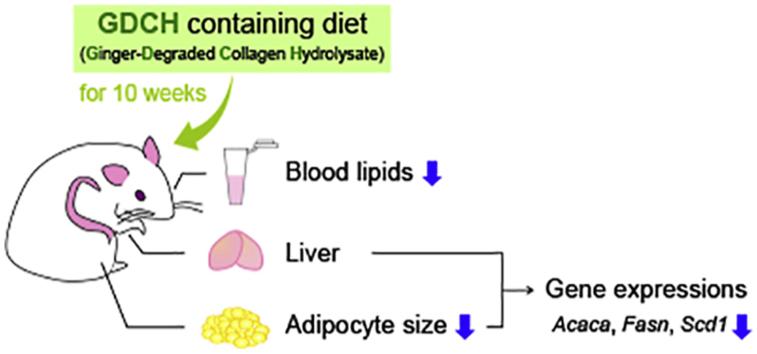
Abstract
Collagen hydrolysate has various beneficial effects, such as bone strengthening, joint/skin protection and lipid metabolism regulation. In this study, the anti-obesity activity of ginger protease-degraded collagen hydrolysate (GDCH) was evaluated in BALB/c mice fed diets containing 14% casein (control group) or 10% casein +4% GDCH (GDCH group) for 10 weeks. In the GDCH group, triglyceride (TG) and cholesterol (CHO) levels in blood and adipocyte size in white adipose tissue were significantly decreased compared with those of the control group. Further, gene expression related to fatty acid synthesis, such as acetyl-CoA carboxylase, fatty acid synthase and stearoyl-CoA desaturase, was decreased in the liver and white adipose tissue of GDCH-fed mice. On the other hand, single oral administration of GDCH did not result in decrease in blood TG and CHO compared with vehicle and casein in ICR mice pre-administered soybean oil. These results suggest that the GDCH-induced decreases in tissue and blood lipids occur through long-term alterations in lipid metabolism, not transient inhibition of lipid absorption. The lipid-lowering effects exhibited by partial substitution of casein with GDCH imply the possibility that daily supplementation of GDCH contributes to prevention/attenuation of obesity and hyperlipidemia.
Keywords: Collagen hydrolysate, Lipid metabolism, Triglyceride, Cholesterol, Adipocyte, Fatty acid synthesis genes
Abbreviations: CHO, cholesterol; E-CHO, esterified CHO; F–CHO, free CHO; GDCH, ginger protease-degraded collagen hydrolysate; Gly, glycine; Hyp, hydroxyproline; PPARα, peroxisome proliferator-activated receptor alpha; Pro, proline; SREBP-1, sterol regulatory element-binding protein 1" to the behind of "prorine; T-CHO, total CHO; TG, triglyceride
Graphical abstract
Highlights
-
•
Long-term feeding of ginger-protease degraded collagen hydrolysate (GDCH) in mice.
-
•
Blood triglycerides and cholesterol were decreased by GDCH intake.
-
•
Adipocyte size of white adipose tissue was reduced by GDCH intake.
-
•
Fatty acid synthesis genes were down-regulated by GDCH intake.
1. Introduction
Collagen is the most abundant protein in the animal body. Collagen forms a triple helix structure composed of three polypeptide chains with repeating glycine (Gly)-X-Y sequences (X and Y are any amino acids). Almost all proline (Pro) residues at the Y position are post-translationally modified to hydroxyproline (Hyp) specific to collagen. The collagen extracted using hot water from tissues, such as skin, tendon, bone and fish scales, is known as gelatin. Collagen hydrolysate (also referred to as gelatin hydrolysate or collagen peptide) is prepared by enzymatic hydrolysis of gelatin and is widely used as a food supplement for its effects on health and beauty. Oral ingestion of collagen hydrolysate is reported to have various beneficial effects, such as enhancement of bone strength (Konig et al., 2018; Wu et al., 2004), attenuation of joint pain (Benito-Ruiz et al., 2009; Clark et al., 2008), improvement of skin conditions (Oba et al., 2013; Proksch et al., 2014) and reduction of blood lipids (Lin et al., 2012; Saito et al., 2009; Tak et al., 2019; Woo et al., 2018). The above reports include clinical studies evaluating bone (Konig et al., 2018), joint (Benito-Ruiz et al., 2009; Clark et al., 2008), skin (Proksch et al., 2014) and lipid metabolism (Tak et al., 2019).
The mechanism of these effects by collagen hydrolysate ingestion had been unclear, but recently, collagen-derived oligopeptides have been considered as key ingredients (Sato, 2018). Due to the presence of Hyp, which confers high resistance to enzymatic digestion (Taga et al., 2018a, Taga et al., 2018b), orally ingested collagen hydrolysate is not completely degraded to free amino acids and can be partly absorbed as di- and tripeptides, such as Pro-Hyp, Hyp-Gly, and X-Hyp-Gly-type tripeptides (Sato, 2018; Taga et al., 2019; Taga et al., 2016). The blood concentration of Hyp-containing peptides (μM level) is markedly higher compared with that of other food-derived peptides (pM level) following ingestion (Sato, 2018). Several studies have indicated that the peptides transported into the blood can reach peripheral tissues (Kawaguchi et al., 2012; Yazaki et al., 2017). Moreover, there are many reports of the bioactivities of these peptides, such as promotion of cell proliferation and/or differentiation (J. A. Minaguchi et al., 2017; Nakatani et al., 2009; Nomura et al., 2019; Ohara et al., 2010; Taga et al., 2018a, Taga et al., 2018b), improvement of skin conditions (Shimizu et al., 2015) and antidepressant effects (Nogimura et al., 2020).
We have investigated the beneficial effects of collagen hydrolysate ingestion and the bioactivities of collagen-derived oligopeptides in vitro and in vivo (Koyama and Kusubata, 2013; Kusubata et al., 2015; J. Minaguchi et al., 2012; Nishikimi et al., 2018; Ohno et al., 2015; Tometsuka et al., 2017). Among them, we reported lipid metabolism-related effects, including a reduction in the size of lipid droplets in mouse adipocytes with Pro-Hyp supplementation to the cell culture (J. Minaguchi et al., 2012) and lowering of blood cholesterol (CHO) level and increase in hepatic lipid-related gene expression following ingestion of collagen hydrolysate in rats and mice (Koyama and Kusubata, 2013; Tometsuka et al., 2017). Other groups have also reported on the anti-obesity effects of collagen hydrolysate. For example, single administration of collagen hydrolysate suppressed the triglyceride (TG) absorption (Saito et al., 2009). Continuous administration of collagen hydrolysate decreased blood and tissue lipid levels in alcohol-induced liver injury model rats (Lin et al., 2012) and mice fed a high-fat diet (Woo et al., 2018). However, there has been little focus on the effect of continuous ingestion of collagen hydrolysate on lipid metabolism in normal animals with mild modification of the diet composition.
We previously developed a novel type of collagen hydrolysate uniquely containing X-Hyp-Gly-type tripeptides using ginger protease (Taga et al., 2016). The ginger protease-degraded collagen hydrolysate (GDCH) and X-Hyp-Gly have various biological effects, including angiotensin-converting enzyme inhibition (Taga et al., 2018a, Taga et al., 2018b), promotion of osteoblast differentiation (Taga et al., 2018a, Taga et al., 2018b) and antidepressant activity (Mizushige et al., 2019). Based on these previous observations, GDCH intake is expected to exert a stronger effect also on lipid metabolism than existing collagen hydrolysates by the involvement of X-Hyp-Gly.
Here, we analyzed blood and tissue lipids of male BALB/c mice after 10-week feeding of a control diet containing 14% casein or a GDCH diet, in which the protein source is partially substituted with GDCH (10% casein plus 4% GDCH). In order to eliminate the influence of dietary factors except GDCH intake, the normal dietary condition is adopted in this study. Furthermore, a single oral administration experiment was also performed to evaluate the effect of GDCH ingestion on lipid absorption.
2. Materials and methods
2.1. Animal experiment ethics
We have read ARRIVE guidelines and confirmed that our experiments complied with these guidelines. All animal studies were conducted using appropriate procedures in accordance with standard guidelines for animal experiments and approved by the Experimental Ethics Committee of Nippi Research Institute of Biomatrix (approval nos. 18002 and 20005).
2.2. Continuous feeding experiment
2.2.1. Feeding mice with the experimental diets
GDCH was prepared from bovine bone gelatin using ginger protease (average molecular weight of 816 Da) (Taga et al., 2016; Taga et al., 2018a, Taga et al., 2018b). The control diet containing 14% casein (AIN-93M) and the AIN-93M-based GDCH diet containing 10% casein plus 4% GDCH (Table 1) were prepared by Oriental Yeast Co., Ltd. (Tokyo, Japan). The protein composition of the two diets was matched based on the nitrogen content of casein and GDCH. Corn starch was used to compensate for the decreased weight of protein in the GDCH diet.
Table 1.
Composition of diets (%).
| Ingredient | Control diet | GDCH diet |
|---|---|---|
| Corn starch | 46.6 | 47.4 |
| Casein (13.3% N a) | 14.0 (14% N) | 10.0 (10% N) |
| GDCH(16.6% N a) | 0.0 | 3.2 (4% N) |
| Pregelatinized corn starch | 15.5 | 15.5 |
| Sucrose | 10.0 | 10.0 |
| Soybean oil | 4.0 | 4.0 |
| Cellulose | 5.0 | 5.0 |
| Mineral mix | 3.5 | 3.5 |
| Vitamin mix | 1.0 | 1.0 |
| L-Cystine | 0.18 | 0.18 |
| Choline bitartrate | 0.25 | 0.25 |
| t-Butylhydroquinone | Trace b | Trace b |
| Total | 100 | 100 |
Nitrogen content of casein or GDCH.
0.0008%.
Male 5-week-old BALB/cCrSlc mice (Japan SLC, Inc., Shizuoka, Japan) were acclimated to breeding conditions for 1 week with the control diet. The mice were then divided into two groups (n = 10/group) on the basis of body weight and fed either the control diet or the GDCH diet for 10 weeks. Mice were housed individually under conventional conditions (20 ± 2 °C, 12 h light-12 h dark cycle) with free access to water and diet. Before sampling procedures, the mice were subjected to fasting for 3 h and anesthetized with 50 mg/kg sodium pentobarbital. Blood was sampled from the right ventricle, and serum was separated by centrifugation at 1200×g for 10 min at 4 °C after standing at room temperature for 30 min. Liver, abdominal white adipose tissue and femurs were carefully removed. All the samples were stored at −80 °C until used for each analysis.
2.2.2. Measurement of blood lipids
Biochemical analysis of serum samples was entrusted to Nagahama Life Science Laboratory, Oriental Yeast Co., Ltd. (Shiga, Japan). The biochemical analyses were performed by absorbance measurements using quantitative assay reagents (L-Type CHO M, L-Type Free Cholesterol and L-Type Triglyceride M; FUJIFILM Wako Pure Chemical Co., Ltd., Osaka, Japan) based on enzymatic methods (Allain et al., 1974; Spayd et al., 1978). The panel of tests included the following: total CHO (T-CHO), free CHO (F–CHO) and TG. The data on esterified CHO (E-CHO) were estimated by the calculation (T-CHO = F–CHO + E-CHO).
2.2.3. Measurement of adipocyte size in white adipose tissue
Paraffin-embedded sections of tissue specimens stained with hematoxylin and eosin were prepared from 20% formalin-fixed white adipose tissues by Sapporo General Pathology Laboratory Co., Ltd. (Hokkaido, Japan). The adipocyte size of the tissue specimens was measured using a BZ-X800 microscope (Keyence, Osaka, Japan) equipped with analytical software. Three measurement areas at 400x magnification were arbitrarily selected per specimen, and adipocytes in each measurement area were color-extracted to measure the cell size. The average cell size of each individual specimen was calculated from the average values of the three measurement areas. A histogram was created based on the cell size and the cell number ratio of each cell size range.
2.2.4. Measurement of liver lipids
Determination of TG and CHO levels in liver samples was entrusted to Skylight Biotech, Inc. (Akita, Japan). Liver tissues were homogenized in chloroform/methanol (2:1, v/v), and lipid extracts were prepared using the Folch method (Folch et al., 1957). Intrahepatic TG and CHO levels were measured using enzymatic assay kits (Cholestest®TG and Cholestest®CHO, respectively; Sekisui Medical Co., Ltd., Tokyo, Japan).
2.2.5. Real-time quantitative RT-PCR analysis
Total RNA of liver or adipose tissue samples was extracted from the centrifuged supernatant (aqueous phase) of each tissue homogenate prepared with ISOGEN® reagent (Nippon Gene Co., Ltd., Toyama, Japan) according to the manufacturer’s protocol. cDNA synthesis of total RNA (1.5 μg/sample) was carried out using a High-capacity cDNA Reverse Transcription Kit (Thermo Fisher Scientific Inc., Waltham, MA, USA) according to the manufacturer’s protocol.
Specific primers and probes for real-time quantitative PCR of mouse acetyl-CoA carboxylase alpha (Acaca), fatty acid synthase (Fasn), stearoyl-CoA desaturase 1 (Scd1) and actin beta (Actb) were purchased from Thermo Fisher Scientific Inc. (TaqMan® Gene Expression Assays; ID: Mm01304257_m1, Mm00662319_m1, Mm00772290_m1, Mm02619580_g1, respectively). The reaction solution for PCR was prepared in volumes of 20 μl/well containing 10 μl TaqPath™ qPCR Master Mix, CG (Thermo Fisher Scientific Inc.), 1 μl primers and probe, and 9 μl cDNA in RNase-free water (250-fold dilution of cDNA synthesis solution). PCR was performed using two wells per sample on a 7300 Real-time PCR System (Thermo Fisher Scientific Inc.). Amplification conditions were 2 min at 50 °C, 10 min at 95 °C and 45 cycles each consisting of 15 s at 95 °C and 1 min at 60 °C. Standard curves were generated by serial dilutions of the cDNA sample mixture. The Ct value obtained by amplification of each analysis target gene was normalized using Actb expression level, and then the expression of each gene was compared between the two groups.
2.3. Single oral administration test
Male 14-week-old Slc:ICR mice (Japan SLC, Inc.) were divided into three groups (n = 6/group) on the basis of body weight. After 16-h fasting, 3.3 ml/kg soybean oil (FUJIFILM Wako Pure Chemical Co., Ltd.) was orally administered by gastric sonde to all groups. Immediately after the procedure, 0.5 g/kg GDCH or 0.5 g/kg casein sodium salt (Sigma-Aldrich, Inc., St. Louis, MO, USA) at a concentration of 20 mg/ml in distilled water was similarly administered to the two groups, respectively. The same volume of distilled water was administered to the remaining group. Blood was collected from the tail vein using heparinized capillary tubes before (0 h) and 2, 4, 6 h after the administration. The blood samples were centrifuged at 10,000×g for 10 min at 4 °C, and prepared plasma was stored at −80 °C until analysis of the blood lipids as described above.
2.4. Statistical analysis
Statistical differences between the two groups in the continuous feeding experiment were assessed by Student’s t-test. In the single oral administration test, the data were assessed by an ANOVA with the Tukey–Kramer post-hoc test. P < 0.05 was considered to indicate significance. Values are represented as the mean ± standard deviation.
3. Results
3.1. Continuous feeding experiment
3.1.1. Dietary intake and growth
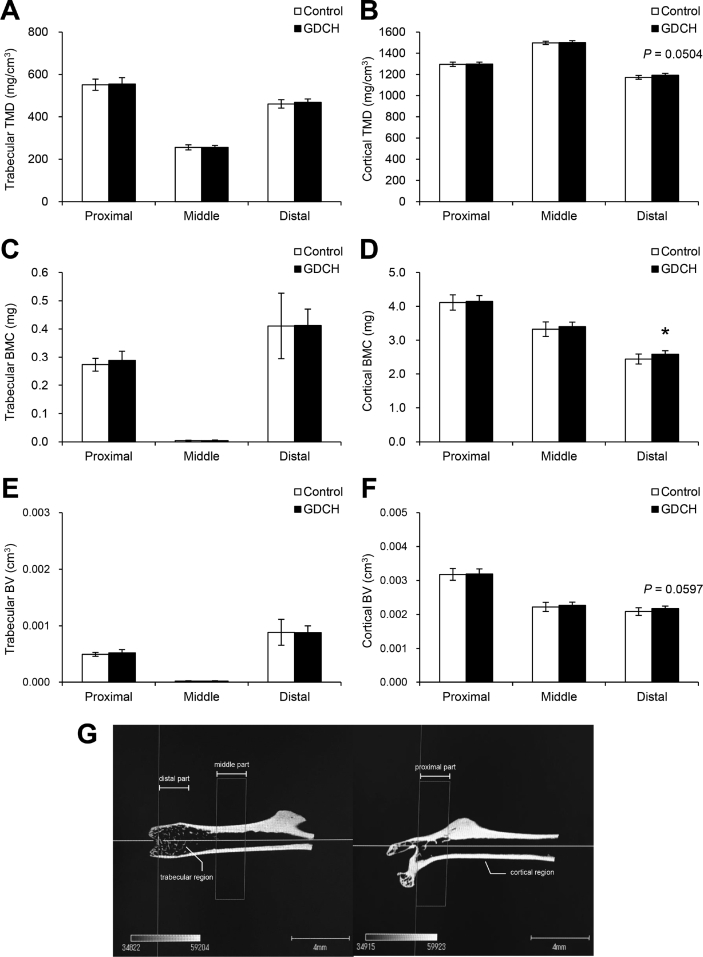
There were no significant differences in total food intake (248.2 ± 28.6 g vs 267.5 ± 18.3 g) and body weight gain (5.1 ± 1.5 g vs 5.6 ± 1.4 g) between the control and GDCH groups after the 10-week experimental period. No significant effect of GDCH intake on bone parameters was observed in this experiment, except for a slight increase of bone mineral content (Supplemental Fig. 1).
3.1.2. Reduction of blood lipids by GDCH intake
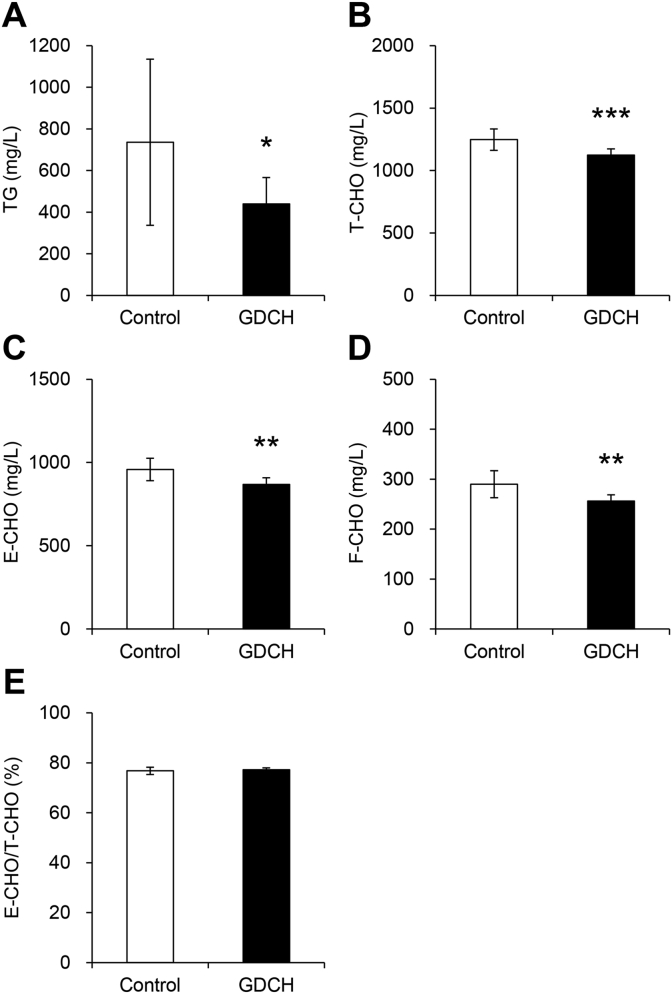
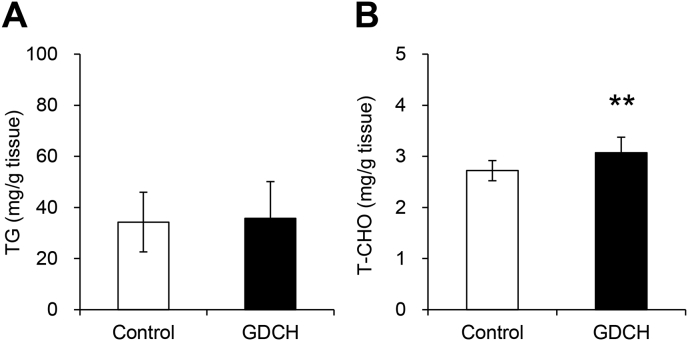
Biochemical analysis of blood serum showed significant reductions in blood levels of TG (P < 0.05) and all CHOs (T-CHO, E-CHO and F–CHO) (P < 0.01) in the GDCH group (Fig. 1A–D), suggesting that GDCH intake affects lipid metabolism. In the GDCH group, lowering of blood lipid levels was also confirmed by blood lipoprotein measurement (Supplemental Table 1). In both groups, the E-CHO/T-CHO ratio showed normal values (Fig. 1E), indicating that no severe liver abnormalities occurred in this experiment. T-CHO in the liver was slightly increased in the GDCH group (P < 0.01), while no significant difference in liver TG was observed (Fig. 2). These findings suggest that GDCH intake reduced blood lipids.
Fig. 1.
Blood concentrations of triglyceride (TG) and cholesterol (CHO).
Blood serum concentrations of TG (A), total CHO (T-CHO) (B), esterified CHO (E-CHO) (C) and free CHO (F–CHO) (D) are shown. The ratio of E-CHO to T-CHO is shown in (E). Significant differences between the two groups (control and GDCH) are indicated by asterisks (∗, P < 0.05; ∗∗, P < 0.01; ∗∗∗, P < 0.001).
Fig. 2.
Lipid contents of triglyceride (TG) and cholesterol (CHO) in the liver.
(A) The amount of liver lipids per tissue weight for TG. (B) The amount of liver lipids per tissue weight for total CHO (T-CHO). Significant differences between the two groups (control and GDCH) are indicated by asterisks (∗∗, P < 0.01).
3.1.3. Reduction of adipocyte size in white adipose tissue by GDCH intake
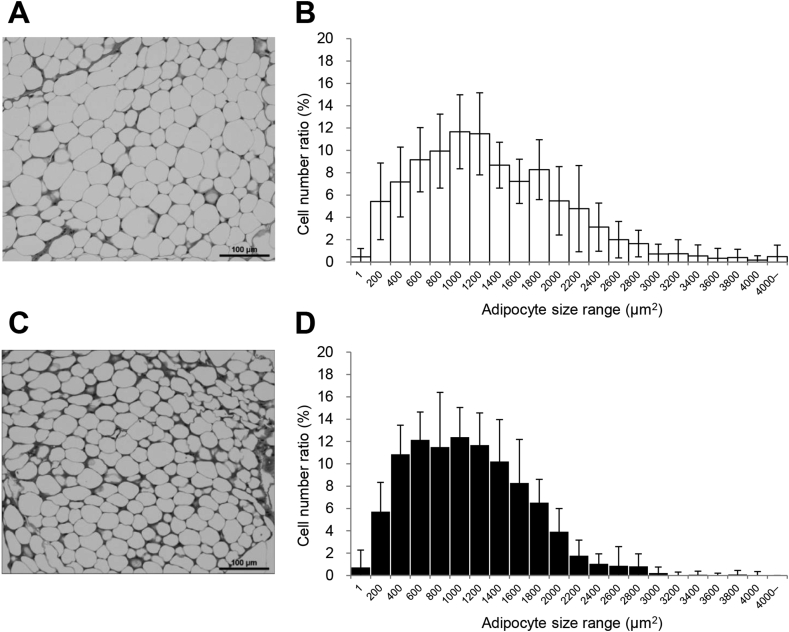
We next hypothesized that GDCH would have an effect on the adipocyte size. To this end, we measured the cell size of white adipose tissue specimens. Adipocyte size was significantly decreased (P < 0.05) in the GDCH group compared with the control group (Fig. 3). The average cell size was significantly lower in the GDCH group (1201.2 ± 144.7 μm2 vs 1424.1 ± 272.0 μm2, P < 0.05). The histogram of the GDCH group shifted to the left with a decreased width, indicating that smaller adipocytes are distributed in the adipose tissue of the mice fed GDCH.
Fig. 3.
Adipocyte size in white adipose tissue.
Representative photomicrographs of adipose tissue specimens in the control group (A) and GDCH group (C). Histograms of adipocyte size in the control group (B) and GDCH group (D). Histograms are based on the cell size and the cell number ratio of each cell size range. Bars, 100 μm.
3.1.4. Decreased expression of genes related to fatty acid synthesis by GDCH intake
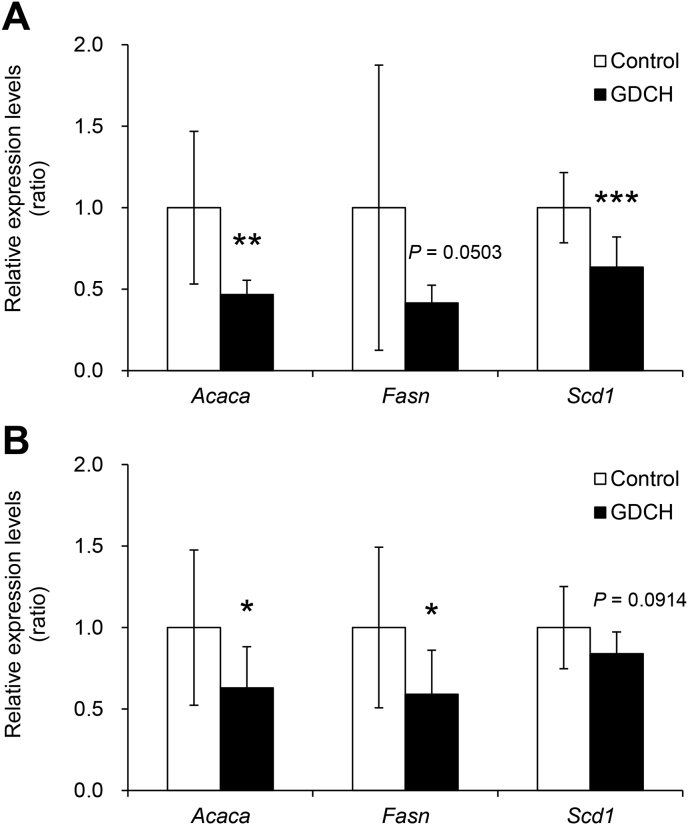
Next, the identity of the gene or genes whose expression is regulated by GDCH intake was investigated. The intake of GDCH resulted in significant down-regulation of fatty acid synthesis genes, including acetyl-CoA carboxylase alpha (Acaca) and stearoyl-CoA desaturase 1 (Scd1) in the liver (Fig. 4A; P < 0.01) and Acaca and fatty acid synthase (Fasn) in the adipose tissue (Fig. 4B; P < 0.05). In addition, the expressions of Fasn in the liver and Scd1 in the adipose tissue were not statistically significantly different between the two groups (control and GDCH), but the P values were close to the cut-off value of significance (P = 0.0503 and P = 0.0914, respectively).
Fig. 4.
Gene expression related to fatty acid synthesis in the liver (A) and adipose tissue (B).
The levels of each gene are expressed relative to Actb and normalized to control. Significant differences between the two groups (control and GDCH) are indicated by asterisks (∗, P < 0.05; ∗∗, P < 0.01; ∗∗∗, P < 0.001). P values are noted where the data are close to the cut-off value of significance.
3.2. Effect of GDCH on lipid absorption by single oral administration
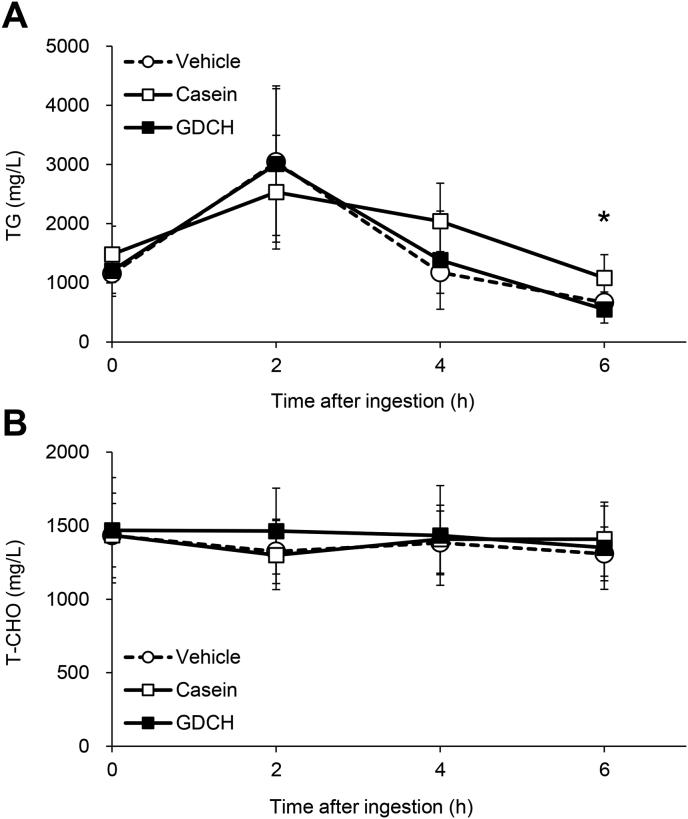
To examine the possibility that GDCH intake immediately decreases blood lipids, a single oral administration experiment was conducted. Generally, the profile of blood TG after oral administration of soybean oil did not change with concomitant administration of GDCH compared to concomitant administration of vehicle or casein, except for a slight, but significant reduction in TG levels in the GDCH-administered group relative to the casein-administered group at 6 h after the administration (Fig. 5A; P < 0.05). These results indicate that the effects of GDCH on blood TG are not due to inhibition of lipid absorption. Moreover, the blood CHO level was not significantly different among the three groups (Fig. 5B).
Fig. 5.
Blood levels of triglyceride (TG) and cholesterol (CHO) following single oral administration of test substances with soybean oil.
Changes in blood TG (A) and total CHO (T-CHO) (B) following oral administration of soybean oil and the three test substances are shown. Significant difference between the casein and GDCH groups is indicated by an asterisk (∗, P < 0.05).
4. Discussion
In this study, we examined the in vivo effect of GDCH and found that long-term intake of GDCH induced remarkable lipid-lowering effects, including decrease of blood lipids and reduction of adipocyte size, in normal mice. On the other hand, there was no significant change in blood lipids by the single oral administration of GDCH. It has been reported that several food ingredients including collagen hydrolysates suppress the absorption of dietary lipids via multiple mechanisms such as inhibition of lipid micelle formation owing to bile acid binding, lipid excretion owing to inhibiting dissolution of lipid micelles or suppression of lipid degradation owing to pancreatic lipase inhibition (de la Garza et al., 2011; Kishimoto et al., 2009; Moriyama et al., 2004; Nagaoka et al., 2001; Saito et al., 2009). Here, the results of a single oral administration test suggested that the lipid-lowering function of GDCH is exerted via its effects on systemic lipid metabolism by long-term ingestion and not through short-term inhibition of lipid absorption. A previous study reported that blood lipids were decreased by single oral administration of collagen hydrolysates (Saito et al., 2009). One of the differences between the previous study and our study is the average molecular weight of the collagen hydrolysates used as the test substance (3,000–10,000 Da and 816 Da, respectively). Collagen-derived peptides may differently affect blood lipids depending on the peptide length. The slight but statistically significant increase of liver CHO in the GDCH group might be due to the maintenance of lipid homeostasis in response to the decrease of blood CHO. However, the CHO level is still low compared with that of the fatty liver model (Kristiansen et al., 2016).
We previously showed that the blood CHO level was decreased by ingestion of collagen hydrolysate (not GDCH) using a high-calorie diet (Koyama and Kusubata, 2013) and a mild low-protein diet condition (Tometsuka et al., 2017). In contrast, the experimental diets used in this study conformed with the composition of the general maintenance feed; thus, the experimental condition is more normal than the conditions in the previous studies in terms of nutrient sufficiency. The weight ratio of GDCH to total protein content in the experimental diet is 24%, which is equivalent to approximately 17 g of GDCH per 60 g of daily protein intake in humans. This amount does not deviate from the general daily amount of collagen hydrolysate for supplemental intake (Jendricke et al., 2019; Zdzieblik et al., 2015). Several studies reported a reduction of either blood TG or CHO under mild experimental conditions (normal animals with low-fat diets) by short- or long-term administration of collagen hydrolysate (Saito et al., 2009; Tometsuka et al., 2017; Wu et al., 2004). However, to the best of our knowledge, no study has shown a reduction of both types of blood lipids under mild experimental conditions until now. It remains unclear how GDCH ingestion reduced blood lipids efficiently; however, X-Hyp-Gly-type tripeptides, which are substantially contained in GDCH, could be involved in the hypolipidemic effects. We previously demonstrated that X-Hyp-Gly as well as Pro-Hyp are efficiently absorbed into the blood after GDCH ingestion (Taga et al., 2016). Since X-Hyp-Gly and Pro-Hyp have various biological activities (J. Minaguchi et al., 2012; J. A. Minaguchi et al., 2017; Nakatani et al., 2009; Nishikimi et al., 2018; Nogimura et al., 2020; Nomura et al., 2019; Ohara et al., 2010; Shigemura et al., 2009; Taga et al., 2018a, Taga et al., 2018b), it is possible that these Hyp-containing oligopeptides are responsible for the effects of GDCH on lipid metabolism. Direct evaluation of collagen-derived oligopeptides for TG storage using adipocytes and for CHO synthesis/catabolism using hepatocytes is currently being planned in order to investigate the key ingredient(s) of the lipid-lowering effects by GDCH.
The size of adipocytes changes depending on the amount of TG stored within the cell as an energy source. Consequently, the reduction of adipocyte size observed in this study indicates that TG storage in the body was decreased by GDCH intake. Active proliferation of adipocytes occurs in the early stage of adipose tissue development, and cell proliferation and division do not occur under normal conditions without intervention such as excessive energy supply and genome editing (Jo et al., 2009). Therefore, it is unlikely that the reduced adipocyte size is due to a change in the number of adipocytes in the adipose tissue. Body weight did not differ significantly despite the significant reduction in adipocyte size in the white adipose tissue. It is presumed that the decrease in lipid storage was not sufficiently large to affect the body weight of the normal mice used in this study. Further studies are needed to clarify whether GDCH intake actually leads to fat mass reduction.
Gene expression analysis of the liver and adipose tissue revealed that GDCH down-regulated the major genes (Acaca, Fasn and Scd1) involved in fatty acid synthesis. The enzymes encoded by these genes catalyze synthesis of malonyl-CoA from acetyl-CoA, palmitic acid from acetyl-CoA and malonyl-CoA, and oleic acid from stearic acid, respectively (Strable and Ntambi, 2010). In the fatty acid synthesis pathway, TG is generated for energy storage. CHO is also synthesized from acetyl-CoA, and lipoproteins containing TG and CHO modulate the lipid balance in the body through the blood circulation (Horie et al., 2013). It is possible that the down-regulation of fatty acid synthesis genes attenuated lipid biosynthesis and accumulation, thereby inducing the phenotypes observed in the GDCH group. Acaca, Fasn and Scd1 are target genes of sterol regulatory element-binding protein 1 (SREBP-1), which is a transcription factor regulating lipogenesis (Horie et al., 2013; Strable and Ntambi, 2010). Scd1 is also the target of peroxisome proliferator-activated receptor alpha (PPARα), which is a nuclear receptor regulating fatty acid catabolism (Pawlak et al., 2015). An interrelationship is known to exist between the transcriptional signaling of SREBP-1 and PPARα (Pawlak et al., 2015; Strable and Ntambi, 2010). However, the expression of PPARα target genes (Acox1, Cyp7a1 and Cyp27a1) previously up-regulated by collagen hydrolysate intake (Tometsuka et al., 2017) did not change by GDCH supplementation (data not shown). It is difficult to explain the mechanism of the effects of GDCH on lipid metabolism solely by these transcription factors; however, the transcriptional cascades from these factors may contribute, at least in part, to the decreased expression of fatty acid synthesis-related genes by GDCH intake, as observed for mice administered collagen hydrolysate with a high-fat diet (Woo et al., 2018). To clarify the mechanism of the hypolipidemic effects of GDCH, further studies are required, such as cell culture experiments using adipocytes/hepatocytes and animal experiments with high-fat diets or obesity models.
In conclusion, our data demonstrated that long-term intake of GDCH exerted hypolipidemic effects in mice, providing the possibility that daily supplementation of GDCH contributes to prevention and attenuation of obesity and hyperlipidemia.
Financial support
This work was supported by the ‘Program to supporting research activities of female researchers (Collaborative type)’ involving Tokyo Medical and Dental University, Juntendo University and Nippi Research Institute of Biomatrix in FY 2018-2020.
CRediT authorship contribution statement
Chisa Tometsuka: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Resources, Writing – original draft, Writing – review & editing, Visualization, Funding acquisition. Noriko Funato: Conceptualization, Methodology, Validation, Investigation, Resources, Writing – review & editing, Funding acquisition. Kazunori Mizuno: Validation, Resources, Writing – review & editing. Yuki Taga: Conceptualization, Methodology, Validation, Investigation, Resources, Writing – review & editing, Supervision, Funding acquisition.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We are grateful to Masashi Kusubata, Yuki Kumazawa and Xiaoyan Yang for their technical assistance with experiments, and the Diversity Diamond Unit Promotional Office for its continued support.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.crfs.2021.03.003.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Figs1.
References
- Allain C.C., Poon L.S., Chan C.S., Richmond W., Fu P.C. Enzymatic determination of total serum cholesterol. Clin. Chem. 1974;20(4):470–475. [PubMed] [Google Scholar]
- Benito-Ruiz P., Camacho-Zambrano M.M., Carrillo-Arcentales J.N., Mestanza-Peralta M.A., Vallejo-Flores C.A., Vargas-Lopez S.V., Villacis-Tamayo R.A., Zurita-Gavilanes L.A. A randomized controlled trial on the efficacy and safety of a food ingredient, collagen hydrolysate, for improving joint comfort. Int. J. Food Sci. Nutr. 2009;60(Suppl. 2):99–113. doi: 10.1080/09637480802498820. [DOI] [PubMed] [Google Scholar]
- Clark K.L., Sebastianelli W., Flechsenhar K.R., Aukermann D.F., Meza F., Millard R.L., Deitch J.R., Sherbondy P.S., Albert A. 24-Week study on the use of collagen hydrolysate as a dietary supplement in athletes with activity-related joint pain. Curr. Med. Res. Opin. 2008;24(5):1485–1496. doi: 10.1185/030079908x291967. [DOI] [PubMed] [Google Scholar]
- de la Garza A.L., Milagro F.I., Boque N., Campion J., Martinez J.A. Natural inhibitors of pancreatic lipase as new players in obesity treatment. Planta Med. 2011;77(8):773–785. doi: 10.1055/s-0030-1270924. [DOI] [PubMed] [Google Scholar]
- Folch J., Lees M., Sloane Stanley G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957;226(1):497–509. [PubMed] [Google Scholar]
- Horie T., Nishino T., Baba O., Kuwabara Y., Nakao T., Nishiga M., Usami S., Izuhara M., Sowa N., Yahagi N., Shimano H., Matsumura S., Inoue K., Marusawa H., Nakamura T., Hasegawa K., Kume N., Yokode M., Kita T., Kimura T., Ono K. MicroRNA-33 regulates sterol regulatory element-binding protein 1 expression in mice. Nat. Commun. 2013;4:2883. doi: 10.1038/ncomms3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jendricke P., Centner C., Zdzieblik D., Gollhofer A., Konig D. Specific collagen peptides in combination with resistance training improve body composition and regional muscle strength in premenopausal women: a randomized controlled trial. Nutrients. 2019;11(4) doi: 10.3390/nu11040892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo J., Gavrilova O., Pack S., Jou W., Mullen S., Sumner A.E., Cushman S.W., Periwal V. Hypertrophy and/or hyperplasia: dynamics of adipose tissue growth. PLoS Comput. Biol. 2009;5(3) doi: 10.1371/journal.pcbi.1000324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi T., Nanbu P.N., Kurokawa M. Distribution of prolylhydroxyproline and its metabolites after oral administration in rats. Biol. Pharm. Bull. 2012;35(3):422–427. doi: 10.1248/bpb.35.422. [DOI] [PubMed] [Google Scholar]
- Kishimoto Y., Yoshikawa Y., Miyazato S., Oga H., Yamada T., Tagami H., Hashizume C., Yamamoto K. Effect of resistant maltodextrin on digestion and absorption of lipids. J. Health Sci. 2009;55(5):838–844. [Google Scholar]
- Konig D., Oesser S., Scharla S., Zdzieblik D., Gollhofer A. Specific collagen peptides improve bone mineral density and bone markers in postmenopausal women-a randomized controlled study. Nutrients. 2018;10(1) doi: 10.3390/nu10010097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama Y.I., Kusubata M. Effects of collagen peptide ingestion on blood lipids in rats fed a high-lipid and high-sucrose diet. Food Sci. Technol. Res. 2013;19(6):1149–1153. [Google Scholar]
- Kristiansen M.N., Veidal S.S., Rigbolt K.T., Tolbol K.S., Roth J.D., Jelsing J., Vrang N., Feigh M. Obese diet-induced mouse models of nonalcoholic steatohepatitis-tracking disease by liver biopsy. World J. Hepatol. 2016;8(16):673–684. doi: 10.4254/wjh.v8.i16.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusubata M., Koyama Y., Tometsuka C., Shigemura Y., Sato K. Detection of endogenous and food-derived collagen dipeptide prolylhydroxyproline (Pro-Hyp) in allergic contact dermatitis-affected mouse ear. Biosci. Biotechnol. Biochem. 2015;79(8):1356–1361. doi: 10.1080/09168451.2015.1027653. [DOI] [PubMed] [Google Scholar]
- Lin B., Zhang F., Yu Y., Jiang Q., Zhang Z., Wang J., Li Y. Marine collagen peptides protect against early alcoholic liver injury in rats. Br. J. Nutr. 2012;107(8):1160–1166. doi: 10.1017/S0007114511004211. [DOI] [PubMed] [Google Scholar]
- Minaguchi J., Tometsuka C., Koyama Y., Kusubata M., Nagayasu A., Sawaya S., Shiga T., Shima H., Hara T., Takehana K. Effects of collagen-derived oligopeptide prolylhydroxyproline on differentiation of mouse 3T3-L1 preadipocytes. Food Sci. Technol. Res. 2012;18(4):593–599. [Google Scholar]
- Minaguchi J.A., Ogata S., Takahashi N., Hirose T., Ueda H., Takehana K. Remodeling of rat stromal-vascular cells to brite/beige adipocytes by prolyl-hydroxyproline. J. Vet. Med. Sci. 2017;79(3):547–553. doi: 10.1292/jvms.16-0163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizushige T., Nogimura D., Nagai A., Mitsuhashi H., Taga Y., Kusubata M., Hattori S., Kabuyama Y. Ginger-degraded collagen hydrolysate exhibits antidepressant activity in mice. J. Nutr. Sci. Vitaminol. 2019;65(3):251–257. doi: 10.3177/jnsv.65.251. [DOI] [PubMed] [Google Scholar]
- Moriyama T., Kishimoto K., Nagai K., Urade R., Ogawa T., Utsumi S., Maruyama N., Maebuchi M. Soybean beta-conglycinin diet suppresses serum triglyceride levels in normal and genetically obese mice by induction of beta-oxidation, downregulation of fatty acid synthase, and inhibition of triglyceride absorption. Biosci. Biotechnol. Biochem. 2004;68(2):352–359. doi: 10.1271/bbb.68.352. [DOI] [PubMed] [Google Scholar]
- Nagaoka S., Futamura Y., Miwa K., Awano T., Yamauchi K., Kanamaru Y., Tadashi K., Kuwata T. Identification of novel hypocholesterolemic peptides derived from bovine milk beta-lactoglobulin. Biochem. Biophys. Res. Commun. 2001;281(1):11–17. doi: 10.1006/bbrc.2001.4298. [DOI] [PubMed] [Google Scholar]
- Nakatani S., Mano H., Sampei C., Shimizu J., Wada M. Chondroprotective effect of the bioactive peptide prolyl-hydroxyproline in mouse articular cartilage in vitro and in vivo. Osteoarthritis Cartilage. 2009;17(12):1620–1627. doi: 10.1016/j.joca.2009.07.001. [DOI] [PubMed] [Google Scholar]
- Nishikimi A., Koyama Y.I., Ishihara S., Kobayashi S., Tometsuka C., Kusubata M., Kuwaba K., Hayashida O., Hattori S., Katagiri K. Collagen-derived peptides modulate CD4(+) T-cell differentiation and suppress allergic responses in mice. Immun Inflamm Dis. 2018;6(2):245–255. doi: 10.1002/iid3.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogimura D., Mizushige T., Taga Y., Nagai A., Shoji S., Azuma N., Kusubata M., Adachi S.I., Yoshizawa F., Kabuyama Y. Prolyl-hydroxyproline, a collagen-derived dipeptide, enhances hippocampal cell proliferation, which leads to antidepressant-like effects in mice. FASEB (Fed. Am. Soc. Exp. Biol.) J. 2020;34(4):5715–5723. doi: 10.1096/fj.201902871R. [DOI] [PubMed] [Google Scholar]
- Nomura K., Kimira Y., Osawa Y., Shimizu J., Kataoka-Matsushita A., Mano H. Collagen-derived dipeptide prolyl hydroxyproline directly binds to Foxg1 to change its conformation and inhibit the interaction with Runx2. Biosci. Biotechnol. Biochem. 2019;83(11):2027–2033. doi: 10.1080/09168451.2019.1642099. [DOI] [PubMed] [Google Scholar]
- Oba C., Ohara H., Morifuji M., Ito K., Ichikawa S., Kawahata K., Koga J. Collagen hydrolysate intake improves the loss of epidermal barrier function and skin elasticity induced by UVB irradiation in hairless mice. Photodermatol. Photoimmunol. Photomed. 2013;29(4):204–211. doi: 10.1111/phpp.12051. [DOI] [PubMed] [Google Scholar]
- Ohara H., Ichikawa S., Matsumoto H., Akiyama M., Fujimoto N., Kobayashi T., Tajima S. Collagen-derived dipeptide, proline-hydroxyproline, stimulates cell proliferation and hyaluronic acid synthesis in cultured human dermal fibroblasts. J. Dermatol. (Tokyo) 2010;37(4):330–338. doi: 10.1111/j.1346-8138.2010.00827.x. [DOI] [PubMed] [Google Scholar]
- Ohno M., Ito K., Lan V.T., Kusubata M., Tometsuka C., Koyama Y., Motoyama T., Ito S., Kawarasaki Y. Synergistic inhibition of human dipeptidyl peptidase IV by combinations of peptides. Peptides. 2015;69:115–117. doi: 10.1016/j.peptides.2015.04.015. [DOI] [PubMed] [Google Scholar]
- Pawlak M., Lefebvre P., Staels B. Molecular mechanism of PPARalpha action and its impact on lipid metabolism, inflammation and fibrosis in non-alcoholic fatty liver disease. J. Hepatol. 2015;62(3):720–733. doi: 10.1016/j.jhep.2014.10.039. [DOI] [PubMed] [Google Scholar]
- Proksch E., Schunck M., Zague V., Segger D., Degwert J., Oesser S. Oral intake of specific bioactive collagen peptides reduces skin wrinkles and increases dermal matrix synthesis. Skin Pharmacol. Physiol. 2014;27(3):113–119. doi: 10.1159/000355523. [DOI] [PubMed] [Google Scholar]
- Saito M., Kiyose C., Higuchi T., Uchida N., Suzuki H. Effect of collagen hydrolysates from salmon and trout skins on the lipid profile in rats. J. Agric. Food Chem. 2009;57(21):10477–10482. doi: 10.1021/jf902355m. [DOI] [PubMed] [Google Scholar]
- Sato K. Structure, content, and bioactivity of food-derived peptides in the body. J. Agric. Food Chem. 2018;66(12):3082–3085. doi: 10.1021/acs.jafc.8b00390. [DOI] [PubMed] [Google Scholar]
- Shigemura Y., Iwai K., Morimatsu F., Iwamoto T., Mori T., Oda C., Taira T., Park E.Y., Nakamura Y., Sato K. Effect of Prolyl-hydroxyproline (Pro-Hyp), a food-derived collagen peptide in human blood, on growth of fibroblasts from mouse skin. J. Agric. Food Chem. 2009;57(2):444–449. doi: 10.1021/jf802785h. [DOI] [PubMed] [Google Scholar]
- Shimizu J., Asami N., Kataoka A., Sugihara F., Inoue N., Kimira Y., Wada M., Mano H. Oral collagen-derived dipeptides, prolyl-hydroxyproline and hydroxyprolyl-glycine, ameliorate skin barrier dysfunction and alter gene expression profiles in the skin. Biochem. Biophys. Res. Commun. 2015;456(2):626–630. doi: 10.1016/j.bbrc.2014.12.006. [DOI] [PubMed] [Google Scholar]
- Spayd R.W., Bruschi B., Burdick B.A., Dappen G.M., Eikenberry J.N., Esders T.W., Figueras J., Goodhue C.T., LaRossa D.D., Nelson R.W., Rand R.N., Wu T.W. Multilayer film elements for clinical analysis: applications to representative chemical determinations. Clin. Chem. 1978;24(8):1343–1350. [PubMed] [Google Scholar]
- Strable M.S., Ntambi J.M. Genetic control of de novo lipogenesis: role in diet-induced obesity. Crit. Rev. Biochem. Mol. Biol. 2010;45(3):199–214. doi: 10.3109/10409231003667500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taga Y., Hayashida O., Ashour A., Amen Y., Kusubata M., Ogawa-Goto K., Shimizu K., Hattori S. Characterization of angiotensin-converting enzyme inhibitory activity of X-Hyp-Gly-type tripeptides: importance of collagen-specific prolyl hydroxylation. J. Agric. Food Chem. 2018;66(33):8737–8743. doi: 10.1021/acs.jafc.8b03648. [DOI] [PubMed] [Google Scholar]
- Taga Y., Iwasaki Y., Shigemura Y., Mizuno K. Improved in vivo tracking of orally administered collagen hydrolysate using stable isotope labeling and LC-MS techniques. J. Agric. Food Chem. 2019;67(16):4671–4678. doi: 10.1021/acs.jafc.9b00571. [DOI] [PubMed] [Google Scholar]
- Taga Y., Kusubata M., Ogawa-Goto K., Hattori S. Efficient absorption of X-hydroxyproline (Hyp)-Gly after oral administration of a novel gelatin hydrolysate prepared using ginger protease. J. Agric. Food Chem. 2016;64(14):2962–2970. doi: 10.1021/acs.jafc.6b00609. [DOI] [PubMed] [Google Scholar]
- Taga Y., Kusubata M., Ogawa-Goto K., Hattori S., Funato N. Collagen-derived X-Hyp-Gly-type tripeptides promote differentiation of MC3T3-E1 pre-osteoblasts. Journal of Functional Foods. 2018;46:456–462. [Google Scholar]
- Tak Y.J., Kim Y.J., Lee J.G., Yi Y.H., Cho Y.H., Kang G.H., Lee S.Y. Effect of oral ingestion of low-molecular collagen peptides derived from skate (Raja Kenojei) skin on body fat in overweight adults: a randomized, double-blind, placebo-controlled trial. Mar. Drugs. 2019;17(3) doi: 10.3390/md17030157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tometsuka C., Koyama Y.I., Ishijima T., Toyoda T., Teranishi M., Takehana K., Abe K., Nakai Y. Collagen peptide ingestion alters lipid metabolism-related gene expression and the unfolded protein response in mouse liver. Br. J. Nutr. 2017;117(1):1–11. doi: 10.1017/S0007114516004384. [DOI] [PubMed] [Google Scholar]
- Woo M., Song Y.O., Kang K.H., Noh J.S. Anti-obesity effects of collagen peptide serived from skate (Raja kenojei) skin through regulation of lipid metabolism. Mar. Drugs. 2018;16(9) doi: 10.3390/md16090306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J., Fujioka M., Sugimoto K., Mu G., Ishimi Y. Assessment of effectiveness of oral administration of collagen peptide on bone metabolism in growing and mature rats. J. Bone Miner. Metabol. 2004;22(6):547–553. doi: 10.1007/s00774-004-0522-2. [DOI] [PubMed] [Google Scholar]
- Yazaki M., Ito Y., Yamada M., Goulas S., Teramoto S., Nakaya M.A., Ohno S., Yamaguchi K. Oral ingestion of collagen hydrolysate leads to the transportation of highly concentrated Gly-Pro-Hyp and its hydrolyzed form of Pro-Hyp into the bloodstream and skin. J. Agric. Food Chem. 2017;65(11):2315–2322. doi: 10.1021/acs.jafc.6b05679. [DOI] [PubMed] [Google Scholar]
- Zdzieblik D., Oesser S., Baumstark M.W., Gollhofer A., Konig D. Collagen peptide supplementation in combination with resistance training improves body composition and increases muscle strength in elderly sarcopenic men: a randomised controlled trial. Br. J. Nutr. 2015;114(8):1237–1245. doi: 10.1017/S0007114515002810. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.