Summary
Calonectria hemileiae, a fungus associated with pustules of the coffee leaf rust (CLR, Hemileia vastatrix) in Brazil, was tested in vitro and in planta to assess its biocontrol potential. The fungus inhibited the germination of rust spores by over 80%. CLR severity was reduced by 93% when Calonectria was applied to coffee leaf discs inoculated with H. vastatrix, whilst a reduction of 70-90% was obtained for in planta experiments. Mycoparasitism was demonstrated through the fulfillment of Koch's postulates. Elucidation of the biochemical interaction between Calonectria and Hemileia on coffee plants indicated that the mycoparasite was able to increase plant resistance to rust infection. Coffee plants sprayed with Calonectria alone showed greater levels of chitinase, β-1,3-glucanase, ascorbate peroxidase and peroxidase. Although effective in controlling the rust, fungicide applications damaged coffee photosynthesis, whereas no harm was caused by Calonectria. We conclude that C. hemileiae shows promise as a biocontrol agent of CLR.
Subject Areas: Biological Sciences, Plant Biology, Plant Pathology
Graphical abstract
Highlights
-
•
Calonectria hemileiae was proven to be an effective mycoparasite of coffee leaf rust
-
•
Calonectria hemileiae reduced significantly the germination and growth of rust spores
-
•
Rust severity on coffee reduced by 70-90% by Calonectria application
-
•
Photosynthetic capacity of coffee unharmed by Calonectria but reduced by fungicides
Biological Sciences ; Plant Biology ; Plant Pathology
Introduction
Brazil is the largest coffee producer and exporter in the world (MAPA, 2018). Coffee is one of the top global commodities, generating around 90 billion US dollars a year (Batista et al., 2012; ICO, 2016). Only two species of the genus Coffea are of economic importance: Coffea arabica (Arabica coffee) and Coffea canephora (Robusta coffee or Conilon), representing 66 and 34%, respectively, of commercially planted coffee (Somarriba et al., 2004). Despite its economic and social relevance, coffee cultivation has always been threatened by negative abiotic and biotic factors, especially drought and fungal diseases, both of which can drastically decrease yields (Rodrigues-Junior, 1990; Menezes-Silva et al., 2017).
The most devastating disease affecting the crop is coffee leaf rust (CLR), caused by Hemileia vastatrix (, Mikronegeriaceae, Pucciniales; McTaggart et al., 2016). At high incidence, CLR can cause defoliation of up to 50% and yield losses between 30 and 50% (Bhat et al., 2000; Capucho et al., 2013a; Zambolim, 2016), whilst economic losses have been estimated at between t 1-2 billion US dollars annually (Talhinhas et al., 2017).
CLR control is based on the use of resistant varieties, the application of contact and systemic fungicides (Zambolim, 2016), as well as disease escape by establishing highland plantations (McCook, 2006). However, there are limitations to each of these approaches. High H. vastatrix variability and the emergence of new races of the rust, as well as the occurrence of a complex of races, challenges the establishment of durable resistance in this crop (Varzea and Marques, 2006; Cabral et al., 2009). The use of fungicides, although effective for the control of CLR (Capucho et al., 2013b), is costly and may be impractical for the challenging terrain of upland plantations. It is also rejected as an option—particularly in the case of systemic fungicides—for the high-value organic coffee market. Additionally, the continuous and repetitive use of systemic fungicides may promote the selection of resistant populations of the rust. Although there is no evidence of fungicide-resistant strains of H. vastatrix, there are records of such events for other rust fungi, such as Phakopsora pachyrhizi—soybean rust (Godoy, 2012), and as chemical control of CLR presently relies on products belonging to two chemical groups only (azoles and strobilurins), there is the added danger of loss of efficiency for these products. Regular application of broad-spectrum fungicides also brings the threat of environmental impact and may, for instance, harm populations of beneficial organisms, including bacterial and fungal antagonists of H. vastatrix (Capucho et al., 2013b; Honorato et al., 2015a).
A series of alternative control strategies for CLR are reported in the literature, such as the combination of cultivation under shade and adequate nitrogen fertilization, as well as the use of resistant varieties (Toniutti et al., 2017). Although it has been suggested that there is an untapped potential in biological control for CLR management, published results of studies aimed at rust control are still relatively few and concentrated on the use of antagonistic bacteria, such as Bacillus thuringiensis, B. subtilis, and Pseudomonas putida to be deployed as biopesticides (Shiomi et al., 2006; Mejía, 2015). Examples of promising results with potential bacterial products for control of CLR have been reported (Bettiol et al., 1994; Cristancho, 1995; Costa et al., 2007; Daivasikamani and Rajanaika, 2009; Haddad et al., 2009, 2013, 2014). As compared with bacteria, fungi antagonistic to H. vastatrix have been poorly investigated.
The only publication of a systematic survey for mycoparasites of H. vastatrix was that of Carrión and Rico-Gray (2002) undertaken in the Mexican state of Veracruz. James et al. (2016) used single-molecule DNA sequencing for evaluating the diversity of fungal communities associated with CLR lesions collected from coffee leaves in Mexico and Puerto Rico. These authors found 69 taxonomic units (putative species), 15 of which were interpreted to be mycoparasitic fungal species belonging to the Cordycipitaceae (Ascomycota) and the Tremellales (Basidiomycota).
No surveys for fungal antagonists of H. vastatrix have been conducted in Brazil or Africa until now, and even the ubiquitous “white colony-forming fungi” found on rust pustules have generally been assigned to Lecanicillium (now Akanthomyces) lecanii without careful examination; missing a significant diversity of these moniliaceous fungi, as well as of other fungal groups (Barreto et al., 2015). A project was initiated in 2014, sponsored by World Coffee Research (WCR, 2020), aimed at surveying the natural enemies (fungi) of H. vastatrix associated with the genus Coffea in its African cent of origin, as well as in Brazil. Surveys were undertaken in Africa in collaboration with local scientists and yielded 1516 isolates; representing a broad diversity of fungi, arbitrarily treated as belonging to two ecological groups: (i) endophytic mutualists growing inside coffee plant tissues (potentially serving as fungal bodyguards); and (ii) rust pustule colonizers (purported mycoparasites). Their potential for use in biological control is currently under evaluation for selected isolates. Although coffee and the CLR fungus are non-native in Brazil, the brief survey conducted in the Brazilian coffee-growing areas produced an unexpectedly large diversity of mycoparasites. One such CLR pustule-colonizing fungus among those which were encountered belonged to Calonectria—a genus not known to include mycoparasitic species—which was found to be new to science and described as Calonectria hemileiae S.S. Salcedo, A.A. Colmán, H.C. Evans, and R.W. Barreto, in Crous et al. (2018). During a preliminary screening for potential biocontrol candidates, C. hemileiae was shortlisted for more detailed evaluation. Given the promising preliminary results obtained with of C. hemileiae as an anti-CLR treatment, the present study aimed at further testing the hypotheses that: (a) C. hemileiae is a mycoparasite of H. vastatrix; (b) C. hemileiae can reduce the severity of CLR; (c) C. hemileiae does not interfere with the photosynthetic capacity of the plants; (d) C. hemileiae can induce disease resistance. These were tested through a series of experiments described herein. All experiments involved the type strain of C. hemileiae (COAD 2544), identified here by our original collecting code AC121.
Results
Effect of Calonectria hemileiae on the germination of urediniospores of Hemileia vastatrix
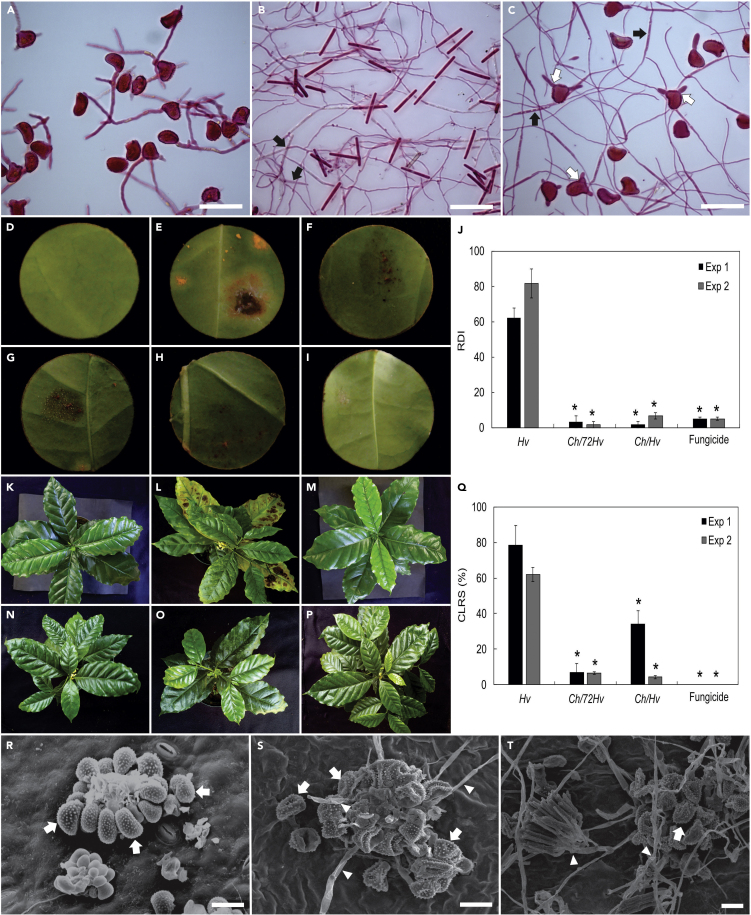
Germination of urediniospores ranged from 60 to 75% for the control treatment (Figure 1A). When mixed with conidial suspensions of C. hemileiae (Figure 1B), germination dropped to between 0 and 20% (Figure 1C). Therefore, inhibition of germination of urediniospores of 80-100% resulted from exposure to C. hemileiae (Figure 1C). The few urediniospores that germinated when exposed to C. hemileiae, were rapidly overgrown by the mycoparasite germ tubes and prevented to further develop. In addition, the germ tubes emerging from urediniospores were abnormal—short and inflated (Figure 1C).
Figure 1.
In vitro and in planta evidence of Calonectria hemileiae antagonistic/biocontrol effect on Hemileia vastatrix.
Urediniospores of H. vastatrix suspended in sterile distilled water (SDW) and germinating after 6 hr incubation in vitro (A, bar 50 μm). Conidia of C. hemileiae suspended in sterile distilled water (SDW) and forming germ tubes and branching – note germinating conidia of C. hemileiae (black arrows) (B, bar 50 μm). C. hemileiae (Ch) in combined suspension in SDW with urediniospores of H. vastatrix—note germinating conidia of C. hemileiae (black arrows) and short and inflated germ tubes emerging from urediniospores (white arrows) (C, bar 50 μm). Coffee leaf discs (D to I) and plants (K to P) from experiments that involved C. hemileiae applications at 72 hr before (G and N) or simultaneously (H and O) with inoculation of H. vastatrix (E and L). Effect of fungicides (trifloxystrobin + tebuconazol) (I and P) on H. vastatrix. Leaf discs and coffee plants sprayed with sterile water (D and K) and C. hemileiae (F and M) without inoculation with H. vastatrix used as controls. Rust disease index (RDI) (J) and coffee leaf rust severity (CLRS) (Q) for coffee plants submitted to different treatments. Asterisks (∗) indicates significant difference (p ≤ 0.05) by the Dunnet-test. Bars represent the standard deviations. n = 3 and 5, respectively, for experiments involving leaf discs and coffee plants. Scanning electron microscopy micrographs showing a healthy pustule of H. vastatrix producing a group of urediniospores (arrowed) on the leaf surface (R, bar 20 μm) and a pustule of H. vastatrix overgown by C. hemileiae, showing collapsing urediniospores (arrowed) (S and T, bar 20 μm) 20 days after spraying with a C. hemileiae conidial suspension.
Interactions of Calonectria hemileiae and Hemileia vastatrix on coffee in vitro and in planta
Based on the in vitro tests (two experiments), the rust disease index (RDI) was significantly reduced by 96, 93 and 96%, respectively, for Ch/72Hv, Ch/Hv, and fungicide treatments when compared to plants that received the rust only (Figure 1J). The presence of pustules and necrotic leaf tissue in the leaf discs treated with the rust alone was evident at 40 days after inoculation (dai) (Figure 1E). Conversely, leaf discs from the Ch/72Hv, Ch/Hv, and fungicide treatments showed none or only a few rust pustules (Figures 1G, 1H, and 1I). No visible harm was observed on the leaf discs treated with C. hemileiae alone (Figure 1F). When compared with healthy untreated controls [discs sprayed with sterile distilled water (SDW) only], no abnormalities were observed on leaves treated with C. hemileiae conidial suspension only (Figures 1D and 1F).
Preliminary in vitro results obtained for biocontrol of H. vastatrix with C. hemileiae were highly significant and suggested a high potential for CLR management. To further evaluate C. hemileiae in planta, experiments under controlled conditions were conducted (as described in the supplemental file ). Significant reductions in CLR severity were also obtained with reductions of 91 and 73%, respectively, for Ch/72Hv and Ch/Hv treatments (Figure 1Q). Control of CLR was complete for fungicide treatment (Figure 1P). Plants inoculated with the rust and treated with C. hemileiae (Ch/72Hv and Ch/Hv treatments) showed reduced CLR severity in comparison to inoculated plants that remained untreated with Ch (Figures 1N, 1O, and 1L). Inoculated plants that were sprayed with the fungicide did not develop any CLR symptoms or exhibited only minor damage (Figure 1P). Likewise, no damage appeared on the control plants and plants sprayed with C. hemileiae (Figures 1K and 1M).
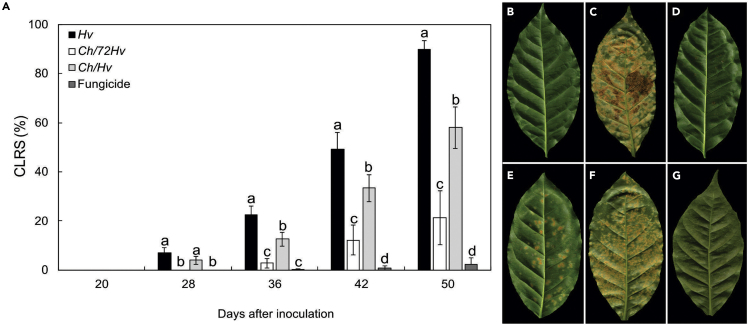
The in planta experiments further confirmed low levels of CLR severity in the disease progress evaluation for plants treated with the fungicide. Fungicide application reduced disease severity by 90% as compared with the untreated control (Figures 2A, 2B, and 2G). Nevertheless, significant reductions of coffee leaf rust severity (CLRS) were also achieved for Ch/72Hv. When compared with the rust treatment, Ch/72Hv caused reductions of CLRS of 100, 87, 75, and 76% at 28, 36, 42, and 50 dai, respectively (Figures 2A, 2C, and 2E). The Ch/Hv treatment was not as effective as Ch/72Hv for CLR control. In this case, the Ch/Hv treatment led to reductions of CLRS at 28, 36, 42, and 50 dai of 43, 44, 32, and 36%, respectively, as compared with the rust treatment (Figures 2A, 2C, and 2F). Again, no damage to coffee plants was observed as a result of the C. hemileiae applications (Figure 2D).
Figure 2.
Calonectria hemileiae reduced coffee leaf rust severity (CLRS) on coffee plants inoculated with Hemileia vastatrix.
CLRS of coffee plants inoculated with H. vastatrix at 20, 28, 36, 42, and 50 days after inoculation. Treatments were, respectively: inoculation with H. vastatrix and spraying of plants with antagonist (C. hemileiae) 72 h before (Ch/72Hv) or simultaneously (Ch/Hv) with the inoculation with H. vastatrix (Hv) and treated with fungicide (A). Abaxial surface of leaves represnting: control (B), Hv (C), Ch only (D), Ch/72Hv (E), Ch/Hv (F), and fungicide treatment (trifloxystrobin + tebuconazol) (G). Pictures taken 50 days after inoculation with H. vastatrix took place. Treatment means followed by the same letter, for each sampling time, are not significantly different (p ≤ 0.05) as determined by Tukey's test. Bars represent the standard deviation. n = 5.
Evidence of mycoparasitism
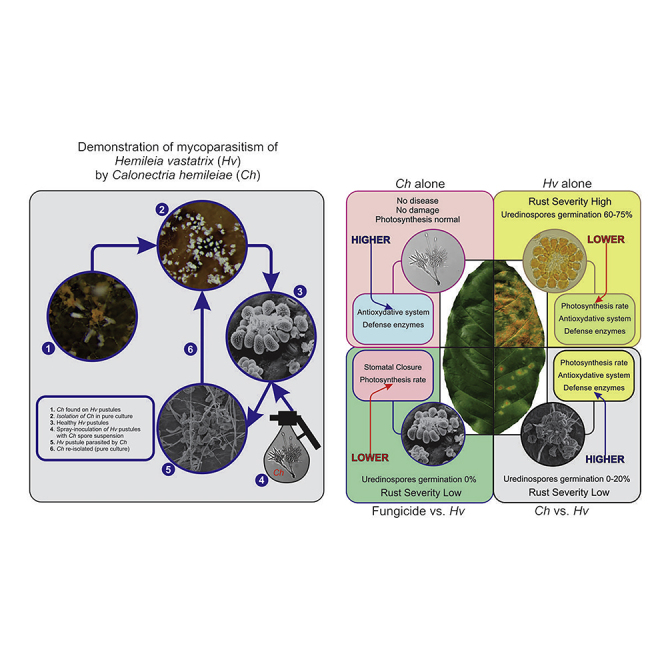
Coffee plants sprayed with rust urediniospores (Figure 1R), which were also treated with Calonectria hemileiae, exhibited CLR symptoms 30 dai. Typical C. hemileiae colonies were observed growing over the abnormal rust pustules, similarly to those seen in the type material under SEM. Colonization of rust pustules by C. hemileiae led to general disorganization of the uredinia with the collapse of sporogenous fascicles, and disruption of sporogenesis (Figures 1S and 1T). The presence of typical structures of C. hemileiae on diseased CLR pustules was confirmed following microscopic examination. This corresponds to the third step of Koch's postulates. Finally, isolations made from mycoparasitized colonies resulted in pure, sporulating colonies, which were identified as C. hemileiae—thereby, fulfilling the fourth step of Koch's postulates and demonstrating that C. hemileiae is a mycoparasite of CLR.
Interactions of Calonectria hemileiae, fungicide mixture and Hemileia vastatrix: effects on enzymatic activity in coffee leaves
At 72 h after spraying the coffee plants with C. hemileiae, the chitinase (CHI), β-1,3-glucanase (GLU), peroxidase (POX), and superoxide dismutase (SOD) activities were significantly different among the treatments. Plants treated with C. hemileiae (Ch and Ch/72Hv treatments) showed a CHI activity 5-times higher than that of the controls. GLU activity had a greater increase in plants treated with C. hemileiae as compared with the controls. APX activity was not significant for all treatments (Table 1). There were significant differences between treatments at 72 h after inoculation (hai) for CHI, GLU, and SOD activities. Meanwhile, at 50 dai, significant differences were detected in the CHI, GLU, APX, POX, and SOD activities (Table 1). At 72 hai, CHI and SOD activities were greater for the Ch/72Hv treatment as compared with other treatments. Significant reductions in the GLU activity were found for treatments involving the application of C. hemileiae, as well as for plants treated with fungicide, in comparison with the rust treatment and the control. SOD activity on plants inoculated with the rust, showed a significant reduction when compared with Ch/72Hv and Ch/Hv treatments (Table 1). At 50 dai, plants sprayed with C. hemileiae (Ch treatment) showed higher CHI, APX, and POX activities in comparison to the other treatments. Conversely, the SOD activity was higher in the Ch/72Hv and fungicide treatments as compared with other treatments, whilst plants inoculated with the rust (Hv treatment) presented a high GLU activity as compared with other treatments.
Table 1.
Defense and antioxidative response in coffee leaves sprayed with Calonectria hemileiae and inoculated with Hemileia vastatrix
| Treatments | 72 hbi |
72 hai |
50 dai |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CHI | GLU | APX | POX | SOD | CHI | GLU | APX | POX | SOD | CHI | GLU | APX | POX | SOD | |
| Control | 2.8b | 3.1b | 15.1a | 0.10b | 1.6a | 5.2ab | 5.7a | 19.6a | 0.2a | 1.8ab | 5.4ab | 1.6b | 26.8b | 0.3ab | 0.6b |
| Hv | – | – | – | – | – | 5.0ab | 4.7a | 21.9a | 0.2a | 0.7b | 7.3ab | 5.0a | 28.2b | 0.2b | 0.6b |
| Ch | 12.7a | 8.6a | 22.2a | 0.07b | 1.5a | 2.2c | 1.4b | 22.2a | 0.2a | 1.5ab | 9.7a | 2.3b | 49.4a | 0.4a | 0.6b |
| Ch/72Hv | 10.7a | 5.4ab | 29.1a | 0.21a | 0.8b | 6.7a | 2.6ab | 24.4a | 0.2a | 2.1a | 2.0b | 0.9b | 2.6c | 0.2ab | 1.4a |
| Ch/Hv | – | – | – | – | – | 4.5abc | 2.6ab | 27.9a | 0.3a | 2.0a | 5.1ab | 2.3b | 29.9b | 0.2b | 0.5b |
| Fungicide | – | – | – | – | – | 2.5bc | 2.7ab | 24.1a | 0.2a | 1.6ab | 4.1ab | 0.4b | 17.4bc | 0.2ab | 1.6a |
| F Values | 26.8∗∗ | 4.56∗ | 2.43ns | 33.6∗∗ | 15.4∗∗ | 6.95∗ | 4.52∗ | 1.47ns | 1.53ns | 3.76∗ | 3.66∗ | 10.9∗∗ | 17.1∗∗ | 3.69∗ | 8.90∗∗ |
Activities of chitinase (CHI, μmol min−1 mg−1 protein), β-1,3-glucanase (GLU, μmol min−1 mg−1 protein), ascorbate peroxidase (APX, μmol min−1 mg−1 protein), peroxidase (POX, μmol min−1 mg−1 protein), and superoxide dismutase (SOD, unit min−1 mg−1 protein) in the leaves of coffee plants that were treated with C. hemileiae at 72 h before as well as at 72 h and 50 days after inoculation with H. vastatrix and coffee plants that were treated with fungicides (trifloxystrobin + tebuconazol). Plants sprayed with sterile water and C. hemileiae without inoculation with H. vastatrix served as the control treatments. Treatment means followed by the same letter, within each experiment, are not significantly different (p ≤ 0.05) as determined by Tukey's test. ∗, ∗∗ = significant differences at 0.05 and 0.01, respectively. ns = not significant. n = 5.
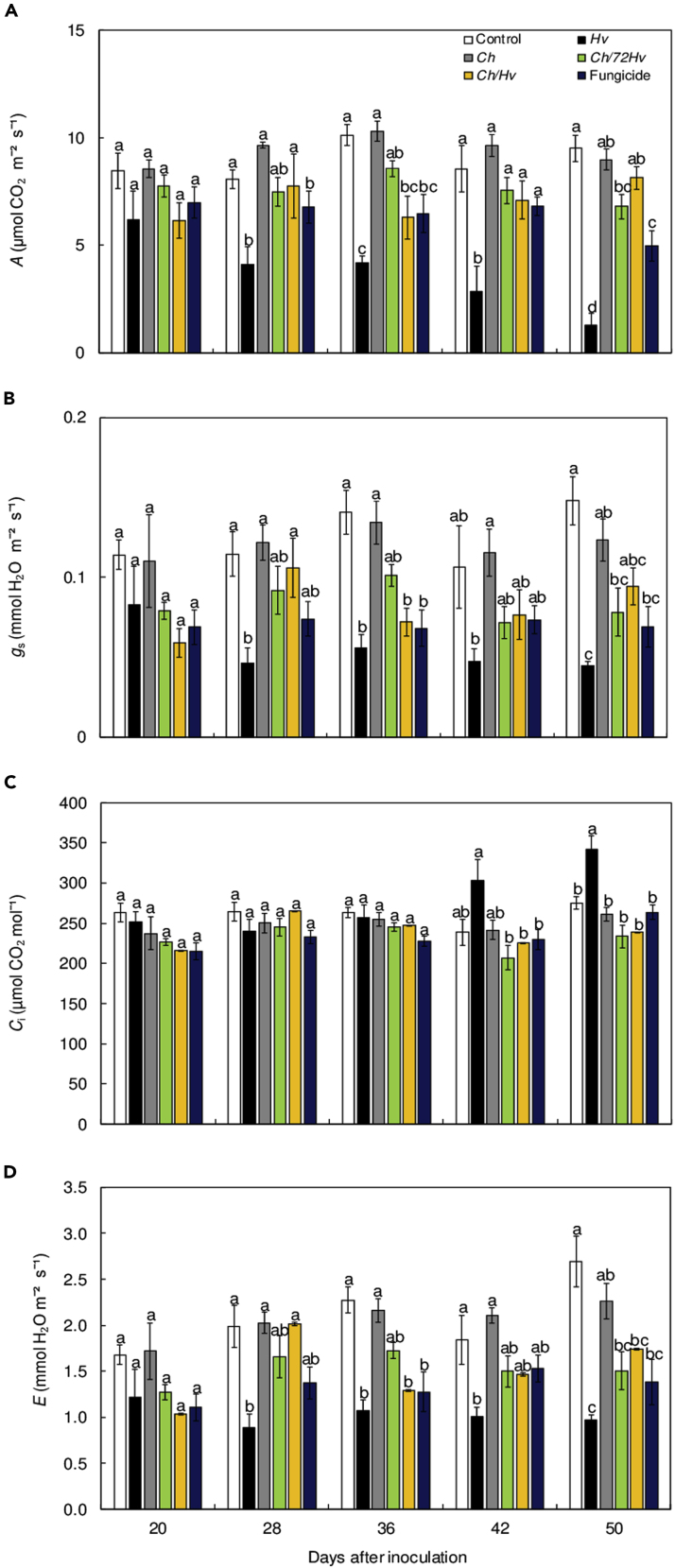
Interactions of Calonectria hemileiae, fungicide mixture, and Hemileia vastatrix: effects on photosynthetic parameters
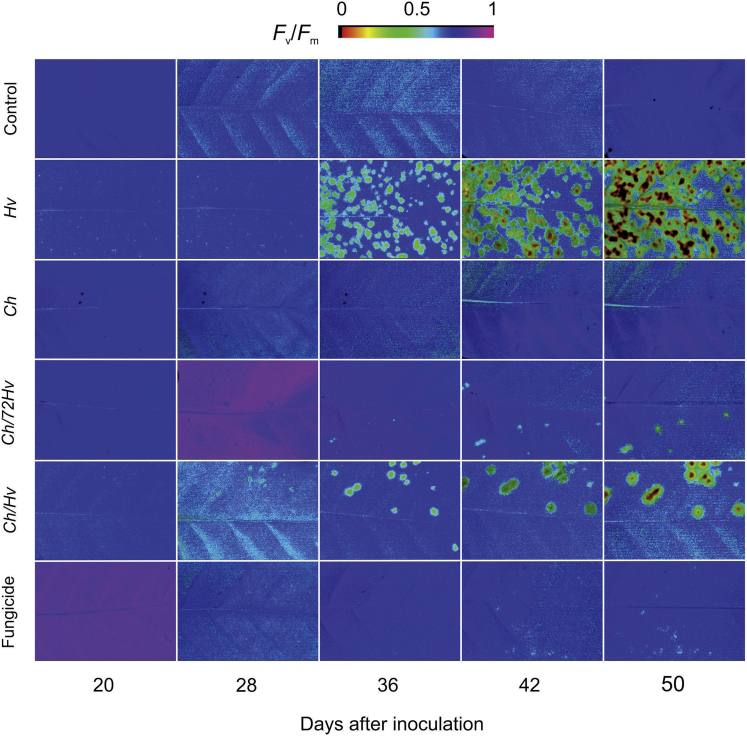
Fluorescence images of maximal photosystem II quantum yield (Fv/Fm) parameter clearly show the alterations caused by the rust on the photosynthetic performance of leaves as CLR developed (Figure 3). This was mirrored by the low Fv/Fm values that were found for inoculated plants (Figure 4A). Additionally, the quantitative analysis of the values of Fv/Fm indicated that after an asymptomatic period, rust infection harmed the photosynthetic process of the leaves (Figure 4A). In the case of the Ch/72Hv, Ch/Hv and fungicide treatments, the values of Fv/Fm (≥0.8) found were not as significantly different from those of healthy non-inoculated plants (Figures 3 and 4A).
Figure 3.
Photosythetic capacity of coffee plants sprayed with Calonectria hemileiae or fungicide mixture was not affected when plants were inoculated with Hemileia vastatrix.
Maximal photosystem II quantum yield (Fv/Fm) determined on leaves of coffee plants at 20, 28, 36, 42, and 50 days after inoculation with H. vastatrix. The treatments used were plants sprayed with C. hemileiae before at 72 h (Ch/72Hv) and simultaneously with H. vastatrix (Ch/Hv). The Ch and Hv treatments were considered as controls for antagonist and pathogen, respectively. Trifloxystrobin + tebuconazol was used as the fungicide treatment. Plants sprayed with sterile distilled water served as the control treatment. The color bar represents the scale from near zero (dark orange) to near one (dark violet).
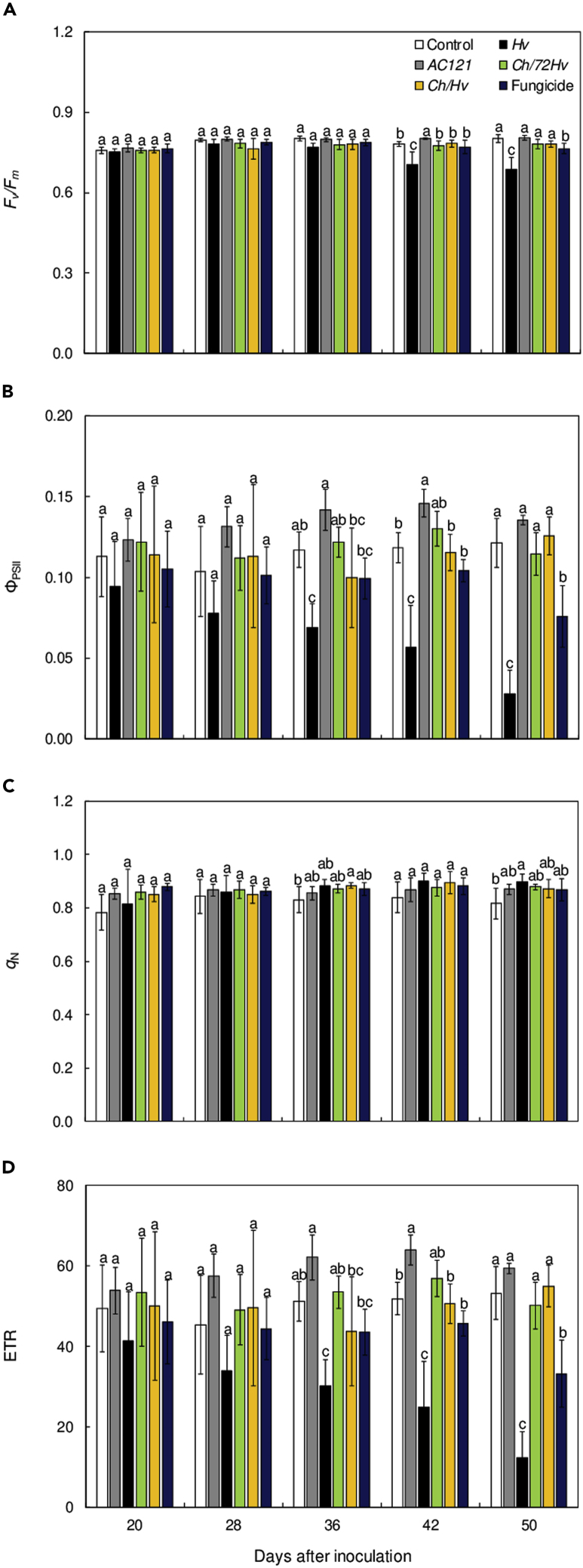
Figure 4.
Photochemical and non-photochemical energy dissipation pathways were preserved on coffee plants inoculated with Hemileia vastatrix but treated with Calonectria hemileiae or fungicide mixture.
Maximal photosystem II quantum yield (Fv/Fm) (A), photochemical yield of photosystem II (ΦPSII) (B) quenching non-photochemical (qN) (C) and electron transport rate (ETR) (D) on leaves of coffee plants at 20, 28, 36, 42, and 50 days after inoculation with H. vastatrix. The treatments used were: plants sprayed with C. hemileiae before at 72 h (Ch/72Hv) and simultaneously with H. vastatrix (Ch/Hv). The Ch and Hv treatments were considered as controls for antagonist and pathogen, respectively. Trifloxystrobin + tebuconazol was used as the fungicide treatment. Coffee plants sprayed with sterile distilled water (Control). Treatments means with the same letters are not significantly different (p ≤ 0.05) as determined by Tukey's test. Bars represent the standard deviations of the means. n = 5.
From 36 dai, onwards, significant differences in the photochemical yield of photosystem II (ΦPSII) and the electron transport rate (ETR) parameters were detected among the treatments (Figures 4B and 4D). For rust-inoculated plants, significant reductions at 36, 42, and 50 dai occurred for ΦPSII and ETR as compared with other treatments. In contrast, for these evaluation times, the Ch/72Hv and Ch/Hv treatments yielded ΦPSII values that stayed at levels that were close to those of coffee plants sprayed only with SDW. At 50 dai, ΦPSII and ETR showed significant reductions for Ch/72Hv and Ch/Hv treatments (Figures 4B and 4D). With regards to qN parameter, these showed a significant increase for plants from the Ch/Hv and Hv treatments at 36 and 50 dai, respectively, as compared to the control treatment (Figure 4C).
The net CO2 assimilation rate (A), stomatal conductance to water vapor (gs), and transpiration rate (E) values were significantly reduced from 28 dai for plants inoculated with the rust (Figures 5A, 5B, and 5D). Losses of up to 70% in the CO2 fixation at 50 dai were observed, whereas the internal CO2 concentration (Ci) showed significant increases after 42 dai (Figure 4C). For the Ch/72Hv, Ch/Hv, and for the fungicide treatments, the A, gs, and E values were significantly higher as compared with those found for coffee plants inoculated with the rust (Figures 5A–5C). However, at 50 dai, the fungicide treatment produced significant reductions in A, gs, and E coupled with significant increases in Ci, as compared with Ch/72Hv and Ch/Hv treatments (Figure 5C). Despite the CLRS reduction in the plants treated with fungicide, the A and E values were significantly reduced in fungicide-treated plants as compared to water-sprayed controls. Reductions were of 18 and 34% at 20 dai, 16 and 31% at 28 dai, 36 and 43% at 36 dai, 20 and 17% at 42 dai, and 48 and 49% at 50 dai. These reductions were in connection with the low gs values, which were as follows: 40% at 20 dai, 35% at 28 dai, 52% at 36 dai, 31% at and 53% at 50 dai (Figures 5A, 5B, and 5D). In contrast, for all the fluorescence and leaf gas exchange parameters evaluated, C. hemileiae did not interfere with the photosynthetic process of the plants (Figures 4 and 5).
Figure 5.
Calonectria hemileiae application did not harm the photosynthetic capacity of the coffee plants whereas spraying with fungicide mixture reduced the photosynthetic capacity.
Net CO2 assimilation rate (A) (A), stomatal conductance to water vapor (gs) (B), internal CO2 concentration (Ci) (C), and transpiration rate (E) (D) determined on the leaves of coffee plants at 20, 28, 36, 42, and 50 days after inoculation with H. vastatrix. The treatments used were plants sprayed with C. hemileiae before at 72 h (Ch/72Hv) and simultaneously with H. vastatrix (Ch/Hv). The Ch and Hv treatments were considered as controls for antagonist and pathogen, respectively, trifloxystrobin + tebuconazol was used as the fungicide treatment. Plants sprayed with sterile distilled water served as the control treatment. Treatments means with the same letters are not significantly different (p ≤ 0.05) as determined by Tukey's test. Bars represent the standard deviations of the means. n = 5.
Discussion
Fungal disease management is one of the most challenging and essential goals in modern agriculture. During the 20th century, the use of fungicides became the dominant method for control of diseases caused by fungi in most crops of economic importance, such as coffee. Nevertheless, genetically homogeneous monocultures and the diminished resistance diversity of crops, became major drivers of change in the genetic profile of pathogen populations and crop resistance to pathogens and fungicide efficacy have been compromised on many fronts (Garrett et al., 2006; Zhan et al., 2014). Different approaches are now needed, and the use of antagonistic fungi and bacteria as plant disease control tools is on the rise (Glare et al., 2012; Qualhato et al., 2013; Nygren et al., 2018), especially against CLR (Shiomi et al., 2006; Haddad et al., 2009, 2013, 2014; Mejía, 2015). Historically, the Central American countries and Colombia escaped CLR through a combination of highland cultivation and chemical control, but global warming has been implicated in recent rust outbreaks at higher altitudes and, since the early 2010s, rust epidemics have devastated coffee plantations in these regions, with crop losses of between 30 and 90% (Avelino et al., 2015). CLR remains by far the worst coffee disease and the cause of great and increasing concern in coffee-producing countries in Central and South America. In countries such as Brazil, planting resistant genotypes and fungicide applications have traditionally been efficient (Zambolim, 2016). However, the burgeoning market demand for pesticide residue-free or organic coffee and the repeated breakdown of resistance, are paving the way for a fresh look at biocontrol for the management of CLR (WCR, 2020).
Here, the recently discovered species Calonectria hemileiae was subjected to in vitro and in planta tests to evaluate its antagonism against H. vastatrix and thus to assess its potential as a biocontrol agent. It was demonstrated that C. hemileiae inhibited the germination of rust urediniospores in vitro, whilst the in planta study revealed that the effect of C. hemileiae was comparable to that of chemical fungicide application.
Calonectria hemileiae was found to induce the production of enzymes known to play a role in the coffee defense system and to reduce the oxidative damage caused by rust infection without causing harmful effects to the plant's photosynthetic performance. This was in contrast to the marked damage caused by a fungicide mixture. Adverse effects of fungicides on carbon metabolism have been reported previously. In the specific case of the azole fungicides, studies have demonstrated that these compounds can cause photosynthesis inhibition (cyazofamid), stomatal closure (triadimefon), Ci increase (cyazofamid and triadimefon), as well as reductions in the oxygen evolution and ETR (epoxiconazole) (Petit et al., 2012). Conversely, other fungicides (paclobutrazol and triadimefon) have been shown to stimulate photosynthesis (Kasele et al., 1995; Gopi et al., 2005; Kishorekumar et al., 2006). It appears that the physiological response of plants to the azole fungicides depends on the compound type, the dosage, and the plant species involved. For the strobilurin fungicides, a highly negative influence on photosynthesis has been reported (Nason et al., 2007). These authors tested five different strobilurin fungicides on barley, soybean, and wheat and found that these caused drastic reductions in the gs (stomatal closure) leading to loss of CO2 influx to carboxylation sites in the chloroplasts (<A), as well as a low transpiration rate for the test plants. Debona et al. (2016) found that rice plants grown under unstressed conditions and sprayed with azoxystrobin had reductions of 19, 36, and 28%, respectively, in A, gs, and E values; confirming the negative impact of this particular strobilurin type on the photosynthetic capacity of rice plants. Here, our results also indicate that adverse effects on the photosynthesis of coffee plants can result from the use of a mixture of tebuconazole and trifloxystrobin.
The plant defense mechanisms against pathogen infection involve pathogenesis-related proteins (PR-proteins), including CHI and GLU, which are responsible for the catalysis of the degradation process of polysaccharides in the pathogens' cell wall (Roberti et al., 2008). These enzymes are abundant in many plant species after infection by pathogens of different lifestyles (Ebrahim et al., 2011). For plants sprayed only with C. hemileiae, CHI and GLU activities were greater than for the controls at 72 hr before rust inoculation. The results suggest that C. hemileiae elicited activation of the genes producing these enzymes, especially CHI, and triggered the plant's defense mechanisms against rust infection. There are examples in the literature of non-pathogenic fungi promoting defense reactions in plants that can lead to their protection against fungal pathogens. For instance, it was found that spraying Curvularia inaequalis conidial suspension on sorghum leaves reduced significantly the severity of anthracnose caused by Colletotrichum sublineolum (Resende et al., 2015). These authors attributed this response to significant increases in the CHI and GLU activities as compared with sorghum plants not treated with C. inaequalis before C. sublineolum inoculation.
In addition, several studies have confirmed that CHI and GLU activities could be elicited in plants due to the presence of microbes (Koike et al., 2001). Proteomic analysis studies of susceptible vs. resistant coffee cultivars indicated increased accumulation of PR-proteins, such as chitinases, osmotin, and a cysteine-rich repeat secretory protein, as a feature present in the resistant cultivar only. This may be related to the induction of the basal defense responses, possibly regulated by salicylic acid (Guerra-Guimarães et al., 2015).
The reactive oxygen species (ROS) production in plants, either during the colonization by pathogenic agents or by symbiotic organisms, is usually very similar in the first stage of the interaction as both can induce oxidative burst (Fester and Hause, 2005; Torres, 2010). In the plant-pathogen interaction, ROS production is activated in the plant for limiting pathogen infection, either directly or indirectly (Møller et al., 2007). However, progressive ROS accumulation may impact on the structure and functions of the plant cells and, in order to avoid such problems, the cell has enzymatic and non-enzymatic mechanisms aimed at helping the metabolism of ROS and hence to ensure cellular homeostasis (Kumar et al., 2009). In our study, SOD activity increased in coffee leaves sprayed with C. hemileiae and inoculated with the rust, especially at 72 hai and 50 dai. This suggests that SOD can actively participate in the O2•- metabolization in response to rust infection. SOD may reduce cellular intoxication by this molecule. A previous study of the maize-Piriformospora indica interaction and the biocontrol agent Fusarium verticillioides posited that induction of SOD by F. verticillioides application may lead to the recognition of P. indica by the plant tissues and consequent activation of plant defense responses (Kumar et al., 2009). Proteomic analysis of the coffee-H. vastratrix interaction demonstrated that the accumulation of copper-zinc superoxide dismutase occurs in tissues of coffee plants that are susceptible to the rust at 48 hai, suggesting that these “PR-like” proteins may co-regulate basal defenses in coffee (Guerra-Guimarães et al., 2015).
APX and POX are involved in H2O2 metabolism (Torres, 2010); APX functions at the level of chloroplasts, peroxisomes, and mitochondria using ascorbate as a specific electron donor to reduce H2O2 to water (Quan et al., 2008); whilst POX is an enzyme with an essential role in plant defense against pathogens due to its participation in lignin biosynthesis (Rauyaree et al., 2001). In our study, increases in APX and POX activities occurred for coffee plants sprayed with C. hemileiae alone at 50 dai, suggesting that the fungus can activate the antioxidant system, keeping it in a “state of alert” to the threat of rust colonization. It is important to note that no sign of damage or disease symptoms appeared as a result of C. hemileiae applications to the coffee plants. In the treatment tested with C. hemileiae combined with the rust, the APX and POX activities were at lower levels as compared with C. hemileiae application alone. The presence of the rust may alter the context of APX and POX activities creating ambiguous scenarios. Biochemical studies have shown that several oxidase enzymes are involved in resistance responses to CLR, constituting one of the components at play and limiting infection by H. vastatrix (Guerra-Guimarães et al., 2009, Guerra-Guimarães et al., 2013).
Studies have demonstrated that CLR causes reduction in the photosynthetic capacity of coffee plants and premature defoliation of trees with direct damage to yield (Avelino et al., 2015; Honorato et al., 2015a, 2015b). In the present study, a progressive increase in CLRS led to detrimental losses in the photosynthetic capacity of the plants. However, our results revealed that coffee plants sprayed with C. hemileiae and inoculated with H. vastatrix showed no damage in the photochemical phase (analyzed as Fv/Fm) of photosynthesis as compared with plants not treated with C. hemileiae. Additionally, ΦPSII and ETR values suggested that there was a preservation of the functional integrity of the photosynthetic machinery in the plants sprayed with C. hemileiae and infected by the rust. In this context, the use of C. hemileiae did not alter the values of A, gs, Ci, and E compared to control plants. This is in sharp contrast to the treatment where the fungicide mixture (chosen as representing commercial products commonly used in coffee plantations for CLR control) was applied. The fungicide treatment was the most effective at reducing CLR.
Nevertheless, the photosynthetic performance of fungicide-treated coffee plants was negatively affected. Studies with pecan-nut plants showed that the application of fungicides containing strobilurins significantly reduced the leaf gas exchange parameters, especially A (Wood and Bock, 2017). Nevertheless, Honorato et al. (2015a, 2015b) concluded that the use of the fungicides triazole and strobilurin did not cause negative physiological alterations in coffee plants. This remains an important issue that needs to be urgently addressed since this fungicide combination has become important for the management of fungal crop diseases. Their use may have a “hidden cost” on plant metabolism, which may not be limited to coffee and pecan-nuts. The maintenance of the photosynthetic process on coffee plants sprayed with C. hemileiae may be attributed to lower CLR severity. Such an effect from antagonistic fungi has been demonstrated previously. In rice, for example, several isolates of Trichoderma asperellum were able to reduce the size of leaf scald lesions and the area under the disease progress curve, minimizing the harmful effects of this disease on A, gs, Ci, and E, as well as on Chl a fluorescence parameters and in the antioxidative metabolism (Bueno et al., 2017). Resende et al. (2015) have also shown that, besides ensuring a reduction in the anthracnose levels in sorghum plants, the application of C. inaequalis did not impair the photosynthetic capacity of the infected plants.
Conclusions
Applying C. hemileiae in advance of the rust promoted host defense responses in the leaves of coffee plants as revealed by high CHI, APX, and POX activities, resulting in reduced CLR severity. CLR control, similar to those of chemical control, was achieved but without any evidence of damage to the photosynthetic capacity of coffee plants. Interestingly, the best CLR control occurred when C. hemileiae was sprayed before rust inoculation (72 hr), for both in vitro and in planta experiments, indicating that C. hemileiae was able to protect the coffee plants against CLR. It may be acting on two fronts, both as a mycoparasite of the rust and by inducing host resistance – as the evidence presented here indicates—and which has been demonstrated for other fungal taxa used in disease biocontrol, such as Trichoderma and Clonostachys (Qualhato et al., 2013; Nygren et al., 2018).
The induction of protection of plants as promoted by biological control agents or their metabolites, is a non-specific form of disease resistance that involves the synthesis and accumulation of several antimicrobial compounds—such as CHI, GLU, callose deposition, and the production of phenolics and phytoalexins—added to a high antioxidant activity triggered through a combination of different modes of host defense against pathogens (Cohen et al., 1993; Köhl et al., 2019). Carrión and Rico-Gray (2002) claimed that the six mycoparasitic fungi found colonizing pustules of H. vastatrix in Mexico were capable of destroying the reproductive structures of the rust and conjectured that these might have an impact on rust populations by decreasing the inoculum potential. Unfortunately, their study did not progress toward a practical biocontrol solution, such as the development of a biofungicide (Loguercio et al., 2009). At present, there are no such products designed explicitly for CLR control. Further studies are underway to determine if Calonectria hemileiae can fit the bill and become a biocontrol tool for use against Hemileia vastatrix.
Limitations of the study
This study identifies C. hemileiae as a promising biocontrol agent of CLR based on laboratory and greenhouse screening. There are other hurdles to overcome before its actual potential can be fully realized. Most notably is the need for a pest risk assessment, especially since the fungus belongs to a genus containing important plant pathogens. Our results, thus far, have shown that C. hemileiae is non-pathogenic to coffee but wider host-range screening is needed to establish whether or not it poses a risk. If C. hemileiae is confirmed to be ecologically restricted to a mycoparasitic lifestyle, then the next phase will be to test its efficacy in the field.
Resource availability
Lead contact
Further information and requests for resources and materials should be directed to and will be fulfilled by the lead contact, Prof. Dr. Robert W, Barreto (rbarreto@ufv.br)
Materials availability
This study did not generate new unique reagents.
Data and code availability
Source data for the figures published in this the paper are available per request.
Methods
All methods can be found in the accompanying transparent methods supplemental file.
Acknowledgments
This work represents part of a research project submitted to the Departamento de Fitopatologia, Universidade Federal de Viçosa (UFV, MG, Brazil) by S.S.-S. as part of the requirement for a DSc in Phytopathology. This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES)—Finance Code 001. S.S.-S. was supported by the “Programa de Estudante-Convênio de Pós-Graduação” (PEC-PG) from CAPES. We thank Prof. Luiz Antônio Dias of the Departamento de Agronomia (UFV) for allowing us the use of scientific equipment. This study was supported by grants from World Coffee Research (WCR), the Coordenacão de Aperfeiçoamento do Pessoal de Nível Superior (CAPES), and the Conselho Nacional do Desenvolvimento Científico e Tecnológico (CNPq).
Author contributions
S.S.-S., C.E.A.-P., F.A.R., and R.W.B designed the experiments. S.S.-S., A.A.C., A.L.S., and P.S.C.M. isolated, multiplied, and inoculated the plants with C. hemileia and H. vastatrix. S.S.-S., C.E.A.-P., A.A.D., A.L.S., and P.S.C.M. evaluated the in vitro and in plant experiments. S.S.-S., C.E.A.-P., A.L.S., and P.S.C.M. performed the leaf gas exchange and chlorophyll a fluorescence measurements. S.S.S., P.R.S., and P.S.C.M. performed the biochemical analysis. S.S.-S and A.A.D. collected and prepared the leaf samples for examination in the scanning electron microscope. S.S.-S., C.E.A.-P., F.A.R., H.C.E., and R.W.B. analyzed the data of the experiments, drafted the manuscript, and prepared its final version for publication. All authors interpreted the results and approved the final version of the manuscript.
Declaration of interests
The authors declare no competing interests.
Published: April 23, 2021
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2021.102352.
Supplemental information
References
- Avelino J., Cristancho M., Georgiou S., Imbach P., Aguilar L., Bornemann G., Läderach P., Anzueto F., Hruska A.J., Morales C. The coffee rust crises in Colombia and Central America (2008–2013): impacts, plausible causes and proposed solutions. Food Sec. 2015;7:303–321. [Google Scholar]
- Barreto R.W., Colmán A.A., Evans H.C. Congress International Botany 2015 - Edmonton, Alberta - July 25–29. 2015. Fungal natural enemies of Hemileia vastatrix and their potential for use in classical biological control of coffee rust.http://2015.botanyconference.org/engine/search/index.php?func=detail&aid=1235 [Google Scholar]
- Batista K.D., Araujo W.L., Antunes W.C., Cavatte P.C., Moraes G.A.B.K., Martins S.C.V., DaMatta F.M. Photosynthetic limitations in coffee plants are chiefly governed by diffusive factors. Trees. 2012;26:459–468. [Google Scholar]
- Bhat S.S., Naidu R., Daivasikamani S., Nirmala K. Integrated disease management in coffee. In: Upadhyay R.K., Mukerji K.G., Dubey O.P., editors. IPM System in Agriculture – Cash Crops. Aditya Books Private Limited; 2000. pp. 233–250. [Google Scholar]
- Bettiol W., Saito M.L., Brandão M.S.B. Controle da ferrugem do cafeeiro com produtos à base de Bacillus subtilis. Summa Phytopathol. 1994;20:119–122. [Google Scholar]
- Bueno A.C.S.O., Castro G.L.S., Rêgo M.C.F., Batista T.F.V., Filippi M.C.C., da Silva G.B. Trichoderma reduces scald and protects the photosynthetic apparatus in rice plants. Biocontrol Sci. Technol. 2017;27:449–460. [Google Scholar]
- Cabral P.G.C., Maciel-Zambolim E., Zambolim L., Lelis T.P., Capucho A.S., Caixeta E.T. Identification of a new race of Hemileia vastatrix in Brazil. Australas. Plant Dis. Notes. 2009;4:129–130. [Google Scholar]
- Capucho A.S., Zambolim L., Cabral P.G.C., Maciel-Zambolim E., Caixeta E.T. Climate favorability to leaf rust in Conilon coffee. Australas. Plant Pathol. 2013;24:511–514. [Google Scholar]
- Capucho A.S., Zambolim L., Milagres N. Chemical control of coffee leaf rust in Coffea canephora cv. Conilon. Australas. Plant Pathol. 2013;42:667–673. [Google Scholar]
- Carrión G., Rico-Gray V. Mycoparasites on the coffee rust in Mexico. Fungal Divers. 2002;11:49–60. [Google Scholar]
- Cohen Y., Gisi U., Niderman T. Local and systemic protection against Phytophthora infestans induced in potato and tomato plants by jasmonic acid and jasmonic methyl ester. Phytopathology. 1993;3:1054–1062. [Google Scholar]
- Costa M.J.N., Zambolim L., Rodrigues F.R. Avaliação de produtos alternativos no controle da ferrugem do cafeeiro. Fitopatol. Bras. 2007;32:147–152. [Google Scholar]
- Cristancho A.M.A. Efecto protector de la bactéria Bacillus thuringiensis em plants de café contra el desarrollo de Hemileia vastatrix. Cenicafé. 1995;46:140–151. [Google Scholar]
- Crous P.W., Luangsa-Ard J.J., Wingfield M.J., Carnegie A.J., Hernández-Restrepo M., Lombard L., Roux J., Barreto R.W., Baseia I.G., Cano-Lira J.F. Calonectria hemileiae. Fungal Planet description sheets: 823. Persoonia. 2018;41:324–325. doi: 10.3767/persoonia.2018.41.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daivasikamani S., Rajanaika A. Biological control of coffee leaf rust pathogen, Hemileia vastatrix Berkeley and Broome using Bacillus subtilis and Pseudomonas fluorescens. J. Biopest. 2009;2:94–98. [Google Scholar]
- Debona D., Nascimento K.J.T., Gomes J.G.O., Aucique-Pérez C.E., Rodrigues F.A. Physiological changes promoted by a strobilurin fungicide in the rice- Bipolaris oryzae interaction. Pest Bioch. Physiol. 2016;130:8–16. doi: 10.1016/j.pestbp.2015.12.006. [DOI] [PubMed] [Google Scholar]
- Ebrahim S., Usha K., Singh B. Pathogenesis related (PR) proteins in plant defense mechanism. Sci. Against Microb. Pathol. 2011;2:1043–1054. [Google Scholar]
- Fester T., Hause G. Accumulation of reactive oxygen species in arbuscular mycorrhizal roots. Mycorrhiza. 2005;15:373–379. doi: 10.1007/s00572-005-0363-4. [DOI] [PubMed] [Google Scholar]
- Garrett K.A., Dendy S.P., Frank E.E., Rouse M.N., Travers S.E. Climate Change effects on plant disease: Genomes to ecosystems. Annu. Rev. Phytopath. 2006;44:489–509. doi: 10.1146/annurev.phyto.44.070505.143420. [DOI] [PubMed] [Google Scholar]
- Glare T., Caradus J., Gelernter W., Jackson T., Keyhani N., Köhl J., Marrone P., Morin L., Stewart A. Have biopesticides come of age? Trends Biotechnol. 2012;30:250–258. doi: 10.1016/j.tibtech.2012.01.003. [DOI] [PubMed] [Google Scholar]
- Godoy C.V. Risk and management of fungicide resistance in crop in the Asian soybean rust fungus. In: Thind T.S., editor. Fungicide Resistance in the Crop Protection: Risk and Management. CABI Publishing; 2012. pp. 87–95. [Google Scholar]
- Gopi R., Sridharan R., Somasundaram R., Lakshmanan G.M.A., Panneerselvam R. Growth and photosynthetic characteristics as affected by triazoles in Amorphophallus campanulatus Blume. Gen. App. Plant Physiol. 2005;31:171–180. [Google Scholar]
- Guerra-Guimarães L., Cardoso S., Martins I., Loureiro A., Bernardes A.S., Varzea V., Silva M.C. Proceedings of the 22nd International Conference on Coffee Science. ASIC; 2009. Differential induction of superoxide dismutase in Coffea arabica–Hemileia vastatrix interactions; pp. 1036–1039. [Google Scholar]
- Guerra-Guimarães L., Tenente R., Pinheiro C., Chaves I., Silva MdoC., Cardoso F.M.H., Planchon S., Barros D.R., Renaut J., Ricardo C.P. Proteomic analysis of apoplastic fluid of Coffea arabica leaves highlights novel biomarkers for resistance against Hemileia vastatrix. Front. Plant Sci. 2015;6:478. doi: 10.3389/fpls.2015.00478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerra-Guimarães L., Vieira A., Chaves I., Queiroz V., Pinheiro C., Renaut J. Proceedings of the 24th International Conference on Coffee Science, AISC. ASIC; 2013. Integrated cytological and proteomic analysis of Coffea arabica - Hemileia vastatrix interactions; pp. 1414–1418. [Google Scholar]
- Haddad F., Maffia L.A., Mizubuti E.S.G., Teixeira H. Biological control of coffee leaf rust by antagonistic bacteria under field conditions in Brazil. Biol. Control. 2009;49:114–119. [Google Scholar]
- Haddad F., Saraiva R.M., Mizubuti E.S.G., Romeiro R.S., Maffia L.A. Antifungal compounds as a mechanism to control Hemileia vastatrix by antagonistic bacteria. Trop. Plant Pathol. 2013;38:398–405. [Google Scholar]
- Haddad F., Saraiva R.M., Mizubuti E.S.G., Romeiro R.S., Maffia L.A. Isolation and selection of Hemileia vastatrix antagonists. Eur. J. Plant Pathol. 2014;139:763–772. [Google Scholar]
- Honorato J.J., Zambolim L., Aucique-Pérez C.E., Resende R.S., Rodrigues F.A. Photosynthetic and antioxidative alterations in coffee leaves caused by epoxiconazole and pyraclostrobin sprays and Hemileia vastatrix infection. Pest Biochem. Physiol. 2015;123:31–39. doi: 10.1016/j.pestbp.2015.01.016. [DOI] [PubMed] [Google Scholar]
- Honorato J.J., Zambolim L., Duarte H.S.S., Aucique-Pérez C.E., Rodrigues F.A. Effects of epoxiconozale and pyraclostrobin fungicides in the infection process of Hemileia vastatrix on coffee leaves as determined by chlorophyll a fluorescence imaging. J. Phytopathol. 2015;163:968–977. [Google Scholar]
- International Coffee Organization (ICO) Statistics 2016. 2016. http://www.ico.org/trade_statistics.asp
- James T.Y., Marino J.A., Perfecto I., Vandermeer J. Identification of putative coffee rust mycoparasites via single-molecule DNA sequencing of infected pustules. Appl. Environ. Microbiol. 2016;82:631–639. doi: 10.1128/AEM.02639-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasele I.N., Shannahan J.F., Nielsel D.C. Impact of growth retardants on corn leaf morphology and gas exchange traits. Crop Sci. 1995;35:190–194. [Google Scholar]
- Kishorekumar A., Jaleel C.A., Manivannan P., Sankar B., Sridharan R., Somasundaram R., Panneerselvam R. Differential effects of hexaconazole and paclobutrazol on the foliage characteristics of Chinese potato (Solenostemon rotundifolius Poir., J.K. Morton) Acta Biol. Szegediensis. 2006;50:127–129. [Google Scholar]
- Köhl J., Kolnaar R., Ravensberg W.J. Mode of action of microbial biological control agents against plant diseases: relevance beyond efficacy. Front. Plant Sci. 2019;10:845. doi: 10.3389/fpls.2019.00845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike N., Hyakumachi M., Kageyama K., Tsuyumu S., Doke N. Induction of systemic resistance in cucumber against several diseases by plant growth promoting fungi: lignification and superoxide generation. Eur. J. Plant Pathol. 2001;107:523–533. [Google Scholar]
- Kumar M., Yadav V., Tuteja N., Johri A.K. Antioxidant enzyme activities in maize plants colonized with Piriformospora indica. Microbiology. 2009;155:780–790. doi: 10.1099/mic.0.019869-0. [DOI] [PubMed] [Google Scholar]
- Loguercio L.L., de Carvalho A.C., Neilla G.R., de Souza J.T., Pomella A.W.V. Selection of Trichoderma stromaticum isolates for efficient biological control of witches’ broom disease in cacao. Biol. Control. 2009;51:130–139. [Google Scholar]
- McCook S. Global rust belt: Hemileia vastatrix and the ecological integration of world coffee production since 1850. J. Glob. Hist. 2006;1:177–195. [Google Scholar]
- McTaggart A.R., Shivas R.G., van der Nest M.A., Roux J., Wingfield B.D., Wingfield M.J. Host jumps shaped the diversity of extant rust fungi [Pucciniales] New Phytol. 2016;209:1149–1158. doi: 10.1111/nph.13686. [DOI] [PubMed] [Google Scholar]
- Mejía L. Memorias del seminario científico internacional: Manejo Agroecológico de la Roya del Café. FAO; 2015. Microbiomas y control biológico como alternativa de manejo de la roya anaranjada del cafeto; pp. 47–53. [Google Scholar]
- Menezes-Silva P.E., Sanglard L.M.P.V., Ávila R.T., Morais L.E., Martins S.C.V., Nobres P., Patreze C.M., Ferreira M.A., Araújo W.L., Fernie A.L., DaMatta F.M. Photosynthetic and metabolic acclimation to repeated drought events play key roles in drought tolerance in coffee. J. Exp. Bot. 2017;68:4309–4322. doi: 10.1093/jxb/erx211. [DOI] [PubMed] [Google Scholar]
- Ministério da Agricultura, Pecuária e Abastecimento (MAPA). (2018) www.agricultura.gov.br.
- Møller I.M., Jensen P.E., Hansson A. Oxidative modifications to cellular components in plants. Annu. Rev. Plant Biol. 2007;58:459–481. doi: 10.1146/annurev.arplant.58.032806.103946. [DOI] [PubMed] [Google Scholar]
- Nason M.A., Farrar J., Bartlett D. Strobilurin fungicides induce changes in photosynthetic gas exchange that do not improve water use efficiency of plants grown under conditions of water stress. Pest Manag. Sci. 2007;63:1191–1200. doi: 10.1002/ps.1443. [DOI] [PubMed] [Google Scholar]
- Nygren K., Dubey M., Zapparata A., Iqbal M., Tzelepis G.D., Durling M.B., Jensen D.F., Karlsson M. The mycoparasitic fungus Clonostachys rosea responds with both common and specific gene expression during interspecific interactions with fungal prey. Evol. Appl. 2018;11:931–949. doi: 10.1111/eva.12609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit A.N., Fontaine F., Vatsa P., Clément C., Vaillant-Gaveau N. Fungicide impacts on photosynthesis in crop plants. Photosyn. Res. 2012;111:315–326. doi: 10.1007/s11120-012-9719-8. [DOI] [PubMed] [Google Scholar]
- Qualhato T.F., Lopes F.A.C., Steindorff A.S., Brandão R.S., Jesuino R.S.A., Ulhoa S.J. Mycoparasitism studies of Trichoderma species against three phytopathogenic fungi: evaluation of antagonism and hydrolytic enzyme production. Biotechnol. Lett. 2013;35:1461–1468. doi: 10.1007/s10529-013-1225-3. [DOI] [PubMed] [Google Scholar]
- Quan L.J., Zhang B., Shi W.W., Li H.Y. Hydrogen peroxide in plants: a versatile molecule of the reactive oxygen species network. J. Integr. Plant Biol. 2008;50:2–18. doi: 10.1111/j.1744-7909.2007.00599.x. [DOI] [PubMed] [Google Scholar]
- Rauyaree P., Choi W., Fang E., Blackmon B., Dean R. Genes expressed during early stages of rice infection with the rice blast fungus Magnaporthe grisea. Mol. Plant Pathol. 2001;2:347–354. doi: 10.1046/j.1464-6722.2001.00085.x. [DOI] [PubMed] [Google Scholar]
- Resende R.S., Milagres C.A., Rezende D., Aucique-Perez C.E., Rodrigues F.A. Bioprospecting of saprobe fungi from the semi-arid north-east of Brazil for the control of anthracnose on sorghum. J. Phytopathol. 2015;163:787–794. [Google Scholar]
- Roberti R., Veronesi A., Cesari A., Cascone A., Di Berardino I., Bertini L., Caruso C. Induction of PR proteins and resistance by the biocontrol agent Clonostachys rosea in wheat plants infected with Fusarium culmorum. Plant Sci. 2008;175:339–347. [Google Scholar]
- Rodrigues-Junior C.J. Coffee rust: history, taxonomy, morphology, distribution and host resistance. Fitopatol. Bras. 1990;15:5–9. [Google Scholar]
- Shiomi H.F., Silva H.S.A., Melo I.S., Nunes F.V., Bettiol W. Bioprospecting endophytic bacteria for biological control of coffee leaf rust. Sci. Agric. 2006;63:32–39. [Google Scholar]
- Somarriba E., Harvey C.A., Samper M., Anthony F., Gonzalez J.E., Staver C., Rice R., Schroth G., da Fonseca G.A.B., Gascon C. Biodiversity conservation in neotropical coffee (Coffea arabica) plantations. In: Schroth G., da Fonseca G.A.B., Harvey C.A., Gascon C., Vasconcelos H.L., Izac A.M.N., editors. Agroforestry and Biodiversity Conservation in Tropical Landscapes. Island Press; 2004. pp. 198–226. [Google Scholar]
- Talhinhas P., Batista D., Diniz I., Vieira A., Silva D.N., Loureiro A., Tavares S., Pereira A.P., Azinheira H.G., Guerra-Guimarães L. The coffee leaf rust pathogen Hemileia vastatrix: one and a half centuries around the tropics. Mol. Plant Pathol. 2017;18:1039–1051. doi: 10.1111/mpp.12512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toniutti L., Breitler J.C., Etienne H., Campa C., Doulbeau S., Urban L., Bertrand B. Influence of environmental conditions and genetic background of Arabica coffee (C. arabica L.) on leaf rust (Hemileia vastatrix) pathogenesis. Front. Plant Sci. 2017;8:2025. doi: 10.3389/fpls.2017.02025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres M.A. ROS in biotic interactions. Physiol. Plant. 2010;138:414–429. doi: 10.1111/j.1399-3054.2009.01326.x. [DOI] [PubMed] [Google Scholar]
- Varzea V.M.P., Marques D.V. Population variability of Hemileia vastatrix vs. coffee durable resistance. In: Zambolim L., Maciel-Zambolim E., Várzea V.M.P., editors. Durable Resistance to Coffee Leaf Rust. UFV, Viçosa; 2006. pp. 53–74. [Google Scholar]
- Wood B.W., Bock C.H. Influence of fungicides on gas exchange of pecan foliage. Plant Pathol. 2017;62:265–276. [Google Scholar]
- World Coffee Research Biocontrol of coffee leaf rust. 2019. https://worldcoffeeresearch.org/work/biocontrol-coffee-leaf-rust/
- Zambolim L. Current status and management of coffee leaf rust in Brazil. Trop. Plant Pathol. 2016;41:1–8. [Google Scholar]
- Zhan J., Thrall P.H., Burdon J.J. Achieving sustainable plant disease management through evolutionary principles. Trends Plant Sci. 2014;19:570–575. doi: 10.1016/j.tplants.2014.04.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Source data for the figures published in this the paper are available per request.