Abstract
BACKGROUND
Multiple surgical techniques to perform minimally invasive intracerebral hemorrhage (ICH) evacuation are currently under investigation. The use of an adjunctive aspiration device permits controlled suction through an endoscope, minimizing collateral damage from the access tract. As with increased experience with any new procedure, performance of endoscopic minimally invasive ICH evacuation requires development of a unique set of operative tenets and techniques.
OBJECTIVE
To describe operative nuances of endoscopic minimally invasive ICH evacuation developed at a single center over an experience of 80 procedures.
METHODS
Endoscopic minimally invasive ICH evacuation was performed on 79 consecutive eligible patients who presented a single Health System between March 2016 and May 2018. We summarize 4 core operative tenets and 4 main techniques used in 80 procedures.
RESULTS
A total of 80 endoscopic minimally invasive ICH evacuations were performed utilizing the described surgical techniques. The average preoperative and postoperative volumes were 49.5 mL (standard deviation [SD] 31.1 mL, interquartile range [IQR] 30.2) and 5.4 mL (SD 9.6, mL IQR 5.1), respectively, with an average evacuation rate of 88.7%. All cause 30-d mortality was 8.9%.
CONCLUSION
As experience builds with endoscopic minimally invasive ICH evacuation, academic discussion of specific surgical techniques will be critical to maximizing its safety and efficacy.
Keywords: Endoscopic, Intracerebral hemorrhage, Hemorrhagic stroke, Minimally invasive, Endoscopic ICH evacuation, Intracerebral hematoma, Minimally invasive clot evacuation
ABBREVIATIONS
- CTA
computed tomographic angiography
- ICES
Intraoperative Stereotactic Computed Tomography Guided Endoscopic Surgery
- ICH
intracerebral hemorrhage
- IQR
interquartile range
- IRB
Institutional Review Board
- MIS
minimally invasive surgery
- mRS
modified Rankin Score
- NIHSS
National Institutes of Health Stroke Scale
- NSICU
neuroscience intensive care unit
- PEG
percutaneous endoscopic gastrostomy
- SCUBA
Stereotactic ICH Underwater Blood Aspiration
- SD
standard deviation
Intracerebral hemorrhage (ICH) is a devastating neurological disease with few proven treatments.1,2 Despite numerous trials, there has yet to be a major improvement in clinical outcome. Recently, new operative techniques have been developed, including stereotactic thrombolysis (MISTIE), endoport-mediated trans-sulcal evacuation, endoscope-assisted evacuation, and endoscopic evacuation, all with varying operative nuances, advantages, and limitations.
Minimally Invasive Evacuation for ICH
Minimally invasive ICH evacuation techniques can be broadly categorized into 2 different treatment strategies: passive and active evacuation. Passive evacuation has been conducted by Chinese clinicians using the YL-1 puncture needle with urokinase, and “stereotactic thrombolysis” has been used by other practitioners using a ventricular catheter with alteplase, which was formally evaluated in the MISTIE trials.3 Active evacuation utilizes suction and attempts complete evacuation at the time of surgery. There is no drainage catheter left in place and no thrombolysis. Active evacuation can be performed using an endoport, a thin endoscope adjacent to a smaller profile sheath housing standard neurosurgical instruments, or endoscopic evacuation, which is performed using an adjunctive aspiration tool entirely through the endoscope through a low profile sheath.4-6
Endoscopic evacuation is a promising option because the low-profile working channel minimizes brain disruption. However, hematoma evacuation and hemostasis can be more challenging working entirely within the endoscope. This strategy requires a different set of skills and techniques than endoport-mediated and endoscope-assisted approaches.
Endoscopic Minimally Invasive Evacuation Techniques
Although one of the first randomized controlled trials evaluating minimally invasive ICH evacuation utilized endoscopic evacuation, there are few technical descriptions of endoscopic ICH evacuation.7 In this particular trial by Auer et al, evacuation was performed with pressurized irrigation through one channel of the endoscope and intermittent suction through the other. More recently, a form of endoscopic evacuation was evaluated in the Intraoperative Stereotactic Computed Tomography Guided Endoscopic Surgery (ICES) trial.8,9 In the ICES study, an endoscope sheath with a side port was introduced stereotactically two-thirds of the way into the clot along the long axis. Suction and irrigation of the hematoma were performed through the endoscopic sheath. The cavity was irrigated and bleeding was addressed with an endoscopic bipolar. The sheath was not manipulated to explore the cavity.
The development of the Apollo & Artemis devices (Penumbra Inc, Alameda, California) permits endoscopic evacuation with live visualization during aspiration. The device consists of a long metal tube 2.3 or 2.9 mm in diameter with a vibrating rod that prevents blood from clotting in the tube and can agitate tough blood clots. The second iteration of the device, Artemis, has the same dimensions, more powerful suction, and a recessed spinning bident to morcellate the clot rather than a vibrating rod. The Apollo and Artemis devices fit down the center port of the Storz Lotta 3-port endoscope and can be used simultaneously with irrigation through one of the endoscope side ports. Suction can be adjusted by a dial on the associated suction apparatus and is activated by covering a hole on the handle of the device, mirroring a common neurosurgical suction.
Working entirely through the endoscope with only the Apollo or Artemis device and the endoscopic cautery, there are unique challenges in evacuation that affect procedure time, evacuation percentage, and potentially functional outcome. While some clots are predominantly liquid and aspirate easily, many have fibrous components that require advanced techniques for evacuation. Some patients have no bleeding arteries while others have multiple, making visualization and cauterization challenging.
Our group has previously described our operative technique called Stereotactic ICH Underwater Blood Aspiration (SCUBA), which divides the endoscopic evacuation procedure into 2 phases (Figure 1 and Video, Supplemental Digital Content 1).10 First, the hematoma is aspirated with full suction and minimal irrigation. Second, when a majority of the clot has been debulked, irrigation is increased and suction is decreased to perform targeted aspiration and cauterization in the fluid-filled cavity. In the 79 patients with 80 clots reported in this study, near-complete clot evacuation was routinely achieved with an average evacuation rate of 88.7% overall. The following are the operative tenets and techniques developed over this experience.
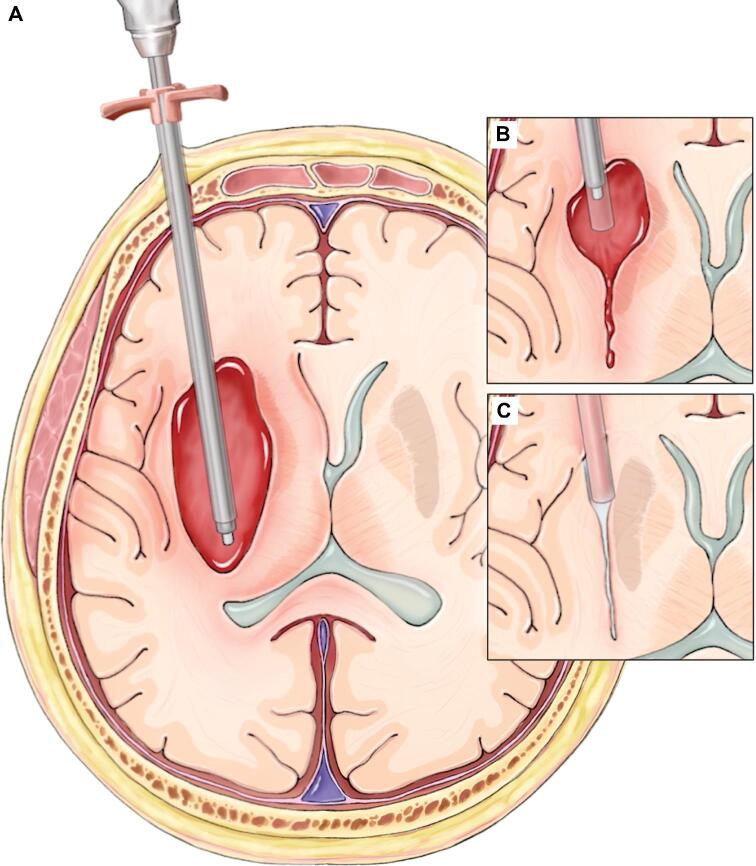
FIGURE 1.
Overview of the SCUBA procedure. A, At the start of the procedure, the sheath, endoscope, and Apollo device are deployed close to the distal aspect of the hematoma. B, The sheath is retracted as the hematoma is debulked and the cavity walls collapse. C, After the hematoma has been debulked, the sheath is withdrawn. Printed with permission from ©Mount Sinai Health System.
SCUBA Protocol
Endoscopic ICH evacuation using the SCUBA technique has been previously described. Eligibility criteria for this procedure were developed by a multidisciplinary group at a single health system based on eligibility criteria from published randomized clinical trials evaluating minimally invasive surgery (MIS) ICH evacuation. Retrospective analysis of clinical data was performed with approval of the Institutional Review Board (IRB) at our institution. A consent waiver was obtained from the IRB as risk was minimal, and many patients were no longer in the standard follow-up window or were lost to follow-up. These criteria have since evolved under the direction of the multidisciplinary group. The full inclusion and exclusion criteria are included here for reference (Appendix, Supplemental Digital Content 2).9 The inclusion criteria included hematoma volume ≥ 15 mL, National Institutes of Health Stroke Scale (NIHSS) ≥ 6, and baseline modified Rankin Score (mRS) ≤ 3. The protocol requires that vascular imaging, including computed tomographic angiography (CTA), magnetic resonance angiography, or angiogram, does not demonstrate an unsecured vascular lesion. Laboratory exclusion guidelines included platelet count ≤ 100 000 cells/mm3, international normalized ratio > 1.4, activated partial thromboplastin time > 36 s, or other documented coagulopathy. Patients with a correctable coagulation abnormality received appropriate reversal, and normal laboratory values were obtained prior to intervention. Patients with symptomatic hydrocephalus received ventriculostomy placement either prior to or during intervention. While a “stability scan” was required in the MISTIE trials, it is not an exclusion criterion for endoscopic ICH evacuation because this technique permits clear visualization and cauterization of intraoperative bleeding vessels.
OPERATIVE TENETS
Minimize Brain Manipulation
To minimize direct injury, the surgical trajectory is optimized for the sheath to pass through the shortest course absent of eloquent brain structures. For supratentorial ICH, the generally avoided entry zones are the supplementary motor area, primary motor cortex, sensory cortex, left-sided perisylvian cortex associated with language function, and primary visual cortex.
The trajectory should also align with the long axis of the hematoma to minimize the torque forces necessary to visualize the full cavity. The lever arm and torque are exponentially increased in relation to the orthogonal target area. Alignment with the long axis through the shortest possible distance not only improves visualization, but also helps to minimize indirect injury to adjacent structures along the access corridor.
Minimize Cavity Distortion
Just as manipulation of the access corridor can cause tissue injury, so too can distortion of the cavity walls. Because the Apollo/Artemis System (Penumbra) utilizes powerful suction, the negative pressure can cause cavity collapse, exposing the brain to potentially traumatic forces.
As a general rule to avoid cavity distortion, suction is maintained at the minimum setting whenever the tip of the device is advanced beyond the end of the working sheath. Suction force generally decreases throughout the procedure as more clot is aspirated and debulked, increasing the available space.
During the initial stage of evacuation, clot is aspirated under maximal suction. During this phase, the clot takes up the entire view in the endoscope and suction forces are applied directly to the clot, minimizing distortion of the cavity wall. Once the clot is brought into the sheath, suction strength is increased to firmly grip the clot. The suction device should be activated on high suction only when the tip is directly touching clot.
The second phase of the operation begins when sufficient clot has been aspirated and the cavity wall becomes visible. At this point, irrigation with lactated ringers is increased to high flow and suction is decreased. Small pieces of clot are aspirated or separated from the cavity wall using low suction.
The Lotta endoscope (Karl Storz, Germany) working channel allows for variable irrigation flow, which is essential for visibility. Constant irrigation helps to maintain slight distention of the cavity and continuous visualization of otherwise disappearing crevices. One of the side access ports is kept in the open position to promote irrigation egress and avoid pressurization and overdistension of the cavity that would otherwise cause collateral injury.
Obtain and Maintain Hemostasis
Effective hematoma evacuation can only be achieved if the risks of intraoperative hemorrhage reaccumulation and postoperative rebleeding can be mitigated. Obtaining and maintaining hemostasis is paramount to successful clot evacuation. It is important to survey all cavity walls for active bleeding throughout the procedure. Residual bleeding is controlled with irrigation or endoscopic bipolar cautery.
In the presence of active bleeding, high flow irrigation can be helpful. Irrigation can be directed towards a bleeding vessel by hovering the sheath immediately over the bleeding site. Bipolar cautery can then be applied to the bleeding vessel under direct visualization when the bleeding clears.
Check Your Work
It is important to utilize intraoperative imaging before completion of the procedure to ensure effective clot evacuation. Given the varied endoscopic appearance of formed blood clot, it can be difficult to differentiate between residual clot and brain tissue. In this series, intraoperative cone-beam CT is always performed after evacuation prior to closing. Ultrasound is also used when possible but does not supplant intraoperative CT.
OPERATIVE TECHNIQUES
Clot consistency is critical to the speed and efficacy of the procedure. It is not yet possible to anticipate the mechanical characteristics of a given hematoma prior to surgery. Adjustment of intraoperative technique based on clot consistency is crucial for successful evacuation, mirroring the tumor consistency-resection technique relationship.
ADAPT Technique
For hematomas with fibrous consistency, pieces may be too large to pass into the lumen of the aspiration device (2.6 mm). In this case, the tip of the aspiration device is placed directly onto the clot and using the minimal necessary suction, the piece of clot is drawn into the lumen of the sheath. Both the endoscope and aspiration device are then withdrawn together from the sheath with the hematoma fragment stuck on the aspiration device and remaining a fixed distance from the tip of the endoscope (Figure 2 and Videos, Supplemental Digital Content 3 and 4). This technique is referred to as the ADAPT technique because of the reliance on aspiration to remove a piece of blood clot corked on the end of the aspiration device, similar to “A Direct Aspiration First Pass Technique” used during thrombectomy for ischemic stroke.11
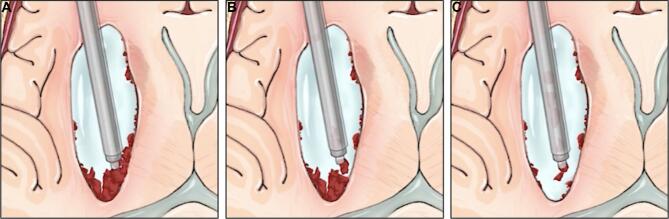
FIGURE 2.
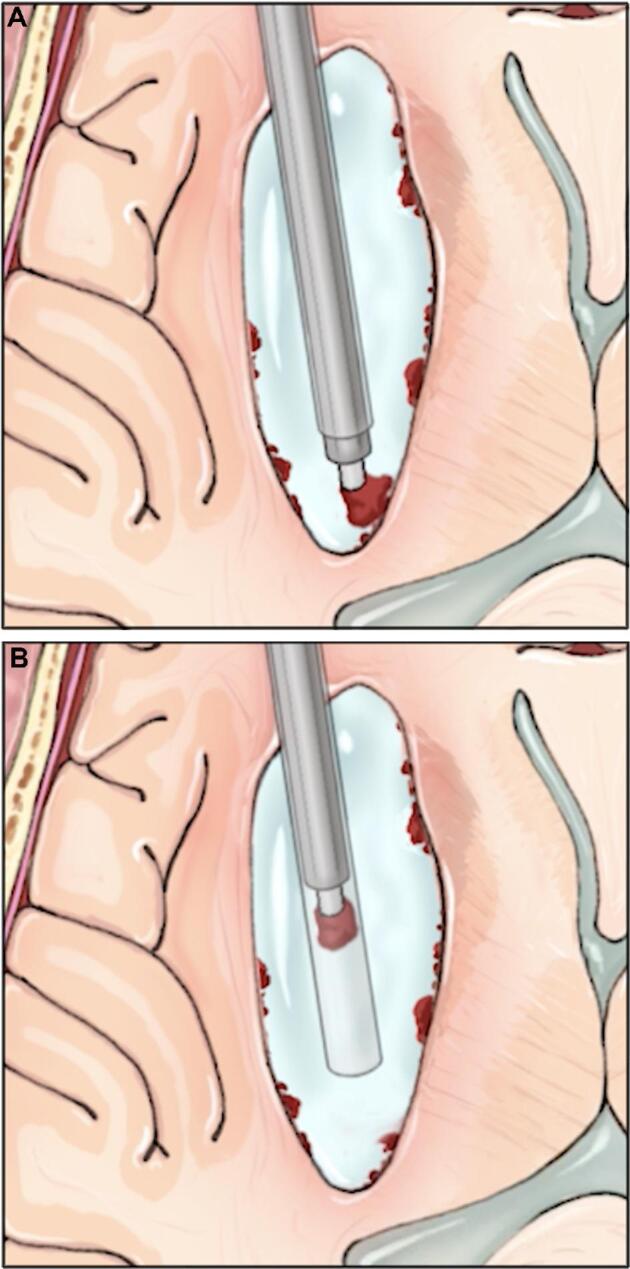
Overview of the ADAPT technique. A, The Apollo device is used to attach to a large piece of tenacious clot. B, The clot piece is brought within the sheath and the Apollo and endoscope are removed as one unit through the sheath with the clot piece in tow. Printed with permission from ©Mount Sinai Health System.
Sheath Dissection Technique
For hematomas with a fibrous capsule, it can be difficult to separate clot from the brain interface. We have found it helpful to use the sheath itself as a blunt dissector for particularly firm segments of capsule. Using the aspiration device, the capsule is drawn into the sheath lumen and dissected off the brain interface by either withdrawing or advancing the sheath. This truncating technique often frees large pieces of fibrous capsule that can be removed using the ADAPT technique (Figure 3 and Videos, Supplemental Digital Content 5 and 6).
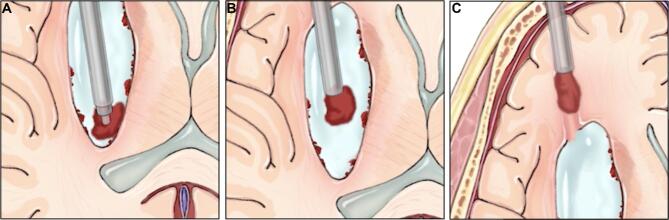
FIGURE 3.
Overview of the sheath dissection technique. A, The Apollo device is used to draw clot towards the sheath edge. B, Clot is dissected and freed along the clot edge. C, Freed clot fragments are evacuated through the Apollo device. Printed with permission from ©Mount Sinai Health System.
Double ADAPT Technique
For particularly large pieces of fibrous capsule, it can be helpful to utilize the working channel of the Lotta endoscope as an additional suction channel. This can be done by attaching a conventional surgical suction to the spare endoscope port (usually the irrigation overflow pathway). Using this maneuver, the large piece of clot can be drawn into the sheath under direct visualization with the suction device. If the clot is too large to fit into the sheath and cannot be morcellated, the entire system (aspiration device, endoscope, and sheath) can be removed gently as a single unit with the clot piece corked on the end of the sheath (Figure 4 and Videos, Supplemental Digital Content 7 and 8). If the piece is pulled off the end of the sheath for any reason during extraction, it is important to immediately stop suction from both sources to minimize exposure of the cavity or access tract to strong suction forces.
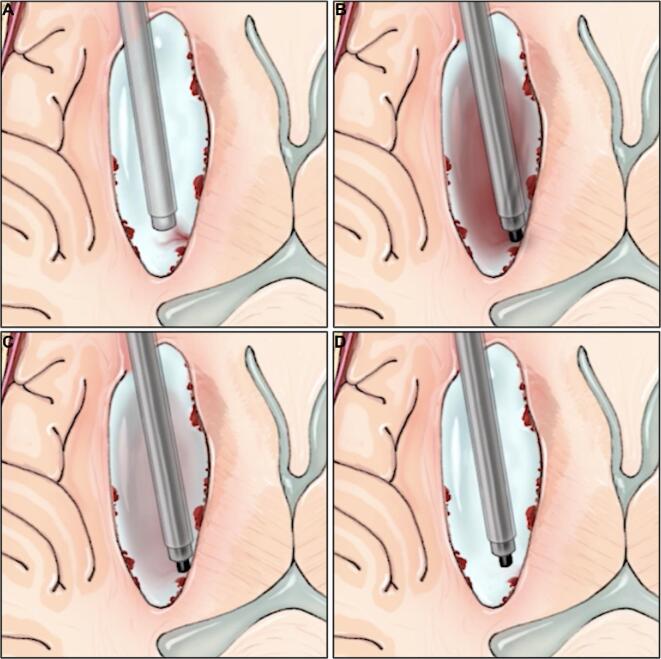
FIGURE 4.
Overview of the double ADAPT technique. A, The Apollo device is used to draw the large clot fragment into the sheath. B, Once the clot is well visualized within the sheath lumen, normal surgical suction is placed onto the second endoscope port, and the entire Apollo/endoscope/sheath apparatus is withdrawn. C, The large clot fragment is able to pass through the surgical corridor and is evacuated. Printed with permission from ©Mount Sinai Health System.
Sheath Hover Technique and Hemostasis
As described above, small bleeding vessels can be difficult to visualize as new blood obscures visualization within the cavity. The constant flow of irrigation can be directed towards a bleeding vessel by hovering immediately over it with the sheath and pulling the scope back from the tip. Bipolar cautery can then be applied to the bleeding area under direct visualization (Figure 5 and Videos, Supplemental Digital Content 9 and 10).
FIGURE 5.
Representation of the sheath hover technique. A, The sheath is hovered over the bleeding site with maximal irrigation to visualize the offending vessel. B, Bipolar cautery is advanced down the endoscope to the site of bleeding. C, Bipolary cautery is applied to the bleeding vessel. D, The surgical cavity is irrigated thoroughly to clear the blood. Printed with permission from ©Mount Sinai Health System.
RESULTS
A total of 79 ICH patients were treated with 80 SCUBA evacuations from March 2016 to May 2018. One patient had 2 hemorrhages which required 2 separate evacuations. Baseline patient characteristics are provided in Table 1. The mean preoperative NIHSS was 17.4. Preoperative active contrast extravasation (CTA spot sign) was observed in 15 patients (19.0%).
TABLE 1.
Baseline Patient Characteristics (n = 79)
| u or # | % or IQR | |
|---|---|---|
| Age (median) | 60 | 52-70 |
| Sex (male) | 52 | 65.8% |
| Preoperative hypertension | 63 | 79.7% |
| Preoperative anticoagulation | 9 | 11.4% |
| Preoperative antiplatelet use | 20 | 25.3% |
| Premorbid mRS = 0 | 63 | 79.7% |
| Glasgow Coma scale (GCS, median) | 9 | 7-13 |
| Intracerebral hemorrhage score (median) | 2 | 1-3 |
| NIHSS (median) | 17 | 12-22 |
| Subcortical location of hemorrhage | 50 | 63.3% |
| Intraventricular hemorrhage (IVH) | 34 | 43.0% |
| Spot sign on CTA | 15 | 19.0% |
The Apollo and Artemis devices (Penumbra) were used in 64 and 16 evacuations, respectively (Table 2). The ADAPT technique was used in 33 (40.7%) cases and the double ADAPT technique was used in 3 (3.7%) cases. The sheath hover technique was used in 35 (43.2%) cases (Table 2).
TABLE 2.
Operative Details (n = 80)
| Number | % | |
|---|---|---|
| Total evacuations | 80 | |
| Device used | ||
| Apollo | 64 | 79.0% |
| Artemis | 16 | 19.8% |
| Number of sheath passes | ||
| 1 | 47 | 58.0% |
| 2 | 24 | 29.6% |
| 3+ | 9 | 11.1% |
| ADAPT | ||
| Yes | 33 | 41.3% |
| No | 47 | 58.8% |
| Double ADAPT | ||
| Yes | 3 | 3.7% |
| No | 77 | 96.3% |
| Active bleeding encountered | 46 | 56.8% |
| Single vessel | 17 | 21.0% |
| Multiple vessels | 29 | 35.8% |
| Irrigation alone was used for hemostasis | 15 | 18.5% |
| Electrocautery applied | 35 | 43.2% |
| Symptomatic procedural hemorrhage | 0 | 0% |
| Asymptomatic procedural hemorrhage | 1 | 1.3% |
| Symptomatic rebleeding within 72 h | 0 | 0% |
| Asymptomatic rebleeding within 72 h | 3 | 3.8% |
| Symptomatic rebleeding within 3-30 d | 0 | 0% |
| Asymptomatic rebleeding within 3-30 d | 0 | 0% |
Operative data (eg, advanced techniques) for patients undergoing SCUBA evacuation.
The mean and median preoperative hematoma volumes were 49.5 mL (standard deviation [SD] 31.1 mL) and 38.8 mL, respectively. The mean and median postoperative volumes were 5.4 mL (SD 9.6 mL) and 1.0 mL, respectively. The average evacuation rate was 88.7% (SD 21.0%); 85% of evacuations had a residual hematoma volume of ≤15 mL (Table 3).
TABLE 3.
Procedural (Evacuation) Outcomes (n = 80)
| Mean or N | SD or % | |
|---|---|---|
| Preoperative volume (mL) | 49.5 | 31.1 |
| Postoperative volume (mL) | 5.4 | 9.6 |
| Evacuation percentage (%) | 88.7% | 21.0% |
| ≤15 mL residual hematoma | 68 | 85.0% |
The mean operative time for endoscopic SCUBA ICH evacuation was 119.2 min (Table 4).
TABLE 4.
Clinical Technical Outcomes (n = 79)
| mean or n | % or SD | |
|---|---|---|
| Ictus to evacuation (h) | 38.4 | 30.2 |
| Hospital admission to evacuation (h) | 28.3 | 28.9 |
| Ventriculostomy placement pre-SCUBA | 24 | 30.5% |
| SCUBA performed for herniation syndrome | 2 | 2.5% |
| SCUBA procedure time (min) | 120 | 68.3 |
| Enlarged craniectomy for hemostasis | 1 | 1.3% |
| Intraoperative or postoperative salvage hemicraniectomy | 0 | 0 |
| NSICU length of stay (d) | 10.5 | 7.7 |
| Hospital length of stay (d) | 25.6 | 30.5 |
| Surgical site infection | 1 | 1.3% |
| Tracheostomy placement | 18 | 22.8% |
| PEG placement | 28 | 35.4% |
| 30-d mortality | 7 | 8.9% |
SCUBA and perioperative ventriculostomy placement were effective in directly addressing intracranial pressure in this patient population, with only 1 patient (1.3%) requiring enlargement of the craniectomy to achieve hemostasis intraoperatively, no patients requiring a salvage hemicraniectomy, and 1 patient requiring postoperative placement of an external ventricular drain (Table 4). A total of 27 patients (34%) underwent SCUBA for urgent management of increased intracranial pressure or brain compression. Surgical site infection rate was 1.3% (1 patient). Eighteen patients (22.8%) required tracheostomy placement and 28 patients (35.4%) required percutaneous endoscopic gastrostomy (PEG) placement (Table 4). Thirty-day all-cause mortality was 8.9% (Table 4).
CASE EXAMPLES
The following case examples demonstrate the utility of the described techniques for endoscopic ICH evacuation.
Case 1
A 47-yr-old man with hypertension and hyperlipidemia presented with acute left hemiplegia with hypoesthesia (NIHSS 6). Head CT demonstrated an 18 mL hemorrhage in the right superior frontal lobe. CTA was negative. The patient was brought to the angiography suite for diagnostic angiography and ultra-early endoscopic ICH evacuation performed 6.5 h after ictus.
The patient was positioned supine on the angiography table and placed under general anesthesia. A single-vessel angiogram was negative for vascular malformation. A right frontal trajectory was planned with stereotactic guidance using the Medtronic StealthStation AxiEM system. A 1.5-cm right frontal incision was made 6 h after ictus. A 1-cm craniectomy was performed with a high-speed drill and the dura was opened. The endoscope was passed to the inferior posterior aspect of the hemorrhage. Clot consistency was predominantly fluid and aspirated without difficulty (Videos, Supplemental Digital Content 11 and 12). A bleeding vessel was encountered in the posteromedial aspect of the cavity. Visibility was achieved with the sheath hover technique, and the vessel was cauterized with bipolar cautery. A complete evacuation was achieved using the Apollo system; the total operative time was 47 min (Video, Supplemental Digital Content 13). Intraoperative cone-beam CT demonstrated near-complete evacuation.
The patient was transferred to the neuroscience intensive care unit (NSICU) and was able to lift and maintain his left leg off the bed (NIHSS 3) 1 h after extubation. The next morning, 12 h after ictus, he was walking with assistance and on postoperative day 1 CT demonstrated near-complete evacuation (Figure 6). He was discharged to acute rehabilitation on hospital day 5. By postbleed day 9, his NIHSS improved to 0.
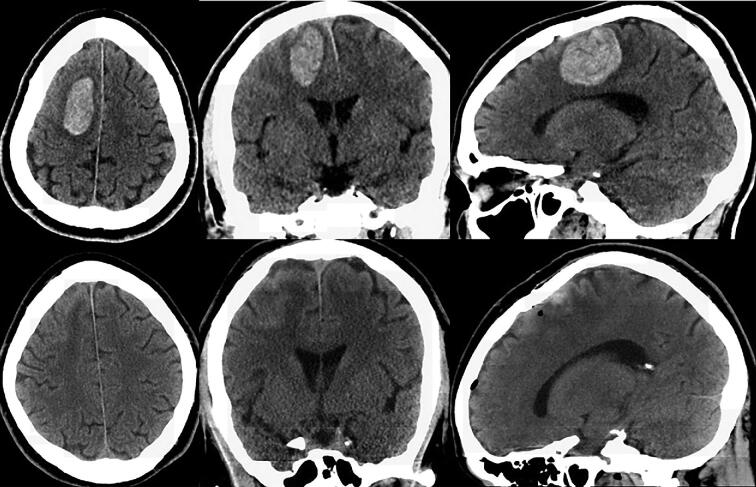
FIGURE 6.
Top: Preoperative axial, coronal, and sagittal CT demonstrating right frontal ICH. Bottom: Postoperative axial, coronal, and sagittal CT demonstrating 97% evacuation.
Case 2
A 57-yr-old woman with a history of hypertension, seizures, and 2 prior ICHs presented to a peripheral hospital with sudden right hemiparesis and aphasia following multiple days of worsening headaches, dizziness, confusion, and nausea (NIHSS 28). Head CT revealed a 38-mL left thalamotemporoparietal hemorrhage. CTA was negative. The patient was transferred to a central hospital 11 h after ictus for MIS ICH evacuation.
Repeat head CT upon arrival demonstrated stable hemorrhage and at 16 h after ictus the patient was brought to the angiography suite and positioned supine with the head turned 45 degrees to the right. Frameless stereotactic navigation was used to plan the trajectory along the long axis of the clot. A 1.5-cm vertical incision was made in the left temporal area. The temporalis muscle was incised and retracted to reveal the temporal bone. A 1-cm craniectomy was performed. A 19F sheath was passed under stereotactic guidance to a point 1 to 2 cm from the medial wall of the clot. The endoscope was inserted into the sheath with the Apollo device inside the working channel of the endoscope. Initially, thick hematoma filled the view and was easily aspirated with the Apollo device at maximal suction (Video, Supplemental Digital Content 14). As the hematoma was debulked, visibility improved and the sheath was re-positioned laterally to explore other parts of the cavity (Video, Supplemental Digital Content 15). More tenacious portions of clot required use of the ADAPT technique (Video, Supplemental Digital Content 16). Lastly, the cavity was evaluated for residual clot and bleeding vessels (Video, Supplemental Digital Content 17).
Postoperative CT demonstrated near-complete evacuation (Figure 7). The patient's NIHSS improved to 13 and she could speak in short sentences by postbleed day 10 when she was discharged to acute rehabilitation. At 6-mo follow-up, she had an mRS of 3 and an NIHSS of 5.
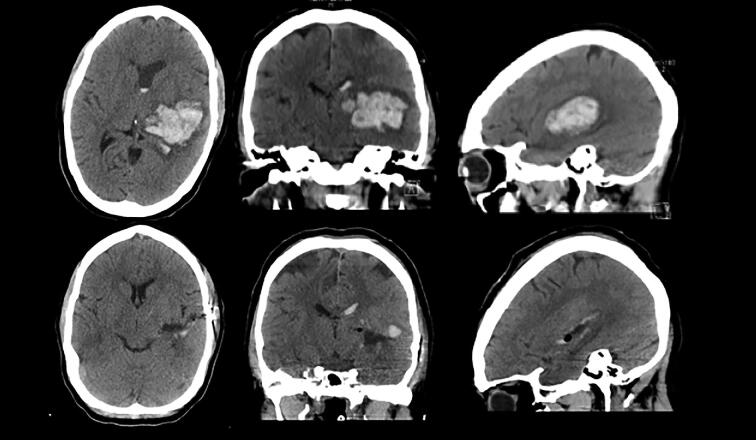
FIGURE 7.
Preoperative axial, coronal, and sagittal CT demonstrating left thalamotemporoparietal ICH. Bottom: Postoperative axial, coronal, and sagittal CT demonstrating 95% evacuation.
DISCUSSION
Minimally invasive ICH evacuation has become the focus of multiple clinical trials aimed at determining which patients benefit from minimally invasive clot removal.18 There currently exists numerous randomized clinical trials that have demonstrated a benefit for minimally invasive ICH evacuation using different techniques and it is from that experience that the inclusion and exclusion criteria for this clinical protocol were identified. The MISTIE III trial recently demonstrated that stereotactic thrombolysis did not result in improved functional outcome overall, although patients with ≤15 mL residual hematoma did appear to demonstrate improved functional outcome when compared to controls. These results suggest that technical success in removing the hematoma may result in improved functional outcome.
Numerous techniques have been described as “minimally invasive” and each has technical nuances, advantages, and disadvantages.12-17 Even endoscopic ICH evacuation has varied techniques ranging from that described in the ICES protocol in which the endoscope was introduced after evacuation was performed through the endoscopic sheath to evaluate and obtain hemostasis to the SCUBA procedure which relies heavily on endoscopic visualization throughout the entire procedure. As experience with endoscopic ICH evacuation grows, new techniques will emerge, requiring discussion and debate to advance the field. This practical, shared experience will maximize the chance of a positive clinical trial as neurosurgeons develop safer and more effective techniques.
In our experience of 80 minimally invasive ICH evacuations using the SCUBA technique, we have identified operative tenets and nuances to manage procedural challenges, some of which are not immediately obvious and others that require balancing operative objectives. The major tenets of our technique aim to minimize brain distortion along the access tract, decrease cavity distortion through elimination of errant suction forces, and reduce collateral bleeding. Other tenets include addressing intraoperative bleeding and utilizing intraoperative imaging. To accomplish our objectives, certain nuanced techniques have proven useful. In addition to the basic technique of the SCUBA procedure, these advanced techniques include the sheath dissection technique for fibrous clot dissection, the ADAPT and double ADAPT techniques for clot extraction, and the sheath hover technique for improved visibility when cauterizing a bleeding vessel.
CONCLUSION
The SCUBA technique, with the tenets and techniques presented here, serves as a viable option to safely and effectively evacuate supratentorial ICH. Further development of this and other techniques will advance the field of MIS for ICH evacuation. Discussion of specific operative tenets and techniques is essential to the success of the procedure. Clinical trials are underway to evaluate the clinical benefit of these strategies.
Disclosures
This research was supported in part by a grant from Arminio and Lucyna Fraga and by a grant from Mr and Mrs Durkovic. Dr Kellner is the director of a CME course titled Endoscopic Minimally Invasive Intracerebral Hemorrhage Evacuation funded by Penumbra and is the site principle investigator for the INVEST and MIND studies, both funded by Penumbra. Dr Mocco is the principal investigator of the INVEST trial funded by Penumbra and has financial interest in Rebound Therapeutics. The authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article.
Supplementary Material
Acknowledgments
All figures and animations created by Jill Gregory and Amy Zhong at the Icahn School of Medicine at Mount Sinai and reproduced with permission. This research was supported in part by a grant from Arminio and Lucyna Fraga and by a grant from Mr and Mrs Durkovic.
Contributor Information
Robert J Rothrock, Department of Neurosurgery, Icahn School of Medicine at Mount Sinai, New York, New York.
Alexander G Chartrain, Department of Neurosurgery, Icahn School of Medicine at Mount Sinai, New York, New York.
Jacopo Scaggiante, Department of Neurosurgery, Icahn School of Medicine at Mount Sinai, New York, New York.
Jonathan Pan, Department of Neurosurgery, Icahn School of Medicine at Mount Sinai, New York, New York.
Rui Song, Department of Neurosurgery, Icahn School of Medicine at Mount Sinai, New York, New York.
Danny Hom, Department of Neurosurgery, Icahn School of Medicine at Mount Sinai, New York, New York.
Adam C Lieber, Department of Neurosurgery, Icahn School of Medicine at Mount Sinai, New York, New York.
Joshua B Bederson, Department of Neurosurgery, Icahn School of Medicine at Mount Sinai, New York, New York.
J Mocco, Department of Neurosurgery, Icahn School of Medicine at Mount Sinai, New York, New York.
Christopher P Kellner, Department of Neurosurgery, Icahn School of Medicine at Mount Sinai, New York, New York.
Supplemental Digital Content 1. Video. Animation of overview of the SCUBA procedure. The sheath, endoscope, and Apollo device are deployed two-thirds into hematoma. The system is pulled back as the clot is evacuated, resulting in complete hematoma evacuation.
Supplemental Digital Content 2. Appendix. Inclusion and exclusion guidelines for endoscopic ICH evacuation performed with the Stereotactic ICH Underwater Blood Aspiration (SCUBA).
Supplemental Digital Content 3. Video. Animation of the ADAPT technique. The Apollo device is used to attach to a large piece of tenacious clot. The clot piece is brought within the sheath, and the Apollo and endoscope are removed as one unit through the sheath with the clot piece in tow.
Supplemental Digital Content 4. Video. Video of the ADAPT technique. A tenacious clot fragment is pulled into the lumen of the sheath using the Apollo device. Under direct visualization, the clot is withdrawn as one unit with the Apollo device and endoscope.
Supplemental Digital Content 5. Video. Animation of the sheath dissection technique. The Apollo device is used to draw clot towards the sheath edge. Clot is dissected and freed along the clot edge and freed clot fragments are evacuated through the Apollo device.
Supplemental Digital Content 6. Video. Video of the sheath dissection Technique. The sheath is manipulated to visualize and free different local areas of tenacious clot to facilitate evacuation.
Supplemental Digital Content 7. Video. Animation of the double ADAPT technique. The Apollo device is used to draw a large clot fragment into the sheath. Once the clot is well visualized within the sheath lumen, normal surgical suction is placed onto the second endoscope port, and the entire Apollo/endoscope/sheath apparatus is withdrawn. The large clot fragment is able to pass through the surgical corridor and is evacuated.
Supplemental Digital Content 8. Video. Video of the double ADAPT technique. A large clot fragment is brought into the sheath. Off screen, normal surgical suction is placed onto the second endoscope port, and the entire Apollo/endoscope/sheath apparatus is withdrawn. The large clot fragment is removed under direct visualization.
Supplemental Digital Content 9. Video. Animation of the sheath hover technique. The sheath is brought to the bleeding site with maximal irrigation to visualize the offending vessel. The bipolar cautery is advanced down the endoscope to the site of bleeding and applied to the bleeding vessel. The surgical cavity is irrigated thoroughly to clear the blood.
Supplemental Digital Content 10. Video. Video of the sheath hover technique. The sheath is brought to the bleeding site with maximal irrigation to visualize the offending vessel. The bipolar cautery is advanced down the endoscope to the site of bleeding and applied to the bleeding vessel.
Supplemental Digital Content 11. Video. Case 1: Aspiration. Liquid and solid clot are aspirated into the Apollo device.
Supplemental Digital Content 12. Video. Case 1: Lateral exploration and aspiration. The cavity is laterally explored by repositioning the sheath and any residual clot is aspirated.
Supplemental Digital Content 13. Video. Case 1: Cavity confirmation. At the end of the procedure, the cavity is thoroughly examined for residual clot and bleeding vessels.
Supplemental Digital Content 14. Video. Case 2: Aspiration. Liquid and solid clot are aspirated into the Apollo device.
Supplemental Digital Content 15. Video. Case 2: Lateral exploration and aspiration. The cavity is laterally explored by repositioning the sheath and any residual clot is aspirated.
Supplemental Digital Content 16. Video. Case 2: ADAPT technique. The Apollo device suctions onto a tenacious fragment of clot and is pulled into the lumen of the sheath. The endoscope, Apollo device, and clot are then pulled out together.
Supplemental Digital Content 17. Video. Case 2 Cavity Confirmation. At the end of the procedure, the cavity is thoroughly examined for residual clot and bleeding vessels.
REFERENCES
- 1. Mendelow A, Gregson B, Fernandes Het al.. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial intracerebral haematomas in the International Surgical Trial in Intracerebral Haemorrhage (STICH): a randomised trial. Lancet. 2005;365(9457):387-397. [DOI] [PubMed] [Google Scholar]
- 2. Mendelow AD, Gregson BA, Rowan ENet al.. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial lobar intracerebral haematomas (STICH II): a randomised trial. Lancet. 2013;382(9890):397-408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hanley DF, Thompson RE, Rosenblum M, Yenokyan G, Lane K. Nichol McBee and the MISTIE trial investigators. Efficacy and safety of minimally invasive surgery with thrombolysis in intracerebral haemorrhage evacuation (MISTIE III): a randomised, controlled, open-label, blinded endpoint phase 3 trial. Lancet. 2019;393(10175):1021-1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mould WA, Carhuapoma JR, Muschelli Jet al.. Minimally invasive surgery plus recombinant tissue-type plasminogen activator for intracerebral hemorrhage evacuation decreases perihematomal edema. Stroke. 2013;44(3):627-634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bauer AM, Rasmussen PA, Bain MD. Initial single-center technical experience with the BrainPath system for acute intracerebral hemorrhage evacuation. Oper Neurosurg. 2017;13(1):69-76. [DOI] [PubMed] [Google Scholar]
- 6. Fiorella D, Gutman F, Woo H, Arthur A, Aranguren R, Davis R. Minimally invasive evacuation of parenchymal and ventricular hemorrhage using the Apollo system with simultaneous neuronavigation, neuroendoscopy and active monitoring with cone beam CT. J Neurointerv Surg. 2015;7(10):752-757. [DOI] [PubMed] [Google Scholar]
- 7. Auer LM, Deinsberger W, Niederkorn Ket al.. Endoscopic surgery versus medical treatment for spontaneous intracerebral hematoma: a randomized study. J Neurosurg. 1989;70(4):530-535. [DOI] [PubMed] [Google Scholar]
- 8. Vespa P, Hanley D, Betz Jet al.. ICES (intraoperative stereotactic computed tomography-guided endoscopic surgery) for brain hemorrhage. Stroke, 2016;47(11):2749-2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Brain Injury Outcomes (BIOS), Johns Hopkins University . MISTIE Surgical Training Module. http://braininjuryoutcomes.com/emissary-college/mistie-training/141. Accessed August 24, 2017. [Google Scholar]
- 10. Kellner CP, Chartrain AG, Nistal DAet al.. The Stereotactic Intracerebral Hemorrhage Underwater Blood Aspiration (SCUBA) technique for minimally invasive endoscopic intracerebral hemorrhage evacuation. J Neurointerv Surg. 2018;10(8):neurintsurg-2017-013719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Turk AS, Spiotta A, Frei Det al.. Initial clinical experience with the ADAPT technique: a direct aspiration first pass technique for stroke thrombectomy. J Neurointervent Surg. 2014;6(3):231-237. [DOI] [PubMed] [Google Scholar]
- 12. Fam MD, Hanley D, Stadnik Aet al.. Surgical performance in minimally invasive surgery plus recombinant tissue plasminogen activator for intracerebral hemorrhage evacuation phase III clinical trial. Neurosurgery, 2017;81(5):860-866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wang W-Z, Jiang B, Liu H-Met al.. Minimally invasive craniopuncture therapy vs. conservative treatment for spontaneous intracerebral hemorrhage: results from a randomized clinical trial in China. Int J Stroke. 2009;4(1):11-16. [DOI] [PubMed] [Google Scholar]
- 14. Ding D, Przybylowski CJ, Starke RMet al.. A minimally invasive anterior skull base approach for evacuation of a basal ganglia hemorrhage. J Clin Neurosci. 2015;22(11):1816-1819. [DOI] [PubMed] [Google Scholar]
- 15. Przybylowski CJ, Ding D, Starke RM, Webster Crowley R, Liu KC. Endoport-assisted surgery for the management of spontaneous intracerebral hemorrhage. J Clin Neurosci. 2015;22(11):1727-1732. [DOI] [PubMed] [Google Scholar]
- 16. Nagasaka T, Tsugeno M, Ikeda H, Okamoto T, Inao S, Wakabayashi T. A novel monoshaft bipolar cautery for use in endoscopic intracranial surgery. A short technical note. Clin Neurol Neurosurg. 2011;113(8):607-611. [DOI] [PubMed] [Google Scholar]
- 17. Nagasaka T, Tsugeno M, Ikeda Het al.. Balanced irrigation-suction technique with a multifunctional suction cannula and its application for intraoperative hemorrhage in endoscopic evacuation of intracerebral hematomas: technical note. Neurosurgery. 2009;65(4):E826-E827; discussion E827. [DOI] [PubMed] [Google Scholar]
- 18. Scaggiante J, Zhang X, Mocco J, Kellner CP. Minimally invasive surgery for intracerebral hemorrhage. Stroke. 2018;49(11):2612-2620. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.