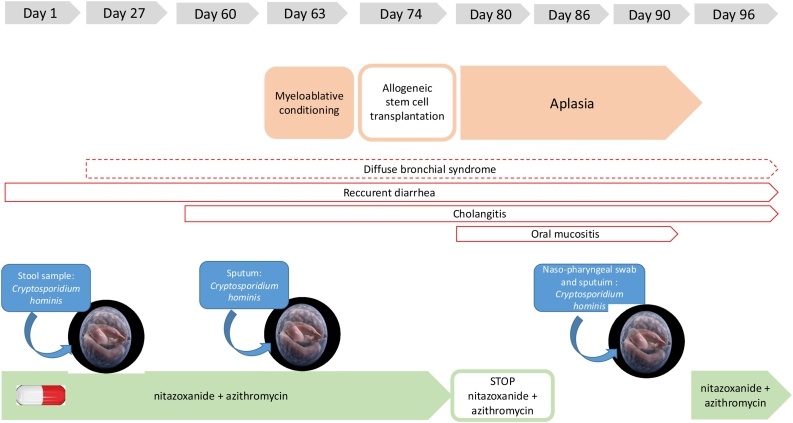
Fig. 1.
Timeline of disseminated cryptosporidiosis.
Case report
A seven-year-old boy, originating from Algeria, was admitted to the pediatric hematology department at the La Timone University Hospital in Marseille France, with a chronic cough and reccurent diarrhea. He was born to non-consanguineous parents and all sibling were healthy. Regarding his medical history, in very early childhood, the boy has exhibited recurrent infections which were presumed to be a primary immune deficiency. Serum IgG and IgA level were significantly reduced (IgG = 4,8 g/L ; cut off = 572 – 1474 g/L and IgA = <0,5 g/L ; cut off = 034 – 305 g/L), and IgM was normal (IgM = 168 g/L; cut off = 031 – 208). Immunophenotyping of lymphocytic subpopulations revealed the absence of CD40 L expression on CD4+ T-lymphocytes; CD40 expression on B-lymphocytes was normal. The definitive diagnosis was a hyper-IgM syndrome with CD40 L deficiency. A prophylactic treatment with antibiotics and immunoglobulin supplementation to prevent opportunistic infections was introduced. A curative treatment based on allo stem cell transplant (SCT) was initiated at the La Timone Hospital in November 2019 in the pediatric hematology department.
During hospitalisation, the child presented profuse diarrhoea and cough. The involvement of intestinal and pulmonary pathogens was investigate using syndromic molecular tools (Film Array ® GI (Gastrointestinal) Panel), which revealed the presence of Cryptosporidium spp. Real-time polymerase chain reaction (Real-time PCR) tests, targetting the 18S rRNA gene, succesfully diagnosed Cryptosporidium hominis infection in three stool samples over a period of 15 days [10]. A specific treatment was initiated involving nitazoxanide (200 mg twice a day) and azithromycine (400 mg, three times a week). Exploration of the chronic cough identified multiple respiratory pathogens including Rhinovirus, Cytomegalovirus, Haemophilus influenzae, Candida glabrata and Geotrichum capitatum. Clinical examination of the lungs, revealed chronic congestion and diffuse crackles, and a tomodensitometry scan showed a diffuse bronchial parietal thickening, leading to the conclusion of diffuse bronchial syndrome with ventilation problems.
During the pre-graft evaluation, laboratory investigations reported mildly abnormal elevated liver enzymes: alanine aminotransferase 87 U/L (cut off <50 U/L) and aspartate aminotransferase 65 U/L (cut-off < 50 U/L). The abdomino-pelvic ultrasound showed a global dilation of the bile ducts with cholangitis. Cholangitis has been imputed to Cryptosporidium hominis. After 10 days of conditioning with immunosuppressive therapy, the patient received allo SCT. During the period of aplasia, nitazoxanide was stopped due to of oral mucositis. A few days later, Cryptosporidium hominis was also isolated in respiratory samples from the nasopharyngeal swab sample and sputum. Taking into account both the positive stool and respiratory samples, diagnosis of disseminated cryptosporidiosis was retained. Nitazoxanide was reintroduced ten days later. With the exeption of hemorrhagic cystitis as a post-transplant complication, the patient’s clinical condition improved and Cryptosporidium was not detected in stool and respiratory samples two months later.
The DNA extracts of stool and respiratory samples were sent to the University Hospital of Dijon as the collaborating laboratory of the National Reference Center - Laboratory Expert (CNR LE) for Cryptosporidioses (Rouen, France) for species determination and sub-typing. Identification of Cryptosporidium hominis subtype Ib A10 G2 was confirmed in both specimens [11].
Conclusion
Cryptosporidiosis causes asymptomatic or with limited diarrhoea infections in immunocompetent persons. However this parasitic infection can lead to diarrhoea that follows a cholera-like course with dehydration and may even result in death in immunodeficient patients. The vast majority of human cases of cryptosporidiosis worldwide are caused by two species, namely C. parvum and C. hominis which differ in host specificity. C. hominis is reported almost exclusively in humans, while C. parvum has a wider potential zoonotic range [9].
Cryptosporidium infections are well described in patients with hyper-IgM syndrome. CD40 L deficiency (X-linked hyper-IgM syndrome type 1) results indeed in altered costimulatory T-lymphocyte function, which impairs B-lymphocyte isotype switching, antibody production and dendritic cell signaling, probably increasing susceptibility to opportunistic infections such as cryptosporidiosis. Recurrent or protracted diarrhoea are the most frequent gastrointestinal complications in HIGM patients, occurring in up to 30 % of patients [12]. In an update of the clinical presentation for 145 HIGM patients from North America, Leven et al. [13] reported gastroenteritis in 20 % of patients and Cryptosporidium spp. was the causal agent for 6 % of them. In addition, in a retrospective analysis of 38 European patients undergoing HSCT for CD40 L deficiency in eight European countries between 1993 and 2002, Gennery et al. [14] described the occurrence of severe Cryptosporidium infection in six patients, all of them dying during the follow up period. Chronic infections with Cryptosporidium spp. are thought to contribute to sclerosing cholangitis and subsequent malignant transformation. Liver disease is a serious complication of HIGM, being observed in more than 80 % of affected males by the age of 20, which may decrease with adequate screening and treatment of Cryptosporidium infection [15]. Interestingly, while C. parvum is the most frequent species reported in HIGM patients suffering from cryptosporidiosis [16], C. hominis was the causative agent identified in stool and respiratory samples in our case report.
Extra intestinal disseminated cryptosporidiosis is rarely observed in immunodeficient patients. Indeed, a limited number of case reports and cases series documenting respiratory tract infections are available. In addition, most of them were published in the 1990s, especially in people living with HIV [17]. To our knowledge, only two cases have reported disseminated Cryptosporidium spp. infections in infants suffering HIGM caused by CD40 L deficiency [18,19]. These young patients presented with severe respiratory infections associated with chronic diarrhea. In this case, we hypothesise that the initial infection occurred in the patient firstly in the digestive tract, with subsequent spread to the respiratory tract either by a fecal-oral route or through hematogenous dissemination. Indeed, the possible routes for disseminated cryptosporidiosis originating from the intestinal tract still remains unclear. Finally the presence of Cryptosporidium spp. in respiratory samples may serve as an viable alternative for parasite propagation, participating in maintaining intestinal pathology, and potential transmission to other individuals. Some cases lacking evidence of gastrointestinal involvement have hinted at the possibility of transmission of cryptosporidiosis via the inhalation of oocytes [20]. It therefore seems important to investigate the pulmonary localisation of Cryptosporidium spp. to avoid person-to-person respiratory transmission of cryptosporidiosis in immunosuppressed individuals, especially in medical care units. The systematic detection in respiratory syndromic molecular panels targeting immunosuppressed patients could, therefore, be considered in order to establish further guidelines for the prevention of cryptosporidiosis. The diagnosis may be established through detection of Cryptosporidium spp. in various upper or lower respiratory specimens from bronchoalveolar lavages, sputa and bronchial biopsies. In this case, the child presented with a chronic cough for three months. Retrospective analysis of repetitive sampling of sputa following the primo-diagnosis of intestinal cryptosporidiosis showed the presence of Cryptosporidium hominis in the respiratory tract up to one month after the initial diagnosis.
It is not clear wether the co-localisation of Cryptosporidium spp. in both pulmonary and gastro-intestinal compartments can worsen outcomes as compared to an exclusive gastro-intestinal infection. Palmieri et al. described a pulmonary cryptosporidiosis in a patient living with HIV who was successfully treated with paromomycin and azithromycin [19]. The authors highlighted the fact that favourable outcomes were linked to an effective immune response recontitution. The first line treatment for our patient was the assocation of azithromycin and nitazoxanide, as recommended in the literature [21]. Nitazoxanide was prematurally stopped due to oral mucositis. Fortunately reconstitution of the immunity began 10 days later, conconmitantly with the re-introduction of nitazoxanide and azithromycin. Thereafter the clinical status of the patient quickly improved.
Pulmonary cryptosporidiosis is likely to be underestimated. The pathogeniesis and clinical course remains still unclear.Clinicians should, therefore, take into account respiratory cryptosporidiosis in children with primary immunodeficiency presenting with a chronic cough.
Disclaimer
“The opinions expressed by authors contributing to this journal do not necessarily reflect the opinions of the Centers for Disease Control and Prevention or the institutions with which the authors are affiliated.”
Funding
This work was supported by the French Government under the « Investissements d’avenir » (Investments for the Future) program managed by the Agence Nationale de la Recherche (ANR, fr: National Agency for Research) (reference: Méditerranée Infection 10-IAHU-03) and by Région Provence Alpes Côte d’Azur and European funding ERDF PRIMI.
Ethical approval
The parents of the patient authorised the publication of the case by a signed agreement during hospitalisation.
Conflict of interest statement
All authors declare any conflicts of interest or sources of funding.
Acknowledgement
The authors gratefully thank the CNR LE cryptosporidiosis for their technical assistance and Sante Publique France for funding our CNR-LE cryptosporidiosis activities.
References
- 1.Chalmers R.M., Davies A.P. Minireview: clinical cryptosporidiosis. Exp Parasitol. 2010;124(January (1)):138–146. doi: 10.1016/j.exppara.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 2.Dillingham R.A., Lima A.A., Guerrant R.L. Cryptosporidiosis: epidemiology and impact. Microbes Infect. 2002;4(August (10)):1059–1066. doi: 10.1016/s1286-4579(02)01630-1. [DOI] [PubMed] [Google Scholar]
- 3.Bouzid M., Kintz E., Hunter P.R. Risk factors for Cryptosporidium infection in low and middle income countries: a systematic review and meta-analysis. PLoS Negl Trop Dis. 2018;12(June (6)) doi: 10.1371/journal.pntd.0006553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang Z.-D., Liu Q., Liu H.-H., Li S., Zhang L., Zhao Y.-K. Prevalence of Cryptosporidium, microsporidia and Isospora infection in HIV-infected people: a global systematic review and meta-analysis. Parasit Vectors. 2018;11(9 January (1)):28. doi: 10.1186/s13071-017-2558-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hadžić N., Nademi Z., Deheragoda M., Zen Y., Elfeky R., Worth A. Chronic cholangiopathy associated with primary immune deficiencies can Be resolved by effective hematopoietic stem cell transplantation. J Pediatr. 2019;209(June):97–106.e2. doi: 10.1016/j.jpeds.2019.01.015. [DOI] [PubMed] [Google Scholar]
- 6.Notarangelo L.D., Hayward A.R. X-linked immunodeficiency with hyper-IgM (XHIM) Clin Exp Immunol. 2000;120(June (3)):399–405. doi: 10.1046/j.1365-2249.2000.01142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parvaneh L., Sharifi N., Azizi G., Abolhassani H., Sharifi L., Mohebbi A. Infectious etiology of chronic diarrhea in patients with primary immunodeficiency diseases. Eur Ann Allergy Clin Immunol. 2019;51(January (1)):32–37. doi: 10.23822/EurAnnACI.1764-1489.77. [DOI] [PubMed] [Google Scholar]
- 8.Bednarska M., Jankowska I., Pawelas A., Piwczyńska K., Bajer A., Wolska-Kuśnierz B. Prevalence of Cryptosporidium, Blastocystis, and other opportunistic infections in patients with primary and acquired immunodeficiency. Parasitol Res. 2018;117(September (9)):2869–2879. doi: 10.1007/s00436-018-5976-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gerace E., Lo Presti V.D.M., Biondo C. Cryptosporidium infection: epidemiology, pathogenesis, and differential diagnosis. Eur J Microbiol Immunol (Bp) 2019;9(25 December (4)):119–123. doi: 10.1556/1886.2019.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mary C., Chapey E., Dutoit E., Guyot K., Hasseine L., Jeddi F. Multicentric evaluation of a new real-time PCR assay for quantification of Cryptosporidium spp. And identification of Cryptosporidium parvum and Cryptosporidium hominis. J Clin Microbiol. 2013;51(August (8)):2556–2563. doi: 10.1128/JCM.03458-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sulaiman I.M., Hira P.R., Zhou L., Al-Ali F.M., Al-Shelahi F.A., Shweiki H.M. Unique endemicity of cryptosporidiosis in children in Kuwait. J Clin Microbiol. 2005;43(June (6)):2805–2809. doi: 10.1128/JCM.43.6.2805-2809.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Winkelstein J.A., Marino M.C., Ochs H., Fuleihan R., Scholl P.R., Geha R. The X-linked hyper-IgM syndrome: clinical and immunologic features of 79 patients. Medicine (Baltimore). 2003;82(November (6)):373–384. doi: 10.1097/01.md.0000100046.06009.b0. [DOI] [PubMed] [Google Scholar]
- 13.Leven E.A., Maffucci P., Ochs H.D., Scholl P.R., Buckley R.H., Fuleihan R.L. Hyper IgM syndrome: a report from the USIDNET registry. J Clin Immunol. 2016;36(July (5)):490–501. doi: 10.1007/s10875-016-0291-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gennery A.R., Khawaja K., Veys P., Bredius R.G.M., Notarangelo L.D., Mazzolari E. Treatment of CD40 ligand deficiency by hematopoietic stem cell transplantation: a survey of the European experience, 1993-2002. Blood. 2004;103(1 February (3)):1152–1157. doi: 10.1182/blood-2003-06-2014. [DOI] [PubMed] [Google Scholar]
- 15.Dunn C.P., de la Morena M.T. X-linked hyper IgM syndrome. In: Adam M.P., Ardinger H.H., Pagon R.A., Wallace S.E., Bean L.J., Stephens K., editors. GeneReviews(®) University of Washington, Seattle; Seattle (WA): 1993. [PubMed] [Google Scholar]
- 16.Xiao L. Molecular epidemiology of cryptosporidiosis: an update. Exp Parasitol. 2010;124(January (1)):80–89. doi: 10.1016/j.exppara.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 17.Sponseller J.K., Griffiths J.K., Tzipori S. The evolution of respiratory Cryptosporidiosis: evidence for transmission by inhalation. Clin Microbiol Rev. 2014;27(July (3)):575–586. doi: 10.1128/CMR.00115-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kutukculer N., Moratto D., Aydinok Y., Lougaris V., Aksoylar S., Plebani A. Disseminated cryptosporidium infection in an infant with hyper-IgM syndrome caused by CD40 deficiency. J Pediatr. 2003;142(February (2)):194–196. doi: 10.1067/mpd.2003.41. [DOI] [PubMed] [Google Scholar]
- 19.Dirim D., Dagci H., Turgay N. Disseminated cryptosporidiosis in Turkey: case report. East Afr Med J. 2003;80(October (10)):550–552. doi: 10.4314/eamj.v80i10.8760. [DOI] [PubMed] [Google Scholar]
- 20.Mercado R., Buck G.A., Manque P.A., Ozaki L.S. Cryptosporidium hominis infection of the human respiratory tract. Emerg Infect Dis. 2007;13(March (3)):462–464. doi: 10.3201/eid1303.060394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Legrand F., Grenouillet F., Larosa F., Dalle F., Saas P., Millon L. Diagnosis and treatment of digestive cryptosporidiosis in allogeneic haematopoietic stem cell transplant recipients: a prospective single centre study. Bone Marrow Transplant. 2011;46(June (6)):858–862. doi: 10.1038/bmt.2010.200. [DOI] [PubMed] [Google Scholar]