Abstract
Trastuzumab, a key treatment for HER2-positive breast cancer, is available in weight-based IV and fixed-dose (600 mg) SC formulations. While the Phase 3 HannaH trial indicated non-inferiority of the SC formulation, there is some concern that the target plasma concentration may not be reached in overweight/obese patients whereas low-body-weight patients may be at risk of toxicity.
This scoping review evaluated whether overweight/obese patients are at risk of below-target exposure with fixed-dose SC trastuzumab, whether low-body-weight patients are at risk of increased toxicity, especially cardiotoxicity, and whether IV and SC trastuzumab are equivalent in terms of treatment-emergent adverse events (TEAEs) (e.g. infections). Thirty-seven publications that met the eligibility criteria were included.
Body weight is not an important determinant of exposure to trastuzumab at steady state (i.e. pre-dose cycle 8); however, real-world evidence suggests that the target concentration (20 μg/mL) may not be reached with the first SC dose in overweight/obese patients. There is no evidence that low-body-weight patients are at increased risk of cardiotoxicity with SC trastuzumab, although this may be confounded by the higher rate of cardiovascular comorbidities in overweight patients. In Phase 3 trials, SC trastuzumab was associated with higher rates of ISRs, ADAs and SAEs, the latter often requiring hospitalization and occurring during adjuvant treatment when patients are not burdened by chemotherapy.
The route of administration of trastuzumab (IV vs SC) in different treatment settings should be discussed with the patient, taking into account the risks and benefits associated with each route.
Keywords: Trastuzumab, Breast cancer, Subcutaneous, Pharmacokinetics, Fixed-dose, Body weight
Abbreviations: ADA, antidrug antibody; ARR, administration-related reaction; EBC, early breast cancer; ISR, injection site reaction; JBI, Joanna Briggs Institute; mAb, monoclonal antibody; MBC, metastatic breast cancer; MGC, metastatic gastric cancer; popPK, population pharmacokinetics; rHuPH20, recombinant human hyaluronidase; RWE, real-world evidence; TEAE, treatment-emergent adverse event
Highlights
-
•
A systematic scoping review of fixed-dose SC trastuzumab and body weight.
-
•
Trastuzumab levels may not reach target with the first dose in overweight patients.
-
•
Low body weight does not seem to increase cardiotoxicity risk with SC trastuzumab.
-
•
Adverse event rates were higher with SC than IV trastuzumab in Phase 3 trials.
-
•
The risks and benefits of IV vs SC trastuzumab should be discussed with the patient.
1. Introduction
Trastuzumab has been a mainstay in the treatment of HER2-positive breast cancer for 30 years. Until recently it was administered by IV infusion at a dose based on body weight, an approach widely used in oncology to overcome interindividual variability in drug exposure [1]. However, a fixed-dosed SC formulation (Hylecta™; Roche) was introduced the EU in 2013 and has recently become available in the USA.
1.1. Development of the IV weight-based dosing strategy
Optimizing the therapeutic index across a target patient population requires understanding of the exposure–effect relationship and interindividual variability in the PK of the therapeutic [2]. The optimal efficacy of monoclonal antibodies (mAbs) requires saturation of the accessible target sites [3]. The minimum target serum trough concentration (Ctrough) for trastuzumab is 20 μg/mL, based on studies in xenograft models and early clinical response data in patients with metastatic breast cancer (MBC) [4]. A population model for IV trastuzumab indicated that HER2 receptors are saturated in the clinical concentration range on the basis of linear PK, with a subsequent plateau in the dose–efficacy relationship [3].
Whilst studies of IV trastuzumab demonstrated that fixed weekly doses ≥250 mg achieved the target Ctrough of >20 μg/mL, with linear PK, a weight-based regimen was adopted after two phase 2 trials (H0551g and H0552g) suggested that intersubject variability in trastuzumab PK was related to body weight. Population PK (popPK) analysis showed that Ctrough 20 μg/mL was achieved in approximately 90% of patients at the approved IV dose, with a mean Ctrough of 25.0 μg/mL after the first dose [5].
1.2. SC versus IV administration
SC trastuzumab contains recombinant human hyaluronidase (rHuPH20) to enhance drug absorption and is administered as a fixed dose (600 mg) over 2–5 min [6]. This dose was determined from modeling and simulation using PK data from the Phase 1 dose-finding study [[7], [8], [9]]. The comparability of the IV and SC formulations in terms of efficacy and PK has been demonstrated in the non-inferiority HannaH trial [[10], [11], [12], [13]].
Whilst PK modeling for mAbs showed similar PK variability with weight-based and fixed doses [14], a PK model for trastuzumab indicated that fixed dosing resulted in greater variability and deviation than weight-based dosing [15]. In their initial review of SC trastuzumab, the FDA noted that transition to fixed dosing from weight-based dosing could lead to under- or over-dosing of patients in the extremes of the body weight spectrum, referring to the popPK model demonstrating that body weight was a significant covariate [16]. This may be a concern in some countries given the prevalence of overweight/obesity, and that obesity is an established risk factor for breast cancer in postmenopausal women [17]. For example, the age-adjusted prevalence of obesity among women aged over 40 years in the USA in 2017–18 was 43.3% [18]. In a US study of adjuvant trastuzumab in HER2-positive early breast cancer (EBC), 43% of 3017 patients were obese (BMI ≥ 30.0 kg/m2) at baseline [19]. The average BMI in women varies widely, for example from 21.8 kg/m2 in Japan, approximately 24 kg/m2 in Switzerland and France, to 29.0 kg/m2 in the USA [20].
The FDA review concluded that body-size based dosing is not required as SC trastuzumab achieved equal or higher Ctrough at pre-dose cycle 8 with no worse efficacy across body weight groups compared with IV trastuzumab [16], as reflected in the current US Prescribing Information [21]. Nevertheless, we wanted to explore whether the “one size fits all“ strategy adopted with SC trastuzumab is appropriate, particularly given that in the real-world setting a broader range of patients is encountered than in clinical trials. We also compared the safety and immunogenicity of the two formulations.
1.3. Choice of method
A scoping review was considered to be more appropriate than a formal systematic review because it is less restrictive – the search strategy is developed iteratively, allowing additional search terms and sources identified during the review process to be incorporated into the final search strategy. As the quality of included sources is not assessed against rigorous criteria, evidence from a variety of sources and study designs can be included. Scoping reviews are ideal for evaluating broader questions than would be possible with a systematic review, such as the nature of the available evidence for a particular intervention [22]. Indeed, scoping reviews are an ideal way to map the literature and identify more specific questions that might be subsequently addressed through a full systematic literature review [23]. The importance of the scoping review as a valid and accepted approach is recognized by Cochrane and PRISMA [22,24].
Direct evidence (e.g., efficacy data from SC trastuzumab studies) was used wherever possible. In the absence of direct evidence, indirect evidence was used, including popPK, exposure–response analyses, and data from dose-escalation studies of IV trastuzumab.
1.4. Objectives
The overall objective was to evaluate the appropriateness of fixed-dose SC trastuzumab across all patients with HER2-positive breast cancer. Three questions were considered:
-
•
Are overweight/obese patients at risk of sub-target exposure with fixed-dose SC trastuzumab?
-
•
Are low-body-weight patients at risk of increased toxicity, especially cardiotoxicity?
-
•
Are the two formulations equivalent in terms of TEAEs?
2. Method
The general principles outlined in the Joanna Briggs Institute (JBI) Reviewer’s Manual for conducting a scoping review [25] were followed. The full method is reported in the Supplementary appendix. The objectives of the scoping review were addressed using the PICOS (population, intervention, comparators, outcomes, study design/setting) criteria. The inclusion and exclusion criteria were developed separately for each review question (Supplementary Tables 1–3).
The search strategy was developed iteratively, as per the JBI-recommended three-step approach, allowing incorporation of search terms and sources identified during the review process [25]. Studies were identified by searching electronic databases, reference lists of relevant articles, regulatory agency websites, and conference proceedings (via the Conference Proceedings Citation Index). The core search strategy is provided in Supplementary Table 4, together with modifications to account for differences in syntax and thesaurus headings for searches of the Cochrane Library, SCOPUS and Web of Science (Supplementary Table 5–7).
The search results and citation screening were managed in an EndNote® library. Titles and abstracts (when available) were reviewed by two independent researchers in a standard two-pass review process. Final inclusion and exclusion was verified by the lead researcher. The final list of studies for inclusion in the review was agreed by the study team.
Data from eligible studies were extracted into a spreadsheet by the lead researcher and quality checked by an independent reviewer. Extracted study-level data included publication details, study, demographic and treatment characteristics, PK and efficacy outcomes, AEs (including cardiotoxicity), and rate of antidrug antibodies (ADAs). The findings were tabulated and a narrative synthesis developed, as per the guidelines [25].
3. Results and discussion
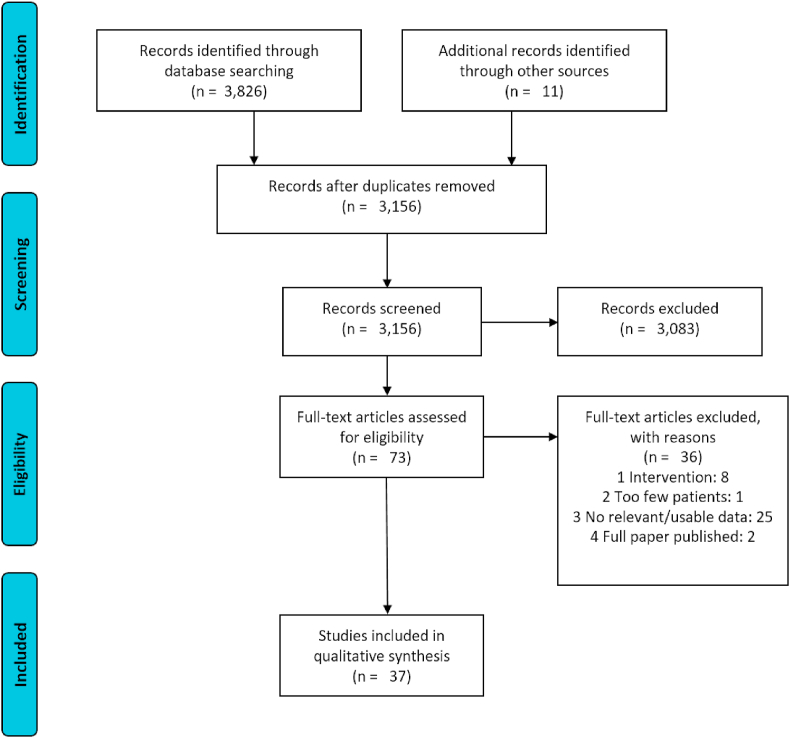
The literature search identified 3826 unique citations and a further 11 were identified from the grey literature/hand search. Thirty-seven publications met the eligibility criteria after de-duplication, screening and full text review, and were included in the scoping review; the PRISMA flow chart is shown in Fig. 1.
Fig. 1.
PRISMA diagram, illustrating the flow of articles though the scoping review. Adapted from [26].
Question 1: Are overweight/obese patients at risk of sub-target exposure with fixed-dose SC trastuzumab?
Relevant information was identified from 16 articles and two regulatory reports. Data directly comparing SC and IV trastuzumab by body weight or PK exposure come from the HannaH study, a Phase 3 non-inferiority study involved 596 patients with stage 1–3 HER2-positive EBC, who received neo/adjuvant trastuzumab once every 3 weeks, either SC (600 mg) or IV (8 mg/kg loading dose; 6 mg/kg maintenance dose) [10]. The co-primary endpoints were serum trough concentration of trastuzumab before surgery (Ctrough pre-dose cycle 8; i.e. steady state) and the pathologic complete response (pCR) rate; the PK profile was a secondary endpoint. Non-inferiority of SC versus IV trastuzumab was reported for both primary endpoints at 12 months [10], 20 months [12], 2 years [11] and 6 years [13]. Exploratory analyses at 20 months did not identify any associations between pCR or event-free survival (secondary endpoint) and body weight or exposure with SC trastuzumab [12].
PopPK models are widely used to determine the influence of different covariates, including body weight, on interindividual variability in PK [2]. PopPK models for trastuzumab consistently identified body weight [7,15,[27], [28], [29], [30], [31], [32]] or BMI [33] as a significant covariate for linear clearance (point estimate > 0.5 [1]) in HER2-positive EBC, MBC and metastatic gastric cancer (MGC). These are summarized in Supplementary Appendix Table 8.
Quartino and colleagues reported a popPK model for SC and IV trastuzumab based on the HannaH data; multiple logistic regression analysis determined that body weight did not affect the primary efficacy endpoint. Estimated odds ratios were similar across both weight and exposure quartiles, prompting the authors to conclude that dose adjustment for patient factors was not required within the ranges studied [7]. However, the point estimate for body weight on CL was 1.04, and body weight explained 8% of the total variability in linear clearance, 10% in the central volume of distribution, and 28% in peripheral volume of distribution [12]. Interpatient variability in clearance is particularly relevant because this strongly influences the serum concentration at the end of the dosing interval [34]. The CHMP reported that 92% of patients weighing ≥90 kg achieved steady-state Ctrough levels (>20 μg/mL), compared with 100% after weight-based IV dosing, and also concluded that an approximately bioequivalent SC dose based on steady state would be 8 mg/kg, which corresponds to fixed doses of about 400 mg, 600 mg and 750 mg for patients weighing <51, 51–90 and > 90 kg, respectively [35].
The authors of the HannaH report highlight the importance of exceeding the target trastuzumab concentration of 20 μg/mL after the first dose, in order to avoid the need for a loading dose [10]. However, the HannaH PK analysis was based on plasma levels at pre-dose cycle 8 (i.e., at steady state, near the end of neoadjuvant treatment) and does not report whether target concentrations of trastuzumab were achieved during the first cycle.
A Phase 1/1b study of weight-based dosing in healthy volunteers (n = 66) reported that 8 mg/kg SC trastuzumab was likely to achieve comparable serum levels at 22 days (end of cycle 1) as 6 mg/kg IV [8]. The mean BMI of patients in the 8 and 12 mg/kg groups was 28.7 and 26.8 kg/m2, respectively (range 17.3–48.3 kg/m2). Mean Ctrough at day 22 was 37.8 μg/mL with 8 mg/kg SC (60.8 μg/mL with 12 mg/kg SC).
Wynne also reported a simulated PK study in patients >75 kg (>75th percentile of the virtual population) comparing 600 mg SC and the approved IV dose. Simulated median Ctrough values for cycle 1 were 36 mg/L (i.e. 36 μg/dL) (5th–95th percentile 33–39 mg/L) and 38 mg/L (34–44 mg/L) respectively, and at cycle 7 were 69 mg/L (33–130 mg/L) and 53 mg/L (25–99 mg/L), respectively, indicating similar plasma levels with the two dosage regimens [9].
The median body weight used in the popPK model simulations was 68 kg (i.e., median body weight of the SC arm in HannaH) [7], which is lower than in patients typically seen in clinical practice. For example, >60% of a clinical sample of 1041 patients were overweight or obese (BMI >30 kg/m2) and > 20% weighed >80 kg [36]. Wynne and colleagues subsequently reported that 29% of the patients weighed >79 kg [9], in line with these real-world data. Nevertheless, a 600 mg SC dose in an 80 kg patient is equivalent to 7.5 mg/kg, which is less than the lower dose evaluated in the Phase 1 study.
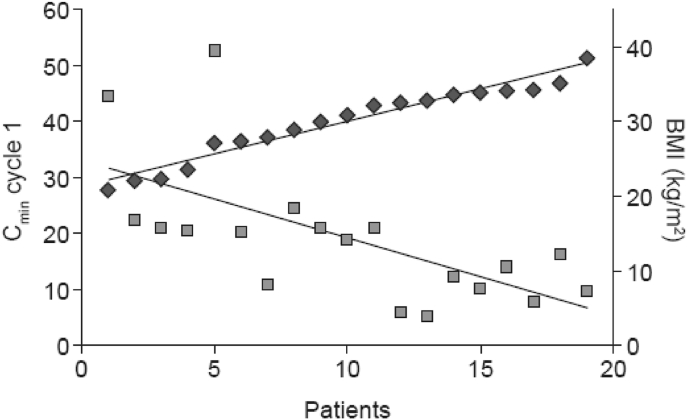
Two real-world studies in HER2-positive EBC looked at the PK from the first dose, and suggested that fewer patients achieve target trastuzumab levels during cycle 1 after SC administration than implied by HannaH findings and that this is influenced by body weight and BMI [37,38]. The HannaH study reported steady-state PK (pre-dose 8) data but not cycle 1 exposure [7,[10], [11], [12], [13]]. The target trastuzumab level in the real-world studies was Cmin ≥20 μg/mL as established in the early studies. In the first study (n = 19), initial (pre-dose cycle 2) exposure (Cmin) with SC trastuzumab decreased with higher body weight, such that half of patients weighing 65–79 kg achieved the target concentration after the first dose of SC trastuzumab, but none of the patients weighing ≥80 kg [37]. Similarly, the target was reached in 89% of patients with BMI ≤30 kg/m2 but only 10% of patients with BMI >30 kg/m2. Median body weight in this study was 75.9 kg (Fig. 2). The mean Cmin was lower in this study than in HannaH (19.0 [SD 12.1] vs 32.7 [18.5] μg/mL), which the authors attributed to the difference in body weight (75.9 vs 68.0 kg), indicating that weight and BMI influence trastuzumab PK [37].
Fig. 2.
Relationship between BMI and Cmin during the first treatment cycle with SC trastuzumab in patients with HER2-positive early breast cancer in real-world practice. Adapted from [37]
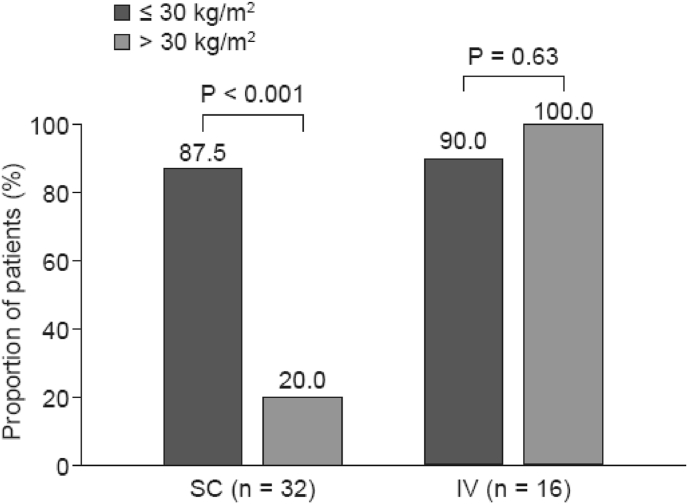
In the second study (n = 50), patients receiving weight-based dosing with IV trastuzumab had significantly higher initial concentrations of trastuzumab than those receiving fixed-dose (600 mg) SC trastuzumab. Significantly more patients in the IV group than in the SC group achieved the target concentration during the first treatment cycle (93.8% vs 67.6%; P = 0.042) [38]. As in the earlier study, body weight influenced whether the target exposure was reached with IV dosing (87.5% for patients with BMI ≤30 kg/m2 versus vs 20% with those >30 kg/m2 P < 0.001) but was not important with SC dosing (Fig. 3). Additional analyses identified BMI as the only covariate that had a significant impact on cycle 1 trastuzumab Cmin (P < 0.05). The authors therefore concluded that the PK profiles of fixed-dose (600 mg) and weight-based IV trastuzumab are not equivalent, particularly in obese patients [38]. However, these were small studies (n = 19 and 50) conducted by the same research group, so the generalizability is not clear.
Fig. 3.
Proportion of patients achieving target exposure in cycle 1 with SC vs IV trastuzumab by BMI in real-world practice. Target exposure was Cmin before cycle 2 ≥ 20 μg/mL. Adapted from [38].
Evidence suggesting that low initial (cycle 1) plasma levels may compromise the efficacy of trastuzumab come from studies in HER2-positive MGC/gastroesophageal cancer [30,[39], [40], [41], [42]], for which IV (but not SC) trastuzumab is indicated. PopPK and exposure–response analysis of data from the Phase 3 ToGA trial (which evaluated the addition of IV trastuzumab to chemotherapy in patients with HER2-positive MGC) found that patients with progressive disease had lower exposure to trastuzumab (based on median AUC, Cmax and Cmin) than patients who had had a partial or complete response or stable disease, although the 1.5 interquartile ranges overlapped [30]. The proportion of patients with progressive disease was highest in the lowest Cmin quartile (<17.3 μg/mL; Table 1) and survival was markedly shorter in patients with the lowest Cmin (≤25th percentile) at cycle 1 compared with the highest centile (overall survival [OS] 7.7 vs 13.8 months) [30]. Whilst patients in the lowest quartile also had the worse risk profile, a case–control comparison that matched patients in the lowest Cmin quartile with patients in the control arm of ToGA (who did not receive trastuzumab) found that the risk factors were no longer significant. Median OS was similar in the two groups, suggesting that trastuzumab conferred no survival benefit when Cmin was <11.8 μg/mL during the first treatment cycle. Whilst it is not appropriate to extrapolate efficacy findings from gastric to breast cancer, these PK results nevertheless indicate that early adequate exposure to trastuzumab may be important in terms of outcomes, which would need to be explored in breast cancer specifically.
Table 1.
Proportions of patients in each response category steady state Cmin quartile in the ToGA trial in MGC.
| Cmin quartile (μg/mL) | Complete response | Partial response | Stable disease | Progressive disease |
|---|---|---|---|---|
| <17.3 | 5.6 | 40.7 | 22.2 | 31.5 |
| ≥17.3 and < 27.6∗ | 5.5 | 49.1 | 36.4 | 9.1 |
| ≥27.6 and < 36.9∗ | 5.5 | 49.1 | 36.4 | 9.1 |
| ≥36.9 | 8.5 | 55.9 | 27.1 | 8.5 |
Values are % of patients in each Cmin (minimum plasma concentration) quartile who achieved each level of response.
∗Reported per published report although one row may be incorrect as it seems unlikely that they would be identical.
Source [30].
The FDA subsequently recommended a post-marketing trial in patients with MGC and higher tumor burden, to determine whether a higher dose of trastuzumab (with acceptable safety) would result in an acceptable OS benefit [39]. The Phase IIb HELOISE trial (n = 248) confirmed that a higher maintenance dose of trastuzumab (10 mg/kg every 3 weeks following the initial 8 mg/kg loading dose) achieved higher serum concentrations than the standard maintenance dose (6 mg/kg) but with no increase in efficacy [41]. However, the ability of this study to distinguish between low trastuzumab exposure and high disease burden as the cause of lower OS in ToGA has been strongly criticized [42] and the issue remains unresolved. The manufacturer of SC trastuzumab has not sought approval for gastric cancer indications because of PK differences expected in this patient population (i.e., lower trastuzumab exposure in MGC patients) [16].
Overall, the clinical trial evidence indicates that body weight does not affect exposure to trastuzumab once steady-steady PK are reached. Importantly, however, real-world evidence suggests that target serum concentrations (20 μg/mL) may not be reached with the first 600 mg SC dose in patients who are overweight or obese – an important consideration when switching from IV to SC dosing without a loading dose [10].
Question 2: Do patients with low body weight have the same risk for cardiotoxicity with SC and IV trastuzumab?
Early reports of increased cardiotoxicity with trastuzumab were largely attributed to co-administration with anthracyclines, and rates are generally lower in monotherapy trials; however, concern about the potential risk of cardiotoxicity with trastuzumab and other HER2-targeted agents persists [43,44]. We therefore explored whether low-body-weight patients are at increased risk of cardiotoxicity with the 600 mg SC dose.
Relevant information was identified from 15 articles reporting 12 studies of wide-ranging designs. In the HannaH study, steady-state AUC was about 80% higher with SC than with IV trastuzumab in patients <50 kg [4,12] but exploratory analyses did not identify any clinically meaningful association between exposure and the incidence of grade ≥3 adverse events (AEs) or serious AEs (SAEs) [12] or body weight [11,13] (Table 2).
Table 2.
Effect of body weight on Grade ≥3 AEs, SAEs, and cardiac AEs in the Phase 3 HannaH study of SC vs IV trastuzumab.
| Grade ≥3 AE, n (%)a |
SAEs, n (%)a |
Cardiac AEs, n/N (%)b,c |
||||
|---|---|---|---|---|---|---|
| Body weight (kg) | SC (n = 297) | IV (n = 298) | SC (n = 297) | IV (n = 298) | SC (n = 297) | IV (n = 298) |
| <59 | 37 (52%) | 50 (65%) | 11 (15%) | 15 (19%) | 6/71 (8.5%) | 9/77 (11.7%) |
| ≥59 to <68 | 37 (53%) | 42 (50%) | 15 (21%) | 8 (10%) | 8/70 (11.4%) | 9/84 (10.7%) |
| ≥68 to <79 | 42 (59%) | 31 (44%) | 17 (24%) | 13 (19%) | 14/71 (19.7%) | 6/70 (8.6%) |
| ≥79 | 43 (51%) | 33 (49%) | 21 (25%) | 6 (9%) | 14/85 (16.5%) | 16/67 (23.9%) |
Similarly, rates of grade ≥3 and cardiac AEs were not increased in lower-weight patients receiving SC trastuzumab in the SafeHer (single-arm SC trastuzumab) study [45].
In its multidisciplinary review of SC trastuzumab, the FDA commented that while the frequency of treatment-emergent cardiac AEs in the HannaH study was numerically highest in the highest weight quartile in the SC arm, the rate was similar in the highest weight quartile in the IV arm [16]. Similarly, in the SafeHer study, the incidence of treatment-emergent cardiac AEs was higher in the highest versus the lowest weight quartile [45]. The FDA suggested that this reflects the known comorbidities and risk associated with the highest weight quartile and that, overall, patients in the lowest weight quartile did not have an increased rate of treatment-emergent cardiac AEs [16]. A retrospective study (n = 260) found that BMI was not a risk factor for cardiotoxicity with SC trastuzumab [46].
Because obesity is associated with an increased risk for cardiotoxicity with both anthracyclines and trastuzumab [47], any potential increase in cardiotoxicity in low-weight patients receiving fixed-dose SC trastuzumab will be confounded by the higher rate of cardiotoxicity in obese patients. We therefore identified publications of early phase trastuzumab dose-escalation studies (Supplementary Table 9) and studies comparing high versus standard loading doses of IV trastuzumab (Supplementary Table 10), in order to investigate whether the rate of cardiotoxicity is increased with higher doses of trastuzumab. The five studies did not provide evidence of any increase in the rate of cardiotoxicity with higher doses of trastuzumab as monotherapy [8,48] or before/after chemoradiotherapy [[49], [50], [51]]. However, by the nature, these studies are short, restrict the maximum administered trastuzumab dose, and include few patients at each dose level. The maximum dose was likely guided by the target Ctrough for therapeutic effectiveness reported in the Phase 1 studies of IV monotherapy in MBC (H0407g and H0453g) but these have not been published so it is not known whether overdosing of trastuzumab per se was associated with an increased frequency of cardiotoxicity.
Three studies showed that higher loading doses of trastuzumab were not associated with an increased frequency of cardiac AEs [41,52,53]. However, any potential increase in rate of cardiotoxicity in low-weight patients receiving fixed-dose SC trastuzumab could be confounded by the higher rate of cardiotoxicity in obese patients.
Overall, this scoping review did not identify any evidence suggesting that patients of low-body-weight have an increased risk of cardiotoxicity with 600 mg SC trastuzumab.
Question 3: Is the risk of adverse events and immunogenicity the same for patients receiving SC and IV trastuzumab?
Relevant information was identified in 14 articles reporting five studies, including the EMA and FDA regulatory reports for SC trastuzumab and one rapid (systematic) review evaluating the efficacy and safety of SC and IV trastuzumab.
3.1. Overall toxicity
Data from the pivotal HannaH study showed similar rates of any-grade AEs with SC and IV trastuzumab (97% vs 94%) [10], and grade ≥3 AEs (54% vs 52%), with a numerically higher rate of treatment discontinuation because of AEs with SC trastuzumab (5.7%, vs 2.7% for IV) [12]. An imbalance in withdrawal of study treatment between groups at 20 months’ follow-up was attributed to cardiac disorders (3.0% [9/297] with SC trastuzumab versus 1.7% [5/298] with IV) but the rate of grade ≥3 cardiac AEs was low (5 [2%] vs 3 (1.0%)]. In the final analysis of HannaH (6 years’ follow-up), the incidence of cardiac AEs was similar in the SC and IV groups (14.8% vs 14.1%) and no new safety signals were identified [13].
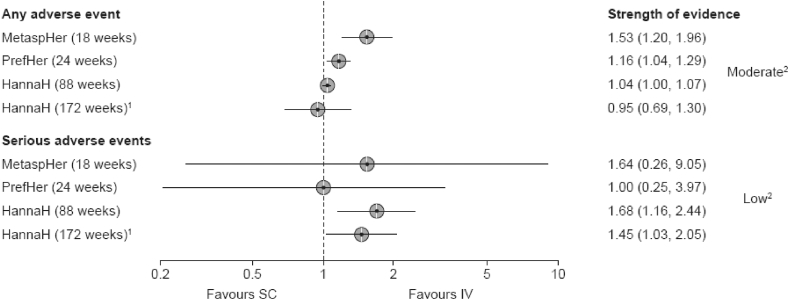
The rapid review of the HannaH, PrefHer [54,55] and MetaPher [56] studies reported a higher relative risk of experiencing an AE or SAE with SC versus IV trastuzumab (Fig. 4), due mainly to injection site-related AEs (e.g., pain and erythema) [57].
Fig. 4.
Forest plot of risk ratios for AEs and with SC vs IV trastuzumab (HannaH, PrefHer, and MetaPHER studies). Values are risk ratio and 95% confidence intervals. 1Drop-out rate 37%; 2Due to high risk of bias.
3.2. Serious adverse events
The rate of SAEs in the HannaH study was double with SC compared with IV trastuzumab (21% vs 12%), driven by a higher number of infections and infestations that required new or prolonged hospitalization (summarized in Supplementary Table 11) [10]. These events were manageable (resolving within a mean of 13 and 17 days in the IV and SC arms, respectively); however, the EMA pointed that the need for hospitalization or IV antibiotics for systemic infections is relevant from the patient’s perspective [4] and may compromise health-related quality of life (HRQL). These events also increase healthcare resource utilization, increasing costs. Importantly, the higher rate of infection-related SAEs and severe events with SC trastuzumab occurred during adjuvant treatment (i.e., during monotherapy), when the balance between benefit and risk of toxicity needs to be carefully considered, given that patients are not burdened by chemotherapy during this treatment phase [4].
3.3. Administration-related reactions
In HannaH, any-grade administration-related reaction (ARRs) occurred more frequently with SC than IV trastuzumab (48%, vs 37%) although the incidence of grade ≥3 reactions was low (around 2%) and there were no grade 4 ARRs [12]. The imbalance between arms was mostly due to disorders of the skin and SC tissue disorders or respiratory, thoracic, and mediastinal disorders [12].
Whilst late-onset ARRs might be expected with SC trastuzumab based on the PK profile [4], a retrospective single-center study did not find this to be the case [58]. Retrospective studies of SC trastuzumab have shown that ARRs with SC trastuzumab usually occur within 2 h of administration and are typically mild and self-limiting [[59], [60], [61]].
3.4. Injection-site reactions
Injection site reactions (ISRs) were experienced by 11% of patients receiving SC trastuzumab in the HannaH study [10]. While ISRs were mostly grade 1, avoiding these events may be relevant to patients who are already receiving other IV chemotherapies via a central line Indeed, in a real-world study in Germany, patients receiving chemotherapy or another IV antibody therapy in addition usually received IV trastuzumab, particularly if a central venous port was already in place, even though SC administration would have significantly reduced the time spent in the center by these patients. In these cases, avoiding additional injections was given preference over potential time savings [62].
3.5. Anti-drug antibodies
The incidence of trastuzumab ADAs was higher with SC than IV trastuzumab in the HannaH study (15% vs 7%), although neutralizing antibodies were detected in only two and one patients, respectively. The rate of rHuPH20 ADAs in the SC group was 16% (48/295). ADAs did not appear to affect PK, efficacy (pCR and EFS) or rate of ARRs [12].
3.6. Summary
Overall, SC administration of trastuzumab was associated with a higher rate of SAEs than IV administration [10], largely infections and infestations that required new or prolonged hospitalization [10], which may compromise HRQL and also incur healthcare costs. SAEs occurred largely during adjuvant treatment when patients are no longer burdened by chemotherapy. Thus, decisions on the appropriate route of administration should take into consideration the increased risk of SAEs, ISRs and ADAs with the SC formulation alongside potential benefits and patient preferences, particularly during adjuvant monotherapy [4] and in patients who already have a central line.
4. Study limitations
A scoping review is a validated method that allows mapping of disparate literature without the potential bias towards randomized controlled trials that is inherent in systematic review. This method is useful to identify issues in the literature that might be subsequently evaluated in a systematic review. Here we comment on the limits of the database in addressing the research questions.
The evidence base supporting the non-inferiority of SC trastuzumab is limited, originating solely from the prospective randomized HannaH study; no other clinical trials were identified in our systematic search.
All statistical comparisons for differences in AE rates between SC and IV trastuzumab in the HannaH study were descriptive.
In terms of immunogenicity, the HannaH study provided only a descriptive analysis whereas a recent narrative review reported a significant two-fold association between development of ADAs and receiving SC trastuzumab (p = 0.003; Fisher’s exact test) [63]. An association with neutralizing ADAs was not reported, and so far there has been no evidence that patients develop long-lasting memory ADAs or that this could affect the safety or efficacy of subsequent treatment with IV trastuzumab or pertuzumab [63]. ADA formation was monitored in the HannaH trial extension as an additional pharmacovigilance activity linked to the potential risk of immunogenicity [4]. The Phase 3b single-arm MetaPHER study, which evaluated the safety and efficacy of SC trastuzumab in combination with IV pertuzumab and docetaxel in the first-line treatment of HER2-positive MBC, reported a higher incidence of post-treatment ADAs to SC trastuzumab (24%) than in HannaH (15%), although this was not associated with an increased frequency or severity of ARRs [10,64]. In addition, a small observational study of 51 patients with non-metastatic HER2-positive breast cancer, published after this scoping review was completed, did not identify ADAs with IV (n = 24; median of 13 treatment cycles) or SC trastuzumab (n = 27; 10 cycles) [65]. However, these results should be interpreted with caution given the small sample.
5. Conclusions
This scoping review highlights the lack of data comparing IV and SC trastuzumab beyond the HannaH study. Real-world evidence suggests that a significant proportion of overweight/obese patients may not reach the target plasma concentration during the first cycle of treatment with 600 mg SC trastuzumab [37,38]. Given the prevalence of obesity in patients with breast cancer [19], this may be a concern in clinical practice, as dosing aims to reach the target concentration from the first cycle of treatment [10].
The scoping review did not identify evidence indicating that low-weight patients who receive a fixed SC dose of trastuzumab are at increased risk of cardiotoxicity.
In terms of safety, SC trastuzumab was associated with higher rates of infection-related and severe events requiring hospitalization, which affects patients and increases healthcare resource utilization and costs. Notably, these events occurred during adjuvant monotherapy when patients are not burdened by chemotherapy. The balance between benefit and risk of toxicity with SC versus IV trastuzumab therefore needs to be considered carefully in this setting [4]. The incidence of ADAs was also higher with SC than IV trastuzumab, although these were rarely neutralizing ADAs. Finally, SC trastuzumab was associated with ISRs in about 11% of patients in HannaH. When discussing treatment in a particular setting, the clinical benefit, risks and appropriate dosing of IV or SC trastuzumab should be discussed with the patient according to their individual needs.
Funding
This work was funded by Amgen Inc.
Declaration of competing interest
Hans-Christian Kolberg has received honoraria from Carl Zeiss meditec, Teva, Theraclion, Novartis, Amgen, AstraZeneca, Pfizer, Janssen-Cilag, GSK, LIV Pharma, Roche, MSD, SurgVision, Onkowissen, Daiichi Sankyo, and Genomic Health.
Christian Jackisch has received honorira from Amgen, AstraZeneca, Roche, Celgene, Pfizer, Novartis, Lilly, and Exact Sciences.
Sara Hurvitz has conducted research (fees paid to institution) for: Ambrx, Amgen, Arvinas, Bayer, Daiichi-Sankyo, Dignitana, Genentech/Roche, GSK, Immunomedics, Lilly, Macrogenics, Novartis, Pfizer, OBI Pharma, Pieris, PUMA, Radius, Sanofi and Seattle Genetics, and performs consultancy and holds stock options for NK Max.
Vladimir Hanes and Delphine Courmier are employees of Amgen, Inc.
Julie Winstone and Helen Barham have no declarations of interest.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.breast.2021.03.003.
Contributor Information
Hans-Christian Kolberg, Email: hans-christian.kolberg@mhb-bottrop.de.
Christian Jackisch, Email: Christian.Jackisch@sana.de.
Sara A. Hurvitz, Email: SHurvitz@mednet.ucla.edu.
Julie Winstone, Email: jwinstone@perscribo.co.uk.
Vladimir Hanes, Email: vhanes@amgen.com.
Delphine Courmier, Email: dcourmie@amgen.com.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Bai S., Jorga K., Xin Y., Jin D., Zheng Y., Damico-Beyer L.A. A guide to rational dosing of monoclonal antibodies. Clin Pharmacokinet. 2012;51:119–135. doi: 10.2165/11596370-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 2.Bajaj G., Suryawanshi S., Roy A., Gupta M. Evaluation of covariate effects on pharmacokinetics of monoclonal antibodies in oncology. Br J Clin Pharmacol. 2019;85:2045–2058. doi: 10.1111/bcp.13996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bittner B., Richter W.F., Hourcade-Potelleret F., McIntyre C., Herting F., Zepeda M.L. Development of a subcutaneous formulation for trastuzumab - nonclinical and clinical bridging approach to the approved intravenous dosing regimen. Arzneimittelforschung. 2012;62:401–409. doi: 10.1055/s-0032-1321831. [DOI] [PubMed] [Google Scholar]
- 4.EMA. reportCHMP assessment report. Herceptin. Procedure No. EMEA/H/C/000278. Available at: https://www.ema.europa.eu/en/documents/variation-report/herceptin-h-c-278-x-0060-epar-assessment-report-extension_en.pdf. 27 June. 2013.
- 5.Cobleigh M.A., Vogel C.L., Tripathy D., Robert N.J., Scholl S., Fehrenbacher L. Multinational study of the efficacy and safety of humanized anti-HER2 monoclonal antibody in women who have HER2-overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease. J Clin Oncol. 1999;17:2639–2648. doi: 10.1200/JCO.1999.17.9.2639. [DOI] [PubMed] [Google Scholar]
- 6.Roche Herceptin® 600 mg solution for injection in vial. Summary of product characteristics. Revised October 2019. 2019.
- 7.Quartino A.L., Hillenbach C., Li J., Li H., Wada R.D., Visich J. Population pharmacokinetic and exposure-response analysis for trastuzumab administered using a subcutaneous "manual syringe" injection or intravenously in women with HER2-positive early breast cancer. Canc Chemother Pharmacol. 2016;77:77–88. doi: 10.1007/s00280-015-2922-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wynne C., Harvey V., Schwabe C., Waaka D., McIntyre C., Bittner B. Comparison of subcutaneous and intravenous administration of trastuzumab: a phase I/Ib trial in healthy male volunteers and patients with HER2-positive breast cancer. J Clin Pharmacol. 2013;53:192–201. doi: 10.1177/0091270012436560. [DOI] [PubMed] [Google Scholar]
- 9.Wynne C., Harvey V., Schwabe C., Waaka D., McIntyre C., Bittner B. Reply to: letter to the editor "subcutaneous herceptin therapy. J Clin Pharmacol. 2013;53:1343–1344. doi: 10.1002/jcph.172. [DOI] [PubMed] [Google Scholar]
- 10.Ismael G., Hegg R., Muehlbauer S., Heinzmann D., Lum B., Kim S.-B. Subcutaneous versus intravenous administration of (neo)adjuvant trastuzumab in patients with HER2-positive, clinical stage I-III breast cancer (HannaH study): a phase 3, open-label, multicentre, randomised trial. Lancet Oncol. 2012;13:869–878. doi: 10.1016/S1470-2045(12)70329-7. [DOI] [PubMed] [Google Scholar]
- 11.Jackisch C., Hegg R., Stroyakovskiy D., Ahn J.-S., Melichar B., Chen S.-C. HannaH phase III randomised study: association of total pathological complete response with event-free survival in HER2-positive early breast cancer treated with neoadjuvant-adjuvant trastuzumab after 2 years of treatment-free follow-up. Eur J Canc. 2016;62:62–75. doi: 10.1016/j.ejca.2016.03.087. [DOI] [PubMed] [Google Scholar]
- 12.Jackisch C., Kim S.B., Semiglazov V., Melichar B., Pivot X., Hillenbach C. Subcutaneous versus intravenous formulation of trastuzumab for HER2-positive early breast cancer: updated results from the phase III HannaH study. Ann Oncol. 2015;26:320–325. doi: 10.1093/annonc/mdu524. [DOI] [PubMed] [Google Scholar]
- 13.Jackisch C., Stroyakovskiy D., Pivot X., Ahn J.S., Melichar B., Chen S.-C. Subcutaneous vs intravenous trastuzumab for patients with ERBB2-positive early breast cancer: final analysis of the HannaH phase 3 randomized clinical trial. JAMA Oncol. 2019;5 doi: 10.1001/jamaoncol.2019.0339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang D.D., Zhang S., Zhao H., Men A.Y., Parivar K. Fixed dosing versus body size-based dosing of monoclonal antibodies in adult clinical trials. J Clin Pharmacol. 2009;49:1012–1024. doi: 10.1177/0091270009337512. [DOI] [PubMed] [Google Scholar]
- 15.Fukushima Y., Charoin J.-E., Brewster M., Jonsson E.N. 16th annual meeting of the population approach group in europe (PAGE) PAGE; Copenhagen, Denmark: 2007. Abstrr 1121 Population pharmacokinetic analysis of trastuzumab (Herceptin) based on three different dosing regimens; p. 16. [Google Scholar]
- 16.FDA. CDER NDA/BLA multi-discipline review and evaluation for Herceptin Hylecta. Application No. 761106Orig1s000. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2019/761106Orig1s000MultidisciplineR.pdf. 01 February. 2016.
- 17.Engin A. Obesity-associated breast cancer: analysis of risk factors. Adv Exp Med Biol. 2017;960:571–606. doi: 10.1007/978-3-319-48382-5_25. [DOI] [PubMed] [Google Scholar]
- 18.Hales C.M., Fryar C.D., Carroll M.D., Freedman D.S., Aoki Y., Ogden C.L. Differences in obesity prevalence by demographic characteristics and urbanization level among adults in the United States, 2013-2016. Jama. 2018;319:2419–2429. doi: 10.1001/jama.2018.7270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Crozier J.A., Moreno-Aspitia A., Ballman K.V., Dueck A.C., Pockaj B.A., Perez E.A. Effect of body mass index on tumor characteristics and disease-free survival in patients from the HER2-positive adjuvant trastuzumab trial N9831. Cancer. 2013;119:2447–2454. doi: 10.1002/cncr.28051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.NCD-RIsC . 2020. NRFC National adult body-mass index (evolution of BMI over time): country-specific data for all countries (age-standardised) [Google Scholar]
- 21.Herceptin Genentech. Genentech, Inc.; South San Fracisco, CA: 2019. HYLECTA™ (trastuzumab and hyaluronidase-oysk) Prescribing Information.https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/761106s000lbl.pdf Available from: [Google Scholar]
- 22.Tricco A.C., Lillie E., Zarin W., O’Brien K.K., Colquhoun H., Levac D. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. 2018;169:467–473. doi: 10.7326/M18-0850. [DOI] [PubMed] [Google Scholar]
- 23.Munn Z., Peters M.D.J., Stern C., Tufanaru C., McArthur A., Aromataris E. Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach. BMC Med Res Methodol. 2018;18:143. doi: 10.1186/s12874-018-0611-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Armstrong R., Hall B.J., Doyle J., Waters E. Cochrane Update. ’Scoping the scope’ of a cochrane review. J Public Health. 2011;33:147–150. doi: 10.1093/pubmed/fdr015. [DOI] [PubMed] [Google Scholar]
- 25.JBI J.B.I. 2019. Reviewer’s manual. [Google Scholar]
- 26.Moher D., Liberati A., Tetzlaff J., Altman D.G., Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6 doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bernadou G., Campone M., Merlin J.-L., Gouilleux-Gruart V., Bachelot T., Lokiec F. Influence of tumour burden on trastuzumab pharmacokinetics in HER2 positive non-metastatic breast cancer. Br J Clin Pharmacol. 2016;81:941–948. doi: 10.1111/bcp.12875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Charoin J.-E., Jacqmin P., Banken L., Lennon S., Jorga K. Population pharmacokinetic analysis of trastuzumab (Herceptin) following long-term administration using different regimens. Population Approach Group in Europe (PAGE) 2004;13 Abstract 489. [Google Scholar]
- 29.Chen X., Li C., Ewesuedo R., Yin D. Population pharmacokinetics of PF-05280014 (a trastuzumab biosimilar) and reference trastuzumab (Herceptin®) in patients with HER2-positive metastatic breast cancer. Canc Chemother Pharmacol. 2019;84:83–92. doi: 10.1007/s00280-019-03850-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cosson V.F., Ng V.W., Lehle M., Lum B.L. Population pharmacokinetics and exposure-response analyses of trastuzumab in patients with advanced gastric or gastroesophageal junction cancer. Canc Chemother Pharmacol. 2014;73:737–747. doi: 10.1007/s00280-014-2400-5. [DOI] [PubMed] [Google Scholar]
- 31.Hourcade-Potelleret F., Lemenuel-Diot A., McIntyre C., Brewster M., Lum B., Bittner B. Use of a population pharmacokinetic approach for the clinical development of a fixed-dose subcutaneous formulation of trastuzumab. CPT Pharmacometrics Syst Pharmacol. 2014;3:e87. doi: 10.1038/psp.2013.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Quartino A.L., Li H., Kirschbrown W.P., Mangat R., Wada D.R., Garg A. Population pharmacokinetic and covariate analyses of intravenous trastuzumab (Herceptin®), a HER2-targeted monoclonal antibody, in patients with a variety of solid tumors. Canc Chemother Pharmacol. 2019;83:329–340. doi: 10.1007/s00280-018-3728-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reijers J.A.A., van Donge T., Schepers F.M.L., Burggraaf J., Stevens J. Use of population approach non-linear mixed effects models in the evaluation of biosimilarity of monoclonal antibodies. Eur J Clin Pharmacol. 2016;72:1343–1352. doi: 10.1007/s00228-016-2101-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oude Munnink T.H., Henstra M.J., Segerink L.I., Movig K.L., Brummelhuis-Visser P. Therapeutic drug monitoring of monoclonal antibodies in inflammatory and malignant disease: translating TNF-α experience to oncology. Clin Pharmacol Ther. 2016;99:419–431. doi: 10.1002/cpt.211. [DOI] [PubMed] [Google Scholar]
- 35.CMP E.M.A. 2013. Assessment report. Herceptin.https://www.ema.europa.eu/en/documents/variation-report/herceptin-h-c-278-x-0060-epar-assessment-report-extension_en.pdf Procedure No. EMEA/H/C/000278. Available at: 27 June. [Google Scholar]
- 36.O’Donoghue N., Quintyne K.I., Woulfe B., Gupta R., O’Reilly S. Subcutaneous herceptin therapy. J Clin Pharmacol. 2013;53:1341–1342. doi: 10.1002/jcph.171. [DOI] [PubMed] [Google Scholar]
- 37.González García J., Gutiérrez Nicolás F., Nazco Casariego G.J., Batista López J.N., Ceballos Lenza I., Ramos Díaz R. Influence of anthropometric characteristics in patients with her2-positive breast cancer on initial plasma concentrations of trastuzumab. Ann Pharmacother. 2017;51:976–980. doi: 10.1177/1060028017715727. [DOI] [PubMed] [Google Scholar]
- 38.González García J., Gutiérrez Nicolás F., Ramos Díaz R., Nazco Casariego G.J., Viña Romero M.M., Llabres Martinez M. Pharmacokinetics of trastuzumab after subcutaneous and intravenous administration in obese patients. Ann Pharmacother. 2020 doi: 10.1177/1060028020902318. 1060028020902318-. 10.1177/1060028020902318. [DOI] [PubMed] [Google Scholar]
- 39.Yang J., Zhao H., Garnett C., Rahman A., Gobburu J.V., Pierce W. The combination of exposure-response and case-control analyses in regulatory decision making. J Clin Pharmacol. 2013;53:160–166. doi: 10.1177/0091270012445206. [DOI] [PubMed] [Google Scholar]
- 40.Shah M.A., Herraez-Baranda L.A., Garg A., Tabernero J. Reply to M. Mo et al. J Clin Oncol. 2018;36:303. doi: 10.1200/jco.2017.75.2758. [DOI] [PubMed] [Google Scholar]
- 41.Shah M.A., Xu R.H., Bang Y.J., Hoff P.M., Liu T., Herráez-Baranda L.A. HELOISE: phase IIIb randomized multicenter study comparing standard-of-care and higher-dose trastuzumab regimens combined with chemotherapy as first-line therapy in patients with human epidermal growth factor receptor 2-positive metastatic gastric or gastroesophageal junction adenocarcinoma. J Clin Oncol. 2017;35:2558-67. doi: 10.1200/JCO.2016.71.6852. [DOI] [PubMed] [Google Scholar]
- 42.Mo M., Li Y., Zhu J. The HELOISE study: concerns about trial design. J Clin Oncol. 2018;36:302. doi: 10.1200/jco.2017.75.2139. [DOI] [PubMed] [Google Scholar]
- 43.Mohan N., Jiang J., Dokmanovic M., Wu W.J. Trastuzumab-mediated cardiotoxicity: current understanding, challenges, and frontiers. Antib Ther. 2018;1:13–17. doi: 10.1093/abt/tby003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Morgan J., Hayes D., Vora S. 2020. Cardiotoxicity of trasuzumab and other HER2-targeted agents. [Google Scholar]
- 45.Jung K.H., Ataseven B., Verrill M., Pivot X., De Laurentiis M., Al-Sakaff N. Adjuvant subcutaneous trastuzumab for HER2-positive early breast cancer: subgroup Analyses of safety and active medical conditions by body weight in the SafeHer phase III study. Oncol. 2018;23:1137–1143. doi: 10.1634/theoncologist.2018-0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.De Sanctis R., D’Antonio F., Agostinetto E., Marinello A., Masci G., Zuradelli M. Real-life data on the cardiac toxicity of adjuvant fixed-dose subcutaneous trastuzumab in HER2-positive breast cancer. Ann Oncol. 2018;29:71. [Google Scholar]
- 47.Kabore E.G., Guenancia C., Vaz-Luis I., Di Meglio A., Pistilli B., Coutant C. Association of body mass index and cardiotoxicity related to anthracyclines and trastuzumab in early breast cancer: French CANTO cohort study. PLoS Med. 2019;16 doi: 10.1371/journal.pmed.1002989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tokuda Y., Watanabe T., Omuro Y., Ando M., Katsumata N., Okumura A. Dose escalation and pharmacokinetic study of a humanized anti-HER2 monoclonal antibody in patients with HER2/neu-overexpressing metastatic breast cancer. Br J Canc. 1999;81:1419–1425. doi: 10.1038/sj.bjc.6690343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fleming G.F., Meropol N.J., Rosner G.L., Hollis D.R., Carson W.E., 3rd, Caligiuri M. A phase I trial of escalating doses of trastuzumab combined with daily subcutaneous interleukin 2: report of cancer and leukemia group B 9661. Clin Canc Res : an official journal of the American Association for Cancer Research. 2002;8:3718–3727. [PubMed] [Google Scholar]
- 50.Safran H., DiPetrillo T., Nadeem A., Steinhoff M., Tantravahi U., Rathore R. Trastuzumab, paclitaxel, cisplatin, and radiation for adenocarcinoma of the esophagus: a phase I study. Canc Invest. 2004;22:670–677. doi: 10.1081/cnv-200032951. [DOI] [PubMed] [Google Scholar]
- 51.Zhou X., Yu J., Wang W., Song G., Wang X., Ren J. A phase I dose-escalation study of a biosimilar trastuzumab in Chinese metastasis breast cancer patients. SpringerPlus. 2015;4:1–6. doi: 10.1186/s40064-015-1603-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Leyland-Jones B., Colomer R., Trudeau M.E., Wardley A., Latreille J., Cameron D. Intensive loading dose of trastuzumab achieves higher-than-steady-state serum concentrations and is well tolerated. J Clin Oncol : official journal of the American Society of Clinical Oncology. 2010;28:960–966. doi: 10.1200/JCO.2009.23.1910. [DOI] [PubMed] [Google Scholar]
- 53.Xu N., Redfern C.H., Gordon M., Eppler S., Lum B.L., Trudeau C. Trastuzumab, in combination with carboplatin and docetaxel, does not prolong the QT interval of patients with HER2-positive metastatic or locally advanced inoperable solid tumors: results from a phase Ib study. Canc Chemother Pharmacol. 2014;74:1251–1260. doi: 10.1007/s00280-014-2603-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pivot X., Gligorov J., Müller V., Curigliano G., Knoop A., Verma S. Patients’ preferences for subcutaneous trastuzumab versus conventional intravenous infusion for the adjuvant treatment of HER2-positive early breast cancer: final analysis of 488 patients in the international, randomized, two-cohort PrefHer study. Ann Oncol. 2014;25:1979–1987. doi: 10.1093/annonc/mdu364. [DOI] [PubMed] [Google Scholar]
- 55.Pivot X., Verma S., Fallowfield L., Müller V., Lichinitser M., Jenkins V. Efficacy and safety of subcutaneous trastuzumab and intravenous trastuzumab as part of adjuvant therapy for HER2-positive early breast cancer: final analysis of the randomised, two-cohort PrefHer study. Eur J Canc. 2017;86:82–90. doi: 10.1016/j.ejca.2017.08.019. [DOI] [PubMed] [Google Scholar]
- 56.Pivot X., Spano J.P., Espie M., Cottu P., Jouannaud C., Pottier V. Patients’ preference of trastuzumab administration (subcutaneous versus intravenous) in HER2-positive metastatic breast cancer: results of the randomised MetaspHer study. Eur J Canc. 2017;82:230–236. doi: 10.1016/j.ejca.2017.05.009. [DOI] [PubMed] [Google Scholar]
- 57.Van den Nest M., Glechner A., Gold M., Gartlehner G. The comparative efficacy and risk of harms of the intravenous and subcutaneous formulations of trastuzumab in patients with HER2-positive breast cancer: a rapid review. Syst Rev. 2019;8:321. doi: 10.1186/s13643-019-1235-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pombo F.H., Passos P.M., Gamboa N.F., Alvellos K.C., Soares M.C.S.T. Safety profile and reduced observation time in outpatients using subcutaneous Herceptin. J Clin Oncol. 2018;36 doi: 10.1200/JCO.2018.36.15_suppl.e18848. [DOI] [Google Scholar]
- 59.Karmali S., Hughes N., Galiauskas R., Cook J., Murphy K., Bird B.R. Is 6 hour monitoring for administration related reactions after first administration of subcutaneous trastuzumab necessary? A single institution audit. Canc Res. 2017;77 doi: 10.1158/1538-7445.Sabcs16-p3-12-04. [DOI] [Google Scholar]
- 60.Karmali S., Hughes N., Kinneally A., Kroes J., Cook J., Killian M. A regional audit of 6-hour monitoring for administration related reactions during the first administration of subcutaneous trastuzumab. Canc Res. 2019;79 10 1158/1538-7445.Sabcs18-pp.4–16-08. [Google Scholar]
- 61.Zambetti M., Montemurro F., Morandi P., Zamagni C., Brandes A.A., Bisagni G. Safety profile of subcutaneous trastuzumab for the treatment of patients with HER2-positive early or locally advanced breast cancer: primary analysis of the SCHEARLY study. Eur J Canc. 2018;105:61–70. doi: 10.1016/j.ejca.2018.09.034. [DOI] [PubMed] [Google Scholar]
- 62.Jackisch C., Muller V., Dall P., Neumeister R., Park-Simon T.W., Ruf-Dordelmann A. Subcutaneous trastuzumab for HER2-positive breast cancer - evidence and practical experience in 7 German centers. Geburtshilfe Frauenheilkd. 2015;75:566–573. doi: 10.1055/s-0035-1546172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pimentel F.F., Morgan G., Tiezzi D.G., de Andrade J.M. Development of new formulations of biologics: expectations, immunogenicity, and safety for subcutaneous trastuzumab. Pharmaceut Med. 2018;32:319–325. doi: 10.1007/s40290-018-0247-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kümmel S., Tondini C., Abraham J., Nowecki Z., Itrych B., Hitre E. 12 August 2020. Subcutaneous trastuzumab with pertuzumab and docetaxel in HER2-positive metastatic breast cancer: final analysis of MetaPHER, A phase 3b single-arm safety study. PREPRINT (Version 1) available at: Research Square. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Padrón IM, García JG, Díaz RR, Lenza IC, Nicolás FG. Anti-drug antibodies anti-trastuzumab in the treatment of breast cancer. J Oncol Pharm Pract.0:1078155220953873. 10.1177/1078155220953873. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.