Abstract
Purpose:
To compare the acute success and complication rates of distal radial (DR) vs proximal radial (PR) artery access for superficial femoral artery (SFA) interventions.
Materials and Methods:
Between 2016 and 2019, 195 consecutive patients with symptomatic SFA stenosis were treated via DR (n=38) or PR (n=157) access using a sheathless guide. Secondary access was achieved through the pedal artery when necessary. The main outcomes were technical success, major adverse events (MAEs), and access site complications. Secondary outcomes were treatment success, fluoroscopy time, radiation dose, procedure time, and crossover rate to another puncture site.
Results:
Overall technical success was achieved in 188 patients (96.4%): 37 of 38 patients (97.3%) in the DR group and 151 of 157 patients (96.2%) in the PR group (p=0.9). Dual (transradial and transpedal) access was used in 14 patients (36.8%) in the DR group and 28 patients (18.9%) in the PR group (p<0.01). Chronic total occlusions were recanalized in 25 of 26 DR patients (96.1%) and in 79 of 81 PR patients (92.6%) (p=0.57). The crossover rate to femoral access was 0% in the DR group vs 3.2% in the PR group (p=0.59). Stents were implanted in the SFA in 15 DR patients (39.4%) and in 39 patients (24.8%) in the PR group (p=0.1). The contrast volume, fluoroscopy time, radiation dose, and procedure time were not statistically different between the DR and PR groups, nor were the rates of access site complications (2.6% and 7.0%, respectively). The cumulative incidences of MAE at 6 months in the DR and PR groups were 15.7% vs 14.6%, respectively (p=0.8).
Conclusion:
SFA interventions can be safely and effectively performed using PR or DR access with acceptable morbidity and a high technical success rate. DR access is associated with few access site complications.
Keywords: angioplasty, femoropopliteal segment, stent, superficial femoral artery, transpedal approach, transradial approach, vascular access
Introduction
The proximal radial artery access is a gold standard for coronary interventions, and it is also gaining popularity for peripheral interventions due to better patient comfort and the low rate of major access site complications.1–8 For percutaneous superficial femoral artery (SFA) interventions, the proximal radial (PR) access is associated with high technical success and low rates of major access site complications, but radial artery occlusion occurs in up to 5%.4–8 Distal radial (DR) access was used first for Cimino fistula access and then for coronary and peripheral interventions.9–15 The main advantages of this puncture site are the ease of compression and low rate of radial artery occlusion. The purpose of this study was to evaluate the acute success and complication rates of the DR approach and to compare the complication rate and procedural success with the conventional PR approach.
Materials and Methods
Patient Population
Between 2016 and 2019, 195 consecutive patients with symptomatic (>70% diameter stenosis) SFA stenosis underwent endovascular interventions via a radial artery access using a sheathless guide. Thirty-eight patients (mean age 68.5±8.5 years; 26 men) were treated via a DR access and were compared to 157 patients (mean age 67.3±9.8 years; 101 men) who had an SFA intervention via a PR access. Patients with bilateral occluded radial arteries or ulnar artery accesses were not included. Patients who had a right transradial access and in whom the 125-cm diagnostic catheter would not reach the common iliac artery were also excluded. The impact of the learning curve was analyzed in each year by comparing the procedural data obtained in the first 20 cases with the remaining patients. The indication for the intervention was intermittent claudication in 85 patients (43.5%) and chronic limb-threatening ischemia (CLTI) in 110 patients (56.4%). Baseline patient and lesion characteristics are summarized in Table 1.
Table 1.
Baseline Patient and Lesion Characteristics.a
| Proximal Radial Access (n=157) | Distal Radial Access (n=38) | |
|---|---|---|
| Patient variables | ||
| Age, y | 67.3±9.8 | 68.5±8.5 |
| Women | 56 (35.4) | 12 (31.6) |
| Weight, kg | 80.1±17.5 | 75.8±16.6 |
| Height, cm | 167.9±8.4 | 167.5±7.9 |
| Hypertension | 150 (95.5) | 36 (94.7) |
| Current smoking | 25 (15.9) | 7 (18.4) |
| IDDM | 28 (17.8) | 6 (15.7) |
| NIDDM | 56 (35.7) | 12 (31.6) |
| COPD | 10 (6.4) | 5 (13.1) |
| Renal insufficiency | 43 (27.3) | 17 (44.7) |
| CAD | 52 (33.1) | 20 (52.6) |
| Previous PTA | 60 (38.2) | 22 (57.8) |
| Previous bypass | 17 (10.8) | 5 (13.1) |
| Lesion variables | ||
| Diameter stenosis, % | 86.7±14.9 | 96±6.3 |
| Length, mm | 88.2±65.1 | 136.6±87.3b |
| Reference vessel diameter, mm | 5.4±0.56 | 5.11±0.47 |
| TASC class | ||
| A | 73 (44.2)c | 4 (10.5) |
| B | 36 (21.8) | 10 (26.3) |
| C | 13 (7.8) | 7 (18.4) |
| D | 35 (21.2) | 17 (44.7)c |
| Chronic total occlusion | 76 (48.7) | 26 (68.4) |
Abbreviations: CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; IDDM, insulin-dependent diabetes mellitus; NIDDM, non-insulin-dependent diabetes mellitus; PAD, peripheral artery disease; PTA, percutaneous transluminal angioplasty; TASC, TransAtlantic Inter-Society Consensus.
Continuous data are presented as the mean ± standard deviation; categorical data are given as the count (percentage).
p<0.05.
p<0.01.
The Institutional Review Committee approved the study (SE TUKEB 212/2016) and all patients provided written informed consent prior to treatment.
Procedure
Two skilled operators trained in bilateral transradial access and SFA interventions performed all cases. The preferred access site was the right radial artery; a contralateral access was used if the right radial artery was occluded. Ultrasound-guided puncture was used routinely for DR access but was optional for PR access. The radial artery diameters and peak systolic velocities were measured at the distal and proximal radial artery sites. Transpedal puncture for dual access cases was done under ultrasound guidance.
The patients were given loading doses of aspirin (325 mg) and clopidogrel (300 mg) on the day of the procedure. After local anesthesia, the DR artery was punctured under ultrasound guidance in the longitudinal view (Figure 1A), while the PR artery was located by palpation (ultrasound was used when the artery was not palpable). A dedicated transradial needle and 5-F transradial sheath (Terumo, Tokyo, Japan) were introduced. Heparin (5000 units), verapamil (2.5 mg), and nitroglycerine (250 µg) were administered directly through the sheath. Additional heparin was given up to 100 U/kg during the procedure, but the activated clotting time was not routinely measured. A J tip guidewire was advanced under fluoroscopy (40° left lateral projection) into the descending aorta along with a 5-F, 125-cm-long pigtail catheter. In a complex aortic arch, the loop technique with the pigtail catheter or Simmons catheter was used. Aortography (Figure 1A) was performed via the pigtail catheter, which was helpful in estimating the distance between the puncture site and the lesion.
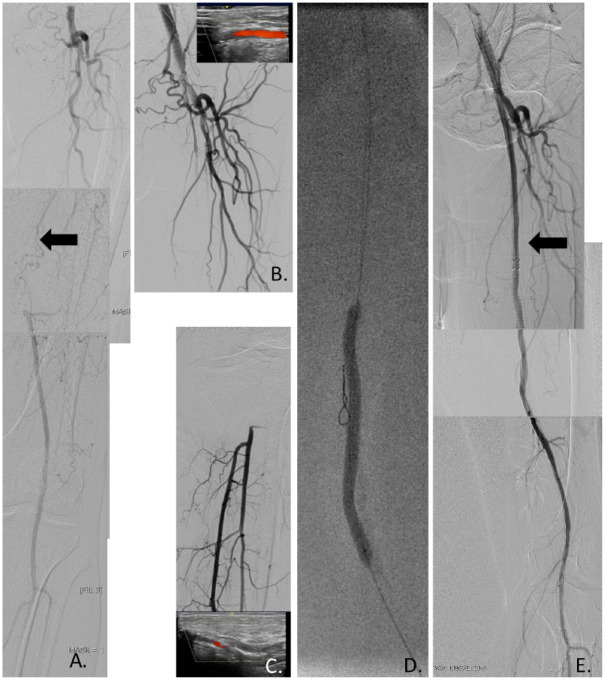
Figure 1.
(A) Digital subtraction angiography shows chronic total occlusion of the left superficial femoral artery (SFA; arrow). (B) Ultrasound-guided distal radial artery puncture and selective cannulation of the right common femoral artery with a long 125-cm multipurpose catheter through a sheathless guiding catheter. (C) Ultrasound-guided pedal artery puncture and retrograde angiography after transpedal puncture. (D) Subintimal V18 guidewire advancement, but failed antegrade guidewire reentry. Retrograde balloon angioplasty of the right SFA after successful passage with a Gladius guidewire. (E) Angiography after retrograde dual 6×120-mm Zilver PTX stent implantation (arrow) and postdilation.
The diagnostic catheter and introducer sheath were exchanged for a dedicated, 6-F, 120-cm-long peripheral transradial sheathless guiding system (SheathlessPV; Asahi Intecc, Aichi, Japan) over a 260-cm-long, 0.035-inch guidewire (Starter or Jindo, Amplatz) in PR cases. For DR cases, a 6-F, 100-cm coronary transradial sheathless guide was used (Eaucath; Asahi Intecc). A short hemostasis valve (Terumo) was employed to decrease the length of the delivery system. After angiography, the common femoral artery was selectively cannulated with a 125-cm-long multipurpose diagnostic catheter (telescopic method), and the guidewire was advanced through the lesion (Figure 1B).
Angioplasty was performed under roadmap imaging superselectively acquired through the diagnostic catheter to minimize contrast use. A balloon with a 180-cm-long shaft was used for dilation (Pacific Extreme; Medtronic, Minneapolis, MN, USA). Stent implantation was done only in cases of flow-limiting dissection or significant recoil. In the PR approach, self-expanding stents with a 180-cm-long shaft (Sinus Superflex; OptiMed, Ettlingen, Germany) were used. In very complex cases, stenting was performed from the transpedal approach using a Supera stent (Abbott Vascular, Santa Clara, CA, USA) or Zilver PTX stent (Cook Medical, Bloomington, IN, USA) via a Terumo slender sheath. All stents were postdilated (Figure 1C). After the procedure, any transpedal sheath was removed before the radial sheath, and the patency of the pedal puncture site was checked with transradial angiography.
The radial sheath was then removed, and hemostasis was achieved in PR cases by applying the Terumo Band for 4 hours. For DR cases, a SealOne device (Perouse Medical, a Vygon company, Ivry le Temple, France) was used for 4 hours after 2 minutes of local compression. The patency of the DR artery was checked with ultrasound in all patients. All patients were immediately mobilized after the procedure and underwent a physical examination. On the first postoperative day, the patency of the radial artery was evaluated if it was not palpable at the puncture site. Patients who underwent balloon angioplasty were prescribed lifelong aspirin therapy. The patients who underwent stenting received dual antiplatelet therapy (aspirin 100 mg and clopidogrel 75 mg) for 2 months.
All patients were scheduled for a detailed clinical follow-up examination at 3 and 12 months after the procedure. Furthermore, patients with CLTI and nonhealing wounds returned for the treatment of outflow disease.
Outcomes and Definitions
The main outcomes were technical success, major adverse events (MAEs), and access site complications. Secondary outcomes were treatment success, angioplasty equipment use, fluoroscopy time, radiation dose, procedure time, and crossover rate to another puncture site.
Technical success was defined as angioplasty resulting in <50% residual stenosis with sufficient antegrade flow; an optimal result was characterized by residual stenosis <30% and fast flow, while a suboptimal result was characterized by sluggish flow and/or a residual stenosis between 30% and 50% after repeated dilation.
MAE was assessed as a composite endpoint consisting of death, stroke, myocardial infarction, major amputation, and repeated revascularization of the target vessel during the hospital stay and at the 6-month follow-up. Major vascular complications were (1) diminished or symptomatic loss of arterial pulse or (2) the presence of any pseudoaneurysm, arteriovenous fistula, or hematoma >2 cm or requiring treatment at the radial access site. Minor vascular complications were asymptomatic loss of the radial artery pulse and hematomas <2 cm in diameter over the radial puncture area requiring no further treatment or <5 cm in diameter over the femoral puncture site. Major bleeding was defined as a drop in the hemoglobin level >3 g/dL, as well as any bleeding requiring transfusions.
Limb salvage was defined as the prevention of major amputation. Any amputation at or distal to the transmetatarsal level was classified as minor.
Statistical Analysis
Continuous variables are expressed as the mean ± standard deviation or the median with interquartile range (Q1, Q3). Categorical variables are presented as the count (percentage). The patient groups were compared using either the Mann-Whitney U test or the Kruskal-Wallis test. The threshold of statistical significance was p<0.05. Statistical analysis was performed using Graph Pad Prism software (version 8.0; GraphPad Software, San Diego, CA, USA).
Results
Characteristics of the procedures and outcome data are summarized in Table 2. Overall technical success was achieved in 188 patients (96.4%): 37 of 38 patients (97.3%) in the DR group and 151 of 157 patients (96.2%) in the PR group (p=0.9). The mean diameter of the DR artery was 1.9±0.36 mm. Crossover to femoral access was not necessary in DR cases, but 5 PR patients (3.2%) were changed to a femoral access (p=0.59). Dual (transradial and transpedal) access was used in 14 DR patients (36.8%) and in 28 PR patients (18.9%; p<0.01). Chronic total occlusions were recanalized in 25 of 26 DR patients (96.1%) and in 79 of 81 PR patients (92.6%; p=0.57). Stents were implanted in the SFA in 15 DR patients (39.4%) and in 39 PR patients (24.8%; p=0.1).
Table 2.
Procedural Data.a
| Proximal Radial Access (n=157) | Distal Radial Access (n=38) | |
|---|---|---|
| Balloon angioplasty in the SFA | 157 (100) | 38 (100) |
| Stenting in the SFA | 39 (24.8) | 15 (39,4) |
| Stent length, mm | 122.2±85 | 164±77.5b |
| Dual access (pedal and radial) | 28 (16.5) | 14 (36.8)b |
| Crossover to femoral access | 5 (3.2) | 0 (0) |
| Success | 151 (96.2) | 37 (97.3) |
| Radiation dose, Gy/cm2 | 33.5 [7.45, 59.5] | 24.1 [16.5, 31.7] |
| Fluoroscopy time, s | 762.5 [659.6, 865.4] | 663 [540, 787] |
| Procedure time, min | 36.4 [32.6, 40.2] | 37.1 [31.1, 43.1] |
| Contrast volume, mL | 119.5 [107.7, 131.3] | 93.3 [80.4, 106.2] |
Abbreviations: SFA, superficial femoral artery.
Continuous data are presented as the mean ± standard deviation or median [interquartile range Q1, Q3]; categorical data are given as the count (percentage).
p<0.05.
Additional procedures were done in the same setting in 2 iliac, 4 popliteal, and 6 below-the-knee arteries in DR patients and in 9 popliteal and 30 below-the-knee arteries in the PR group. There were no significant differences in the radiation dose, fluoroscopy time, procedure time, or contrast volume between the DR and PR groups.
Complications and outcomes are summarized in Table 3. One patient in the PR group suffered a distal embolization that was successfully treated with manual thrombus aspiration; no major procedural complications were encountered in the DR group. The rates of access site complications in the DR and PR group were 2.6% and 7%, respectively (p=0.46). The only access site complication in the DR group was a minor hematoma, while 1 PR patient had a hematoma >2 cm in the forearm. Ten other patients in the PR group had minor access sequelae including 7 asymptomatic radial artery occlusions and 3 forearm hematomas. Six DR patients (15.7%) and 23 PR patients (14.6%) had MAEs at 6 months. Three patients (7.8%) in the DR group died vs 8 in the PR group (5.1%; p=0.38).
Table 3.
Complications and Outcomes in Follow-up.a
| Variables | Proximal Radial Access (n=157) | Distal Radial Access (n=38) |
|---|---|---|
| Procedural complications Distal embolization Perforation Acute vessel closure |
1 (0.6) 1 (0.6) 0 0 |
0 0 0 0 |
| Access site complications Major RAO (symptomatic) Forearm hematoma (>2 cm) Minor RAO (asymptomatic) Forearm hematoma (<2 cm) |
11 (7.0) 1 (0.6) 0 1 10 (6.3) 7 (4.4) 3 (1.9) |
1 (2.6) 0 0 0 1 (2.6) 0 1 (2.6) |
| Major adverse events at 6 months Death Major amputation Redo PTA Myocardial infarction Stroke Total of all events Patients with events |
8 (5.1) 11 (6.5) 9 (5.7) 0 1 (0.6) 29 (18.4) 23 (14.6) |
3 (7.8) 1 (0.6) 1 (0.6) 0 1 (0.6) 6 (15.7) 6 (15.7) |
Abbreviations: PTA, percutaneous transluminal angioplasty; RAO, radial artery occlusion.
Data are given as the number (percentage).
The impact of the learning curve is summarized in Table 4. Over time, there were no significant differences in the procedure times, fluoroscopy times, radiation doses, or contrast volumes despite the higher number of complex cases after the first 20 cases in either group. There were significant decreases in fluoroscopy time and contrast volume over the years, but the procedure times and radiation dose were not statistically different. The crossover rate was significantly lower after the first year in the last 158 patients (p=0.01).
Table 4.
Impact of the Learning Curve.a
| 2015 (n=47) | 2016 (n=75) | 2017 (n=35) | 2018 (n=38) | 2019 (n=10) | |
|---|---|---|---|---|---|
| Procedure time, min | 28.8 [24, 33] | 37.3 [30, 43] | 44.6 [37, 51] | 37.1 [31, 43] | 36.5 [26, 46] |
| Fluoroscopy time, s | 784 [671, 897] | 689.1 [501, 876]b | 490.5 [708, 1072]c | 663.6 [540, 787]b | 570.9 [368, 773]c |
| Radiation dose, Gy/cm2 | 29.6 [7, 65] | 18.3 [12, 24] | 18.2 [14, 22] | 13.2 [9.9, 16.5] | 14.2 [5, 24] |
| Contrast volume, mL | 100.3 [82, 118] | 115.1 [99, 130] | 93.3 [80, 106]b | 92.3 [80, 106]b | 84.5 [51, 117]c |
| Crossover | 3 (6.3)c | 0 | 0 | 0 | 0 |
| TASC A,B TASC C,D |
45 (95.7)b
2 (4.2) |
56 (74.6)b
19 (25.3)b |
18 (51.4)b
17 (48.6)b |
31 (81.6)b
7 (18.4)b |
3 (30) 7 (70)b |
Abbreviations: TASC, TransAtlantic Inter-Society Consensus.
Continuous data are presented as the median [interquartile range Q1, Q3]; categorical data are given as the count (percentage).
p<0.05.
p<0.01.
Discussion
PR access has been used as an alternative access site for SFA angioplasty in several studies.3–8 The main advantages of PR access are the low rates of bleeding and major access site complications, improved patient comfort, and decreased procedure costs.1–8 From a technical point of view, the PR technique offers good support for the devices and easy cannulation of the iliac arteries. The most important disadvantages are the limited number of compatible dedicated devices, the need for dual access, and a high rate of minor vascular complications.
The hybrid approach has been reported for SFA interventions utilizing the radial and pedal approach,8 with high technical success and few complications. However, hybrid approaches utilizing the brachial and femoral access sites are associated with high vascular complication rates despite the use of ultrasound-guided access in femoral cases.16–18 Patel et al8 have reported that primary transradial and transpedal crossings were successful in 74% and 54% of their cases, but the hybrid strategy was successful in 99% of the failed cases.
The radial artery has two branches at the wrist level, the superficial palmar branch and the distal radial artery.15 The main advantage of the DR puncture is the superficial course of the artery, which allows easy puncture and hemostasis. Due to the nonaggressive hemostasis, DR artery occlusions are rare (1 vs 3 in our PR group) and usually asymptomatic, but small bleedings can occur. Bleedings are also different because the DR artery lies in subcutaneous tissue, but the PR artery is in a compartment and can cause compartment syndrome. Theoretically, DR artery occlusion might more often be asymptomatic because the occlusion is mostly focal, and the main branch of the radial artery is patent. Though there was a 4.8% rate of radial artery occlusion reported,8 there was no major access site complication in our study despite the use of large bore sheaths.
Other reported complications during DR access are local hematoma, pseudoaneurysm formation, and arteriovenous fistula formation.19 Arteriovenous fistula is asymptomatic; it needs only local compression, and intervention is not necessary.13 Pseudoaneurysm formation can be treated with ultrasound-guided compression.14 The most important disadvantage of the technique is the difficult guidewire advancement in the proximal radial artery due to the smaller size and acute angulation of the DR artery, which limits the use of available materials. One more technical limitation of the distal puncture site is the need for longer devices (2–3 cm) than PR cases, but despite this, the procedure success was the same in DR and PR cases in our cohort.
Ultrasound-guided puncture is recommended to overcome these limitations because the size and course of the radial artery and the severity of atherosclerosis can be investigated before puncture.20 We have used ultrasound guidance in all cases because an anterior single wall puncture can be done very safely with this technique, avoiding multiple punctures, and the site of the puncture can be selected in a nonangulated and nondiseased segment. Intraprocedural complications, such as dissection, hematoma formation, and spasm can also be detected, and a second puncture can be more accurate in a more proximal location.21
Roberts et al22 suggest that operators with no prior ultrasound experience for PR access guidance can acquire and integrate this skill into their practice without significant difficulty. In our learning curve analysis, there were no significant differences in terms of procedure-related factors after our first 20 cases. Two meta-analyses23,24 support the use of ultrasound guidance for conventional radial artery access vs blind puncture with palpation as this leads to higher first pass success,23 quicker puncture time, and less hematoma formation.24
Left radial access with PR puncture is often very uncomfortable and can increase the radiation exposure for the interventionist, therefore changing the patient’s position or lateral preparation of the hand is recommended.4–6 DR puncture is very comfortable for the patients because the hand lies on the abdomen in a very comfortable position and the hand can be moved so the interventionist can work as if from a femoral access. Left hand access shortens the device route and also might have fewer silent embolizations in the cerebral system.25
Limitations
The primary limitation of the study is the lack of randomization with the PR approach, so a randomized analysis of left vs right radial access would be worthwhile in future studies. Also, this study did not measure puncture time.
Conclusion
Femoral artery intervention can be safely and effectively performed using PR and DR access with an acceptable morbidity and a high technical success rate. DR access is associated with a very low access site complication rate.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Zoltán Ruzsa  https://orcid.org/0000-0002-2474-5723
https://orcid.org/0000-0002-2474-5723
Ádám Csavajda  https://orcid.org/0000-0002-7184-7853
https://orcid.org/0000-0002-7184-7853
References
- 1. Staniloae CS, Korabathina R, Coppola JT. Transradial access for peripheral vascular interventions. Catheter Cardiovasc Interv. 2013;81:1194–1203. [DOI] [PubMed] [Google Scholar]
- 2. Ruzsa Z, Tóth K, Nemes B, et al. Transradial and transulnar access for iliac artery interventions using sheathless guiding systems: a feasibility study. Catheter Cardiovasc Interv. 2016;88:923–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sanghvi K, Kurian D, Coppola J. Transradial intervention of iliac and superficial femoral artery disease is feasible. J Interv Cardiol. 2008;21:385–387. [DOI] [PubMed] [Google Scholar]
- 4. Lorenzoni R, Lisi C, Corciu A, et al. Tailored use of transradial access for above-the-knee angioplasty. J Endovasc Ther. 2014;21:635–640. [DOI] [PubMed] [Google Scholar]
- 5. Lorenzoni R, Mazzoni A, Lazzari M, et al. Radial artery access for above the knee angioplasty: a feasibility study. EuroIntervention. 2011;7:924–949. [DOI] [PubMed] [Google Scholar]
- 6. Meertens MM, Ng E, Loh SEK, et al. Transradial approach for aortoiliac and femoropopliteal interventions: a systematic review and meta-analysis. J Endovasc Ther. 2018;25:599–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ruzsa Z, Bellavics R, Nemes B, et al. Combined transradial and transpedal approach for femoral artery interventions. JACC Cardiovasc Interv. 2018;11:1062–1071. [DOI] [PubMed] [Google Scholar]
- 8. Patel A, Parikh R, Htun W, et al. Transradial versus tibiopedal access approach for endovascular intervention of superficial femoral artery chronic total occlusion. Catheter Cardiovasc Interv. 2018;92:1338–1344. [DOI] [PubMed] [Google Scholar]
- 9. Wolowczyk L, Williams AJ, Donovan KL, et al. The snuffbox arteriovenous fistula for vascular access. Eur J Vasc Endovasc Surg. 2000;19:70–76. [DOI] [PubMed] [Google Scholar]
- 10. Kiemeneij F. Left distal transradial access in the anatomical snuffbox for coronary angiography (ldTRA) and interventions (ldTRI). EuroIntervention. 2017;13:851–857. [DOI] [PubMed] [Google Scholar]
- 11. Mizuguchi Y, Izumikawa T, Hashimoto S, et al. Efficacy and safety of the distal transradial approach in coronary angiography and percutaneous coronary intervention: a Japanese multicenter experience. Cardiovasc Interv Ther. 2020;35:162–167. [DOI] [PubMed] [Google Scholar]
- 12. Wretowski D, Krakowian M, Łabyk A, et al. Very distal transradial approach (VITRO) for coronary interventions. Postepy Kardiol Interwencyjnej. 2019;15:42–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nardai S, Végh E, Óriás V, et al. Feasibility of distal radial access for carotid interventions: the RADCAR-DISTAL pilot study. EuroIntervention. 2020;15:1288–1290. [DOI] [PubMed] [Google Scholar]
- 14. van Dam L, Geeraedts T, Bijdevaate D, et al. Distal radial artery access for noncoronary endovascular treatment is a safe and feasible technique. J Vasc Interv Radiol. 2019;30: 1281–1285. [DOI] [PubMed] [Google Scholar]
- 15. Sgueglia GA, Di Giorgio A, Gaspardone A, et al. Anatomic basis and physiological rationale of distal radial artery access for percutaneous coronary and endovascular procedures. JACC Cardiovasc Interv. 2018;11:2113–2119. [DOI] [PubMed] [Google Scholar]
- 16. Alvarez-Tostado JA, Moise MA, Bena JF, et al. The brachial artery: a critical access for endovascular procedures. J Vasc Surg. 2009;49:378–385. [DOI] [PubMed] [Google Scholar]
- 17. Seto AH, Abu-Fadel MS, Sparling JM. Real-time ultrasound guidance facilitates femoral arterial access and reduces vascular complications: FAUST (Femoral Arterial Access With Ultrasound Trial). JACC Cardiovasc Interv. 2010;3:751–758. [DOI] [PubMed] [Google Scholar]
- 18. Gutzeit A, Graf N, Schoch E, et al. Ultrasound-guided antegrade femoral access: comparison between the common femoral artery and the superficial femoral artery. Eur Radiol. 2011;21:1323–1328. [DOI] [PubMed] [Google Scholar]
- 19. Mizuguchi Y, Yamada T, Taniguchi N, et al. Pseudoaneurysm formation after cardiac catheterization using the distal transradial approach. J Invasive Cardiol. 2019;31:E257. [PubMed] [Google Scholar]
- 20. Hadjivassiliou A, Kiemeneij F, Nathan S, et al. Ultrasound-guided access of the distal radial artery at the anatomical snuffbox for catheter-based vascular interventions: a technical guide. EuroIntervention. Published online August 6, 2019. doi: 10.4244/EIJ-D-19-00555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Thakor AS, Alshammari MT, Liu DM, et al. Transradial access for interventional radiology: single centre procedural and clinical outcome analysis. Can Assoc Radiol J. 2017;68:318–327. [DOI] [PubMed] [Google Scholar]
- 22. Roberts J, Manur R. Ultrasound-guided radial artery access by a non-ultrasound trained interventional cardiologist improved first-attempt success rates and shortened time for successful radial artery cannulation. J Invasive Cardiol. 2013;25:676–679. [PubMed] [Google Scholar]
- 23. Moussa Pacha H, Alahdab F, Al-Khadra Y, et al. Ultrasound-guided versus palpation-guided radial artery catheterization in adult population: a systematic review and meta-analysis of randomized controlled trials. Am Heart J. 2018;204:1–8. [DOI] [PubMed] [Google Scholar]
- 24. Tang L, Wang F, Li Y, et al. Ultrasound guidance for radial artery catheterization: an updated meta analysis of randomized controlled trials. PLoS One. 2014;9:e111527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pacchioni A, Versaci F, Mugnolo A, et al. Risk of brain injury during diagnostic coronary angiography: comparison between right and left radial approach. Int J Cardiol. 2013; 167:3021–3026. [DOI] [PubMed] [Google Scholar]