Highlights
-
•
Rapidly growing mycobacteria (RGM) causing infections by biofilm formation.
-
•
Semi-quantitative method of biofilm formation was adapted for macrotechnics.
-
•
Sulphonamides complexed with metals is a promising anti-adhesion agent.
-
•
Sulphonamides complexed with metal is a possible inhibitor of signaling of biofilm formation.
Keywords: Rapidly growing mycobacteria, Sulfonamides, Metals, Biofilm, c-di-GMP
Abstract
Rapidly growing mycobacteria (RGM) are found in non-sterile water and often associated with severe post-surgical infections and affect immunocompromised patients. In addition, RGM can prevent the host's immune response and have the ability to adhere to and form biofilms on biological and synthetic substrates, making pharmacological treatment difficult because conventional antimicrobials are ineffective against biofilms. Thus, there is an urgent need for new antimicrobial compounds that can overcome these problems. In this context, sulfonamides complexed with Au, Cd, Ag, Cu, and Hg have shown excellent activity against various microorganisms. Considering the importance of combating RGM-associated infections, this study aimed to evaluate the activity of sulfonamide metal complexes against RGM biofilm. The sulfonamides were tested individually for their ability to inhibit mycobacterial formation and destroy the preformed biofilm of standard RGM strains, such as Mycobacterium abscessus, M. fortuitum, and M. massiliense. All sulfonamides complexed with metals could reduce, at subinhibitory concentrations, the adhesion and biofilm formation of three RGM species in polystyrene tubes. It is plausible that the anti-biofilm capacity of the compounds is due to the inhibition of c-di-GMP synthesis, which is an important signal for RGM biofilm formation. Hence, the impacts and scientific contribution of this study are based on the discovery of a potential new therapeutic option against RGM-associated biofilm infections. Sulfonamides complexed with metals have proven to be a useful and promising tool to reduce microbial adhesion on inert surfaces, stimulating the improvement of methodologies to insert compounds as new antibacterial and coating agents for medical and hospital materials.
1. Introduction
Rapidly growing mycobacteria (RGM) have been gaining prominence in clinical laboratories, being the species M. abscessus, M. chelonae, M. fortuitum, and M. smegmatis the most clinically reported RGM Misch et al. [24]. Associated with post-surgical, post-traumatic, and device-related infections, these organisms tend to form biofilms in surgical equipment, catheters, and prostheses, thus increasing disease resistance [31]. Moreover, research has demonstrated that 75% of patients with disseminated infections on the skin have been infected by M. fortuitum and M. chelonae. Similarly, M. abcessus was recently isolated from an infection related to knee prosthesis placement in a postoperative procedure [22]. The most common cutaneous or subcutaneous manifestations are caused by disseminated infections in immunocompromised patients, skin and soft tissue, and post-surgical infections [18].Table 1.
Table 1.
Values of the minimum inhibitory concentration (MIC) for the standard strains of rapidly growing mycobacteria.
| Compound |
M. fonuitum |
M. abscesses |
M. massiliense |
|---|---|---|---|
| MIC (pg/ml) | |||
| Sulfadiazine Au-PPh3 | 19.53 | 9.76 | 9.76 |
| Sulfadiazine Ph2P-Au-Au-PPh2 | 19.53 | 9.76 | 9.76 |
| Sulfamethoxazole Au-PPh3 | 39.06 | 19.53 | 19.53 |
| Sulfamethoxazole Ph2P-Au-Au-PPh2 | 39.06 | 19.53 | 19.53 |
| Sulfamethoxazolate Au | 19.53 | 19.53 | 9.76 |
| Sulfamethoxazole Ag | 39.06 | 19.53 | 9.76 |
| Sulfamethoxazole Cd | 19.53 | 4.88 | 4.88 |
| Sulfamethoxazole Hg | 9.76 | 4.88 | 4.88 |
| Sulfamethoxazole Cu | 9.76 | 39.06 | 19.53 |
| Sulfamethoxazole | 32 | 8 | 64 |
| Trimethoprim | 256 | 16 | 1 |
RGM-associated skin and soft tissue infections are deep and can result in progressive tenosynovitis. Traumatic injuries, surgical wounds, and environmental exposures (e.g., water) are major causes of RGM infections [18]. Despite RGM infections being common in immunocompromised patients and those with antecedent chronic diseases, recent studies have shown that RGM infections can occur in healthy patients with a history of surgical procedures such as liposuction and fat grafting [20].
Contributing factors may include using alternative medicine and procedures performed in freestanding surgical outpatient facilities that are not routinely monitored by infection-control committees or equivalent supervisory bodies. These establishments typically use tap water (non-sterile) for medical procedures and inappropriate instrument cleaning methodsDe Groote and Huitt [12].
Detection of mycobacteria in biofilm samples from different water systems have been reported, and rapidly growing species such as M. fortuitum and M. chelonae have been described as part of these polymicrobial biofilms [31], [14]. Furthermore, biofilm development capacity is related to the pathogenicity of these bacteria and antimicrobial resistance. The RGM can form biofilms in vitro, with differences regarding the importance of biofilms in the pathogenesis of human diseases [26].
Most bacteria can shift between two modes of action: single cells (planktonic mode) and biofilm (a sessile microbial community). Biofilm and planktonic cells differ significantly in their physiology, gene expression, and morphology. Biofilm cells are characterized by high production of adhesion factors and extracellular polysaccharides (EPS), lower sensitivity to antibiotics, and high resistance to environmental stress [13].
Mycobacteria in biofilms resist high antibiotic concentrations, unlike their planktonic counterparts [32], [9]. Biofilms formed by RGM were treated with amikacin, ciprofloxacin, clarithromycin, doxycycline, and sulfamethoxazole, and none of the antimicrobials could completely eradicate the bacterial biofilms. Furthermore, M. fortuitum was the microorganism that showed resistance to the antimicrobial inhibitory action when applied before the formation of mature biofilms [15].
Metal complexes with sulfamethoxazole have shown promising results against gram-positive and gram-negative bacteria and fungiAnacona and Osorio [3]. Furthermore, new sulfonamides complexed with metals have demonstrated in vitro activity against Mycobacterium tuberculosis, M. abscessus, M. fortuuitum, and M. massiliense, and its effects were potentiated with the combination trimethoprim [1], in addition to showing inhibitory activity against Escherichia coli biofilms [25]. These results are significant because they demonstrate that the coordination of antimicrobials with metals may be a promising new strategy to discover new anti-infective agents, especially with antibiofilm efficacy. Thus, these new sulfonamides were tested, for the first time, against RGM biofilms.
2. Materials and methods
2.1. Compounds
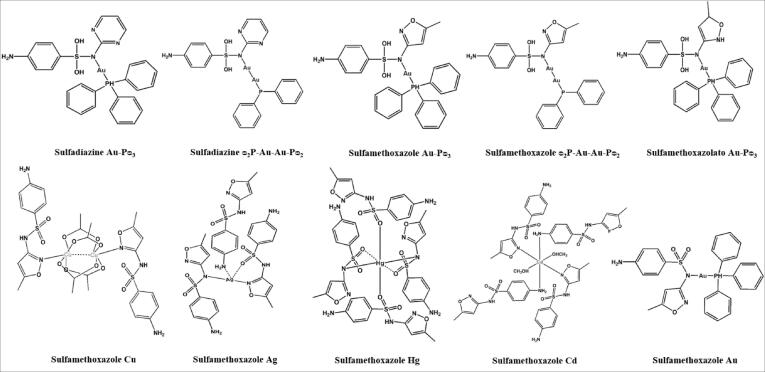
Compounds sulfadiazine Au-Pᴓ3, sulfadiazine ᴓ2P-Au-Au-Pᴓ2, sulfamethoxazole Au-Pᴓ3, sulfamethoxazole ᴓ2P-Au-Au-Pᴓ2, sulfamethoxazolato Au, sulfamethoxazole Ag, sulfamethoxazole Hg, sulfamethoxazole Cd and sulfamethoxazole Cu were synthesized in Laboratório de Materiais Inorgânicos (LMI) of Departamento de Química of Universidade Federal de Santa Maria [23]. The Fig. 1 shows the structures of the compounds. Sulfamethoxazole and trimethoprim were purchased from Sigma Chemical Company. Initial stock solutions of these drugs and sulfonamide compounds were dissolved in dimethyl sulphoxide (DMSO) at 50 mg/ml.
Fig. 1.
Structures of sulfonamide-derived compounds.
Dilutions were made in Mueller Hinton (Merck) broth (39.06 – 0.153 µg/ml for sulfonamides e 64 – 0,125 µg/ml for sulfamethoxazole). These concentration range were used in the tests based on the results of the minimum inhibitory concentrations (MICs) of the isolated compounds and in combination with the metals obtained by [2].
2.2. Strains and growth media
Three ATCC strains of Rapidly Growing Mycobacteria (RGM) were used, including M. abscessus (ATCC 19977), M. fortuitum (ATCC 6841) and M. massiliense (ATCC 48898). Standard strains were maintained on Löwenstein-Jensen (HiMedia Laboratories Pvt. Ltd, India) agar until needed.
2.3. Biofilm inhibition test
The sulfonamides were tested individually for their ability to inhibit biofilm formation of a mycobacterial species. The concentrations of the sulfonamides used were equal and lower than the MICs. The biofilm formation was adapted to macro-technique, maintaining the proportions of medium, antibacterial and inoculum. In polystyrene test tubes with a 5 mL capacity were added 1 mL of Middlebrook 7H9 medium containing 1x107 CFU /mL of each bacterial species to be tested and 1 mL of the dilution of the sulfonamides to be evaluated. The tubes were covered with parafilm® and incubated at 30 °C for 7 days [8].
2.4. Biofilm destruction test
Using the adapted technique, 1 mL of Middlebrook 7H9 medium containing 1x107 CFU/mL of the bacterial species were added in polystyrene tubes, which were covered with parafilm ® and incubated at 30 °C for 7 days [8]. After biofilm formation, 1 mL of sulfonamides was added to each tube in concentrations equal or higher than the MICs. The tube was covered with parafilm® and it was incubated at 30 °C for 24 h.
2.5. Biofilms quantification
The biofilm was quantified as described, adapted to macro-technique [6]. The cells that were weakly adhered to the biofilm were removed by the rinsing with saline and the remainder was dried at room temperature for a few minutes. After this, it was added 2 mL of a suspension of 0.1% crystal violet and the tubes were kept at rest for 10 min to further rinsing with saline to remove remaining planktonic cells and the excess dye. 2 mL of 95% ethanol were added to each test tube, kept for 15 min, and transferred to disposable cuvettes for a later reading in optical density (OD) of 570 nm. The biofilm formation was determined by the significant difference between the averages of absorbance obtained in the positive control (culture medium and bacteria) and the average obtained by the negative control (culture medium only). The experiments were performed in triplicate in three different times.
2.6. Statistical analysis
The optical density readings obtained in the biofilm formation assay was recorded as mean ± SE and submitted to the t test (compared with the positive control). A P value < 0.05 was considered to indicate statistical significance. Graphs were prepared using GraphPad Prism version 5.01 (GraphPad Software, La Jolla, CA).
3. Results and discussion
Bacterial biofilms can be formed in vivo and in vitro on biotic or abiotic surfaces, such as minerals, dead organisms, air–water interfaces, medical devices, plants, and other microbes and animals. This fact causes great concern, especially for health areas, due to the limited efficacy of antimicrobial treatments against biofilm infections Costerton et al. [11].
Several mycobacterial species are known to form biofilms and lipids in their cell wall, including mycolic acid, lipooligosaccharides, phytocoldoldiocycearates, phenolic glycolipids, lipoarabinomannans (LAM), and glycopeptidolipids (GPL), which are closely related to biofilm formation and influence colony morphology, antibiotic resistance, and mycobacterial virulence [32], [9], [15]. Moreover, in hydrophobic microorganisms, such as those of the genus Mycobacterium, biofilm formation is a successful survival strategy Costerton et al. [11].
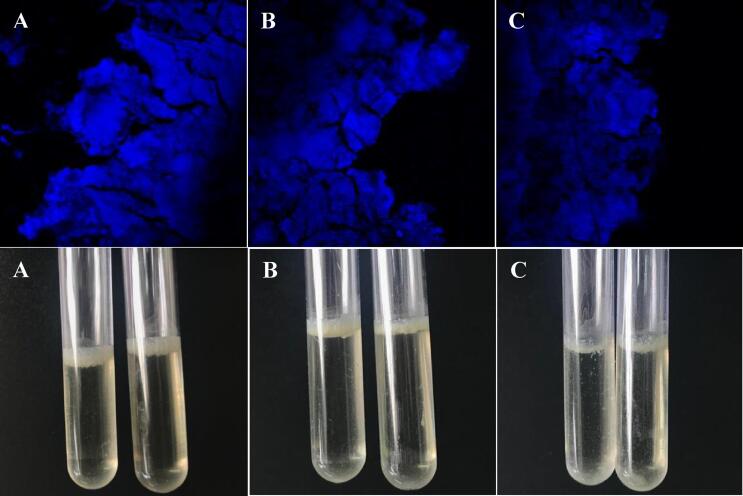
In this study, the three species of RGM could form highly dense and compact biofilms on the air–liquid interface and surface of polystyrene tubes (Fig. 2). Biofilms formed by mycobacteria have been reported in environmental studies, mainly in water systems. In addition, acute infections caused by microorganisms of the genus Mycobacterium are usually associated with contamination by planktonic organisms, while chronic infections appear to be closely associated with biofilm formation Maunders and Welch [21].
Fig. 2.
Biofilms formed on the liquid–air interface in polystyrene tubes by Mycobacterium abscessus (A), Mycobacterium fortuitum (B) and Mycobacterium massiliense (C). Viewed by microscope (Olympus Fluwil FV 10i) without using dyes and only with the autofluorescence produced by mycobacteria (upper images) and photos of the biofilm in the tubes (lower images).
In this sense, genes responsible for biofilm formation and metabolic and signal transduction pathways are potential targets for new drugs. In addition, these inhibitors can be used alone or in combination with conventional antimicrobial agents [19]. Therefore, we tested the sulfonamides associated with metals since they can enhance sulfonamide action, which is a class of drugs that damages bacterial metabolism and is safe, despite being ineffective against biofilms if used alone.
Sulfamethoxazole is a broad-spectrum sulfonamide and highly relevant in controlling infections caused by different pathogens, including RGMBrasil [7]. The clinical indication for sulfamethoxazole alone also persists, although activity decreases significantly when the infectious agent is in the form of biofilms or inside host macrophages [15].
Subinhibitory sulfonamide concentrations were used for testing in order to maintain bacterial cell viability. Therefore, the inoculum microorganisms remained after treatment with the drugs and were available to adhere to the tube surface and form the sessile structure.
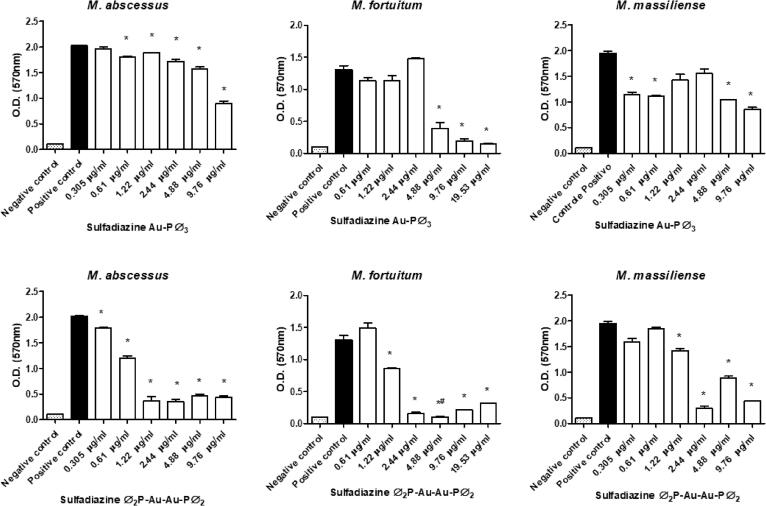
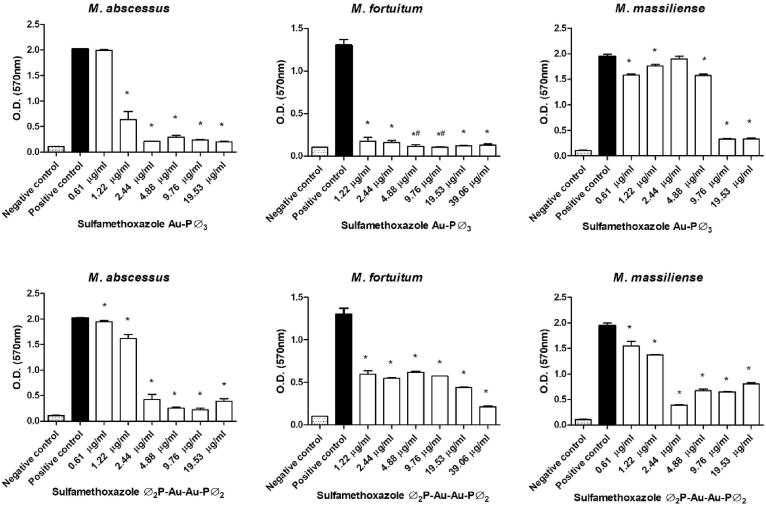
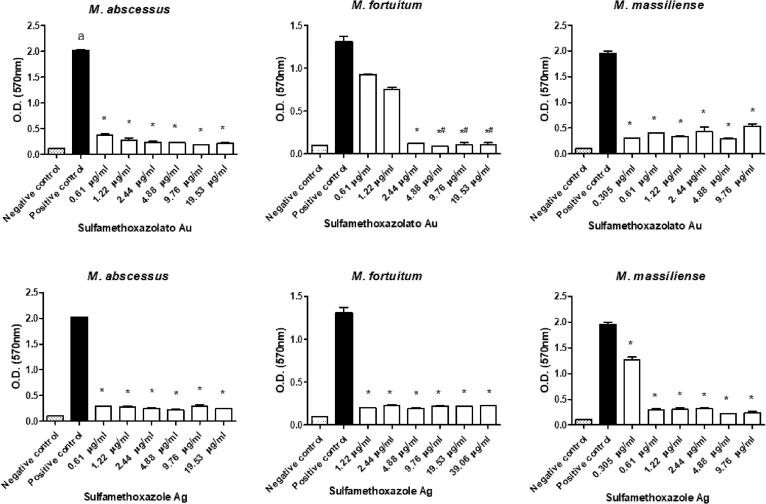
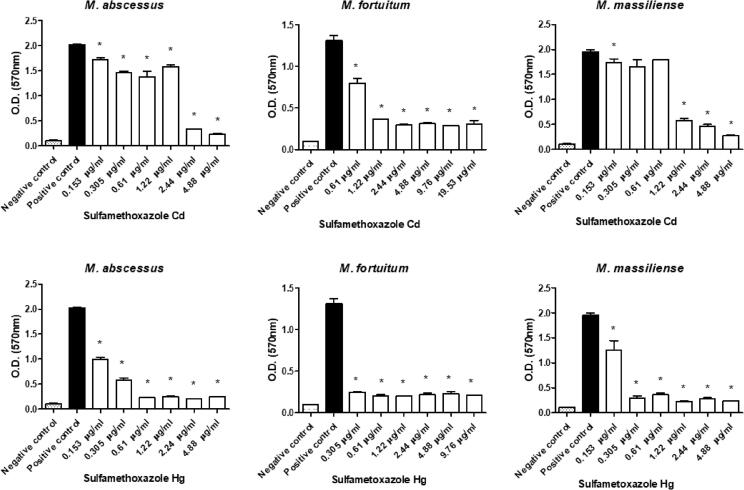
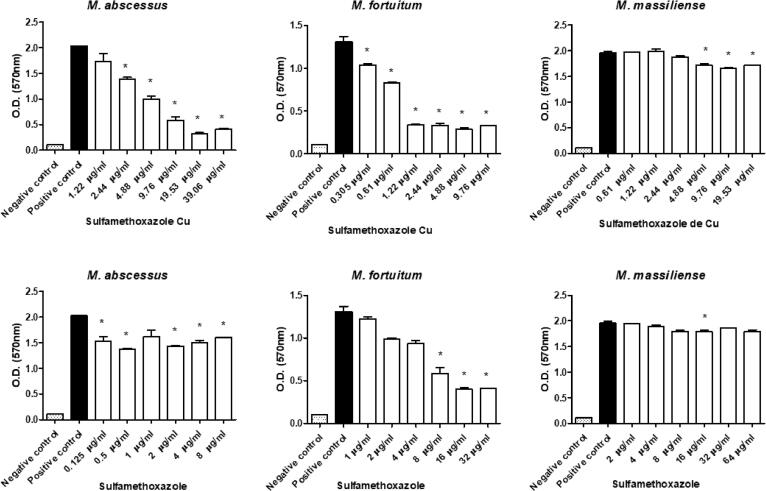
All sulfonamides complexed with metals showed activity and inhibited biofilm formation to some degree when used at subinhibitory concentrations. In addition, free sulfamethoxazole did not inhibit biofilm formation, while the sulfadiazine ᴓ2P-Au-Au-Pᴓ2 and sulfamethoxazole Au-Pᴓ3 completely inhibited M. fortuitum biofilm formation at some concentrations (Fig. 3, Fig. 4, Fig. 5, Fig. 6, Fig. 7). Nonetheless, sulfonamides complexed with metals could not destroy the previously formed biofilm (data not shown).
Fig. 3.
Optical densities (OD) of biofilms formed by a semi-quantitative technique using a violet crystal. The images show the differences in biofilm formation by M. abscessus, M. fortuitum, and M. massiliense at increasing concentrations of sulfadiazine Au-Pᴓ3 (upper images) and sulfadiazine ᴓ2P-Au-Au-Pᴓ2 (lower images). (*)Statistically significant difference between the positive control and the corresponding concentration. (#) No statistically significant difference between the negative control and the corresponding concentration.
Fig. 4.
Optical densities (OD) of biofilms formed by a semi-quantitative technique using a violet crystal. The images show the differences in biofilm formation by M. abscessus, M. fortuitum, and M. massiliense at increasing concentrations of sulfamethoxazole Au-Pᴓ3 (upper images) and sulfamethoxazole ᴓ2P-Au-Au-Pᴓ2 (lower images). (*)Statistically significant difference between the positive control and the corresponding concentration. (#) No statistically significant difference between the negative control and the corresponding concentration.
Fig. 5.
Optical densities (OD) of biofilms formed by a semi-quantitative technique using a violet crystal. The images show the differences in biofilm formation by M. abscessus, M. fortuitum, and M. massiliense at increasing concentrations of sulfamethoxazolato Au (upper images) and sulfamethoxazole Ag (lower images). (*)Statistically significant difference between the positive control and the corresponding concentration. (#) No statistically significant difference between the negative control and the corresponding concentration.
Fig. 6.
Optical densities (OD) of biofilms formed by a semi-quantitative technique using a violet crystal. The images show the differences in biofilm formation by M. abscessus, M. fortuitum, and M. massiliense at increasing concentrations of sulfamethoxazole Cd (upper images) and sulfamethoxazole Hg (lower images). (*)Statistically significant difference between the positive control and the corresponding concentration.
Fig. 7.
Optical densities (OD) of biofilms formed by a semi-quantitative technique using a violet crystal. The images show the differences in biofilm formation by M. abscessus, M. fortuitum, and M. massiliense at increasing concentrations of sulfamethoxazole Cu (upper images) and sulfamethoxazole free (lower images). (*)Statistically significant difference between the positive control and the corresponding concentration.
High antimicrobial activity coordinated with the ions Ag, Au, and Hg is shown in Fig. 5, Fig. 6. These complexes prevented mycobacterial biofilm formation even at the lowest concentrations tested, with values 5 times lower than the MIC of the strains under study. This higher performance may have occurred due to the slow release of these ions to the environment, which allowed a more significant interaction with the different bacterial macromolecules and, consequently, resulted in possible changes in the metabolism, damage to cellular structures such as membranes, proteins, and DNA, affecting adhesion and biofilm formation [16], [17].
Moreover, our results demonstrate that, for all strains evaluated, sulfamethoxazole showed low effectiveness in inhibiting biofilm formation (Fig. 7). The drug presented moderate and irregular inhibition for the M. abcessus biofilm, effectiveness at one of the concentrations for M. fortuitum, and only the highest concentrations were effective for M. massiliense. These data corroborate findings in the literature that report the low efficacy of sulfamethoxazole in inhibiting the sessile structure formed by M. abcessus, M. fortuitum, and M. massiliense and confirm that sulfamethoxazole is ineffective in destroying biofilm [15], [29].
In this context, it is known that biofilm formation is often regulated by a coordinated process called quorum sensing (QS), a cell to cell communication and involved in modulating the social behavior of bacteria. A variety of small molecules mediate QS, such as cyclic-di-GMP (c-di-GMP) [28], discovered in 1987 by Benziman and coworkers, and an activator of cellulose synthesis in Acetobacter xylinus. Since then, several other bacterial species have been discovered, including mycobacteria. The cellular level of c-di-GMP in M. smegmatis may also be involved in biofilm formation under specific growth conditions [28].
Intracellular levels of c-di-GMP are determined by two classes of enzymes with opposite activities: diguanylate cyclases (DGC), which synthesize c-di-GMP, and c-di-GMP phosphodiesterase (PDE), which hydrolyze it into the inactive diguanylate phosphate (pGpG) form [28]. Genes involved in c-di-GMP biosynthesis and the number of molecules are conserved in all eubacteria while absent in animal species, suggesting that enzymes involved in c-di-GMP biosynthesis may be interesting targets for anti-biofilm agents [19]. A screening study of compounds capable of interfering with c-di-GMP synthesis demonstrated that sulfathiazole could inhibit c-di-GMP biosynthesis and prevent biofilm formation at subinhibitory concentrations in E. coli [4].
Therefore, as previously suggested herein, the sulfonamides complexed with metals prevent biofilm formation at concentrations that do not affect planktonic cell growth. These sulfonamides exert their effects by inhibiting the dihydropteroate synthase (DHPS), the target of sulfonamides. Furthermore, increasing the antimicrobial activity of the compounds coordinated with metal ions occurs is likely due to the presence of an electron donor present in the system in coordination compounds. These donor groups and the relocation of electrons within the chelate reduce the polarity of the complexed metal. The chelation process increases the lipophilic character of the central metal atom, favoring the penetration of the complex through the lipid layer of the cell membrane of the microorganism [10].
Sulfathiazole belongs to the sulfonamide class and is an inhibitor of di- and tetrahydrofolate biosynthesis via DHPS interactionVilchèze and Jacobs [30]. The depletion of intracellular tetrahydrofolate, in turn, affects various metabolic pathways, including purine nucleotide biosynthesis. The antimicrobial activity of sulfonamides can be overcome by growing bacteria in complex media, thus providing tetrahydrofolate metabolism products. Furthermore, sulfathiazole affects tetrahydrofolate biosynthesis and nucleotide metabolism. Nevertheless, it is probable that c-di-GMP biosynthesis inhibition by sulfathiazole does not occur through direct inhibition of DGC activity but indirect effects, such as alteration of nucleotide pools, affecting the availability of the DGC substrate [4].
Nucleotide biosynthesis inhibition can block the production of modified nucleotides that act as signaling molecules for biofilm formation, such as c-di-GMP. This fact stimulates the degradation of nucleotides and recycling triphosphate for DNA and RNA production. Another possibility is partial nucleotide biosynthesis inhibition, as seen at subinhibitory concentrations of sulfathiazole and fluorouracil, and may result in failure to deoxyribonucleotides for DNA replication. The bacterial cell may react by suppressing “non-essential” DNA synthesis, such as extracellular DNA production [19]. Extracellular DNA is an essential component of the RGM biofilm matrix; thus, the DNase treatment can prevent biofilm formation, which must be explored in future studies [5].
The resistance of bacterial biofilms to antimicrobials limits therapeutic options. Thus, biofilm inhibition and dispersion are interesting research topics for the scientific community. Innovative methods that explore biosynthesis and biofilm dispersion mediated by c-di-GMP should elucidate new targets for action, resulting in a more significant number of biofilm inhibitors that can be used directly or provide the starting material to develop new drugs [19].
In this context, we suggest that sulfonamide complexes with metals prevent biofilm formation, probably by inhibition of c-di-GMP synthesis. These results are significant and suggest that the coordination of metals with sulfonamides seems to be a new strategy for discovering new antimicrobial agents. The coordination of the metal with the sulfonamides should be well explored since there is a possibility of drug development, mainly for topical use, as in the case of coordinated silver ions for sulfadiazine [27].
Since many persistent infections occur due to biofilm formation in medical devices, formulations of metal sulfonamides may be incorporated as coating agents on the surfaces of medical-hospital materials, acting as prophylactic agents of microbial adhesion [32]. Hence, this study provides substantial results that complement the scientific basis for future research to combat persistent infections caused by RGM.
4. Conclusion
The present study demonstrated that all metals complexed with sulfonamides inhibited, to some degree, RGM biofilm formation at concentrations below the MIC values. However, none of the metal complexed sulfonamides could destroy RGM formed biofilms at concentrations above MIC values. Thus, these preliminary results justify further studies to elucidate the mechanisms of biofilm formation in mycobacteria and present new effective therapeutic possibilities to combat infections associated with the adhesion of these microorganisms.
Ethical statement
Our work did not involve studies with animals and humans
CRediT authorship contribution statement
Pauline Cordenonsi Bonez: Investigation, Data curation, Writing - review & editing. Vanessa Albertina Agertt: Investigation, Data curation, Writing - review & editing. Grazielle Guidolin Rossi: Investigation, Data curation, Writing - review & editing. Fallon dos Santos Siqueira: Writing - review & editing. Josiéli Demétrio Siqueira: Software. Lenice Lorenço Marques: Resources. Gelson Noe Manzoni de Oliveira: Resources. Roberto Christ Vianna Santos: Supervision. Marli Matiko Anraku de Campos: Supervision.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We Appreciate to the Laboratório de Materiais Inorgânicos (LMI) of Departamento de Química of Universidade Federal de Santa Maria by the synthesis of the compounds and CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico) and CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior) for scholarships.
References
- 1.Agertt V.A., Marques L.L., Bonez P.C., Dalmolin T.V., Manzoni de Oliveira G.N., de Campos M.M.A. Evaluation of antimycobacterial activity of a sulphonamide derivative. Tuberculosis. 2013;93(3):318–321. doi: 10.1016/j.tube.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 2.Agertt V.A., Bonez P.C., Rossi G.G., Flores V.d.C., Siqueira F.D.S., Mizdal C.R., Marques L.L., de Oliveira G.N.M., de Campos M.M.A. Identification of antimicrobial activity among new sulfonamide metal complexes for combating rapidly growing mycobacteria. Biometals. 2016;29(5):807–816. doi: 10.1007/s10534-016-9951-3. [DOI] [PubMed] [Google Scholar]
- 3.Anacona J.R., Osorio I. Synthesis and antibacterial activity of copper(II) complexes with sulphathiazole and cephalosporin ligands. Transition Met Chem. 2008;33(4):517–521. doi: 10.1007/s11243-008-9074-y. [DOI] [Google Scholar]
- 4.Antoniani D., Bocci P., Maciag A., Raffaelli N., Landini P. Monitoring of diguanylate cyclase activity and of cyclic-di-GMP biosynthesis by whole-cell assays suitable for high-throughput screening of biofilm inhibitors. Appl Microbiol Biotechnol. 2010;85(4):1095–1104. doi: 10.1007/s00253-009-2199-x. [DOI] [PubMed] [Google Scholar]
- 5.Aung TT, Yam JKH, Lin S, Salleh SM, Givskov M, Liu S, et al. Biofilms of pathogenic nontuberculous mycobacteria targeted by new therapeutic Approaches. Antimicrob Agents Chemother. 2016; 60 (1): 24-35. https://doi.org/10.1128/AAC.01509-15. [DOI] [PMC free article] [PubMed]
- 6.Bonez P.C., dos Santos Alves C.F., Dalmolin T.V., Agertt V.A., Mizdal C.R., Flores V.d.C., Marques J.B., Santos R.C.V., Anraku de Campos M.M. Chlorhexidine activity against bacterial biofilms. Am J Infect Control. 2013;41(12):e119–e122. doi: 10.1016/j.ajic.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 7.Brasil. Ministério da Saúde. Secretaria de Vigilância em Saúde. Departamento de Vigilância Epidemiológica. Manual nacional de vigilância laboratorial da tuberculose e outras micobactérias [Internet]. Brasília, 2008. Disponível em: http://bvsms.saude.gov.br/bvs/publicacoes/manual_vigilancia_laboratorial_tuberculose.pdf. Accessed 10 may 2020.
- 8.Carter G., Wu M., Drummond D.C., Bermudez L.E. Characterization of biofilm formation by clinical isolates of Mycobacterium avium. J Med Microbiol. 2003;52(9):747–752. doi: 10.1099/jmm.0.05224-0. [DOI] [PubMed] [Google Scholar]
- 9.Chakraborty P, Kumar A. The extracellular matrix of mycobacterial biofilms: could we shorten the treatment of mycobacterial infections? Microbial Cell. 2019; 6 (2): 105-22. doi: 10.15698/mic2019.02.667. [DOI] [PMC free article] [PubMed]
- 10.Chohan Z.H., Shad H.A., Youssoufi M.H., Ben Hadda T. Some new biologically active metal-based sulfonamide. Eur J Med Chem. 2010;45(7):2893–2901. doi: 10.1016/j.ejmech.2010.03.014. [DOI] [PubMed] [Google Scholar]
- 11.Costerton J.W., Stewart P.S., Greenberg E.P. Bacterial biofilms: A common cause of persistent infections. Science. 1999;284(5418):1318–1322. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- 12.De Groote M.A., Huitt G. Infections Due to Rapidly Growing Mycobacteria. Clin Infect Dis. 2006;42(12):1756–1763. doi: 10.1086/504381. [DOI] [PubMed] [Google Scholar]
- 13.Donlan R. Biofilm Formation: A Clinically Relevant Microbiological Process. CLIN INFECT DIS. 2001;33(8):1387–1392. doi: 10.1086/322972. [DOI] [PubMed] [Google Scholar]
- 14.Esteban J., Martín-de-Hijas N.Z., Kinnari T.J., Ayala G., Fernández-Roblas R., Gadea I. Biofilm development by potentially pathogenic non-pigmented rapidly growing mycobacteria. BMC Microbiol. 2008;8(1):184. doi: 10.1186/1471-2180-8-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flores V.d.C., Siqueira F.D.S., Mizdal C.R., Bonez P.C., Agertt V.A., Stefanello S.T., Rossi G.G., Campos M.M.A.d. Antibiofilm effect of antimicrobials used in the therapy of mycobacteriosis. Microb Pathog. 2016;99:229–235. doi: 10.1016/j.micpath.2016.08.017. [DOI] [PubMed] [Google Scholar]
- 16.Frei A. Metal Complexes, an Untapped Source of Antibiotic Potential? Antibiotics (Basel). 2020;9(2):90. doi: 10.3390/antibiotics9020090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kostenko V., Lyczak J., Turner K., Martinuzzi R.J. Impact of Silver-Containing Wound Dressings on Bacterial Biofilm Viability and Susceptibility to Antibiotics during Prolonged Treatment. AAC. 2010;54(12):5120–5131. doi: 10.1128/AAC.00825-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kothavade R.J., Dhurat R.S., Mishra S.N., Kothavade U.R. Clinical and laboratory aspects of the diagnosis and management of cutaneous and subcutaneous infections caused by rapidly growing mycobacteria. Eur J Clin Microbiol Infect Dis. 2013;32(2):161–188. doi: 10.1007/s10096-012-1766-8. [DOI] [PubMed] [Google Scholar]
- 19.Landini P., Antoniani D., Burgess J.G., Nijland R. Molecular mechanisms of compounds affecting bacterial biofilm formation and dispersal. Appl Microbiol Biotechnol. 2010;86(3):813–823. doi: 10.1007/s00253-010-2468-8. [DOI] [PubMed] [Google Scholar]
- 20.Lim J.M., Kim J.H., Yang H.J. Management of Infections with rapidly growing mycobacteria after unexpected complications of skin and subcutaneous surgical procedures. Arch Plast Surg. 2012;39(1):18–24. doi: 10.5999/aps.2012.39.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maunders E., Welch M. Matrix exopolysaccharides: the sticky side of biofilm formation. FEMS Microbiol Lett. 2017;364(13):1–20. doi: 10.1093/femsle/fnx120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Malhotra R., Bala K., Gautam D., Bhattacharya A., Xess A.B., Pandey P., Verma S., Singh U.B. Mycobacterium abscessus Periprosthetic joint infection following bilateral Total Knee arthroplasty. IDCases. 2019;17:e00542. doi: 10.1016/j.idcr.2019.e00542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marques L.L., Manzoni de Oliveira G., Schulz Lang E., Anraku de Campos M.M., Soccol Gris L.R. New gold(I) and silver(I) complexes of sulfamethoxazole: Synthesis, X-ray structural characterization and microbiological activities of triphenylphosphine(sulfamethoxazolato-N2)gold(I) and (sulfamethoxazolato)silver(I) Inorg Chem Commun. 2007;10(9):1083–1087. doi: 10.1016/j.inoche.2007.06.005. [DOI] [Google Scholar]
- 24.Misch E.A., Saddler C., Davis J.M. Skin and soft tissue infections due to nontuberculous mycobacteria. Curr Infect Dis Rep. 2018;20(4):6. doi: 10.1007/s11908-018-0611-3. [DOI] [PubMed] [Google Scholar]
- 25.Mizdal C., Stefanello S., Bonez P., Agertt V., Flores V., Rossi G., Siqueira F., Marques L., Campos M. Anti-biofilm and Antibacterial Effects of Novel Metal-coordinated Sulfamethoxazole Against Escherichia coli. LDDD. 2017;14(3):339–344. doi: 10.2174/1570180813666160930101653. [DOI] [Google Scholar]
- 26.Muñoz-Egea M.-C., García-Pedrazuela M., Mahillo I., García M.J., Esteban J. Autofluorescence as a Tool for Structural Analysis of Biofilms Formed by Nonpigmented Rapidly Growing Mycobacteria. Appl Environ Microbiol. 2013;79(3):1065–1067. doi: 10.1128/AEM.03149-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rocha D.P., Pinto G.F., Ruggiero R., de Oliveira C.A., Guerra W., Fontes A.P.S. Coordination of metals to antibiotics as a strategy to combat bacterial resistance. Quim Nova. 2011;34(1):111–118. doi: 10.1590/S0100-40422011000100022. [DOI] [Google Scholar]
- 28.Sharma I.M., Petchiappan A., Chatterji D. Quorum sensing and biofilm formation in mycobacteria: Role of c-di-GMP and methods to study this second messenger: QS, biofilm formation and c-di-GMP in mycobacteria. IUBMB Life. 2014;66(12):823–834. doi: 10.1002/iub.1339. [DOI] [PubMed] [Google Scholar]
- 29.Siqueira F.D.S., Rossi G.G., Machado A.K., Alves C.F.S., Flores V.C., Somavilla V.D., Agertt V.A., Siqueira J.D., Dias R.d.S., Copetti P.M., Sagrillo M.R., Back D.F., de Campos M.M.A. Sulfamethoxazole derivatives complexed with metals: a new alternative against biofilms of rapidly growing mycobacteria. Biofouling. 2018;34(8):893–911. doi: 10.1080/08927014.2018.1514497. [DOI] [PubMed] [Google Scholar]
- 30.Vilchèze C., Jacobs W.R., Jr. The Combination of Sulfamethoxazole, Trimethoprim, and Isoniazid or Rifampin Is Bactericidal and Prevents the Emergence of Drug Resistance in Mycobacterium tuberculosis. Antimicrob Agents Chemother. 2012;56(10):5142–5148. doi: 10.1128/AAC.00832-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xess AB, Bala K, Panigrahy A, Singh U. Mycobacterium fortuitum as a cause of acute CNS infection in an immune-competent girl undergoing repeated VP shunt surgeries. BMJ Case Rep. 2019; 12 (4): e226900. http://dx.doi.org/10.1136/bcr-2018-226900. [DOI] [PMC free article] [PubMed]
- 32.Xiang X., Deng W., Liu M., Xie J. Mycobacterium biofilms : factors involved in development, dispersal, and therapeutic strategies against biofilm-relevant pathogens. Crit Rev Eukaryot Gene Expr. 2014;24(3):269–279. doi: 10.1615/CritRevEukaryotGeneExpr.2014010545. [DOI] [PubMed] [Google Scholar]