Abstract
Background
Intracranial atherosclerotic stenosis is a highly prevalent cause of stroke worldwide with important ethnic disparities. Widely considered to be a common cause of stroke in Asian and Afro-Caribbean populations, relatively less is known about the burden and significance of intracranial atherosclerotic stenosis in Caucasians.
Aims
We aim to highlight recent insights and advances into the prevalence, prognosis, and treatment of symptomatic and asymptomatic atherosclerotic intracranial atherosclerotic stenosis in Caucasian patients.
Summary of review
We identified 48 articles studying intracranial atherosclerotic stenosis in Caucasian patients with ischemic stroke or transient ischemic attack. Most studies were on hospital-based cohorts of consecutive patients and half were graded as “fair” quality. There was significant variation between studies in the definition of intracranial atherosclerotic stenosis and in the imaging modalities used to detect intracranial atherosclerotic stenosis. Overall, 12.1% of Caucasian patients were found to have any intracranial atherosclerotic stenosis, 6.4% symptomatic intracranial atherosclerotic stenosis and 11.1% asymptomatic intracranial atherosclerotic stenosis, with higher rates at older ages. In studies reporting prognosis, there were 61 and 10 same-territory ischemic strokes in 1000 person-years in patients with symptomatic and asymptomatic intracranial atherosclerotic stenosis, respectively. Percutaneous stenting and angioplasty have not proven superior to intensive medical management in patients with symptomatic intracranial atherosclerotic stenosis.
Conclusions
Intracranial atherosclerotic stenosis has previously been neglected as a cause of stroke in Caucasians but is highly prevalent at older ages and frequently discovered with the growing use of noninvasive angiography. Intensive medical therapy is the treatment of choice, but there is a need to develop novel treatments or therapeutic approaches to lower the risk of stroke in higher risk patients.
Keywords: Angiography, epidemiology, ischemic stroke, secondary prevention, stenosis, stroke prognosis
Introduction
Ischemic stroke is a heterogeneous disease and up to a fifth of cases are caused by atherosclerosis of the aortic arch, neck, or intracranial arteries.1 There is significant ethnic variation in the location of large artery disease; Asian, Hispanic, and Black populations have a high burden of intracranial atherosclerotic stenosis (ICS), accounting for a third of ischemic cerebrovascular events,2–6 whereas relatively less is known about the burden of ICS in Caucasians, in whom extracranial carotid artery atherosclerosis is considered predominant and ICS is only attributed to 5–10% of all ischemic strokes.2,7
Given the perceived lack of importance of ICS in Caucasians, routine screening for extracranial internal carotid artery stenosis is recommended by US and European guidelines but there is no consensus on the value of routine screening for ICS. Furthermore, the most appropriate screening modality with adequate sensitivity, specificity, and practicability remains contested.
ICS are more frequently detected by the increasing use of intracranial angiography in the assessment of acute stroke patients, posing a challenge to clinicians to accurately counsel patients about the likely prognosis and optimal treatment strategy. Although intensive medical therapy has been established as standard secondary prevention therapy by randomized trials,8,9 it remains to be seen whether risk of recurrent stroke can be further reduced by percutaneous angioplasty and stenting or novel surgical approaches in high-risk subgroups of patients.
In this review, we highlight recent insights and advances into the prevalence, detection, prognosis, and treatment of symptomatic and asymptomatic ICS in Caucasian patients. Details of the literature search strategy and inclusion criteria are outlined in the Supplementary material, and a flow diagram of article exclusions is shown in Figure 1.
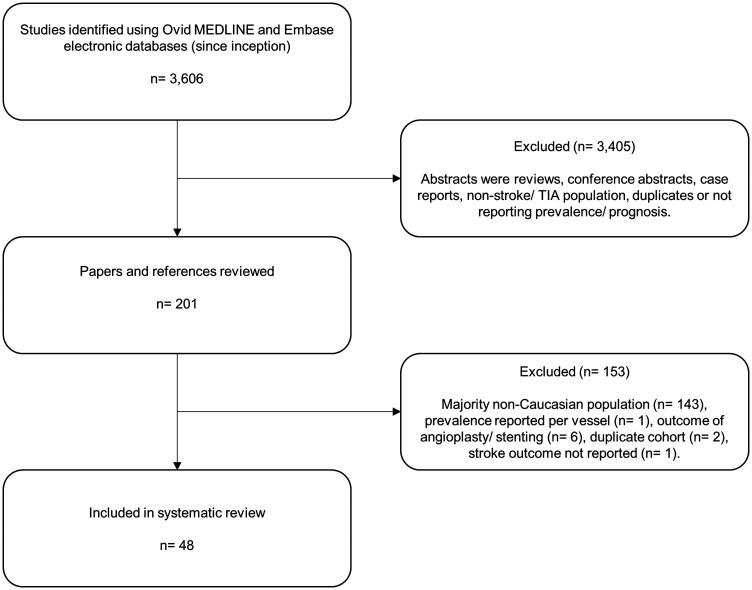
Figure 1.
Flow diagram of systematic review article exclusions for ICS prevalence and prognosis in Caucasian TIA/stroke patients.
Systematic review results
The systematic review identified 48 articles which fulfilled criteria studying ICS in Caucasian patients with ischemic stroke or transient ischemic attack (TIA) (Tables 1 and 2). Studies were hospital-based cohorts of consecutive ischemic stroke and TIA patients (39/81.3%: prospective n = 22, retrospective n = 17), two (4.2%) population-based studies of minor stroke TIA patients and the medical arms of clinical trials (n = 7/14.6%).
Table 1.
Prevalence of intracranial stenosis in Caucasian TIA/stroke patients
| Study | Location | Sample size | Mean age (years) | Caucasian (%) | IS/TIA | Definition of ICS |
Prevalence of ICS |
|||
|---|---|---|---|---|---|---|---|---|---|---|
| Screening imaging modality | Criteria | Any ICS n (%) | Asympt ICS n (%) | Sympt ICS n (%) | ||||||
| All ICS | ||||||||||
| Sacco (NOMASS) 1995w8 | USA | 82 | 70 | 100 | IS | TCD | Velocity criteria | – | – | 8 (1.0%) |
| Wityk 1996w9 | USA | 108 | 75 | 100 | IS/TIA | TCD/MRA/CA | Velocity criteria or 50–100% stenosis | 26 (24.0) | – | 10 (9.0%)a |
| Thijs 2000w13 | USA | 1344 | – | – | IS/TIA | CA/TCD/MRA | Velocity criteria or 50–99% stenosis | 54 (4.0) | – | 36 (2.7) |
| Weimar 2006w20 | Germany | 4157 | 67 | – | IS/TIA | TCD/MRA/ CTA/CA | Velocity criteria or 50–100% stenosis | 1259 (30.3) | – | 611 (14.7) |
| Nahab (WASID)b 2008w22 | USA | 312 | ∼64 | 50b | IS/TIA | CA/MRA | 50–99% stenosis | – | 79 (25.3%) | – |
| Holzer 2009w23 | Germany | 163 | 63 | 100 | TIA | TCD | Velocity criteria | 15 (9.2) | – | – |
| Meseguer 2010w27 | France | 1823 | 61 | – | TIA | TCD | Velocity criteria | 161 (8.8) | – | 67 (3.7) |
| Weber 2010w28 | Germany | 13,584 | 67 | – | IS/TIA | TCD/MRA | Velocity criteria or 50–99% stenosis | 736 (5.4) | – | 304 (2.2) |
| Homburg 2011w30 | Netherlands | 786 | 62 | 90 | IS/TIA | CTA | 50–100% stenosis | 77 (9.8) | – | 18 (2.3) |
| Von Weitzel-Mudersbach 2012w31 | Denmark | 195 | 66 | 100 | TIA | TCD | Velocity criteria | 24 (12.3) | – | 16 (8.2) |
| Lau 2013w32 | USA | 539 | 66 | 83 | IS/TIA | CTA | Any grade of stenosis | 212 (39.3) | – | 176 (32.7) |
| Ovesen 2013w34 | Denmark | 652 | 67 | 95 | IS/TIA | CTA | ≥30% stenosis | 101 (15.5) | – | 3 (0.5) |
| Ssi-Yan-Kai 2013w36 | France | 129 | 64 | – | IS/TIA | MRA | 50–100% stenosis | – | – | 16 (12.4) |
| Wolff 2014w37 | France | 159 | 37 | 99 | Young IS (age 18–45) | MRA/CTA/ DSA | ≥50% stenosis | – | – | 49 (31.2) |
| Logallo 2014w38 | Norway | 575 | 73 | – | IS/TIA | TCD/MRA/ CTA | Velocity criteria or any degree stenosis | 69 (12.0) | 44 (7.7) | 45 (7.8) |
| Tsivgoulis 2014w39 | Greece | 467 | 58 | 98 | IS/TIA | TCD | Velocity criteria | 51 (10.9) | 9c (1.9) | 43 (9.2) |
| Gouveia 2014w42 | Portugal | 1302 | 72 | – | IS/TIA | TCD/CTA | Velocity criteria or ≥50% stenosis | 158 (12.1) | 83 (6.3) | 75 (5.8) |
| Baracchini 2016w44 | Italy | 1134 | 71 | 97 | IS | TCD | Velocity criteria | – | – | 99 (8.7) |
| Hoshino 2018w46 | France | 403 | 62 | – | IS | TCD/MRA/ CTA | Velocity criteria or 50–100% stenosis | 146 (36.2) | 74c (18.3) | 72 (17.9) |
| Uchiyama 2019w48 | France | 3317 | 66 | 78 | IS/TIA | TCD/MRA/ CTA | Velocity criteria or 50–100% stenosis | 424 (12.8) | – | – |
| Hurford 2020w49, w50 | UK | 1368 | 62 | 94 | Minor IS/TIA | MRA/CTA | 50–100% stenosis | 260 (19.0) | 202 (14.8) | 105 (7.7) |
| Anterior circulation ICS only | ||||||||||
| Kappelle (NASCET)1999w10 | USA | 2589 | 66 | 95 | IS/TIA | CA | 50–100% ACA, MCA, intracranial ICA stenosis | – | – | 14d (0.5) |
| Baumgartnere 2003w15 | Switzerland | 244 | 65 | 98 | Lacunar stroke syndrome | TCD | Velocity criteria | 30 (12.3) | – | 16 (6.6%) |
| von Sarnowskie 2013w35 | Pan-European | 1561 | 46 | – | Young IS/TIA (age 18–55) | TCD | Velocity criteria | 184 (11.8) | – | 137 (8.8) |
| Mattioni 2014w40 | Netherlands | 220 | 65 | – | IS/TIA | CTA | Intracranial ICA and MCA; any degree stenosis or occlusion | 85 (38.6) | – | 80 (36.4) |
| Posterior circulation ICS only | ||||||||||
| Shin 1999w12 | USA | 430 | 60 | 88 | Vertebrobasilar IS/TIA | CTA/MRA/CA | 50–100% intracranial VA or BA stenosis | – | – | 119 (27.7) |
| Baumgartnere 2003w15 | Switzerland | 244 | 65 | 98 | Lacunar stroke syndrome | TCD | Velocity criteria | 19 (7.8) | – | 13 (5.3) |
| Marquardt 2009w24 | UK | 141 | 69 | 95 | Vertebrobasilar IS/TIA | MRA | ≥50% intracranial VA or BA stenosis | – | – | 14 (9.9) |
| Klein 2010w26 | France | 41 | 66 | – | Pontine IS | MRA | ≥30% BA stenosis | – | – | 7 (17.1) |
| von Sarnowskie 2013w35 | Pan-European | 1511 | 46 | – | Young IS/TIA (age 18–55) | TCD | Velocity criteria | 75 (5.0) | – | 45 (3.0) |
W: reference cited in supplementary material. Figures in italics derived from available data. IS: ischemic stroke; ICS: intracranial stenosis; CA: catheter angiography; TCD: transcranial Doppler; MRA: magnetic resonance angiography; CTA: computed tomography angiography; Asympt: asymptomatic; Sympt: symptomatic.
Of entire cohort, but no racial/sex differences.
WASID patients reviewed for coexistent asympt ICS; no significant racial differences in prevalence.
Patients with asympt ICS reported but not how many with isolated asympt ICS or coexistent sympt ICS unknown.
Ipsilateral to symptomatic ICA stenosis.
Study reports anterior and posterior circulation ICS separately without noting duplicate patients hence are reported separately in this table.
Table 2.
Prognosis of intracranial stenosis in medically-treated Caucasian TIA/stroke patients
| Study | Location | Sample size | Mean age (years) | Caucasian (%) | IS/TIA | Definition of ICS |
Prognosis of ICS |
|||
|---|---|---|---|---|---|---|---|---|---|---|
| Screening imaging modality | Criteria | Mean follow-up (months) | Any IS n (%) | Same territory IS n (%) | ||||||
| Symptomatic ICS | ||||||||||
| Hinton 1979w2 | USA | 16 | 62 | – | IS/TIA | CA | 40–100% MCA stenosis | 36 | – | 2 (12.5) |
| EC/IC bypass study group 1985w3 | Worldwide | 714 | 56 | – | IS/TIA | CA | Any degree of MCA and intracranial ICA stenosis | 56 | – | ∼10.0% at 1 year |
| Wechler 1986w4 | USA | 12 | 62 | – | IS/TIA | CA | 50–100% carotid siphon stenosis | 51 | – | 3 (25.0) |
| Moufarrijj 1986w5 | USA | 44 | ∼58 | – | Vertebrobasilar IS/TIA | CA | ≥50% intracranial VA or BA stenosis | 73 | – | 5 (11.4) |
| Pessin 1987w6 | USA | 9 | 62 | 56 | Vertebrobasilar IS/TIA | CA | ≥40% distal BA stenosis | 21 | – | 1 (11.1) |
| Pessin 1987w7 | USA | 6 | 70 | – | PCA territory IS/TIA | CA | ≥50% PCA stenosis | 9 | 0 | 0 |
| Kappelle (NASCET) 1999w10 | USA | 2589a | 66 | 93 | IS/TIA | CA | 50-100% ACA, MCA, intracranial ICA stenosis | – | – | 19.4–45.7% at 3 years |
| Woolfenden 1999w11 | USA | 26 | 72 | 82 | Vertebrobasilar IS/TIA | CA/MRA | 50–99% BA stenosis | 23 | 5 (19.2) | 4 (15.4) |
| Thijs 2000w13 | USA | 52 | 67 | 65 | IS/TIA | CA/TCD/MRA | Velocity criteria or 50–99% stenosis | 17 | 6 (11.5) | – |
| Arenillas 2001w14 | Spain | 40 | 62 | – | IS/TIA | TCD | MCA velocity criteria | 27 | – | 8 (20.0) |
| Qureshi 2003w16 | USA | 102 | 64 | 54 | Vertebrobasilar IS/TIA | CA/MRA | 50–99% intracranial VA or BA stenosis | 15 | 14 (13.7) | 8 (7.8) |
| Kern 2005w18 | Germany | 46 | 57 | – | IS/TIA | TCD | Velocity criteria | 31 | 15b (32.6) | 11b (23.9) |
| Chimowitz (WASID) 2005w19 | USA | 280c | 63 | 58 | IS/TIA | TCD/MRA/ CTA | 50–99% stenosis | 22 | 57 (20.4) | 42 (15.0) |
| Weimar 2006w20 | Germany | 272 | 67 | – | IS/TIA | TCD/MRA/ CTA/CA | Velocity criteria or 50–100% stenosis | – | 26 (9.6) at 1 year | – |
| Mazighi 2006w21 | France | 102 | 63 | 97 | IS/TIA | MRA/CTA/CA | 50–99% stenosis | 23 | – | 14 (13.7) |
| Samaniego 2009w25 | USA | 58 | 65 | 83 | IS/TIA | MRA/CTA/CA | Undefined | 14 | 3 (5.1) | – |
| Weber 2010w28 | Germany | 197 | 65 | – | IS/TIA | TCD/MRA | Velocity criteria or 50–99% stenosis | 24 | 23.3% at 3 years | – |
| Kozak 2011w29 | USA | 25 | 61 | 72 | IS/TIA | CA | 50–99% stenosis | 16 | – | 11 (44.0) |
| Nahab 2013w33 | USA | 22 | 66 | 59 | IS/TIA | CA/CTA | 50–99% stenosis | 14 | 0 | 0 |
| Ssi-Yan-Kai 2013w36 | France | 129 | 64 | – | IS/ TIA | MRA | 50–100% stenosis | – | 0 | 0 |
| Gouveia 2014w42 | Portugal | 72 | 73 | – | IS/TIA | TCD/CTA | Velocity criteria or ≥50% stenosis | 14 | – | 14 (19.4) |
| Derdeyn (SAMMPRIS) 2014w41 | USA | 227a | 60 | 71 | IS/TIA | CA | 70–99% stenosis | 32 | – | 31 (13.7) |
| Zaidat (VISSIT) 2015w43 | USA | 53a | 62 | 72 | IS/TIA | CA | 70–99% stenosis | – | – | 5 (9.4) at 1 year |
| Markus (VIST) 2017w45 | UK | 88a | 67 | – | Vertebrobasilar IS/TIA | MRA/CTA/CA | ≥50% intracranial VA stenosis | 29 | – | 4 (4.6) |
| Caliandro 2018w47 | Italy | 48 | 65 | – | IS/TIA | TCD | Velocity criteria | – | – | 5c (10.4) at 1 year |
| Hoshino 2018w46 | France | 72 | 65 | – | IS | TCD/MRA/CTA | Velocity criteria or 50–100% stenosis | – | 9b (13.2) at 4 years | – |
| Hurford 2020w50 | UK | 94 | 74 | 94 | Minor IS/TIA | MRA/CTA | 50–99% stenosis | 29 | 12 (12.8) | 8 (8.5) |
| Asymptomatic ICS | ||||||||||
| Kremer 2004w17 | Switzerland | 53 | 67 | – | IS/TIA | TCD | Velocity criteria | 68 | – | 0 |
| Kern 2005w18 | Germany | 56 | 66 | – | IS/TIA | TCD | Velocity criteria | 30 | 4b (7.1) | 2b (3.6) |
| Nahab 2008w22 | USA | 100 | 66 | 50 | IS/TIA | CA/MRA | 50–99% stenosis | 18 | – | 5 (5.0) |
| Gouveia 2014w42 | Portugal | 47 | 77 | – | IS/TIA | TCD/CTA | Velocity criteria or ≥ 50% stenosis | 14 | – | 1 (2.1) |
| Hoshino 2018w46 | France | 74 | 65 | – | IS | TCD/MRA/CTA | Velocity criteria or 50–100% stenosis | – | 8b (11.5) at 4 years | – |
| Hurford 2020w49 | UK | 155 | 77 | 94 | Minor IS/TIA | MRA/CTA | 50–100% stenosis | 40 | 8 (5.2) | 5 (3.2) |
W: reference cited in supplementary material. IS: ischemic stroke, ICS: intracranial stenosis; CA: catheter angiography; TCD: transcranial Doppler; MRA: magnetic resonance angiography; CTA: computed tomography angiography; MCA: middle cerebral artery; ICA: internal carotid artery; VA: vertebral artery; BA: basilar artery; PCA: posterior cerebral artery.
Medical arm of trial.
Ischemic stroke and TIA.
Patients randomized to receive aspirin.
Of the 28 studies reporting ICS prevalence, 21 (75.0%) included all ICS, whereas seven studies (25.0%) only reported anterior or posterior circulation ICS (two studies reported both but did not identify duplicate patients so have been included twice in Table 1). Of the 29 studies reporting ICS prognosis, 23 (79.3%) reported the prognosis of symptomatic ICS only, 2 (6.9%) of asymptomatic ICS only, and 4 (13.8%) of both symptomatic and asymptomatic ICS (presented separately in Table 2). Seven studies (24.1%) did not report the mean follow-up time and were excluded from analyses of prognosis.
The study quality outcomes are shown in Supplementary Table 1; 24 studies (50.0%) were graded as fair, 14 poor (29.2%), and 10 good (20.8%) quality. The most frequent limitations were incomplete description of ICS definition, predominant use of TCD only, and lack of follow-up information.
Definition and diagnosis of intracranial stenosis
ICS is a narrowing or occlusion of an intracranial (intradural or subarachnoid) arterial lumen due to atherosclerotic plaque (Figure 2). Atherosclerosis can be limited to the intracranial arteries or part of more systemic disease also affecting the coronary, renal, or peripheral arteries.10 It is important to distinguish non-atherosclerotic causes of intracranial vascular stenosis, including arterial dissection, moyamoya disease, intracranial vasculitis (idiopathic, infectious, or inflammatory), and vasospasm, as these conditions have different treatments and prognoses.11
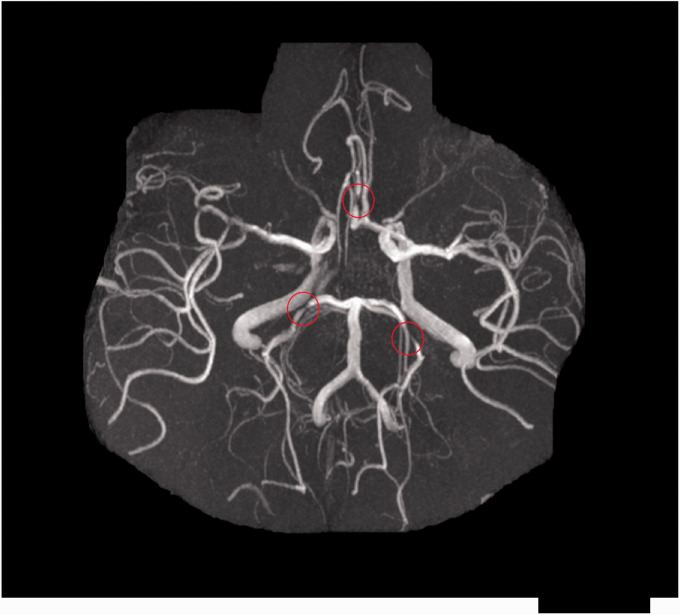
Figure 2.
Time-of-flight MR angiogram of the large intracranial arteries showing multifocal atherosclerotic stenoses, including bilateral posterior cerebral arteries and right anterior cerebral artery (indicated by red circles).
There is variation between studies in the degree of luminal restriction and its method of measurement used to define ICS with cross-sectional angiography. The Warfarin–Aspirin Symptomatic Intracranial Disease (WASID) trial method12 compares the narrowest luminal diameter with the closest normal luminal diameter proximal to the stenosis (or distal if the proximal artery is also stenosed) and is most commonly used. Some investigators use a method analogous to the North American Symptomatic Carotid Endarterectomy Trial (NASCET), which considers the diameter of a site distal to the lesion as normal.13 Reliability of these methods has not been extensively investigated in ICS. One study of 25 patients with symptomatic middle cerebral artery (MCA) ICS found a significant difference in the degree of narrowing as determined by NASCET and WASID methodology on catheter angiography, but not CT angiography (CTA).14
In our systematic review, all studies using TCD defined ICS according to velocity parameters based on the Baumgartner criteria.15 In studies using cross-sectional angiography (n = 38), 20 studies (52.6%) calculated the degree of stenosis using the WASID trial method, 3 studies (7.9%) used criteria based on the NASCET, and 15 studies (39.5%) did not report the methodology used.
Greater degrees of luminal narrowing have been associated with higher risks of recurrent same-territory ischemic stroke.16 Consequently, although ≥50% stenosis is most commonly used in observational studies, randomized trials have typically recruited patients with 70–99% stenosis in order to enrich the study population.9,17 In our review, the degree of luminal narrowing used to define ICS with cross-sectional angiography (n = 38) was ≥50% in 27 studies (71.1%; 50–100% in 17, and 50–99% in 10), 70–99% in two studies (5.3%), ≥30% in two studies (5.3%), ≥40% in two studies (5.3%), any grade of luminal narrowing in four studies (10.6%), and unknown in one study (2.7%).
The gold standard imaging modality for detecting ICS is digital subtraction angiography (DSA) as it provides high-resolution visualization of the intracranial vasculature and, as a dynamic procedure, permits assessment of flow rates and direction in addition to assessment of collateral supply.18 However, DSA is invasive and is associated with a risk of serious complications in up to 1% of procedures and is therefore not appropriate for routine screening or research.19,20
Noninvasive angiography, such as transcranial Doppler ultrasound, MR angiography (MRA) or CTA, is safer, quicker, and more accessible in routine practice, but the available methods have differing sensitivities and specificities for ICS detection. Moreover, no single modality is suitable for all patients.
Transcranial Doppler ultrasound (TCD) identifies ICS by detecting increased flow velocity distal to the ICS. The Stroke Outcomes and Neuroimaging of Intracranial Atherosclerosis (SONIA) trial assessed the accuracy of TCD in detecting 50–99% ICS compared with DSA and reported negative and positive predictive values of 85% and 36%, respectively.21 Therefore, TCD can be a useful initial screening tool for ≥50–99% ICS but is limited to the major proximal intracranial arteries and to patients with adequate temporal acoustic bone windows. Also, there is some evidence the sensitivity and specificity of TCD is greater in the anterior than posterior circulation.22 TCD was the most commonly used modality identified in our systematic review; either as the sole modality (n = 12) or alongside cross-sectional angiography (n = 9).
CTA can detect and quantify ICS by opacification following administration of an iodine-based contrast, it is easily performed in routine practice, and can detect perfusion deficits when combined with CT-perfusion sequences. Studies comparing CTA with DSA for identification of ≥50% ICS have reported high sensitivities and specificities and a good inter-operator reliability.13,23–25 Patient-related limitations include the requirement for ionizing radiation and intravenous contrast. Technical limitations include the reduced spatial resolution of smaller intracranial vessels (particularly <2 mm),26 obscuration by extensive mural calcification,27 or susceptibility gradients, for example of the internal carotid artery near the sphenoid sinus.26 However, improving post-processing techniques mitigates many of these shortfalls.28
MRI can detect ICS either by time-of-flight (TOF) or contrast-enhanced sequences and has the advantage of offering detailed parenchymal imaging which may indicate the likely infarct mechanism. TOF-MRA does not use any radiation or contrast material to visualize the intracranial arteries and has variable sensitivity and specificity for ICS, but different magnet strengths and post-processing techniques have been used.13,29,30 One study comparing TOF-MRA and CTA in ICS detection concluded CTA was superior, with a higher sensitivity (98% vs. 70%) and positive predictive value (93% vs. 65%).13 The main limitation of TOF-MRA is the susceptibility to artifact because of flow abnormalities—low flow may mimic stenosis and turbulent or loss of laminar flow through stenosis may over- or underestimate its degree.31–33
Unlike TOF-MRA, gadolinium-based contrast enhanced MRA (CE-MRA) is not vulnerable to signal-intensity flow artifacts and can assess the origins of the major intracranial arteries. However, CE-MRA is more costly and increases the complexity of imaging, in particular requiring accurate timing of the contrast bolus, which is contraindicated in some patients.34,35 Older coil systems were limited by poor spatial resolution,36 but modern techniques have a similar sensitivity and specificity to TOF-MRA in detecting ICS.33,37,38
With the exception of TCD, the modalities discussed so far only allow diagnosis of ICS as defined by a degree of arterial luminal restriction, which may limit risk stratification.39 Although not yet widely adopted by clinical practice, novel post-processing techniques can be used to assess the downstream hemodynamic impact of an ICS, for example by noninvasive angiography to measure peri-stenotic flow by parameters such as fractional flow and translesional wall shear stress ratio.39,40 Similarly, modalities such as high-resolution MRI (HR-MRI) and intravascular ultrasonography, can provide direct assessment of plaque composition and detection of non-stenotic intracranial atheroma which may have clinical relevance.41,42 Recently symptomatic, unstable plaques have been shown to have a higher lipid content, intra-plaque hemorrhage and inflammatory cell infiltration,43 properties which can be detected by HR-MRI.44 Intravascular ultrasonography can detect fibrous, lipid, and calcific plaque constituents, but is rarely used as it is invasive and technically challenging.45
Epidemiology of intracranial stenosis
Intracranial stenosis in stroke/TIA patients
The importance of ICS as a cause of ischemic stroke in Asian, Black, and Hispanic populations is well recognized.46 The Northern Manhattan Stroke study has reported higher rates of ICS in Afro-Caribbean and Hispanic compared to Caucasian patients, with ICS attributed to 9% of strokes in Caucasians, 17% of African Americans, and 15% of Hispanics.47 Potential reasons for the differences seen in the prevalence of ICS between racial groups include genetic factors, such as ring finger protein 213 (RNF213)48 or salt sensitivity associated polymorphisms (e.g., α-adducin, angiotensinogen, and aldosterone synthase).49 There are also interracial differences in lifestyle and risk factor profiles,50,51 and due to a thinner media and adventitia and fewer elastic medial fibers compared to extracranial arteries, intracranial arteries are more vulnerable to hypertension-induced hemodynamic stress.52 Furthermore, ICS develops at younger ages in Asians than Caucasians with the reverse is seen with extracranial artery atherosclerosis53 and it has been postulated that protective antioxidant enzyme activity is greater in the intracranial arteries compared to the extracranial arteries at a younger age.54
Our review identified 28 studies of ICS prevalence in Caucasian stroke and TIA patients (Table 1). In these studies, 4166 of 34,563 patients (12.1%) were found to have any ICS, 2198 of 35,788 (6.4%) symptomatic ICS and 490 of 4427 (11.1%) asymptomatic ICS. There were significantly different rates of ICS in the pooled prospective (including trials and population-based studies) versus retrospective data: 12.2% versus 10.8% (p = 0.01) any ICS, and 5.8% versus 9.0% (p < 0.0001) symptomatic ICS.
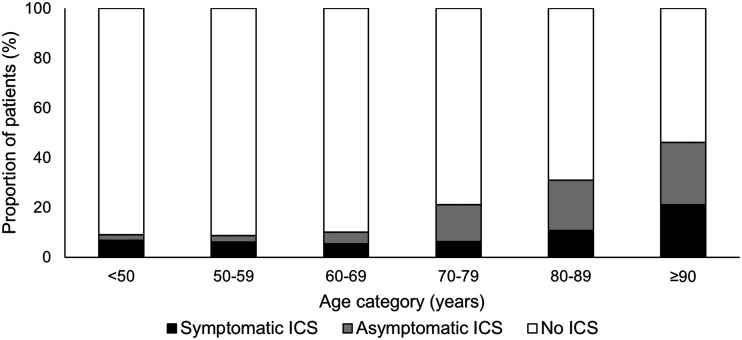
In an Oxford population-based study of 1368 Caucasian patients with TIA and minor ischemic stroke, 6.9% had symptomatic 50–99% ICS and this was heavily age dependent; increasing from 4.7% at <50 years to 19.6% at ≥90 years (Figure 3).55 In addition to being older, the patients with ICS had a higher burden of hypertension, diabetes mellitus, hyperlipidemia, atrial fibrillation, previous stroke, peripheral vascular disease, and ischemic heart disease.55
Figure 3.
Age-specific prevalence of any symptomatic, only asymptomatic and no intracranial stenosis in minor ischemic stroke and TIA patients in the Oxford Vascular Study.
There have been few studies of the prevalence of asymptomatic, “incidental” ICS in a stroke and TIA population. In a post hoc analysis of WASID, coexistent asymptomatic ICS was identified in a quarter of participants,56 and in one hospital-based study of 403 stroke patients admitted to a single French center the asymptomatic ICS rate was 18.4%.57 In the aforementioned Oxford population-based study, 202 patients (14.8%) had any asymptomatic ICS similarly increasing with age; from 3.8% at <50 years to 34.6% at ≥90 years. Of note in this study asymptomatic ICS were more common than asymptomatic extracranial internal carotid artery disease. Older age, hypertension, and prior stroke/TIA were independent predictors of any asymptomatic ICS.58
Intracranial stenosis in healthy participants
There are relatively few studies examining the prevalence of ICS in Caucasian patients without cerebrovascular disease. One large study of 1765 community-dwelling individuals estimated the US prevalence of ≥50% asymptomatic ICS for Caucasian 65–90 year olds as 8% using high-resolution MRA.59 The Barcelona-Asymptomatic Intracranial Atherosclerosis (AsIA) population-based study investigated 933 Spanish participants over the age of 50 years with transcranial color Doppler (TCCD) and reported a prevalence of moderate to severe ICS of 3.3%.60
Prognosis of intracranial stenosis
Mechanisms of stroke
The pathophysiology of infarction due to ICS is analogous to the mechanisms of extracranial internal carotid artery atherosclerosis-related infarction, and includes artery-to-artery embolism, in situ thrombo-occlusion, hypoperfusion due to subocclusive plaque and small perforating artery occlusion. The pattern of ischemia seen on neuroimaging can be suggestive of particular mechanisms. Border zone infarctions result from hypoperfusion due to a stenosed artery, territorial infarctions result from artery-to-artery embolism, and occlusion of small branching perforating arteries can cause subcortical strokes resembling lacunar infarcts.61 It is unclear whether the specific mechanism of ICS-related infarction has prognostic value, although in a post hoc analysis of the Stenting versus Aggressive Medical Therapy for Preventing Recurrent Stroke in Intracranial Stenosis (SAMMRPIS) trial data, patients with border zone infarctions were more likely to have poor collateral supply and were at the highest risk of recurrent stroke.62
Prognosis of intracranial stenosis
Symptomatic ICS had been considered to convey a high risk of recurrent ischemic stroke; the SAMMPRIS trial sample size estimates were based on a primary endpoint rate of 29% at two years for comparable medically treated patients in WASID.17 The observed two-year primary endpoint rate in medically treated SAMMPRIS participants, 70% of who were Caucasian, was 14.1%, attributed to the more intensive secondary prevention therapy and lifestyle interventions.8,63
Our systematic review identified 29 studies that reported the prognosis of ICS in Caucasian minor stroke and TIA patients (Table 2). Of these, 19 (65.5%) reported the mean duration of patient follow-up and number of patients with recurrent ischemic stroke. In these studies, there were 89 (95% confidence interval (CI) = 74–108) any-territory ischemic strokes in 1000 person-years and 61 (95% CI = 52–71) same-territory ischemic strokes in 1000 person-years in patients with symptomatic ICS, and 10 (95% CI = 6–19) same-territory ischemic strokes in 1000 patient-years in patients with asymptomatic ICS.
There are few studies of ICS prognosis in Caucasian patients without cerebrovascular disease. One Spanish community-based cohort of 80 stroke-free participants with a high burden of vascular risk factors reported a rate of 2.9% and 12.6% of ischemic stroke and any vascular event/vascular death respectively during seven years follow-up.64 Intracranial carotid artery calcification volume (ICAC) was used as a surrogate marker of ICS in a sample population of the Rotterdam study, a population-based study of predominantly Caucasian community-dwelling individuals. The study included 2,323 stroke-free individuals of mean age 70 years; during 14,055 person-years of follow-up, 74 (3.2%) had an ischemic stroke and a larger ICAC volume was associated with a higher risk of stroke, independent of vascular risk factors.65
Treatment of intracranial stenosis
Medical management
Antiplatelet therapy is the principle antithrombotic treatment for patients with symptomatic ICS since the WASID trial demonstrated no benefit of warfarin over aspirin and higher rates of major hemorrhage in the warfarin arm.66 However, the role of direct oral anticoagulants has yet to be examined in patients with symptomatic ICS, and the combination of rivaroxaban and aspirin has been shown to reduce stroke risk in patients with systemic atherosclerosis.67
The optimal antiplatelet regime for treatment of recently symptomatic ICS has not been investigated by randomized trials. The Platelet-Oriented Inhibition in New TIA and Minor Ischemic Stroke (POINT) and Clopidogrel in High-Risk Patients with Acute Nondisabling Cerebrovascular Events (CHANCE) trials showed short-term dual antiplatelet therapy (DAPT) to be safe and effective in patients with high-risk TIA and minor ischemic stroke,68,69 and a subgroup analysis of CHANCE showed greater benefit in patients with symptomatic or asymptomatic ICS.70 A pooled analysis of these trials showed that the greatest benefits in stroke risk reduction were in the first 21 days,71 with longer term DAPT shown to increase the risk of major hemorrhage in the Management of Atherothrombosis with Clopidogrel in High-Risk Patients (MATCH)72 and Clopidogrel for High Atherothrombotic Risk and Ischemic Stabilization, Management, and Avoidance (CHARISMA)73 trials.
Short-term DAPT was used in the SAMMPRIS trial which reported a lower rate of recurrent ischemic stroke than expected based on the older WASID trial.66 Based on this, the American Heart Association/American Stroke Association (AHA/ASA) secondary stroke prevention guidelines state that treatment of recently (within 30 days) symptomatic 70–99% ICS with dual antiplatelet therapy for 90 days might be reasonable.74 However, the independent contribution of the antiplatelet regimen is unclear as the SAMMPRIS treatment protocol also included intensive risk factor management and lifestyle advice.63
Alternative antiplatelet agents, such as ticagrelor or prasugrel, may be more efficacious in patients with symptomatic atherosclerosis, particularly in cases of clopidogrel resistance. In a subgroup analysis of the Acute Stroke or Transient Ischemic Attack Treated with Aspirin or Ticagrelor and Patient Outcomes (SOCRATES) trial, ticagrelor was superior to aspirin in prevention of vascular events or death 90 days in patients with acute ischemic stroke or TIA due to ipsilateral extra- or ICS.75
Management of the primary risk factors for atherosclerosis (elevated blood pressure, poor glycemic control, and elevated low-density lipoprotein (LDL) cholesterol) has been shown to be effective secondary prevention of ischemic stroke of any etiology.76,77 Evidence in patients with symptomatic ICS is derived indirectly from randomized trials. Risk factor management in WASID was not standardized, but subgroup analysis revealed improved outcomes in patients with a mean systolic blood pressure <140 mmHg,78 total mean cholesterol < 200 mg/dL79 and HbA1c of <7%.80 These findings were the basis of the intensive medical management protocol of SAMMPRIS, which aimed for systolic blood pressure <140 mmHg (or <130 mmHg in patients with diabetes mellitus), an LDL cholesterol level <70 mg/dL (1.81 mmol/L) and HbA1c of <7%.63 The Treat Stroke to Target (TST) trial recently confirmed this LDL target to be more effective at reducing recurrent vascular events than patients with a target of 90–110 mg/dL (2.3–2.8 mmol/L).81 In addition, SAMMPRIS employed a lifestyle modification program for increased physical activity, optimized nutrition, and weight loss and smoking cessation advice.80
There are no randomized trials informing the management of asymptomatic or remotely symptomatic ICS. As described previously, they can be a common finding in older patients with cerebrovascular disease and in those with vascular risk factors.58,60 The risk of recurrent ischemic stroke in patients found to have incidental, asymptomatic ICS is low, and management should follow standard secondary prevention guidelines.82
Endovascular therapy
Until the Food and Drug Administration approved the self-expanding Wingspan stent (Stryker Neurovascular) for treatment of recently symptomatic 50–99% ICS, there had only been published case series demonstrating high periprocedural complication rates.83 SAMMPRIS commenced shortly after the approval and randomized patients with 70–99% recently (within 30 days) symptomatic ICS (TIA or minor ischemic stroke) to percutaneous transluminal angioplasty and stenting (PTAS) with the Wingspan stent and intensive medical management or intensive medical management alone.17
Recruitment to SAMMPRIS was stopped early due to a significantly higher rate of post-procedure stroke (due to perforating vessel occlusion) or death; 14% versus 6% in the non-stenting arm.17 Post hoc analyses concluded that the higher degrees of ICS and earlier treatment windows (compared to the previous Wingspan registries), but not operator experience, may have increased this periprocedural risk.84,85 Furthermore, an old infarct in the territory of the ICS on baseline imaging, a new stroke presentation, and the absence of statin use at enrollment were independently associated with a high risk of recurrent stroke.86 There were no risk differences between Caucasian and Black patients or other subgroups in a preplanned sensitivity analysis.87
The Vitesse Intracranial Stent Study for Ischemic Stroke Therapy (VISSIT) trial started shortly after SAMMPRIS and had a similar protocol and patient mix (70% Caucasian), with the exception of investigating the PHAROS Vitesse balloon-expandable stent (Codman Neurovascular). As with SAMMPRIS, VISSIT was stopped early as the 30-day rate of ischemic stroke or TIA was higher in the intervention arm (24.1% vs. 9.4%), and at one year, 36.2% in the stent group had a stroke or TIA, versus 15.1% in the non-stenting group.9
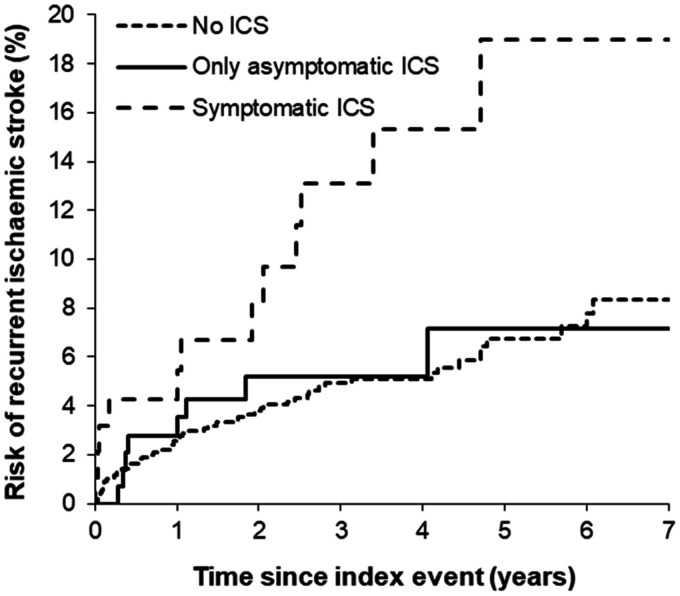
Aside from the high procedural risks, SAMMPRIS and VISST were criticized for the lower than expected rates of recurrent stroke in the non-stenting arms and the relatively young cohort (mean age < 60 years). However, a validation study of symptomatic ICS prognosis in an older, population-based TIA and minor stroke cohort confirmed a low rate of recurrent stroke on intensively treated medical patients (Figure 4; one-year risk of recurrent ischemic stroke 5.6%).55
Figure 4.
Kaplan–Meier graph showing the seven-year risks of recurrent ischemic stroke in minor ischemic stroke/ TIA patients with 50–99% symptomatic, asymptomatic, or no intracranial stenosis in the Oxford Vascular Study.
Patients with recently symptomatic posterior circulation ICS are at particularly high risk of early recurrent stroke.88 The Vertebral Artery Ischaemia Stenting Trial (VIST) sought to compare vertebral artery (VA) PTAS and medical management with medical treatment alone for recently symptomatic extra- or intracranial VA stenosis, but was stopped after 182 participants because of slow recruitment. Although underpowered, there were no significant differences in outcome between arms in patients with intracranial VA stenosis, but overall a nonsignificant 60% lower risk of recurrent stroke in the PTAS arm during a median follow-up of 3.5 years, driven by fewer complications in the extracranial VA stenting group.89
Although the Wingspan Stent System Post Market Surveillance (WEAVE) trial, has shown an improved periprocedural complication rate with Wingspan stents,90 current AHA/ASA guidelines do not recommend PTAS for patients with symptomatic ICS even if the event occurred while receiving antiplatelet therapy. For patients with recurrent events despite optimal medical treatment, the benefit of PTAS is unclear and should be considered investigational.82
Improved patient selection may improve the safety and efficacy of PTAS in symptomatic ICS. The ongoing China Angioplasty and Stenting for Symptomatic Intracranial Severe Stenosis (CASSISS) trial is comparing best medical therapy with/without PTAS in patients with 70–99% symptomatic ICS. The investigators exclude patients with perforator stroke without MRI appearances of distal hypoperfusion or artery-to-artery embolism and delay stenting for three weeks following the index event in order to reduce periprocedural risks.91
Surgical therapy
The Extracranial to Intracranial (EC/IC) Bypass Study was an international, randomized controlled trial which failed to show the superiority of arterial bypass (superficial temporal artery to the MCA) and medical therapy over medical therapy alone in patients with extracranial carotid occlusion, intracranial carotid, or MCA stenosis.92 The procedure is no longer routinely performed for symptomatic atherosclerotic ICS but the indirect revascularization technique, encephaloduroarteriosynangiosis (EDAS), is of emerging interest.93
Conclusion and future directions
ICS is a highly prevalent cause of stroke worldwide with important ethnic disparities. ICS has previously been neglected as a cause of stroke in Caucasians but is highly prevalent at older ages and frequently discovered with the growing use of non-invasive angiography. Intensive medical therapy, including antiplatelet medication, risk factor control, and lifestyle advice, is the treatment of choice. However, a subgroup of patients with ICS experience recurrent ischemic stroke despite medical therapy. Future research should aim at establishing standard approaches to detecting ICS, elucidating the ethnic differences in risk and developing biomarkers to identify high-risk patients. Furthermore, there is a need to develop novel treatments or therapeutic approaches to lower the risk of stroke in these higher risk patients.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Robert Hurford https://orcid.org/0000-0002-4226-7681
References
- 1.Kolominsky-Rabas PL, Weber M, Gefeller O, Neundoerfer B, Heuschmann PU. Epidemiology of ischemic stroke subtypes according to TOAST criteria: incidence, recurrence, and long-term survival in ischemic stroke subtypes: a population-based study. Stroke 2001; 32: 2735–2740. [DOI] [PubMed] [Google Scholar]
- 2.Sacco RL, Kargman DE, Gu Q, Zamanillo MC. Race-ethnicity and determinants of intracranial atherosclerotic cerebral infarction. The Northern Manhattan Stroke Study. Stroke 1995; 26: 14–20. [DOI] [PubMed] [Google Scholar]
- 3.Wityk RJ, Lehman D, Klag M, Coresh J, Ahn H, Litt B. Race and sex differences in the distribution of cerebral atherosclerosis. Stroke 1996; 27: 1974–1980. [DOI] [PubMed] [Google Scholar]
- 4.Huang YN, Gao S, Li SW, et al. Vascular lesions in Chinese patients with transient ischemic attacks. Neurology 1997; 48: 524–525. [DOI] [PubMed] [Google Scholar]
- 5.Liu HM, Tu YK, Yip PK, Su CT. Evaluation of intracranial and extracranial carotid steno-occlusive diseases in Taiwan Chinese patients with MR angiography: preliminary experience. Stroke 1996; 27: 650–653. [DOI] [PubMed] [Google Scholar]
- 6.Wong KS, Li H. Long-term mortality and recurrent stroke risk among Chinese stroke patients with predominant intracranial atherosclerosis. Stroke 2003; 34: 2361–2366. [DOI] [PubMed] [Google Scholar]
- 7.Suwanwela NC, Chutinetr A. Risk factors for atherosclerosis of cervicocerebral arteries: intracranial versus extracranial. Neuroepidemiology 2003; 22: 37–40. [DOI] [PubMed] [Google Scholar]
- 8.Derdeyn CP, Chimowitz MI, Lynn MJ, et al. Aggressive medical treatment with or without stenting in high-risk patients with intracranial artery stenosis (SAMMPRIS): the final results of a randomised trial. Lancet 2014; 383: 333–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zaidat OO, Fitzsimmons B-F, Woodward BK, et al. Effect of a balloon-expandable intracranial stent vs medical therapy on risk of stroke in patients with symptomatic intracranial stenosis: the VISSIT randomized clinical trial. JAMA 2015; 313: 1240–1248. [DOI] [PubMed] [Google Scholar]
- 10.Qureshi AI, Caplan LR. Intracranial atherosclerosis. Lancet 2014; 383: 984–998. http://www.sciencedirect.com/science/article/pii/S0140673613610880. [DOI] [PubMed] [Google Scholar]
- 11.Bang OY, Toyoda K, Arenillas JF, Liu L, Kim JS. Intracranial large artery disease of non-atherosclerotic origin: recent progress and clinical implications. J Stroke 2018; 20: 208–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Samuels OB, Joseph GJ, Lynn MJ, Smith HA, Chimowitz MI. A standardized method for measuring intracranial arterial stenosis. AJNR Am J Neuroradiol 2000; 21: 643–646. [PMC free article] [PubMed] [Google Scholar]
- 13.Bash S, Villablanca JP, Jahan R, et al. Intracranial vascular stenosis and occlusive disease: evaluation with CT angiography, MR angiography, and digital subtraction angiography. AJNR Am J Neuroradiol 2005; 26: 1012–1021. [PMC free article] [PubMed] [Google Scholar]
- 14.Huang J, Degnan AJ, Liu Q, et al. Comparison of NASCET and WASID criteria for the measurement of intracranial stenosis using digital subtraction and computed tomography angiography of the middle cerebral artery. J Neuroradiol 2012; 39: 342–345. [DOI] [PubMed] [Google Scholar]
- 15.Baumgartner RW, Mattle HP, Schroth G. Assessment of >/=50% and <50% intracranial stenoses by transcranial color-coded duplex sonography. Stroke 1999; 30: 87–92. [DOI] [PubMed] [Google Scholar]
- 16.Kasner SE, Chimowitz MI, Lynn MJ, et al. Predictors of ischemic stroke in the territory of a symptomatic intracranial arterial stenosis. Circulation 2006; 113: 555–563. [DOI] [PubMed] [Google Scholar]
- 17.Chimowitz MI, Lynn MJ, Derdeyn CP, et al. Stenting versus aggressive medical therapy for intracranial arterial stenosis. N Engl J Med 2011; 365: 993–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barr JD. Cerebral angiography in the assessment of acute cerebral ischemia: guidelines and recommendations. J Vasc Interv Radiol 2004; 15: S57–S66. [DOI] [PubMed] [Google Scholar]
- 19.Willinsky RA, Taylor SM, TerBrugge K, Farb RI, Tomlinson G, Montanera W. Neurologic complications of cerebral angiography: prospective analysis of 2,899 procedures and review of the literature. Radiology 2003; 227: 522–528. [DOI] [PubMed] [Google Scholar]
- 20.Kaufmann TJ, Huston 3rd, J, Mandrekar JN, Schleck CD, Thielen KR, Kallmes DF. Complications of diagnostic cerebral angiography: evaluation of 19,826 consecutive patients. Radiology 2007; 243: 812–819. [DOI] [PubMed] [Google Scholar]
- 21.Mturi N, Alcock K, Carter JA, Newton CR, Lange JH, LaPorte RE, Talbott EO, Chang YF, Monsurrò MR, Aiello I, Morgante L. Stroke outcome and neuroimaging of intracranial atherosclerosis (SONIA): design of a prospective, multicenter trial of diagnostic tests. Neuroepidemiology 2004; 23: 23–32. [DOI] [PubMed] [Google Scholar]
- 22.Feldmann E, Wilterdink JL, Kosinski A, et al. The Stroke Outcomes and Neuroimaging of Intracranial Atherosclerosis (SONIA) trial. Neurology 2007; 68: 2099–2106. [DOI] [PubMed] [Google Scholar]
- 23.Nguyen-Huynh MN, Wintermark M, English J, et al. How accurate is CT angiography in evaluating intracranial atherosclerotic disease?. Stroke 2008; 39: 1184–1188. [DOI] [PubMed] [Google Scholar]
- 24.Roubec M, Kuliha M, Jonszta T, et al. Detection of intracranial arterial stenosis using transcranial color-coded duplex sonography, computed tomographic angiography, and digital subtraction angiography. J Ultrasound Med 2011; 30: 1069–1075. [DOI] [PubMed] [Google Scholar]
- 25.Duffis EJ, Jethwa P, Gupta G, Bonello K, Gandhi CD, Prestigiacomo CJ. Accuracy of computed tomographic angiography compared to digital subtraction angiography in the diagnosis of intracranial stenosis and its impact on clinical decision-making. J Stroke Cerebrovasc Dis 2013; 22: 1013–1017. [DOI] [PubMed] [Google Scholar]
- 26.Skutta B, Fürst G, Eilers J, Ferbert A, Kuhn FP. Intracranial stenoocclusive disease: double-detector helical CT angiography versus digital subtraction angiography. AJNR Am J Neuroradiol 1999; 20: 791–799. [PMC free article] [PubMed] [Google Scholar]
- 27.Marquering HA, Nederkoorn PJ, Bleeker L, van den Berg R, Majoie CB. Intracranial carotid artery disease in patients with recent neurological symptoms: high prevalence on CTA. Neuroradiology 2013; 55: 179–185. [DOI] [PubMed] [Google Scholar]
- 28.Saba L, Sanfilippo R, Montisci R, Mallarini G. Assessment of intracranial arterial stenosis with multidetector row CT angiography: a postprocessing techniques comparison. AJNR Am J Neuroradiol 2010; 31: 874–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hirai T, Korogi Y, Ono K, et al. Prospective evaluation of suspected stenoocclusive disease of the intracranial artery: combined MR angiography and CT angiography compared with digital subtraction angiography. AJNR Am J Neuroradiol 2002; 23: 93–101. [PMC free article] [PubMed] [Google Scholar]
- 30.Choi CG, Lee DH, Lee JH, et al. Detection of intracranial atherosclerotic steno-occlusive disease with 3D time-of-flight magnetic resonance angiography with sensitivity encoding at 3T. AJNR Am J Neuroradiol 2007; 28: 439–446. [PMC free article] [PubMed] [Google Scholar]
- 31.Korogi Y, Takahashi M, Mabuchi N, et al. Intracranial vascular stenosis and occlusion: diagnostic accuracy of three-dimensional, Fourier transform, time-of-flight MR angiography. Radiology 1994; 193: 187–193. [DOI] [PubMed] [Google Scholar]
- 32.Heiserman JE, Drayer BP, Keller PJ, Fram EK. Intracranial vascular stenosis and occlusion: evaluation with three-dimensional time-of-flight MR angiography. Radiology 1992; 185: 667–673. [DOI] [PubMed] [Google Scholar]
- 33.Nederkoorn PJ, Elgersma OEH, Mali WPTM, Eikelboom BC, Kappelle LJ, van der Graaf Y. Overestimation of carotid artery stenosis with magnetic resonance angiography compared with digital subtraction angiography. J Vasc Surg 2002; 36: 806–813. [PubMed] [Google Scholar]
- 34.Leclerc X, Gauvrit JY, Nicol L, Pruvo JP. Contrast-enhanced MR angiography of the craniocervical vessels: a review. Neuroradiology 1999; 41: 867–874. [DOI] [PubMed] [Google Scholar]
- 35.Yang CW, Carr JC, Futterer SF, et al. Contrast-enhanced MR angiography of the carotid and vertebrobasilar circulations. AJNR Am J Neuroradiol 2005; 26: 2095–2101. [PMC free article] [PubMed] [Google Scholar]
- 36.van den Wijngaard IR, Holswilder G, van Walderveen MAA, et al. Treatment and imaging of intracranial atherosclerotic stenosis: current perspectives and future directions. Brain Behav 2016; 6: e00536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wutke R, Lang W, Fellner C, et al. High-resolution, contrast-enhanced magnetic resonance angiography with elliptical centric k-space ordering of supra-aortic arteries compared with selective X-ray angiography. Stroke 2002; 33: 1522–1529. [DOI] [PubMed] [Google Scholar]
- 38.Willinek WA, von Falkenhausen M, Born M, et al. Noninvasive detection of steno-occlusive disease of the supra-aortic arteries with three-dimensional contrast-enhanced magnetic resonance angiography: a prospective, intra-individual comparative analysis with digital subtraction angiography. Stroke 2005; 36: 38–43. [DOI] [PubMed] [Google Scholar]
- 39.Liebeskind DS. Understanding blood flow: the other side of an acute arterial occlusion. Int J Stroke 2007; 2: 118–120. [DOI] [PubMed] [Google Scholar]
- 40.Liebeskind DS, Feldmann E. Fractional flow in cerebrovascular disorders. Interv Neurol 2013; 1: 87–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Arenillas JF. Intracranial atherosclerosis: current concepts. Stroke 2011; 42: S20–S23. [DOI] [PubMed] [Google Scholar]
- 42.Klein IF, Lavallee PC, Mazighi M, Schouman-Claeys E, Labreuche J, Amarenco P. Basilar artery atherosclerotic plaques in paramedian and lacunar pontine infarctions: a high-resolution MRI study. Stroke 2010; 41: 1405–1409. [DOI] [PubMed] [Google Scholar]
- 43.Chen XY, Wong KS, Lam WWM, Zhao H-L, Ng HK. Middle cerebral artery atherosclerosis: histological comparison between plaques associated with and not associated with infarct in a postmortem study. Cerebrovasc Dis 2008; 25: 74–80. [DOI] [PubMed] [Google Scholar]
- 44.Turan TN, Bonilha L, Morgan PS, Adams RJ, Chimowitz MI. Intraplaque hemorrhage in symptomatic intracranial atherosclerotic disease. J Neuroimag 2011; 21: e159–e161. [DOI] [PubMed] [Google Scholar]
- 45.Diethrich EB, Pauliina Margolis M, Reid DB, et al. Virtual histology intravascular ultrasound assessment of carotid artery disease: the Carotid Artery Plaque Virtual Histology Evaluation (CAPITAL) study. J Endovasc Ther Off J Int Soc Endovasc Spec 2007; 14: 676–686. [DOI] [PubMed] [Google Scholar]
- 46.Wong LKS. Global burden of intracranial atherosclerosis. Int J Stroke 2006; 1: 158–159. [DOI] [PubMed] [Google Scholar]
- 47.White H, Boden-Albala B, Wang C, et al. Ischemic stroke subtype incidence among whites, blacks, and Hispanics: the Northern Manhattan Study. Circulation 2005; 111: 1327–1331. [DOI] [PubMed] [Google Scholar]
- 48.Liao X, Zhang T, Li B, et al. Rare RNF213 variants and the risk of intracranial artery stenosis/occlusion disease in Chinese population: a case-control study. BMC Med Genet 2019; 20: 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kokubo Y. Prevention of hypertension and cardiovascular diseases: a comparison of lifestyle factors in Westerners and East Asians. Hypertension 2014; 63: 655–660. [DOI] [PubMed] [Google Scholar]
- 50.Kim JS, Bonovich D. Research on intracranial atherosclerosis from the East and west: why are the results different?. J Stroke 2014; 16: 105–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Forouhi NG, Sattar N. CVD risk factors and ethnicity—a homogeneous relationship?. Atheroscler Suppl 2006; 7: 11–19. [DOI] [PubMed] [Google Scholar]
- 52.Ritz K, Denswil NP, Stam OCG, van Lieshout JJ, Daemen MJAP. Cause and mechanisms of intracranial atherosclerosis. Circulation 2014; 130: 1407–1414. [DOI] [PubMed] [Google Scholar]
- 53.Kim JS, Kim Y-J, Ahn S-H, Kim BJ. Location of cerebral atherosclerosis: why is there a difference between East and West?. Int J Stroke 2018; 13: 35–46. [DOI] [PubMed] [Google Scholar]
- 54.D'Armiento FP, Bianchi A, de Nigris F, et al. Age-related effects on atherogenesis and scavenger enzymes of intracranial and extracranial arteries in men without classic risk factors for atherosclerosis. Stroke 2001; 32: 2472–2479. [DOI] [PubMed] [Google Scholar]
- 55.Hurford R, Wolters FJ, Li L, Lau KK, Küker W, Rothwell PM. Prevalence, predictors, and prognosis of symptomatic intracranial stenosis in patients with transient ischaemic attack or minor stroke: a population-based cohort study. Lancet Neurol 2020; 19: 413–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nahab F, Cotsonis G, Lynn M, et al. Prevalence and prognosis of coexistent asymptomatic intracranial stenosis. Stroke 2008; 39: 1039–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hoshino T, Sissani L, Labreuche J, et al. Prevalence of systemic atherosclerosis burdens and overlapping stroke etiologies and their associations with long-term vascular prognosis in stroke with intracranial atherosclerotic disease. JAMA Neurol 2018; 75: 203–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hurford R, Wolters FJ, Li L, Lau KK, Küker W, Rothwell PM. Prognosis of asymptomatic intracranial stenosis in patients with transient ischemic attack and minor stroke. JAMA Neurol 2020; 77: 947–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Suri MFK, Qiao Y, Ma X, et al. Prevalence of intracranial atherosclerotic stenosis using high-resolution magnetic resonance angiography in the general population: the atherosclerosis risk in communities study. Stroke 2016; 47: 1187–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lopez-Cancio E, Dorado L, Millan M, et al. The Barcelona-asymptomatic intracranial atherosclerosis (AsIA) study: prevalence and risk factors. Atherosclerosis 2012; 221: 221–225. [DOI] [PubMed] [Google Scholar]
- 61.Feng X, Chan KL, Lan L, et al. Stroke mechanisms in symptomatic intracranial atherosclerotic disease: classification and clinical implications. Stroke 2019; 50: 2692–2699. [DOI] [PubMed] [Google Scholar]
- 62.Wabnitz AM, Derdeyn CP, Fiorella DJ, et al. Hemodynamic markers in the anterior circulation as predictors of recurrent stroke in patients with intracranial stenosis. Stroke 2018. STROKEAHA118020840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chaturvedi S, Turan TN, Lynn MJ, et al. Do patient characteristics explain the differences in outcome between medically treated patients in SAMMPRIS and WASID?. Stroke 2015; 46: 2562–2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Planas-Ballve A, Crespo AM, Aguilar LM, et al. The Barcelona-asymptomatic intracranial atherosclerosis study: subclinical intracranial atherosclerosis as predictor of long-term vascular events. Atherosclerosis 2019; 282: 132–136. [DOI] [PubMed] [Google Scholar]
- 65.Bos D, Portegies MLP, van der Lugt A, et al. Intracranial carotid artery atherosclerosis and the risk of stroke in whites: the Rotterdam study. JAMA Neurol 2014; 71: 405–411. [DOI] [PubMed] [Google Scholar]
- 66.Chimowitz MI, Lynn MJ, Howlett-Smith H, et al. Comparison of warfarin and aspirin for symptomatic intracranial arterial stenosis. N Engl J Med 2005; 352: 1305–1316. [DOI] [PubMed] [Google Scholar]
- 67.Sharma M, Hart RG, Connolly SJ, et al. Stroke outcomes in the COMPASS trial. Circulation 2019; 139: 1134–1145. [DOI] [PubMed] [Google Scholar]
- 68.Wang Y, Wang Y, Zhao X, et al. Clopidogrel with aspirin in acute minor stroke or transient ischemic attack. N Engl J Med 2013; 369: 11–19. [DOI] [PubMed] [Google Scholar]
- 69.Johnston SC, Easton JD, Farrant M, et al. Clopidogrel and aspirin in acute ischemic stroke and high-risk TIA. N Engl J Med 2018; 379: 215–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liu L, Wong KSL, Leng X, et al. Dual antiplatelet therapy in stroke and ICAS: subgroup analysis of CHANCE. Neurology 2015; 85: 1154–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pan Y, Elm JJ, Li H, et al. Outcomes associated with clopidogrel-aspirin use in minor stroke or transient ischemic attack: a pooled analysis of clopidogrel in high-risk patients with acute non-disabling cerebrovascular events (CHANCE) and platelet-oriented inhibition in new TIA and minor ischemic stroke (POINT) trials. JAMA Neurol 2019; 76: 1466–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Diener H-C, Bogousslavsky J, Brass LM, et al. Aspirin and clopidogrel compared with clopidogrel alone after recent ischaemic stroke or transient ischaemic attack in high-risk patients (MATCH): randomised, double-blind, placebo-controlled trial. Lancet 2004; 364: 331–337. [DOI] [PubMed] [Google Scholar]
- 73.Bhatt DL, Fox KAA, Hacke W, et al. Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events. N Engl J Med 2006; 354: 1706–1717. [DOI] [PubMed] [Google Scholar]
- 74.Kernan WN, Ovbiagele B, Black HR, et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2014; 45: 2160–2236. [DOI] [PubMed] [Google Scholar]
- 75.Amarenco P, Albers GW, Denison H, et al. Efficacy and safety of ticagrelor versus aspirin in acute stroke or transient ischaemic attack of atherosclerotic origin: a subgroup analysis of SOCRATES, a randomised, double-blind, controlled trial. Lancet Neurol 2017; 16: 301–310. [DOI] [PubMed] [Google Scholar]
- 76.PROGRESS Collaborative Group. Randomised trial of a perindopril-based blood-pressure-lowering regimen among 6,105 individuals with previous stroke or transient ischaemic attack. Lancet 2001; 358: 1033–1041. . [DOI] [PubMed] [Google Scholar]
- 77.Amarenco P, Bogousslavsky J, Callahan A, 3rd, et al. High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med 2006; 355: 549–559. [DOI] [PubMed] [Google Scholar]
- 78.Turan TN, Cotsonis G, Lynn MJ, Chaturvedi S, Chimowitz M. Relationship between blood pressure and stroke recurrence in patients with intracranial arterial stenosis. Circulation 2007; 115: 2969–2975. [DOI] [PubMed] [Google Scholar]
- 79.Chaturvedi S, Turan TN, Lynn MJ, et al. Risk factor status and vascular events in patients with symptomatic intracranial stenosis. Neurology 2007; 69: 2063–2068. [DOI] [PubMed] [Google Scholar]
- 80.Turan TN, Lynn MJ, Nizam A, et al. Rationale, design, and implementation of aggressive risk factor management in the Stenting and Aggressive Medical Management for Prevention of Recurrent Stroke in Intracranial Stenosis (SAMMPRIS) trial. Circ Cardiovasc Qual Outcomes 2012; 5: e51–e60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Amarenco P, Kim JS, Labreuche J, et al. A comparison of two LDL cholesterol targets after ischemic stroke. N Engl J Med 2020; 382: 9. [DOI] [PubMed] [Google Scholar]
- 82.Kernan WN, Ovbiagele B, Black HR, et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack. Stroke 2014; 45: 2160–2236. [DOI] [PubMed] [Google Scholar]
- 83.Cruz-Flores S, Diamond AL. Angioplasty for intracranial artery stenosis. Cochrane Database Syst Rev 2006, pp. CD004133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Derdeyn CP, Fiorella D, Lynn MJ, et al. Impact of operator and site experience on outcomes after angioplasty and stenting in the SAMMPRIS trial. J Neurointerv Surg 2013; 5: 528–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Banerjee C, Chimowitz MI. Stroke caused by atherosclerosis of the major intracranial arteries. Circ Res 2017; 120: 502–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Waters MF, Hoh BL, Lynn MJ, et al. Factors associated with recurrent ischemic stroke in the medical group of the SAMMPRIS trial. JAMA Neurol 2016; 73: 308–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lutsep HL, Lynn MJ, Cotsonis GA, et al. Does the stenting versus aggressive medical therapy trial support stenting for subgroups with intracranial stenosis?. Stroke 2015; 46: 3282–3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gulli G, Marquardt L, Rothwell PM, Markus HS. Stroke risk after posterior circulation stroke/transient ischemic attack and its relationship to site of vertebrobasilar stenosis: pooled data analysis from prospective studies. Stroke 2013; 44: 598–604. [DOI] [PubMed] [Google Scholar]
- 89.Markus HS, Larsson SC, Kuker W, et al. VIST Investigators. Stenting for symptomatic vertebral artery stenosis: the Vertebral Artery Ischaemia Stenting Trial. Neurology 2017; 89: 1229–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Alexander MJ, Zauner A, Chaloupka JC, et al. WEAVE trial: final results in 152 on-label patients. Stroke 2019; 50: 889–894. [DOI] [PubMed] [Google Scholar]
- 91.Gao P, Zhao Z, Wang D, et al. China Angioplasty and Stenting for Symptomatic Intracranial Severe Stenosis (CASSISS): a new, prospective, multicenter, randomized controlled trial in China. Interv Neuroradiol 2015; 21: 196–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.EC/IC Bypass Study Group*. Failure of extracranial-intracranial arterial bypass to reduce the risk of ischemic stroke. Results of an international randomized trial. N Engl J Med 1985; 313: 1191–1200. . [DOI] [PubMed] [Google Scholar]
- 93.Gonzalez NR, Dusick JR, Connolly M, et al. Encephaloduroarteriosynangiosis for adult intracranial arterial steno-occlusive disease: long-term single-center experience with 107 operations. J Neurosurg 2015; 123: 654–661. [DOI] [PubMed] [Google Scholar]