Abstract
Objective
To compare the value of the subcutaneous tunneling technique versus the normal technique in improving the outcomes of patients undergoing chemotherapy with peripherally inserted central catheters (PICCs).
Methods
One hundred thirty patients were randomly divided into an experimental group (subcutaneous tunneling technique) and control group (normal technique) according to the PICC placement technique, and clinical data were compared between the groups.
Results
In total, 129 PICCs were successfully inserted. Compared with the control group, the experimental group had a lower occurrence of complications after placement (especially catheter dislodgement: 3.1% vs. 15.4%, venous thrombosis: 3.1% vs. 15.4%, and wound oozing: 14.1% vs. 27.7%), lower occurrence of unscheduled PICC removal (3.1% vs. 13.8%), greater comfort during placement (14.16 ± 2.21 vs. 15.09 ± 2.49 on a scale ranging from 6 to 30 points, with higher scores indicating lower degrees of comfort), and lower costs of PICC maintenance (median (interquartile range) per-day maintenance cost: 13.90 (10.99–32.83) vs. 15.69 (10.51–57.46) Yuan). The occurrence of complications and amount of bleeding during placement were not significantly different between the two groups.
Conclusions
The subcutaneous tunneling technique can improve PICC placement by reducing complications and costs of maintenance with better patient comfort during placement.
Keywords: Subcutaneous tunneling technique, peripherally inserted central catheter, chemotherapy, complication, randomized controlled trial, patient comfort
Introduction
Peripherally inserted central catheters (PICCs) have become widely used in China for patients requiring chemotherapy, parenteral nutrition, and medication administration, especially with the introduction of power-injectable PICCs. Power-injectable PICCs supply rapid intravenous infusion, high-pressure injection of contrast media for radiological examinations, easy central venous pressure monitoring, and a multilumen option for clinical selection that truly realizes a “one-needle” approach to completing all intravenous therapy for patients.1 Although power-injectable PICCs have further expanded the clinical use of PICCs, PICC use still has limitations and complications associated with patients’ health status, catheter maintenance, catheter implanting techniques, and other factors. Multiple lumen catheters ranging from 4 to 6 Fr are currently available on the market, but the need for an adequate vein size in the upper mid-arm limits their use.2 The incidence of wound oozing, which is still the most common complication, can be as high as 24.7%.3 Other complications also remain a matter of concern, including infection, skin injury, catheter dislodgement, and even catheter dislocation.
The subcutaneous tunneling technique has been advocated to improve the outcomes of patients with PICCs and has been proven effective for tunneled central catheters inserted in the internal jugular and subclavian veins.4 With this method, a short PICC tract can be tunneled subcutaneously to simultaneously obtain both a higher venipuncture site in the upper mid-arm and a more safely positioned exit site. Additionally, this technique creates distance between the puncture site and the exit site, which minimizes micromotion and offers mechanical stability for the long and heavy end of the PICC, thus reducing the risk of catheter dislodgement.5
The use of subcutaneous tunnels in vascular access is not a new concept, and its feasibility and safety are well documented.4,6,7 However, only a few reports have described the use of this technique for PICC placement. The method was first reported by Selby et al.8 using a hemostat and scalpel for PICC placement in 2001. Thereafter, Pittiruti and Scoppettuolo9 and Elli et al.2 successively created a quick tunnel with a needle cannula, which was associated with no significant complications. Saijo et al.10 reported that the use of a tunneled PICC for a small-diameter basilic vein was safe and feasible. However, these previous studies were single-center clinical trials with small samples.8,10,11 Moreover, the tunneling devices used in previous studies, such as hemostats, puncture needles, and 14G trocars, are inappropriate5,12–14; such devices have a limited effect on catheter fixation and may even exert more trauma to the subcutaneous tissue. Therefore, the present randomized controlled trial was performed to evaluate the impact of the subcutaneous tunneling technique using a proper metal tunneller on the outcomes of patients with PICCs.
Methods
Trial design and ethical considerations
This prospective randomized controlled trial with long-term follow-up was conducted from July 2019 to January 2020 at Sun Yat-Sen University Cancer Center in Guangzhou, China. Ethical approval was provided by the ethics committee of Sun Yat-Sen University Cancer Center (approval no. B2018-111-01). Written informed consent was obtained from all patients in advance. This study has been registered in the Chinese Clinical Trial Registry (ChiCTR1900021624, http://www.chictr.org.cn/showproj.aspx?proj=34929).
Participants
Eligible participants who were candidates for PICC insertion were recruited for the trial. The inclusion criteria were an age of 18 to 75 years, the ability to understand and communicate in Chinese, first-time PICC placement, and scheduled to regularly receive catheter maintenance at our hospital. Patients with any contraindications for PICC placement were excluded.
Sample size and randomization
The sample size was calculated based on the incidence of wound oozing between the two groups in a previous study (experimental group: 13.3%, control group: 36.7%).15 According to the two-sided PICC calculations and the formula for comparing a two-sample rate and considering a dropout rate of 20%, 130 patients were needed to achieve 80% power with a two-tailed alpha of 5%, and 65 patients were required in each group. Eligible patients were assigned to the groups in a 1:1 manner according to computer-generated random numbers. The randomization scheme was hidden using the envelope method and managed by a researcher who was not involved in participant recruitment.
Intervention
The PICCs used in this study were open-ended, power-injectable polyurethane PICCs with a proximal valve (PowerPICC SOLO 2; Bard Access Systems, Inc., Salt Lake City, UT, USA). All PICCs were inserted by one experienced PICC specialist, and maintenance was conducted by a uniformly trained intravenous catheter team at our hospital. In the control group, non-tunneled PICC catheterization was performed using the normal PICC placement technique under B-mode ultrasound guidance combined with the modified Seldinger technique. In the experimental group, the PICC was placed using a subcutaneous tunneling technique combined with the normal PICC placement technique for which the puncture site was located 5 cm above the catheter exit site on the patient’s upper arm. First, the vein was evaluated by ultrasound, and the catheter exit site and puncture site (5 cm above) were marked on the patient’s arm. Second, we used a scalpel to create a 1- to 2-mm skin incision at the puncture site after inserting the guide wire into the vein. Third, using local anesthesia at the puncture and exit sites, a metal tunneller with a blunt end to penetrate the subcutaneous tissue and another end to connect the catheter was inserted into the subcutaneous tissue from the exit site to the puncture site. Fourth, the catheter was introduced from the tunnel to the puncture site through the tunneller, which was subsequently removed. Finally, we used reinforced sterile skin closures (3M, Saint Paul, MN, USA) to close the skin incision at the puncture site after catheterization. All PICCs were placed under electrocardiographic guidance, and a chest radiograph was obtained to confirm the correct catheter tip position.
Definitions and measures of outcomes
The primary outcome in this study was the incidence of wound oozing. Complications after placement included wound oozing, venous thrombosis, mechanical phlebitis, medical adhesive-related skin injury, occlusion, infection, catheter dislodgement, and unscheduled PICC removal. Immediate complications during placement were venipuncture failure, injuries to nerves or arteries, primary malpositioning, bleeding from the exit site, and difficulties in catheter or guide wire progression. Other outcomes were the success rate of placement, the duration of cannulation, the patients’ degree of comfort during placement, and the patients’ costs of PICC maintenance.
Immediate complications during PICC placement, as well as the catheter cannulation length, punctured vein diameter and depth, duration of cannulation, and amount of bleeding, were assessed and recorded by the researcher during cannulation. Complications after PICC placement were inspected and recorded by the uniformly trained intravenous catheter team at each dressing change. The patients’ degree of comfort during placement was measured using a revision of the patient degree of comfort questionnaire designed by Li et al.16 The questionnaire contained six items consisting of pain and fatigue issues that influence the patient’s degree of comfort during insertion. Scoring was performing using a 5-point Likert scale, with 1 representing “strongly disagree” and 5 representing “strongly agree” (Item 2 is scored in reverse). The total score ranged from 6 to 30 points, with higher scores corresponding to lower degrees of comfort. Patients’ PICC-related costs included the costs for placement and maintenance. The costs of placement consisted of expenditures related to consumable material, medical devices, and drugs used during the PICC placement procedure. The costs of maintenance included payments for dressing changes, diagnostic examinations, and treatment for complications. The definitions of outcomes and complications of PICC placement are shown in Table 1.
Table 1.
Definitions of complications and outcomes of PICC placement.
| Outcomes | Definitions |
|---|---|
| Successful placement | Successful placement on first puncture attempt in the control group or successful placement by tunneling on first attempt in the experimental group. |
| Wound oozing | Oozing that lasted >24 hours after placement. Classified into three grades according to severity: Grade 1, (bleeding lasting for 2 to 3 days), Grade 2, (bleeding lasting for 4 to 5 days), and Grade 3 (bleeding lasting >6 days). |
| Bleeding from insertion site during placement | Measured by the difference in the weight of sterile gauze before and after insertion. During placement, all blood was absorbed by the sterile gauze. |
| Depth and diameter of puncture vein | Measured using the same B-ultrasound instrument used to assess the vessel. |
| Duration of cannulation | Measured from first puncture to complete fixation. |
| Catheter-related venous thrombosis | The presence of an intraluminal thrombus as confirmed by color Doppler ultrasound. Classified as symptomatic or asymptomatic (symptomatic thrombosis was diagnosed when symptoms occurred). Ultrasound evaluation was performed before catheter removal to check whether asymptomatic thrombosis had occurred. |
| Mechanical phlebitis | Symptoms of vein irritation including induration, warmth, pain, or tenderness existing around the insertion site. Classified into five grades according to the standards of the Infusion Nurses Society. |
| Medical adhesive-related skin injury | Skin itching, erythema, bulla, or tearing that persisted for ≥30 minutes after removal of adhesive dressing.18 Divided into three grades: slight, (slight skin itching and erythema (less than 5 × 5 cm)), moderate, (obvious skin itching, erythema, and papules (more than 5 × 5 cm)), and severe (unbearable itching accompanied by blisters, corrosion, or exudation (more than 10 × 10 cm) that affected sleep, daily life, and even therapy). |
| Catheter occlusion | Inability to infuse any fluid into the catheter or aspirate blood from the PICC. |
| Catheter-related infection | Defined according to the Centers for Disease Control and Prevention and classified as local infection or central line-associated bloodstream infection. |
| Catheter dislodgement | Exposed portion of PICC prolapsed by >2 cm. |
| Unplanned extubation | Removal of PICC because of complications or for reasons other than completion of the patient’s intended treatment. |
PICC, peripherally inserted central catheter.
Statistical analysis
The statistical analysis was performed with IBM SPSS Statistics for Windows, Version 25.0 (IBM Corp., Armonk, NY, USA). Continuous variables are expressed as mean and standard deviation and were assessed using the chi-square test or Fisher’s exact test. Categorical variables are reported as percentages and were assessed using the independent-samples t-test. The level of statistical significance was set at 0.05.
Results
Demographic and disease data
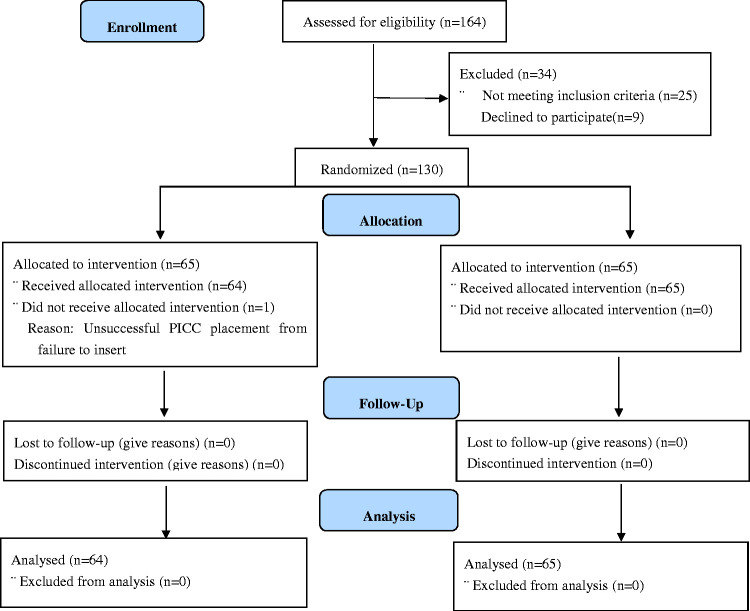
In total, 130 patients were recruited and randomized from July 2019 to January 2020. Of these 130 patients, 129 underwent successful PICC insertion; the exception was 1 patient in the experimental group whose procedure was considered a technical failure because of inability to puncture. All PICCs were used for chemotherapy, and all patients had cancer. Most patients had nasopharyngeal carcinoma, followed by lymphoma or osteosarcoma, with no significant differences between the two groups. Additionally, there were no significant differences in the patients’ demographic data, disease-related data, or coagulation function. This detailed information is shown in Table 2. A CONSORT diagram of patient recruitment is shown in Figure 1.
Table 2.
Basic characteristics of participants.
| Characteristics | Categories | Experimental group (n = 64) | Control group (n = 65) | t/χ2 | P |
|---|---|---|---|---|---|
| Age, years | 45.64 ± 11.59 | 47.95 ± 11.96 | −1.115 | 0.267 | |
| Sex | Male | 35 (54.7) | 39 (60.0) | 0.372 | 0.595 |
| Female | 29 (45.3) | 26 (40.0) | |||
| Diagnosis | Nasopharyngeal carcinoma | 38 (59.4) | 37 (56.9) | 3.879 | 0.923 |
| Lymphoma or osteosarcoma | 10 (15.6) | 11 (16.9) | |||
| Upper gastrointestinal cancer | 8 (12.5) | 9 (13.9) | |||
| Gynecological or breast cancer | 8 (12.5) | 8 (12.3) | |||
| Treatment | Chemotherapy | 10 (15.6) | 13 (20.1) | 3.184 | 0.374* |
| Chemotherapy with radiotherapy | 50 (78.1) | 50 (76.9) | |||
| Surgery with chemotherapy | 4 (6.3) | 1 (1.5) | |||
| Surgery with chemotherapy and radiotherapy | 0 (0.0) | 1 (1.5) | |||
| Body mass index, kg/m2 | ≤18.4 | 5 (7.8) | 4 (6.2) | 0.964 | 0.617 |
| 18.5–24.9 | 45 (70.3) | 42 (64.6) | |||
| ≥25.0 | 14 (21.9) | 19 (29.2) | |||
| Coagulation functiona | |||||
| Platelet count | Low | 1 (1.6) | 0 (0.0) | 2.636 | 0.268 |
| Normal | 55 (85.9) | 61 (93.8) | |||
| High | 8 (12.5) | 4 (6.2) | |||
| APTT | Low | 4 (6.3) | 11 (16.9) | 3.575 | 0.097 |
| Normal | 60 (93.7) | 54 (83.1) | |||
| TT | Low | 1 (1.6) | 0 (0.0) | 1.024 | 0.496 |
| Normal | 63 (98.4) | 65 (100) | |||
| PT | Normal | 64 (100) | 64 (98.5) | 0.992 | 0.319 |
| High | 0 (0.0) | 1 (1.5) | |||
| Fibrinogen | Normal | 56 (87.5) | 60 (92.3) | 0.823 | 0.397 |
| High | 8 (12.5) | 5 (7.7) | |||
| D-dimers | Normal | 58 (90.6) | 62 (95.4) | 1.126 | 0.324 |
| High | 6 (9.4) | 3 (4.6) |
Data are presented as mean ± standard deviation or n (%).
aReference ranges: platelet count, 100–300 × 109/L; APTT, 22.5–34 s; TT, 14.0–21.0 s; PT, 9.8–13.5 s;
fibrinogen, 1.80–4.00 g/L; D-dimers, 0.00–0.55 μg/mL.
*Fisher’s exact test.
APTT, activated partial thromboplastin time; TT, thrombin time; PT, prothrombin time.
Figure 1.
CONSORT diagram of recruited patients.
PICC, peripherally inserted central catheter.
Data related to PICC placement
The data related to PICC placement in the two groups are shown in Table 3. The total duration of catheter placement was 5451 days with a median (interquartile range) of 88 (8–191) days in the experimental group and 5532 days with a median (interquartile range) of 72 (15–192) days in the control group, with no significant difference between the two groups. In both groups, most PICCs were placed in the right arm with the insertion site in the basilic vein. The exposure length of most PICCs was 4 cm, and the tip position was parallel to the seventh dorsal vertebra in both groups. No significant difference in vein depth was found between the groups. There was also no significant difference in the vein diameter at the exit site between the two groups.
Table 3.
PICC characteristics of participants.
| Characteristics | Categories | Experimental group(n = 64) | Control group(n = 65) | P |
|---|---|---|---|---|
| Arm for PICC placement | Left | 11 (17.2) | 8 (12.3) | 0.434 |
| Right | 53 (82.8) | 57 (87.7) | ||
| Vein for puncture | Basilic | 47 (73.4) | 51 (78.5) | 0.82 |
| Brachial | 10 (15.6) | 13 (20.0) | ||
| Axillary | 7 (10.9) | 1 (1.5) | ||
| Basal upper-arm circumference, cm | 27.258 ± 3.330 | 26.623 ± 2.330 | 0.211 | |
| Upper arm length, cm | 21.961 ± 1.429 | 21.746 ± 1.335 | 0.379 | |
| Vein diameter, cm | At exit site | 0.40 (0.15–0.80) | 0.50 (0.20–0.70) | 0.160 |
| At puncture site | 0.50 (0.30–1.00) | 0.50 (0.20–0.70) | 0.001 | |
| Vein depth, cm | At exit site | 0.725 (0.25–2.00) | 0.60 (0.20–2.00) | 0.101 |
| At puncture site | 0.70 (0.25–1.50) | 0.60 (0.20–2.00) | 0.297 | |
| Distance from puncture site to cubital crease, cm | 14.739 ± 1.528 | 9.415 ± 1.304 | <0.001 | |
| Insertion length, cm | 34.44 ± 3.162 | 38.72 ± 3.075 | <0.001 | |
| Exposure length, cm | 4.00 (0.00–17.00) | 4.00 (1.00–6.00) | 0.644 | |
| Amount of bleeding, mL | 2.32 (0.15–8.37) | 1.42 (0.11–7.84) | 0.060 | |
| Duration of placement, minutes | 10.51 (5.57–20.00) | 6.43 (3.97–53.13) | <0.001 | |
| Tip position | T6 | 16 (25.0) | 12 (18.5) | 0.570 |
| T7 | 31 (48.4) | 37 (56.9) | ||
| T8 | 17 (26.6) | 16 (24.6) | ||
| Cannulation duration, days | 88.0 (8.0–191.0) | 72.0 (15.0–192.0) | 0.993 |
Data are presented as n (%), mean ± standard deviation, or median (interquartile range).
PICC, peripherally inserted central catheter.
The mean length from the puncture site to the cubital crease was significantly longer in the experimental group (14.739 ± 1.528 cm) than in the control group (9.415 ± 1.304 cm, P < 0.001). Similarly, the median vein diameter at the puncture site was significantly larger in the experimental group (0.50 cm (0.30–1.00 cm)) than in the control group (0.50 cm (0.20–0.70 cm), P = 0.001). The mean insertion length was also significantly shorter in the experimental group than in the control group (34.44 ± 3.162 cm) than in the control group (38.72 ± 3.075 cm, P < 0.001). The median amount of bleeding was slightly higher in the experimental group (2.32 mL (0.15–8.37 mL)) than in the control group (1.42 mL (0.11–7.84 mL)), but the difference was not statistically significant. The median duration of the maneuver was significantly longer in the experimental group (10.51 minutes (5.57–20.00) minutes) than in the control group (6.43 minutes (3.97–53.13 minutes), P < 0.001).
Complications
Table 4 shows the incidence of complications in both groups. The incidence of complications during placement was not significantly different between the two groups. However, the incidence of complications after placement was significantly lower in the experimental group (32.8%) than in the control group (58.5%, P = 0.003). Venous thrombosis occurred in 2 (3.1%) patients in the experimental group and in 10 (15.4%) patients in the control group, with a significant difference (P = 0.030). The main outcome of wound oozing was significantly lower in the experimental group (14.1%) than in the control group (27.7%, P = 0.032). Catheter dislodgement occurred in 2 (3.1%) patients in the experimental group and in 10 (15.4%) patients in the control group, with a significant difference (P = 0.030). The incidence of infection was slightly lower in the experimental than control group, although the difference was not statistically significant (1.6% vs. 6.1%, respectively). The incidence of unscheduled PICC removal was significantly lower in the experimental group (3.1%) than in the control group (13.8%, P = 0.029). Other complications after placement were not significantly different between the groups.
Table 4.
Complications of the participants.
| Category | Experimental group (n = 64) | Control group (n = 65) | Z/χ2 | P |
|---|---|---|---|---|
| Complications during placement | 16 (25.0) | 19 (29.2) | 0.292 | 0.589 |
| Primary malposition | 14 (21.9) | 16 (24.6) | 3.003 | 0.391 |
| Nerve or artery injuries | 1 (1.6) | 1 (1.5) | – | 1.000* |
| Difficulties in catheter or guide wire propulsion | 3 (4.8) | 8 (12.3) | 2.429 | 0.274* |
| Complications after placement | 21 (32.8) | 38 (58.5) | 8.548 | 0.003 |
| Venous thrombosis | 2 (3.1) | 10 (15.4) | 5.745 | 0.030* |
| Symptomatic | 2 (3.1) | 5 (7.7) | 6.697 | 0.027* |
| Asymptomatic | 0 (0.0) | 5 (7.7) | ||
| Wound oozing | 9 (14.1) | 18 (27.7) | −2.140 | 0.032* |
| Grade 1 | 9 (14.1) | 8 (12.3) | 10.679 | 0.008* |
| Grade 2 | 0 (0.0) | 8 (12.3) | ||
| Grade 3 | 0 (0.0) | 2 (3.1) | ||
| Catheter dislodgement | 2 (3.1) | 10 (15.4) | 5.745 | 0.030* |
| Medical adhesive-related skin injury | 12 (18.8) | 11 (16.9) | 0.073 | 0.786 |
| Slight | 11 (17.2) | 5 (7.7) | −0.046 | 0.963* |
| Moderate | 1 (1.6) | 4 (6.2) | ||
| Severe | 0 (0.0) | 2 (3.1) | ||
| Infection | 1 (1.6) | 4 (6.1) | 1.825 | 0.365* |
| Local | 1 (1.6) | 3 (4.6) | 1.894 | 0.491* |
| Central line-associated bloodstream infection | 0 (0.0) | 1 (1.5) | ||
| Phlebitis | 2 (3.1) | 1 (1.5) | 0.357 | 0.619* |
| Catheter occlusion | 5 (7.9) | 2 (3.0) | 1.409 | 0.273* |
| Unplanned extubation | 2 (3.1) | 9 (13.8) | 4.572 | 0.029* |
Data are presented as n (%).
*Fisher’s exact test.
Degree of comfort during PICC placement
The degree of comfort during PICC placement in the two groups is shown in Table 5. The mean scores for most items were slightly lower in the experimental group than in the control group, although the differences were not statistically significant. However, the patients’ mean total score was significantly lower in the experimental group than in the control group (P = 0.026).
Table 5.
Questionnaire used to assess degree of comfort during PICC placement.
| Item | Experimental group(n = 64) | Control group(n = 65) | P |
|---|---|---|---|
| 1. The pain at the puncture site is unbearable. | 1.81 ± 1.31 | 2.18 ± 1.52 | 0.139 |
| 2. I can understand the nurse’s explanations well and cooperate with the nurse to complete the PICC catheterization. | 3.95 ± 0.68 | 3.95 ± 0.60 | 0.995 |
| 3. The PICC catheterization took a long time, and it was difficult for me to finish because of my discomfort. | 2.13 ± 0.49 | 2.31 ± 0.56 | 0.050 |
| 4. The position in which the PICC is placed is very uncomfortable. | 2.20 ± 0.51 | 2.38 ± 0.68 | 0.088 |
| 5. The first puncture failed and the puncture had to be repeated many times, which caused me a lot of pain. | 1.95 ± 0.21 | 2.03 ± 0.35 | 0.133 |
| 6. I had to keep my jaw close to my collarbone for a long time while the nurse was placing the PICC. | 2.11 ± 0.62 | 2.23 ± 0.55 | 0.243 |
| Total score | 14.16 ± 2.21 | 15.09 ± 2.49 | 0.026 |
PICC, peripherally inserted central catheter.
Costs of PICC placement and maintenance
Table 6 shows the costs for PICC placement and per-day maintenance. The PICC placement cost was 17.87 Yuan higher in the experimental group than in the control group. However, the median per-day maintenance cost was significantly lower in the experimental group (13.90 Yuan (10.99–32.83 Yuan)) than in the control group (15.69 Yuan (10.51–57.46 Yuan), P < 0.05).
Table 6.
Costs of proximal valve power-injectable PICC placement and maintenance.
| Item | Experimental group (n = 64) | Control group (n = 65) | Z | P |
|---|---|---|---|---|
| PICC placement cost, Yuan | 3358.87 | 3341 | −11.314 | <0.001 |
| PICC per-day maintenance cost, Yuan | 13.90 (10.99–32.83) | 15.69 (10.51–57.46) | −2.124 | 0.034 |
Data are presented as total cost or median (interquartile range).
PICC, peripherally inserted central catheter.
Discussion
In this study, a subcutaneous tunneling technique was applied for improved PICC placement, and the results showed that the technique was safe, feasible, and effective. No difference was found in the success rate of catheterization or amount of bleeding between the subcutaneous tunneling technique and the normal technique. However, the PICC-related costs and complications after placement were significantly lower in the subcutaneous tunneling technique group.
The success rate of tunneled PICC placement was 95.3%, which is consistent with previous studies.5,13 In 2019, Dai et al.5 reported that the subcutaneous tunneling technique increased the amount of bleeding through creation of the tunnel. However, our study showed no difference in the amount of bleeding between the two groups. The difference in these findings might be attributed to the difference in the tunneling instrument. A trocar, hemostat, and puncture needle5,12–14 might exert more trauma to the subcutaneous tissue. In our study, the tunneller had a blunt tip at one end to penetrate the subcutaneous tissue and a connection system at the other end to connect to a 3- to 6-Fr PICC; this involved fewer incisions and provided better fixation of the catheter. Moreover, the subcutaneous tissue in the experimental group was expanded by infusing local anesthetic before the passage of the tunneler to reduce the severity of trauma, which also helped to reduce the amount of bleeding.9
Multilumen power-injectable PICCs ranging in size from 4 to 6 Fr are being increasingly used in the clinical setting, especially in patients with cancer or severe illness who require administration of many contemporary therapies. According to one study, the catheter-to-vein ratio should range from 33% to 45% to reduce the incidence of thrombosis.17 However, it is often difficult to find sufficiently large vessels in the middle third of the arm, which limits the use of multilumen PICCs. Our results affirmed that the subcutaneous tunnel technique can reduce the incidence of thrombosis and expand the use of multilumen PICCs. The subcutaneous tunnel technique allows a higher puncture site in which the vein diameter is larger and maintains an exit site in the middle third of the arm for suitable fixation. The large vessel diameter not only helps to reduce the incidence of thrombosis but also enables use of the multilumen catheter, which otherwise exceeds the optimal catheter-to-vein ratio for suitable vessels.
In our study, the incidence of wound oozing was significantly lower in patients with subcutaneous tunnels than in those who underwent PICC placement using the normal method; this finding is consistent with previous studies.4,5,14 In the control group, the puncture site was located above the vessels, and the puncture point of the vessels and skin was located at the same position. The tip of the needle caused mechanical cutting injury to the vessels. Moreover, the diameter of the steel needle trocar was larger than that of the PICC. When the needle was withdrawn, blood seeped out around the PICC. However, the distance between the puncture site and exit site created by the subcutaneous tunnel technique not only increased the friction force of pipe sliding but also compressed the site to prevent bleeding. Therefore, the subcutaneous tunnel technique can reduce the incidence of wound oozing.
Catheter dislodgement caused by increased intrathoracic or intra-abdominal pressure, rapid injection, and too much arm movement13 is a common complication of PICCs. In the present study, the incidence of catheter dislodgement was higher than that reported in other studies.5,12 The reason for this outcome might be the different types of PICCs used. The power-injectable PICC used in the present study was made of polyurethane, and the epitaxial tube of the catheter was relatively hard, which stimulates the local muscle tissue and is not conducive to healing. In this study, the incidence of catheter dislodgement was significantly lower in the experimental group than in the control group. This suggests that the subcutaneous tunneling technique can reduce the incidence of catheter dislodgement after placement. The catheter was exported to the outside of the body through the subcutaneous tunnel, which can minimize freedom of movement and supply fixation of the PICC position to reduce catheter dislodgement.
In 2001, Selby et al.,8 who first reported the subcutaneous tunneling technique, presumed that tunneling may not effectively reduce the infection rate. However, the results of a multicenter retrospective study suggested that subcutaneous tunneling could significantly decrease the rate of central line-associated bloodstream infection.12 In the present study, only one case of central line-associated bloodstream infection and four cases of local infection occurred in the control group, whereas no cases of central line-associated bloodstream infection and two cases of local infection occurred in the experimental group. We believe that there are two explanations for this low incidence of infection. First, the incidence of infection was low in our patients because of the short duration of catheter use and the strict infection control measures in our hospital. Second, the sample size in this study might not have been sufficiently large to detect significant differences in the low incidence of infection between the two techniques. Further research is needed to determine whether this technology can reduce the incidence of infection.
This study is the first to compare the degree of comfort during placement of a tunneled versus non-tunneled PICC. Interestingly, the overall degree of comfort with the tunneled PICC was not lower than that with the non-tunneled PICC; instead, it was slightly higher. The reason for this result might be the injection of a few milliliters of local anesthetic, which expanded the subcutaneous tissue to reduce trauma before the passage of the tunneller and might have also helped to reduce patient pain and tension during cannulation. We consider that although the subcutaneous tunnel technique is more complicated than the normal method, it does not result in greater pain or affect the degree of comfort during placement.
The cost of PICC placement was 17.87 Yuan higher in the experimental group than in the control group. This difference was due to the cost of the 3M reinforced sterile skin closures in the experimental group (17.87 Yuan) to close the nick at the tunneled puncture site. However, the per-day maintenance cost was significantly lower in the experimental group than in the control group. The difference was primarily due to the cost related to management of complications and unscheduled PICC removal. Subcutaneous tunnel technology placement increased the cost by only 17.87 Yuan while reducing the incidence of adverse events and the cost of PICC maintenance.
Limitations
Despite our careful preparation before the study began, certain limitations remain. The first is that double blinding was not possible in our study because the wounds and surgical procedures were different between the groups, which might have influenced the degree of comfort in the two groups. However, blinding was possible for follow-up and diagnosis of complications. The second limitation is the insufficient sample size, which prevented us from determining whether the subcutaneous tunnel technique was effective for reducing the incidence of catheter-related infection. This outcome might have been due to the small sample size in each group, which we calculated based on the incidence of complications after PICC placement; however, it could also have been caused by the low incidence of catheter-related infection in our hospital. The third limitation is that our study focused only on patients with cancer. Further studies involving patients undergoing PICC placement for different reasons are needed.
Conclusions
In this study, we evaluated the effect of the subcutaneous tunneling technique on improving outcomes in patients with PICCs. We demonstrated that the subcutaneous tunneling technique is a safe, feasible, and efficient method to expand the use of multilumen PICCs by allowing insertion of a larger PICC without increasing pain during placement. Moreover, this technique can reduce the cost of PICC maintenance and reduce complications after placement, especially with respect to catheter dislodgement, venous thrombosis, wound oozing, and unscheduled PICC removal. Therefore, the subcutaneous tunneling technique should be recommended to improve patient outcomes of PICC insertion.
Footnotes
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by the Medical Scientific Research Foundation of Guangdong Province of China (A2019007).
ORCID iDs: Jia Li https://orcid.org/0000-0003-0543-664X
Hui-ying Qin https://orcid.org/0000-0001-7079-6022
References
- 1.Zhang S, Sun X, Lei Y. The microbiological characteristics and risk factors for PICC-related bloodstream infections in intensive care unit. Sci Rep 2017; 7: 15074. DOI: 10.1038/s41598-017-10037-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Elli S, Abbruzzese C, Cannizzo L, et al. “Extended subcutaneous route” technique: a quick subcutaneous tunnelling technique for PICC insertion. J Vasc Access 2017; 18: 269–272. DOI: 10.5301/jva.5000647. [DOI] [PubMed] [Google Scholar]
- 3.Leung TK, Lee CM, Tai CJ, et al. A retrospective study on the long-term placement of peripherally inserted central catheters and the importance of nursing care and education. Cancer Nurs 2011; 34: E25–E30. DOI: 10.1097/NCC.0b013e3181f1ad6f. [DOI] [PubMed] [Google Scholar]
- 4.Al-Hwiesh AK, Abdul-Rahaman IS. Tunneled femoral vein catheterization for long term hemodialysis: a single center experience. Saudi J Kidney Dis Transpl 2007; 18: 37–42. [PubMed] [Google Scholar]
- 5.Dai C, Li J, Li QM, et al. Effect of tunneled and nontunneled peripherally inserted central catheter placement: a randomized controlled trial. J Vasc Access 2020: 21: 511–519. 1129729819888120. DOI: 10.1177/1129729819888120. [DOI] [PubMed] [Google Scholar]
- 6.Jenkins GW, Kelly M, Anwar S, et al. The S-Tunnel for tunnelled dialysis catheter: an alternative approach to the prevention of displacement. J Vasc Access 2015; 16: 527–529. DOI: 10.5301/jva.5000455. [DOI] [PubMed] [Google Scholar]
- 7.Yamamoto K, Tanikake M, Arimoto H, et al. Scheme for creating a subcutaneous tunnel to place an indwelling implantable central venous access system in the forearm. Cardiovasc Intervent Radiol 2008; 31: 1215–1218. DOI: 10.1007/s00270-008-9370-0. [DOI] [PubMed] [Google Scholar]
- 8.Selby JB, Jr, Cohn DJ, Koenig G. Peripherally inserted tunnelled catheters: a new option for venous access. Minim Invasive Ther Allied Technol 2001; 10: 231–234. DOI: 10.1080/136457001753334521. [DOI] [PubMed] [Google Scholar]
- 9.Pittiruti M, Scoppettuolo G. The GAVeCeLT manual of PICC and midline. Indications, insertion, management. 1st edition. New York: Edra Publishing, 2017. [Google Scholar]
- 10.Saijo F, Odaka Y, Mutoh M, et al. A novel technique of axillary vein puncture involving peripherally inserted central venous catheters for a small basilic vein. J Vasc Access 2018; 19: 311–315. DOI: 10.1177/1129729818757974. [DOI] [PubMed] [Google Scholar]
- 11.Fabiani A, Dreas L, Sanson G. Tunnelling a midline catheter: when the traffic light shifts from yellow to green. J Vasc Access 2018; 19: 667–671. DOI: 10.1177/1129729818769032. [DOI] [PubMed] [Google Scholar]
- 12.Kim IJ, Shim DJ, Lee JH, et al. Impact of subcutaneous tunnels on peripherally inserted catheter placement: a multicenter retrospective study. Eur Radiol 2019; 29: 2716–2723. DOI: 10.1007/s00330-018-5917-x. [DOI] [PubMed] [Google Scholar]
- 13.Ostroff MD, Moureau NL. Report of modification for peripherally inserted central catheter placement: subcutaneous needle tunnel for high upper arm placement. J Infus Nurs 2017; 40: 232–237. DOI: 10.1097/NAN.0000000000000228. [DOI] [PubMed] [Google Scholar]
- 14.Maria K, Theodoros K, Maria B, et al. Implementation of tunneled versus not tunneled peripherally inserted central catheters. J Vasc Nurs 2019; 37: 132–134. DOI: 10.1016/j.jvn.2018.11.007. [DOI] [PubMed] [Google Scholar]
- 15.Jiang HM, Peng L. Observation on the effect of subcutaneous tunneling in PICC catheterization. World Health Digest Medical Periodical 2013: 73–73,74. [Chinese text] [Google Scholar]
- 16.Li J, Fan YY, Xin MZ, et al. A randomised, controlled trial comparing the long-term effects of peripherally inserted central catheter placement in chemotherapy patients using B-mode ultrasound with modified Seldinger technique versus blind puncture. Eur J Oncol Nurs 2014; 18: 94–103. DOI: 10.1016/j.ejon.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 17.Sharp R, Cummings M, Fielder A, et al. The catheter to vein ratio and rates of symptomatic venous thromboembolism in patients with a peripherally inserted central catheter (PICC): a prospective cohort study. Int J Nurs Stud 2015; 52: 677–685. DOI: 10.1016/j.ijnurstu.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 18.Kim MJ, Jang JM, Kim HK, et al. medical adhesives-related skin injury in a pediatric intensive care unit: a single-center observational study. J Wound Ostomy Continence Nurs 2019; 46: 491–496. DOI: 10.1097/WON.0000000000000592. [DOI] [PubMed] [Google Scholar]