Summary
This protocol outlines a reliable and versatile approach to isolate stromal vascular fraction cells from different adipose tissues across human and mouse species. A number of downstream applications can then be performed to gain an appreciation of the functional activity of unique adipose tissue-resident cell populations.
For complete details on the use and execution of this protocol, please refer to Macdougall et al. (2018).
Subject areas: Cell Biology, Cell isolation, Flow Cytometry/Mass Cytometry, Immunology, Metabolism
Graphical abstract
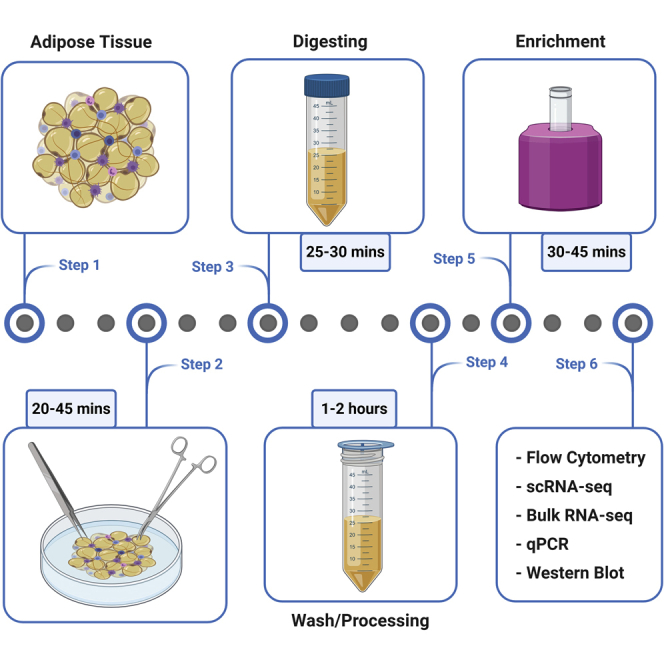
Highlights
-
•
Isolation of viable stromal vascular fraction cells from adipose tissue
-
•
Applicable to multiple adipose tissue depots from human and mouse
-
•
Highly reproducible adipose tissue digestion for various downstream assays
This protocol outlines a reliable and versatile approach to isolate stromal vascular fraction cells from different adipose tissues across human and mouse species. A number of downstream applications can then be performed to gain an appreciation of the functional activity of unique adipose tissue-resident cell populations.
Before you begin
This protocol outlines a highly consistent and versatile method of isolating stromal vascular cell populations from human and mouse adipose tissues. Compared with previously published protocols, we illustrate its applicability across species and different types of adipose tissue (visceral and subcutaneous) and highlight critical points such as the importance of measuring collagenase enzymatic activity when adding to the adipose tissue digestion suspension. Furthermore, given the exhaustively detailed nature of the protocol framework, we feel this makes it highly reproducible, easy-to-follow with clear examples of the benefits, potential pitfalls, and relevant troubleshooting steps.
Before attempting this protocol, ensure all reagents and buffers are made up to a suitable volume (see materials and equipment). To avoid unnecessary waiting during steps that are reliant on enzymatic activity, we recommend turning on your shaker-incubator and setting the temperature to 37°C beforehand.
For the duration of this protocol (with the exception of the incubation step during digestion), keep all reagents, buffers, and samples on ice. This is crucial for preserving cell yield. It is also important if you are trying to isolate macrophage populations (which can stick to plasticware). Keeping samples cold can minimize this.
The use of animals and human tissue for research requires ethical approval. Therefore, ethical approval from the relevant institutional or other regulatory body must be obtained prior to the use of animal or human tissue for research purposes.
Harvesting adipose tissue - mouse (epididymal fat pad)
Timing: [30–45 min]
-
1.
Sacrifice mouse using an appropriate method as per local animal license regulations.
Note: Typically, males will have more abdominal visceral adipose tissue than females. Likewise, mice on western or high fat diets will yield much more visceral adipose tissue than those on a normal chow diet.
-
2.
Spray 70% ethanol over the fur in order to sterilize the external surface.
-
3.
Placing the mouse on its back in the supine position, and using forceps and scissors, make a lateral incision across the abdomen just beneath the diaphragm. From the midpoint of the first incision make a further cut along the midline towards the rectum.
-
4.
Dissect the epididymal fat pads from both sides of the mouse, taking care to avoid the testes or ovaries, and any lymph nodes.
-
5.
Transfer the adipose tissue directly into ice cold Wash/Digestion buffer and proceed to digestion protocol.
Pause point: While it is best to proceed directly to the digestion protocol, you can leave the adipose tissue in the fridge at this point for 1–12 h, which is better than pausing during the digestion protocol.
Note: Pausing here will result in decreased cell yield.
Obtaining adipose tissue – human (e.g., epicardial, mediastinal, and subcutaneous adipose tissue)
Timing: [20–30 min]
-
6.
Prepare a 50 mL Falcon tube containing 20 mL of Wash/Digestion buffer ready for the transfer of human adipose tissue samples acquired from surgical procedures.
-
7.
Immediately transfer human adipose tissue samples to ice cold Wash/Digestion buffer and keep on ice. Minimum adipose tissue recommended for the protocol is 0.15 g.
Note: Depending on the type of adipose tissue collected (e.g., subcutaneous, visceral) residual blood may be present. Ensure samples are thoroughly washed with Wash/Digestion buffer. The wash steps involve serial (two) immersions of the tissues in fresh Wash/Digestion buffer to remove residual blood.
-
8.
Using sterile forceps and scissors, carefully dissect out any additional non-adipose tissue (e.g., connective tissue, cauterized tissue) (Figure 1A).
-
9.
Proceed to digestion protocol.
Pause point: While it is best to proceed directly to the digestion protocol, you can leave the adipose tissue in the fridge at this point for 1–12 h, which is better than pausing during the digestion protocol.
Note: Pausing here will result in decreased cell yield.
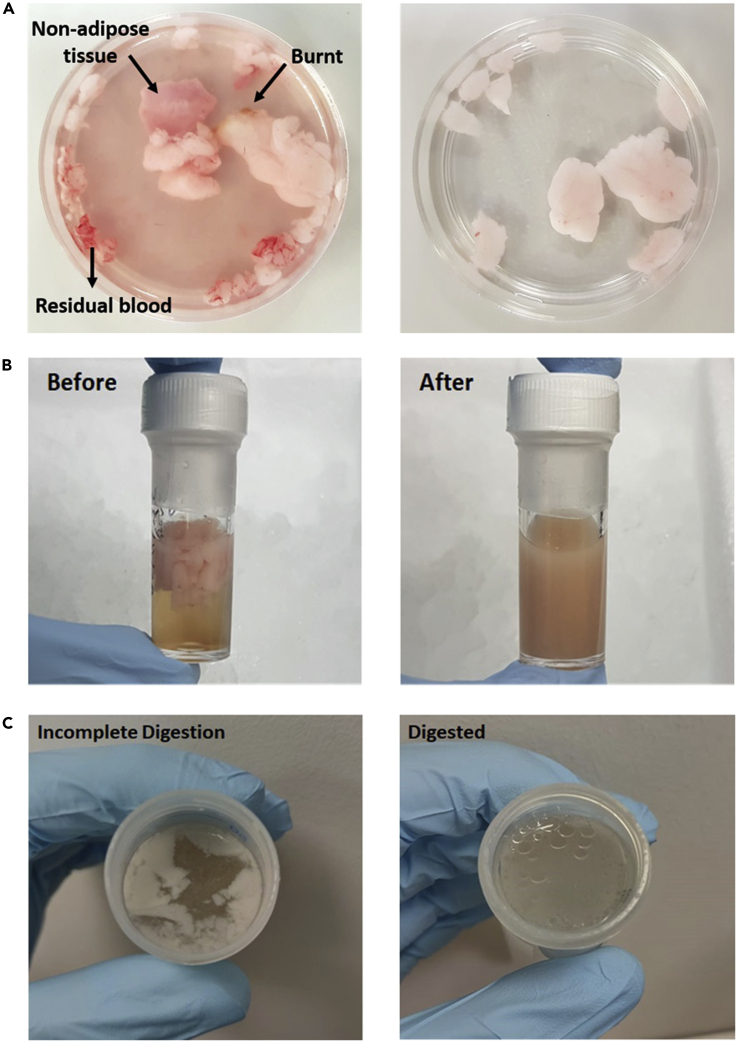
Figure 1.
Examples of how samples look before and after different steps of the adipose tissue digestion protocol
(A) Ensure adipose tissue samples are thoroughly washed and processed to remove residual blood and non-adipose tissue (e.g., connective tissue, cauterized tissue).
(B) If there are still lumps of adipose tissue visible within the sample (Left) after step 3 of the digestion protocol, then return samples to the shaker-incubator until they form a homogeneous mixture (Right).
(C) If samples look partially digested (Left) after step 5, with adipose tissue still visible, then carry out the additional re-digestion step before proceeding to step 6. If samples look completely digested (Right), with only lipid droplets visible, then proceed to step 6 of the adipose tissue digestion protocol.
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Anti-human CCR7 | BioLegend | Cat# 353216; RRID: AB_10916386 |
| Anti-human CD19 | BioLegend | Cat# 302228; RRID: AB_893272 |
| Anti-human CD45 | BioLegend | Cat# 304048; RRID: AB_2563129 |
| Anti-human CD3 | BioLegend | Cat# 300318;RRID: AB_314054 |
| Anti-human CD4 | BioLegend | Cat# 357410; RRID: AB_2565662 |
| Anti-human CD8α | BioLegend | Cat# 300920; RRID: AB_528885 |
| Anti-human CD45RO | BioLegend | Cat# 304224; RRID: AB_2563817 |
| Anti-human CD14 | BioLegend | Cat# 325608; RRID: AB_830681 |
| Anti-human CD16 | BioLegend | Cat# 302026; RRID: AB_2278418 |
| Anti-human CD206 | BioLegend | Cat# 321126; RRID: AB_2563839 |
| Anti-human CD141 | BioLegend | Cat# 344110; RRID: AB_2561623 |
| Anti-human Clec9A | BioLegend | Cat# 353804; RRID: AB_10965546 |
| Anti-human CD1c | BioLegend | Cat# 331520; RRID: AB_10644008 |
| Anti-mouse CD45 | BioLegend | Cat# 103146; RRID: AB_2564003 |
| Anti-mouse CD11c | BioLegend | Cat# 117317; RRID: AB_493569 |
| Anti-mouse CD11b | Thermo Fisher Scientific | Cat# 47-0112-82; RRID: AB_1603193 |
| Anti-mouse MHCII | BioLegend | Cat# 107622; RRID: AB_493727 |
| Anti-mouse CD8α | Thermo Fisher Scientific | Cat# 17-0081-82; RRID: AB_469335 |
| Chemicals, peptides, and recombinant proteins | ||
| Deoxyribonuclease I from bovine pancreas | Sigma | Cat# D4527 |
| Collagenase from Clostridium histolyticum (type II) | Sigma | Cat# C6885 |
| Fetal bovine serum | SeraLab | Cat# EU-000-F |
| Dulbecco's phosphate buffered saline pH 7.2 (without CaCl2 and MgCl2) | Sigma | Cat# D8537-500ML |
| EDTA solution | Sigma | Cat# E7889-100ML |
| NH4Cl | Sigma | Cat# A9434-1KG |
| KHCO3 | Sigma | Cat# 237205-100g |
| Na3EDTA | Sigma | Cat# ED3SS-1KG |
| TES | Sigma | Cat# T1375-100G |
| CaCl2 | Sigma | Cat# C7902-500G |
| NaCl | Sigma | Cat# S7653-1KG |
| Critical commercial assays | ||
| Dead Cell Removal Kit | Miltenyi Biotec | Cat# 130-090-101 |
| CD11c MicroBeads UltraPure, mouse | Miltenyi Biotec | Cat# 130-125-835 |
| Other | ||
| 7 mL Bijou container | Thermo Fisher Scientific | Cat# 129A |
| 50 mL Falcon tubes | Corning | Cat# 430829 |
| 100 μm Cell strainer | Fisher | Cat# 11517532 |
| 70 μm Cell strainer | Greiner | Cat# 542070 |
| V-Bottom 96-well plate | Sarstedt | Cat# 83.3926.500 |
| LIVE/DEAD Fixable Aqua Stain | Invitrogen | Cat# L34966 |
| Shaker-incubator (225 rpm, 37°C) | N/A | N/A |
| Refrigerated centrifuge (800 ×g, 4°C) | N/A | N/A |
Materials and equipment
DNase
| Reagent | Final Concentration (mM or μM) | Amount |
|---|---|---|
| Deoxyribonuclease I from Bovine Pancreas | 11,100 U/mL | Varies by batch |
| NaCl | 150 mM | 8.766 mg |
| ddH2O | n/a | 1 mL |
| Total | n/a | 1 mL |
Store at −20°C for up to 1 year.
Collagenase II
| Reagent | Final Concentration (mM or μM) | Amount |
|---|---|---|
| Collagenase from Clostridium Histolyticum (Type II) | 14,220 U/mL | Varies by batch |
| TES | 50 mM | 572.5 mg |
| CaCl2 | 360 μM | 2.645 mg |
| ddH2O | n/a | 50 mL |
| Total | n/a | 50 mL |
Store at −20°C for up to 1 year. Use 1M NaOH to adjust to pH of 7.4.
Wash/Digestion Buffer
| Reagent | Final Concentration (mM or μM) | Amount |
|---|---|---|
| Fetal Bovine Serum | 2% | 10 mL |
| Dulbecco's Phosphate Buffered Saline pH 7.2 (without CaCl2 and MgCl2) | 1× | 490 mL |
| Total | n/a | 500 mL |
Store at 4°C for up to 3 months.
MACS Buffer
| Reagent | Final Concentration (mM or μM) | Amount |
|---|---|---|
| Fetal Bovine Serum | 2% | 10 mL |
| EDTA (0.5 M) | 2 mM | 2 mL |
| Dulbecco's Phosphate Buffered Saline pH 7.2 (without CaCl2 and MgCl2) | 1× | 488 mL |
| Total | n/a | 500 mL |
Store at 4°C for up to 3 months.
ACK Lysis Buffer (1×)
| Reagent | Final Concentration (mM or μM) | Amount |
|---|---|---|
| NH4Cl | 150 mM | 8.29 g |
| KHCO3 | 10 mM | 1.0 g |
| Na3EDTA | 0.1 mM | 37.2 mg |
| ddH2O | n/a | 1000 mL |
| Total | n/a | 1000 mL |
Store at 20°C–25°C for 6 months.
Step-by-step method details
Processing and digesting the adipose tissue
Timing: [1–2 h]
During this step, the adipose tissue is processed and digested. This isolates the stromal vascular fraction cells from the extracellular matrix whilst ensuring their viability.
-
1.
Weigh the adipose tissue and add 2 mL of ice-cold Wash/Digestion buffer per gram of sample.
Note: If samples are <1g, add 2 mL of ice-cold Wash/Digestion and continue with all steps.
Note: If the adipose tissue weighs up to 2 g then we suggest using 7 mL bijou tubes. However, if the samples exceed 2 g then use larger tubes (e.g., 15 mL tubes) or split the sample into two smaller tubes and weigh each separately.
-
2.
Using sterile scissors, mince the adipose tissue and add 200 μL Collagenase II (2844 U) and 2.5 μL DNase (27.75 U) per mL of Wash/Digestion buffer.
CRITICAL: Always check LOT number and corresponding enzymatic activity in Units per mg when making up the Collagenase II and DNase stock solutions, as this will vary. As a result, it is not practical to state the enzyme concentrations in mg/mL as we must standardise to U/mL.
CRITICAL: Ensure at this point that all the adipose tissue is floating. Any tissue present sinking to the bottom of the tube is likely to be non-adipose tissue and should be removed.
Note: Store the Collagenase II and DNase enzyme stock solutions in small aliquots to minimise the effect of repeated freeze/thaw cycles, which will decrease the enzymatic activity.
-
3.
Place samples in a shaker-incubator at 37°C and 225 rpm for 25–30 min.
Note: Digested samples should look homogeneous and pinkish white in colour, larger samples can take slightly longer than smaller samples (Figure 1B).
-
4.
Transfer the samples through a 100 μm cell sieve into a 50 mL tube pre-washed with ice cold Wash/Digestion buffer. Then top up the Falcon tube to 30 mL with Wash/Digestion buffer.
Note: Ensure you rinse the sample tube used for digestion with Wash/Digestion buffer to remove any remaining sample and strain through the same 100 μm cell sieve.
-
5.
Centrifuge samples at 800 × g for 10 min at 4°C.
CRITICAL: After centrifugation, open the tubes and check that the floating portion of the sample is composed of transparent lipid droplets. If you can see a white layer of adipose tissue floating, then this top layer will need removing with a Pasteur pipette and re-digestion (Figure 1C). Re-digest in 2 mL Wash/Digestion buffer, with half the original collagenase volume used i.e., 200 μL Collagenase II in 2 mL Wash/Digestion buffer, with 5 μL DNase for 10 min (as in step 3). This will ensure you obtain all the stromal cells possible, particularly macrophages, from within each sample.
-
6.
Discard supernatant and the remaining pellet contains the stromal vascular fraction cells.
Note: Proceed directly to wash step 8 if you will use bead enrichment (recommended), otherwise carry out red blood cell lysis described in step 7.
-
7.
Optional: Resuspend pellets in 500 μl of red blood cell lysis buffer (ACK Lysis Buffer) for about 1 min.
-
8.
Top up with ice cold Wash/Digestion buffer and transfer the samples through a 70 μm cell sieve into a 50 mL tube pre-washed with Wash/Digestion buffer. Then fill the tube to the top with Wash/Digestion buffer.
-
9.
Centrifuge samples at 800 × g for 10 min at 4°C.
-
10.
Discard supernatant and the remaining pellet contains the stromal vascular fraction cells.
Magnetic bead enrichment of conventional dendritic cells (recommended)
Timing: 30–45 min
This step enriches cells from the population of interest within a sample and as a result can change for any given experiment. We recommend this step is completed to reduce time required for flow cytometry assisted cell sorting. In this example we enrich live cells from the stromal vascular fraction of mouse adipose tissue followed by positive enrichment of CD11c + cells to increase the relative proportion of conventional dendritic cells (cDCs) within our samples (Figure 2A).
-
11.
Re-suspend the cell pellet in ice cold 1× binding buffer (Miltenyi Biotec) containing Dead Cell Removal MicroBeads (Miltenyi Biotec) at 200 μL per 107 cells and follow manufacturer’s instructions.
Note: Transferring the sample into fresh tubes at this stage prevents excess lipids which are stuck to the inside of the previous tube from affecting the sample.
Note: We use twice the concentration recommended by the manufacturer for dead cell removal, as there is a higher abundance of dead cells compared to cDCs (our cells of interest).
-
12.
The effluent from the magnetic column is your live cell enriched population. Top-up these tubes with MACs buffer and centrifuge at 350 × g for 5 min at 4°C.
-
13.
Discard supernatant and resuspend pellet in ice cold MACs buffer containing CD11c MicroBeads (Miltenyi Biotec) following manufacturer’s instructions. Depending on adipose tissue site and metabolic status, a total of 105 to 106 CD45+ cells/g of fat are expected.
-
14.
The sample you are left with is a single cell suspension enriched for live cells and CD11c+ cells. Proceed to next step.
Optional: You can change these enrichment steps to suit your experiment. However, you may need to optimise bead concentrations.
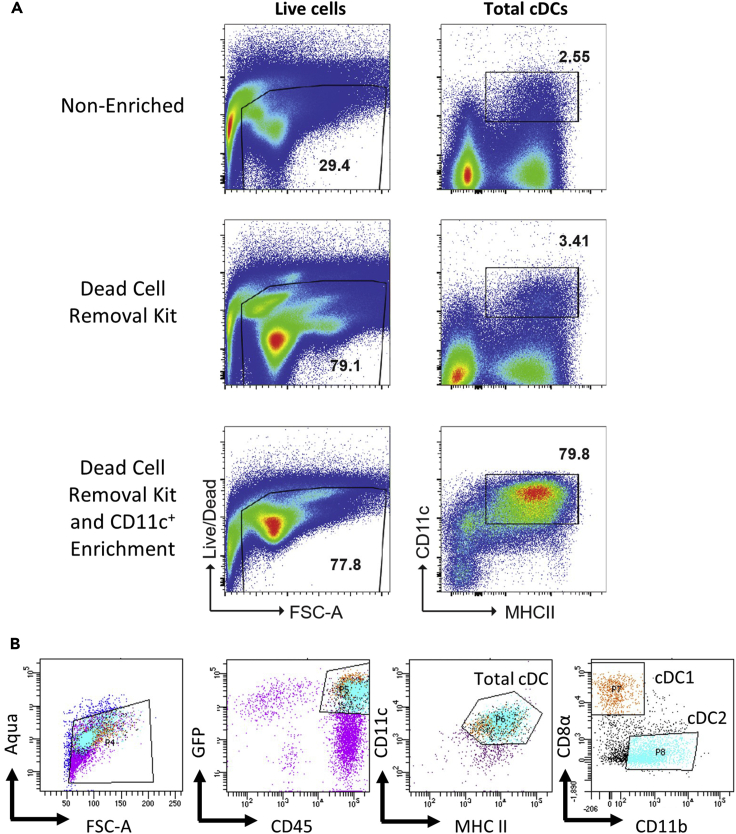
Figure 2.
Bead enrichment and flow cytometry gating strategy for isolation of conventional dendritic cell subsets from mouse adipose tissue
(A) The beneficial effects of magnetic bead enrichment (dead cell removal and CD11c+ enrichment) on live cells and conventional dendritic cell populations within a sample.
(B) An example flow cytometry gating strategy for the isolation of conventional dendritic cell subsets (cDC1 and cDC2). Note that stromal vascular fractions taken from mouse adipose tissue were pooled (to increase yield) and cDCs expressing a Zbtb46-GFP reporter are shown in this example.
Extracellular staining and cell sorting
During this step, you isolate a particular cell population of interest from the stromal vascular fraction by flow cytometry assisted cell sorting. Alternatively, if you do not need to obtain pure cell suspensions, you can add experimental readout markers and analyze the cells present in the stromal vascular fraction directly by regular flow cytometry.
-
15.
Take single cell suspensions and centrifuge at 350 × g for 5 min at 4°C.
-
16.
Resuspend cell pellets in 75 μL of PBS containing diluted fluorescently conjugated extracellular antibody markers following manufacturer’s instructions.
Note: See Figures 2B and 3 for examples of immune cell populations isolated from human and mouse adipose tissues and their gating strategies.
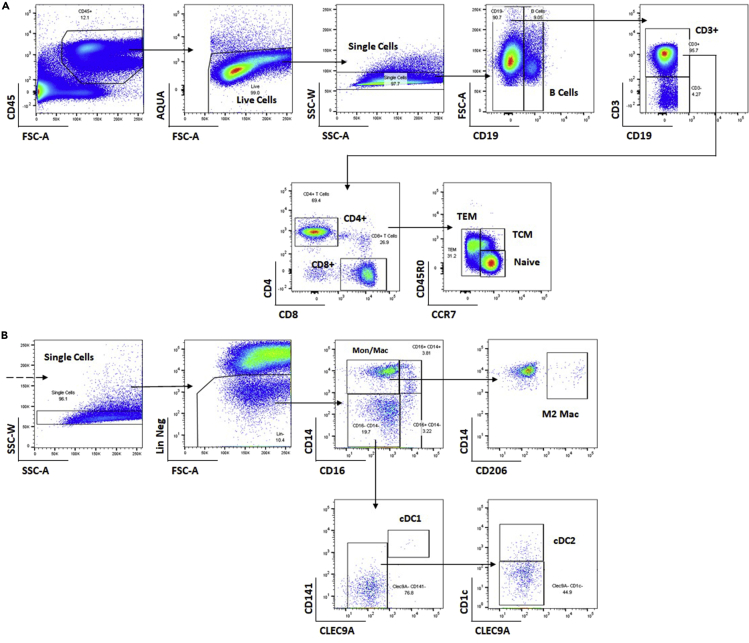
Figure 3.
Example flow cytometry gating strategy for quantifying immune cell populations from digested adipose tissues
Lymphocyte populations (A) and myeloid populations (B) analyzed from the stromal vascular fraction of human adipose tissue via multi-color flow cytometry.
-
17.
Incubate cells in the dark at 4°C for 18 min.
-
18.
Add ice cold PBS and centrifuge at 350 × g for 5 min at 4°C.
-
19.
Resuspend pellet in ice cold MACs buffer as a wash and centrifuge at 350 × g for 5 min at 4°C.
-
20.
Resuspend pellet in a small volume of MACs buffer and proceed to flow cytometry assisted cell sorting.
Note: We usually resuspend cells taken from one mouse in around 500 μL for cell sorting on either a BD FACS Aria II or a BD FACS fusion cell sorter.
Expected outcomes
Upon the completion of this protocol, viable single cell suspensions containing the stromal vascular fraction of adipose tissue should be obtained. With further processing, pure suspensions of immune cells (e.g., macrophages, T cells, B cells, and dendritic cell subsets) in addition to other stromal populations such as pre-adipocytes, endothelial cells, fibroblasts, and mesenchymal progenitor/stem cells can be acquired.
From these single cell suspensions, there are several potential downstream applications (Macdougall et al., 2018). For instance, selective cell populations could be cultured for in vitro assays, flow cytometry (Figure 3), RNA-sequencing (e.g., sc-RNAseq), total RNA extraction for real-time PCR, or submitted for proteomic or metabolomic analyses. Additionally, cell samples can be lysed to evaluate protein expression through western blot analysis.
The consistent nature of this protocol makes it uniquely versatile and as a result, it can better facilitate comparison between species and anatomically distinct adipose depots in contrast to other protocols where different digestion methods are routinely required.
Limitations
Adipose depots can harbor unique cellular profiles based on their anatomical location and factors such as adiposity state (as alluded to earlier mice on high fat diets tend to yield more visceral adipose tissue). These differences can be readily observed in the stromal vascular fraction when subcutaneous and visceral adipose tissues are analyzed comparatively (Peinado et al., 2010)(Silva et al., 2017). Under normal homeostatic conditions, these stromal cells help maintain the functional activity of adipose tissue from its metabolic duties as a site for energy storage, to its role as an endocrine organ for various biologically active compounds (Macdougall et al., 2018)(Mahlakõiv et al., 2019). Therefore, depending on the type of adipose tissue used for this protocol, the abundance of different stromal cell populations can vary. This should be taken into consideration when acquiring samples as you may need to digest more adipose tissue in order to obtain and sufficiently define rarer cell populations.
Troubleshooting
Problem 1
Adipose tissue is partially digested (Figures 1B and 1C).
Potential solution
If you find that you frequently only achieve partial digestions, try to reconsider the tubes you are using. The best sized tubes to perform the digest in are those which are only around half full; this allows for optimal mixing and facilitates physical movement of the sample in the shaker-incubator.
Problem 2
Low cell viability after isolating stromal vascular fraction.
Potential solution
It is possible that you may observe a larger number of dead cells after digestion (particularly if processing mouse tissue). If this becomes an issue, it is important that you check your collagenase lot number and verify that the activity (measured in units) is comparable to other batches used. It is also important that you check the conditions used for digestion e.g., incubation periods greater than 30 min will ultimately reduce cell viability.
Problem 3
No visible digestion has taken place.
Potential solution
If no digestion has taken place after greater incubation times (e.g., 30 min) it is possible that either no enzyme was added, or the collagenase II used has impaired enzymatic activity. Ensure all enzymes are stored correctly and try to avoid repeated freeze thaw cycles.
Problem 4
Small white lumps are floating in the digested samples.
Potential solution
If you accidentally include any non-adipose tissue with the sample you are digesting (such as the testes or ovarian tissue in mice), your sample can become contaminated and as a result should be removed from your experiment and repeated with freshly obtained adipose tissue. In order to prevent this from occurring, ensure that all of the tissue in your tube (prior to adding digestion enzymes), is floating (Figure 4A). If there is white tissue sinking within your sample, then it is not adipose tissue and should be removed before proceeding to the next stage.
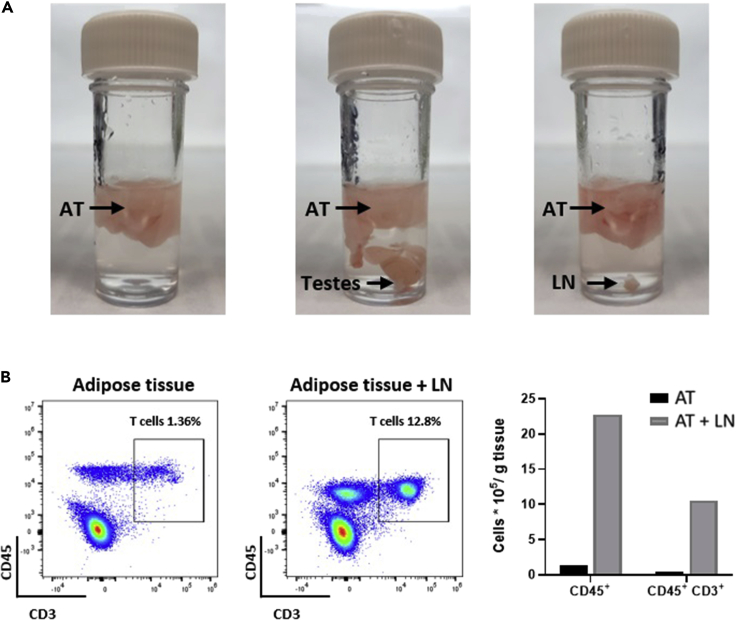
Figure 4.
Samples contaminated with different non-adipose tissues can affect experimental data
(A) Tissue samples that sink in Wash/Digestion buffer are most likely not adipose tissue. When acquiring adipose tissue (AT) from mice, if testes (middle) or lymph nodes (LN) (right) are accidentally acquired, ensure they are removed before digestion.
(B) Tissue contaminants can distort different cell populations. As shown via flow cytometry analysis, when lymph nodes from a mouse are present during digestion, samples have increased CD45+ cells. This can compromise studies investigating T cell (CD45+ CD3+) abundance (right).
Problem 5
More cells are obtained unexpectedly when cell sorting or analyzing via flow cytometry (Figure 4B).
Potential solution
It is possible that during the dissection there was a lymph node amongst the adipose tissue in the sample. This would mean that your sample contains more immune cells than it should. In this event you will need to disregard the data you obtain, as the cells in the sample will be predominantly derived from the lymph node rather than the adipose tissue. To avoid this, prior to adding the digestion enzymes, inspect the samples and ensure that any areas of the adipose sample which appear slightly darker and vascularized are indeed adipose tissue. A quick way to test this is to cut the darker section out and test whether it floats (Figure 4A). If it sinks, then it is not adipose tissue.
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Dr M. Paula Longhi (m.longhi@qmul.ac.uk).
Materials availability
This study did not generate new unique reagents.
Data and code availability
This study did not generate any unique dataset or code.
Acknowledgments
R.H. is supported by the Medical Research Council (MR/N014308/1). B.S. is supported by the British Heart Foundation (FS/19/62/34901). V.V. is supported by Barts Charity (MGU0413), Abbott, and the Medical Research Council (MR/T008059/1). M.P.L. is supported by Barts Charity (MGU0413). Some graphical illustrations were created using Biorender.com.
Author contributions
R.H. and B.S. wrote the protocol and created the figures. V.V. reviewed, edited, and made revisions to the protocol. M.P.L. is the senior author and edited, reviewed, and revised the protocol.
Declaration of interests
The authors declare no competing interests.
References
- Macdougall C.E., Wood E.G., Loschko J., Scagliotti V., Cassidy F.C., Robinson M.E. Visceral adipose tissue immune homeostasis is regulated by the crosstalk between adipocytes and dendritic cell subsets. Cell Metab. 2018;27:588–601.e4. doi: 10.1016/j.cmet.2018.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahlakõiv T., Flamar A.L., Johnston L.K., Moriyama S., Putzel G.G., Bryce P.J. Stromal cells maintain immune cell homeostasis in adipose tissue via production of interleukin-33. Sci. Immunol. 2019;4:eaax0416. doi: 10.1126/sciimmunol.aax0416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peinado J.R., Jimenez-Gomez Y., Pulido M.R., Ortega-Bellido M., Diaz-Lopez C., Padillo F.J. The stromal-vascular fraction of adipose tissue contributes to major differences between subcutaneous and visceral fat depots. Proteomics. 2010;10:3356–3366. doi: 10.1002/pmic.201000350. [DOI] [PubMed] [Google Scholar]
- Silva K.R., Côrtes I., Liechocki S., Carneiro J.R.I., Souza A.A.P., Borojevic R. Characterization of stromal vascular fraction and adipose stem cells from subcutaneous, preperitoneal and visceral morbidly obese human adipose tissue depots. PLoS One. 2017;12:e0174115. doi: 10.1371/journal.pone.0174115. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This study did not generate any unique dataset or code.