Abstract
Apoptosis, programmed cell death, is a critical component of neurodevelopment occurring in temporal, spatial, and at times, sex-specific, patterns across the cortex during the early postnatal period. During this time, the brain is particularly susceptible to environmental influences that are often used in animal models of neurodevelopmental disorders. In the present study, the timing of peak cell death was assessed by the presence of pyknotic cells in the male and female rat medial prefrontal cortex (mPFC), a cortical region that in humans, is often involved in developmental disorders. One male and one female rat per litter were sacrificed at the following ages: postnatal day (P)2, 4, 6, 8, 10, 12, 14, 16, 18, and 25. The mPFC was Nissl-stained, the densities of pyknotic cells and live neurons were stereologically collected, and the number of pyknotic cells per 100 live neurons, pyknotic cell density, and neuron density were analyzed. Males and females showed a significant peak in the ratio of pyknotic to live neurons on P8, and in females, this elevation persisted through P12. Likewise, the density of pyknotic cells peaked on P8 in both sexes and persisted through P12 in females. The timing of cell death within the rat mPFC will inform study design in experiments that employ early environmental manipulations that might disrupt this process.
Keywords: Apoptosis, Pyknosis, MPFC, Neurodevelopment
Highlights
-
•
The number of pyknotic cells per live neuron was quantified.
-
•
Postnatal cell death peaked on P8 in the male rat medial prefrontal cortex.
-
•
In females, postnatal cell death peaked from P8 to P12.
1. Introduction
Apoptosis, a process of regulated cell death, is a critical component of early neurodevelopment in both rodents (Buss et al., 2006) and humans (Rakic and Zecevic, 2000) that occurs in spatially and temporally distinct patterns across the cortex. Evidence from rodent studies suggests two distinct waves of apoptosis (Blaschke et al., 1996). The first occurs embryonically, with the highest density of apoptotic cells observed on embryonic day 16, specifically within proliferative zones (Thomaidou et al., 1997). The second surge in apoptosis occurs just after birth during the first two postnatal weeks (Ferrer et al., 1990, Mosley et al., 2017, Nuñez et al., 2001, Spreafico et al., 1995, Verney et al., 2000). As an important developmental process, postnatal cortical apoptosis contributes to neural circuit formation (Forger, 2009) and disrupting this process, for example, as a result of maternal separation stress results in altered neuron number and behaviors observed in adolescence (Majcher-Maślanka et al., 2019). Apoptosis in the cortex can also be influenced by gonadal hormones (Nuñez et al., 2000). This has been well-established in hypothalamic nuclei where differing rates of apoptosis between the sexes, influenced by perinatal gonadal hormone environment, lead to sexual dimorphisms (Forger, 2009).
While the presence of postnatal apoptosis has been established in the cortex, the rates and timing of this process can vary by region. For example, programmed cell death in the rat somatosensory cortex reaches the highest levels between postnatal days (P)5-8 (Ferrer et al., 1990, Spreafico et al., 1995), and a similar rise during the first postnatal week is seen in mice (Verney et al., 2000). Our laboratory has quantified apoptosis in the visual cortex of rats of both sexes, finding distinct timing of elevated levels in males compared to females (Nuñez et al., 2001). To quantify apoptosis, both the ratio of pyknotic cells, cells displaying condensed nuclear chromatin, and TUNEL-labeled cells, which are marked by fragmented DNA, to the number of live neurons were used and showed a similar pattern. Both measures were highest in males at P7 while females showed a smaller peak at P7, and additional peaks at P11 and P25. Moreover, this developmental process is promoted, at least in part, by androgens, not estrogen (Nuñez et al., 2000). It is therefore important to assess patterns of cortical cell death in both sexes.
The timing of cell death in the postnatal medial prefrontal cortex (mPFC) is unknown. The rodent mPFC is analogous to the primate dorsolateral prefrontal cortex (Uylings et al., 2003) and this region undergoes protracted development compared to other cortical regions in both rats (van Eden et al., 1991) and humans (Gogtay et al., 2004). The prefrontal cortex is involved in high-level cognition including decision-making, working memory, cognitive flexibility, and impulse control in both rodents and humans (Dalley et al., 2004, Euston et al., 2012, Uylings et al., 2003), and aberrant development of the prefrontal cortex is implicated in several psychiatric disorders in humans (Gunaydin and Kreitzer, 2016). Therefore, rodent models employing environmental manipulations during early postnatal development are commonly used to address the underlying neural changes that might underlie psychopathology. An understanding of the patterns of cell death during early development is critical for effective experimental design as manipulations (i.e. exposure to stress or toxicants) may produce their effects by acting on this process. Therefore, the present study quantified the number of pyknotic cells in the male and female rat mPFC across early postnatal development, between P2 and P25, to identify the periods of peak cell death.
2. Materials and methods
2.1. Animals
Male and female Long Evans rats were bred in the vivarium of the Psychology Department at the University of Illinois, and offspring were used as experimental subjects. Subjects were kept on a 12:12 h light-dark cycle and housed with the dam for the duration of the experiment. Dams were given ad libitum access to food (Harlan 2020x; Teklad Diets, Madison, WI) and water. All procedures adhered to the National Institute of Health guidelines on the ethical use of animals and were approved by the University of Illinois Institutional Animal Care and Use Committee.
2.2. Tissue collection
Day of birth was recorded as P0. One male and one female pup per litter were sacrificed at the following timepoints: P2, 4, 6, 8, 10, 12, 14, 16, 18, and 25. A total of 13 litters and 86 subjects were used resulting in 3–5 subjects per sex at each age (Table 1). On the appropriate postnatal day between 10:00 a.m. and 2:00 p.m., subjects were deeply anesthetized with sodium pentobarbital and then intracardially perfused with 0.1 M phosphate-buffered saline (PBS) followed by a fixative solution composed of 4% paraformaldehyde in PBS. Brains were extracted, left in the fixative solution for 24 h, transferred to a 30% sucrose solution in PBS for 3 days, and then cut into 40-micron coronal sections on a freezing microtome.
Table 1.
Number of subjects across age groups.
| P2 | P4 | P6 | P8 | P10 | P12 | P14 | P16 | P18 | P25 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Males | 4 | 4 | 4 | 5 | 5 | 5 | 4 | 5 | 3 | 4 |
| Females | 4 | 4 | 4 | 5 | 5 | 5 | 4 | 5 | 3 | 4 |
Both pyknotic cells and neurons can be visualized using a Nissl stain, which labels cell bodies and nuclear chromatin. Per subject, 2–3 sections of the mPFC between Bregma 4.2 mm and 3.00 mm (Paxinos and Watson, 2006) were selected. Sections were washed in 0.1 M PBS, mounted on electrostatically charged slides, and washed in 70% followed by 50% ethanol for 2 min each. Sections were then washed in 0.2 M PBS (7 mins), followed by periodic acid (5 mins) and stained with Methylene Blue/Azure II (3 mins) to label cell bodies (Lillie, 1997). Slides were rinsed with 0.2 M PBS (5 mins), 50% ethanol (2 mins), 70% ethanol (20 s), 95% ethanol (2 mins), and 100% ethanol (10 s) before being placed in CitriSolv (2 mins) and coverslipped with Permount.
2.3. Quantification
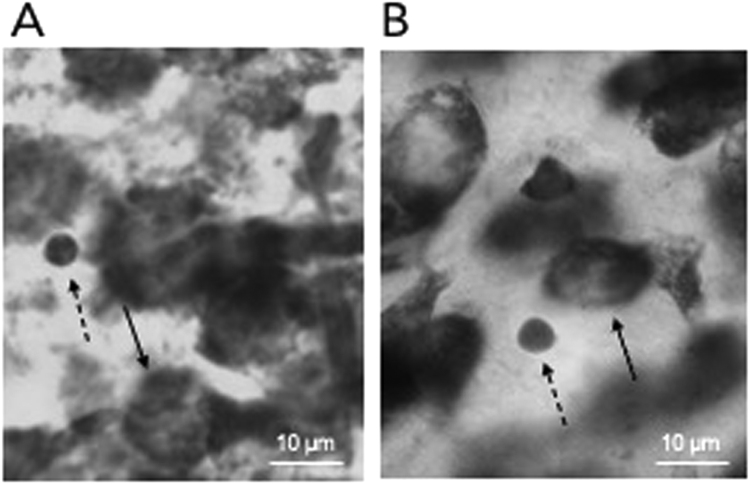
Apoptotic cells undergoing pyknosis, a stage of cell death when the nuclear chromatin becomes condensed, appear as small, symmetric, dark spheres with sharp boundaries in Nissl-stained tissue that can be readily identified by an experimenter (Ferrer et al., 1990) (Fig. 1). While apoptotic cells can also be visualized with terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining, which labels the 3′-hydroxyl end of nuclear DNA that forms when DNA fragmentation occurs in the final stage of apoptosis, both methods reveal similar patterns when quantifying cell death on the same cell populations (Ferrer et al., 1994, Nuñez et al., 2001). A Nissl stain allows for simultaneous quantification of neurons in the same tissue sections.
Fig. 1.
Pyknotic cells. A Nissl-stained section of layers 2/3 in the mPFC on P8 (A) and P25 (B) under 63x objective where the solid arrows point to neurons and the dashed arrows denote pyknotic cells. The scale bar denotes 10 micrometers.
Neurons and pyknotic cells within the mPFC were counted using similar methods to those previously described by our laboratory (Drzewiecki et al., 2020, Kougias et al., 2018, Willing and Juraska, 2015) using the StereoInvestigator optical disector, which allows for unbiased stereology. In the mature mPFC, the dorsal border is distinguished by a thinning layer I and increased cell density in layer III while the ventral is marked by a blurring of lamina borders. However, at most ages examined here, the lamina and boundaries are not clearly defined (van Eden and Uylings, 1985). Therefore, a conservative area was traced around the mPFC to include layers 2–6 of both infralimbic and prelimbic cortices. White matter was used as a guide in that parcellated regions did not extend above or below the dorsal and ventral edges of the white matter. Then Stereoinvestigator randomly and uniformly laid counting frames (40 µm × 40 µm × 10 µm depth) across the parcellated region. The counting frame had two “inclusion” edges and two “exclusion” edges. Neurons and pyknotic cells that came into focus and fell within the boundaries of the counting frame were counted, while those that fell outside the counting frame, touched the exclusion edges, or did not come into focus within the 10 µm depth were not. Both the number of pyknotic cells and number of live neurons per unit volume (40 µm × 40 µm × 10 µm × number of counting sites) were quantified and are reported here as pyknotic cell density and neuron density respectively. Because the density of all cells in the region are changing as cells die and dendrites grow, the ratio of pyknotic cells to 100 live cells was calculated (Nuñez et al., 2001).
2.4. Statistical analysis
Data were analyzed using RStudio statistical software. The sexes were analyzed separately in all measures. The densities of neurons and pyknotic cells as well as the ratio of pyknotic cells to live neurons were analyzed in an ANOVA on a mixed linear model (using the “lmer” package) using Satterthwaite’s method to estimate degrees of freedom and setting age as a fixed factor. As two experimenters performed the counts of pyknotic cells, the experimenter was included as a random factor along with litter. Significant main effects were investigated with post hoc pairwise-comparisons in which neighboring ages were compared and p values were adjusted using a Bonferroni correction (9 comparisons).
3. Results
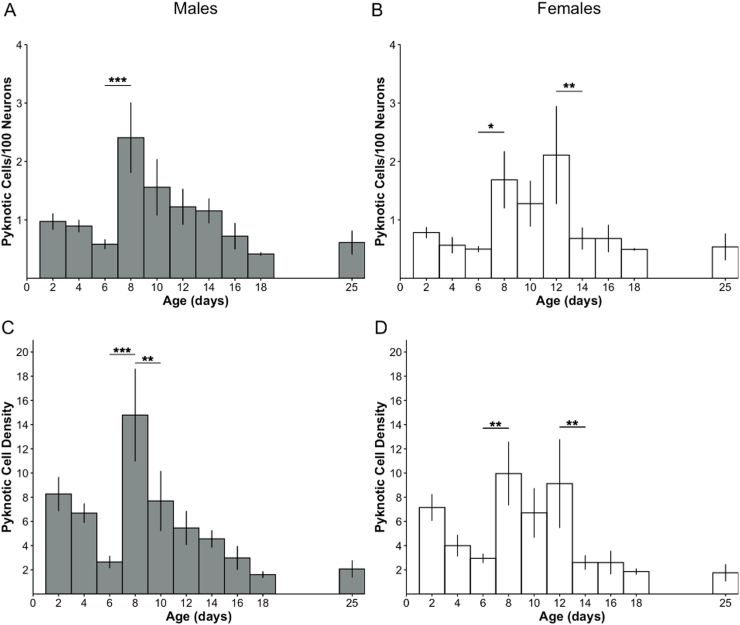
There was a main effect of age on the ratio of pyknotic cells per 100 live neurons, in males [F(9, 25.3) = 6.08, p < 0.0001] (Fig. 2A) and females [F(9, 26.7) = 3.15, p = 0.01] (Fig. 2B). P8 appeared as a peak, defined as a significant difference between ages, in both sexes as post hoc tests showed a significantly higher pyknotic cell ratio on P8 compared to P6 (p < 0.0001 in males; p = 0.049 in females). Females showed a prolonged peak through at P12 where the ratio of pyknotic cells to live neurons was significantly higher than that seen on P14 (p = 0.009).
Fig. 2.
Ratio of pyknotic cells to live neurons and pyknotic cell density. The ratio of the number of pyknotic cells per 100 live neurons, in male (A) and female (B) rats and the pyknotic cell density (number of pyknotic cells per unit volume (µm3 ×106)) in male (C) and female (D) rats across postnatal days 2–25. *p < 0.05; **p < 0.01; *** p < 0.001.
Pyknotic cell density (number of cells per unit volume) followed a similar pattern to that seen in the ratio of pyknotic cells to live neurons, with a main effect of age in males [F(9, 25.3) = 8.18, p < 0.001] (Fig. 2C) and females [F(9, 26.7) = 4.17, p = 0.002] (Fig. 2D). In males, P8 again appeared as a peak with post hoc tests showing a significantly higher density of pyknotic cells compared to that seen on neighboring ages, P6 (p < 0.0001) and P10 (p = 0.004). In females, a longer relative peak again emerged where pyknotic cell density was significantly higher on P8 compared to P6 (p = 0.003) with no significant change between P8 and P10 nor between P10 and P12 before decreasing significantly between P12 and P14 (p = 0.007).
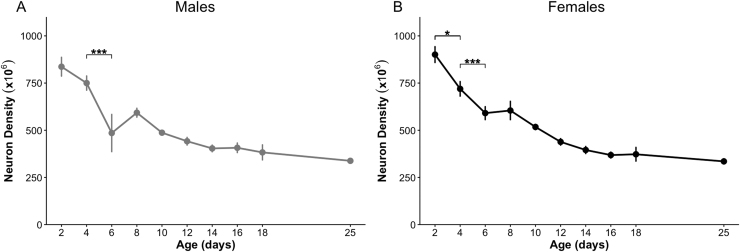
Neuron density decreased steadily from a maximum at P2 through P25, revealing a significant effect of age in both males [F(9, 32.1) = 18.5, p < 0.001] and females [F(9, 33) = 34.6, p < 0.001] (Fig. 3). Post hoc tests confirm a significant decrease in neuron density between P4 and P6 in both males (p < 0.001) and females (p < 0.001). Females showed an additional significant drop between P2 and P4 (p = 0.03).
Fig. 3.
Neuron density. Neuron density as the number of neurons per unit volume (µm3 ×106) in the male (A) and female (B) mPFC from ages P2 through P25. *p < 0.05; ***p < 0.001.
4. Discussion
We found elevated levels of pyknotic cells in both the male and female mPFC during the first two postnatal weeks, similar to levels of cell death seen in other cortical regions. We expect the pattern of pyknotic cell density represents apoptosis occurring in the cortex at this time as several studies have shown similar patterns of cell death using Nissl-stained pyknotic cell detection, as we do here, compared to TUNEL detection methods (Ferrer et al., 1994, Nuñez et al., 2001). Both the number of pyknotic cells per 100 live neurons, which accounts for changing neuron density during this time, and the pyknotic cell density peaked at P8 in both sexes. In males, these measures fell two days later, on P10, whereas females displayed a continued elevation through P12. The observed decrease in neuron density in both sexes across this early postnatal period is driven primarily by extensive dendritic growth and synapse formation, which has been previously reported in the visual cortex during the first two postnatal weeks (Juraska and Fifková, 1979, Miller, 1981).
The pattern of cell death observed in the mPFC closely aligned with that previously reported by our laboratory in the visual cortex, where one main peak in apoptosis appears in males on P7 (Nuñez et al., 2001). In the female visual cortex, however, two relative peaks are seen during the first two postnatal weeks on P7 and P11. Comparatively, in the mPFC, we did not observe two distinct peaks in the ratio of pyknotic cells to live neurons but instead saw an elevation on P8 that persisted until P12, after which levels dropped. Additionally, unlike the visual cortex however, there did not appear to be a relative peak in cell death on P25 in the female mPFC. While it appears that testosterone, not estrogen, mediates the first peak in cell death in the male visual cortex, it is unknown whether this mechanism generalizes to other cortical regions, like the mPFC, given the regional difference in androgen receptor expression (Nuñez et al., 2003). Additionally, the mechanism behind the later peaks seen in females is not known.
In order to model human neurodevelopmental disorders including those linked to early life experience (such as exposure to environmental chemicals or stress), knowledge of the rate and timing of critical processes like apoptosis is important (Majcher-Maślanka et al., 2019). While data from human cortical tissue is limited, examination of frontal cortex indicates that apoptosis continues through gestational week 32, the oldest age assessed (Chan and Yew, 1998). By examining neuronal density and estimating approximate cortical volume (Rabinowicz et al., 1996), calculations showed that there were more cells in the human cerebral cortex at weeks 28–32 of gestation than at postnatal weeks 0–13 by about 70%, indicating considerable cell death at the end of gestation. The developmental timeline differs between rats and humans with rats born significantly more immature, so the timing of neurodevelopment occurring during the third trimester in humans roughly corresponds to the first 10 postnatal days in the rat (Semple et al., 2013). Therefore, the postnatal rise in cortical apoptosis observed in the rat in the present and past studies (Ferrer et al., 1994; Nuñez et al., 2001; Spreafico et al., 1995) may represent a comparable event to that observed in humans during late gestation.
4.1. Conclusions
In this study, we quantified pyknotic cell and neuron density in the mPFC and demonstrated elevated levels of cell death during the first two postnatal weeks in male and female rats. These findings ultimately contribute to our understanding of neurodevelopment as the processes involved in brain maturation can show distinct regional and temporal patterns. The data presented here can be applied to the design of future studies involving the early environmental impact or other perinatal insult, such as hypoxic-ischemic lesions, on mPFC development or those modeling neurodevelopmental disorders implicating apoptosis.
Funding
E.P Sellinger and J. Willing were supported by NIH T32 ES007326, and R21 ES026896 to J.M. Juraska.
Compliance with ethical standards
The authors certify that they were carried out in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80-23) revised 1996. The authors also certify that formal approval to conduct the experiments described has been obtained from the animal subjects review board of their institution and could be provided upon request. The authors further attest that all efforts were made to minimize the number of animals used and their suffering.
CRediT authorship contribution statement
Elli Sellinger: Investigation, Formal analysis, Writing - original draft, Visualization. Carly Drzewiecki: Conceptualization, Methodology. Jari Willing: Investigation, Conceptualization, Methodology. Janice Juraska: Conceptualization, Funding acquisition, Writing - review & editing.
Conflicts of Interest
None.
Acknowledgments
We thank the Beckman Institute Imaging Technology Group for use of the stereology microscopy suite.
Contributor Information
Elli P. Sellinger, Email: ellenps2@illinois.edu.
Carly M. Drzewiecki, Email: carly.drzewiecki@gmail.com.
Jari Willing, Email: jwillin@bgsu.edu.
Janice M. Juraska, Email: jjuraska@illinois.edu.
References
- Blaschke A.J., Staley K., Chun J. Widespread programmed cell death in proliferative and postmitotic regions of the fetal cerebral cortex. Development. 1996;122:1165–1174. doi: 10.1242/dev.122.4.1165. [DOI] [PubMed] [Google Scholar]
- Buss R.R., Sun W., Oppenheim R.W. Adaptive roles of programmed cell death during nervous system development. Annu. Rev. Neurosci. 2006;29:1–35. doi: 10.1146/annurev.neuro.29.051605.112800. [DOI] [PubMed] [Google Scholar]
- Chan W.Y., Yew D.T. Apoptosis and Bcl-2 oncoprotein expression in the human fetal central nervous system. Anat. Rec. 1998;252:165–175. doi: 10.1002/(SICI)1097-0185(199810)252:2<165::AID-AR2>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Dalley J.W., Cardinal R.N., Robbins T.W. Prefrontal executive and cognitive functions in rodents: neural and neurochemical substrates. Neurosci. Biobehav. Rev. 2004;28:771–784. doi: 10.1016/j.neubiorev.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Drzewiecki C.M., Willing J., Juraska J.M. Influences of age and pubertal status on number and intensity of perineuronal nets in the rat medial prefrontal cortex. Brain Struct. Func. 2020;225:2495–2507. doi: 10.1007/s00429-020-02137-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Eden C.G., Kros J.M., Uylings H.B.M. Chapter 8 The development of the rat prefrontal cortex: Its size and development of connections with thalamus, spinal cord and other cortical areas. Prog. Brain Res. 1991;85:169–183. doi: 10.1016/S0079-6123(08)62680-1. [DOI] [PubMed] [Google Scholar]
- van Eden C.G., Uylings H.B.M. Cytoarchitectonic development of the prefrontal cortex in the rat. J. Comp. Neurol. 1985;241:253–267. doi: 10.1002/cne.902410302. [DOI] [PubMed] [Google Scholar]
- Euston D.R., Gruber A.J., McNaughton B.L. The role of medial prefrontal cortex in memory and decision making. Neuron. 2012;76:1057–1070. doi: 10.1016/j.neuron.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrer I., Bernet E., Soriano E., del Rio T., Fonseca M. Naturally occurring cell death in the cerebral cortex of the rat and removal of dead cells by transitory phagocytes. Neuroscience. 1990;39:451–458. doi: 10.1016/0306-4522(90)90281-8. [DOI] [PubMed] [Google Scholar]
- Ferrer I., Tortosa A., Blanco R., Martin F., Serrano T., Planas A., Macaya A. Naturally occurring cell death in the developing cerebral cortex of the rat. Evidence of apoptosis-associated internucleosomal DNA fragmentation. Neurosci. Lett. 1994;182:77–79. doi: 10.1016/0304-3940(94)90210-0. [DOI] [PubMed] [Google Scholar]
- Forger N.G. Control of cell number in the sexually dimorphic brain and spinal cord. J. Neuroendocrinol. 2009;21:393–399. doi: 10.1111/j.1365-2826.2009.01825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N., Giedd J.N., Lusk L., Hayashi K.M., Greenstein D., Vaituzis A.C., Nugent T.F., Herman D.H., Clasen L.S., Toga A.W., Rapoport J.L., Thompson P.M. Dynamic mapping of human cortical development during childhood through early adulthood. Proc. Natl. Acad. Sci. U. S. A. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunaydin L.A., Kreitzer A.C. Cortico–Basal Ganglia circuit function in psychiatric disease. Annu. Rev. Physiol. 2016;78:327–350. doi: 10.1146/annurev-physiol-021115-105355. [DOI] [PubMed] [Google Scholar]
- Juraska J.M., Fifková E. A Golgi study of the early postnatal development of the visual cortex of the hooded rat. J. Comp. Neurol. 1979;183:247–256. doi: 10.1002/cne.901830203. [DOI] [PubMed] [Google Scholar]
- Kougias D.G., Sellinger E.P., Willing J., Juraska J.M. Perinatal exposure to an environmentally relevant mixture of phthalates results in a lower number of neurons and synapses in the medial prefrontal cortex and decreased cognitive flexibility in adult male and female rats. J. Neurosci. 2018:38. doi: 10.1523/JNEUROSCI.0607-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillie R.D. nineth ed. Williams & Wilkins; Baltimore: 1997. H. J. Conn’s Biological Stains. [Google Scholar]
- Majcher-Maślanka I., Solarz A., Chocyk A. Maternal separation disturbs postnatal development of the medial prefrontal cortex and affects the number of neurons and glial cells in adolescent rats. Neuroscience. 2019;423:131–147. doi: 10.1016/j.neuroscience.2019.10.033. [DOI] [PubMed] [Google Scholar]
- Miller M. Maturation of rat visual cortex. I. A quantitative study of Golgi-impregnated pyramidal neurons. J. Neurocytol. 1981;10:859–878. doi: 10.1007/BF01262658. [DOI] [PubMed] [Google Scholar]
- Mosley M., Shah C., Morse K.A., Miloro S.A., Holmes M.M., Ahern T.H., Forger N.G. Patterns of cell death in the perinatal mouse forebrain. J. Comp. Neurol. 2017;525:47–64. doi: 10.1002/cne.24041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuñez J.L., Huppenbauer C.B., McAbee M.D., Juraska J.M., DonCarlos L.L. Androgen receptor expression in the developing male and female rat visual and prefrontal cortex. J. Neurobiol. 2003;56:293–302. doi: 10.1002/neu.10236. [DOI] [PubMed] [Google Scholar]
- Nuñez J.L., Jurgens H.A., Juraska J.M. Androgens reduce cell death in the developing rat visual cortex. Dev. Brain Res. 2000;125:83–88. doi: 10.1016/S0165-3806(00)00126-7. [DOI] [PubMed] [Google Scholar]
- Nuñez J.L., Lauschke D.M., Juraska J.M. Cell death in the development of the posterior cortex in male and female rats. J. Comp. Neurol. 2001;436:32–41. doi: 10.1002/cne.1051. [DOI] [PubMed] [Google Scholar]
- Paxinos G., Watson C. fifth ed. Elsevier; 2006. The Rat Brain in Stereotaxic Coordinates. [Google Scholar]
- Rabinowicz T., de Courten-Meyers G.M., McDonald-Comber Petetot J., Xi G., de Los Reyes E. Human cortex development: estimtes of neuronal numbers indicate major loss late during gestation. J. Neuropathol. Exp. Neurol. 1996;55:320–328. [PubMed] [Google Scholar]
- Rakic S., Zecevic N. Programmed cell death in the developing human telencephalon. Eur. J. Neurosci. 2000;12:2721–2734. doi: 10.1016/S0008-6363(99)00401-0. [DOI] [PubMed] [Google Scholar]
- Semple B.D., Blomgren K., Gimlin K., Ferriero D.M., Noble-Haeusslein L.J. Brain development in rodents and humans: identifying benchmarks of maturation and vulnerability to injury across species. Prog. Neurobiol. 2013;106–107:1–16. doi: 10.1016/j.pneurobio.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreafico R., Frassoni C., Arcelli P., Selvaggio M., de Biasi S. In situ labeling of apoptotic cell death in the cerebral cortex and thalamus of rats during development. J. Comp. Neurol. 1995;363:281–295. doi: 10.1002/cne.903630209. [DOI] [PubMed] [Google Scholar]
- Thomaidou D., Mione M.C., Cavanagh J.F.R., Parnavelas J.G. Apoptosis and its relation to the cell cycle in the developing cerebral cortex. J. Neurosci. 1997;17:1075–1085. doi: 10.1523/jneurosci.17-03-01075.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uylings H.B.M., Groenewegen H.J., Kolb B. Do rats have a prefrontal cortex? Behav. Brain Res. 2003;146:3–17. doi: 10.1016/j.bbr.2003.09.028. [DOI] [PubMed] [Google Scholar]
- Verney C., Takahashi T., Bhide P.G., Nowakowski R.S., Caviness V.S. Independent controls for neocortical neuron production and histogenetic cell death. Dev. Neurosci. 2000;22:125–138. doi: 10.1159/000017434. [DOI] [PubMed] [Google Scholar]
- Willing J., Juraska J.M. The timing of neuronal loss across adolescence in the medial prefrontal cortex of male and female rats. Neuroscience. 2015;301:268–275. doi: 10.1016/j.neuroscience.2015.05.073. [DOI] [PMC free article] [PubMed] [Google Scholar]