Abstract
Background
Malignant peripheral nerve sheath tumor (MPNST) is an aggressive and poorly understood malignant neoplasm. Even in the setting of multimodal therapy, the clinical course of MPNST is frequently marked by metastatic conversion and poor overall prognosis, with optimal treatment paradigms for this rare tumor unknown.
Methods
We reviewed the medical records and histopathology of 54 consecutive patients who were treated at University of California San Francisco between 1990 and 2018.
Results
Our cohort consisted of 24 male and 30 female patients (median age 38 years). Fédération Nationale des Centres de Lutte Contre Le Cancer (FNCLCC) sarcoma grading criteria segregated patients into groups with differences in overall survival (OS) (P = .02). Increasing Ki-67 labeling index was associated with poor OS (hazard ratio [HR] 1.36 per 10%, P = .0002). Unsupervised hierarchical clustering-based immunohistochemical staining patterns identified 2 subgroups of tumors with differences in H3K27me3, Neurofibromin, S100, SOX10, p16, and EGFR immunoreactivity. In our cohort, cluster status was associated with improved locoregional failure-free rate (P = .004) in response to radiation.
Conclusions
Our results lend support to the FNCLCC sarcoma grading criteria as a prognostic scheme for MPNST, although few cases of grade 1 were included. Further, we identify increased Ki-67 labeling as a strong predictor of poor OS from MPNST. Finally, we identify a subset of MPNSTs with a predictive immunohistochemical profile that has improved local control with adjuvant radiotherapy. These data provide insights into the grading and therapy for patients with MPNST, although further studies are needed for independent validation.
Keywords: clinical outcomes, Fédération Nationale des Centres de Lutte Contre Le Cancer, immunohistochemistry, malignant peripheral nerve sheath tumor, radiotherapy
Key Points.
Our results corroborate the validity of the FNCLCC sarcoma grading criteria as a prognostic scheme for MPNST.
We identify a subset of MPNSTs with a distinct immunophenotypic profile that have improved local control with adjuvant radiotherapy.
Importance of the Study.
Due to the rarity of MPNST, optimal treatment paradigms remain controversial. The FNCLCC grading system has been proposed to risk-stratify sarcomas, although its prognostic value for MPNST is debated. Additionally, few immunohistochemical markers are prognostic and none are currently used to inform treatment for MPNST patients. Thus, there is an urgent, unmet need for prognostic and predictive markers to identify patients who may benefit from adjuvant therapy. These data provide insights into the grading, immunohistochemical markers, and adjuvant treatment for patients with MPNST, shedding light on MPNST biology and treatment.
Malignant peripheral nerve sheath tumor (MPNST) is an aggressive and rare neoplasm involving peripheral nerves and extraneural soft tissues. MPNST represents 5% of soft tissue sarcomas, with an incidence of 0.2 cases per 100,000 persons each year in the United States.1–3 They exhibit Schwann or perineurial cell differentiation and are classically sporadic, associated with neurofibromatosis type 1 (NF1), or prior radiotherapy.4 Treatment options for patients with MPNST are limited, and their clinical course is marked by high metastatic risk and poor overall prognosis.1,5–12 Surgical resection is the mainstay of MPNST treatment, with ionizing radiation and chemotherapy primarily reserved for high risk features—such as large size, deep location, or subtotal resection—unresectable disease, or salvage treatment.1,5–7,10,13–17 However, due to the rarity of MPNST, optimal treatment paradigms remain controversial. Thus, there is an urgent, unmet need for prognostic and predictive markers to identify patients who may benefit from adjuvant therapy.
MPNST is mostly a hypercellular spindled cell tumor with fusiform nuclei arranged in streaming and intersecting fascicles, histologically mimicking various sarcomas, spindle cell melanomas, and poorly differentiated carcinomas. Neural crest differentiation can be confirmed using immunohistochemical (IHC) markers such as SOX10 or S100, but these have limited diagnostic sensitivities and specificities, often being lost in MPNST18 and expressed to various degrees in many other tumors. Thus, the diagnosis of MPNST is often predicated on exclusion of other spindle cell neoplasms through an extensive IHC panel. Moreover, the distinction of malignant transformation of peripheral nerve sheath tumors in patients with NF1 is dependent on integration of various clinicopathologic findings, and no highly sensitive and specific biomarkers are available.19 Further, few immunohistochemical markers are prognostic and none are currently used to inform treatment paradigms for MPNST patients. Interestingly, the Fédération Nationale des Centres de Lutte Contre le Cancer (FNCLCC) grading system has been proposed to risk-stratify sarcomas, although its prognostic value for MPNST is debated.20,21 Grading is not currently included in diagnostic reports at many institutions, although the fifth edition WHO Classification of Soft Tissue and Bone Tumours implies prognostic significance to histologic grade.22
Here, we describe a retrospective series of 54 patients diagnosed with MPNST at a single institution with comprehensive histopathologic characterization and clinical follow-up. We report long-term outcomes, prognostic markers, and a predictive classification scheme to identify MPNST patients who are most likely to benefit from adjuvant radiotherapy.
Methods
Patient Cohort and Tumor Characteristics
This is a retrospective cohort study of patients who were treated for MPNST between 1990 and 2018 at a single center. Fifty-four consecutive patients were included in this study. Inclusion criteria were UCSF patients with available pathology materials and clinical follow-up. Pathology material for all cases was reviewed for diagnostic accuracy and various histologic features outlined below. Tumors in which melanoma was a consideration, neoplasms in which sarcoma diagnoses (eg, fibrosarcoma, fibromyxoid sarcoma, and leiomyosarcoma) remained in the differential, and nerve sheath tumors with equivocal features of malignancy were excluded based on consensus review between an expert soft tissue pathologist (A.E.H.) and board-certified neuropathologists (A.P. and M.P.). Tumors were categorized using the FNCLCC system grading criteria as a composite of tumor differentiation, necrosis, and mitotic count scores as previously described.20 Tumor differentiation was scored on a scale of 1–3 based on the most prominent morphology on H&E-stained sections as follows: well-differentiated peripheral nerve sheath tumor such as neurofibroma-like morphology with cytologic atypia and increased mitotic activity (1); monomorphic spindle cell neoplasm with intersecting fascicles with moderate atypia and at least focal suggestion of morphologic Schwannian differentiation (2); and undifferentiated pleomorphic sarcoma-like morphology without any morphologic Schwannian differentiation, and/or those with a rhabdomyosarcomatous component (3). Necrosis was scored on a scale of 0–2 based on total area of available diagnostic material: no necrosis (0), less than 50% tumor necrosis (1), and greater than or equal to 50% tumor necrosis (2). Mitotic count was performed in the most proliferative tumor focus across a contiguous area of approximately 2 mm2 (roughly 10 high-power fields at 400× magnification) and scored on a scale of 1–3: 0–9 mitoses (1), 10–19 mitoses (2), and 20 or more mitoses (3). The overall grade was calculated from a sum of the tumor differentiation, necrosis, and mitotic count scores: FNCLCC Grade 1 (total score of 2 or 3), FNCLCC Grade 2 (total score of 4 or 5), and FNCLCC Grade 3 (total score of 6, 7, or 8). An additional 2-tier grading system was generated with tumors dichotomized in to low-grade (FNCLCC Grade 1 and Grade 2 tumors; total score of 5 or less) and high-grade (FNCLCC Grade 3 tumors; total score of 6 or more) using the above mentioned FNCLCC criteria. The Institutional Review Board, Human Research Protection Program Committee at the University of California San Francisco approved this study (CHR# 18-24633).
Immunohistochemistry
IHC was performed using formalin-fixed, paraffin-embedded tissue sections on a combination of whole slide sections and tissue microarrays using the following antibodies: Ki-67 (Ventana, clone 30–9, 1:1 dilution), trimethylated lysine 27 on histone-H3 i.e. H3K27me3 (Cell Signaling Technology, clone C36B11, 1:50 dilution), SOX2 (EMD Millipore, clone AB5603, 1:200 dilution), SOX10 (Cell Marque, 1:50 dilution), p16 (Santa Cruz, clone JC8, 1:50 dilution), p53 (Dako, clone DO-7, 1:50), epidermal growth factor receptor, that is, EGFR (Ventana, clone 3C6, 1:20 dilution), p75NTR (Abcam, clone NGFR5, 1:100 dilution), S100 (Ventana, 1:2 dilution), and Neurofibromin (DKFZ, clone NFC, 1:4 dilution). All immunostaining was performed on Ventana Benchmark XT automated stainer (Roche Diagnostics) using standard techniques.18 IHC studies that were previously performed as part of clinical diagnostic workup, or stains obtained as part of prior research studies were also reviewed.18,23 Percent staining for H3K27me3, SOX2, SOX10, p16, p53, EGFR, p75NTR, S100, and Neurofibromin was estimated as the percentage of positive tumor cells on available stained tissue. H3K27me3 staining was also dichotomized as “retained” and “complete loss,” with the latter defined as staining in less than 5% of tumor cells in the presence of internal positive control. The Ki-67 proliferation index was estimated as percentage of positive nuclei in the area of highest labeling across an area of approximately 2 mm2 (10 high-power fields at 400× magnification). Ki-67 values greater than the median labeling value of this cohort (60%) were classified as “significantly elevated.”
Statistical Analysis
Locoregional failure-free rate (LFFR), metastasis-free survival (MFS), and overall survival (OS) were estimated using the Kaplan–Meier method and visualized in GraphPad Prism. LFFR was defined as the time to local recurrence in the setting of a gross total resection or local progression in the setting of a subtotal resection based on increased size on surveillance imaging. MFS was defined as time to a radiographically identified metastasis. Survival analysis including the log-rank test and Cox Proportional Hazards (CPH) regression were computed using the “survival” package in R. Variables reaching P < .1 on univariate analysis using the log-rank test were included in multivariate analysis.
Hierarchical clustering of IHC staining percentages per sample was performed using the “heatmaply” package in R, with default setting and ward linkage. The differences between mean percentage staining of each IHC stain between identified clusters was tested for significance using the nonparametric Mann–Whitney U test (Wilcoxon rank-sum test). Unless specified, all tests used were 2-tailed.
Results
A summary of the patient characteristics within our cohort is provided in Table 1. Our cohort consisted of 24 male (44%) and 30 female (56%) patients. The median age of MPNST patients at the time of initial diagnosis was 38 years (range 5–83), and 32 patients (59%) had a clinical diagnosis of NF1. Two patients (4%) had prior radiation treatment to the tumor sites for unrelated causes, both 19 years prior to the diagnosis of MPNST, suggestive of radiation-induced MPNST. Three patients (6%) received neoadjuvant chemotherapy. A total of 16 patients (30%) underwent subtotal resection, and the remaining 38 patients (70%) underwent gross total resection. Margin status was assessed in 49 cases (5 cases of piecemeal resection were excluded), with positive margins in 29 (59%) and negative margins in 20 (41%) patients. Postoperative therapy was delivered at the discretion of the treating physician, with a total of 25 patients (46%) receiving adjuvant radiotherapy and 16 patients (30%) receiving adjuvant chemotherapy. A summary of patient characteristics sorted by adjuvant radiotherapy status is provided in Supplementary Table S1. Tumor location was the only baseline characteristic that differed between patients who received adjuvant radiotherapy and patients who did not (P = .02). By FNCLCC grading criteria, there were 6 Grade 1 (11%), 16 Grade 2 (30%), and 32 Grade 3 (59%) tumors in our cohort. Using a modified 2-tier FNCLCC grading scheme, there were 22 lower-grade tumors (41%) and 32 high-grade tumors (59%). Histologically, tumors demonstrated haphazardly arranged elongate cells with varying degrees of nuclear atypia, consistent with a diagnosis of MPNST (Figure 1A–A’).
Table 1.
Patient and Tumor Characteristics of 54 Patients With MPNST
| Variable | Value/n | % | |
|---|---|---|---|
| Sex | Male | 24 | 44 |
| Female | 30 | 56 | |
| Age | Median | 38 | - |
| Min | 5 | ||
| Max | 83 | ||
| Clinical Neurofibromatosis Type 1 | Yes | 32 | 59 |
| No | 22 | 41 | |
| Tumor location | Head and neck | 9 | 17 |
| Trunk | 28 | 52 | |
| Upper extremities | 7 | 13 | |
| Lower extremities | 10 | 18 | |
| Tumor dimensions (cm) | Median | 8 | - |
| Min | 0.5 | ||
| Max | 26 | ||
| Prior history of radiation therapy | Yes | 2 | 4 |
| No | 52 | 96 | |
| Neoadjuvant chemotherapy | Yes | 3 | 6 |
| No | 51 | 94 | |
| Adjuvant radiation therapy | Yes | 25 | 46 |
| No | 29 | 54 | |
| Adjuvant chemotherapy | Yes | 16 | 30 |
| No | 38 | 70 | |
| Extent of resection | Sub total | 16 | 30 |
| Gross total | 38 | 70 | |
| Margin status | Negative | 20 | 41 |
| Positive | 29 | 59 | |
| n/a | 5 | - | |
| FNCLCC grade | Grade 1 | 6 | 11 |
| Grade 2 | 16 | 30 | |
| Grade 3 | 32 | 59 | |
| Modified FNCLCC grade | Low grade | 22 | 41 |
| High grade | 32 | 59 |
FNCLCC, Fédération Nationale des Centres de Lutte Contre Le Cancer; MPNST, Malignant peripheral nerve sheath tumor.
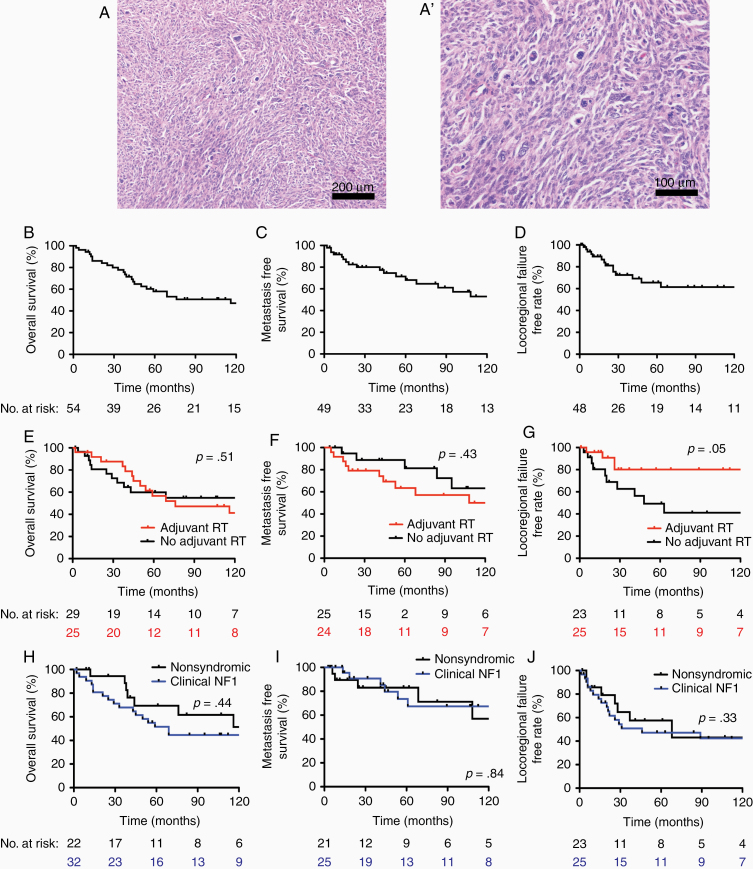
Figure 1.
Malignant peripheral nerve sheath tumor (MPNST) clinical outcomes. Representative H&E images of MPNSTs at (A) low and (A’) high magnification. Kaplan–Meier curves for all patients in the MPNST cohort shows (B) overall survival (OS), (C) metastasis-free survival, and (D) locoregional failure-free rate. (E) OS, (F) metastasis-free survival, and (G) locoregional failure-free rate (LFFR) based on adjuvant radiation therapy reveals significant improvement in LFFR associated with radiation (P = .05). (H) OS, (I) metastasis-free survival, and (J) locoregional failure-free rate based on based on clinical NF1 status reveals no significant differences in patients with NF1-associated MPNST.
In our cohort, 5-year OS was 58% (Figure 1B), 5-year MFS was 68% (Figure 1C), and 5-year LFFR was 66% (Figure 1D). Patients who received adjuvant radiotherapy demonstrated no significant benefit with regard to OS (Figure 1E; P = .5) or MFS (Figure 1F; P = .4), but adjuvant radiotherapy improved LFFR (Figure 1G; P = .05). Patients who received adjuvant chemotherapy demonstrated no significant benefit to OS (P = .3), MFS (P = .2), or LFFR (P = .9). There were no significant differences between NF1-associated and sporadic MPNSTs for OS (Figure 1H; P = .44), MFS (Figure 1I; P = .90), or LFFR (Figure 1J; P = .33). On univariate CPH analysis for these baseline clinical parameters (Table 2), increased tumor size was significantly associated with poor MFS (hazard ratio [HR] 1.09 per cm, P = .01) and poor OS (HR 1.07 per cm, P = .03), male sex was associated with poor MFS (HR 6.23, P = .002), and gross total resection (HR 0.37, P = .06) and adjuvant radiotherapy (HR 0.36, P = .05) trended toward improved LFFR.
Table 2.
Univariate and Multivariate Cox Proportional Hazards Analysis for Clinical and Histopathologic Features
| UVA - LFFR | UVA - MFS | UVA - OS | MVA - LFFR | MVA - MFS | MVA - OS | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR | P-value (Log-rank) | HR | P-value (Log-rank) | HR | P-value (Log-rank) | HR | P-value | HR | P-value | HR | P-value | |
| Male | 1.001 (0.35–2.89) | 1 | 6.23 (1.72–22.52) | .002 | 1.17 (0.53–2.57) | .7 | 5.61 (1.35–23.37) | .02 | ||||
| Age | 1.003 per year (0.97–1.04) | .9 | 1.02 per year (0.99–1.05) | .2 | 1.01 per year (0.99–1.04) | .2 | ||||||
| Neurofibromatosis Type 1 | 1.69 (0.21–1.70) | .3 | 0.96 (0.34–2.73) | .9 | 1.38 (0.60–3.18) | .4 | ||||||
| Maximum tumor dimension | 0.94 per cm (0.83– 1.06) | .3 | 1.09 per cm (1.02–1.18) | .01 | 1.07 per cm (1.01–1.13) | .03 | - | .9 | - | .4 | ||
| Adjuvant RT | 0.36 (0.12–1.06) | .05 | 1.63 (0.56–4.79) | .4 | 1.29 (0.56–2.96) | .5 | 0.37 (0.13–1.10) | .07 | ||||
| Adjuvant chemotherapy | 0.96 (0.33–2.81) | .9 | 1.89 (0.69–5.23) | .2 | 1.50 (0.66–3.37) | .3 | ||||||
| GTR | 0.37 (0.13–1.09) | .06 | 1.88 (0.61–5.71) | .3 | 0.52 (0.24–1.13) | .1 | 0.40 (0.14–1.17) | .09 | ||||
| Positive margin | 1.19 (0.15–9.50) | .8 | 1.03e8 (0-Inf) | .2 | 1.55 (0.36–6.73) | .4 | ||||||
| FNCLCC grade | ||||||||||||
| 2 vs 1 | 3.26 (0.37–28.50) | .3 | 9.5e8 (0-Inf)* | .2 | 1.7e8 (0-Inf)* | .08 | ||||||
| 3 vs 1 | 3.16 (0.40–25.02) | .3 | 2.8e8 (0-Inf)* | .06 | 3e8 (0-Inf)* | .01 | ||||||
| 3 vs 2 | 0.96 (0.31–2.95) | .9 | 2.67 (0.75–9.57) | .1 | 1.86 (0.75–4.66) | .18 | ||||||
| Modified FNCLCC grade | 1.34 (0.47–3.79) | .6 | 3.95 (1.10–14.16) | .02 | 2.97 (1.19–7.39) | .01 | - | .4 | - | .8 | ||
| Ki-67 labeling index | 1.14 per 10% increase (0.93–1.39) | .2 | 1.20 per 10% (0.98–1.48) | .07 | 1.36 per 10% (1.14–1.63) | .0002 | - | .7 | 1.29 per 10% increase (1.05–1.59) | .02 | ||
| H3K27me3 loss | 0.68 (0.19–2.44) | .5 | 0.81 (0.13–4.92) | .8 | 0.52 (0.16–1.61) | .2 | ||||||
Bold, P < 0.1. FNCLCC, Fédération Nationale des Centres de Lutte Contre Le Cancer; GTR, gross total resection; MPNST, Malignant peripheral nerve sheath tumor; LFFR, locoregional failure-free rate; MFS, metastasis-free survival; MVA, multivariate analysis; OS, overall survival; UVA, univariate analysis.
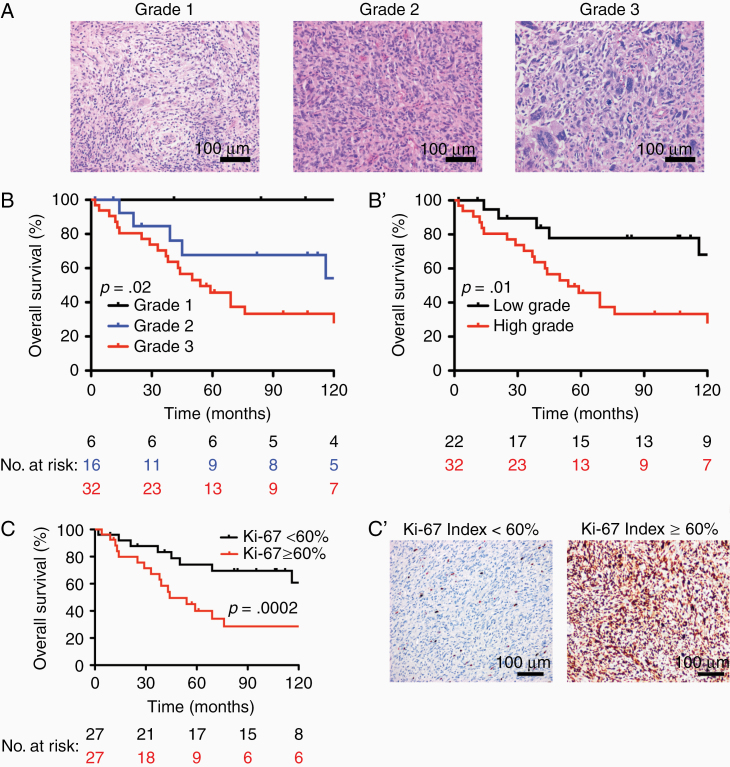
We next explored histopathologic and IHC characteristics within our cohort. FNCLCC grading was performed as mentioned above across a histologically diverse spectrum of MPNST (Figure 2A). FNCLCC grade segregated patients into groups with significant differences in OS (Figure 2B; P = .02, log-rank test). Of note, FNCLCC grade 1 tumors appeared to have both improved OS and a trend toward improved MFS on univariate analysis, although there were only 6 cases meeting this histologic criterion. We generated a 2-tier system combining grades 1 and 2 as “lower-grade” and assigning grade 3 as “high-grade,” and showed that patients with high-grade tumors have worse MFS (P = 0.02) and OS (Figure 2B’; P = .01) compared with patients with low-grade tumors. We then evaluated the components of the FNCLCC grading separately (Table 3) and showed that tumor differentiation score of 1 trended toward better OS (P = .07), and tumor differentiation score of 3 was associated with worse MFS (P = .03). Presence of necrosis was associated with worse OS (P = .0001) and MFS (.03). There was no difference in OS or MFS based on a necrosis score of 1 versus 2 on univariate analysis (P = .80 and P = .40, respectively). Higher mitotic activity score was associated with worse OS (P = .04), but there was no association between mitotic activity score and MFS. LFFR was not associated with any of the FNLCC components. Immunohistochemically, significantly elevated Ki-67 labeling index (defined as greater than the cohort median of 60%; Figure 2C–C’; P = .0002) was associated with significantly worse OS. Similarly, on univariate CPH analysis, increasing Ki-67 labeling index was associated with poor OS (HR 1.36 per 10%, P = .0002) and trended toward worse MFS (HR 1.2 per 10%, P = .07). Loss of H3K27me3 was not associated with OS (P = .2), MFS (P = .8), or LFFR (P = .5).
Figure 2.
Malignant peripheral nerve sheath tumor (MPNST) clinical outcomes stratified by immunohistochemical staining and Fédération Nationale des Centres de Lutte Contre Le Cancer (FNCLCC) grade. (A) Overall survival (OS) based on FNCLCC grade with (A) representative images of FNLCC grades 1, 2, and 3. OS based on (B) 3-tier FNCLCC grade and (B’) 2-tier modified FNCLCC grade. OS based on (C) Ki-67 labeling index with (C’) representative images of Ki-67 <60 versus Ki-67 ≥ 60.
Table 3.
Univariate Cox Proportional Hazards Analysis for FNCLCC Categories
| UVA - LFFR | UVA - MFS | UVA - OS | ||||
|---|---|---|---|---|---|---|
| HR | P-value (Log-rank) | HR | P-value (Log-rank) | HR | P-value (Log-rank) | |
| FNLCC Differentiation | ||||||
| 2 vs 1 | 2.13 (0.24–18.68) | .7 | 5.0e7 (0-Inf)* | .4 | 7.0e8 (0-Inf)* | .07 |
| 3 vs 1 | 1.17 (0.13–10.29) | .9 | 1.6e8 (0-Inf)* | .2 | 9.6e8 (0-Inf)* | .14 |
| 3 vs 2 | 0.55 (0.18–1.71) | .3 | 3.17 (1.08–9.32) | .03 | 1.37 (0.63–2.98) | .4 |
| FNLCC differentiation - continuous | 0.83 (0.39–1.79) | .6 | 3.48 (1.25–9.69) | .01 | 1.82 (0.94–3.54) | .07 |
| FNLCC Necrosis | ||||||
| 1 vs 0 | 1.58 (0.52–4.80) | .4 | 2.63 (0.69–10.09) | .1 | 8.96 (2.08–38.60 | .0004 |
| 2 vs 0 | 0.67 (0.08–5.74) | .7 | 4.60 (1.00–21.18) | .03 | 8.19 (1.58–42.57) | .001 |
| 2 vs 1 | 0.44 (0.06–3.57) | .4 | 1.67 (0.48–5.81) | .4 | 0.86 (0.43–3.14) | .8 |
| Necrosis - present or absent | 1.59 (0.53–4.71) | .4 | 3.58 (0.99–12.94) | .03 | 10.4 (2.45–44.4) | .0001 |
| FNLCC Mitosis | ||||||
| 2 vs 1 | 0.70 (0.06–7.77) | .9 | 2.86 (0.26–31.60) | .35 | 2.17 (0.36–13.02) | .3 |
| 3 vs 1 | 2.58 (0.57–11.66) | .2 | 4.14 (0.54–32.02) | .15 | 3.78 (0.89–16.16) | .07 |
| 3 vs 2 | 3.92 (0.50–30.81) | .2 | 1.44 (0.32–6.47) | .64 | 1.74 (0.51–5.89) | .4 |
| FNLCC mitosis- continuous | 1.80 (0.82–3.94) | .1 | 1.87 (0.80–4.37) | .1 | 1.90 (.0–3.61) | .04 |
| Mitoses/10hpf - continuous | 1.01 (0.99–1.03) | .4 | 1.01 (0.99–1.04) | .4 | 1.01 (0.99–1.03) | .1 |
Bold, P < 0.05. FNCLCC, Fédération Nationale des Centres de Lutte Contre Le Cancer; MPNST, Malignant peripheral nerve sheath tumor; LFFR, locoregional failure-free rate; MFS, metastasis-free survival; OS, overall survival; UVA, univariate analysis.
To identify prognostic variables for LFFR, MFS, and OS, we performed multivariate CPH analysis using variables with P values <.1 on univariate analysis, excluding the separate FNCLCC categories (Table 2). For LFFR, multivariate analysis revealed a trend toward improved outcomes for both adjuvant radiotherapy (P = .07) and gross total resection (P = .09). For MFS, only male sex remained significantly associated with poor MFS (P = .02) on multivariate analysis. Ki-67 labeling index remained a significant independent parameter associated with poor OS on multivariate analysis (P = .02). Taken together, these results identify adjuvant radiation therapy and resection status as important parameters in achieving local control and suggest that high Ki-67 index can be used as a histologic surrogate to predict OS.
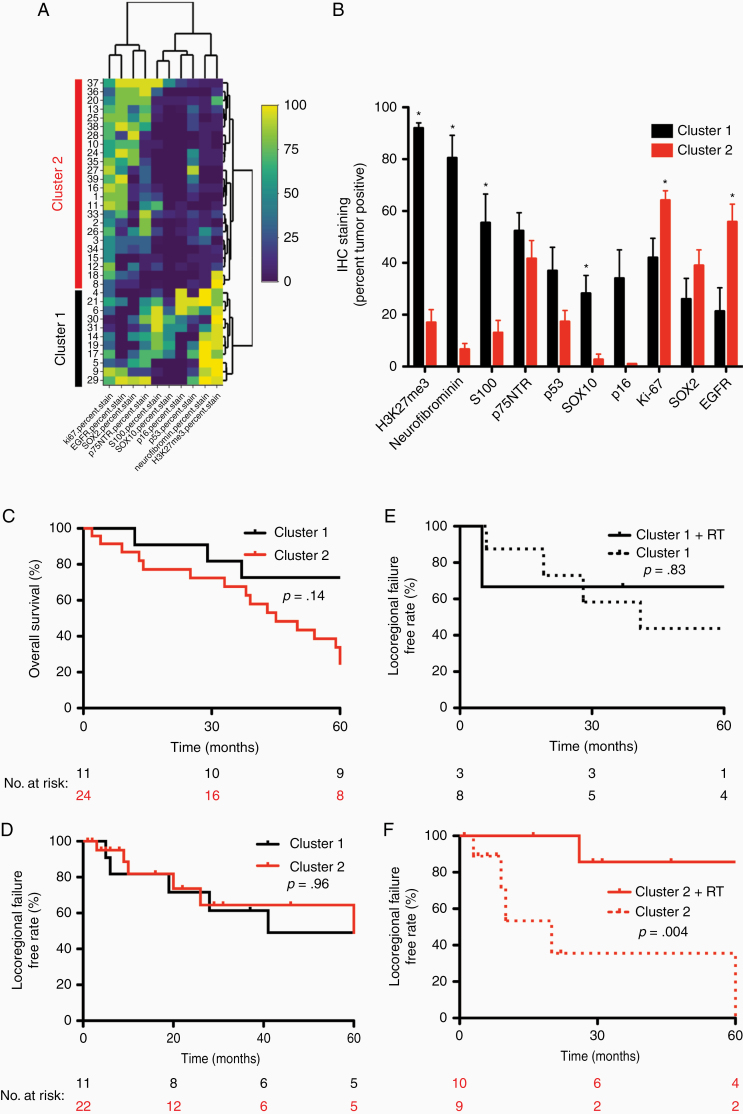
Finally, we hypothesized that examining a more diverse array of IHC stains targeting numerous proteins involved in various pathways implicated in MPNST biology may outperform any single stain and potentially identify prognostic and predictive immunohistochemical signatures.23–31 To that end, we performed staining for H3K27me3, EGFR, SOX2, p75NTR, S100, SOX10, p53, p16, and Neurofibromin on MPNSTs with sufficient available tissue and quantitatively scored the stains as percent positive tumor cells (n = 35). Unsupervised hierarchical clustering based on this larger IHC panel identified 2 subgroups of tumors (Figure 3A). Cluster 1 (n = 11) was characterized by retention of H3K27me3 and Neurofibromin staining, relative increased S100, SOX10, and p16 immunoreactivity, and relatively limited EGFR staining (Figure 3B). In contrast, Cluster 2 (n = 24) was characterized by decreased Neurofibromin (7% vs 80%, P = 4.7E−06), H3K27me3 (17% vs 92%, P = 2.7E−05), SOX10 (3% vs 28%, P = .001), S100 (13% vs 55%, P = .01), and p16 staining (3% vs 34%, P = .07), as well as increased EGFR staining (56% vs 21%, P = .01) and higher Ki-67 labeling (64% vs 42%, P = .02) (Supplementary Figure S1; Supplementary Table S2). Consistent with IHC results, clinical NF1 status was the only clinical characteristic that differed between subgroups, with Cluster 2 enriched for NF1 patients (Supplementary Table S3). With regard to clinical outcomes, no significant differences were observed between clusters with regard to OS (Figure 3C; P = .15) or LFFR (Figure 3D; P = .96). However, to determine whether MPNST clusters harbored predictive utility, we compared responses to adjuvant therapy between subgroups. While there was no difference in response to chemotherapy with regard to LFFR, MFS, or OS (data not shown), we found cluster status was predictive of response to radiation in our cohort. Although Cluster 1 patients appeared to have no LFFR benefit with adjuvant radiotherapy (Figure 3E; P = .83), Cluster 2 patients who received adjuvant radiotherapy showed significantly improved LFFR (Figure 3F; P = .004). Thus, our data broadly suggest that immunohistochemical profiles are prognostic and predictive for MPNST outcomes and incorporating these staining patterns into clinical decision-making may help guide treatment of MPNST patients.
Figure 3.
Malignant peripheral nerve sheath tumor (MPNST) clinical outcomes stratified by immunohistochemical clustering. (A) Hierarchical clustering heatmap of immunohistochemical (IHC) stains (x-axis) in MPNSTs (y-axis). (B) Histogram of IHC labeling distribution based on cluster. (C) Overall survival (OS) based on cluster. (D) Locoregional failure-free rate based on cluster. (E) OS for Cluster 1 stratified by adjuvant radiation therapy. (F) Locoregional failure-free rate for Cluster 2 stratified by adjuvant radiation therapy.
Discussion
Here, we report a single-institution MPNST experience with comprehensive clinical follow-up and histopathologic characterization. In this cohort, outcomes are similar to previously reported cohorts, with long-term OS of ~50% and high rates of metastasis and local failure.14,16,32–34 While adjuvant radiotherapy does not appear to affect OS or MFS, we demonstrate a trend toward improved LFFR with adjuvant radiotherapy. From a clinical perspective, MPNSTs arising in patients with a clinical NF1 diagnosis do not show significant differences in outcome, but univariate regression analysis did identify clinical factors associated with differences in MFS (male sex and tumor size) and OS (tumor size).
From a histopathologic perspective, univariate analysis demonstrates that increased grade, as determined by current FNCLCC criteria or using a modified 2-tier system, correlates with poor MFS and OS in MPNST. FNCLCC grading has been shown to be a reliable predictor of metastatic potential in many soft tissue tumors. However, in a review of 1240 patients with soft tissue sarcomas, FNCLCC grading did not show any significant predictive value in a subset of 72 MPNSTs.20 Other contemporary series have shown conflicting data regarding the prognostic significance of FNCLCC grading in MPNST.15,35,36 Thus, the current recommendation by the College of American Pathologists is to not employ the FNCLCC grading scheme in MPNSTs. While our cohort is relatively small, especially for tumors on the lower end of the grading scheme, our findings lend support to the use of FNCLCC grading criteria as a clinically prognostic score that may contribute to clinical decision-making in the treatment of individuals with MPNST.
Although mitotic score calculated by FNCLCC criteria was associated with poor OS, proliferation index calculated by Ki-67 labeling appears to be a better prognostic marker of OS on univariate and multivariate analysis. Increased Ki-67 labeling index was one of the first reported independent prognostic IHC markers for MPNST.37,38 While benign peripheral nerve sheath tumors such as schwannomas or neurofibromas may show an increased mitotic rate, the Ki-67 labeling index is significantly elevated in MPNST compared to benign peripheral nerve sheath tumors.18,37,39 Our results reiterate the predictive value of Ki-67 labeling as an independent prognostic marker for OS in MPNST.
Unsupervised hierarchical clustering of immunohistochemical profiles identifies 2 MPNST subgroups with significant differences in H3K27me3, Neurofibromin, S100, SOX10, p16, EGFR, and Ki-67 labeling. Cluster 2 shows significant loss of H3K27me3, Neurofibromin, S100, SOX10, and p16 labeling, with increased EGFR and Ki-67 labeling when compared with Cluster 1. Loss-of-function mutations in the genes encoding polycomb repressive complex 2 (PRC2) subunits EED and SUZ12 have been reported as oncogenic drivers in MPNST.40,41 PRC2 is a histone-modifying complex that functions as a protein lysine methyltransferase responsible for producing H3K27me3 which is often a repressive transcriptional mark.42 Complete loss of the H3K27me3 signature by IHC has been reported as a moderately sensitive and relatively specific marker for the diagnosis of MPNST.23,25–27,30 In addition to use as a candidate diagnostic marker, loss of H3K27me3 has been shown to have prognostic significance. Patients with MPNSTs that have loss of H3K27me3 have inferior survival compared with patients with MPNSTs that have intact H3K27me3.26 While we did note a spectrum of H3K27me3 labeling in our cohort of MPNST patients, we did not find a significant association between the retention of H3K27me3 staining and LFFR, MFS, or OS on univariate analysis. However, Cluster 2 (the group with decreased H3K27me3 labeling) exhibits improved LFFR associated with adjuvant radiotherapy while Cluster 1 did not show any benefit from adjuvant radiotherapy. While H3K27me3 status in isolation may not be a significant predictor of outcomes, integration of immunohistochemical results in MPNST may help predict response to adjuvant radiotherapy and help to stratify patients into different treatment paradigms.
In addition to decreased H3K27me3, Cluster 2 is characterized by decreased labeling with Neurofibromin as well as the lineage markers SOX10 and S100 and cell cycle regulator p16. Neurofibromin is a GTPase-activating protein encoded by NF1 and is involved in various cell proliferation and differentiation pathways in neural crest and mesenchymal stem cell–derived tissues.43 Loss of immunoreactivity of Neurofibromin has been reported in MPNST and implies dysregulation of protein production or expression.24 SOX10 is a transcription factor that regulates neural crest multipotency and is necessary for Schwann cell and melanocyte differentiation.44,45 Similarly, the S100 family of proteins are expressed in neural crest derived cells and immunoreactivity for S100 is often used diagnostically to support neural crest derivation. While SOX10 and S100 are usually preserved in benign peripheral nerve sheath tumors, they are often lost in MPNST.18 Similarly, damaging mutations to the CDKN2A locus encoding the p16 protein have been associated with malignant transformation of peripheral nerve sheath tumors.29,31 Taken together, the relative decrease of Neurofibromin, SOX10, S100, and p16 immunoreactivity in this cluster imply dysregulation of differentiation and regulatory factors important in neural crest-derived tissues. Increased EGFR expression by IHC has been reported in a minority of MPNST.18,28 Upregulation of EGFR in this cluster implies a specific targetable oncogenic driver, although this has not been confirmed with sequencing studies in this cohort. These data suggest that there is a biologically distinct group of MPNST with loss of Neurofibromin, S100, SOX10, and p16 labeling, in addition to loss of H3K27me3, that also have increased EGFR expression. We find that this group of tumors is enriched for patients with NF1, suggesting distinct tumor biology when compared to tumors arising sporadically.
Numerous factors underlie radiosensitivity of tumors, and in the particular case of MPNST Cluster 2, the observation of increased Ki-67 labeling in these tumors provides a putative biologic basis for increased radiosensitivity. With regard to Ki-67, radiation therapy has long been known to induce more efficient cell kill in cells actively undergoing mitosis in M phase, and recent reports have demonstrated a correlation between Ki-67 index and radiosensitivity.46,47 Additionally, while alterations in genes involved in double-strand break repair are not common in MPNST, and immunoreactivity for p53 was not increased in across our entire cohort, there is a trend toward decreased p53 expression in Cluster 2, potentially suggesting an aberrant DNA damage response underlying the observed difference in radiosensitivity.48 In summary, differences in cell proliferation and DNA damage response likely contribute to the observed radiosensitivity of Cluster 2 tumors although further work is needed to confirm and elucidate any such mechanisms of crosstalk.
In summary, we identify high FNCLCC grade and increased proliferation as determined by Ki-67 labeling as strong predictors of poor OS from MPNST. Furthermore, hierarchical clustering of immunohistochemical markers segregates MPNST into 2 subgroups demonstrating predictive significance with regard to LFFR in response to adjuvant radiotherapy. These data provide insights into the grading, immunohistochemical markers, and adjuvant treatment for patients with MPNST, shedding light on MPNST biology and treatment.
Supplementary Material
Supplementary material is available at Neuro-Oncology Advances online.
Supplementary Fig 1. Immunohistochemical pattern differences between Cluster 1 and Cluster 2.
Funding
This study is supported in part by the Residents’ Teaching and Research Endowment from the University of California San Francisco, Department of Anatomic Pathology. D.R.R. is supported by NIH NCI award K08 CA212279-01 and the UCSF Physician Scientist Scholar Program. H.N.V. is supported by a Children’s Tumor Foundation Young Investigator Award.
Conflict of interest statement. None of the authors have any conflicts of interest to disclose.
Authorship Statement. C.-H.G.L., H.N.V., and W.C.C. designed the study, performed experiments, and analyzed the data. C.-H.G.L., H.N.V., M.P., and D.R.R. wrote the manuscript. S.T.M. and L.J. provided samples and appraised the manuscript with S.E.B., S.D., F.J.R., and A.P. M.P. and D.R.R. designed and supervised the study.
References
- 1. Carli M, Ferrari A, Mattke A, et al. Pediatric malignant peripheral nerve sheath tumor: the Italian and German soft tissue sarcoma cooperative group. J Clin Oncol. 2005;23(33):8422–8430. [DOI] [PubMed] [Google Scholar]
- 2. Ng VY, Scharschmidt TJ, Mayerson JL, Fisher JL. Incidence and survival in sarcoma in the United States: a focus on musculoskeletal lesions. Anticancer Res. 2013;33(6):2597–2604. [PubMed] [Google Scholar]
- 3. Martin E, Muskens IS, Coert JH, Smith TR, Broekman MLD. Treatment and survival differences across tumor sites in malignant peripheral nerve sheath tumors: a SEER database analysis and review of the literature. Neurooncol Pract. 2019;6(2):134–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Evans DG, Baser ME, McGaughran J, Sharif S, Howard E, Moran A. Malignant peripheral nerve sheath tumours in neurofibromatosis 1. J Med Genet. 2002;39(5):311–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ducatman BS, Scheithauer BW, Piepgras DG, Reiman HM, Ilstrup DM. Malignant peripheral nerve sheath tumors. A clinicopathologic study of 120 cases. Cancer. 1986;57(10):2006–2021. [DOI] [PubMed] [Google Scholar]
- 6. Wong WW, Hirose T, Scheithauer BW, Schild SE, Gunderson LL. Malignant peripheral nerve sheath tumor: analysis of treatment outcome. Int J Radiat Oncol Biol Phys. 1998;42(2):351–360. [DOI] [PubMed] [Google Scholar]
- 7. Anghileri M, Miceli R, Fiore M, et al. Malignant peripheral nerve sheath tumors: prognostic factors and survival in a series of patients treated at a single institution. Cancer. 2006;107(5):1065–1074. [DOI] [PubMed] [Google Scholar]
- 8. Moretti VM, Crawford EA, Staddon AP, Lackman RD, Ogilvie CM. Early outcomes for malignant peripheral nerve sheath tumor treated with chemotherapy. Am J Clin Oncol. 2011;34(4):417–421. [DOI] [PubMed] [Google Scholar]
- 9. Farid M, Demicco EG, Garcia R, et al. Malignant peripheral nerve sheath tumors. Oncologist. 2014;19(2):193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kahn J, Gillespie A, Tsokos M, et al. Radiation therapy in management of sporadic and neurofibromatosis type 1-associated malignant peripheral nerve sheath tumors. Front Oncol. 2014;4:324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Reilly KM, Kim A, Blakely J, et al. Neurofibromatosis type 1-associated MPNST state of the science: outlining a research agenda for the future. J Natl Cancer Inst. 2017;109(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Widemann BC, Lu Y, Reinke D, et al. Targeting sporadic and neurofibromatosis type 1 (NF1) related refractory malignant peripheral nerve sheath tumors (MPNST) in a phase II study of everolimus in combination with Bevacizumab (SARC016). Sarcoma. 2019;2019:7656747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wanebo JE, Malik JM, VandenBerg SR, Wanebo HJ, Driesen N, Persing JA. Malignant peripheral nerve sheath tumors. A clinicopathologic study of 28 cases. Cancer. 1993;71(4):1247–1253. [DOI] [PubMed] [Google Scholar]
- 14. Stucky CC, Johnson KN, Gray RJ, et al. Malignant peripheral nerve sheath tumors (MPNST): the Mayo Clinic experience. Ann Surg Oncol. 2012;19(3):878–885. [DOI] [PubMed] [Google Scholar]
- 15. Valentin T, Le Cesne A, Ray-Coquard I, et al. Management and prognosis of malignant peripheral nerve sheath tumors: the experience of the French Sarcoma Group (GSF-GETO). Eur J Cancer. 2016;56:77–84. [DOI] [PubMed] [Google Scholar]
- 16. Bishop AJ, Zagars GK, Torres KE, Bird JE, Feig BW, Guadagnolo BA. Malignant peripheral nerve sheath tumors: a single institution’s experience using combined surgery and radiation therapy. Am J Clin Oncol. 2018;41(5):465–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Martin E, Coert JH, Flucke UE, et al. A nationwide cohort study on treatment and survival in patients with malignant peripheral nerve sheath tumours. Eur J Cancer. 2020;124:77–87. [DOI] [PubMed] [Google Scholar]
- 18. Pekmezci M, Reuss DE, Hirbe AC, et al. Morphologic and immunohistochemical features of malignant peripheral nerve sheath tumors and cellular schwannomas. Mod Pathol. 2015;28(2):187–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Miettinen MM, Antonescu CR, Fletcher CDM, et al. Histopathologic evaluation of atypical neurofibromatous tumors and their transformation into malignant peripheral nerve sheath tumor in patients with neurofibromatosis 1-a consensus overview. Hum Pathol. 2017;67:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Coindre JM, Terrier P, Guillou L, et al. Predictive value of grade for metastasis development in the main histologic types of adult soft tissue sarcomas: a study of 1240 patients from the French Federation of Cancer Centers Sarcoma Group. Cancer. 2001;91(10):1914–1926. [DOI] [PubMed] [Google Scholar]
- 21. Coindre JM. Grading of soft tissue sarcomas: review and update. Arch Pathol Lab Med. 2006;130(10):1448–1453. [DOI] [PubMed] [Google Scholar]
- 22. Nielsen GP, Chi P. Malignant peripheral nerve sheath tumor. In: WHO Classification of Tumours Editorial Board. Soft Tissue and Bone Tumours. 5th ed. Lyon, France: International Agency for Research on Cancer; 2020. [Google Scholar]
- 23. Pekmezci M, Cuevas-Ocampo AK, Perry A, Horvai AE. Significance of H3K27me3 loss in the diagnosis of malignant peripheral nerve sheath tumors. Mod Pathol. 2017;30(12):1710–1719. [DOI] [PubMed] [Google Scholar]
- 24. Reuss DE, Habel A, Hagenlocher C, et al. Neurofibromin specific antibody differentiates malignant peripheral nerve sheath tumors (MPNST) from other spindle cell neoplasms. Acta Neuropathol. 2014;127(4):565–572. [DOI] [PubMed] [Google Scholar]
- 25. Prieto-Granada CN, Wiesner T, Messina JL, Jungbluth AA, Chi P, Antonescu CR. Loss of H3K27me3 expression is a highly sensitive marker for sporadic and radiation-induced MPNST. Am J Surg Pathol. 2016;40(4):479–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cleven AH, Al Sannaa GA, Briaire-de Bruijn I, et al. Loss of H3K27 tri-methylation is a diagnostic marker for malignant peripheral nerve sheath tumors and an indicator for an inferior survival. Mod Pathol. 2016;29(9):1113. [DOI] [PubMed] [Google Scholar]
- 27. Mito JK, Qian X, Doyle LA, Hornick JL, Jo VY. Role of histone H3K27 trimethylation loss as a marker for malignant peripheral nerve sheath tumor in fine-needle aspiration and small biopsy specimens. Am J Clin Pathol. 2017;148(2):179–189. [DOI] [PubMed] [Google Scholar]
- 28. Jour G, Andeen NK, Al-Rohil R, et al. Novel enriched pathways in superficial malignant peripheral nerve sheath tumours and spindle/desmoplastic melanomas. J Pathol. 2018;244(1):97–106. [DOI] [PubMed] [Google Scholar]
- 29. Kaplan HG, Rostad S, Ross JS, Ali SM, Millis SZ. Genomic profiling in patients with malignant peripheral nerve sheath tumors reveals multiple pathways with targetable mutations. J Natl Compr Canc Netw. 2018;16(8):967–974. [DOI] [PubMed] [Google Scholar]
- 30. Lu VM, Marek T, Gilder HE, et al. H3K27 trimethylation loss in malignant peripheral nerve sheath tumor: a systematic review and meta-analysis with diagnostic implications. J Neurooncol. 2019;144(3):433–443. [DOI] [PubMed] [Google Scholar]
- 31. Rhodes SD, He Y, Smith A, et al. Cdkn2a (Arf) loss drives NF1-associated atypical neurofibroma and malignant transformation. Hum Mol Genet. 2019;28(16):2752–2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zou C, Smith KD, Liu J, et al. Clinical, pathological, and molecular variables predictive of malignant peripheral nerve sheath tumor outcome. Ann Surg. 2009;249(6):1014–1022. [DOI] [PubMed] [Google Scholar]
- 33. Kolberg M, Høland M, Agesen TH, et al. Survival meta-analyses for >1800 malignant peripheral nerve sheath tumor patients with and without neurofibromatosis type 1. Neuro Oncol. 2013;15(2):135–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fan Q, Yang J, Wang G. Clinical and molecular prognostic predictors of malignant peripheral nerve sheath tumor. Clin Transl Oncol. 2014;16(2):191–199. [DOI] [PubMed] [Google Scholar]
- 35. Chou YS, Liu CY, Chang YH, et al. Prognostic factors of primary resected retroperitoneal soft tissue sarcoma: analysis from a single Asian tertiary center and external validation of Gronchi’s nomogram. J Surg Oncol. 2016;113(4):355–360. [DOI] [PubMed] [Google Scholar]
- 36. Singh HP, Grover S, Garg B, Sood N. Histopathological spectrum of soft-tissue tumors with immunohistochemistry correlation and FNCLCC grading: A North Indian Experience. Niger Med J. 2017;58(5):149–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Watanabe T, Oda Y, Tamiya S, Kinukawa N, Masuda K, Tsuneyoshi M. Malignant peripheral nerve sheath tumours: high Ki67 labelling index is the significant prognostic indicator. Histopathology. 2001;39(2):187–197. [DOI] [PubMed] [Google Scholar]
- 38. Yuan Z, Xu L, Zhao Z, et al. Clinicopathological features and prognosis of malignant peripheral nerve sheath tumor: a retrospective study of 159 cases from 1999 to 2016. Oncotarget. 2017;8(62):104785–104795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kindblom LG, Ahldén M, Meis-Kindblom JM, Stenman G. Immunohistochemical and molecular analysis of p53, MDM2, proliferating cell nuclear antigen and Ki67 in benign and malignant peripheral nerve sheath tumours. Virchows Arch. 1995;427(1):19–26. [DOI] [PubMed] [Google Scholar]
- 40. Lee W, Teckie S, Wiesner T, et al. PRC2 is recurrently inactivated through EED or SUZ12 loss in malignant peripheral nerve sheath tumors. Nat Genet. 2014;46(11):1227–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zhang M, Wang Y, Jones S, et al. Somatic mutations of SUZ12 in malignant peripheral nerve sheath tumors. Nat Genet. 2014;46(11):1170–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kadoch C, Copeland RA, Keilhack H. PRC2 and SWI/SNF chromatin remodeling complexes in health and disease. Biochemistry. 2016;55(11):1600–1614. [DOI] [PubMed] [Google Scholar]
- 43. Abramowicz A, Gos M. Neurofibromin in neurofibromatosis type 1 - mutations in NF1gene as a cause of disease. Dev Period Med. 2014;18(3):297–306. [PubMed] [Google Scholar]
- 44. Britsch S, Goerich DE, Riethmacher D, et al. The transcription factor Sox10 is a key regulator of peripheral glial development. Genes Dev. 2001;15(1):66–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kim J, Lo L, Dormand E, Anderson DJ. SOX10 maintains multipotency and inhibits neuronal differentiation of neural crest stem cells. Neuron. 2003;38(1):17–31. [DOI] [PubMed] [Google Scholar]
- 46. Koelbl O, Rosenwald A, Haberl M, Müller J, Reuther J, Flentje M. p53 and Ki-67 as predictive markers for radiosensitivity in squamous cell carcinoma of the oral cavity? An immunohistochemical and clinicopathologic study. Int J Radiat Oncol Biol Phys. 2001;49(1): 147–154. [DOI] [PubMed] [Google Scholar]
- 47. Takeuchi H, Ozawa S, Ando N, Kitagawa Y, Ueda M, Kitajima M. Cell-cycle regulators and the Ki-67 labeling index can predict the response to chemoradiotherapy and the survival of patients with locally advanced squamous cell carcinoma of the esophagus. Ann Surg Oncol. 2003;10(7):792–800. [DOI] [PubMed] [Google Scholar]
- 48. Menon V, Povirk L. Involvement of p53 in the repair of DNA double strand breaks: multifaceted Roles of p53 in homologous recombination repair (HRR) and non-homologous end joining (NHEJ). Subcell Biochem. 2014;85:321–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.