Abstract
Background
This randomised controlled trial investigates the dosing effect of neuromuscular electrical stimulation (NMES) in patients with chronic venous disease (CVD).
Methods
Seventy-six patients with CEAP C3-C5 were randomised to Group A (no NMES), B (30 minutes of NMES daily) or C (60 minutes of NMES daily). Primary outcome was percentage change in Femoral Vein Time Averaged Mean Velocity (TAMV) at 6 weeks. Clinical severity scores, disease-specific and generic quality of life (QoL) were assessed.
Results
Seventy-six patients were recruited - mean age 60.8 (SD14.4) and 47:29 male. Six patients lost to follow-up. Percentage change in TAMV (p<0.001) was significantly increased in Groups B and C. Aberdeen Varicose Veins Questionnaire Score (-6.9, p=0.029) and Venous Clinical Severity Score (-4, p-0.003) improved in Group C, and worsened in Group A (+1, p=0.025).
Conclusions
Daily NMES usage increases flow parameters, with twice daily usage improving QoL and clinical severity at 6 weeks in CVD patients.
Keywords: Venous stasis, venous reflux, venous severity score, venous disease, CEAP clinical class
Introduction
In the era of an ageing population, where the prevalence of obesity is increasing, chronic venous disease (CVD) is an increasing burden.1 CVD can be divided into three main sub-types – superficial venous insufficiency (SVI - commonly known as varicose veins), deep venous insufficiency (DVI) and deep venous obstruction (DVO). SVI affects up to 80% of the population in its mildest form as graded by the CEAP clinical staging system (C1 - dilated cutaneous spider veins), up to 40% of the populationwith superficial varicose vein incompetence (C2) and up to 1% of individuals in its most severe form (C6 - venous ulceration).2–4 DVI and DVO can be a primary or secondary condition with significant associations with deep venous thrombosis (DVT). DVT can be a manifestation of DVO and can lead to DVI. DVT leads to the development of the post thrombotic syndrome (PTS) in up to 50% of patients, which can be extremely challenging to treat.5,6 Modern treatment methods for DVO include endovenous stenting and open venous reconstruction, though these both require significant interventions, medication regimes and surveillance post-operatively.
Untreated, varicose veins progress to more severe forms of CVD in 32% of patients over 6 years,7 while the incidence of DVI or DVO is expected to increase by 33% over 5 years,8 highlighting the potential future burden of venous disease. CVD yields important negative effects on quality of life and result in a significant expenditure of the national healthcare budget,9,10 particularly if it progresses to ulcerative disease. As such, interventions to help manage these conditions and their symptoms, are required.
The progression of venous disease is thought to be due to persistently high ambulatory venous pressures, which can be offloaded by treating superficial venous incompetence.11,12 Activating the calf muscle pump with activities such as walking, wearing compression stockings and intermittent pneumatic compression are thought to be beneficial in reducing this chronic venous hypertension, even in deep venous obstruction.13–15
Assessment of venous haemodynamics has been carefully investigated over the past few decades with ultrasound derived measurements, with the effects of different neuromuscular stimulation devices, intermittent pneumatic compression devices and graduated compression stockings (GCS).16–22 The main haemodynamic parameters investigated include Time Averaged Mean Velocity (TAMV), Venous Volume Flow (VF) and Peak Venous Velocity (PV), which have been reported in previous venous flow studies.18,21,23–27
TAMV is the venous flow equivalent to mean arterial blood pressure and is an attempt to provide a steady state estimation of a sinusoidal flow pattern over multiple cycles.28,29 The femoral vein is the main exit conduit for venous blood in the leg, and therefore, an increase in average flow in this conduit should correspond to improved venous reservoir emptying and reduced venous pressure.24,30,31
The Society for Vascular Surgery (SVS) and the National Institute for Health and Care Excellence (NICE) recommend endovenous ablation for the treatment of SVI32,33 and endovenous ablation and surgical ablation have success rates of greater than 90%.32–35 The management of DVI and DVO involves preventing disease progression by reducing ambulatory venous pressure, preventing recurrent thromboembolism using antithrombotic agents or flavonoids, with surgical or endovascular options in severe cases.6,36 Interventions for deep venous disease have promising but mixed results.37
Neuromuscular electrical stimulation (NMES) refers to the use of electrical impulses to elicit muscle contraction. The REVITIVE IX is a Class IIa medical device that administers NMES via two footplate electrodes. It has been shown to increase venous blood flow parameters by artificially activating the muscle pumps of the lower limb in healthy individuals21 and patients with CEAP C2-4 CVD.23 It has also been shown to prevent orthostatic oedema during use and improve disease specific quality of life (QoL) outcome measures in the pilot trial on patients with CVD.23 Recent publications have asessed its use in claudicants.38,39 Despite limitations of the pilot data, mainly pertaining the small sample size, the positive results compared to a sham device were encouraging. Previous work has suggested that graduated compression stockings (GCS) worn for 6 hours have a significantly larger effect on limb volume compared to this NMES device (a single session of 30 minutes of treatment).40 Recent work by Gianesini et al. in healthy subjects found that sitting comfortably for 30 minutes leads to no significant increase in limb volume.41 Additionally, recent work indicates that heel-rise test repetition may have a significant effect on clinical stage of venous disease and this is the exact movement elicited by the NMES in this device.42
This trial aimed to assess the effect of increasing the “dosage” of NMES treatment, by increasing the duration of use per day from the routine 30 minutes to 60 minutes (1 session to 2 sessions).
Hypothesis
The hypothesis of this study was that two treatment sessions of NMES per day would lead to increased venous flow (as measured by Time Averaged Mean Velocity - TAMV) compared to a single session treatment.
Methods
Ethical approval
This study was a single-centre randomised controlled trial conducted at Charing Cross Hospital, London between November 2015 and October 2016. Ethical approval for the study was obtained from the National Research Ethics Committee (NRES ref: 15/LO/0620). Clinical Trials Registration number NCT03850496.
Patient recruitment
Patients with Clinical Etiological Anatomical and Pathological (CEAP) (Supplementary Appendix 1) clinical class C3-C5 and duplex ultrasound scan-confirmed diagnosis of superficial and/or deep venous disease due to reflux, obstruction or mixed aetiology were recruited from the vascular outpatient department at Charing Cross Hospital. All patients had normal arterial exams and an ankle brachial pressure index of greater than 0.8. Patients with a target for superficial venous treatment were not included, so as not to delay interventional treatment, unless they had declined that intervention. Patients with a target for deep venous obstruction treatment were not included, so as to not delay interventional treatment, unless they had declined that intervention. The study recruitment team were separate to the clinical treating team to prevent conflict of interest.
Patients with varicose veins only (CEAP C2 disease) were excluded as the pilot trial had demonstrated minimal effect in this patient cohort. Patients with C6 disease were excluded as their normal clinical management would include multilayer bandaging for treating ulceration, which is precluded in the use of this NMES device which requires skin to device contact. Patients were screened according to a priori agreed criteria (Supplementary Appendix 2). Medical history, medications and anthropometric measurements were recorded at baseline. A urine test was performed to exclude pregnancy in patients of reproductive age. CEAP assessment, venous flow parameters and limb volumes were measured in the affected or worse affected limb (for bilateral symptoms).
Randomisation
Eligible participants were assigned a study number prior to randomisation and randomised on a 1:1:1 ratio, using a web-based simple non-stratified randomisation service (www.sealedenvelope.com) to one of three groups:
Group A: no electrical stimulation (control group)
Group B: One 30minute session of electrical stimulation a day
Group C: Two 30minute sessions of electrical stimulation a day
Patients were required to continue with the established compression stockings and best medical therapy as prescribed by the clinical team, which was independent from the trial. Compression hosiery as standard in the unit is European Class 2 below knee. Compliance with compression hosiery was not formally assessed for pragmatic reasons and to avoid performance bias. Patients who underwent surgical treatment for varicose veins were excluded from the trial. The investigator was not blinded to the allocation at the point of ultrasound assessment as it was not possible due to the design of the study (two groups of patients would have a moving limb).
Sample size
The target sample size for the study was planned at 90 patients, based on data from the pilot study.23 This was based on the Time Averaged Mean Velocity (TAMV) as the primary outcome (-9.1% vs 102.4%, sham versus active, with an active range of -34.7%-507.6%).
At 5% significance and 90% power, using repeated measures between factors ANOVA assessment, 25 patients per group would be required to detect a statistical difference between all three groups. Based on pilot compliance, 15% loss to follow-up was anticipated, requiring a target recruitment of 90 patients (30 per group) to allow for attrition.
The neuromuscular electrical stimulation (NMES) device: REVITIVE® IX
The device investigated (REVITIVE®, Actegy Ltd) is a class IIa medical device, CE marked for treating disorders of the lower limb. The device is used in the seated position, with the users’ bare feet placed on a pair of conductive footplate electrodes. Electrical impulses are delivered to the muscles and nerves of the feet, which cause foot and calf muscle contraction at a sufficient intensity. Direct contact between skin and electrodes is required for stimulation, precluding the use of compression stockings. Both feet have to be placed on the conductive footplates for the device to work.
The device runs a 30-minute programme of NMES consisting of 15 different waveform patterns, each with varying electrical output characteristics. The intensity of stimulation ranges from 1–99 units, delivering a maximum current of 15mA (r.m.s., root mean square) at 500Ω resistance. Stimulation intensity is increased by the subject until visible contraction is seen. The minimum threshold intensity level that should be used is double the stimulation intensity. Patients were advised to use the highest intensity that was comfortable for them. The intensity of stimulation varies for each individual, and is affected by oedema and moisture. The additional IsoRocker feature involves a fulcrum across the middle of the device over which the device can pivot to an angle of up to 15°. This allows the foot to remain in contact with the conductive footplate electrodes during ankle flexion and dorsiflexion. Patients were taught to use the device and instructed to utilise the both the electrical stimulation and the IsoRocker feature. The stimulation can be uncomfortable initially, however during the teaching phase patients were reassured that acclimatisation and tolerance develops.
Trial arms
Group A
Patients did not receive the device during the 6-week trial duration. Measurements of venous flow parameters and microcirculatory blood flow were performed whilst sitting for 30 minutes (to simulate the effect of orthostatic oedema). Measurement of limb volume was performed before and after this period of quiet sitting. Questionnaires were administered at week 0 and week 6. No sham device was provided based on feedback and design assessment from the pilot study.23 Compression hosiery was not utilised in this group during assessment times based on previous work by Clark Moloney et al.16
Group B
Patients received the NMES device and were taught how to use it. Measurements of venous flow parameters and microcirculatory blood flow were performed whilst using the device in a seated position for 30 minutes. Measurement of limb volume was performed before and after using the device. Questionnaires were administered at week 0 and week 6. Patients were advised to use the device at home for 30 minutes a day over the 6-weeks. This was monitored using a patient reported diary.
Group C
Patients received the NMES device and were taught how to use it. Measurements of venous flow parameters and microcirculatory blood flow were performed whilst using the device in a seated position for 30 minutes only. Measurement of limb volume was performed before and after using the device. Questionnaires were administered at week 0 and week 6. Patients were advised to use the device at home for 60 minutes a day, either consecutively or at two different times over the 6-weeks. This was monitored using a patient reported diary.
Outcome assessments
The primary outcome measure was percentage change in the Time Average Mean Velocity (TAMV) venous flow parameters compared to baseline.
Secondary outcome measures were limb volume (measured using an optoelectronic limb volumeter (Perometer®)), microcirculatory flow (measured using laser dopper fluximetry (LDF, Moor Instruments, UK)), clinical severity score (Venous Clinical Severity Score – VCSS), generic quality of life outcome measures (Euroqol 5 Level – EQ-5D-5L and EQ VAS and Short-Form 12 – SF-12) and disease specific quality of life measure, (Aberdeen Varicose Vein Questionnaire - AVVQ), from baseline. Outcome measures for venous flow parameters and limb volume were performed on the affected or worse affected limb. Limb volume was measured before and after using the device at week 0 and week 6. Quality of life outcome measure questionnaires were compared between week 0 and week 6. The timeline for measurements is illustrated in Supplementary Appendix 3. All assessments were performed in the same room, and serial assessments were performed at the same time of day. The trial was completed during the same season.
Venous flow parameters
A Philips iU22 duplex ultrasound machine was used to measure venous flow parameters. Time averaged mean velocity (TAMV), peak venous velocity (PV) and volume flow (VF) were the venous flow parameters selected as outcome measures at the outset. These parameters have been described in other trials reporting venous flow parameters as an outcome measure.18,21,23–27 Patients were scanned in the seated position with their bare feet either on the floor for Group A or on the REVITIVE IX footplates for groups B and C. Measurements of venous flow parameters were taken from the femoral vein of the examined limb, 3-5cm from the saphenofemoral junction or as proximal as possible, depending on the patients’ body habitus. The limb was marked for repeated measurements. Venous flow parameter measurements were taken at baseline (after 10 minutes of quiet sitting) and during the “stimulation” period (group B and C: 30 min of NMES whilst using the device, group A: a 30 min period of quiet sitting). An average of the six repeat venous flow parameter measurements (of TAMV, PV and VF) were taken whilst using the device. All studies were performed by one operator. Inter-rater and intra-rater reliability data for venous flow parameters measurements were published in the pilot trial.12 Venous flow parameters were reported as percentage change in venous flow parameters from baseline. Fifteen-second screenshots were saved and analysed offline. TAMV and VF were calculated using built-in software. PV was measured with image J 1.47V programme (Wayne Rasband, National Institutes of Health USA). The highest single peak in each 15 second screenshot was used taken as the peak venous velocity.
Measurement of microcirculatory flow
A Laser Doppler Fluximeter (LDF) (Moor Instruments, UK) was used to measure microcirculatory blood flow. Two cutaneous leads were used, one positioned on the gaiter area of the patient’s most affected limb and the other on the dorsum of the contralateral hand. Data was recorded and analysed offline. The flux (arbitrary units) and temperature (°C) from each lead was calculated using the Moor-VMS Software (Moor Instruments, UK). Measurements were taken during the 10-minute resting period and throughout the “stimulation” period. Two regions of interest (ROI) were selected representing the last 5 min of the resting period (ROI1) and the last 15 minutes of “stimulation” (ROI2). Percentage change from baseline was reported for the flux and temperature in the hand and leg.
Measurement of limb volume
Limb volume was measured using an optoelectronic limb volumeter (Perometer® 350 NT, Pero-System Meβgeräte GmbH). Patients using compression stockings were advised to remove stockings 2 hours prior to appointment. The room temperature, and timing of the appointment were controlled to provide as little variation as possible. Measurements were taken with the patient seated and the affected leg in a horizontal position. Limb volume measurements were taken at four timeframes:
prior to “stimulation” at week 0
following “stimulation at week 0
prior to “stimulation” at week 6
following “stimulation at week 6
Five readings were taken at each time frame. The average (x̄) of each time frame was used to calculate the percentage change in limb volume. Results were reported comparing:
the percentage change in limb volume before and after “stimulation” at week 0
the percentage change in limb volume before and after “stimulation” at week 6
the percentage change in limb volume before “stimulation” at week 0 compared to week 6
Quality of life outcome measurements
Clinical severity was compared for each group at week 0 and week 6 using the Venous Clinical Severity Score (VCSS) and Venous Disability Score (VDS). Comparison and multiple comparisons were also made across the three groups at week 0 and week 6. Quality of life outcome measures were assessed with disease specific (AVVQ) and generic quality of life questionnaires (Euro-Qol 5D (EQ5D) and Short Form 12 (SF12) version 2) at week 0 and week 6. The EQ5D assesses generic QOL on a scale from 1 (perfect health) to -0.594 (worst possible state) with 0 equating to death. AVVQ assesses symptomatology due to venous disease on a scale from 0-100, with higher score indicating worse disease. The SF-12 creates a health profile based on 12 questions. Comparisons were made between the week 0 and week 6 scores for each group, and across the groups for both week 0 and week 6.
Patient diary
Compliance to protocol was determined using a patient diary over the 6 weeks of device usage. This did not assess stocking compliance, as this was considered standard care out with the remit of the study.
Statistical analysis
Statistical analysis was performed according to the intention to treat principle, and all analysis was performed offline in a blinded fashion. All raw and scored data was transcribed on to a database in Excel (Microsoft, Redmond, Washington, USA) Data was analysed using Prism 8 software (GraphPad Software, La Jolla, California, USA), Wizard Pro (Evan Miller, Chicago, Illinois, USA) and STATA 15 (StataCorp, College Station, Texas, USA). Where appropriate, following visual inspection for distribution normality, D’Agostino and Pearson formula and the Shapiro-Wilk test for normality confirmation was undertaken. Intergroup comparison of patient demographics were analysed using one-way analysis of variance (ANOVA) for parametric and Kruskal-Wallis test for non-parametric data to exclude baseline variance despite randomisation. Comparison of venous flow parameters comparing all three groups and group B versus group C was analysed using Kruskal-Wallis and Mann-Whitney test, respectively. Data on limb volumes were analysed using Wilcoxon matched pairs signed rank test. Questionnaire scores measuring quality of life outcome measures were analysed comparing week 0 and week 6 using the paired t-test for parametric data and Wilcoxon matched pairs signed rank test for non-parametric data. Comparisons between groups were made using the one-way ANOVA for parametric and Kruskall-Wallis test for non-parametric data. Corrections for multiple comparisons were made using Dunn’s correction for parametric and Tukey’s correction for non-parametric data. A value of p<0.05 was taken as being statistically significant. Missing data was dealt with case exclusion.
Results
Baseline characteristics
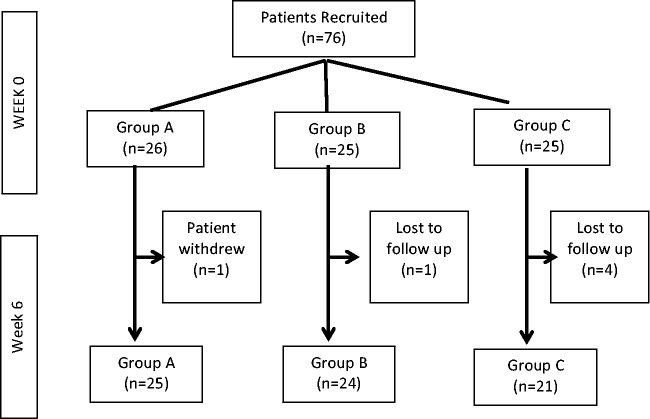
Seventy-six patients with C3-C5 disease were recruited into the trial. Twenty-six patients were randomised to group A, twenty-five patients to group B and Group C, each. One patient withdrew from group A following randomisation. One patient in group B and 4 patients in group C were lost to follow up (See Figure 1).
Figure 1.
CONSORT flow diagram for trial.
The mean age of patients in the trial was 60.8 years (standard deviation 14.4), and mean body mass index (BMI) of 29.9 (standard deviation 6.1). There was a 47:29 male preponderance, however the groups were otherwise well-matched. There was no significant difference in the CEAP classification of patients between the groups. Data on patient demographics, CEAP classification and duplex findings are tabulated in Table 1.
Table 1.
Patient demographics.
| A | B | C | P-value | |
|---|---|---|---|---|
| No patients (n=) | 26 | 25 | 25 | |
| Age (years) | 59 | 59 | 63 | 0.631 ns ϕ |
| Gender (F:M) | 8:18 | 9:16 | 12:13 | 0.443 ns ϕ |
| Height (m) | 1.74 | 1.71 | 1.70 | 0.349 ns ϕ |
| Weight (kg) | 92.4 | 89.4 | 84.7 | 0.516 ns ϕ |
| BMI | 30.4 | 30.3 | 29.15 | 0.753 ns ϕ |
| Co-morbidities | ||||
| DVT (n=) | 12 | 9 | 14 | 0.362 ns ϕ |
| DM | 5 | 2 | 2 | 0.365 ns ϕ |
| HT | 2 | 5 | 5 | 0.384 ns ϕ |
| IHD | 4 | 2 | 4 | 0.656 ns ϕ |
| Heart failure | 2 | 1 | 1 | 0.799 ns ϕ |
| History malignancy | 2 | 3 | 2 | 0.872 ns ϕ |
| Obese (BMI≥30) | 14 | 11 | 8 | 0.348 ns ϕ |
| CEAPmedian (Clinical classification) | 4 | 4 | 4 | 0.203 ns ϕ |
| VCSSmedian | 6 | 6 | 7 | 0.414 ns γ |
BMI body mass index, CEAP Clinical Aetiological Anatomical Pathological criteria, VCSS Venous Clinical Severity Score, ns – non-significant, ϕ One Way ANOVA, γ Kruskal-Wallis test.
Statistical significance defined as P<0.05.
25.64% patients had SVI only, 20.51% had DVI only, 43.59% had mixed SVI and DVI, 7.79% had mixed SVI and DVI disease with DVO, 6.49% had DVO and DVI only and 8.97% had no venous incompetence demonstrable on duplex ultrasound. The full breakdown of duplex results is in Table 2. Analysis of the duplex data, including number of trunks involved indicates no difference between groups except for a higher incidence of iliac vein reflux and obstruction in Groups B and Group C (p=0.006). This suggests worsened anatomical venous disease in Group B and Group C compared to Group A despite randomisation. A full breakdown is in Supplementary Appendix 7.
Table 2.
Breakdown of duplex findings in percentages.
| GSV | SSV | ATV | Non-truncal varicosities | Iliac vein | SFV | PFV | Calf veins | |
|---|---|---|---|---|---|---|---|---|
| Overall | ||||||||
| Competent | 67.61 | 83.10 | 90.14 | 45.07 | 82.86 | 45.07 | 85.92 | 57.75 |
| Reflux | 32.39 | 15.49 | 9.86 | 54.93 | 8.57 | 43.66 | 12.68 | 42.25 |
| Obstruction | 0 | 1.41 | 0 | 0 | 8.57 | 11.27 | 1.41 | 0 |
| Group A | ||||||||
| Competent | 62.50 | 79.16 | 96.15 | 34.62 | 96.15 | 57.69 | 83.33 | 62.50 |
| Reflux | 37.50 | 16.67 | 3.85 | 65.38 | 3.85 | 30.77 | 16.67 | 37.50 |
| Obstruction | 0 | 4.17 | 0 | 0 | 0 | 11.54 | 0 | 0 |
| Group B | ||||||||
| Competent | 78.26 | 86.96 | 96.00 | 44.00 | 80.00 | 36.00 | 86.95 | 69.57 |
| Reflux | 21.74 | 13.04 | 4.00 | 56.00 | 0 | 48.00 | 8.70 | 30.43 |
| Obstruction | 0 | 0 | 0 | 0 | 20.00 | 16.00 | 4.35 | 0 |
| Group C | ||||||||
| Competent | 62.50 | 83.33 | 80.77 | 38.46 | 76.92 | 50.00 | 86.96 | 41.67 |
| Reflux | 37.50 | 16.67 | 19.23 | 61.54 | 19.23 | 45.83 | 13.04 | 58.33 |
| Obstruction | 0 | 1.41 | 0 | 0 | 3.85 | 4.17 | 0 | 0 |
GSV: great saphenous veins; SSV: small saphenous vein; ATV: anterior thigh vein; SFV: superficial femoral vein; PFV: profunda femoral vein.
Primary outcome - venous flow parameters – time averaged mean velocity
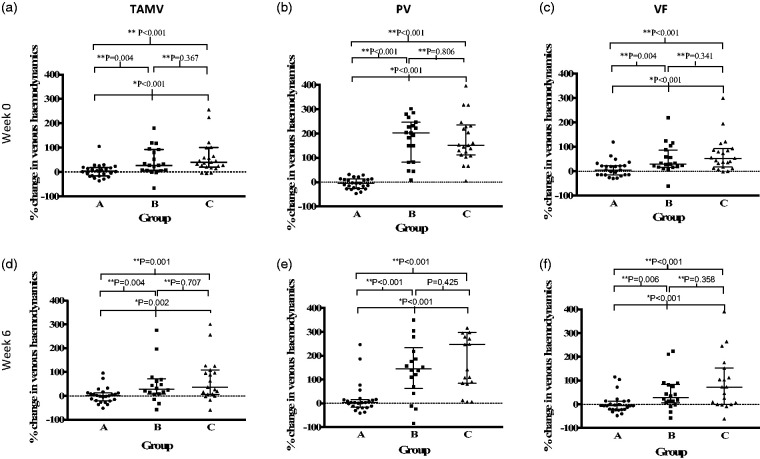
At week 0, there was a significant difference in the percentage change in Time Averaged Mean Velocity (TAMV) between group A (median 3.36% (-16.48 – 18.54%)), group B (median 26.58% (6.65 – 92.32%)) and group C (median 39.49% (20.48 – 99.75%)) (p<0.001) (see Figure 2(a)). There was a significant difference in the percentage change in TAMV between group A and B (P=0.004) and group A and C (P<0.001), but not between group B and C (p=0.367).
Figure 2.
Scatter plot demonstrating percentage change in time averaged mean velocity (TAMV), peak velocity (PV) and volume flow (VF) for each group at week 0 and 6. Error bars demonstrate interquartile range. Statistical test used: *Kruskal-Wallis test **Mann-Whitney test (statistical significance defined as P<0.05).
At week 6, there was a significant difference in the percentage change in TAMV between group A (median 0.46% (-20.81 – 12.26%)), group B (median 27.83% (8.01 – 70.93%)) and group C (median 36.38% ( 6.45 – 108.80%)) (p<0.002) (see Figure 2(d)). There was a significant difference in the percentage change in TAMV between group A and B (p=0.004) and group A and C (p=0.001). However, there was no significant difference in TAMV with between group B and group C (p=0.707).
There was no significant difference in percentage change in TAMV between week 0 and week 6 in any group.
Venous flow parameters – peak velocity and venous flow
At week 0, there was a significant difference in the percentage change in peak velocity (PV) between group A (median -4.76% (-25.84 – 12.61%)), group B (median 202.7% (82.63 – 247.1%)) and group C (median 151.9% (111.3 – 234.7%)) (p=0.0001) (see Figure 2(b)). There was a significant difference in the PV between group A and B (p<0.001) and group A and C (p<0.001), but not between group B and C (p=0.806).
At week 0, there was a significant difference in the percentage change in VF between group A (median 5.53% (-14.76 - 22.48%)), group B (median 28.81% (16.59-87.42%)) and group C (median 52.82% (16.99-92.54%)) (p<0.001) (see Figure 2(c)). There was a significant difference in the VF between group A and B (p=0.004) and group A and C (p<0.001), but not between group B and C (p=0.341).
The pattern was similar at week 6 as demonstrated in see Figure 2(e) and (f). There was no significant difference in the PV or VF parameters at week 0 and week 6 for each group.
Effect of NMES on disease specific QoL
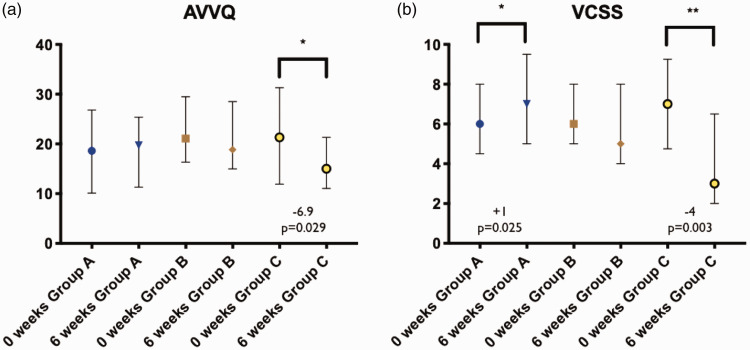
There was a significant 6.3point decrease (improvement) in the AVVQ score between week 0 and week 6 in group C (P=0.029), but not in group A (p=0.684) and group B (p=0.458) (see Figure 2). There was a significant difference in score change between the two groups over the 6 weeks (group A (-1.16 (-3.65 – 2.41) vs group C -5.68 (-15.82 – 0.53), p=0.025). Full details in Supplementary Appendix 4.
Effect of NMES on clinical severity scores
There was a significant increase (worsening) in the VCSS score between week 0 and week 6 in group A and a significant decrease (improvement) in VCSS score in group C (see Figures 2 and 3). The increase in score for group A was 1 point, whereas the decrease in score in group C was 4 points. There was no significant difference between groups A, B and C at week 0 (p=0.570) but a significant difference at week 6 (p=0.002). At week 6, there was a significant difference between the scores of group A and C (p=0.001), but not between group A and B (p=0.458) and groups B and C (p=0.132) (Kruskal Wallis using Dunn’s correction for multiple comparison). Full details in Supplementary Appendix 4.
Figure 3.
Box plot of (a) Aberdeen Varicose Vein Questionnaire (AVVQ) and (b) Venous Clinical Severity Score (VCSS) for all groups at 0 and 6 weeks, showing median and interquartile range. Statistical significance defined as significant, *P<0.05, very significant **P<0.01.
There was a significant decrease in median VDS overall across all groups between week 0 and week 6 (median 2 to 1, p=0.045). However, there was no significant difference in the VDS score in any group between week 0 and week 6: group A (p=0.531), group B (p=0.531), group C (p=0.183). There was also no significant difference between the three groups at week 0 and week 6 (p=0.351 and p=0.432, respectively). Full details in Supplementary Appendix 4.
Microcirculatory blood flow
There was a significant difference in microcirculatory flux in the feet (p<0.001) compared to the hands (p=0.465) between the groups at week 0. There was a reduction in microcirculatory flux in group A (-3.0 (-21.5 – 15.1)) compared to an increase in group B (167.7 (45.5 – 446.8)) and group C (254.5 (141.9 – 580.5)). This was associated with a significant difference in temperature in the foot (p=0.039) compared to the hand (p=0.381). The pattern was similar with a decrease in temperature in group A (–0.7 (-2.1 – 1.1)) compared to an increase in temperature in group (B 0.9 (-1.4 – 2.6)) and group C (0.9 (-0.1 – 2.2)).
Limb volumes
The immediate effect of NMES on limb volumes was assessed by comparing the pre- and post-treatment (or quiet sitting in group A) limb volumes. At week 0, group A demonstrated a significant median increase in limb volume of 73ml, (p<0.001). Patients in group B had a median significant reduction in limb volume of 18ml, (p=0.046). Patients in group C demonstrated a significant increase in limb volume 11ml, (p=0.0451).
At week 6, limb volume increased significantly post-treatment in group A by 46ml (+1%, p<0.001), but remained static in groups B (p=0.726) and group C (p=0.681).
There was no significant difference in limb volume over the 6 weeks, comparing pre-stimulation limb volumes between week 0 and week 6.
Full details are presented in Supplementary Appendix 5.
Effect of NMES on generic quality of life measures
There was no significant difference in the EQ-5D score in any group between week 0 and week 6 (Group A 0.68 vs 0.74, p=0.989; Group B 0.64 vs 0.69, p=0.246; Group C 0.73 vs 0.75, p>0.999). Similarly, treatment with NMES had no effect on the EQ5D Visual Analogue Score (EQ5D-VAS) between week 0 and week 6 (Group A 67.72 vs 66.28, p=0.627; Group B 64.68 vs 63.61, p=0.572; Group C 67.08 vs 71.20, p=0.274).
There was also no effect of NMES treatment over the 6 week trial on SF12 results: Physical Component Score (SF12:PCS) (week 0 vs week 6: Group A 42.03 vs 43.73, p=0.359; Group B 42.02 vs 43.32, p=0.285; Group C 47.3 vs 49.9, p=0.459) and SF12: Mental Component Score (SF12: MCS) (week 0 vs week 6: Group A 55.11 vs 53.93, p=0.345; Group B 52.38 vs 50.37, p=0.092; Group C 47.96 vs 50.03, p=0.823)
There was no significant intra- or inter-group difference in the EQ5D, EQ5D VAS, SF12:PCS or SF12:MCS scores at any time points.
Full details are presented in Supplementary Appendix 6.
Patient diary
A patient reported diary was completed by 20 (83%) patients in group B and 16 (76%) patients in group C. The average diary completion in group B was 94% (79 – 100%), with average usage of 28 minutes per day. The average diary completion in group C was 92% (67 – 100%), with average usage of 55 minutes per day.
Discussion
This trial demonstrates that the use of the REVITIVE IX device significantly increases venous flow parameters during device usage. No difference was seen between Groups B and C, indicating that maximum benefit was achieved during each intervention session, which is understandable from a mechanistic viewpoint. The effect of NMES on venous flow parameters is akin to the effect of exercise of moderate intensity. Although not measured directly, an increase in venous flow parameters would reduce ambulatory venous pressure, as is seen in deep venous stenting for outflow obstruction.14 A significant improvement was also demonstrated in the lower limb microcirculatory blood flux whilst using the device. Poor microcirculatory flow is thought to be connected to venous ulceration.43
Although the trial showed a significant difference in pre and post NMES limb volume in all three groups in week 0, at week 6, the change in limb volume was only significant in group A, suggesting that device usage prevents oedema associated with sitting still (orthostatic oedema), however there was no change in resting limb volume over the 6-week trial period, this requires further investigation to assess whether this is secondary to the short timescale of the study, or actually a peak improvement.
There was a significant improvement in disease severity as measured by the VCSS in patients using the device for two 30-minute sessions a day (Group C), compared to a significant worsening in the VCSS score in patients in the control group (Group A). The VCSS is a clinician-completed questionnaire, scoring 10 hallmarks of venous disease in order of severity from 0 to 3 with a maximum score of 30, and is recommended by the Society of Vascular Surgery and American Venous Forum as a clinical outcome measure. This study demonstrated a clinically and statistically significant 4-point reduction in VCSS score in the group receiving 60 minutes of treatment a day, suggesting an improvement in clinical severity of CVD following treatment with NMES, in line with that seen in randomised clinical trials after surgical intervention.44–46
There was also a significant 6.29 point improvement in the disease specific AVVQ score in group C, which is above the accepted minimum important difference of 5 points in AVVQ. The control group demonstrated a non-significant worsening of their AVVQ score. The AVVQ is a validated patient completed QoL assessment tool comprising of 13 questions with domains including physical symptoms, social effects and cosmesis. Each question is graded in terms of severity or presence of symptoms, with a higher the score indicative of worse symptoms. In the multi-centre CLASS interventional study assessing surgical treatments of varicose veins, the improvement in AVVQ after invasive treatment at 6-weeks was greater than 5 points,46,47 and the results from this study appear to offer a reasonable treatment pathway for those for whom interventions such as in CLASS are not available.
The VDS results show an overall improvement, however, the study was not powered to show a significant difference between groups.
The findings of this study with respect to clinical severity and disease specific quality of life suggests that twice daily usage of the NMES device may produce similar benefits to surgical intervention.
There was no significant improvement in the generic quality of life outcome measures, which may be explained by the duration of the study. Generic quality of life measures may be relatively insensitive to short term change in symptomatology, and longer-term intervention duration may have led to produced similar findings as seen in the disease specific scores.
The increase in microcirculatory flow indicated by increase in laser Doppler fluxmetre is to be expected with the increase in venous flow seen in groups B and C, and is in agreement with previous studies24,48,49 as is the large confidence intervals due to the inevitable structural heterogeneity of the microvasculature.50
Self-reported patient compliance with the NMES device appears extremely robust, which when coupled to the beneficial effects suggest that patients find the device feasible and satisfactory to use, with disease benefit.
There were no adverse events reported in patients undergoing this trial.
The results of this trial support the pilot trial findings, which were limited by the small sample population, leading to a biased difference in patient demographics in favour of the test group.23
This dose finding study suggests that 2 sessions of treatment per day (1 hour in total) is needed to achieve the maximal improvements in QoL and clinical severity seen.
Limitations of this trial include a short trial duration of 6 weeks. It is possible that the changes seen at 6 weeks may or may not persist over longer follow-up, and it is unknown whether changes persist after cessation of therapy. Indeed, it may be that the changes seen by 6 weeks are in fact only early changes and that a longer study would provide greater changes. This study did not fully recruit the target patient number, however, 84% of target were recruited (76 of 90), with 92% of those recruited returning for 6-week follow-up. The attrition rate was therefore below the planned 15%, providing appropriate statistical power.
This study did not specifically assess lifestyle or working habits, which may lead to confounding variable. However, randomisation will have minimised the effect as much as possible.
No formal venoactive drugs are licensed in the United Kingdom or available on the NHS, and they were not utilised in this study. It may be their usage alters the outcome of this study.
The intensity of electrical stimulation deemed acceptable by each patient may have been an uncontrolled confounder, however initial training with the device highlighted the target intensity for use, and this was gauged as double the sensation threshold level.
The study utilised optoelectronic limb volumetry rather than the gold standard of water plethysmography, and this may have slightly reduced the limb volume accuracy.
The timing of the two sessions each day was at the discretion of the participant, with the options of two sessions in a row or two separate sessions, for pragmatic reasons as it was felt few study participants would comply with routine 1-hour sessions of NMES. This may have been a further confounder, but a further dedicated study would be required to investigate timings of sessions.
Venous flow parameters were selected as a key primary outcome measure as they demonstrate an improvement in circulation. The variability in venous flow parameters can be attributed to a combination of factors. The varying electrical waveform patterns, which change every minute, result in different maximum strength of muscle contraction as well as duration of contraction and relaxation phases. Hence, certain rapid contractions may not be beneficial as it is akin to trying to pump water from an empty well. In addition, operator reliability, pressure by the ultrasound probe or operators’ hand on vein diameter, breathing and movement influence venous flow parameters.
Movement caused by the powerful muscle contractions results in a reduction of vein diameter during the contraction phase as well as movement artefact. Due to these limitations, repeat venous haemodynamic measurements were taken at the same five-minute intervals to minimise device related variations.
NMES has a variable effect on patients due to several factors. Pain thresholds affects the intensity tolerated by patients. Oedema and dry skin are a barrier to stimulation. Patients were advised to apply moisturiser if the effect of NMES was diminished. Patients with asymmetric disease (e.g. post-thrombotic syndrome) receive a lower intensity of stimulation on the affected limb, which reduces both the strength of muscle contraction and the improvement in venous flow parameters in the affected side. The intensity of stimulation is often limited by pain due to the stronger muscle contractions on the unaffected limb. However, tolerance for the treatment develops and so users also have to adjust the intensity to continue effective interventions.
A larger study with a primary endpoint of AVVQ or VCSS improvement at 6 months would be beneficial to support this study’s short-term findings of benefit from NMES. This would potentially place NMES as an additional conservative management for CVD, to be used in conjunction with other measures such as compression hosiery. Compression hosiery can be difficult to apply, can be poorly tolerate and requires quarterly replacement, whereas in principle the NMES device should have a significantly longer lifespan. In those unable to wear compression stockings NMES may offer a long-term option.
Supplemental Material
Supplemental material, sj-pdf-1-phl-10.1177_0268355520968640 for A randomised controlled trial of neuromuscular stimulation in non-operative venous disease improves clinical and symptomatic status by Raveena Ravikumar, Tristan RA Lane, Adarsh Babber, Sarah Onida and Alun H Davies in Phlebology
Acknowledgements
Imperial College London was the study sponsor. The research was supported by the National Institute for Health Research (NIHR) Biomedical Research Centre based at Imperial College Healthcare NHS Trust and Imperial College London. The views expressed are those of the authors and not necessarily those of the NHS, NIHR or the Department of Health.
Authors' Note: Previously presented at: a) UIP World Congress of Phlebology, Australia, 4-8th February 2018 and b) Vascular Society Annual Scientific Meeting 2018.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Actegy Ltd funded this study and provided the footplate NMES devices. Actegy Ltd are the manufacturer and developer of the Revitive Medic IX footplate NMES device.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by an unrestricted educational grant from Actegy Ltd, managed by Imperial College London. Provision of footplate NMES machines was funded by Actegy Ltd.
Ethical approval: Ethical approval for the study was obtained from the National Research Ethics Committee (NRES ref: 15/LO/0620).
Guarantor: Professor Alun Davies is the Guarantor.
Supplemental material: Supplemental material for this article is available online.
Contributorship
RR and TRAL should be recognised as joint first authors. RR, AB and AHD conceived and designed the study. RR, AB and AHD conducted the study
RR, AB, and TRAL conducted the statistical analysis. RR and TRAL drafted the manuscript. RR, TRAL, AB, SO and AHD critically reviewed and adjusted the manuscript.
ORCID iDs
Tristan RA Lane https://orcid.org/0000-0002-8681-7075
Alun H Davies https://orcid.org/0000-0001-5261-6913
References
- 1.Onida S, Davies AH. Predicted burden of venous disease. Phlebology 2016; 31: 74–79. [DOI] [PubMed] [Google Scholar]
- 2.Beebe-Dimmer JL, Pfeifer JR, Engle JS, et al. The epidemiology of chronic venous insufficiency and varicose veins. Ann Epidemiol 2005; 15: 175–184. [DOI] [PubMed] [Google Scholar]
- 3.Robertson L, Evans CJ, Fowkes FGR. Epidemiology of chronic venous disease. Phlebology J Venous Dis 2008; 23: 103–111. [DOI] [PubMed] [Google Scholar]
- 4.Lurie F, Passman M, Meisner M, et al. The 2020 update of the CEAP classification system and reporting standards. J Vasc Surg Venous Lymphat Disord 2020; 8: 342–352. [DOI] [PubMed] [Google Scholar]
- 5.Heit JA, Rooke TW, Silverstein MD, et al. Trends in the incidence of venous stasis syndrome and venous ulcer: a 25-year population-based study. J Vasc Surg 2001; 33: 1022–1027. [DOI] [PubMed] [Google Scholar]
- 6.Baldwin MJ, Moore HM, Rudarakanchana N, et al. Post-thrombotic syndrome: a clinical review. J Thromb Haemost 2013; 11: 795–805. [DOI] [PubMed] [Google Scholar]
- 7.Rabe E, Pannier F, Ko A, et al. Incidence of varicose veins, chronic venous insufficiency, and progression of the disease in the bonn vein study II. J Vasc Surg 2010; 51: 791–791. [Google Scholar]
- 8.Deitelzweig SB, Johnson BH, Lin J, et al. Prevalence of clinical venous thromboembolism in the USA: current trends and future projections. Am J Hematol 2011; 86: 217–220. [DOI] [PubMed] [Google Scholar]
- 9.Carradice D, Mazari FAK, Samuel N, et al. Modelling the effect of venous disease on quality of life. Br J Surg 2011; 98: 1089–1098. [DOI] [PubMed] [Google Scholar]
- 10.Guest JF, Ayoub N, McIlwraith T, et al. Health economic burden that different wound types impose on the UK’s National Health Service. Int Wound J 2017; 14: 322–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shepherd AC, Lane TRA, Davies AH. The natural progression of chronic venous disorders: an overview of available information from longitudinal studies. Phlebolymphology 2012; 19: 138–147. [Google Scholar]
- 12.Kostas TI, Ioannou CV, Drygiannakis I, et al. Chronic venous disease progression and modification of predisposing factors. J Vasc Surg 2010; 51: 900–907. [DOI] [PubMed] [Google Scholar]
- 13.Bogachev VY, Golovanova OV, Kuznetsov AN, et al. Electromuscular stimulation with VEINOPLUS® for the treatment of chronic venous edema. Int Angiol 2011; 30: 567–590. [PubMed] [Google Scholar]
- 14.Raju S, Knepper J, May C, et al. Ambulatory venous pressure, air plethysmography, and the role of calf venous pump in chronic venous disease. J Vasc Surg Venous Lymphat Disord 2019; 7: 428–440. [DOI] [PubMed] [Google Scholar]
- 15.Araki CT, Back TL, Padberg FT, et al. The significance of calf muscle pump function in venous ulceration. J Vasc Surg 1994; 20: 872–877. [DOI] [PubMed] [Google Scholar]
- 16.Clarke Moloney M, Lyons GM, Breen P, et al. Haemodynamic study examining the response of venous blood flow to electrical stimulation of the gastrocnemius muscle in patients with chronic venous disease. Eur J Vasc Endovasc Surg 2006; 31: 300–305. [DOI] [PubMed] [Google Scholar]
- 17.Corley GJ, Breen PP, Grace PA, et al. The effect of surface neuromuscular electrical stimulation and compression hosiery applied to the lower limb, on the comfort and blood flow of healthy subjects. Conf Proc IEEE Eng Med Biol Soc 2008; 2008: 703–706. [DOI] [PubMed] [Google Scholar]
- 18.Corley GJ, Breen PP, Bîrlea SI, et al. Hemodynamic effects of habituation to a week-long program of neuromuscular electrical stimulation. Med Eng Phys 2012; 34: 459–465. [DOI] [PubMed] [Google Scholar]
- 19.Delis KT, Slimani G, Hafez HM, et al. Enhancing venous outflow in the lower limb with intermittent pneumatic compression. A comparative haemodynamic analysis on the effect of foot vs. calf vs. foot and calf compression. Eur J Vasc Endovasc Surg 2000; 19: 250–260. [DOI] [PubMed] [Google Scholar]
- 20.Izumi M, Ikeuchi M, Mitani T, et al. Prevention of venous stasis in the lower limb by transcutaneous electrical nerve stimulation. Eur J Vasc Endovasc Surg 2010; 39: 642–645. [DOI] [PubMed] [Google Scholar]
- 21.Varatharajan L, Williams K, Moore H, et al. The effect of footplate neuromuscular electrical stimulation on venous and arterial haemodynamics. Phlebology 2015; 30: 648–650. [DOI] [PubMed] [Google Scholar]
- 22.Williams KJ, Moore HM, Davies AH. Haemodynamic changes with the use of neuromuscular electrical stimulation compared to intermittent pneumatic compression. Phlebology 2015; 30: 365–372. [DOI] [PubMed] [Google Scholar]
- 23.Ravikumar R, Williams KJ, Babber A, et al. Randomised controlled trial: potential benefit of a footplate neuromuscular electrical stimulation device in patients with chronic venous disease. Eur J Vasc Endovasc Surg 2017; 53: 114–121. [DOI] [PubMed] [Google Scholar]
- 24.Jawad H, Bain DS, Dawson H, et al. The effectiveness of a novel neuromuscular electrostimulation method versus intermittent pneumatic compression in enhancing lower limb blood flow. J Vasc Surg Venous Lymphat Disord 2014; 2: 160–165. [DOI] [PubMed] [Google Scholar]
- 25.Kaplan R, Czyrny J, Fung T, et al. Electrical foot stimulation and implications for the prevention of venous thromboembolic disease. Thromb Haemost 2002; 88: 200–204. [PubMed] [Google Scholar]
- 26.Reed B. The physiology of neuromuscular electrical stimulation. Pediatr Phys Therapy 1997; 9: 96–102. [Google Scholar]
- 27.Tucker AT, Maass A, Bain DS, et al. Augmentation of venous, arterial and microvascular blood supply in the leg by isometric neuromuscular stimulation via the peroneal nerve. Int J Angiol 2010; 19: e31–e37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li S, Hoskins PR, Anderson T, et al. Measurement of mean velocity during pulsatile flow using time-averaged maximum frequency of Doppler ultrasound waveforms. Ultrasound Med Biol 1993; 19: 105–113. [DOI] [PubMed] [Google Scholar]
- 29.Thrush A, Hartshorne T. Vascular ultrasound: how, why, and when. 3rd ed. Churchill Livingstone, 2009, p. 320.
- 30.Raju S, Knight A, Lamanilao L, et al. Peripheral venous hypertension in chronic venous disease. J Vasc Surg Venous Lymphat Disord 2019; 7: 706–714 [DOI] [PubMed] [Google Scholar]
- 31.Williams KJ, Ravikumar R, Gaweesh AS, et al. A review of the evidence to support neuromuscular electrical stimulation in the prevention and management of venous disease. Adv Exp Med Biol 2017; 906: 377–386. [DOI] [PubMed] [Google Scholar]
- 32.Gloviczki P, Comerota AJ, Dalsing MC, et al. The care of patients with varicose veins and associated chronic venous diseases: clinical practice guidelines of the Society for Vascular Surgery and the American Venous Forum. J Vasc Surg 2011; 53: 2S–48S. [DOI] [PubMed] [Google Scholar]
- 33.NICE National Clinical Guideline Centre. Varicose veins in the legs: the diagnosis and management of varicose veins. London: National Institute for Health and Care Excellence, 2013 [PubMed] [Google Scholar]
- 34.Belramman A, Bootun R, Lane TRA, et al. Endovenous management of varicose veins. Angiology 2019; 70: 388–396. [DOI] [PubMed] [Google Scholar]
- 35.Bootun R, Onida S, Lane TRA, et al. Varicose veins and their management. Surgery (Oxford) 2016; 34: 165–171. [Google Scholar]
- 36.Vedantham S, Kahn SR, Goldhaber SZ, et al. Endovascular therapy for advanced post-thrombotic syndrome: proceedings from a multidisciplinary consensus panel. Vasc Med 2016; 21: 400–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Writing Committee, Wittens C, Davies AH, et al. Editor’s choice – management of chronic venous disease. Eur J Vasc Endovasc Surg 2015; 49: 678–737. [DOI] [PubMed] [Google Scholar]
- 38.Babber A, Ravikumar R, Onida S, et al. Effect of footplate neuromuscular electrical stimulation on functional and quality-of-life parameters in patients with peripheral artery disease: pilot, and subsequent randomized clinical trial. Br J Surg 2020; 107: 355–363. [DOI] [PubMed] [Google Scholar]
- 39.Lawton R, Babber A, Braithwaite B, et al. A multicenter randomized controlled study to evaluate whether neuromuscular electrical stimulation improves the absolute walking distance in patients with intermittent claudication compared with best available treatment. J Vasc Surg 2019; 69: 1567–1573. [DOI] [PubMed] [Google Scholar]
- 40.Wou J, Williams KJ, Davies AH. Compression stockings versus neuromuscular electrical stimulation devices in the management of occupational leg swelling. Int J Angiol 2016; 25: 104–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gianesini S, Raffetto JD, Mosti G, et al. Volume control of the lower limb with graduated compression during different muscle pump activation conditions and the relation to limb circumference variation . J Vasc Surg Venous Lymphat Disord 2020; 8: 814–820. [DOI] [PubMed] [Google Scholar]
- 42.Pereira DAG, Furtado SRC, Amâncio GPDO, et al. Association between heel-rise test performance and clinical severity of chronic venous insufficiency. Phlebology 2020; 35: 631–636. [DOI] [PubMed] [Google Scholar]
- 43.Mayrovitz HN, Larsen PB. Periwound skin microcirculation of venous leg ulcers. Microvasc Res 1994; 48: 114–123. [DOI] [PubMed] [Google Scholar]
- 44.Lane TRA, Kelleher D, Shepherd AC, et al. Ambulatory varicosity avulsion later or synchronized (AVULS): a randomized clinical trial. Ann Surg 2015; 261: 654–661. [DOI] [PubMed] [Google Scholar]
- 45.Rasmussen L, Lawaetz M, Bjoern L, et al. Randomized clinical trial comparing endovenous laser ablation and stripping of the great saphenous vein with clinical and duplex outcome after 5 years. J Vasc Surg 2013; 58: 421–426. [DOI] [PubMed] [Google Scholar]
- 46.Brittenden J, Cotton SC, Elders A, et al. A randomized trial comparing treatments for varicose veins. N Engl J Med 2014; 371: 1218–1227. [DOI] [PubMed] [Google Scholar]
- 47.Brittenden J, Cooper D, Dimitrova M, et al. Five-year outcomes of a randomized trial of treatments for varicose veins. N Engl J Med 2019; 381: 912–922. [DOI] [PubMed] [Google Scholar]
- 48.Evans DRS, Williams KJ, Strutton PH, et al. The comparative hemodynamic efficacy of lower limb muscles using transcutaneous electrical stimulation. J Vasc Surg Venous Lymphat Disord 2016; 4: 206–214. [DOI] [PubMed] [Google Scholar]
- 49.Bahadori S, Immins T, Wainwright TW. The effect of calf neuromuscular electrical stimulation and intermittent pneumatic compression on thigh microcirculation. Microvasc Res 2017; 111: 37–41. [DOI] [PubMed] [Google Scholar]
- 50.Pries AR, Secomb TW. Origins of heterogeneity in tissue perfusion and metabolism. Cardiovasc Res 2009; 81: 328–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-phl-10.1177_0268355520968640 for A randomised controlled trial of neuromuscular stimulation in non-operative venous disease improves clinical and symptomatic status by Raveena Ravikumar, Tristan RA Lane, Adarsh Babber, Sarah Onida and Alun H Davies in Phlebology