Abstract
Objectives
To 1) report prevalence of ‘osteosarcopenia’ (OS) and osteosarcopenic obesity (OSO) entities using evidence-based diagnostic techniques and definitions, 2) examine if OSO offers additional predictive value of functional decline over its components, and 3) identify associated factors in a multi-racial Southeast Asian population.
Methods
We performed a cross-sectional study of a representative sample of 542 community-dwelling adults (21–90 years old), and assessed anthropometry, cognition, functional performance, and self-report sociodemographic, health and lifestyle questionnaires. Low muscle mass, and the Asian Working Group for Sarcopenia (AWGS) 2019 criteria, were used to assess sarcopenia. Obesity was defined using percentage body fat and fat mass index. Osteopenia/osteoporosis was determined using lumbar spinal bone mineral density. Associated factors were examined using logistic regression, and OSO’s value investigated using linear regressions with functional performance.
Results
OS and OSO prevalence were 1.8% and 0% (21–59 years), 12.9% and 2.8% (≥ 60 years), 17.3% and 4.1% (≥ 65 years), and 25.5% and 7.0% (≥75 years), respectively. OSO entity as defined was not a significant predictor (P > 0.05) and did not improve explanations for functional decline over sarcopenia or sarcopenic obesity. Age, sex, race and body mass index (BMI) were associated with OS, while age, sex, race and alcoholism were associated with OSO.
Conclusions
Our results do not support OSO as a distinct entity in relation to functional decline. Aside from biological age, sex, and race, amenable lifestyle factors such as BMI and alcohol intake are important variables that can influence the co-existence of osteopenia/osteoporosis, sarcopenia and obesity.
Keywords: Osteosarcopenic obesity, Osteopenia, Sarcopenia, Obesity, Prevalence
1. Introduction
With older persons (≥ 65 years old) set to grow two-fold to 1.5 billion within the next 3 decades [1], many societies will need to better manage and delay conditions related to physiological ageing. The loss of muscle mass, strength and function with age (sarcopenia) [2], disproportionate accrual of body fat (obesity) [3], and decline in bone density and bone health (osteopenia/osteoporosis) [4] commonly accompany normal ageing. Due to their shared pathophysiological risk factors, these conditions are often found to be overlapping and mutually-aggravating. A concurrent occurrence of sarcopenia and obesity is characterized as ‘sarcopenic obesity’ (SO) [3], sarcopenia and osteopenia/osteoporosis known as ‘osteosarcopenia’ (OS) [5] while coexistence of all 3 conditions has been referred to as ‘osteosarcopenic obesity’ (OSO) [6].
The heterogeneity of diagnostic techniques and operational definitions in one or more of these components has resulted in problematic and wide-ranging reported prevalences of OS (4.7–40%) [7,8] and OSO (0.4–38%) [9,10]. Although the clinical cut-offs for osteopenia and osteoporosis have been standardized as T-scores of −1 and −2.5 for bone mineral density (BMD), respectively, measurements are often done at different regions (ie, lumbar spine, total hip or femoral neck) [4]. The identification of sarcopenia has been progressively unified by consensus working groups [2], but researchers are still divided on whether to use the full diagnostic algorithm (ie, muscle mass, strength and function) [10,11] or focus solely on a low muscle mass criterion [9] for defining sarcopenia. Perhaps the most polarized of the 3 components, obesity currently has no unified or universally-accepted definition. Body mass index (BMI), waist circumference (WC), percentage body fat (PBF), fat mass index (FMI) and fat mass to fat-free mass ratio (FM/FFM) are just some of the more commonly used measures, each assessing a distinct concept of obesity [12]. To better comprehend its fundamental pathophysiology, estimate its prevalence and explore possible treatments, it is important to advance towards an integrated criteria for the identification and characterisation of OSO.
In recent years, OSO has been proposed as a cumulative triad of the adverse effects of its components: impaired bone health, muscle health, and excess adiposity [6]. We have recently reported population prevalence of osteopenia, osteoporosis [13], sarcopenia [14], obesity and SO [15], and provided reference values for BMD [13], skeletal muscle, strength and function [14], and compared different adiposity measures [15]. Additionally, we have also shown that SO further exacerbates poor physical function when compared to sarcopenia or obesity alone [15]. However, there is contrasting evidence regarding the value of OSO in predicting for functional decline (ie, physical performance and frailty) [5,16], risk of adverse clinical outcomes (ie, fractures and falls risks) [17,18] and mortality [19] over sarcopenia, osteopenia/osteoporosis, or SO alone. Therefore, more studies on the characteristics and value of OSO are needed [6].
The main objective of this cross-sectional study is to report OS and OSO prevalence using evidence-based diagnostic techniques and definitions. We further examined if OSO offers additional predictive value of functional decline over sarcopenia and sarcopenic obesity, and identified the risk factors for OS and OSO in a multi-racial Southeast Asian population of community-dwelling adults.
2. Methods
2.1. Setting
Community-dwellers (≥ 21 years old) were recruited from Yishun, one of the largest north-residential towns in Singapore. It has a resident population of 220,320 (50.6% females), 12.2% of which are older than 65 years, comparable to the general Singapore population of 4,026,210 (51.1% females), 14.4% of which older than 65 years [20].
2.2. Participants
A representative sample of around 300 men and 300 women was recruited through random sampling, with 20–40 in each age- and sex-group (10-year intervals for 21–60 years; 5-year intervals for ≥ 60 years). Detailed recruitment methods and exclusion criteria have been reported previously [14]. Research ethics were approved by the National Healthcare Group DSRB (2017/00212). All participants undertook informed consent prior to study recruitment.
2.3. Questionnaires
Self-report questionnaires were administered, relating to education, accommodation, living arrangement, marital status, tobacco and alcohol use, history of disease and conditions, mini nutritional assessment (MNA) short form [21], and the global physical activity questionnaire (GPAQ) [22] and Longitudinal Aging Study Amsterdam (LASA) physical activity questionnaires [23].
2.4. Anthropometry
Height (m) and weight (kg) were assessed with a digital balance and stadiometer (Seca, GmbH & Co. KG, Hamburg, Germany). Waist and hip circumferences were determined with a tape measure encircling the narrowest waist and widest hip regions accordingly. Body mass index (BMI) was computed as weight over height squared (kg/m2).
2.5. Cognition
The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) was administered to evaluate cognitive functions including memory, visuospatial, language and attentional domains, totalling to 0–321 points [24]. Higher points indicate greater global cognition.
2.6. Functional performance
To assess functional deterioration or limitation, participants performed the short physical performance battery (SPPB). The SPPB is an objective and validated assessment comprising the components of balance, gait speed, and sit-to-stands [25].
2.7. Sarcopenia, obesity and osteopenia/osteoporosis
Sarcopenia was assessed and defined in 2 ways: the Asian Working Group for Sarcopenia criteria (AWGS 2019; ie, AWGS-based) [2], and according to poor skeletal muscle alone, ie, appendicular lean mass index (ALMI-based). ALMI, muscle strength and physical function were measured using a dual-energy X-ray absorptiometry (DXA) scan, maximal handgrip strength and habitual gait speed tests respectively, with detailed methods described in an earlier paper [14]. Obesity was assessed using the DXA scan, and defined using the 2 upper quintiles of population-derived sex-specific percentage body fat (PBF) and fat mass index (FMI) values [15]. We have previously shown that FMI is the more favourable approach for assessing adiposity as a component of sarcopenic obesity [15]. Osteopenia and osteoporosis were indicated by a population-derived lumbar spinal bone mineral density (BMDLS) T-score of −1 to −2.5 and ≤ −2.5 respectively [4]. Detailed methods, along with osteopenia/osteoporosis prevalence and reference values, have been described elsewhere [13].
2.8. Osteosarcopenia and osteosarcopenic obesity
Participants who were sarcopenic and osteopenic/osteoporotic only were classified as ‘osteosarcopenic’ (OS), while those who were osteosarcopenic and obese were classified as ‘osteosarcopenic obese’ (OSO).
2.9. Statistical analysis
Data analysis was done using SPSS version 22 (Chicago, Illinois, USA). Continuous and categorical variables were presented as mean (SD) and number (%) respectively. Prevalence approximations of OS and OSO were adjusted to the overall Singapore population by age-group weights. Multiple linear regressions were performed against SPPB to compare the effects of the respective phenotypes on physical function. Backward stepwise logistic regressions (removal threshold: P = 0.05) were employed to identify variables correlated with OS and OSO, with no correction for multiple significance testing (significance level: P < 0.05).
3. Results
Among the 542 participants recruited (21–90 years old; 57.9% females), n = 6 and n = 1 had inadequate sarcopenia and obesity data respectively, while n = 72 were excluded due to poor DXA scan imaging (ie, body portions cut-off, or participants with considerably large metal implants including hip/knee replacements). Therefore, data analysis was performed for the remaining 463 participants, aged 57.9 (18.5) years on average, 82.1% of which were Chinese, 8.6% Malay, 6.5% Indians, and 2.8% of other races. ‘Race’ was defined as Chinese, Malay, Indian, or others by biological origin (ie, skin color), rather than by social, national or cultural contexts.
3.1. Prevalence
Population-adjusted prevalence of AWGS-based OS was 4.7%, OSO–PBF 2.1%, and OSO-FMI 0.7% (Table 1). For the ALMI-based definitions, overall population-adjusted prevalence of OS was 14.5%, OSO–PBF 3.9%, and OSO-FMI 1.9%. The trendlines of OS and OSO with age are displayed in Fig. 1, Fig. 2. Descriptive statistics and corresponding OSO categories are presented in Table 2.
Table 1.
Prevalence estimates adjusted to the Singapore general population by age group weights.
| Variable | ALMI-based |
AWGS-based |
||||
|---|---|---|---|---|---|---|
| OS | OSO–PBF | OSO-FMI | OS | OSO–PBF | OSO-FMI | |
| Total Residents | 14.5 | 3.9 | 1.9 | 4.7 | 2.1 | 0.7 |
| 21–59 Years | 9.8 | 2.2 | 0.6 | 1.8 | 1.0 | 0.0 |
| ≥ 60 Years | 27.8 | 8.7 | 5.3 | 12.9 | 5.3 | 2.8 |
| ≥ 65 Years | 32.2 | 10.2 | 6.2 | 17.3 | 7.9 | 4.1 |
| ≥ 75 Years | 34.2 | 11.0 | 8.7 | 25.5 | 8.6 | 7.0 |
| Total Male Residents | 10.3 | 2.9 | 2.3 | 1.7 | 0.9 | 0.4 |
| 21–59 Years | 6.5 | 1.3 | 1.3 | 0.0 | 0.0 | 0.0 |
| ≥ 60 Years | 21.1 | 7.6 | 5.3 | 6.7 | 3.5 | 1.6 |
| ≥ 65 Years | 22.8 | 7.8 | 4.3 | 8.3 | 5.3 | 2.4 |
| ≥ 75 Years | 24.9 | 5.8 | 4.1 | 13.1 | 4.1 | 4.1 |
| Total Female Residents | 18.5 | 4.9 | 1.5 | 7.6 | 3.3 | 1.0 |
| 21–59 Years | 12.9 | 3.1 | 0.0 | 3.6 | 2.0 | 0.0 |
| ≥ 60 Years | 34.0 | 9.7 | 5.4 | 18.6 | 6.9 | 3.9 |
| ≥ 65 Years | 40.5 | 12.4 | 7.9 | 25.3 | 10.2 | 5.7 |
| ≥ 75 Years | 41.5 | 15.2 | 12.3 | 35.3 | 12.1 | 9.2 |
ALMI, appendicular lean mass index; AWGS, Asian Working Group for Sarcopenia; OS, osteosarcopenia; OSO, osteosarcopenic obesity; PBF, percentage body fat; FMI, fat mass index.
Values are presented as percentages (%).
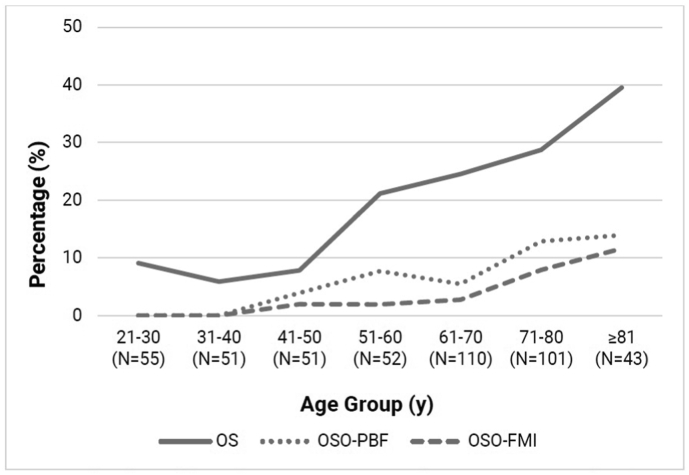
Fig. 1.
Age-trends of ALMI-based osteosarcopenia and osteosarcopenic obesity prevalence.
y: years, OS: osteosarcopenia, OSO: osteosarcopenic obesity, ALMI: appendicular lean mass index, AWGS: Asian Working Group for Sarcopenia, PBF: percentage body fat, FMI: fat mass index, N: sample size.
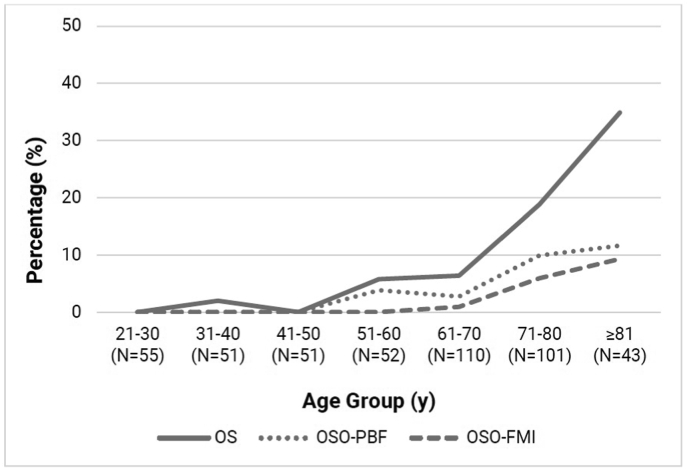
Fig. 2.
Age-trends of AWGS-based osteosarcopenia and osteosarcopenic obesity prevalence.
y: years, OS: osteosarcopenia, OSO: osteosarcopenic obesity, ALMI: appendicular lean mass index, AWGS: Asian Working Group for Sarcopenia, PBF: percentage body fat, FMI: fat mass index, N: sample size.
Table 2.
Participant characteristics and osteosarcopenic obesity statuses.
| Characteristics | Total n = 463 | ALMI-based |
AWGS-based |
||
|---|---|---|---|---|---|
| OSO–PBF n = 31 | OSO-FMI n = 18 | OSO–PBF n = 20 | OSO-FMI n = 11 | ||
| Age, yr | |||||
| 21-40 | 106 (22.9) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 41-60 | 103 (22.2) | 6 (19.4) | 2 (11.1) | 2 (10.0) | 0 (0.0) |
| 61-80 | 211 (45.6) | 19 (61.3) | 11 (61.1) | 13 (65.0) | 7 (63.6) |
| ≥ 81 | 43 (9.3) | 6 (19.4) | 5 (27.8) | 5 (25.0) | 4 (36.4) |
| Sex | |||||
| Male | 204 (44.1) | 10 (32.3) | 7 (38.9) | 5 (25.0) | 3 (27.3) |
| Female | 259 (55.9) | 21 (67.7) | 11 (61.1) | 15 (75.0) | 8 (72.7) |
| Race | |||||
| Chinese | 380 (82.1) | 24 (77.4) | 13 (72.2) | 15 (75.0) | 7 (63.6) |
| Malay | 40 (8.6) | 1 (3.2) | 1 (5.6) | 1 (5.0) | 1 (9.1) |
| Indian | 30 (6.5) | 5 (16.1) | 3 (16.7) | 3 (15.0) | 2 (18.2) |
| Others | 13 (2.8) | 1 (3.2) | 1 (5.6) | 1 (5.0) | 1 (9.1) |
| Highest Qualification | |||||
| ≤ Primary | 141 (30.5) | 12 (38.7) | 7 (38.9) | 10 (50.0) | 5 (45.5) |
| Secondary | 147 (31.7) | 11 (35.5) | 5 (27.8) | 8 (40.0) | 5 (45.5) |
| Tertiary | 100 (21.6) | 5 (16.1) | 4 (22.2) | 2 (10.0) | 1 (9.1) |
| ≥ Degree | 75 (16.2) | 3 (9.7) | 2 (11.1) | 0 (0.0) | 0 (0.0) |
| Housing Type | |||||
| 1–2 rooms | 56 (12.1) | 4 (12.9) | 2 (11.1) | 3 (15.0) | 1 (9.1) |
| 3 rooms | 97 (21.0) | 10 (32.3) | 4 (22.2) | 8 (40.0) | 4 (36.4) |
| 4–5 rooms | 270 (58.3) | 14 (45.2) | 9 (50.0) | 7 (35.0) | 4 (36.4) |
| High-end Public/Private | 40 (8.6) | 3 (9.7) | 3 (16.7) | 2 (10.0) | 2 (18.2) |
| Living Arrangement (n = 421) | |||||
| Alone | 36 (8.6) | 5 (16.7) | 3 (16.7) | 4 (20.0) | 2 (18.2) |
| Not Alone | 385 (91.4) | 25 (83.3) | 15 (83.3) | 16 (80.0) | 9 (81.8) |
| Marital Status (n = 440) | |||||
| Married | 307 (69.8) | 21 (67.7) | 12 (66.7) | 12 (60.0) | 6 (54.5) |
| Single | 71 (16.1) | 1 (3.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Divorced/Separated | 15 (3.4) | 1 (3.2) | 1 (5.6) | 0 (0.0) | 0 (0.0) |
| Widowed | 47 (10.7) | 8 (25.8) | 5 (27.8) | 8 (40.0) | 5 (45.5) |
| Medical Conditions | |||||
| Diabetes | 66 (14.3) | 4 (12.9) | 3 (16.7) | 3 (15.0) | 2 (18.2) |
| Hypertension | 161 (34.8) | 16 (51.6) | 12 (66.7) | 13 (65.0) | 9 (81.8) |
| High Cholesterol | 175 (37.8) | 18 (58.1) | 12 (66.7) | 13 (65.0) | 8 (72.7) |
| Smoking and Drinking | |||||
| Smokers/Ex-smokers | 102 (22.0) | 6 (19.4) | 4 (22.2) | 4 (20.0) | 3 (27.3) |
| Alcoholics/Ex-alcoholics | 38 (8.2) | 4 (12.9) | 3 (16.7) | 3 (15.0) | 3 (27.3) |
| Smoke and Drink | 21 (4.5) | 1 (3.2) | 1 (5.6) | 1 (5.0) | 1 (9.1) |
ALMI, appendicular lean mass index; AWGS, Asian Working Group for Sarcopenia; OSO, osteosarcopenic obesity; PBF, percentage body fat; FMI, fat mass index.
Values are presented as mean (SD) or number (%).
Across age groups, AWGS-based OS prevalence were 1.8% (21–59 years), 12.9% (≥ 60 years), 17.3% (≥ 65 years) and 25.5% (≥ 75 years); OSO–PBF prevalence were 1.0%, 5.3%, 7.9% and 8.6%; and OSO-FMI prevalence were 0%, 2.8%, 4.1% and 7.0%, respectively. For the ALMI-based definitions, OS prevalence were 9.8% (21–59 years), 27.8% (≥60 years), 32.2% (≥65 years) and 34.2% (≥75 years); OSO–PBF prevalence were 2.2%, 8.7%, 10.2% and 11.0%; and OSO-FMI prevalence were 0.6%, 5.3%, 6.2% and 8.7%, respectively.
3.2. Multiple linear regression
In multiple linear regression analyses of the respective conditions for SPPB (dependent variable), we adjusted for age, sex, race, education, accommodation, living arrangement, marriage status, diabetes, hypertension, high cholesterol, self-rated health, smoking, alcoholism, MNA, GPAQ, LASA, BMI, waist and hip circumferences, and RBANS (independent variables; Table 3). Across all models, the OSO phenotypes did not explain for any increase in variance (R2 = 0.206–0.209) of SPPB functional performance over the sarcopenic (R2 = 0.206–0.211) and sarcopenic obese (R2 = 0.209–0.221) phenotypes. Ordinal logistic regressions were additionally performed across all 6 models for SPPB to confirm our findings that the OSO phenotypes did not increase the predictive value of the models on functional performance (estimated Cox and Snell, Nagelkerke, and McFadden R2 values) compared to the sarcopenic and sarcopenic obese phenotypes. None of the models violated the assumption of multicollinearity.
Table 3.
Multiple linear regression analysis for SPPB.
| ALMI-based |
AWGS-based |
||||||
|---|---|---|---|---|---|---|---|
| Model | Adjusted R2 | β | P-value | Model | Adjusted R2 | β | P-value |
| S | 0.206 | 0.015 | 0.807 | S | 0.211 | −0.081 | 0.130 |
| SO–PBF | 0.209 | −0.056 | 0.250 | SO–PBF | 0.214 | −0.095 | 0.049∗ |
| SO-FMI | 0.209 | −0.060 | 0.211 | SO-FMI | 0.221 | −0.131 | 0.006∗∗ |
| OSO–PBF | 0.206 | −0.011 | 0.813 | OSO–PBF | 0.207 | −0.031 | 0.492 |
| OSO-FMI | 0.207 | −0.024 | 0.596 | OSO-FMI | 0.209 | −0.055 | 0.230 |
SPPB, short physical performance battery; ALMI, appendicular lean mass index; AWGS, Asian Working Group for Sarcopenia; β, standardized coefficient; S, sarcopenia; SO, sarcopenia obesity; OSO, osteosarcopenic obesity; PBF, percentage body fat; FMI, fat mass index.
∗ denotes P < 0.05; ∗∗ denotes P < 0.01; ∗∗∗ denotes P < 0.001.
Model includes Age, Sex, Race, Education Level, Housing Type, Living Arrangement, Marital Status, Diabetes, Hypertension, High Cholesterol, Self-rated Health, Smoking, Alcoholism, Mini Nutritional Assessment, Global Physical Activity Questionnaire, Longitudinal Aging Study Amsterdam Physical Activity Questionnaire, Body Mass Index, Waist Circumference, Hip Circumference, and Repeatable Battery for the Assessment of Neuropsychological Status.
3.3. Associated factors
Variables associated with AWGS-based OS and OSO are shown in Table 4. Increasing age, female sex, non-Chinese race and lower BMI were risk factors of OS (P < 0.01), while increasing age, female sex, non-Chinese race and alcoholism (P < 0.05) were risk factors of OSO. For OS, the regression model explained for 37.7% (Nagelkerke R2) of the variance and correctly classified 90.5% of the cases, while for OSO-FMI, the model explained for 32.6% of the variance and correctly classified 97.4% of the cases. Multicollinearity was not violated in any of the models.
Table 4.
Factors associated with AWGS-based OS and OSO using logistic regression.
| Characteristics | OS |
OSO-FMI |
OSO–PBF |
||||||
|---|---|---|---|---|---|---|---|---|---|
| R2 | Percentage Correct (%) | Odds Ratio (95% CI) | R2 | Percentage Correct (%) | Odds Ratio (95% CI) | R2 | Percentage Correct (%) | Odds Ratio (95% CI) | |
| 37.7 | 90.5 | 32.6 | 97.4 | 16.7 | 95.2 | ||||
| Age, yr | 1.11 (1.07–1.15)∗∗∗ | 1.16 (1.06–1.26)∗∗ | 1.08 (1.03–1.13)∗∗ | ||||||
| Sex | |||||||||
| Female | 1 | 1 | 1 | ||||||
| Male | 0.20 (0.09–0.47)∗∗∗ | 0.17 (0.03–0.94)∗ | 0.32 (0.11–0.91)∗ | ||||||
| Race | |||||||||
| Non-Chinese | 1 | 1 | – | ||||||
| Chinese | 0.24 (0.09–0.66)∗∗ | 0.17 (0.04–0.72)∗ | – | ||||||
| Alcoholism | |||||||||
| No | – | 1 | – | ||||||
| Yes | – | 9.88 (1.76–55.58)∗∗ | – | ||||||
| BMI, kg/m2 | 0.74 (0.65–0.84)∗∗∗ | – | – | ||||||
AWGS, Asian Working Group for Sarcopenia; OS, osteosarcopenia; OSO, osteosarcopenic obesity; FMI, fat mass index; PBF, percentage body fat; BMI, body mass index.
∗ denotes P < 0.05; ∗∗ denotes P < 0.01; ∗∗∗ denotes P < 0.001.
4. Discussion
As the first population-based study to present ‘osteosarcopenia’ (OS) and ‘osteosarcopenic obesity’ (OSO) prevalence among younger (21–59 years) and older (≥ 60 years) community-dwellers in a multi-racial, Southeast Asian population, our findings contribute important information to the knowledge base on OS and OSO entities. It should be noted that while OS and OSO are conditions relating to older people, we applied the same criteria in younger adults in order to estimate prevalence across adult age groups, and so this would have likely been an underestimate for the younger adults.
4.1. Prevalence
In a similar study done on community-dwelling Korean adults (≥ 50 years) [9], the overall prevalence of OSO based on low bone mineral density (BMD), high percentage body fat (PBF) and low skeletal muscle was 6.6%. Similarly, among our representative sample of Singaporean adults (≥ 50 years), OSO prevalence as defined by the same diagnostic methods was comparable at 7.1%. In another study on Japanese men and women (≥40 years) [11], the overall OSO prevalence among the community-dwellers based on low BMD, high PBF and sarcopenia based on the 2014 Asian Working Group for Sarcopenia criteria (AWGS 2014) [26] was 0.9%. This was lower compared to the prevalence of 3.3% among our representative Singaporean sample (≥40 years). However, it should be noted that the AWGS 2014 criteria used in the Japanese study has lower cut-off values for muscle strength and function as compared to the updated AWGS 2019 criteria [2] used in our study, thus leading to a lower prevalence of sarcopenia and OSO. We have recently shown that by using the AWGS 2019 versus the AWGS 2014 criteria, sarcopenia prevalence alone was inflated by more than two-fold [14].
4.2. No evidence of osteosarcopenic obesity as a distinct entity
Multiple linear regression analyses of SPPB showed that, across all models, functional deterioration was not more severe in individuals with OSO compared to those from the sarcopenic-only or sarcopenic obese groups. A recent critical literature review [6] highlighted the concerns regarding the utility of OSO as a distinct entity, and questioned its value in predicting increased risks of adverse outcomes over and above that of its constituents. Osteopenia/osteoporosis did not elevate falls and frailty risks any more than sarcopenia [16,18,27]. Additionally, although some researchers have shown significant correlation between OSO and poor functional performance when compared to non-OSO individuals [11,28], our findings revealed that this association with decreased function is attributable to the sarcopenia and obesity components of OSO. OSO itself, or the addition of osteopenia/osteoporosis to sarcopenic obesity, did not correlate with functional performance. Therefore, our results do not support OSO as a discrete entity in relation to physical function. Nevertheless, the concurrence of osteopenia/osteoporosis, sarcopenia and/or obesity among community-dwelling adults still presents a noteworthy public health concern. To further determine the importance of OSO, future studies should evaluate the severity of other adverse clinical outcomes that are associated with OSO (ie, fractures [17,27] disability [29], hospitalization [28], and mortality [19]) in comparison to that of its components alone.
4.3. Associated factors
Backward stepwise logistic regression, for AWGS-based definitions, identified increasing age, female sex, non-Chinese race and lower BMI as risk factors for OS. Furthermore, increasing age, female sex, non-Chinese race as well as alcoholism were risk factors for OSO. In sensitivity analyses, forward stepwise and full saturated logistic regression models identified the same variables for OS. With OSO, forward stepwise model found one less variable (ie, sex), while the full saturated model found one different variable (ie, self-rated health instead of alcoholism) than the backward model. One shortcoming of the forward selection is “suppressor effects”, while allowing evidently insignificant variables to remain as with the saturated model can influence the significance of other variables.
Not surprisingly, increasing age and the female sex elevated the risks of OS and OSO. Due to the effects of progressive physiological decline, deterioration in bone mass, bone health, muscle mass, strength, function, and increase in fat mass, are natural occurrences even with ‘normal aging’ [6]. Additionally, women at all ages inherently have much higher relative fat mass [30] and much lower skeletal muscle and bone mass [30] compared with men, attributed mainly to disparities in hormonal profiles. Elevated testosterone in men promotes bone and muscle anabolism [31], while women have inflated estrogen that encourages fat storage around the chest, hips and thighs [32].
Non-Chinese races had an increased risk of OS and OSO compared to the Chinese, even after adjusting for all available sociodemographic, lifestyle, and medical factors. Observations of racial differences arising from genetic predispositions can be abstruse and difficult to explain. A study on quality of life among Singaporean adolescents reported significant racial differences regardless of health and socioeconomic profiles, indicating key differences among the different races [33]. Chinese Singaporeans also had the lowest BMI, lowest HbA1c levels, and were least likely to have diabetes compared to their Malay and Indian counterparts – even after adjusting for factors like age, sex and exercise [34]. Other studies have reported that Chinese Singaporeans were least likely to suffer a myocardial infarction, or to die from one, compared to Malays and Indians, attributable to unfavourable racial predispositions that cannot be fully explained by dietary intake [35]. In addition, Malays and Indians were more than twice as likely as Chinese to develop dementia, even with adjustments for age, sex and education, suggesting fundamental variations in heredity among the different races [36].
Racial predispositions have sometimes been attributed to modifiable lifestyle factors (ie, physical activity, diet, and obesity). Particularly, significant associations were reported previously between diet quality and OSO [37,38]. Malay Singaporeans are known to consume the largest amounts of saturated and total fat, and fewest servings of vegetables and fruits [39], modifiable lifestyle factors that translated to significantly lower life expectancies compared to the Chinese and Indians [40]. According to FMI and PBF definitions, our findings showed that 60.2% and 55.4% of non-Chinese were obese, almost double that of the Chinese at 33.2% and 34.7% respectively. Obesity alone is associated with functional decline [41], morbidity [42], and metabolic syndrome [43], and its pro-inflammatory effects are known to perpetuate insulin resistance and contribute to a decline in muscle and bone health [3]. Furthermore, obesity is associated with lower activity levels, further exacerbating the reduction in bone mass, muscle mass, and strength [30]. Although activity levels and nutritional status, measured by GPAQ and MNA short form, were not found to be significant predictors of OS and OSO, GPAQ quantifies activity by MET hours (ie, calories burned per kg of body weight), and does not account for exercise intensity (higher intensity preserves muscle mass and strength) or impact (greater impact preserves bone density) [22]. Additionally, MNA short form does not assess nutritional intake – such as protein (to preserve muscle) and calcium or vitamin D (for bone health) [21]. Therefore, more specific measures of physical activity and dietary intake could exemplify our understanding of the effects of modifiable lifestyle factors on OS and OSO.
Alcoholism was associated with OSO. Alcohol consumption in excess perpetuates systemic inflammation, resulting in adverse health outcomes including movement inhibition and diminished functional efficiency [44].
Interestingly, lower BMI increased the risk of OS but not OSO. Higher BMI is an indicator of better nourishment, where greater protein, nutrient and caloric intake translate to improved muscle and bone health compared with the undernourished [30,45]. Since BMI is a direct indicator of obesity, the effects of lower BMI on elevated disease risk disappeared in the OSO model.
The strengths of this study are its population-based nature, thoroughness of data collection and application of up-to-date and evidence-based consensus. There are also several limitations. Although race was identified as a risk factor in multivariate logistic regression, our study was not adequately powered for racial comparisons as we did not have the same proportion of participants by race (ie, Malays and Indians are under-represented in our sample). Further studies may be required to exemplify our understanding of the effects of biological race on OS and OSO risk. With a cross-sectional design, causality of disease from risk factors also cannot be inferred, and age-associated changes may not truly represent the longitudinal trajectories of bone health, muscle health and adiposity. Without BMD of the hip or femoral neck, we used only lumbar spinal BMD to determine osteopenia and osteoporosis, which could have led to an under-detection of OS and OSO. Finally, the study subjects were community-dwellers, which may implicate the generalizability of the results to the disabled, hospitalized or institutionalized.
5. Conclusions
This study contributes important information to improve the understanding and identification of OS and OSO among community-dwellers. We did not find evidence to bolster OSO as a distinct entity with regards to physical function. To further determine its importance, future studies should evaluate the severity of other adverse clinical outcomes associated with OSO (ie, fractures, disability, hospitalization and mortality) in comparison to that of its components alone. Apart from increasing age, female sex, and biological race, amenable lifestyle factors including BMI and alcohol intake are important variables that can influence the development of osteosarcopenia and osteosarcopenic obesity.
CRediT author statement
Benedict Wei Jun Pang: Conceptualization, Methodology, Formal analysis, Investigation, Resources, Data curation, Writing - original draft, Writing - review & editing, Project administration. Shiou-Liang Wee: Conceptualization, Methodology, Writing - review & editing, Supervision, Project administration, Funding acquisition. Kenneth Kexun Chen: Formal analysis, Investigation, Resources, Data curation. Lay Khoon Lau: Formal analysis, Investigation, Resources, Data curation, Project administration. Khalid Abdul Jabbar: Formal analysis, Investigation, Resources, Data curation. Wei Ting Seah: Formal analysis, Investigation, Resources, Data curation. Daniella Hui Min Ng: Formal analysis, Investigation, Resources, Data curation. Queenie Lin Ling Tan: Formal analysis, Investigation, Resources, Data curation. Mallya Ullal Jagadish: Conceptualization, Methodology, Supervision. Tze Pin Ng: Conceptualization, Methodology, Supervision.
Conflicts of interest
The authors declare no competing interests.
Acknowledgments
This research was supported as part of a core funding from the Ministry of Health of Singapore to Geriatric Education and Research Institute (GERI/1609).
The authors gratefully acknowledge the strong support of Prof. Pang Weng Sun in making this Yishun Study possible, and the support of Dr. Lilian Chye, Sylvia Ngu Siew Ching, Aizuriah Mohamed Ali, Mary Ng Pei Ern, Chua Xing Ying and Shermaine Thein in this study.
ORCID Benedict Wei Jun Pang: 0000-0002-7655-9834. Shiou Liang Wee: 000-0002-7853-4112. Kenneth Kexun Chen: 0000-0002-6280-0391. Lay Khoon Lau: 0000-0002-1761-3431. Khalid bin Abdul Jabbar: 0000-0002-9620-2875. Wei Ting Seah: 0000-0002-9329-4014. Daniella Hui Min Ng: 0000-0003-1128-5219. Queenie Lin Ling Tan: 0000-0002-0926-389X. Mallya Ullal Jagadish: 0000-0003-0049-2052. Tze Pin Ng: 0000-0001-9585-855X.
Footnotes
Peer review under responsibility of The Korean Society of Osteoporosis.
References
- 1.United Nations DoEaSA, Population Division World population prospects 2019: highlights. New York: United Nations. 2019. https://population.un.org/wpp/Publications/Files/WPP2019_Highlights.pdf Available from:
- 2.Chen L.-K., Woo J., Assantachai P., Auyeung T.-W., Chou M.-Y., Iijima K. Asian working group for sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J Am Med Dir Assoc. 2020;21 doi: 10.1016/j.jamda.2019.12.012. 300-7.e2. [DOI] [PubMed] [Google Scholar]
- 3.Kalinkovich A., Livshits G. Sarcopenic obesity or obese sarcopenia: a cross talk between age-associated adipose tissue and skeletal muscle inflammation as a main mechanism of the pathogenesis. Ageing Res Rev. 2017;35:200–221. doi: 10.1016/j.arr.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 4.Kanis J.A., Adachi J.D., Cooper C., Clark P., Cummings S.R., Diaz-Curiel M. Standardising the descriptive epidemiology of osteoporosis: recommendations from the epidemiology and quality of life working group of IOF. Osteoporos Int. 2013;24:2763–2764. doi: 10.1007/s00198-013-2413-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Drey M., Sieber C.C., Bertsch T., Bauer J.M., Schmidmaier R. The FiAT ig. Osteosarcopenia is more than sarcopenia and osteopenia alone. Aging Clin Exp Res. 2016;28:895–899. doi: 10.1007/s40520-015-0494-1. [DOI] [PubMed] [Google Scholar]
- 6.Bauer J.M., Cruz-Jentoft A.J., Fielding R.A., Kanis J.A., Reginster J.-Y., Bruyère O. Correction to: is there enough evidence for osteosarcopenic obesity as a distinct entity? A critical literature review. Calcif Tissue Int. 2019;105:125–126. doi: 10.1007/s00223-019-00587-0. [DOI] [PubMed] [Google Scholar]
- 7.Hirschfeld H.P., Kinsella R., Duque G. Osteosarcopenia: where bone, muscle, and fat collide. Osteoporos Int. 2017;28:2781–2790. doi: 10.1007/s00198-017-4151-8. [DOI] [PubMed] [Google Scholar]
- 8.Kirk B., Al Saedi A., Duque G. Osteosarcopenia: a case of geroscience. Aging Med. 2019;2:147–156. doi: 10.1002/agm2.12080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee K. Association of osteosarcopenic obesity and its components: osteoporosis, sarcopenia and obesity with insulin resistance. J Bone Miner Metabol. 2020;38:695–701. doi: 10.1007/s00774-020-01104-2. [DOI] [PubMed] [Google Scholar]
- 10.Keramidaki K., Tsagari A., Hiona M., Risvas G. Osteosarcopenic obesity, the coexistence of osteoporosis, sarcopenia and obesity and consequences in the quality of life in older adults ≥65 years-old in Greece. J Frailty Sarcopenia Falls. 2019;4:91–101. doi: 10.22540/JFSF-04-091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sasaki K-i, Kakuma T., Sasaki M., Ishizaki Y., Fukami A., Enomoto M. The prevalence of sarcopenia and subtypes in cardiovascular diseases, and a new diagnostic approach. J Cardiol. 2020;76:266–272. doi: 10.1016/j.jjcc.2020.03.004. [DOI] [PubMed] [Google Scholar]
- 12.Khor E.Q., Lim J.P., Tay L., Yeo A., Yew S., Ding Y.Y. Obesity definitions in sarcopenic obesity: differences in prevalence, agreement and association with muscle function. J Frailty Aging. 2020;9:37–43. doi: 10.14283/jfa.2019.28. [DOI] [PubMed] [Google Scholar]
- 13.Chen K.K., Wee S.-L., Pang B.W.J., Lau L.K., Jabbar K.A., Seah W.T. Bone mineral density reference values in Singaporean adults and comparisons for osteoporosis establishment – the Yishun study. BMC Muscoskel Disord. 2020;21:633. doi: 10.1186/s12891-020-03646-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pang B.W.J., Wee S.L., Lau L.K., Jabbar K.A., Seah W.T., Ng D.H.M. Prevalence and associated factors of sarcopenia in Singaporean adults-the Yishun study. J Am Med Dir Assoc. 2020 Jul 19 doi: 10.1016/j.jamda.2020.05.029. [Online ahead of print] S1525-8610(20)30436-9. [DOI] [PubMed] [Google Scholar]
- 15.Pang B.W.J., W S.-L., Lau L.K., Jabbar K.A., Seah W.T., Ng D.H.M. Obesity measures and definitions of sarcopenic obesity in Singaporean adults – the Yishun study. J Frailty Aging. 2020 doi: 10.14283/jfa.2020.65. in press. [DOI] [PubMed] [Google Scholar]
- 16.Zaslavsky O., Li W., Going S., Datta M., Snetselaar L., Zelber-Sagi S. Association between body composition and hip fractures in older women with physical frailty. Geriatr Gerontol Int. 2017;17:898–904. doi: 10.1111/ggi.12798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chalhoub D., Cawthon P.M., Ensrud K.E., Stefanick M.L., Kado D.M., Boudreau R. Risk of nonspine fractures in older adults with sarcopenia, low bone mass, or both. J Am Geriatr Soc. 2015;63:1733–1740. doi: 10.1111/jgs.13605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scott D., Seibel M., Cumming R., Naganathan V., Blyth F., Le Couteur D.G. Does combined osteopenia/osteoporosis and sarcopenia confer greater risk of falls and fracture than either condition alone in older men? The concord health and ageing in men project. J Gerontol: Series A. 2019;74:827–834. doi: 10.1093/gerona/gly162. [DOI] [PubMed] [Google Scholar]
- 19.Yoo J.-I., Kim H., Ha Y.-C., Kwon H.-B., Koo K.-H. Osteosarcopenia in patients with hip fracture is related with high mortality. J Kor Med Sci. 2018;33(4) doi: 10.3346/jkms.2018.33.e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.(DOS) SDoS. Base. 2020. https://www.singstat.gov.sg Available from:
- 21.Kaiser M.J., Bauer J.M., Ramsch C., Uter W., Guigoz Y., Cederholm T. Validation of the mini nutritional assessment short-form (MNA®-SF): a practical tool for identification of nutritional status. J Nutr Health Aging. 2009;13:782. doi: 10.1007/s12603-009-0214-7. [DOI] [PubMed] [Google Scholar]
- 22.Armstrong T., Bull F. Development of the world health organization global physical activity questionnaire (GPAQ) J Public Health. 2006;14:66–70. [Google Scholar]
- 23.Stel V.S., Smit J.H., Pluijm S.M.F., Visser M., Deeg D.J.H., Lips P. Comparison of the LASA physical activity questionnaire with a 7-day diary and pedometer. J Clin Epidemiol. 2004;57:252–258. doi: 10.1016/j.jclinepi.2003.07.008. [DOI] [PubMed] [Google Scholar]
- 24.Collinson S.L., Fang S.H., Lim M.-L., Feng L., Ng T.-P. Normative data for the repeatable battery for the assessment of neuropsychological status in elderly Chinese. Arch Clin Neuropsychol. 2014;29:442–455. doi: 10.1093/arclin/acu023. [DOI] [PubMed] [Google Scholar]
- 25.Puthoff M.L. Research corner:Outcome measures in cardiopulmonary physical therapy: short physical performance battery. Cardiopulm Phys Ther J. 2008;19 [PMC free article] [PubMed] [Google Scholar]
- 26.Chen L.-K., Liu L.-K., Woo J., Assantachai P., Auyeung T.-W., Bahyah K.S. Sarcopenia in Asia: consensus report of the Asian working group for sarcopenia. J Am Med Dir Assoc. 2014;15:95–101. doi: 10.1016/j.jamda.2013.11.025. [DOI] [PubMed] [Google Scholar]
- 27.Harris R., Chang Y., Beavers K., Laddu-Patel D., Bea J., Johnson K. Risk of fracture in women with sarcopenia, low bone mass, or both. J Am Geriatr Soc. 2017;65:2673–2678. doi: 10.1111/jgs.15050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Szlejf C., Parra-Rodríguez L., Rosas-Carrasco O. Osteosarcopenic obesity: prevalence and relation with frailty and physical performance in middle-aged and older women. J Am Med Dir Assoc. 2017;18:733. doi: 10.1016/j.jamda.2017.02.023. e1-e5. [DOI] [PubMed] [Google Scholar]
- 29.Ormsbee M.J., Prado C.M., Ilich J.Z., Purcell S., Siervo M., Folsom A. Osteosarcopenic obesity: the role of bone, muscle, and fat on health. J Cachexia Sarcopenia Muscle. 2014;5:183–192. doi: 10.1007/s13539-014-0146-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cheng Q., Zhu X., Zhang X., Li H., Du Y., Hong W. A cross-sectional study of loss of muscle mass corresponding to sarcopenia in healthy Chinese men and women: reference values, prevalence, and association with bone mass. J Bone Miner Metabol. 2014;32:78–88. doi: 10.1007/s00774-013-0468-3. [DOI] [PubMed] [Google Scholar]
- 31.Flöter A., Nathorst-böös J., Carlström K., Ohlsson C., Ringertz H., von Schoultz B. Effects of combined estrogen/testosterone therapy on bone and body composition in oophorectomized women. Gynecol Endocrinol. 2005;20:155–160. doi: 10.1080/09513590400021193. [DOI] [PubMed] [Google Scholar]
- 32.Lizcano F., Guzmán G. Estrogen deficiency and the origin of obesity during menopause. BioMed Res Int. 2014;2014:757461. doi: 10.1155/2014/757461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ng T.P., Lim L.C.C., Jin A., Shinfuku N. Ethnic differences in quality of life in adolescents among Chinese, Malay and Indians in Singapore. Qual Life Res. 2005;14:1755–1768. doi: 10.1007/s11136-005-1741-2. [DOI] [PubMed] [Google Scholar]
- 34.Hong C.Y., Chia K.S., Hughes K., Ling S.L. Ethnic differences among Chinese, Malay and Indian patients with type 2 diabetes mellitus in Singapore. Singap Med J. 2004;45:154–160. [PubMed] [Google Scholar]
- 35.Mak K.H., Chia K.S., Kark J.D., Chua T., Tan C., Foong B.H. Ethnic differences in acute myocardial infarction in Singapore. Eur Heart J. 2003;24:151–160. doi: 10.1016/s0195-668x(02)00423-2. [DOI] [PubMed] [Google Scholar]
- 36.Sahadevan S., Saw S.M., Gao W., Tan L.C.S., Chin J.J., Hong Cy. Ethnic differences in Singapore’s dementia prevalence: the stroke, Parkinson’s disease, epilepsy, and dementia in Singapore study. J Am Geriatr Soc. 2008;56:2061–2068. doi: 10.1111/j.1532-5415.2008.01992.x. [DOI] [PubMed] [Google Scholar]
- 37.Kim J., Lee Y., Kye S., Chung Y.-S., Kim J.-H., Chon D. Diet quality and osteosarcopenic obesity in community-dwelling adults 50 years and older. Maturitas. 2017;104:73–79. doi: 10.1016/j.maturitas.2017.08.007. [DOI] [PubMed] [Google Scholar]
- 38.Owen J.K., Jennifer C.G., Youjin K., Jasminka Z.I. Micronutrient intake in the etiology, prevention and treatment of osteosarcopenic obesity. Curr Aging Sci. 2016;9:260–278. doi: 10.2174/1874609809666160509122001. [DOI] [PubMed] [Google Scholar]
- 39.Health Promotion Board S Report of the national nutrition survey 2004. 2004. https://www.hpb.gov.sg Available from:
- 40.Lim R.B.T., Zheng H., Yang Q., Cook A.R., Chia K.S., Lim W.Y. Ethnic and gender specific life expectancies of the Singapore population, 1965 to 2009 – converging, or diverging? BMC Publ Health. 2013;13:1012. doi: 10.1186/1471-2458-13-1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Prado C.M.M., Wells J.C.K., Smith S.R., Stephan B.C.M., Siervo M. Sarcopenic obesity: a critical appraisal of the current evidence. Clin Nutr. 2012;31:583–601. doi: 10.1016/j.clnu.2012.06.010. [DOI] [PubMed] [Google Scholar]
- 42.Lee Y.S., Biddle S., Chan M.F., Cheng A., Cheong M., Chong Y.S. Health promotion board-Ministry of health clinical practice guidelines: Obesity. Singap Med J. 2016;57:292–300. doi: 10.11622/smedj.2016103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu P., Ma F., Lou H., Liu Y. The utility of fat mass index vs. body mass index and percentage of body fat in the screening of metabolic syndrome. BMC Publ Health. 2013;13:629. doi: 10.1186/1471-2458-13-629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cawthon P.M., Fink H.A., Barrett-Connor E., Cauley J.A., Dam T.-T., Lewis C.E. Alcohol use, physical performance, and functional limitations in older men. J Am Geriatr Soc. 2007;55:212–220. doi: 10.1111/j.1532-5415.2007.01062.x. [DOI] [PubMed] [Google Scholar]
- 45.Cederholm T., Jensen G.L., Correia M.I.T.D., Gonzalez M.C., Fukushima R., Higashiguchi T. GLIM criteria for the diagnosis of malnutrition - a consensus report from the global clinical nutrition community. Clin Nutr. 2019;38(1):1–9. doi: 10.1016/j.clnu.2018.08.002. [DOI] [PubMed] [Google Scholar]