Abstract
Purpose
To report imaging findings at computed tomography angiography (CTA) and venography (CTV) of the abdomen and pelvis in evaluation of hemorrhagic and thrombotic lesions in hospitalized patients with COVID-19.
Methods
In this retrospective observational study, patients admitted to a single tertiary care center from April 1 to July 20, 2020, who tested positive for SARS-CoV-2 and developed acute abdominal pain or decreasing hemoglobin levels over the course of hospitalization were included. Abdominal CTA/CTV imaging studies performed in these patients were reviewed, and acute hemorrhagic or thromboembolic findings were recorded.
Results
A total of 40 patients (mean age, 59.7 years; 20 men, 20 women) were evaluated. Twenty-five patients (62.5%) required intensive care unit (ICU) admission and 15 patients (37.5%) were treated in the medical ward. Hemorrhagic complications were detected in 19 patients (47.5%), the most common was intramuscular hematoma diagnosed in 17 patients; It involved the iliopsoas compartment unilaterally in 10 patients, bilaterally in 2 patients and the rectus sheath in 5 cases. Pelvic extraperitoneal hemorrhage was found in 3 patients, and mesenteric hematoma in one patient. Thromboembolic events were diagnosed in 8 patients (20%) including; arterial thrombosis (n = 2), venous thrombosis (n = 2), splenic infarct (n = 1), bowel ischemia (n = 1) and multiple sites of thromboembolism (n = 2).
Conclusion
Our study highlights that both hemorrhagic and thromboembolic complications can be seen in hospitalized patients with COVID-19. It is important that radiologists maintain a high index of suspicion for early diagnosis of these complications.
Keywords: Coronavirus disease 2019, Computed tomography angiography, Computed tomography venography
1. Introduction
In December 2019, a large outbreak of a novel coronavirus infection occurred in Wuhan, China. The novel coronavirus was named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) by the International Committee on Taxonomy of Viruses.1 The pneumonic disease caused by this virus is called Coronavirus disease 2019 (COVID-19) by the World Health Organization (WHO). The WHO recognized the (COVID-19) as a worldwide pandemic on March 11, 2020.2 , 3
With the increasing number of cases, different imaging findings have been found related to extra-pulmonary affection including cardiac, gastrointestinal, and central nervous system involvement. While the effect of SARS-CoV-2 on different organs other than the lungs is not yet fully understood, several studies reported that the coagulation pathway may be adversely affected resulting in acute venous and arterial thrombotic events, as venous thromboembolism, acute stroke, acute myocardial infarction, and clotting of the ECMO (extracorporeal membrane oxygenation) circuit.4 , 5
Thromboembolic complications have been shown to be an important cause of morbidity and mortality in COVID-19 patients both in the general ward and in the ICU, even in patients receiving therapeutic anticoagulation. At the same time, it remains unclear whether the increased intensity of thrombosis prophylaxis provides clinical benefit in patients without confirmed acute thromboembolism. Hemorrhagic complications have been reported in COVID-19 patients despite achieving low therapeutic anticoagulation. This raises the question about the exact mechanism of interaction between coagulopathy and the effect of the SARS-CoV-2 on the vascular endothelium.6 , 7
Gastrointestinal symptoms including diarrhea, vomiting, and abdominal pain were seen in 12–50% of patients with COVID-19. SARS-CoV-2 is thought to enter the cell via the Angiotensin-converting enzyme 2 (ACE2) receptors. The high expression of these receptors in the bowel could explain these GIT symptoms.6., [7], [8], 9. Many studies have evaluated abdominal imaging findings in COVID-19 patients that helped to understand the disease course and potential complications, yet, to our knowledge; limited data are available about the potential abdominal vascular complications in COVID-19 patients. Although thrombotic events are one of the main complications of COVID-19, bleeding risk is also increased in these patients.10
Therefore, our purpose was to describe both hemorrhagic and thromboembolic findings on computed tomography angiography (CTA) and venography (CTV) of the abdomen and pelvis in evaluation of hemorrhagic and thrombotic lesions in hospitalized patients with COVID-19.
2. Patients and methods
2.1. Patients and study design
This was a retrospective observational study performed at our tertiary care institution. Institutional review board approval was obtained and informed consent was waived. We included adult patients (>18 years) who were admitted to our institution in the duration between April 1 to July 20, 2020, who tested positive for SARS-CoV-2. Patients who developed acute abdominal pain or decreasing hemoglobin levels (fall in hemoglobin level of 2 g/dL or more) over the course of hospitalization and underwent CTA/CTV of the abdomen and pelvis were included in our study. Demographics, clinical data (presence of acute abdominal pain, distention), intensive care unit (ICU) admission, and surgical or endovascular intervention notes, duration between admission and imaging, and outcome of the patients during hospitalization, were collected from electronic medical records. Laboratory data within 3 days before CTA/CTV were considered. Laboratory data collected included D-dimer level (ng/mL, reference range less than 500 ng/mL) prothrombin time (PT, s, reference range 13.2–16.4 s), and platelets (×103/μL, reference range 150–400 × 103/μL).
2.2. Image acquisition
All scans were performed on 128-slice multidetector CT scanner (SOMATOM Definition Edge, Siemens, Germany). The examination was performed by acquiring non contrast images followed by acquisition during the arterial, and portal venous phases. Axial CT Images for the abdomen and pelvis were acquired with the following parameters: 120 kVp; mAs 200–650 adjusted according to patient size and section thickness of 0.625 mm. All images were reconstructed with a section thickness of 2.5–5 mm. Multiplanar reconstructed images were created in sagittal and coronal planes, with a reconstructed section thickness of 3 mm. For CTA, the patient received intravenous administration of 80–120 mL of nonionic iodinated contrast material (using 350 mg iodine/mL) at a rate of 3–4 mL/s followed by a 40-mL saline flush at the same rate. Delay times of 30 s for the arterial phase and 70 s for the venous phase were obtained. The bolus-triggered method was used to optimize the phase of acquisition, with the enhancement threshold set at 150 Hounsfield units.
2.3. Image analysis
All CTA/CTV studies of the abdomen and pelvis were interpreted in a clinical setting by two abdominal radiologists (10 and 15 years of experience). The CTA studies were retrospectively reviewed and intraabdominal pathology was classified as hemorrhagic or thromboembolic findings. If hemorrhage was present, the site and extent of hemorrhage and the presence of active contrast extravasation were assessed. If thrombosis was present, the location of thromboembolism whether arterial or venous and solid organ or bowel infarction were reviewed.
2.4. Statistical analysis
Descriptive statistical analysis is presented as counts and percentages for categorical variables and as mean and standard deviation, or median and range for continuous variables. Patients were divided into three groups, group 1 included patients with hemorrhagic complications, group 2 included patients with thromboembolic complications, and the third group included patients with no acute findings at CTA/CTV. Proportions for categorial variables were compared using Chi-Square test. P values for categorical variables were calculated with the Mann Whitney U test. P value less than 0.05 represented a significant difference.
3. Results
3.1. Demographic and clinical data of the study cohort
A total of 736 adult patients who tested positive for SARS-CoV-2 were admitted to our institution during the study period. In this retrospective study; CTA/CTV imaging for the abdomen and pelvis were done for 40 confirmed COVID-19 patients who developed acute abdominal symptoms during their hospital stay. There were 20 males (50%) and 20 females (50%), their ages ranged from 24 to 81 years, with a mean age of 59.7 years. Twenty-five patients (62.5%) required ICU admission and 15 patients (37.5%) were treated in the medical ward. The most common clinical presentations were acute abdominal pain (9/40; 22.5%) and acute abdominal distention (9/40; 22.5%). Decreasing hemoglobin level suggestive of hypovolemic shock was the main indication of CTA in 21 patients (21/40; 52.5%). Demographic, clinical and laboratory data of the patients were illustrated in Table 1 .
Table 1.
Demographic, clinical and laboratory data of the study cohort.
| Demographic and clinical data | Patients with hemorrhagic complications (n = 19) | Patients with thromboembolic complications (n = 8) | P value1 | Patients with negative CTA (n = 13) | P value2 | P value3 |
|---|---|---|---|---|---|---|
| Age mean ± SD, years | 60.79 ± 13.10 | 60.0 ± 10.06 | 0.89 | 56.38 ± 15.71 | 0.37 | 0.55 |
| Male sex, n (%) | 9 (47.4) | 4 (50) | 0.901 | 7 (53.8) | 0.719 | 1.0 |
| Duration between positive swab and CTA, days, median (range) | 14 (6–38) | 11 (4–38) | 0.558 | 14 (5–34) | 0.908 | 0.537 |
| ICU admission, n (%) | 12 (63.2) | 5 (62.5) | 1.0 | 8 (61.5) | 0.926 | 0.965 |
| Invasive mechanical ventilation, n (%) | 9 (47.4) | 4 (50) | 0.767 | 6 (46.2) | 0.687 | 0.383 |
| Laboratory data, median (range) | ||||||
| D-dimer, ng/ml | 1398 (278–3757) | 2414 (718–5821) | 0.075 | 989 (432–7401) | 0.788 | 0.218 |
| Prothrombin time, s | 15 (13.1–20.2) | 17.5 (14.7–23.4) | 0.093 | 15.3 (11.9–18.5) | 0.347 | 0.063 |
| Platelet count (×103/μL) | 243 (56–530) | 166 (32–254) | 0.063 | 193 (82–420) | 0.466 | 0.111 |
| Death, n (%) | 11 (57.9) | 5 (62.5) | 0.824 | 6 (46.2) | 0.513 | 0.466 |
P values indicated statistical difference between patients with thromboembolic complications and patients with hemorrhagic complications.
P values indicated statistical difference between patients with hemorrhagic complications and patients with negative CTA.
P values indicated statistical difference between patients with thromboembolic complications and patients with negative CTA.
3.2. Use of thromboprophylaxis
During hospitalization, standard prophylactic dose (40 mg enoxaparin daily) was used in 16 patients, intermediate-dose thromboprophylaxis (enoxaparin 0.5 mg/kg every 12 h) was used in 14 patients, and full therapeutic anticoagulation (1 mg/kg enoxaparin every 12 h, or heparin infusion) was used in 10 patients. In patients with diagnosis of thromboembolism; thromboprophylaxis was escalated to therapeutic anticoagulation (8 patients).
3.3. Imaging findings
Out of 40 CTA/CTV examinations; 27 examinations revealed acute vascular abnormalities (27/40; 67.5%) and 13 CTA studies (13/40; 32.5%) showed no acute vascular findings. Acute hemorrhagic complications were detected in 19 patients (19/40; 47.5%), and thromboembolic complications were diagnosed in 8 patients (8/40; 20%). Imaging findings are summarized in Table 2 .
Table 2.
Hemorrhagic and thrombotic complication diagnosed at CTA in hospitalized patients with COVID-19.
| Imaging findings | Number of patients |
|---|---|
| Hemorrhagic complications | 19 (47.5%) |
| Iliopsoas compartment | 11 |
| Rectus sheath | 4 |
| Pelvic extraperitoneal | 1 |
| Pelvic extraperitoneal and intramuscular hematoma | 2 |
| Mesenteric hematoma | 1 |
| Thrombotic complications | 8 (20%) |
| Venous | 2 |
| Arterial | 2 |
| Splenic infarct | 1 |
| Bowel ischemia | 1 |
| Multiple sites | 2 |
| Normal findings | 13 (32.5%) |
3.4. Hemorrhagic complications
Hemorrhagic complications were diagnosed in 19 patients. The intramuscular hematoma was detected in 17 patients. It involved the iliopsoas compartment unilaterally in 10 patients (Fig. 1 ), bilaterally in 2 patients (Fig. 2 ), and the rectus sheath in 5 cases (Fig. 3 ). Out of these 17 patients, 11 patients were admitted to the ICU. On CT scans, fresh hemorrhage was seen as a discrete mass of high attenuation within the muscle (Fig. 1a). Active contrast extravasation in the arterial phase was detected in 6 patients (Fig. 1b). Pelvic extraperitoneal hemorrhage was found in 3 patients (Fig. 4 ). CT scan in these patients showed large pelvic blood collection. A fluid-fluid level was found in one patient mostly owing to the hematocrit effect (Fig. 4a). Active contrast extravasation in the arterial phase was detected in one patient. Out of these 3 patients with pelvic extraperitoneal hemorrhage, 2 patients had associated intramuscular hematoma (one in the rectus sheath and one in iliopsoas compartments bilaterally). The mesenteric hematoma was diagnosed in one ICU patient. Regarding thromboprophylaxis, 5 patients received the standard prophylactic dose, 4 patients (21.1%) received intermediate-dose thromboprophylaxis and 10 patients (52.6%) received therapeutic dose of anticoagulation.
Fig. 1.
Intramuscular hematoma-Psoas hematoma. (a) Axial non-contrast CT revealed hyperdense fresh hematoma in the right psoas muscle. (b) Axial CTA image revealed sizable left psoas hemorrhage with contrast extravasation suggesting active bleeding.
Fig. 2.
Intramuscular hematoma-Bilateral iliopsoas hematoma. (a) Axial CTA image revealed large right psoas compartment hemorrhage, showing blood fluid level (arrow) (b) Follow up in the same patient 3 weeks later revealed sizable bilateral psoas compartment hematomas as well as a large pelvic hematoma. (c) Axial CTV revealed left iliopsoas hematoma and iliacus hematoma on the right side.
Fig. 3.
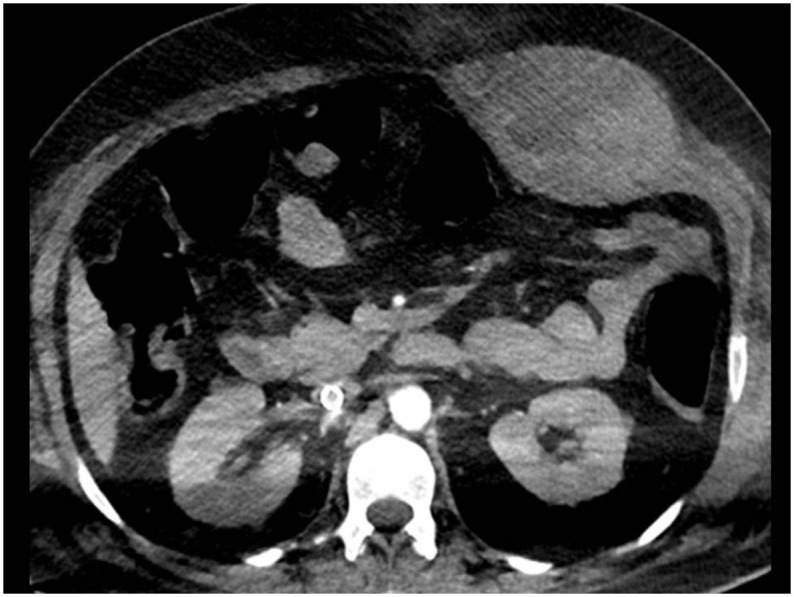
Intramuscular hematoma-Rectus sheath hematoma. (a) Axial CTA image revealed hematoma involving the left rectus sheath, no active bleeding.
Fig. 4.
Pelvic extraperitoneal hemorrhage. (a) Axial CTA image revealed a large amount of pelvic extraperitoneal hemorrhage, showing blood fluid level (long arrow), the patient also had left rectus sheath hematoma with foci of active bleeding (small arrows). (b) Axial CTA image revealed a large amount of pelvic extraperitoneal hemorrhage, showing foci of active bleeding (arrows).
3.5. Thromboembolic complications
Thromboembolic events occurred in 8 patients.
3.6. Arterial thrombus
Arterial occlusion was diagnosed in 2 patients; both of them were admitted to the ICU. CTA of the abdomen and pelvis in both cases revealed thrombus totally occluding the distal abdominal aorta just before bifurcation, and extending to involve the left common and external iliac arteries (Fig. 5 ).
Fig. 5.
Arterial thrombosis. (a) Axial CTA image revealed acute thrombus partially occluding the distal abdominal aorta. (b) Axial CTA image at a lower level revealed an extension of the thrombus to the left common iliac and external iliac arteries in (c).
3.7. Venous thromboembolism
Venous thrombosis involving the distal inferior vena cava (IVC) and common iliac veins was diagnosed in 4 patients. Three patients were admitted to the ICU, one of them required extracorporeal membrane oxygenation (ECMO) (Fig. 6 ).
Fig. 6.
Venous thrombosis. (a) Coronal CTA image in the venous phase showed filling defect with near-complete occlusion of the infrarenal inferior vena cava (IVC) (arrows). Extracorporeal membrane oxygenation device (ECMO) is inserted. (b) Axial CT chest in the same patient revealed COVID-19 pneumonia in the form of bilateral large consolidations and ground-glass opacities.
3.8. Organ infarction
Intra-abdominal organ infarct was diagnosed in 4 patients; 2 patients had splenic infarcts, only 1 patient developed small bowel ischemia, and one patient had both splenic and small bowel infarcts. Splenic infarcts appeared as single large wedge-shaped hypodensity in two cases, and multiple wedge-shaped areas of hypoattenuation in one case (Fig. 7 ). Splenic vessels were patent. In both patients with bowel ischemia, CTA revealed the absence of mucosal enhancement, and luminal dilatation (Fig. 8 ), mesenteric vasculature was patent. Two patients had iliac vein thrombosis and in addition, they developed other thrombotic complications (one patient had splenic infarct and the other patient had splenic infarct and ischemic bowel changes).
Fig. 7.
Splenic infarction. (a) Axial CTV revealed multiple wedge-shaped areas of hypoattenuation in the spleen indicative of multifocal splenic infarcts. (b) Axial CTA image revealed hypodensity with the spleen suggestive of the splenic infarct.
Fig. 8.
Bowel ischemia. (a) Axial CTA image revealed mural thickening and non- enhancement of the small bowel loops in the left side (white arrows) with a mild intraperitoneal collection. The patient died before surgical exploration. (b) A large wedge-shaped splenic infarct is also noted. (c) Axial CT chest in the same patient revealed features of late COVID-19 pneumonia in the form of predominantly bilateral peripheral consolidations and few ground-glass opacities.
3.9. Comparison of patient groups
D-dimer values were higher in patients with thromboembolic complications compared to the other groups 989 (median value was 2414 vs. 1398 and 989), but not statistically significant. Overall mortality was 57.9%. A slightly higher proportion of patients with thrombotic complications died when compared with the other two groups (62.5% vs. 57.9% and 46.2%) but this was not statistically significant (Table 1).
4. Discussion
In our study, different hemorrhagic and thromboembolic complications have been found in hospitalized patients with COVID-19. The study included 40 patients who presented with acute abdominal pain or decreasing hemoglobin levels over the course of hospitalization and underwent CTA/CTV of the abdomen and pelvis. Among our cohort, acute hemorrhagic complications were detected in 19 patients (47.5%), and thromboembolic complications were diagnosed in 8 patients (20%). Out of 40 patients, 25 patients (62.5%) required ICU admission.
Intra-abdominal hemorrhagic complications were detected in 19 patients in our study most of them (18/19) were extraperitoneal. Intramuscular hematoma was detected in 17 patients. It involved the iliopsoas compartment unilaterally in 10 patients, bilaterally in 2 patients, and the rectus sheath in 5 cases. Out of these 17 patients, 11 patients were admitted to the ICU. Iliopsoas hematoma is considered a potentially lethal condition. It usually occurs as a result of trauma, or hematologic disorders. Patel et al reported a case of retroperitoneal hemorrhage in a COVID-19 patient involving the entire length of right psoas muscle.11 Conti et al described two cases of spontaneous abdominal bleeding, at the iliopsoas and pelvic extraperitoneal regions in hospitalized patients with bilateral interstitial pneumonia and SARS-CoV-2.12 In our study, 3 patients developed pelvic extraperitoneal hemorrhage, 2 of them had associated intramuscular hematoma (one in the rectus sheath and one in iliopsoas muscle). Major bleeding in critically ill patients with COVID-19 is significant clinically given the high morbidity and mortality associated with it.13
As therapeutic anticoagulation may be required in patients with COVID-19 to combat the hypercoagulable state, awareness of life-threatening bleeding complications such as retroperitoneal hemorrhage is of great importance.13 , 14 The psoas muscle can accumulate up to 10 times its own volume and can present with abdominal pain or decrease in hemoglobin as seen in our patients. Hemodynamically unstable patients on anticoagulation therapy with large soft tissue hematomas can be treated with arterial embolization as it is minimally invasive with quick therapeutic effect when compared with surgical treatment. When active psoas hematoma bleeding is seen, arterial embolization of third and fourth lumbar arteries is usually required.11 It requires selective catheterization of the bleeding artery, embolization, and post-embolization studies to document successful treatment.11
Anticoagulant drugs are the cornerstone for venous thromboembolism prophylaxis; however, patients with COVID-19 can develop complications which can affect their bleeding status.15 Wang et al found that among the patients with COVID-19 who were at high risk of venous thromboembolism in their cohort, 11% also had a high risk of bleeding.16 In a study included 187 critically ill COVID-19 patients, 8% of the patients experienced hemorrhagic complications more than half were classified as major bleeding; one third of them received therapeutic anticoagulation.17 Fraissé et al reported 21% (19/92) rate of significant hemorrhage among COVID-19 patients admitted to the ICU, five of these events occurred in deep muscles, and most of these patients (84%) received full-dose anticoagulation.18 Escalation of thromboprophylaxis may be associated with increased incidence of major and fatal bleeding, which has been reported to occur in some COVID-19 Italian patients.19
It is important to keep in mind that patients with COVID-19 may develop thrombosis of various vascular systems and there could be concomitant involvement of one or more venous or arterial vascular beds. Therefore, if evidence of thrombosis is detected, careful assessment of the entire imaged vascular system, as well as correlated organ or structure is essential.5 , 20
There is growing evidence that COVID-19 is associated with an increased incidence of coagulopathy, which may predispose patients to different serious complications sequel to arterial and venous thrombosis. Multiple factors have been implicated, including inflammatory cytokine release, and endothelial dysfunction.20 In addition, hypoxia, immobility, the use of mechanical ventilation and extracorporeal circuits all contribute to the increased risk of thrombosis. The occurrence of macrovascular thrombosis in COVID-19 has been reported in a few studies. Klok et al reported an incidence of 31% venous thrombosis and thromboembolism in ICU patients.21 In our study 4 patients had IVC and iliac vein thrombosis, one of them required extracorporeal membrane oxygenation (ECMO). CT angiography/venography helps for adequate evaluation of thrombus extension into central veins. The presence of a filling defect within a vessel, with or without occlusion, is the characteristic finding of venous thrombosis.20
In a study of 199 hospitalized patients with COVID-19, proximal and distal deep vein thrombosis (DVT) was recorded in 7.1% and 5.6% of the patients, mostly in ICU patients.22 In another study, screening of hospitalized COVID-19 patients by lower limb duplex ultrasound revealed lower limb DVT in 15% of the patients; over half of them showed a proximal extension. Importantly, signs or symptoms leading to the clinical suspicion of DVT were present in only four of the patients screened (2%).23 Lodigiani et al in their study showed that, despite the use of anticoagulant prophylaxis, the rate of venous and arterial thromboembolic complications in hospitalized COVID-19 patients was approximately 8%.24
Aorto-iliac thrombus was detected in two cases in our study. Wengerter et al found major vessel arterial thrombosis occurred at a rate of 1% (4/773) or less in patients hospitalized with COVID-19.25 CT angiography aids in the assessment of the extent of thrombosis and its potential complications. Goldman et al found that 94% of patients with COVID-19 who had lower extremity arterial thrombus, had proximal thrombi compared with 47% of control patients; suggesting a large thrombus burden and high frequency of thromboses involving proximal vessels in these patients.26 Another study reported arterial thrombosis in two patients with COVID-19 involving the descending thoracic aorta and infrarenal aorta extending into common iliac arteries without evidence of atherosclerosis.27 A study included patients from 4 tertiary hospitals in UK, reported a relatively higher incidence of arterial ischemic complications (6.4%) compared to 2.6% in other studies.17 , 28 These cases illustrate that the prothrombotic sequela of COVID-19 are not confined to the venous circulation, and macrovascular thrombi in the arterial circulation can occur in during SARS-CoV-2 infection.
To date, only a few imaging findings of splenic infarction related to COVID-19 have been reported, including solitary or multifocal splenic infarcts. Generally, the splenic infarct is usually attributed to systemic hypercoagulability or cardiac thromboembolism.20 , 29 In our study, 3 patients developed splenic infarction. Two patients in our study had iliac vein thrombosis and in addition, they developed other thrombotic complications (one patient had splenic infarct and the other patient had splenic infarct and ischemic bowel changes).
In patients with COVID-19, bowel wall ischemia occurs in the setting of either arterial macro- or microthrombosis, and in many cases the main mesenteric vasculature was patent at imaging as in our cases. On CT, early signs of bowel ischemia include bowel wall thickening and edema and dilated loops (>3 cm), followed by the presence of non-enhancing thickened bowel loops suggesting bowel infarction later, pneumatosis, and portal venous gas have been described.30 Two patients in our study showed findings suggestive of bowel ischemia (absence of mucosal enhancement, and luminal dilatation). It has been described that areas of bowel necrosis have demonstrated unique characteristics at surgery, including yellow discoloration and clear demarcation of the borders of ischemia without anatomic transition zones, with circumferential and patchy antimesenteric involvement. It has been suggested that this phenomenon may a result from microvascular thrombosis and associated inflammation in COVID-19 patients.31 , 32
Thromboembolic complications were reported in many studies at abdominopelvic CT. Goldberg-Stein et al in their cohort, among 80 patients with positive finding at CT of the abdomen and pelvis; 4 had splenic infarcts, 3 had deep vein thrombosis, 3 had arterial occlusion and no cases of intestinal ischemia were reported in their study.9 Dane et al detected thromboembolism at abdominopelvic CT in 6 patients (15.8%), 2 patients had splenic infarctions with patent vasculature similar to our cases, and 3 patients had arterial thromboembolism in the aorta or major branches.29 Bhayana et al described among 42 patients undergone abdominopelvic CT, 3 patients with bowel ischemia, and 2 patients developed solid organ infarction.30
Many studies showed that patients who have severe COVID-19 have a tendency to develop thromboembolic complications, and they also can less frequently have bleeding complications. A multicenter study reported that radiographically confirmed venous thromboembolic rate was about 4.8% (7.6% in critically ill patients), in patients managed with standard doses of prophylactic anticoagulation. The same study observed an overall bleeding rate of about 4.8% (7.6% in the critically ill).10 , 13 , 33 With the expectation that another wave of COVID-19 may hit the world in the following few months, detailed knowledge about various complications related to COVId-19 is essential. We currently know that there is an increased risk of thrombosis in COVID- 19. However, the current use of therapeutic anticoagulants may possibly contribute to the development of various hemorrhagic complications.
There are some limitations of this study. First, this was a single-center retrospective study, which limits its generalizability. Further multicenter studies are recommended. Second, the pathologic correlation with imaging abnormalities was not available for our patients.
5. Conclusion
Both hemorrhagic and thromboembolic complications can be seen at CTA of the abdomen and pelvis in hospitalized COVID-19 patients. Our study highlights the importance that radiologists should maintain a high index of suspicion for thromboembolic complications. We reported higher rates of hemorrhagic complications, this restates the need for better evaluation of the risk-benefit ratio of different anticoagulation strategies.
Ethics approval and consent to participate
Approval for this study was obtained from the Research Ethics Committee of Kuwait ministry of health Ass undersecretary for planning and quality (1520/2020) All study procedures were carried out in accordance with the Declaration of Helsinki regarding research involving human subjects. Consent to participate was waived (retrospective study).
Declaration of competing interest
The authors have no potential conflicts of interest to disclose.
References
- 1.Revzin M.V., Raza S., Warshawsky R., et al. Multisystem imaging manifestations of COVID-19, part 1: viral pathogenesis and pulmonary and vascular system complications. Radiographics. 2020;40:1574–1599. doi: 10.1148/rg.2020200149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Behzad S., Aghaghazvini L., Radmard A.R., Gholamrezanezhad A. Extrapulmonary manifestations of COVID-19: radiologic and clinical overview. Clin Imaging. 2020;66:35–41. doi: 10.1016/j.clinimag.2020.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Laya BF, Cledera THC, Lim TRU, Baluyut JMP, Medina JMP, Pasia NV. Cross-sectional imaging manifestations of extrapulmonary involvement in COVID-19 disease. JComput Assist Tomogr. 2020; Nov 12. [DOI] [PubMed]
- 4.Oudkerk M, Büller HR, Kuijpers D, van Es N, Oudkerk SF, McLoud TC, et al. Diagnosis, prevention, and treatment of thromboembolic complications in COVID-19: report of the National Institute for Public Health of the Netherlands. Radiology 2020; Apr 23:201629. [DOI] [PMC free article] [PubMed]
- 5.O’Shea A., Parakh A., Hedgire S., Lee S.I. Multisystem assessment of the imaging manifestations of coagulopathy in hospitalized patients with COVID-19. AJR Am J Roentgenol. 2021 Feb;3:1–9. doi: 10.2214/AJR.20.24132. [DOI] [PubMed] [Google Scholar]
- 6.Lui K, Wilson MP, Low G. Abdominal imaging findings in patients with SARS-CoV-2 infection: a scoping review. Abdom Radiol 2020; Sep 14:1–7. [DOI] [PMC free article] [PubMed]
- 7.Shiralkar K., Chinapuvvula N., Ocazionez D. Cross-sectional abdominal imaging findings in patients with COVID-19. Cureus. 2020;12 doi: 10.7759/cureus.9538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaafarani H.M.A., El Moheb M., Hwabejire J.O., et al. Gastrointestinal complications in critically ill patients with COVID-19. Ann Surg. 2020;272:e61–e62. doi: 10.1097/SLA.0000000000004004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goldberg-Stein A, Fink A, Paroder V, Kobi M, Yee J, Chernyak V. Abdominopelvic CT findings in patients with novel coronavirus disease 2019 (COVID-19). Abdom Radiol (NY).2020; Aug 6:1–11. [DOI] [PMC free article] [PubMed]
- 10.Al-Samkari H., Karp Leaf R.S., Dzik W.H., et al. COVID and coagulation: bleeding and thrombotic manifestations of SARS-CoV2 infection. Blood. 2020;136:489–500. doi: 10.1182/blood.2020006520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patel I., Akoluk A., Douedi S., et al. Life-threatening psoas hematoma due to retroperitoneal hemorrhage in a COVID-19 patient on enoxaparin treated with arterial embolization: a case report. J Clin Med Res. 2020;12(7):458–461. doi: 10.14740/jocmr4256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Conti C., Henchi S., Coppeta G., Testa S., Grassia R. 2020. Bleeding in COVID-19 severe pneumonia: the other side of abnormal coagulation pattern? Eur J Intern Med. 2020;77:147–149. doi: 10.1016/j.ejim.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dogra S., Jain R., Cao M., et al. Hemorrhagic stroke and anticoagulation in COVID-19. J Stroke Cerebrovasc Dis. 2020;28:104984. doi: 10.1016/j.jstrokecerebrovasdis.2020.104984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Joob B., Wiwanitkit V. Hemorrhagic problem among the patients with COVID-19: clinical summary of 41 Thai infected patients. Clin Appl Thromb Hemost. 2020 Jan-Dec;26 doi: 10.1177/1076029620918308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Erdinc B, Raina JS. Cureus. Spontaneous retroperitoneal bleed coincided with massive acute deep vein thrombosis as initial presentation of COVID-19. 2020 Aug 15;12(8): e9772. [DOI] [PMC free article] [PubMed]
- 16.Wang T., Chen R., Liu C., et al. Attention should be paid to venous thromboembolism prophylaxis in the management of COVID-19. Lancet Haematol. 2020 May;7(5):e362–e363. doi: 10.1016/S2352-3026(20)30109-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shah A., Donovan K., McHugh A., et al. Thrombotic and haemorrhagic complications in critically ill patients with COVID-19: a multicentre observational study. Crit Care. 2020 Sep 18;24(1):561. doi: 10.1186/s13054-020-03260-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fraissé M., Logre E., Pajot O., Mentec H., Plantefève G., Contou D. Thrombotic and hemorrhagic events in critically ill COVID-19 patients: a French monocenter retrospective study. Crit Care. 2020 Jun 2;24(1):275. doi: 10.1186/s13054-020-03025-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cattaneo M., Bertinato E.M., Birocchi S., et al. Pulmonary embolism or pulmonary thrombosis in COVID-19? Is the recommendation to use high-dose heparin for thromboprophylaxis justified? Thromb Haemost. 2020 Aug;120(8):1230–1232. doi: 10.1055/s-0040-1712097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Revzin M.V., Raza S., Srivastava N.C., et al. Multisystem imaging manifestations of COVID-19, part 2: from cardiac complications to pediatric manifestations. Radiographics. 2020;40:1866–1892. doi: 10.1148/rg.2020200195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klok F.A., Kruip M.J.H.A., van der Meer N.J.M., et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Middeldorp S., Coppens M., van Haaps T.F., et al. Incidence of venous thromboembolism in hospitalized patients with COVID-19. J Thromb Haemost. 2020;18:1995–2002. doi: 10.1111/jth.14888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baccellieri D., Bertoglio L., Apruzzi L., Ardita V., D’Angelo A., Bossi M. Incidence of deep venous thrombosis in COVID-19 hospitalized patients during the first peak of the Italian outbreak. Phlebology. 2020 Nov;26 doi: 10.1177/0268355520975592. [DOI] [PubMed] [Google Scholar]
- 24.Lodigiani C., Iapichino G., Carenzo L., et al. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb Res. 2020 Jul;191:9–14. doi: 10.1016/j.thromres.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wengerter S., Wengerter K., Masoudpoor H., et al. Acute aortoiliac and infrainguinal arterial thrombotic events in four patients diagnosed with the novel Coronavirus 2019 (COVID-19) J Vasc Surg Cases Innov Tech. 2020 Dec;6(4):698–702. doi: 10.1016/j.jvscit.2020.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goldman I.A., Ye K., Scheinfeld M.H. Lower-extremity arterial thrombosis associated with COVID-19 is characterized by greater thrombus burden and increased rate of amputation and death. Radiology. 2020 Nov;297(2):E263–E269. doi: 10.1148/radiol.2020202348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vulliamy P., Jacob S., Davenport R.A. Acute aorto-iliac and mesenteric arterial thromboses as presenting features of COVID-19. Br J Haematol. 2020 Jun;189(6):1053–1054. doi: 10.1111/bjh.16760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Helms J., Tacquard C., Severac F., et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020 Jun;46(6):1089–1098. doi: 10.1007/s00134-020-06062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dane B., Smereka P., Wain R., Kim D., Katz D.S. Hypercoagulability in COVID-19: identification of arterial and venous thromboembolism in the abdomen, pelvis, and lower extremities. AJR Am J Roentgenol. 2020 doi: 10.2214/AJR.20.23617. [DOI] [PubMed] [Google Scholar]
- 30.Bhayana R, Som A, Li MD, Carey DE, Anderson MA, Blake MA, et al. Abdominal imaging findings in COVID-19: preliminary observations. Radiology 2020; May 11:201908. [DOI] [PMC free article] [PubMed]
- 31.Singh B., Mechineni A., Kaur P., et al. Acute intestinal ischemia in a patient with COVID-19 infection. Korean J Gastroenterol. 2020 Sep 25;76(3):164–166. doi: 10.4166/kjg.2020.76.3.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Olson M.C., Lubner M.G., Menias C.O., et al. Update: venous thrombosis and hypercoagulability in the abdomen and pelvis-findings in COVID-19. Radiographics. 2020;40:E24–E28. doi: 10.1148/rg.2020200119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bikdeli B., Madhavan M.V., Jimenez D., et al. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up: JACC state-of-the-art review. J Am Coll Cardiol. 2020 Jun 16;75(23):2950–2973. doi: 10.1016/j.jacc.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]









