Abstract
Objectives
Calcaneal quantitative ultrasound measurement (QUS) has been considered an alternative to dual-energy X-ray absorptiometry (DXA) based bone mineral density (BMD) for assessing bone health. This study sought to examine the utility of QUS as an osteoporosis screening tool by evaluating the correlation between QUS and DXA.
Methods
The study was a part of the Vietnam Osteoporosis Study that involved 1270 women and 773 men aged 18 years and older. BMD at the femoral neck, total hip and lumbar spine was measured using DXA. Osteoporosis was diagnosed based on the femoral neck T-score using World Health Organization criteria. Broadband ultrasound attenuation (BUA) at the calcaneus was measured by QUS. The concordance between BUA and BMD was analyzed by the linear regression model.
Results
In all individuals, BUA modestly correlated with femoral neck BMD (r = 0.35; P < 0.0001) and lumbar spine BMD (r = 0.34; P < 0.0001) in both men and women. In individuals aged 50 years and older, approximately 16% (n = 92/575) of women and 3.2% (n = 10/314) of men were diagnosed to have osteoporosis. Only 0.9% (n = 5/575) women and 1.0% (n = 3/314) men were classified as “Low BUA”. The kappa coefficient of concordance between BMD and BUA classification was 0.09 (95% CI, 0.04 to 0.15) for women and 0.12 (95% CI, 0.03 to 0.22) for men.
Conclusions
In this population-based study, QUS BUA modestly correlated with DXA BMD, suggesting that BUA is not a reliable method for screening of osteoporosis.
Keywords: Bone mineral density, DXA, Osteoporosis, QUS, BUA, Vietnamese population
1. Introduction
Osteoporosis remains a significant public health problem among the aged population, because of its high prevalence and association with reduced life expectancy [1]. Our previous study [2] has shown that among individuals aged 50 years and older, ∼25% of women and 10% of men were having osteoporosis, and these prevalence estimates are quite comparable with those observed in Caucasian populations [3]. The ultimate consequence of osteoporosis is a fragility fracture. It is little known that fracture is associated with increased risk of mortality, with men having higher risk than women [4]. Among women with a hip fracture, the risk of mortality is equivalent to that among patients with breast cancer. With the ongoing aging of population around the world, it is expected that osteoporosis and its clinical consequences will impose a significant health care burden to the public health system worldwide.
Among those without a fragility fracture, the operational definition of osteoporosis is based on a measurement of bone mineral density (BMD) at the femoral neck [5] by dual-energy X-ray absorptiometry (DXA). BMD changes with age: rapid increase during adolescence; reaching a peak level between the age of 20 and 30; and declining after the age of 45. Therefore, the measured BMD of an individual is commonly standardized by the T-score, which is defined as the number of standard deviations away from the peak level. According to the World Health Organization’s recommendation, an individual’s T-score ≤ −2.5 is diagnosed to have osteoporosis. While this practice is standard in economically advanced countries, it presents a significant problem in developing countries where DXA availability is limited. In Vietnam, for example, DXA densitometers are currently available in large teaching hospitals in cities, not in rural regions where 70% of the population reside. Thus, the problem of underdiagnosis of osteoporosis is common in economically less advanced countries.
Quantitative ultrasound measurement (QUS) is a technology that measures the transmission of ultrasound through the distal end of the proximal phalangeal diaphysis of the hand or the heel [6]. Because QUS reflects the characteristics of the electrical signal generated by ultrasound after going through the phalanx soft tissues and bone, it can provide information that may be relevant for determining fracture risk. Indeed, several studies have indicated that individuals with lower QUS measurements have higher risk of fragility fracture [7], and this increased risk was independent of BMD. QUS has several advantages over BMD: it is inexpensive, easy to use, safe (eg, radiation-free) and portable. The low-cost and portability of QUS makes it an attractive tool for screening purpose in settings where DXA is not available. In Vietnam (and perhaps in other developing countries), many health care centers, including pharmacies, use QUS for the diagnosis of osteoporosis. Nevertheless, the use of QUS in clinical settings remains controversial, as the correlation between QUS and BMD has not been examined in population based studies [8]. This study seeks to evaluate the utility of QUS as an osteoporosis screening tool in low-resource settings by assessing the concordance between BUA and BMD in the diagnosis of osteoporosis.
2. Methods
2.1. Participants
This study is a part of the longitudinal population study based on the Vietnam Osteoporosis Study [9] that involved more than 4000 men and women aged 18 years and older. The study’s procedure and protocol were approved by the research and ethics committee of the People’s Hospital 115 on August 6, 2015 (Approval Number 297/BV-NCKH). The study was conducted according to the ethical principles of the Declaration of Helsinki, and all participants gave written informed consent.
Participants were randomly drawn from various districts who were living in Ho Chi Minh City and surrounding rural areas. We contacted community organizations to solicit a list of members, and from the list we ran a computer program of randomly selected individuals who met the age and gender criteria. A letter was then sent to the selected individuals to invite them and their friends or family members to participate in the study. In the second approach, we recruited participants via television, the Internet, and flyers in universities. The flyers (in Vietnamese) described the Study’s purposes, procedures, benefits and potential risks of participants. Individuals agreed to participate in the study were then transported to the Bone and Muscle Research Laboratory at the Ton Duc Thang University for clinical assessment and evaluation. Participants did not receive any financial incentive, but they received a free health check-up, and lipid analyses. We excluded individuals deemed to have impaired cognitive function or who are not willing to give informed consent or were physically unable to complete the clinical tests.
2.2. Measurements
Extensive data were collected at baseline. Demographic and life style data were ascertained by a structured questionnaire administered by a trained interviewer. Height and weight were measured by an electronic portable, wall-mounted stadiometer (Seca Model 769; Seca Corp, CA, USA) without shoes, ornaments, hats or heavy layers of clothing. Body mass index (BMI) was derived as the weight in kilograms divided by the square of the height in meters (kg/m2). Current smoking was ascertained from the self-report.
We measured BMD at the femoral neck and lumbar spine (L2-L4) by a Hologic Horizon densitometer (Hologic Corp., Bedford, MA, USA). BMD at the femoral neck (FNBMD) and lumbar spine (LSBMD) was measured in gram per cm2. The densitometer was standardized before each measurement with a phantom. The process was done by a qualified radiology technologist. The coefficient of variation of BMD measurements was 1.5% for the lumbar spine and 1.7% for the femoral neck. BMD was also expressed in terms of T-scores at the femoral and lumbar spine, using the previously published reference range [2]. We calculated the T-score for each individual based on gender-specific peak bone mineral density and standard deviation that have been published previously [2]. The T-score was determined at the femoral neck, total hip, and lumbar spine. An individual was classified into one of 3 groups based on the femoral neck T-score [5]: normal BMD (T-score of −1.00 or above); osteopenia (T-score of −1.01 to 2.49); and osteoporosis (T-score of −2.50 or below).
In addition to BMD assessment, QUS was measured using a portable ultrasound (Sahara, Hologic Corp., Bedford, MA, USA). Sahara measures broadband ultrasound attenuation transmitted through the calcaneus. The output is presented in terms of T-scores, derived from an Asian reference population, from which we classified individuals into 3 groups: those with low BUA if T-scores -2.5, and those with medium BUA if T-scores range between −2.5 and −1.0; and normal BUA if T-scores -1.0.
2.3. Data analysis
We used the linear regression model to assess the sex-specific correlation between BMD and BUA, taking into account 2 main covariates: age and weight. In addition, we used the Kappa coefficient to assess the concordance in diagnostic categories (eg, osteoporosis, osteopenia, and normal) between DXA and BUA among those aged 50 years and older. All analyses were conducted using the R statistical environment Version 3.6.3 for Windows (R Foundation for Statistical Computing, Vienna, Austria) [10], and the variance contribution of each variable was estimated using “relaimpo” package [11].
3. Results
The study included 2043 individuals (1270 women and 773 men) whose average age (standard deviation) was 45.2 years (14.7), with approximately 45.2% of women and 40.6% of men aged 50 years and older. The mean BMI was 22.4 (3.06) kg/m2 in women and 23.0 (2.85) kg/m2 in men. Approximately 7% of participants were obese, and 36% were overweight (Table 1).
Table 1.
Baseline characteristics stratified by sex.
| Variable | Women (n = 1270) | Men (n = 773) | P-value |
|---|---|---|---|
| Age, yr | 45.9 (14.6) | 44.0 (14.7) | 0.005 |
| Weight, kg | 52.9 (7.5) | 62.3 (8.5) | < 0.001 |
| Height, cm | 154 (5.4) | 165 (5.6) | < 0.001 |
| Body mass index, kg/m2 | 22.4 (3.1) | 23.0 (2.9) | < 0.001 |
| BMI group, n,% | < 0.001 | ||
| Under-weight | 32 (2.5%) | 13 (1.7%) | |
| Normal-weight | 744 (58.6%) | 391 (50.6%) | |
| Over-weight | 412 (32.4%) | 315 (40.8%) | |
| Obese | 82 (6.5%) | 54 (7.0%) | |
| Femoral neck BMD, g/cm2 | 0.70 (0.12) | 0.79 (0.12) | < 0.001 |
| Total hip BMD, g/cm2 | 0.81 (0.12) | 0.90 (0.12) | < 0.001 |
| Lumbar spine BMD, g/cm2 | 0.90 (0.14) | 0.94 (0.12) | < 0.001 |
| Calcaneus BUA, dB/MHz | 66.7 (9.60) | 68.0 (9.16) | 0.004 |
Values are mean (SD), unless otherwise specified.
BMD, bone mineral density; DXA: dual-energy X-ray absorptiometry; BUA, broadband ultrasound attenuation; QUS, quantitative ultrasound; SD, standard deviation; P-values were derived from t-test for continuous variables and Chi-squared test for categorical variables.
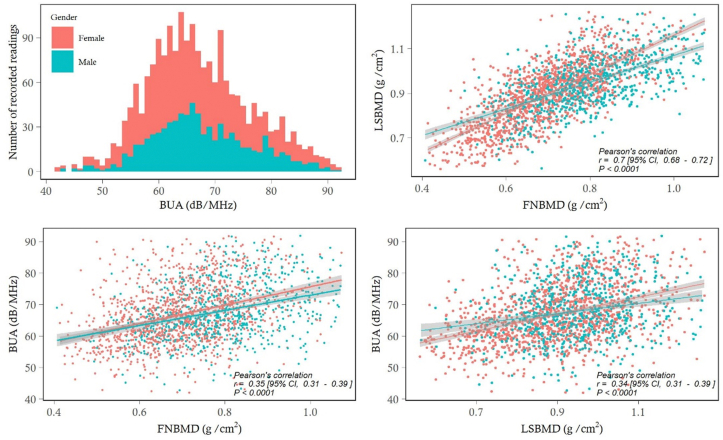
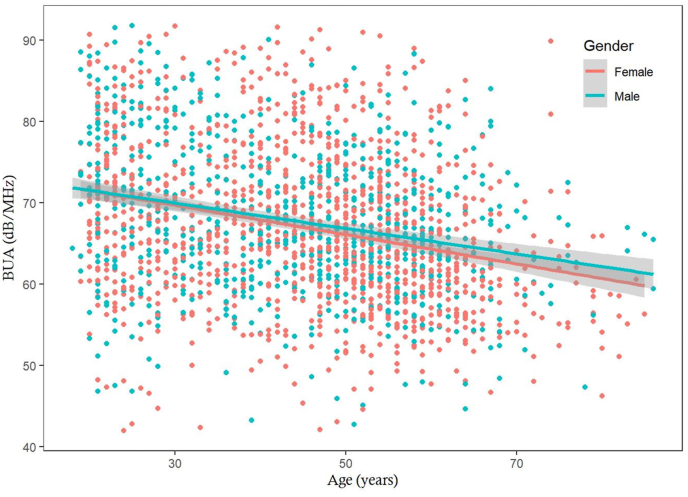
The distribution of BUA approximately followed the normal distribution, with mean in men being 1.3 dB/MHz higher than women (Fig. 1). In either sex, there was an inverse association between BUA and advancing age (Fig. 2). Using linear regression analysis, each year increase in age was associated with a decrease of 0.18 dB/MHz in women and 0.154 dB/MHz in men. Apart from gender and age, weight was also a significant predictor of BUA, with estimated regression coefficient being 0.54 (SE 0.13). The 3 factors (gender, age, and weight) collectively ‘explained’ ∼8% of total variance in BUA. Analysis of relative importance indicated that age was the most imporant factor, followed by weight and gender (Table 2).
Fig. 1.
The distribution of BUA values for 1270 women and 773 men (top left); the correlation between femoral neck BMD and lumbar spine BMD (top right), femoral neck BMD and BUA (bottom left), and lumbar spine BMD and BUA (bottom right) stratified by gender.
BUA, broadband ultrasound attenuation; BMD, bone mineral density.
Fig. 2.
Association between age and BUA for men (blue dots) and women (red dots) in 1270 women and 773 men.
BUA, broadband ultrasound attenuation.
Table 2.
Predictors of Broadband Ultrasound Attenuation in 1270 women and 773 men.
| Predictor | Dependent variable: BUA | Relative Importance (R2) |
|---|---|---|
| Regression coefficients | ||
| Gender (men vs women) | −0.103 (0.480) | 0.002 |
| Age (+5 yr) | −0.869 (0.069) | 0.071 |
| Weight (+5 kg) | 0.537 (0.127) | 0.008 |
| Goodness-of-fit index | ||
| Mean squared error | 82 | – |
| R-squared | 0.08 | – |
Values in brackets are standards error of estimates.
BUA, broadband ultrasound attenuation.
There was a statistically significant correlation between BUA and BMD at the femoral neck and lumbar spine. The linear correlation coefficient for BUA and FNBMD was 0.35 (95% CI, 0.31 to 0.39; P < 0.0001), and LSBMD 0.34 (95% CI, 0.31 to 0.39; P < 0.0001). In linear regression analysis, after accouting for age and gender, each unit increment in BUA was associated with 0.003 g/cm2 (SE 0.0002) increase in FNBMD, and with 0.003 g/cm2 (SE 0.0003) in LSBMD (Table 3).
Table 3.
Association between BUA and BMD in 1270 women and 773 men.
| Predictors | Dependent variable: Femoral neck BMD | Dependent variable: Lumbar spine BMD |
|---|---|---|
| Regression coefficients | ||
| Gender (men vs women) | 0.032 (0.005)∗ | 0.0006 (0.006) |
| Age (+5 yr) | −0.017 (0.001)∗ | −0.014 (0.001)∗ |
| Weight (+5 kg) | 0.024 (0.001)∗ | 0.019 (0.002)∗ |
| BUA (+dB/MHz) | 0.003 (0.0002)∗ | 0.003 (0.0002)∗ |
| Goodness-of-fit index | ||
| Mean squared error | 0.009 | 0.014 |
| R-squared | 0.43 | 0.27 |
Values in brackets are standards error of estimates.
∗Statistical significance at the level of P < 0.001.
BUA, broadband ultrasound attenuation; BMD, bone mineral density.
Among those aged 50 years and older, using the T-score data, 0.9% (n = 5/575) of women and 1.0% (n = 3/314) of men were classified as having “low BUA” measurement. Using the femoral neck BMD T-scores, 16% (n = 92/575) of women and 3.2% (n = 10/314) of men had osteoporosis. Among those diagnosed to have osteoporosis by DXA, only 2 (in women) and none (in men) were classified as “low BUA”. The kappa coefficient of concordance between DXA and BUA classification was 0.09 (95% CI, 0.04 to 0.15) for women and 0.12 (95% CI, 0.03 to 0.22) for men (Table 4).
Table 4.
Concordance between BMD- and BUA-based classification of bone status in 575 women and 314 men aged 50 years and older, stratified by gender.
| FNBMD: Osteoporosis |
FNBMD: Osteopenia |
FNBMD: Normal |
Total | |
|---|---|---|---|---|
| Women | ||||
| BUA: low | 2 (2.2) | 2 (0.6) | 1 (0.6) | 5 (0.9) |
| BUA: medium | 60 (65.2) | 126 (40.0) | 37 (22.2) | 223 (38.8) |
| BUA: normal | 30 (32.6) | 188 (59.4) | 129 (77.2) | 347 (60.3) |
| Total | 92 (100.0) | 316 (100.0) | 167 (100.0) | 575 (100.0) |
| Men | ||||
| BUA: low | 0 | 3 (2.1) | 0 | 3 (1.0) |
| BUA: medium | 4 (40.0) | 45 (31.0) | 30 (18.9) | 79 (25.1) |
| BUA: normal | 6 (60.0) | 97 (66.9) | 129 (81.1) | 232 (73.9) |
| Total | 10 (100.0) | 145 (100.0) | 159 (100.0) | 314 (100.0) |
Numbers in brackets are columnwise percentages.
BUA, broadband ultrasound attenuation; FN, femur neck; BMD, bone mineral density.
4. Discussion
In the absence of a fragility fracture, measurement of BMD by DXA has been the ‘gold standard’ method for the diagnosis of osteoporosis. This practice is justified because the magnitude of association between low BMD and fracture risk is equivalent to that of between blood pressure and stroke. However, in settings where DXA is not available, the diagnosis of osteoporosis is not possible, and alternative method is required. One alternative method is QUS measured by ultrasonography, because this is a versatile and relative inexpensive technology for bone health assessment. In this study, we evaluated the utility of QUS in the screening of osteoporosis in a developing country, and found that the concordance between BUA (a QUS measurement) and BMD was very low, suggesting that QUS is not a reliable method for identifying individuals at high risk of osteoporosis, and this conclusion is agreeable with the recommendation by the International Society of for Bone Densitometry (https://www.iscd.org/official-positions/2019-iscd-official-positions-adult, access date: July 10, 2020).
Our finding of weak correlation between BUA and BMD confirms previous observations that BUA poorly correlated with BMD [12], including in men [13], women [14], and children [15]. Nevertheless, some previous studies suggested that BUA was a sensitive tool for the assessment of osteoporosis [16]. Indeed, a meta-analysis of 15 original studies concluded that heel ultrasound “should be considered to be as accurate as densitometry in diagnosing osteoporosis” but with a different T-score threshold [17]. However, most previous studies were based on small sample sizes (eg, mostly under 500 individuals) [17], or focused on a specific group of patients [18], and different QUS technologies were used. A review of QUS in the assessment of fracture risk called for population-based studies to determine the etiological correlation between QUS and BMD [8]. To date our study represents the largest population-based study to address the question of QUS and BMD correlation, and our data clearly showed that among those diagnosed to have osteoporosis by BMD, BUA identified only 2%, suggesting that this BUA measurement missed the majority of patients.
Theoretically, QUS measures the speed of sound of an ultrasound wave as it is propagated through the bone. The speed of propagation through bone is influenced by elasticity modulus and the thickness of bone which is partly measured by bone mineral density. Therefore, a correlation between BUA and BMD is expected, but this study showed that the correlation was weak. The weak correlation implies that BUA measures bone properties that are not captured by BMD. One such property is bone quality or microarchitecture of bone. Still, a recent study found that BUA did not to predict the mechanical properties of high-density trabecular bone [19]. Taken together, although it is possible that BUA identified people at high risk of fracture by different bone properties, it is not suitable as an osteoporosis screening tool.
The present finding has important implications in the prevention of osteoporosis at the population level. Osteoporosis affects about 25%–30% of post-menopausal women and 10% of men aged 50 years and older [2,3]. The significance of osteoporosis lies not just in its high prevalence, but the clinical consequences and economic costs. Patients with osteoporosis have higher risk of fracture, which is associated with reduced life expectancy [4]. In the United States alone, the cost associated with osteoporosis has been estimated to be $25.3 billion [20]. In developing countries, with the rapid ageing of the population, the burden of osteoporosis is also high [2]. However, DXA technology is not widely available in developing countries, and the identification of high risk individuals has to be based on non-DXA technologies. In many developing countries, QUS has been advocated as a screening tool for osteoporosis, and it has been installed in pharmacies, shopping centers, and community groups. In Vietnam, many people have been treated on the basis of QUS results. In this study, we found that QUS missed most osteoporotic patients, suggesting that some individuals in Vietnam have probably been under-diagnosed and under-treated.
The present study’s finding should be interpreted within context of strengths and potential weaknesses. A major strength of this study is that it was a population-based investigation with a large sample size for both men and women who were recruited from the general community. We used the state-of-the-art DXA technology (Hologic Horizon) for the measurement of bone mineral density. It is likely that participants in this study had a better health status than the general population, and this could have affected the prevalence of osteoporosis. However, the study population was mainly from a major city of Vietnam, which is not representative of the Vietnam population with 70% living in rural areas. The prevalence of osteoporosis in men aged 50 years and older was only 1%, suggesting that the men in our sample were probably not representative of the population where the prevalence would be expected to be around 5–10%.
5. Conclusions
In summary, this population-based study found that there was a weak correlation between BUA and bone mineral density at the femoral neck and lumbar spine, and that the BUA would miss the majority of women and men with osteoporosis by DXA. Thus, we conclude that heel ultrasound is not a reliable tool for screening of osteoporosis.
CRediT author statement
Huy G. Nguyen: Conceptualization, Formal analysis, Writing - Original Draft. Khanh B. Lieu: Investigation, Resources, Data Curation. Thao P. Ho-Le: Formal analysis. Lan T. Ho-Pham: Conceptualization, Methodology, Investigation, Validation, Formal analysis, Writing – Review & Editing. Tuan V. Nguyen: Conceptualization, Methodology, Formal analysis, Writing – Review & Editing.
Conflicts of interest
The authors declare no competing interests.
Acknowledgments
This research is partly funded by the Foundation for Science and Technology Development of Ton Duc Thang University (FOSTECT, http://fostect.tdt.edu.vn), Grant number FOSTECT.2014.BR.09, and a grant from the Department of Science and Technology of Ho Chi Minh City. We sincerely thank MS Tran Thi Ngoc Trang and Fr Pham Ba Lam for coordinating the recruitment of participants. We also thank doctors and medical students of the Pham Ngoc Thach University of Medicine for the data collection and clinical measurements.
ORCID Huy G. Nguyen: 0000-0002-6545-7242. Khanh B. Lieu: 0000-0002 -0873-0265. Thao P. Ho-Le: 0000-0002-8387-1893. Lan T. Ho-Pham: 0000-0001-8382-5080. Tuan V. Nguyen: 0000-0002-3246-6281.
Footnotes
Peer review under responsibility of The Korean Society of Osteoporosis.
References
- 1.Center J.R., Nguyen T.V., Schneider D., Sambrook P.N., Eisman J.A. Mortality after all major types of osteoporotic fracture in men and women: an observational study. Lancet. 1999;353:878–882. doi: 10.1016/S0140-6736(98)09075-8. [DOI] [PubMed] [Google Scholar]
- 2.Ho-Pham L.T., Nguyen U.D., Pham H.N., Nguyen N.D., Nguyen T.V. Reference ranges for bone mineral density and prevalence of osteoporosis in Vietnamese men and women. BMC Muscoskel Disord. 2011;12:182. doi: 10.1186/1471-2474-12-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wright N.C., Looker A.C., Saag K.G., Curtis J.R., Delzell E.S., Randall S. The recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spine. J Bone Miner Res. 2014;29:2520–2526. doi: 10.1002/jbmr.2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bliuc D., Nguyen N.D., Milch V.E., Nguyen T.V., Eisman J.A., Center J.R. Mortality risk associated with low-trauma osteoporotic fracture and subsequent fracture in men and women. J Am Med Assoc. 2009;301:513–521. doi: 10.1001/jama.2009.50. [DOI] [PubMed] [Google Scholar]
- 5.Who Study Group . WHO; Geneva: 1994. Assessment of fracture risk and its application to screening for post-menopausal osteoporosis. [Google Scholar]
- 6.Morita R., Yamamoto I., Yuu I., Hamanaka Y., Ohta T., Takada M. Quantitative ultrasound for the assessment of bone status. Osteoporos Int. 1997;7:S128–S134. doi: 10.1007/BF03194358. [DOI] [PubMed] [Google Scholar]
- 7.Marin F., Gonzalez-Macias J., Diez-Perez A., Palma S., Delgado-Rodriguez M. Relationship between bone quantitative ultrasound and fractures: a meta-analysis. J Bone Miner Res. 2006;21:1126–1135. doi: 10.1359/jbmr.060417. [DOI] [PubMed] [Google Scholar]
- 8.Gregg E.W., Kriska A.M., Salamone L.M., Roberts M.M., Anderson S.J., Ferrell R.E. The epidemiology of quantitative ultrasound: a review of the relationships with bone mass, osteoporosis and fracture risk. Osteoporos Int. 1997;7:89–99. doi: 10.1007/BF01623682. [DOI] [PubMed] [Google Scholar]
- 9.Ho-Pham L.T., Nguyen T.V. The Vietnam osteoporosis study: rationale and design. Osteoporos Sarcopenia. 2017;3:90–97. doi: 10.1016/j.afos.2017.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.R Core Team A language and environment for statistical computing. dim (ca533) 2019;1:34. [Google Scholar]
- 11.Grömping U. Estimators of relative importance in linear regression based on variance decomposition. Am Statistician. 2007;61:139–147. [Google Scholar]
- 12.Faulkner K.G., McClung M.R., Coleman L.J., Kingston-Sandah E. Quantitative ultrasound of the heel: correlation with densitometric measurements at different skeletal sites. Osteoporos Int. 1994;4:42–47. doi: 10.1007/BF02352260. [DOI] [PubMed] [Google Scholar]
- 13.Adler R.A., Funkhouser H.L., Holt C.M. Utility of heel ultrasound bone density in men. J Clin Densitom. 2001;4:225–230. doi: 10.1385/jcd:4:3:225. [DOI] [PubMed] [Google Scholar]
- 14.Taal M.W., Cassidy M.J., Pearson D., Green D., Masud T. Usefulness of quantitative heel ultrasound compared with dual-energy X-ray absorptiometry in determining bone mineral density in chronic haemodialysis patients. Nephrol Dial Transplant. 1999;14:1917–1921. doi: 10.1093/ndt/14.8.1917. [DOI] [PubMed] [Google Scholar]
- 15.Weeks B.K., Hirsch R., Nogueira R.C., Beck B.R. Is calcaneal broadband ultrasound attenuation a valid index of dual-energy x-ray absorptiometry-derived bone mass in children? Bone Joint Res. 2016;5:538–543. doi: 10.1302/2046-3758.511.BJR-2016-0116.R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sweeney A.T., Malabanan A.O., Blake M.A., Weinberg J., Turner A., Ray P. Bone mineral density assessment: comparison of dual-energy X-ray absorptiometry measurements at the calcaneus, spine, and hip. J Clin Densitom. 2002;5:57–62. doi: 10.1385/jcd:5:1:057. [DOI] [PubMed] [Google Scholar]
- 17.Tabor E., Pluskiewicz W., Tabor K. Clinical conformity between heel ultrasound and densitometry in postmenopausal women: a systematic review. J Ultrasound Med. 2018;37:363–369. doi: 10.1002/jum.14340. [DOI] [PubMed] [Google Scholar]
- 18.Yang N.P., Jen I., Chuang S.Y., Chen S.H., Chou P. Screening for low bone mass with quantitative ultrasonography in a community without dual-energy X-ray absorptiometry: population-based survey. BMC Muscoskel Disord. 2006;7:24. doi: 10.1186/1471-2474-7-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Toyras J., Nieminen M.T., Kroger H., Jurvelin J.S. Bone mineral density, ultrasound velocity, and broadband attenuation predict mechanical properties of trabecular bone differently. Bone. 2002;31:503–507. doi: 10.1016/s8756-3282(02)00843-8. [DOI] [PubMed] [Google Scholar]
- 20.Dempster D.W. Osteoporosis and the burden of osteoporosis-related fractures. Am J Manag Care. 2011;17:S164–S169. [PubMed] [Google Scholar]