Abstract
Objectives
To determine the prevalence of sarcopenia obesity (SO) in healthy Indian adults and delineate the relative impact of the 3 indices of obesity [body mass index (BMI), waist circumference (WC), fat mass percent (FM%)] with regards to inter-definitional agreement and their relationship with usual gait speed (GS).
Methods
Apparently healthy adults (aged ≥ 20 years) with no background history of comorbidities were enrolled from the community by door-to-door survey. Following blood investigations, individuals with biochemical abnormalities were excluded. Enrolled participants underwent dual-energy X-ray absorptiometry (DXA). Sarcopenia was defined according to EWGSOP2 consensus based on indigenous cut-offs obtained from the Sarcopenia-Chandigarh Urban Bone Epidemiological Study (Sarco-CUBES). Obesity was defined based on BMI (≥ 25.0 kg/m2) or WC (> 90 cm in men, > 80 cm in women) or DXA-derived FM% (> 32% in men, > 40% in women).
Results
Data of 804 participants were analyzed after exclusion. The mean ± SD for BMI, WC, and FM% were 26.5 ± 2.7 kg/m2, 86.8 ± 9.6, and 34.7 ± 7.3%, respectively. Prevalence of sarcopenia was 3.2%. Based on BMI, WC, and FM%, the prevalence of SO in elderly subjects (≥65 years) was 5.4%, 5.4%, and 6.3%, respectively. Using Cohen’s kappa, inter-definitional agreement between the 3 groups was ‘almost perfect’. FM%, and not BMI/WC, emerged as a significant predictor of GS on multiple linear regression analysis.
Conclusions
The prevalence of SO in healthy elderly Indian adults is 5.4%–6.3%. Either BMI/WC/FM% can be used to correctly identify individuals with SO.
Keywords: India, Sarcopenic obesity, Waist circumference, Body mass index, Fat mass percent
1. Introduction
Sarcopenic obesity (SO) is an increasing global public health concern that has resulted from a confluence of 2 epidemiological trends, namely, obesity epidemic and population aging [1]. It is best characterized as a combined clinical and functional entity marked by the co-existence of surplus fat mass and sarcopenia in an individual [2]. The latter is described as a “progressive and generalized skeletal muscle disorder that is associated with an increased likelihood of adverse outcomes including falls, fractures, physical disability, and mortality.” For decades, low muscle mass had been the mainstay for the diagnosis of sarcopenia; however, recently, more emphasis has been placed on muscle strength as the latter correlates with adverse outcomes better than the former [3].
Although traditionally regarded as a geriatric syndrome, SO can be found in younger obese patients with underlying comorbidities like chronic kidney disease (CKD), chronic obstructive pulmonary disease (COPD), congestive cardiac failure, cancer, or occasionally following bariatric surgery [2]. From a clinical standpoint, SO predisposes an individual to the combined risk of the 2 independent body phenotypes, adversely affecting health, resulting in disability, loss of functional independence, poor quality of life, and eventually increased mortality [1,[4], [5], [6], [7]]. Besides, SO also contributes to insulin resistance with the resultant increase in the risk of metabolic syndrome (MS) and type 2 diabetes mellitus (T2DM) [8,9].
Multiple thresholds have been used to define SO; hence, the hitherto reported prevalence of SO in the aging population ranges from as low as 0.9% to as high as 30.1% [10,11]. Moreover, a globally acclaimed definition, diagnostic criterion and cut-off to describe SO are still lacking to date. A recently conducted systematic review showed significant heterogeneity in the definitions and approaches to define SO. It was attributed to the marked polarity in the definitions of “sarcopenia” and “obesity”, variability in the techniques used to estimate body composition, muscle strength, and physical performance, and lastly, the reference values used as thresholds [2,7]. In short, there still exists no granularity in the definition of SO.
India is home to over 1.3 billion people and its population is aging rapidly [12]. Besides, the country has a high prevalence of T2DM and MS [13,14]. Hence, the prevalence rate of SO is also expected to be high in the country. However, there is no data on the prevalence of SO in Indians, nor has diagnostic criteria for defining SO in India been established. The present study was conducted to define the diagnosis and establish the prevalence of SO in healthy Indian men and women residing in the community.
2. Methods
The present study was an extension of the recently published “The Chandigarh Urban Bone Epidemiological Study (CUBES)” and “The Sarcopenia-Chandigarh Urban Bone Epidemiological Study (Sarco-CUBES)” [15,16]. The CUBES was an observational cross-sectional study conducted in Chandigarh, a Union Territory in North India, wherein healthy adult men and women were recruited from the community by door-to-door surveys over the study duration of 2 ½ years (December 2016 to June 2019). The study was approved by the Institute Ethics Committee, Post Graduate Institute of Medical Education and Research, Chandigarh, India (Reference No. – NK/3339/DM). The study was conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from all the participants prior to enrollment.
Detailed methodology of the CUBES and the Sarco-CUBES have hitherto already been published [15,16]. In brief, healthy community-dwelling adults aged ≥ 20 years were surveyed from four sectors in Chandigarh. The selection of sectors and houses within each sector were randomized. Kish selection method was used to select a potential participant from a household [17]. Apart from demographic details, potential participants were enquired about the presence of any comorbidity, chronic drug intake, and addictions. Besides, routine diet and physical activity were assessed by the 24-h dietary recall method and the Global Physical Activity Questionnaire (GPAQ), respectively [18]. Individuals having a medical history of any underlying renal, gastrointestinal/hepatic, respiratory, rheumatological, endocrine (namely, diabetes mellitus, Cushing’s syndrome, hyperthyroidism, hyperparathyroidism that could potentially affect muscle mass and/or fat mass), infective or neoplastic disorders, addictions, chronic drug use (especially corticosteroids, complementary and alternative medications, protein supplements), inadequate nutrition (defined as total daily calorie intake < 2100 kcal) [19] and inadequate physical activity (defined as < 250 MET-minutes/week) [20] were excluded. Besides, individuals with contraindications to dual-energy X-ray absorptiometry (DXA) scan (pregnancy, implant placement) were also not included.
Following an overnight fast, participants who meet the aforementioned preliminary inclusion criteria underwent an array of blood tests that included estimation of hemoglobin, renal function (and subsequent calculation of estimated glomerular filtration rate, eGFR), liver function, fasting blood glucose, albumin, calcium, inorganic phosphate, total alkaline phosphatase (ALP), glycated hemoglobin, thyroid function, 25-hydroxyvitamin D, intact parathyroid hormone (iPTH), testosterone (in men), and IgA tissue transglutaminase (IgA tTg) antibody. Participants with anemia, renal dysfunction (eGFR < 90 ml/min), hypoalbuminemia, diabetes mellitus, hyperthyroidism, hypercalcemia, serum 25-hydroxyvitamin D < 10 ng/ml, hypogonadism (in men) and celiac disease were excluded at this step [16].
Healthy participants finally underwent DXA scan using the HOLOGIC Discovery A (QDR 4500; Hologic, Inc., Bedford, MA, USA) scanner for assessment of body composition (lean mass and fat mass). Prior to DXA scan, anthropometry was performed. Height was measured thrice by a wall-mounted stadiometer to the nearest centimeter (cm); the mean of the 3 readings was considered as the final height. Likewise, weight was measured thrice by a digital weighing machine to the nearest of 0.1 kg; the average of the 3 individual recordings was taken as the final weight. Body mass index (BMI) was calculated using the standard formula: weight (in kg)/height (in meter)2. Participants found to have a BMI < 18.5 kg/m2 (qualifying as underweight) were excluded before the DXA scan. Besides, waist circumference (WC) was also measured. The participant was made to stand on a level ground with both the feet placed close together with the body weight being uniformly distributed, arms kept by the side of the body, and with minimal clothing on. The participant was then asked to relax. Waist circumference was then measured at the end of a normal expiration at a specific point corresponding to the midpoint between the lower margin of the last palpable rib and the top of the iliac crest, using a stretch-resistant tape. The measurement was repeated twice; if they were within 1 cm of each other, the average of the 2 was calculated as the final WC. On the contrary, if the difference between the 2 measurements was more than 1 cm, both were repeated [21].
As in the Sarco-CUBES, handgrip strength (HGS) was estimated in the dominant arm of each participant using the Jamar Plus Digital Hand Dynamometer (Jamar®, Patterson Medical, Warrenville, IL, USA). Physical performance was measured in terms of usual gait speed using a 4-meter walk test [16].
2.1. Statistical analysis
Statistical analysis was performed using the Statistical Package for Social Sciences 23.0 (SPSS 23.0) software program (SPSS Inc., Chicago, IL, USA). Kolmogorov-Smirnov test was used to verify the normality of data. Mean ± standard deviation (SD) was used to represent normally distributed data, while nonparametric data were presented as median (interquartile range, IQR). Muscle strength and muscle mass were expressed as dominant handgrip strength (HGS, in kg) and appendicular skeletal muscle index (ASMI, in kg/m2), respectively. The cut-offs used to define low muscle strength and low muscle mass were derived from the Sarco-CUBES. In short, HGS < 27.5 kg in men and < 18.0 kg in women defined low muscle strength, while ASMI < 6.11 kg/m2 in men and < 4.61 kg/m2 in women defined low muscle mass [16]. Participants with a usual gait speed (GS) ≤ 0.8 m/s were categorized as having poor physical performance [3,22,23]. Sarcopenia was defined based on the European Working Group on Sarcopenia in Older People (EWGSOP2) consensus.
Obesity was described according to the 3 widely used indices: BMI, WC, and fat mass percent (FM%, derived from DXA measurement). For BMI, we employed the standard cut-off of ≥ 25.0 kg/m2 to define obesity as is recommended for the Asian-Indian population [24]. Likewise, well-accepted Asian cut-offs for WC of > 90 cm and > 80 cm were used for men and women, respectively [25]. For FM%, thresholds were derived from the study by Marwaha and colleagues. The study that had included 2347 healthy Indian adults had shown that a BMI of 25 kg/m2 corresponded to a FM% of 32.0% in men and 40.0% in women [26]. We employed the same cut-offs to define obesity. Based on each of the 3 criteria, the study cohort was categorized into “sarcopenic non-obese (sarcopenic)” and “sarcopenic obese (SO)” phenotypes. Agreement between the 3 definitions of SO based on BMI, WC, and FM%, respectively, were assessed using Cohen’s kappa [27]. Correlations between BMI, WC, and FM% with GS were performed using Spearman’s rank-order correlation. Subsequently, multiple linear regression analysis was performed with GS as the dependent variable and BMI, WC, FM%, age, and ASMI as the independent variables. A P-value of < 0.05 was considered significant.
Scatterplot with LOESS (locally weighted scatterplot smoothing) curve was used to depict the relationship between FM% and GS. LOESS, also known as LOWESS, is a nonparametric tool used in regression analysis to fit a local regression function. It creates a smooth fitted line through a scatterplot and assesses the graphical relationship between two variables.
3. Results
As in the Sarco-CUBES, 804 healthy participants were included as a part of the final analysis. Amongst the 804 participants, 339 were men, 302 were pre-menopausal and 163 were post-menopausal women. The mean ± SD age of the participants was 44.4 ± 15.4 years (range: 20–85 years) with no statistically significant difference between men and women. The mean ± SD for BMI, WC, and FM% of the group were 26.5 ± 2.7 kg/m2, 86.8 ± 9.6 cm, and 34.7 ± 7.3%, respectively. The baseline characteristics have been described in Table 1. There was a positive correlation between age and BMI (r = 0.412, P < 0.001), WC (r = 0.310, P < 0.001) and FM% (r = 0.462, P < 0.001). The 3 yardsticks used to define obesity also had a positive correlation with each other (BMI and WC: r = 0.823, P < 0.001; BMI and FM%: r = 0.735, P < 0.001; WC and FM%: r = 0.573, P < 0.001).
Table 1.
BMI, WC and FM% in men, premenopausal, and postmenopausal women.
| Variable | Men |
Premenopausal women |
Postmenopausal women |
P-value |
|---|---|---|---|---|
| (n = 339) | (n = 302) | (n = 163) | ||
| BMI, kg/m2 | 26.0 ± 2.2 | 26.1 ± 3.0 | 28.2 ± 2.8 | < 0.001a |
| Mean ± SD | ||||
| WC, cm | 87.4 ± 9.5 | 85.0 ± 9.8 | 89.0 ± 8.7 | < 0.001b |
| Mean ± SD | ||||
| FM% (%) | 29.6 ± 5.7 | 37.9 ± 6.1 | 39.3 ± 7.3 | < 0.001c |
| Mean ± SD |
Values are presented as mean ± standard deviation.
BMI, body mass index; WC, waist circumference; FM%, fat mass percent; SD, standard deviation.
Postmenopausal women vs men: P-value < 0.001, Postmenopausal women vs premenopausal women: P-value < 0.001.
Premenopausal women vs men: P-value = 0.004, Postmenopausal women vs men: P-value < 0.001.
Premenopausal women vs men: P-value < 0.001, Postmenopausal women vs men: P-value < 0.001, Postmenopausal women vs Premenopausal women: P-value = 0.048.
The biochemical parameters of all the participants have been summarized in the Sarco-CUBES [16].
3.1. Prevalence of sarcopenic obesity
The prevalence of sarcopenia in the study population was 3.2% [16]. Subsequently, the prevalence of sarcopenic non-obese and sarcopenic obese individuals using BMI, WC or FM% as the diagnostic criteria is shown in Table 2. Notably, with BMI as a diagnostic criterion, SO was identified in 6 participants; WC and FM% were able to identify 6 and 7 participants with SO, respectively. All individuals with SO were above 65 years of age. Thus, the prevalence of SO in elderly participants (≥ 65 years, n = 111) was 5.4%, 5.4%, and 6.3% based on BMI, WC, and FM%, respectively. The prevalence of SO in elderly subjects stratified according to age and sex is represented in Table 3. Likewise, in patients with sarcopenia (n = 26), the prevalence of SO was 23%–27% based on any of the 3 criteria used. We found no statistically significant differences in HGS or GS between the sarcopenic non-obese and sarcopenic obese groups based either on BMI or WC or FM%. Similarly, there was no difference in HGS or GS between subjects classified as having SO based on any of the 3 criteria. As assessed using Cohen’s kappa, the inter-definitional agreement between the 3 groups was ‘almost perfect’ (Table 4).
Table 2.
Prevalence of sarcopenic non-obese and sarcopenic obese phenotypes based on the criteria used to define obesity (n = 804).
| Variable | Sarcopenic non-obese | Sarcopenic obese |
|---|---|---|
| BMI ≥ 25, kg/m2 | 20 (2.5%) | 6 (0.7%) |
| WC | 20 (2.5%) | 6 (0.7%) |
| > 90 cm in men | ||
| > 80 cm in women | ||
| FM% | 19 (2.3%) | 7 (0.9%) |
| > 32% in men | ||
| > 40% in women |
Values are presented as number (%).
BMI, body mass index; WC, waist circumference; FM%, fat mass percent.
Table 3.
Prevalence of sarcopenic obesity (SO) in elderly subjects (≥ 65 years) based on age and sex of the study participants.
| Age group | Prevalence of SO based on BMI |
Prevalence of SO based on WC |
Prevalence of SO based on FM% |
|||
|---|---|---|---|---|---|---|
| Men | Women | Men | Women | Men | Women | |
| 65–69 | 1 (4.0%) | 0 (0.0%) | 1 (4.0%) | 0 (0.0%) | 1 (4.0%) | 0 (0.0%) |
| (n = 54) | ||||||
| 70–74 | 1 (9.1%) | 1 (9.1%) | 1 (9.1%) | 1 (9.1%) | 1 (9.1%) | 1 (9.1%) |
| (n = 22) | ||||||
| 75–79 | 1 (7.1%) | 1 (9.1%) | 1 (7.1%) | 1 (9.1%) | 1 (7.1%) | 2 (18.2%) |
| (n = 25) | ||||||
| 80–85 | 1 (11.1%) | 0 (0.0%) | 1 (11.1%) | 0 (0.0%) | 1 (11.1%) | 0 (0.0%) |
| (n = 10) | ||||||
| Total | 4 (6.7%) | 2 (3.8%) | 4 (6.7%) | 2 (3.8%) | 4 (6.7%) | 3 (5.7%) |
| (n = 111) | ||||||
Values are presented as number (%).
BMI, body mass index; WC, waist circumference; FM%, fat mass percent; SO, sarcopenic obesity.
Table 4.
Agreement between the 3 criteria used to define sarcopenic obesity.
| First definition | Second definition | Cohen’s kappa (κ) | Magnitude of agreement |
|---|---|---|---|
| BMI ≥ 25, kg/m2 | WC | 1.000 | Almost perfect |
| > 90 cm in men | |||
| > 80 cm in women | |||
| BMI ≥ 25, kg/m2 | FM% | 0.922 | Almost perfect |
| > 32% in men | |||
| > 40% in women | |||
| WC | FM% | 0.922 | Almost perfect |
| > 90 cm in men | > 32% in men | ||
| > 80 cm in women | > 40% in women |
BMI, body mass index; WC, waist circumference; FM%, fat mass percent.
3.2. Correlations between BMI, WC, and FM% with muscle strength and physical performance
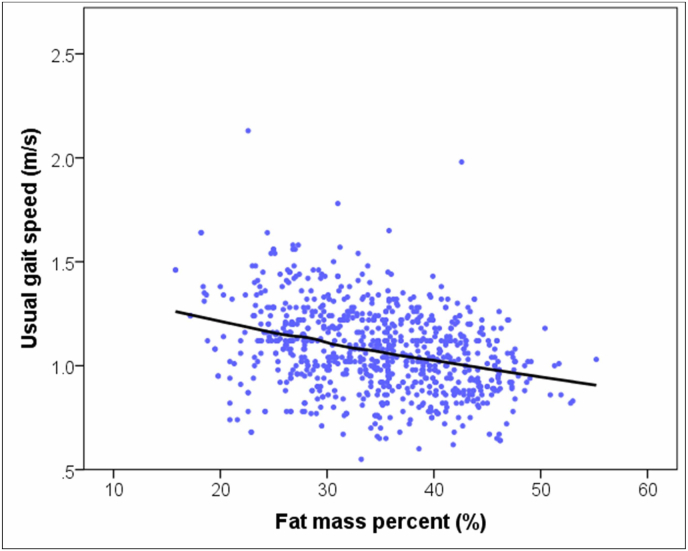
Muscle strength, expressed as HGS, did not have any statistically significant correlations between BMI, WC, or FM%. On the other, GS (a measure of physical performance) negatively correlated with BMI (r = −0.124, P < 0.001), WC (r = −0.072, P = 0.041), and FM% (r = −0.326, P < 0.001). Fig. 1 depicts the scatterplot with LOESS curve showing the relation between GS and FM%. Multiple linear regression analysis (with age, BMI, WC, FM%, and ASMI as independent variables) showed that only age (β = −0.007, P < 0.001), FM% (β = −0.006, P < 0.001) and ASMI (β = 0.027, P < 0.001) were significant predictors of GS.
Fig. 1.
Scatterplot with locally weighted smoothing curve depicting the relation between usual gait speed and fat mass percent.
3.3. Correlations between HGS, ASMI, GS, BMI, WC, and FM% with biochemical parameters
Univariate analysis (using Pearson/Spearman correlation) showed that serum albumin had a significant positive correlation with HGS (r = 0.114, P = 0.001). Likewise, serum testosterone (only in men) positively correlated with HGS (rs = 0.348, P < 0.001), ASMI (rs = 0.111, P = 0.041) and GS (rs = 0.366, P < 0.001). Serum calcium, 25-hydroxyvitamin D and iPTH did not significantly correlate with any of the 3 parameters. On multiple linear regression analyses, only serum testosterone (in men) was a positive predictor of HGS, ASMI, and GS.
As far as the indices of obesity were concerned, only serum testosterone negatively correlated with BMI (rs = −0.190, P < 0.001), WC (rs = −0.244, P < 0.001) and FM% (rs = −0.276, P < 0.001) in men. However, other biochemical parameters, notably calcium, 25-hydroxyvitamin D, and iPTH, did not correlate with BMI/WC or FM% in either men or women.
4. Discussion
To the best of our knowledge, this is the first study evaluating the prevalence of sarcopenic obesity in the Indian population. As an extension of the Sarco-CUBES, we found that using BMI, WC or FM% as criteria for defining obesity, the prevalence of SO ranges from 5.4% to 6.3% in healthy Indian adults aged ≥ 65 years residing in the community. Either BMI or WC or FM% can be used to define obesity with an almost perfect inter-definitional agreement between the 3 parameters.
Sarcopenic obesity is a clinical-cum-functional entity typified by the co-occurrence of sarcopenia and obesity in an individual. The co-existence of obesity and sarcopenia exerts a synergistic effect that predisposes an individual to the risk of MS, T2DM, physical disability, morbidity and eventual mortality compared to either of the 2 entities alone [7,9]. Hence, SO poses a major public health challenge and demands identification and intervention at an early stage. Notably, certain lifestyle interventions, including calorie restriction, protein supplementation, and graded physical activity, are potential therapies in SO with improvement in physical activity and reduction in mortality [7,28,29].
The diagnosis of SO is based on the discrete definitions of sarcopenia and obesity; however, at present, no generalized consensus exists that defines the cut-off points for either of these entities. Hence, making an accurate diagnosis of SO is challenging [2,7]. Most studies have used muscle mass as a sole parameter to define sarcopenia in SO; only a handful of studies have considered both muscle mass and muscle strength/physical performance as a criterion to define sarcopenia based on the EWGSOP (2010) or International Working Group on Sarcopenia (IWGS) or Federation for the National Institutes of Health (FNIH) consensus [2]. Hitherto, very few studies have defined sarcopenia (in SO) based on the EWGSOP2 consensus that lays more emphasis on muscle strength than muscle mass [30]. Similarly, obesity has been variably defined based on BMI or WC or fat mass percent [2]. The prevalence of SO in an American population varied up to 26-fold using 8 different definitions [11].
More than 1.3 billion people live in India, with over 87 million people aged 65 years or above accounting for 6.4% of the population. By 2030, the figure is expected to increase to more than 128 million [31]. By 2050, the proportion of the population aged > 60 years is estimated to reach 19%. Finally, at the end of the century, a mammoth fraction (34%) of the Indian population would be constituted by the elderly [32]. Hence, sarcopenia and SO are of increasing clinical relevance in the country. Nevertheless, there is limited data on sarcopenia and a complete dearth of data on SO in India.
We had recently shown that using indigenous (rather than Caucasian) cut-offs, the prevalence of sarcopenia in community-dwelling healthy Indian adults, as per the revised EWGSOP2 consensus, was 3.2% [16]. Applying the same cut-offs to describe sarcopenia and using either BMI or WC or FM% for defining obesity, we found that the SO was prevalent in 5.4%–6.3% of healthy Indian men and women aged ≥ 65 years. The reported prevalence of SO in the elderly population ranges from 0.9% to 30.1% [10,11]. In a cross-sectional study (WCHAT study) conducted in 4 Chinese provinces, SO was prevalent in 2.6% of individuals aged 50 years and older [33]. In our study, the prevalence of SO in individuals aged ≥ 50 years (n = 288) would be 2.1%–2.4%, very similar to the WCHAT study. Nevertheless, although the prevalence of SO in the aging Indian population is low and comparable to the neighboring Asian nations, nearly one-fourth of the sarcopenic subjects had accompanying obesity, necessitating screening for SO in all patients with sarcopenia.
Regarding the definition of obesity, we found that BMI, WC, and FM% had almost perfect inter-definitional agreement implying that either BMI or WC or FM% can be reliably used as a diagnostic criterion for SO in Indians. However, only FM% was a negative predictor of GS amongst the 3 variables, even after adjustment for age and ASMI. Prior studies have also shown an association between high body fat percentage and slow GS [34,35]. Thus, FM% may be a better diagnostic criterion for SO compared with either BMI or WC as the former predicts the functional status of an individual. This is in contrast to a study by Khor et al who found that among 200 community-dwelling, functionally-independent older adults residing in Singapore, only WC, and not BMI or DXA-derived fat mass percent, was best associated with poor muscle strength and function in SO [36]. Nevertheless, body mass index may not be suitable for recognizing poor physical performance in SO as it does not assess body fat distribution and fails to account for the loss of lean body mass with age [7].
We do respect the inherent limitations of the present study. First, we did not perform any prior statistical analysis to calculate the sample size. For sample size calculation, the prevalence of the disease entity being studied (sarcopenic obesity in this case) needs to be known. In the absence of any data on SO from India, prior sample size collection was not possible. Instead, we used the same cohort as was used for the Sarco-CUBES [16]. The sample size of Sarco-CUBES was estimated based on a prior study that had reported a prevalence of low muscle mass of 15% in healthy Indian women [37]. Second, the proportion of elderly subjects (≥ 65 years), especially those aged 75 years and older, was limited and probably underrepresented in the study cohort; the meticulous and stringent exclusion criteria led to the elimination of a significant proportion of older adults with comorbidities that could have contributed to (secondary) sarcopenia. The inclusion of subjects with comorbidities would have inflated the prevalence of sarcopenia (and probably SO); however, our intention was not to include subjects with secondary sarcopenia. The same should be regarded as a strength rather than a drawback as most of the large-scale studies evaluating the prevalence of SO in the community have not excluded secondary causes of sarcopenia/SO.
Third, we did not compare the metabolic parameters (like fasting blood glucose, fasting lipid profile) among participants with and without SO. Fourth, we assessed only usual gait speed by 4-m walk test as a measure of physical performance. A single physical performance measure may not be able to identify sarcopenia correctly, often requiring a combination of tests like Short Physical Performance Battery, chair stand test (CST), timed up and go (TUG), and 4-m walk test [38]. However, GS is a time-tested and dependable measure of physical performance and has been shown to predict major health-related outcomes reliably [39]. Lastly, being a cross-sectional study, the clinical outcomes of the participants classified as having sarcopenia or sarcopenic obesity could not be assessed due to lack of follow-up.
5. Conclusions
In conclusion, the prevalence of SO in healthy elderly Indian adults (≥ 65 years) is 5.4%–6.3% using indigenous Asian-Indian cut-offs. Nearly one-fourth of subjects with sarcopenia have accompanying obesity, necessitating screening for SO in all adults with sarcopenia. Either BMI or WC or FM% (derived from DXA) can be used to identify individuals with SO; however, FM% may be a better predictor of physical performance and functional outcomes and may be clinically more relevant. In the absence of any data on SO from the Indian subcontinent, the present study is likely to lead the way to large-scale observational and interventional studies in the future.
Availability of data and material
Anonymous data sheets will be made available on reasonable request to the corresponding author.
CRediT author statement
Rimesh Pal: Conceptualization, Data curation, Formal analysis, Writing - original draft. Sanjay K. Bhadada: Conceptualization, Resources, Writing - review & editing. Anshita Aggarwal: Data curation, Writing - review & editing. Tulika Singh: Investigation, Writing - review & editing.
Conflicts of interest
The authors declare no competing interests.
Acknowledgments
ORCID Rimesh Pal: 0000-0003-4859-9393. Sanjay K. Bhadada: 0000-0002-0410-8778. Anshita Aggarwal: 0000-0002-6969-2530. Tulika Singh: 0000-0002-2270-1639.
Footnotes
Peer review under responsibility of The Korean Society of Osteoporosis.
References
- 1.Roubenoff R. Sarcopenic obesity: the confluence of two epidemics. Obes Res. 2004;12:887–888. doi: 10.1038/oby.2004.107. [DOI] [PubMed] [Google Scholar]
- 2.Donini LM, Busetto L, Bauer JM, Bischoff S, Boirie Y, Cederholm T. Critical appraisal of definitions and diagnostic criteria for sarcopenic obesity based on a systematic review. Clin Nutr. 2019;39:2368–2388. doi: 10.1016/j.clnu.2019.11.024. [DOI] [PubMed] [Google Scholar]
- 3.Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48:16–31. doi: 10.1093/ageing/afy169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baumgartner RN. Body composition in healthy aging. Ann N Y Acad Sci. 2006;904:437–448. doi: 10.1111/j.1749-6632.2000.tb06498.x. [DOI] [PubMed] [Google Scholar]
- 5.Baumgartner RN, Wayne SJ, Waters DL, Janssen I, Gallagher D, Morley JE. Sarcopenic obesity predicts instrumental activities of daily living disability in the elderly. Obes Res. 2004;12:1995–2004. doi: 10.1038/oby.2004.250. [DOI] [PubMed] [Google Scholar]
- 6.Villareal DT, Shah K. Obesity in older adults – a growing problem. In: Bales C.W., Ritchie C.S., editors. Handb. Clin. Nutr. Aging. Humana Press; Totowa, NJ: 2009. pp. 263–277. [Google Scholar]
- 7.Batsis JA, Villareal DT. Sarcopenic obesity in older adults: aetiology, epidemiology and treatment strategies. Nat Rev Endocrinol. 2018;14:513–537. doi: 10.1038/s41574-018-0062-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khadra D, Itani L, Tannir H, Kreidieh D, Masri DE, Ghoch ME. Association between sarcopenic obesity and higher risk of type 2 diabetes in adults: a systematic review and meta-analysis. World J Diabetes. 2019;10:311–323. doi: 10.4239/wjd.v10.i5.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lim S, Kim JH, Yoon JW, Kang SM, Choi SH, Park YJ. Sarcopenic obesity: prevalence and association with metabolic syndrome in the Korean longitudinal study on health and aging (KLoSHA) Diabetes Care. 2010;33:1652–1654. doi: 10.2337/dc10-0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kemmler W, von Stengel S, Engelke K, Sieber C, Freiberger E. Prevalence of sarcopenic obesity in Germany using established definitions: baseline data of the FORMOsA study. Osteoporos Int. 2016;27:275–281. doi: 10.1007/s00198-015-3303-y. [DOI] [PubMed] [Google Scholar]
- 11.Batsis JA, Barre LK, Mackenzie TA, Pratt SI, Lopez-Jimenez F, Bartels SJ. Variation in the prevalence of sarcopenia and sarcopenic obesity in older adults associated with different Research definitions: dual-energy X-ray absorptiometry data from the national health and nutrition examination survey 1999-2004. J Am Geriatr Soc. 2013;61:974–980. doi: 10.1111/jgs.12260. [DOI] [PubMed] [Google Scholar]
- 12.Chatterji S, Kowal P, Mathers C, Naidoo N, Verdes E, Smith JP. The health of aging populations in China and India. Health Aff. 2008;27:1052–1063. doi: 10.1377/hlthaff.27.4.1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anjana RM, Deepa M, Pradeepa R, Mahanta J, Narain K, Das HK. Prevalence of diabetes and prediabetes in 15 states of India: results from the ICMR–INDIAB population-based cross-sectional study. Lancet Diabetes Endocrinol. 2017;5:585–596. doi: 10.1016/S2213-8587(17)30174-2. [DOI] [PubMed] [Google Scholar]
- 14.Deepa M, Farooq S, Datta M, Deepa R, Mohan V. Prevalence of metabolic syndrome using WHO, ATPIII and IDF definitions in Asian Indians: the Chennai urban rural epidemiology study (CURES-34) Diabetes Metab Res Rev. 2007;23:127–134. doi: 10.1002/dmrr.658. [DOI] [PubMed] [Google Scholar]
- 15.Aggarwal A, Ram S, Garg A, Pal R, Bhansali A, Singh P. Metabolic Bone profile of healthy adult North Indian population from Chandigarh urban Bone epidemiological study (CUBES) Indian J Clin Biochem. 2019 doi: 10.1007/s12291-019-00857-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pal R, Aggarwal A, Singh T, Sharma S, Khandelwal N, Garg A. Diagnostic cut-offs, prevalence, and biochemical predictors of sarcopenia in healthy Indian adults: the Sarcopenia-Chandigarh Urban Bone Epidemiological Study (Sarco-CUBES) Eur Geriatr Med. 2020;11:725–736. doi: 10.1007/s41999-020-00332-z. [DOI] [PubMed] [Google Scholar]
- 17.Kish L. A procedure for objective respondent selection within the household. J Am Stat Assoc. 1949;44:380–387. [Google Scholar]
- 18.Armstrong T, Bull F. Development of the world health organization global physical activity Questionnaire (GPAQ) J Public Health. 2006;14:66–70. [Google Scholar]
- 19.Srivastava SK, Chand R. Tracking transition in calorie-intake among Indian households: insights and policy implications. Agric Econ Res Rev. 2017;30:23. [Google Scholar]
- 20.Janssen I, Ross R. Vigorous intensity physical activity is related to the metabolic syndrome independent of the physical activity dose. Int J Epidemiol. 2012;41:1132–1140. doi: 10.1093/ije/dys038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Waist circumference and waist–hip ratio. Report of a WHO expert consultation; Geneva: December 2008. pp. 8–11. n.d. [Google Scholar]
- 22.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F. Sarcopenia: European consensus on definition and diagnosis: report of the European working group on sarcopenia in older people. Age Ageing. 2010;39:412–423. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Studenski SA, Peters KW, Alley DE, Cawthon PM, McLean RR, Harris TB. The FNIH sarcopenia project: rationale, study description, conference recommendations, and final estimates. J Gerontol A Biol Sci Med Sci. 2014;69:547–558. doi: 10.1093/gerona/glu010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Misra A, Chowbey P, Makkar BM, Vikram NK, Wasir JS, Chadha D. Consensus statement for diagnosis of obesity, abdominal obesity and the metabolic syndrome for Asian Indians and recommendations for physical activity, medical and surgical management. J Assoc Phys India. 2009;57:163–170. [PubMed] [Google Scholar]
- 25.Alberti KGMM, Zimmet P, Shaw J. Metabolic syndrome-a new world-wide definition. A consensus statement from the international diabetes federation. Diabet Med. 2006;23:469–480. doi: 10.1111/j.1464-5491.2006.01858.x. [DOI] [PubMed] [Google Scholar]
- 26.Marwaha RK, Tandon N, Garg MK, Narang A, Mehan N, Bhadra K. Normative data of body fat mass and its distribution as assessed by DXA in Indian adult population. J Clin Densitom. 2014;17:136–142. doi: 10.1016/j.jocd.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 27.McHugh ML. Interrater reliability: the kappa statistic. Biochem Med. 2012;22:276–282. [PMC free article] [PubMed] [Google Scholar]
- 28.Villareal DT, Aguirre L, Gurney AB, Waters DL, Sinacore DR, Colombo E. Aerobic or resistance exercise, or both, in dieting obese older adults. N Engl J Med. 2017;376:1943–1955. doi: 10.1056/NEJMoa1616338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kritchevsky SB, Beavers KM, Miller ME, Shea MK, Houston DK, Kitzman DW. Intentional weight loss and all-cause mortality: a meta-analysis of randomized clinical trials. PloS One. 2015;10 doi: 10.1371/journal.pone.0121993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.von Berens Å, Obling SR, Nydahl M, Koochek A, Lissner L, Skoog I. Sarcopenic obesity and associations with mortality in older women and men – a prospective observational study. BMC Geriatr. 2020;20 doi: 10.1186/s12877-020-01578-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.World population ageing 2019 highlights. United Nations, New York.
- 32.Caring for our elders: Early responses. India ageing report - 2017. United Nations Population Fund, New Delhi.
- 33.Liu X, Hao Q, Yue J, Hou L, Xia X, Zhao W. Sarcopenia, obesity and sarcopenia obesity in comparison: prevalence, metabolic profile, and key differences: results from WCHAT study. J Nutr Health Aging. 2020;24:429–437. doi: 10.1007/s12603-020-1332-5. [DOI] [PubMed] [Google Scholar]
- 34.Fujita T, Kono A. Association of high body fat percentage with slow gait speed in elderly Japanese. Gerontol. 2015;55:463. [Google Scholar]
- 35.Falsarella G, Gasparotto LPR, Barcelos CC, Moretto MC, Pascoa MA, Ferreira TCBR. Body composition as a frailty marker for the elderly community. Clin Interv Aging. 2015:1661. doi: 10.2147/CIA.S84632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Khor EQ, Lim JP, Tay L, Yeo A, Yew S, Ding YY. Obesity definitions in sarcopenic obesity: differences in prevalence, agreement and association with muscle function. J Frailty Aging. 2020;9:37–43. doi: 10.14283/jfa.2019.28. [DOI] [PubMed] [Google Scholar]
- 37.Marwaha RK, Garg MK, Bhadra K, Mithal A, Tandon N. Assessment of lean (muscle) mass and its distribution by dual energy X-ray absorptiometry in healthy Indian females. Arch Osteoporos. 2014;9:186. doi: 10.1007/s11657-014-0186-z. [DOI] [PubMed] [Google Scholar]
- 38.Looijaard SMLM, Oudbier SJ, Reijnierse EM, Blauw GJ, Meskers CGM, Maier AB. Single physical performance measures cannot identify geriatric outpatients with sarcopenia. J Frailty Aging. 2018;7:262–267. doi: 10.14283/jfa.2018.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peel NM, Kuys SS, Klein K. Gait speed as a measure in geriatric assessment in clinical settings: a systematic review. J Gerontol Ser A. 2013;68:39–46. doi: 10.1093/gerona/gls174. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymous data sheets will be made available on reasonable request to the corresponding author.