Abstract
Background
Enhancing detection of unrecognized atrial fibrillation among acute ischemic stroke patients is crucial for secondary stroke prevention.
Aim
To evaluate whether the detection rate of new atrial fibrillation in acute ischemic stroke patients without known atrial fibrillation could be improved by doing serial 12-lead electrocardiograms once daily for five days, compared with conventional 24-h Holter monitoring (24-h Holter).
Methods
We conducted a randomized clinical trial to compare the detection rates of paroxysmal atrial fibrillation between serial electrocardiograms versus 24-h Holter from October 2015 to October 2018 at six hospitals. Eligible participants were acute ischemic stroke patients with aged ≥65 years, with neither atrial fibrillation history nor any presence of atrial fibrillation on baseline electrocardiogram at admission. The primary outcome was newly detected electrocardiogram in the serial electrocardiograms and 24-h Holter group.
Results
Among 826 patients, baseline characteristics were similar between both groups. In the intention-to-treat analysis, there was no statistical difference between serial electrocardiograms versus 24-Holter to detect atrial fibrillation (8.4% vs. 6.9%; adjusted odds ratio 1.17, 95% confidence interval 0.69–2.01). Stepwise multivariate logistic regression revealed age ≥80 years and history of heart failure were associated with detection of paroxysmal atrial fibrillation whereas patients with lacunar infarction had lower odds for detection of paroxysmal atrial fibrillation.
Conclusions
Serial electrocardiograms had comparable detection rate of paroxysmal atrial fibrillation compared with 24-h Holter and might be a viable alternative to 24-h Holter as a first-line approach to survey for potential paroxysmal atrial fibrillation among elderly patients with acute ischemic stroke.
Clinical Trial Registration: URL https://clinicaltrials.gov/ct2/show/NCT02578979
Unique Identifiers: NCT02578979
Keywords: Atrial fibrillation, stroke, electrocardiography, 24-h Holter, randomized controlled trial
Introduction
Atrial fibrillation (AF) is the most common chronic cardiac arrhythmia in the elderly and is known to be associated with fivefold risk of future ischemic stroke in general population.1,2 A new diagnosis of AF after discharge for stroke is associated with an increased risk of recurrent stroke, compared with patients without AF and with known AF.3 Even when an index stroke is presumed to be caused by another pathophysiologic mechanism, such as lacunar infarct or large artery atherosclerosis, AF may still exist. Once AF is found, oral anticoagulant therapy, rather than antiplatelet therapy, is recommended for secondary stroke prevention because the initiation of oral anticoagulation is the most effective strategy for secondary stroke prevention in stroke patients with AF.4
While approaches for detecting AF vary,5 doing a conventional 12-lead electrocardiogram (ECG) or 24-h Holter monitoring are typically seen as the standard and cost-effective methods for detecting occult AF.6 Prolonged ECG monitoring may improve the detection rate of AF, but it is relatively expensive and inconvenient, thereby limiting its widespread use in clinical practice.7,8 A standard 12-lead ECG is a basic and mandatory device in all hospitals, and remains a gold standard modality for the diagnosis of AF.9 A retrospective study suggested that serial ECGs could improve the detection rate of paroxysmal AF when compared to a single ECG in patients with acute ischemic stroke, and the sensitivity of AF detection was not different between serial ECGs and 24-h Holter monitoring.10
The objective of the Atrial Fibrillation Trial to Evaluate Real-world Procedures for their Utility in helping to Lower Stroke Events (AFTER-PULSE) was to compare the detection rate of AF with serial 12-lead ECGs once daily for five days versus 24-h Holter monitoring in elderly patients with acute ischemic stroke and no known AF at the time of admission.
Methodology
Trial design
Details of the study design and methods have been published previously.11 In brief, we conducted a multicenter, randomized clinical trial at six hospitals in Taiwan to compare the detection rates of paroxysmal AF between serial 12-lead ECGs once daily for five days vs. 24-h Holter monitoring in acute ischemic stroke patients, without known AF, from 1 October 2015 to 31 October 2018. This trial followed CONSORT guidelines for reporting clinical trial protocols12 and was approved by the local Institutional Review Board of Chang Gung Memorial Hospital, Chiayi Branch, Taiwan (103-7597B and 104-9611C).
Trial participants
Eligible participants were patients admitted to a hospital for an ischemic stroke within two days of stroke symptom onset, and aged ≥ 65 years, with neither a history of AF nor any presence of AF on baseline ECG at the time of hospital admission. Patents were excluded if they had an indication for oral anticoagulation at randomization, intracerebral hemorrhage by medical history, pacemaker or implantable cardioverter–defibrillator device, or end-stage renal disease.
Trial procedure
We randomly allocated eligible patients in a 1:1 ratio to receive 12-lead ECGs once daily for five days (intervention group) or 24-h Holter monitoring (comparator group). Allocation occurred when a study participant met the inclusion criteria and did not meet any exclusion criterion and signed the informed consent forms. Randomization was done in blocks of random sizes of 4 and 6 at each site by using of a web-based randomization service. One investigator (YLW) conducted computer-generated site-specific randomization lists, wrote an assigned-group on a small paper, put it in an envelope in sequence, and then sent it to a research assistant at each participated hospital in advance. Once an eligible patient agreed to participate in this trial, an investigator would then open up an envelope to discover to which group the patient was randomly assigned. Investigators and patients were aware of study group allocation. The interpretations of findings for both the 12-lead ECG and 24-h Holter groups were made by attending cardiologists who were unaware of the objectives of this study.
Trial intervention
If participants were randomly assigned to the serial ECG group, they first received 12-lead ECG within two days of stroke onset and then a 12-lead ECG was repeated once daily for five days during hospitalization. If five exams of 12-lead ECGs could not be completed during hospitalization, the remaining 12-lead ECGs would be done when the patient returned to an outpatient clinic and all 12-lead ECG exams were supposed to be done within three months after index stroke. Participants who were randomly assigned to the 24-h Holter group received Holter monitoring at each hospital site during their hospitalization. Participants in both randomized groups did not receive any specific additional medical treatment. All secondary stroke prevention therapies and rehabilitation treatments were determined at the discretion of the patients treating physicians.
Sample size calculation
We calculated sample size based on our preliminary data preceding the actual conduct of this trial. We evaluated 193 patients comprising 96 patients in a serial 12-lead ECG group and 97 patients a Holter monitoring group. AF was detected in five patients in the serial ECG group and in two patients in the Holter monitoring group. For a two-tailed t-test at the 5% level and with 80% power, 576 patients would be required per group. To allow for dropouts, 600 patients would be recruited in each group. Sample size calculation was performed using G Power software by one investigator (YLW)
Statistical analysis
Primary outcome was newly detected AF on a 12-lead ECG in the serial ECG group and 24-h Holter group after randomization based on intention-to-treat analysis. We also conducted additional analyses based on per-protocol. For the intention-to-treat analysis, all newly detected AF was counted regardless of the modality (e.g., stroke patients assigned to the 24-h Holter monitoring group may not have AF detected by 24-h Holter monitoring, but have AF detected by transthoracic echocardiogram, and in such a situation, AF would not be counted in the per-protocol analysis, but would be counted in the intention-to-treat analysis). For the per-protocol analysis, newly detected AF was counted only when AF was detected by the modality to which the patient was randomly assigned. Subgroup analyses to explore whether certain baseline characteristics (e.g., age, sex, National Institutes of Health Stroke Scale (NIHSS) score, stroke type (lacunar infarction vs. non-lacunar infarction) hypertension, diabetes mellitus, chronic kidney disease, coronary artery disease, heart failure, and previous stroke or transient ischemic attack) would influence overall results were conducted. Oral anticoagulants therapy, including warfarin or non-vitamin K oral anticoagulant (NOAC), in each group was recorded at discharge and at 90 days among stroke patients with newly detected AF. Baseline characteristics were compared using chi-square (categorical variables), Mann–Whitney U (continuous variables which were not normally distributed) or Student t-test (continuous variables which were normally distributed). Detection rates for paroxysmal AF between the two groups were compared using chi-square test with adjustment of age, sex, NIHSS score, lacunar stroke, hypertension, diabetes mellitus, chronic kidney disease, coronary artery disease, heart failure, and previous stroke or transient ischemic attack. Stepwise multivariate logistic regression was conducted to explore the association between baseline factors and newly detected AF using the entire trial data. All statistical analyses were performed with IBM SPSS statistics 19 for Windows.
Results
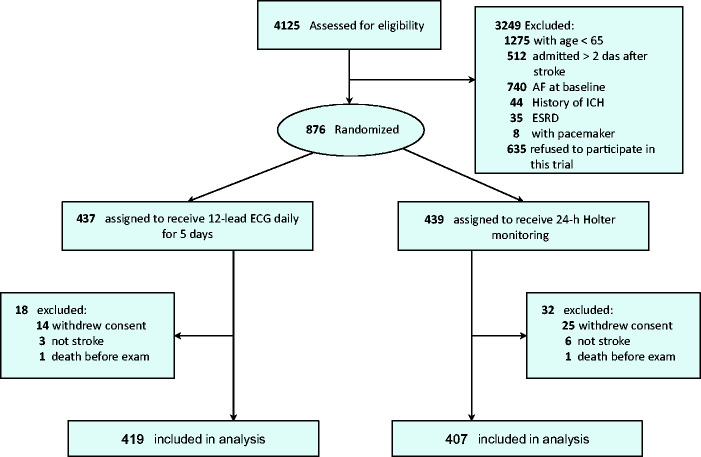
Between 1 October 2015 and 31 July 2018, 874 patients were randomized and 826 patients were included for analysis with 419 patients received 12-lead ECGs once daily for five days and 407 patients received 24-h Holter monitoring at six study hospitals (Figure 1). Baseline characteristics of both groups are summarized in Table 1 and there were no significant differences between the groups in terms of age, sex, NIHSS score, length of hospitalization, stroke type (lacunar infarction vs. non-lacunar infarction), comorbidity, and laboratory data. The duration of each 12-lead ECG recording was ∼5 min and the formal result was reported by a cardiologist within one day after examination. The median duration of 24-h Holter report was three days after examination (interquartile range two to five days). Overall, a higher frequency of newly detected paroxysmal AF was found in ischemic stroke patients ≥80 years vs. 65–79 years (12.6% vs. 5.3%).
Figure 1.
Study flowchart of patient selection.
Table 1.
Baseline demographics and clinical characteristics of patients with acute ischemic stroke, divided by the strategies for detection of atrial fibrillation
| ECG once daily for five days (n = 419) | 24-h Holter monitoring (n = 407) | |
|---|---|---|
| Age (years) | 76 (69–82) | 75 (69–81) |
| Male | 242 (57.8%) | 256 (62.9%) |
| Height (cm) | 160 (153–165) | 160 (153–165) |
| Weight (kg) | 62 (54–70) | 62 (55–70) |
| Body mass index (kg/m2) | 24.5 (22.3–27.2) | 24.5 (22.3–27.0) |
| NIHSS score at admission | 4 (3–7) | 4 (2–7) |
| Length of hospitalization | 9 (6–16) | 9 (7–16) |
| Stroke type | ||
| Lacunar infarction | 253 (60.4%) | 236 (58.0%) |
| Non-lacunar infarction | 166 (39.6%) | 171 (42.0%) |
| Comorbidity | ||
| Hypertension | 318 (75.9%) | 316 (77.6%) |
| Diabetes mellitus | 171 (40.8%) | 153 (37.6%) |
| Hypercholesterolemia | 127 (30.3%) | 121 (29.7%) |
| Valvular heart disease | 4 (1.0%) | 5 (1.2%) |
| Chronic kidney disease | 39 (9.3%) | 29 (7.1%) |
| Coronary artery disease | 46 (11.0%) | 30 (7.4%) |
| Heart failure | 8 (1.9%) | 4 (1.0%) |
| Previous ischemic stroke or TIA | 89 (21.2%) | 98 (24.1%) |
| Cancer | 24 (5.7%) | 23 (5.7%) |
| Lab data | ||
| Glycohemoglobin (%) | 6.2 (5.8–7.3) | 6.0 (5.7–7.0) |
| Creatinine (mg/dL) | 1.0 (0.8–1.2) | 1.0 (0.8–1.2) |
| Total cholesterol (mg/dL) | 180 (152–205) | 175 (151–200 |
| Triglyceride (mg/dL) | 120 (77–150) | 107 (79–144) |
| LDL cholesterol (mg/dL) | 110 (76–162) | 111 (89–131) |
| HDL cholesterol (mg/dL) | 42 (35–49) | 41 (34–48) |
ECG: electrocardiogram; HDL: high-density lipoprotein; LDL: low-density lipoprotein; NIHSS: National Institutes of Health Stroke Scale; TIA: transient ischemic attack.
Data are presented as median (interquartile range) or n (%).
p < 0.05, Chi-square or Mann–Whitney U test.
In the intention-to-treat analysis, of the 419 patients whom received serial 12-lead ECGs once daily for 5 days, 35 (8.4%) patients were identified as having newly detected AF, whereas of the 407 patients who received 24-h Holter monitoring, 28 (6.9%) patients were identified as having newly detected AF. There was no statistical difference between serial 12-lead ECG and 24-Holter to detect AF (adjusted odds ratio (OR) 1.17, 95% confidence interval (CI) 0.69–2.01). In subgroup analysis, all baseline characteristics did not influence new AF detection between these two modalities (Table 2).
Table 2.
Intention-to-treat analysis for the detection rate of atrial fibrillation, ECG once daily for five days versus 24-h Holter monitoring
| ECG once daily for five days, event/total (%) | 24-h Holter, event/total (%) | OR (95% CI) | Adjusted OR (95% CI)a | P for interaction | |
|---|---|---|---|---|---|
| Overall | 35/419 (8.4%) | 28/407 (6.9%) | 1.23 (0.74–2.07) | 1.17 (0.69–2.01) | |
| Age | |||||
| ≥80 years | 16/141 (11.3%) | 17/120 (14.2%) | 0.78 (0.37–1.61) | 0.74 (0.34–1.60) | 0.14 |
| 65–79 years | 19/278 (6.8%) | 11/287 (3.8%) | 1.84 (0.86–3.94) | 2.03 (0.92–4.49) | |
| Sex | |||||
| Men | 19/242 (7.9%) | 18/256 (7.0%) | 1.13 (0.58–2.20) | 1.07 (0.53–2.15) | 0.67 |
| Women | 16/177 (9.0%) | 10/151 (6.6%) | 1.40 (0.62–3.19) | 1.81 (0.72–4.55) | |
| NIHSS | |||||
| 0–4 | 11/221 (5.0%) | 14/209 (6.7%) | 0.73 (0.32–1.65) | 0.76 (0.33–1.77) | 0.07 |
| ≥5 | 24/198 (12.1%) | 14/198 (7.1%) | 1.81 (0.91–3.62) | 1.61 (0.77–3.35) | |
| Stroke type | |||||
| Lacunar infarction | 11/253 (4.3%) | 13/236 (5.5%) | 0.78 (0.34–1.78) | 0.92 (0.39–2.16) | 0.07 |
| Non-lacunar infarction | 24/166 (14.5%) | 15/171 (8.8%) | 1.76 (0.89–3.48) | 1.64 (0.80–3.36) | |
| Hypertension | |||||
| Yes | 28/318 (8.8%) | 21/316 (6.6%) | 0.89 (0.30–2.65) | 1.30 (0.70–2.43) | 0.51 |
| No | 7/101 (6.9%) | 7/91 (7.7%) | 0.89 (0.30–2.65) | 1.04 (0.33–3.31) | |
| Diabetes mellitus | |||||
| Yes | 10/171 (5.8%) | 6/153 (3.9%) | 1.52 (0.54–4.29) | 1.61 (0.51–5.09) | 0.89 |
| No | 25/248 (10.1%) | 22/254 (8.7%) | 1.18 (0.65–2.16) | 1.15 (0.61–2.15) | |
| Chronic kidney disease | |||||
| Yes | 4/39 (10.3%) | 5/29 (17.2%) | 0.55 (0.13–2.26) | 0.17 (0.01–2.83) | 0.18 |
| No | 31/380 (8.2%) | 23/378 (6.1%) | 1.37 (0.78–2.40) | 1.40 (0.79–2.48) | |
| Coronary artery disease | |||||
| Yes | 3/46 (6.5%) | 3/30 (10%) | 0.63 (0.12–3.34) | –b | 0.41 |
| No | 32/373 (8.6%) | 25/377 (6.6%) | 1.32 (0.77–2.28) | 1.29 (0.73–2.25) | |
| Heart failure | |||||
| Yes | 4/8 (50%) | 1/4 (25%) | 3.00 (0.21–42.62) | –b | 0.14 |
| No | 31/411 (7.5%) | 27/403 (6.7%) | 1.14 (0.67–1.94) | 1.16 (0.67–2.00) | |
| Previous stroke or TIA | |||||
| Yes | 6/89 (6.7%) | 5/98 (5.1%) | 1.35 (0.40–4.57) | 1.28 (0.33–4.92) | 0.95 |
| No | 29/330 (8.8%) | 23/309 (7.4%) | 1.20 (0.68–2.12) | 1.17 (0.64–2.11) | |
CI: confidence interval; ECG: electrocardiogram; OR: odds ratio; TIA: transient ischemic attack.
Data are presented as n (%).
Adjustment of age, sex, National Institutes of Health Stroke Scale (NIHSS) score, lacunar stroke, hypertension, diabetes mellitus, chronic kidney disease, coronary artery disease, heart failure, and previous stroke or transient ischemic attack.
Unable to obtain results due to small sample size.
In the per-protocol analysis, of the 419 patients who received serial 12-lead ECGs once daily for five days, 30 (7.2%) patients were identified as having newly detected AF, whereas of the 407 patients who received 24-h Holter monitoring, 22 (5.4%) patients were identified as having newly detected AF. There was no statistical difference between serial 12-lead ECG and 24-Holter to detect AF (adjusted OR 1.29, 95% CI 0.71–2.32). Most baseline characteristics did not influence new AF detection between these two modalities (Table 3).
Table 3.
Per-protocol analysis for the detection rate of atrial fibrillation, ECG once daily for five days versus 24-h Holter monitoring
| ECG once daily for five days, event/total (%) | 24-h Holter, event/total (%) | OR (95% CI) | Adjusted (OR 95% CI)a | P for interaction | |
|---|---|---|---|---|---|
| Overall | 30/419 (7.2%) | 22/407 (5.4%) | 1.35 (0.77–2.38) | 1.29 (0.71–2.32) | |
| Age | |||||
| ≥80 years | 15/141 (10.6%) | 15/120 (12.5%) | 0.83 (0.39–1.78) | 0.80 (0.36–1.79) | 0.18 |
| 65–79 years | 15/278 (5.4%) | 7/287 (2.4%) | 2.28 (0.92–5.68) | 2.59 (1.01–6.66) | |
| Sex | |||||
| Men | 16/242 (6.6%) | 13/256 (5.1%) | 1.32 (0.62–2.81) | 1.21 (0.55–2.64) | 0.91 |
| Women | 14/177 (7.9%) | 9/151 (6.0%) | 1.36 (0.57–3.23) | 1.70 (0.64–4.50) | |
| NIHSS | |||||
| 0–4 | 9/221 (4.1%) | 11/209 (5.3%) | 0.76 (0.31–1.88) | 0.87 (0.34–2.20) | 0.07 |
| ≥5 | 21/198 (10.6%) | 11/198 (5.6%) | 2.02 (0.95–4.30) | 1.76 (0.78–3.99) | |
| Stroke type | |||||
| Lacunar infarction | 8/253 (3.2%) | 10/236 (4.2%) | 0.74 (0.29–1.90) | 0.81 (0.31–2.15) | 0.03 |
| Non-lacunar infarction | 22/166 (13.3%) | 12/171 (7.0%) | 2.02 (0.97–4.24) | 1.86 (0.85–4.05) | |
| Hypertension | |||||
| Yes | 23/318 (7.2%) | 18/316 (5.7%) | 1.29 (0.68–2.44) | 1.24 (0.63–2.43) | 0.80 |
| No | 7/101 (6.9%) | 4/91 (4.4%) | 1.62 (0.46–5.73) | 1.79 (0.48–6.72) | |
| Diabetes mellitus | |||||
| Yes | 8/171 (4.7%) | 4/153 (2.6%) | 1.83 (0.54–6.20) | 2.09 (0.51–8.52) | 0.94 |
| No | 22/248 (8.9%) | 18/254 (7.1%) | 1.28 (0.67–2.44) | 1.23 (0.62–2.43) | |
| Chronic kidney disease | |||||
| Yes | 4/39 (10.3%) | 5/29 (17.2%) | 0.55 (0.13–2.26) | 0.17 (0.01–2.83) | 0.13 |
| No | 26/380 (6.8%) | 17/378 (4.5%) | 1.56 (0.83–2.91) | 1.62 (0.85–3.09) | |
| Coronary artery disease | |||||
| Yes | 2/46 (4.3%) | 3/30 (10%) | 0.41 (0.06–2.61) | –b | 0.17 |
| No | 28/373 (7.5%) | 19/377 (5.0%) | 1.53 (0.84–2.79) | 1.53 (0.82–2.85) | |
| Heart failure | |||||
| Yes | 3/8 (37.5%) | 1/4 (25%) | 1.80 0.12–26.20) | –b | 0.45 |
| No | 27/411 (6.6%) | 21/403 (5.2%) | 1.28 (0.71–2.30) | 1.33 (0.73–2.45) | |
| Previous stroke or TIA | |||||
| Yes | 6/89 (6.7%) | 4/98 (4.1%) | 1.70 (0.46–6.23) | 1.73 (0.41–7.27) | 0.77 |
| No | 24/330 (7.3%) | 18/309 (5.8%) | 1.27 (0.67–2.39) | 1.23 (0.64–2.38) |
CI: confidence interval; ECG: electrocardiogram; OR: odds ratio; TIA: transient ischemic attack.
Data are presented as n (%).
Adjustment of age, sex, National Institutes of Health Stroke Scale (NIHSS) score, lacunar stroke, hypertension, diabetes mellitus, chronic kidney disease, coronary artery disease, heart failure, and previous stroke or transient ischemic attack.
Unable to obtain results due to small sample size.
Of the patients who had newly detected AF, 37.1% of patients in serial ECG group versus 39.3% of patients in 24-Holter group were on an oral anticoagulant at discharge, while 51.4% of patients in serial ECG group versus 64.3% of patients in 24-Holter group were on an oral anticoagulant at three months after their index ischemic stroke (Table 4).
Table 4.
Oral anticoagulation in patients with atrial fibrillation detected in serial ECGs and 24-h Holter monitoring groups, respectively, at discharge and at three months
| Oral anticoagulation | ECG once daily for five days (n/N) | 24-h Holter (n/N) |
|---|---|---|
| At discharge | 13/35 (37.1%) | 11/28 (39.3%) |
| Three months | 18/35 (51.4%) | 18/28 (64.3%) |
N: number of patients that paroxysmal atrial fibrillation was detected, n: number of patients that an oral anticoagulant was used.
Stepwise multivariate logistic regression revealed age ≥ 80 years (adjusted OR 2.14, 95% CI 1.24–3.69) and history of heart failure (adjusted OR 8.10, 95% CI 2.21–29.74) were associated with detection of paroxysmal AF, whereas patients with lacunar infarction had lower odds for detection of paroxysmal AF (adjusted OR 0.48, 95% CI 0.26–0.91) (Table 5).
Table 5.
Logistic regression for predictors of new-onset atrial fibrillation in patients with acute ischemic stroke using the whole trial cohort
| Variables | Univariate logistic regression, odds ratio (95% CI) | Stepwise multivariate logistic regression, odds ratio (95% CI) |
|---|---|---|
| Age ≥ 80 years | 2.58 (1.54–4.33) | 2.14 (1.24–3.69) |
| Male | 0.93 (0.55–1.57) | |
| Height | 1.00 (0.97–1.03) | |
| Weight | 1.00 (0.98–1.02) | |
| Body mass index | 0.99 (0.92–1.06) | |
| NIHSS score ≥ 5 | 1.72 (1.02–2.91) | |
| Length of stay | 1.01 (0.99–1.03) | |
| Lacunar infarction | 0.39 (0.23–0.67) | 0.48 (0.26–0.91) |
| Hypertension | 1.07 (0.57–1.97) | |
| Diabetes mellitus | 0.50 (0.28–0.90) | |
| Hypercholesterolemia | 0.78 (0.43–1.40) | |
| Valvular heart disease | 0.00 (0.00–) | |
| Chronic kidney disease | 1.99 (0.94–4.23) | |
| Coronary artery disease | 1.04 (0.43–2.50) | |
| Heart failure | 9.31 (2.87–30.25) | 8.10 (2.21–29.74) |
| Previous stroke or TIA | 0.71 (0.36–1.38) | |
| Cancer | 0.25 (0.03–1.85) | |
| Glycohemoglobin | 0.72 (0.57–0.93) | |
| Creatinine | 1.07(0.78–1.46) | |
| Total cholesterol | 0.99 (0.98–1.00) | |
| Triglyceride | 1.00 (0.99–1.00) | |
| LDL cholesterol | 0.99 (0.98–1.00) | |
| HDL cholesterol | 1.01 (0.99–1.04) |
CI: confidence interval; TIA: transient ischemic attack; NIHSS: National Institutes of Health Stroke Scale; LDL: low density lipoprotein; HDL: high density lipoprotein.
Discussion
To our knowledge, this is the first randomized clinical trial to evaluate the detection rate of paroxysmal AF by serial 12-lead ECGs versus 24-h Holter monitoring in elderly ischemic stroke patients without known AF at baseline. The results suggested serial 12-lead ECGs once daily for five days compared with 24-h Holter monitoring, had comparable detection rate of paroxysmal AF among hospitalized elderly ischemic stroke patients without baseline known AF. The new AF detection rate for serial 12-lead ECGs in the current study among Taiwanese patients was similar to a previously published observational study among Canadian patients10 and provides some external validation for our findings. Since it is not uncommon for paroxysmal AF to be undetected in a single ECG on admission among ischemic stroke patients,13 additional cardiac rhythm workup is usually necessary. Our results imply that serial 12-lead ECGs might be a viable alternative to 24-h Holter monitoring as a first-line approach to survey for potential paroxysmal AF in acute ischemic stroke patients.
Two recent large clinical trials showed that NOACs, rivaroxaban,14 and dabigatran15 failed to show superiority over aspirin to prevent recurrent stroke after embolic stroke of undetermined source, which further highlights the importance of identifying AF before use of NOACs. Since 25–40% of AF is paroxysmal and clinically silent,16 it can be a major challenge to detect paroxysmal AF in ischemic stroke patients and a comprehensive scrutiny is often necessary. A recent study indicated that among ischemic stroke patients without AF diagnosed at discharge, 4% actually had an unrecognized history of transient AF and these patients had higher risk of recurrent strokes at one-year follow-up compared to ischemic stroke patients without AF.17 Therefore, a careful history review to uncover a paroxysmal AF history is important for ischemic stroke patients. Since the 12-lead ECG is an inexpensive, readily available, diagnostic modality and the current study showed that serial 12-lead ECGs had comparable AF detection rate compared with 24-h Holter monitoring, it may not be unreasonable to do serial 12-lead ECGs in all elder ischemic stroke patients without known AF by history or baseline ECG at admission. For ischemic stroke patients without AF identified by careful AF history review and serial 12-lead ECGs, but in whom paroxysmal AF is still suspected, prolonged ECG monitoring may be necessary to identify potential paroxysmal AF.7,8 Also, we found that ischemic stroke patients with age ≥ 80 years, non-lacunar stroke, or heart failure were significantly more likely have paroxysmal AF, and these patients in particular, may benefit from more intensive AF surveillance.
We found 37.1% and 39.3% of patients with newly detected AF took oral anticoagulants in serial ECG and 24-h Holter groups, respectively, at discharge. There were several reasons that may explain the relatively low rate of oral anticoagulation use in these people who were supposed to take oral anticoagulants for secondary stroke prevention. First, the ratio of hemorrhagic stroke to ischemic stroke is higher in Asian than non-Asian populations18 and there is a higher risk for anticoagulation-related intracranial hemorrhage among Asian patients with AF.19 Second, Taiwan National Health Bureau only reimburses NOACs after two weeks of ischemic stroke in AF patients. Therefore many attending neurologists hesitate to use oral anticoagulants during acute stage of ischemic stroke even AF exists. Furthermore, it was possible that in certain cases, research assistants did not relay the information of newly detected AF to attending neurologists in time and therefore an oral anticoagulant would not be used at discharge.
AF is the main underlying heart disease of cardioemoblic stroke20,21 and is associated with a higher in-hospital rate among ischemic stroke patients generally as well as among cardioembolic stroke subtype patients specifically.22 Among patients with cardioembolic stroke, in-hospital mortality was 32% for those with AF versus 15% for those without AF, while among patients with atherothrombotic stroke, in-hospital mortality was 29% in those with AF versus 19% in those without AF.23 Presence of AF is associated with a poor clinical outcome in both cardioembolic and atherothrombotic infarction, which may be due to a higher frequency of heart failure in the group of cardioembolic stroke with AF, and a higher frequency of ischemic heart disease in the group of atherothrombotic stroke with AF.22
Lacunar infarcts are the ischemic stroke subtype with a better functional prognosis and a cardiac source of embolism or AF is not typical with this ischemic stroke subtype.24 In this study, we found that patients with lacunar infarction had lower odds for detection of paroxysmal AF. Although autopsy-based evidence shows that cardioembolic occlusion can be an unusual cause of lacunar infarction,25 in patients aged ≥ 85 years vs. under 85 years, the higher occurrence of AF and the lower prevalence of hypertension and diabetes mellitus, suggest that the cardioembolic pathogenic mechanism may be more frequent than lacunar syndromes in very elderly stroke patients.26 Furthermore, a large clinical trial involving patients with recent symptomatic lacunar infarcts showed that about 9% of the recurrent ischemic strokes were caused by cardioembolism27 and therefore survey of potential abnormality of heart, rhythm, and structure, is still crucial in patients with lacunar infarction.
This study has several limitations. First, some patients with paroxysmal AF may remain undetected by doing serial 12-lead ECGs once daily or 24-h Holter monitoring. However, since neither serial 12-lead ECGs nor 24-h Holter monitoring is the most powerful modality for identifying new AF, our study purpose was actually to establish the best first-line tool that can be applied universally to acute ischemic stroke patients without baseline known AF. Second, different underlying stroke mechanisms might influence the detection rate of paroxysmal AF, these varied among our enrolled patients. Since we did not collect detailed data on precise stroke mechanisms among the enrolled stroke patients, further analysis could not be conducted. Nonetheless, underlying stroke mechanisms were not likely to be different between the two groups because we randomly assigned patients to each group. Finally, lack of trial blinding on the part of investigators and patients may have introduced some bias, but the final results were interpreted by cardiologists unaware of the objectives of the study, which likely minimized this issue.
Conclusions
In conclusion, this randomized clinical trial showed that serial 12-lead ECGs once daily for five days compared with 24-h Holter monitoring had comparable detection rate of paroxysmal AF among elder patients with acute ischemic stroke and without known baseline AF, which suggests that serial 12-lead ECGs once daily for five days could be a viable alternative as a first-line approach to detect paroxysmal AF in these patients. Further studies may be warranted to assess whether detection of paroxysmal AF is eventually associated with a lower risk of stroke recurrence, due to an increased timely initiation of oral anticoagulants.
Acknowledgements
Participated hospitals (number recruited):
Chang Gung Memorial Hospital Chiayi branch (311): Meng Lee, Jiann-der Lee, Yen-Chu Huang, Ying-Chih Huang, Huan-Lin Hsu, Ya-Hui Lin, Chia-Yu Hsu, Yi-Ting Pan, Chun-Hsien Lin, Chang-Min Chung, Tsung-Ta Hsieh, Meng-Hsin Lin.
Chang Gung Memorial Hospital Keelung branch (276): Wen-Yi Huang, Tsung-I Peng, Wei-Chieh Weng, Chia-Lun Wu, Yi-Chia Wei.
Chang Gung Memorial Hospital Linkou branch (58): Tsong-Hai Lee, Kuo-Hsuan Chang, Yi-Chun Chen, Yeu-Jhy Chang, Chien-Hung Chang, Hsiu-Chuan Wu, Kuo-Lun Huang, Ting-Yu Chang, Chi-Hung Liu, Long-Sun Ro, Hong-Shiu Chang, Rong-Kuo Lyu, Chiung-Mei Chen, Yih-Ru Wu.
National Taiwan University Hospital (32): Jiann-Shing Jeng, Sung-Chun Tang, Li-Kai Tsai, Shin-Joe Yeh
Chiayi Christian Hospital (129): Sheng-Feng Sung, Cheung-Ter Ong, Chi-Shun Wu, Yung-Chu Hsu, Yu-Hsiang Su, and Ling-Chien Hung.
Buddhist Dalin Tzu Chi General Hospital Chiayi branch (20): Yung-Sung Huang.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and or publication of this article: Study was founded by grants from Ministry of Science and Technology Taiwan (MOST104-2314-B-182-019 and MOST105-2628B-182-008-MY2) and Chang Gung Memorial Hospital (CORPG6D0103). The sponsors played no role in the study design, data collection and analysis, or decision to submit the article for publication.
ORCID iD: Meng Lee https://orcid.org/0000-0002-7491-0571
References
- 1.January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association task force on practice guidelines and the heart rhythm society. J Am Coll Cardiol 2014; 64: e1–76. [DOI] [PubMed] [Google Scholar]
- 2.Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke 1991; 22: 983–988. [DOI] [PubMed] [Google Scholar]
- 3.Kamel H, Johnson DR, Hegde M, et al. Detection of atrial fibrillation after stroke and the risk of recurrent stroke. J Stroke Cerebrovasc Dis 2012; 21: 726–731. [DOI] [PubMed] [Google Scholar]
- 4.Diener HC, Eikelboom J, Connolly SJ, et al. Apixaban versus aspirin in patients with atrial fibrillation and previous stroke or transient ischaemic attack: a predefined subgroup analysis from averroes, a randomised trial. Lancet Neurol 2012; 11: 225–231. [DOI] [PubMed] [Google Scholar]
- 5.Taggar JS, Coleman T, Lewis S, Heneghan C, Jones M. Accuracy of methods for detecting an irregular pulse and suspected atrial fibrillation: a systematic review and meta-analysis. Eur J Prev Cardiol 2016; 23: 1330–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sacco RL, Adams R, Albers G, et al. Guidelines for prevention of stroke in patients with ischemic stroke or transient ischemic attack: a statement for healthcare professionals from the American Heart Association/American Stroke Association council on stroke: co-sponsored by the Council on Cardiovascular Radiology and Intervention: The American Academy of Neurology affirms the value of this guideline. Stroke 2006; 37: 577–617. [DOI] [PubMed] [Google Scholar]
- 7.Gladstone DJ, Spring M, Dorian P, et al. Atrial fibrillation in patients with cryptogenic stroke. N Engl J Med 2014; 370: 2467–2477. [DOI] [PubMed] [Google Scholar]
- 8.Sanna T, Diener HC, Passman RS, et al. Cryptogenic stroke and underlying atrial fibrillation. N Engl J Med 2014; 370: 2478–2486. [DOI] [PubMed] [Google Scholar]
- 9.Kligfield P, Gettes LS, Bailey JJ, et al. Recommendations for the standardization and interpretation of the electrocardiogram: part I: the electrocardiogram and its technology: a scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and The Heart Rhythm Society: endorsed by the International Society for Computerized Electrocardiology. Circulation 2007; 115: 1306–1324. [DOI] [PubMed] [Google Scholar]
- 10.Douen AG, Pageau N, Medic S. Serial electrocardiographic assessments significantly improve detection of atrial fibrillation 2.6-fold in patients with acute stroke. Stroke 2008; 39: 480–482. [DOI] [PubMed] [Google Scholar]
- 11.Hsieh TT, Lee M, Huang WY, et al. Atrial fibrillation trial to evaluate real-world procedures for their utility in helping to lower stroke events (AFTER-PULSE): study protocol for a randomized controlled trial. Contemp Clin Trials Commun 2017; 6: 127–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMC Med 2010; 8: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morris JG, Duffis EJ, Fisher M. Cardiac workup of ischemic stroke: can we improve our diagnostic yield?. Stroke 2009; 40: 2893–2898. [DOI] [PubMed] [Google Scholar]
- 14.Hart RG, Sharma M, Mundl H, et al. Rivaroxaban for stroke prevention after embolic stroke of undetermined source. N Engl J Med 2018; 378: 2191–2201. [DOI] [PubMed] [Google Scholar]
- 15.Diener HC, Sacco RL, Easton JD, et al. Dabigatran for prevention of stroke after embolic stroke of undetermined source. N Engl J Med 2019; 380: 1906–1917. [DOI] [PubMed] [Google Scholar]
- 16.Chiang CE, Naditch-Brule L, Murin J, et al. Distribution and risk profile of paroxysmal, persistent, and permanent atrial fibrillation in routine clinical practice: insight from the real-life global survey evaluating patients with atrial fibrillation international registry. Circ Arrhythm Electrophysiol 2012; 5: 632–639. [DOI] [PubMed] [Google Scholar]
- 17.Hsu CY, Singer DE, Kamel H, et al. Unrecognized history of transient atrial fibrillation at the time of discharge from an index stroke hospitalization is associated with increased recurrent stroke risk. J Stroke 2019; 21: 190–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klatsky AL, Friedman GD, Sidney S, Kipp H, Kubo A, Armstrong MA. Risk of hemorrhagic stroke in Asian American ethnic groups. Neuroepidemiology 2005; 25: 26–31. [DOI] [PubMed] [Google Scholar]
- 19.Shen AY, Yao JF, Brar SS, Jorgensen MB, Chen W. Racial/ethnic differences in the risk of intracranial hemorrhage among patients with atrial fibrillation. J Am Coll Cardiol 2007; 50: 309–315. [DOI] [PubMed] [Google Scholar]
- 20.Syme PD, Byrne AW, Chen R, Devenny R, Forbes JF. Community-based stroke incidence in a Scottish population: the Scottish borders stroke study. Stroke 2005; 36: 1837–1843. [DOI] [PubMed] [Google Scholar]
- 21.Grau AJ, Weimar C, Buggle F, et al. Risk factors, outcome, and treatment in subtypes of ischemic stroke: the German stroke data bank. Stroke 2001; 32: 2559–2566. [DOI] [PubMed] [Google Scholar]
- 22.Arboix A, Alio J. Acute cardioembolic stroke: an update. Expert Rev Cardiovasc Ther 2011; 9: 367–379. [DOI] [PubMed] [Google Scholar]
- 23.Arboix A, Garcia-Eroles L, Massons JB, Oliveres M, Pujades R, Targa C. Atrial fibrillation and stroke: clinical presentation of cardioembolic versus atherothrombotic infarction. Int J Cardiol 2000; 73: 33–42. [DOI] [PubMed] [Google Scholar]
- 24.Arboix A, Blanco-Rojas L, Marti-Vilalta JL. Advancements in understanding the mechanisms of symptomatic lacunar ischemic stroke: translation of knowledge to prevention strategies. Expert Rev Neurother 2014; 14: 261–276. [DOI] [PubMed] [Google Scholar]
- 25.Lodder J, Bamford JM, Sandercock PA, Jones LN, Warlow CP. Are hypertension or cardiac embolism likely causes of lacunar infarction?. Stroke 1990; 21: 375–381. [DOI] [PubMed] [Google Scholar]
- 26.Arboix A, Garcia-Eroles L, Massons J, Oliveres M, Targa C. Lacunar infarcts in patients aged 85 years and older. Acta Neurol Scand 2000; 101: 25–29. [DOI] [PubMed] [Google Scholar]
- 27.Benavente OR, Hart RG, McClure LA, Szychowski JM, Coffey CS, Pearce LA. SPS3 Investigators. Effects of clopidogrel added to aspirin in patients with recent lacunar stroke. N Engl J Med 2012; 367: 817–825. [DOI] [PMC free article] [PubMed] [Google Scholar]