Abstract
Background/methods: Although the prognosis of metastatic breast cancer (BC) has improved, some patients still develop high burden metastases or visceral crisis (VC) and polychemotherapy is commonly used in these cases. Data reporting the real effectiveness of this strategy are scanty. Therefore, the outcomes of patients with metastatic BC treated with platinum-based chemotherapy (P-ChT) at the Jules Bordet Institute during the period of January 2008 and December 2018 were retrospectively reviewed. The presence of VC was defined according to ABC 4 criteria.
Results
441 patients were identified: visceral metastases were observed in 430 (97.5%) while 261 (59.2%) presented VC. As for metastatic BC subtype, 255 (57.8%) had ER-positive/HER2-negative, 41 (9.3%) ER-positive/HER2-positive, 34 (7.7%) ER-negative/HER2-positive and 111 (25.1%) triple-negative BC. Median number of prior treatment lines was 3.8 (0–12). Median OS with P-ChT in the entire cohort was 6.13 months. Patients with VC had lower OS than patients without VC (8.6 vs 3.7 months; p < 0.001). On multivariate analysis, the variables correlated with worse OS were hyperbilirubinemia (HR 1.90; 95% CI 1.34–2.75), ECOG ≥2 (HR 1.77; 95% CI 1.13–2.78) and ECOG ≥3 (HR 2.52; 95% CI 1.48–4.28), and >3 previous treatment lines (HR 2.27; 95% CI 1.53–3.21). Of the 261 patients with VC, 106 (40.5%) presented a resolution of the VC which correlated with better OS (9.3 vs 2.0 months, HR 0.27; 95% CI 0.21–0.36).
Conclusion
Patients who overcome VC benefit from P-ChT with OS similar to patients without VC. In this analysis, hyperbilirubinemia, poor ECOG and >3 previous treatment lines were significant prognostic factors in the overall study population.
Keywords: Metastatic breast cancer, Visceral crisis, Platinum-based chemotherapy
Highlights
-
•
Outcomes of pts with advanced BC treated with platinum-based chemotherapy at our Institution were reviewed.
-
•
Our results highlight the poor prognosis of pts with BC and visceral crisis (VC).
-
•
Hyperbilirubinemia, poor ECOG PS, and >3 prior treatment lines may be factors associated with inferior OS.
-
•
Prospective studies in pts with VC are needed to provide better guidance towards treatment decisions.
1. Introduction
The prognosis of metastatic breast cancer has evolved considerably during the last years [1,2]. This is mainly related to the deepening of knowledge about the different breast cancer molecular subtypes [[3], [4], [5]], which has led to the development of specific target therapies (e.g. anti-HER2 agents [6], CDK 4/6 inhibitors [7,8], PARP inhibitors [9,10], and immunotherapy [11]) and also to the understanding of important mechanisms and pathways of resistance (e.g. PI3CKA [12], AKT-1[13], mTOR [14]). Currently, as we are still not able to cure metastatic patients, the selection of systemic treatment for metastatic breast cancer should consider both survival benefit and impact on the quality of life [15]. For these reasons, the use of effective minimally toxic therapies has been preferred whenever possible, and chemotherapy is usually postponed as much as possible during the course of the disease [15]. Moreover, sequencing of chemotherapy agents, rather than chemotherapy combinations (polychemotherapy) is advised by international guidelines [15]. Notwithstanding, there is a particular clinical situation known as visceral crisis, where physicians commonly prescribe polychemotherapy.
According to international guidelines, visceral crisis is defined as severe organ dysfunction as assessed by signs and symptoms, laboratory work-up, and rapid disease progression implying important organ compromise [15,16]. For visceral crisis, current guidelines still recommend treatment with polychemotherapy to achieve rapid disease control, mainly due to the high response rates obtained with these regimens [16,17]. ‘‘Real world’’ data regarding the efficacy and outcomes related to this approach is, nonetheless, limited. Currently, this information would be of great interest, considering the growing and widespread use of target therapies (e.g. CDK 4/6 inhibitors + endocrine therapy), that elicit high response rates for patients with visceral disease, possibly at the expenses of less toxicities when compared to chemotherapy [18]. Moreover, the recent 5th ESO-ESMO Consensus Guidelines for Advanced Breast Cancer (ABC 5) included a statement clarifying that although visceral crisis requires the use of the most rapidly efficacious therapy, this would not necessarily be chemotherapy in all situations [16].
The aim of this study is to evaluate the clinical outcomes (in terms of overall survival and response rates) of patients with metastatic breast cancer treated with polychemotherapy containing a platinum compound (standard of care in our institution in the scenario of high burden disease and/or visceral crisis particularly in the liver) and to identify prognostic factors for patients receiving this treatment.
2. Patients and methods
2.1. Study design, patient selection, and data extraction
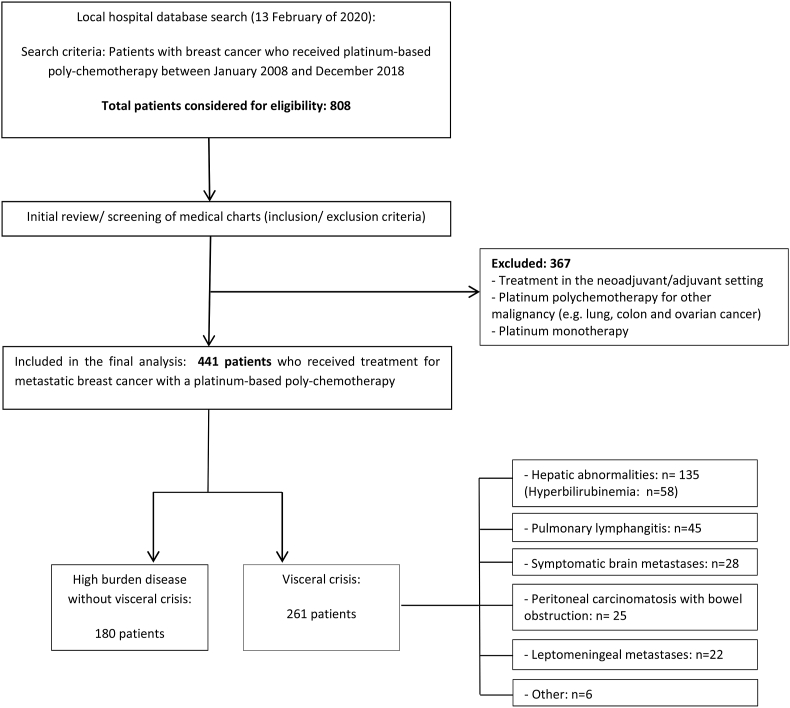
This retrospective cohort study included data extracted from medical records of patients who were treated with a platinum-based chemotherapy regimen for the treatment of metastatic breast cancer at the Jules Bordet Institute, during the period of January 2008 until December 2018. From the initially identified 808 patients, 441 patients were included in this analysis (Fig. 1). This study was approved by an internal ethics committee (CE3105), and informed consent was not required as all patient-related data were retrieved from medical records only, and patient identification were kept confidential. This study was conducted according to the: Strengthening the reporting of observational studies in epidemiology (STROBE) statement [19].
Fig. 1.
Flow chart of study population and patient selection.
2.2. Definition of visceral crisis and visceral crisis resolution
(Table 3) The presence of visceral crisis at the moment of treatment with platinum-based chemotherapy was confirmed through the revision of medical charts based on ABC 4 criteria (a severe organ dysfunction, which involves severe symptoms and rapid disease progression and can also be assessed by laboratory values). For this study, we classified the patients in 6 groups of visceral crisis: a) hepatic metastases with hepatic tests abnormalities (hyperbilirubinemia >1.5 x and/or AST and ALT > 1.5x of normal value due to rapid disease progression and liver burden associated with clinical symptoms), b) symptomatic brain metastases, c) leptomeningeal involvement, d) pulmonary lymphangitis with clinical dyspnoea, e) peritoneal carcinomatosis with symptoms of bowel obstruction and f) other types (including superior cava vein syndrome, cardiac tamponade, bone marrow invasion, and malignant hypercalcemia).
Table 3.
Median OS following platinum polychemotherapy according to baseline characteristics - univariate analysis using the Log rank Test (Mantel-Cox) and multivariate analysis using Cox regression.
| Median OS months (95% CI) | Univariate analyses (P value- Log rank test) |
Multivariate analysis Hazard ratio (95% CI) |
|
|---|---|---|---|
|
BC subtype: HR+/HER2- |
4.8. (3.9–5.7) | 0.078 | – |
| HR+/HER2+ | 9.9 (7.1–12.7 | – | |
| HER2+/HR- | 6.9 (1.7–12.0) | – | |
| TNBC | 6.9 (5.5–8.2) | – | |
|
Visceral crisis: No |
8.6 (7.4–9.8) | <0.001 | Reference |
| Yes | 3.7 (2.6–4.7 | 1.74 (1.43–2.12) | |
|
Metastatic status: De novo |
7.7 (6.1–9.3) | 0.397 | – |
| Disease relapse | 5.8 (4.8–6.8) | – | |
|
Age groups <40 years |
7.3(4.7–9.8) | 0.054 | Reference |
| 40–65 years | 6.7 (5.4–7.9) | 0.78 (0.55–1.11) | |
| >65 years | 4.9 (3.8–6.1) | 0.95 (0.64–1.41) | |
|
ECOG PS: 0 |
10.2 (6.3–14.1) | <0.001 | Reference |
| 1 | 7.8 (6.5–9.7) | 1.04 (0.65–1.55) | |
| 2 | 2.9 (1.7–4.0) | 1.77 (1.13–2.78) | |
| 3 | 2 (1.0–2.9) | 2.52 (1.48–4.28) | |
|
Prior treatment lines: 0 |
7.7 (4.5–10.8) | 0.011 | Reference |
| 1–3 | 6.9 (5.2–8.5) | 1.70 (1.18–2.44) | |
| >3 | 5.4 (4.4–6.4) | 2.27 (1.53–3.21) | |
|
Type of visceral crisis: No visceral crisis |
8.6 (7.4–9.8) | <0.001 | Reference |
| Hepatic abnormalities | 3.6 (2.3–9.8) | 0.67 (0.97–4.74) | |
| Brain metastases | 7.2 (4.5–9.8) | 0.59 (0.08–4.23) | |
| Leptomeningeal metastases | 3.3 (0–8.0) | 0.71 (0.99–5.18) | |
| Pulmonary lymphangitis | 3.6 (1.5–5.8) | 0.58 (0.82–4.16) | |
| Peritoneal carcinomatosis | 2.4 (1.61–6.3) | 0.66 (0.92–4.80) | |
|
Presence of hyperbilirubinemia: No |
7.0 (6.1–7.9) | <0.001 | Reference |
| Yes | 1.4 (0.5–2.3) | 1.90 (1.33–2.72) | |
|
Visceral crisis solved: Yes |
9.3 (8.3–10.2) | <0.001 | 0.27 (0.21–0.36) |
| No | 2.0 (1.7–2.2) | Reference | |
| Year of treatment with platinum combination <2010 |
8.1 (3.9–12.3) | 0.645 | – |
| 2010–2014 | 5.4 (4.3–6.4) | – | |
| 2015–2018 | 6.4 (5.2–7.5) | – |
Abbreviations: ECOG: Eastern Cooperative Oncology Group; HR+: Hormone receptor-positive; HER2: human epidermal growth factor receptor 2; TNBC: triple-negative breast cancer.
2.3. Definitions of overall survival
Overall survival was measured as the time-interval between the first day of treatment with platinum-based chemotherapy until death. Patients who were still alive at the date of last data collection were censored at the last date of follow up.
2.4. Assessing responses
Responses to platinum-based chemotherapy were retrieved from medical charts (as for clinical progression) and radiology reports (CT-scans and/or PET-CT and/or MRI), and further categorized as resolution of visceral crisis vs. non-resolution. The resolution of the visceral crisis was defined as clinical and/or radiological and/or laboratorial improvement according to medical charts review: a) normalization of hepatic blood tests, b) normalization of neurological symptoms, c) improvement of respiratory function without the need of supplementary oxygen, d) normalization of the bowel function, e) resolution of cava vein syndrome and normalization of blood cell counts for the cases of bone marrow invasion).
2.5. Chemotherapy during the last month of life
For this analysis, only patients who died were included. The last date of platinum-based chemotherapy administration was captured and cross checked with the date of death. If the time period elapsed between both dates was ≤30 days, the patient was classified as receiving treatment during the last month of life.
2.6. Statistical analysis
Descriptive statistics were used to examine patient baseline characteristics. Quantitative variables were described in means and compared using paired samples T-test. Overall survival, calculated from the time of treatment initiation until death, was estimated and plotted using the Kaplan-Meier method. Survival rates were compared using the Log-rank test for univariate analysis. For any variable that showed significant or marginally significant association with overall survival, multivariate analysis was performed using the Cox regression model adjusting for breast cancer subtype, age, number of previous treatment lines, presence of visceral crisis, and type of visceral crisis, ECOG-performance status, and type of platinum combination. Subgroup analyses were only performed in subgroups with at least 10 events (deaths). 2-sided p-values <0.05 were considered statistically significant. Given the descriptive nature of this work and the absence of formal hypothesis testing, no sample size was calculated upfront.
All analyses were performed using SPSS Statistics version 25.
3. Results
3.1. Baseline patient characteristics
A total of 441 patients with a mean age of 54.5 years (range 26–85) were eligible for this analysis, of which 261 (59.2%) presented with a visceral crisis at the moment of treatment with platinum-based chemotherapy. Regarding metastatic breast cancer subtype, 57.8% had hormone receptor-positive/HER2-negative, 9.3% hormone receptor-positive/HER2-positive, 25.1% triple-negative and 7.7% hormone receptor-negative/HER2-positive BC. Most patients presented metastatic recurrence (n = 370), and the majority had received more than 3 previous treatment lines for metastatic disease (n = 216). The 3 most common types of visceral crisis were hepatic abnormalities (51.3%), pulmonary lymphangitis (17.2%), and symptomatic brain metastases (10.7%). Besides platinum-based chemotherapy, patients with symptomatic brain metastases also received treatment with corticosteroids and local therapy with radiotherapy (stereotactic-ablative radiotherapy or whole-brain radiotherapy, when indicated). Information on patient baseline characteristics is described in Table 1. Table 2 shows the time elapsed between initial diagnosis of metastatic breast cancer and the diagnosis of visceral crisis as well as median number of prior treatment lines before platinum-based chemotherapy according to breast cancer subtype. The type of platinum combinations administrated are reported in Supplementary Table 1./
Table 1.
Baseline patient characteristics.
| Clinical Characteristics (all cohort n = 441)– N (%) | Visceral crisis (n = 261) | No visceral crisis (n = 180) | ||
|---|---|---|---|---|
| Mean age – years | 54.5 (range 26–85) | 54.5 (26–81) | 54.6 (29–86) | |
| Age groups | <40 years | 46 (10.4%) | 18 (6.9%) | 28 (15.6%) |
| 40–65 years | 303 (68.7%) | 191 (73.2%) | 112 (62.2%) | |
| >65 years | 92 (20.9%) | 52 (19.9) | 40 (22.2%) | |
| Median number of prior treatment lines for metastatic breast cancer | 3.8 (0–12) | 3 (0–12) | 4.35 (0–12) | |
| Treatment line | No prior treatment lines | 48 (10.9%) | 32 (12.3%) | 16 (8.9%) |
| 1-3 prior treatment lines | 177 (40.1%) | 116 (44.4%) | 61 (33.9%) | |
| >3 treatment prior lines | 216 (49.0%) | 113 (43.3%) | 103 (57.2%) | |
| Previous therapy | Anthracyclines∗ | 355 (80.4%) | 218 (83.5%) | 137(76.1%) |
| Taxanes | 280 (63.4%) | 172 (65.9%) | 108 (60%) | |
| Capecitabine | 255(57.8%) | 144 (55.1%) | 111 (61.6%) | |
| Eribulin | 116 (26.3%) | 54 (20.6%) | 62 (34.4%) | |
| Platinum∗∗ | 35 (7.9%) | 13 (6.1%) | 22 (12.2%) | |
| Endocrine therapy | 259 (58.7%) | 155 (59.3%) | 104 (46.1%) | |
| Anti-HER2 agents | 68 (15.4%) | 36 (13.7%) | 32 (17.7%) | |
| Breast cancer subtype | HRa/HER2- | 255 (57.8%) | 166 (63.6%) | 89 (49.4%) |
| HRa/HER2a | 41 (9.3%) | 17 (6.5%) | 24 (13.3%) | |
| HR-/HER2a | 34 (7.7%) | 21 (8%) | 13 (7.2%) | |
| TNBC | 111 (25.1%) | 57 (21.8%) | 54 (30%) | |
| Burden of disease | Locala lymph nodes | 8 (1.8%) | 0 | 8 (4.4%) |
| Bone | 3 (7.0%) | 0 | 3 (1.7%) | |
| Visceral | 430 (97.5%) | 261 (100%) | 169 (93.9%) | |
| ECOG at the moment of platinum | 0 | 32 (7.3%) | 6 (2.3%) | 36 (14.4%) |
| 1 | 157 (35.6%) | 63 (24.1%) | 94 (52.2%) | |
| 2 | 98 (22.2%) | 75 (24.1%) | 23 (12.8%) | |
| 3 | 45 (10.2%) | 40 (15.3%) | 5 (2.8%) | |
| 4a | 3 (0.7%) | 3 (1.1%) | – | |
| Not available (missing) | 106 (24.0%) | 74 (28.4%) | 32 (17.8%) | |
| BRCA status | Negative or not done | 425 (96.4%) | 252 (96.6%) | 173 (96.1%) |
| Mutated | 16 (3.6%) | 9 (3.4%) | 7 (3.9%) | |
| Visceral crisis | No | 180 (40.8%) | – | – |
| Yes | 261 (59.2%) | – | – | |
| Visceral crisis type (n = 261) | ||||
| Hepatic abnormalities | 135 (51.3%) | |||
| Brain metastases | 28 (10.7%) | |||
| Leptomeningeal metastases | 22 (8.4%) | |||
| Pulmonary lymphangitis | 45 (17.2%) | |||
| Peritoneal carcinomatosis | 25 (9.5%) | |||
| Othersb | 6 (2.6%) | |||
∗Anthracyclines in the (neo) adjuvant or metastatic setting ∗∗ Platinum in the (neo) adjuvant setting.
Abbreviations: ECOG: Eastern Cooperative Oncology Group; HR: hormone receptor; HER2: human epidermal growth factor receptor 2; TNBC: triple-negative breast cancer; BRCA: Breast Cancer gene.
3 young patients with triple-negative breast cancer and no prior treatment lines for metastatic disease.
Superior vena cava syndrome, cardiac tamponade, bone marrow invasion and malignant hypercalcemia.
Table 2.
Time from initial diagnosis of metastatic breast cancer to visceral crisis and prior treatment lines for metastatic breast cancer according to breast cancer subtypes.
| BC subtype (number of pts with visceral crisis) | Median time (months) from metastatic BC diagnosis to visceral crisis | Median number of prior treatment lines for metastatic BC |
|---|---|---|
| HR+/HER2- (n = 166) | 29.62 (0–135) | 4 (0–11) |
| HR+/HER2+ (n = 17) |
33.3 (0–118) | 4 (0–12) |
| HR-/HER2+ (n = 21) |
25.5 (0–129) | 3 (0–9) |
| TNBC (n = 57) |
11.56 (0–62) | 2 (0–9) |
| All subtypes (n = 262) |
25.07 (0–135) | 3 (0–12) |
Legend: BC: breast cancer; pts: patients; mBC: metastatic breast cancer; HR+/HER2-: hormone receptor-positive, HER2-negative; HR+/HER2+: hormone receptor-positive, HER2-positive; TNBC: triple-negative breast cancer.
3.2. Overall survival with platinum-based chemotherapy
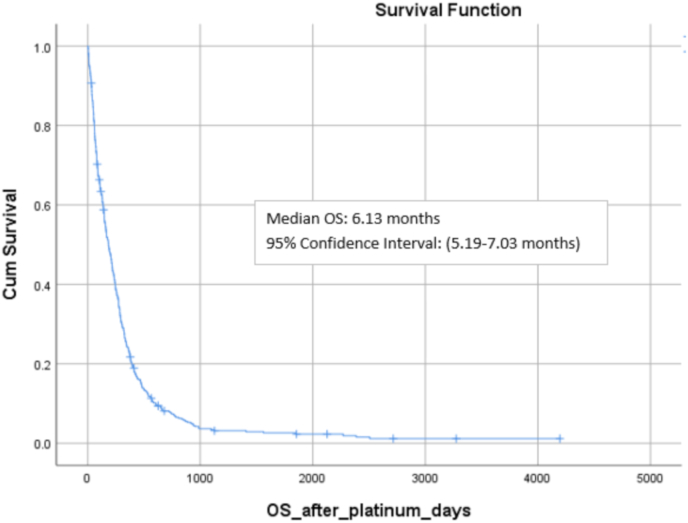
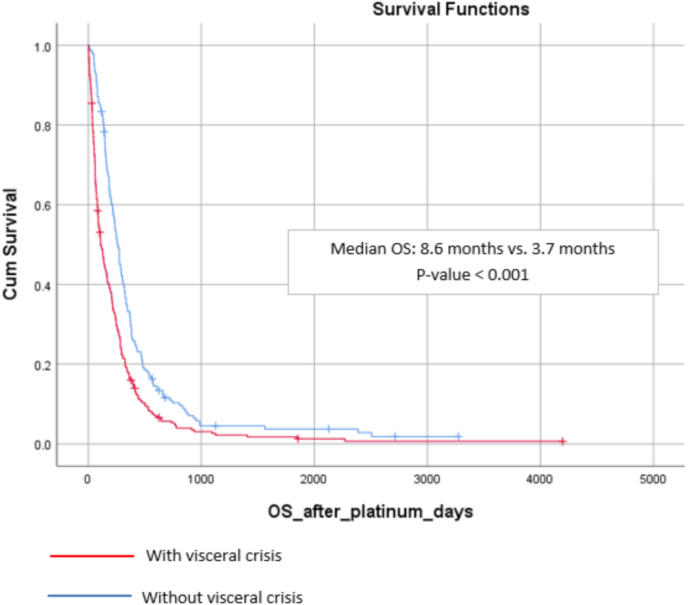
Of the 441 patients included in this analysis, 424 were dead (96.1%), 14 were alive (3.2%) and 3 lost to follow-up. Median overall survival with platinum-based chemotherapy in the entire cohort was 6.1 months (range: 5.2–7.0) (Fig. 2. Patients with visceral crisis had statistically lower overall survival than patients without visceral crisis (8.6 months vs. 3.7 months, p < 0.001) (Fig. 3
Fig. 2.
Median overall survival with platinum-based polychemotherapy in the whole cohort.
Fig. 3.
Median overall survival with platinum-based polychemotherapy patients with and without visceral crisis.
Univariate and multivariate analyses of the association of baseline factors with overall survival with platinum-based chemotherapy for subgroups with at least 10 events are reported in Table 3. In the univariate analysis, there was no statistical difference in median overall survival according to breast cancer subtypes, year of treatment with platinum combination, and de novo vs. metastatic recurrence; a numerically lower overall survival was observed for patients with hormone receptor-positive/HER2-negative breast cancer (Table 3). There was a trend for worse prognosis in elderly patients (p = 0.054- Table 3). The number of previous treatment lines, ECOG performance status, type of visceral crisis, presence of hyperbilirubinemia, and resolution of visceral crisis were factors statistically associated with overall survival in univariate analysis (Table 3). Regarding treatment lines, median overall survival in patients with just 1 prior treatment line was similar to the overall survival of patients with 1–3 treatment lines (7.03 vs. 6.9 months, respectively).
In multivariate analysis, poor ECOG performance status, hyperbilirubinemia, and increased number of previous treatment lines persisted as significant prognostic factors for overall survival with platinum-based chemotherapy in the entire cohort (Table 3).
An exploratory analysis of prognostic factors was performed within breast cancer subtypes, for patients with hormone receptor-positive/HER2-negative, HER2-positive and triple-negative breast cancer (Supplementary Tables 2, 3 and 4, respectively). For patients with hormone receptor-positive/HER2-negative breast cancer, none of the analyzed variables persisted as a significant prognostic factor in the multivariate analysis. In the HER2-positive cohort, resolution of visceral crisis persisted as a prognostic factor in the multivariate analysis, whereas for triple-negative breast cancer ECOG and number of prior treatment lines.
3.3. Visceral crisis resolution and first response to platinum
Of the 441 patients, 73 did not have an imaging exam to measure platinum-based chemotherapy efficacy due to rapidly progressive disease and/or fast clinical deterioration. Among the remaining 368 patients, imaging exams were performed within a median of 41.5 days after start of treatment (range 10–63 days). Overall response rates were 28.1%, 27.2%, and 31.7% for the entire cohort, patients with visceral crisis and without visceral crisis, respectively (Supplementary Table 5).
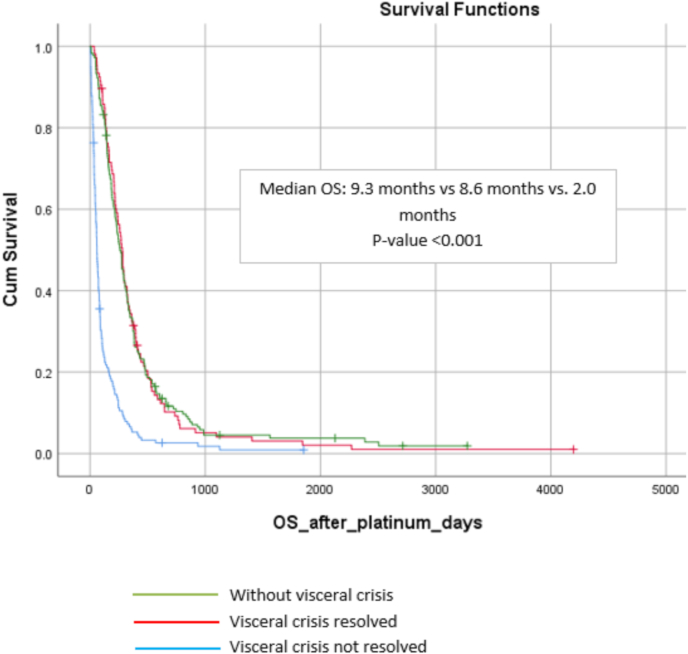
Of the 261 patients with visceral crisis, 106 (40.5%) presented a resolution of visceral crisis, through normalization of laboratorial values and/or clinical symptoms. The resolution of visceral crisis correlated with better overall survival in univariate and multivariate analysis (9.3 vs. 2.0 months – adjusted HR 0.25; 95% CI 0.19–0.34 – p < 0.001) (Fig. 4).
Fig. 4.
Kaplan Meier curves according to visceral crises resolved, not resolved and without visceral crisis.
3.4. Chemotherapy during the last 30 days of life
Data regarding the administration of platinum-based chemotherapy during the last 30 days of life in the entire cohort and according to the presence of visceral crisis is presented in the Supplementary Table 6. In total, 424 patients died and 130 (30.6%) received platinum-based chemotherapy during the last month of life. Of note, this proportion was higher for patients presenting visceral crisis (42.6%).
4. Discussion
Despite substantial progress in early diagnosis and curative treatments [1,2] in developed countries, about 20–30% of patients with breast cancer will develop metastatic disease, and a significant proportion will develop high burden disease and/or visceral crisis (as a first presentation or as a consequence of disease progression) [15].
Our results demonstrate the bad prognosis of metastatic breast cancer patients who develop a visceral crisis, as in our cohort of patients treated with a platinum-based chemotherapy regimen, the difference in median overall survival was 4.9 months (8.6 vs. 3.7 months, p < 0.001). There is a paucity of data in the literature reporting efficacy of existing therapies in patients with breast cancer and visceral crisis, most coming from case reports [20]. One previous small retrospective study (n = 35) also highlighted the poor prognosis of this condition, reporting a median overall survival of 4.6 weeks for patients with hormone receptor-positive breast cancer and visceral crisis according to ABC 4 definition [21].
In our institution, the standard of care for patients presenting metastatic breast cancer and visceral crisis (particularly in the liver) consists of a platinum-based chemotherapy regimen (especially for patients previously exposed to anthracyclines and taxanes). Although a Cochrane review concluded that there is little or no survival benefit, altogether with an excess of toxicities with platinum-based regimens in patients with non-triple negative metastatic breast cancer, this review did not take into account the setting of visceral crisis [22]. In our Institute, the choice of platinum-based chemotherapy is justified by the advantage of not requiring any dose-adjustments according to liver function alterations, which ultimately allows treatment with full-dose chemotherapy, at a moment when a fast response is needed [23]. About half of the patients with metastatic breast cancer will have liver involvement [24] and, are consequently at risk for impaired liver function. In this sense, our study further confirms liver metastases as the most frequent etiology of visceral crisis. Other advantages of using platinum-salts is the potential clinical activity in central-nervous system metastases [25], yet another important cause of visceral crisis, and also its high response rates in triple-negative and BRCA mutated breast cancer patients [26].
Interestingly, 40.8% of the patients in our cohort received a platinum-based chemotherapy regimen even though they were not classified as presenting with a visceral crisis, as per ABC 4 criteria. We hypothesized that this might reflect the challenge in characterizing the term “visceral crisis” and the fact that international guidelines still recommend the use of polychemotherapy in this situation [15,17,27].
As a matter of fact, even though a definition is necessary in order to refine the meaning of visceral crisis, frequently, this definition might not exactly reflect the scenario in which the oncologist perceives that a patient is developing an aggressive disease. Such an ill-defined scenario is informally categorized as ‘‘impending visceral crisis’’, characterized by a high burden of disease, a rapid progression under a previous treatment regimen (for instance endocrine therapy), or a rapid clinical deterioration in the performance status of the patient, among others. This predicament in defining visceral crisis might lead to subjectivity in treatment decision, while precluding a more precise indication for polychemotherapy [16,28]. Importantly, the definition of visceral crisis was revisited in the latest ABC 5 guidelines and some examples were given in order to improve the differentiation of the existence of visceral metastases and the presence of visceral crisis [16]. In addition, the existence of ‘‘impending visceral crisis’’ was also acknowledged [16] which may improve the quality of future studies regarding these definitions as well as clinical practice care.
Another important aspect in clinical practice is that due to the characteristically rapid onset of visceral crises and the need to promptly initiate treatment, to obtain metastatic tissue and perform its molecular characterization in order to identify potential targets for more successful treatments, poses a great challenge. For the same reasons, these patients are commonly excluded from clinical trials testing new therapeutic agents or combinations that could provide better results than chemotherapy alone. In addition, data comparing polychemotherapy regimens in this specific situation is lacking, as well as data from targeted treatments. For example, with respect to the combination of CDK 4/6 inhibitors and endocrine therapy, which confers impressive response rates of ∼50% for patients with visceral metastases [18,29], visceral crisis and high disease’s burden were explicit exclusion criteria in the PALOMA 2[30], PALOMA 3[31], MONALEESA-3[32] and MONARCH 3 trials [33]. Moreover, ECOG performance status 0/1 was an eligibility criteria for inclusion into the MONALEESA 2[34] and MONALEESA 7 trials [35]. Regarding cytotoxic agents, single-arm studies investigating platinum-based chemotherapy regimens in patients with visceral metastases have also yielded response rates of 50%, but common exclusion criteria in those studies included abnormal hepatic function [[36], [37], [38], [39], [40]].
As there is a great need for effective treatments in case of visceral crisis, and data from clinical trials specifically addressing this condition are lacking, real-world data reporting on the efficacy of currently approved treatments (cytotoxic and target therapies) in patients with visceral crisis might, therefore, partially mitigate this gap in knowledge. Of note, an ongoing prospective, multicenter observational study (ABEMACARE) is recruiting patients with symptomatic visceral metastases/and or high tumor burden to receive treatment with abemaciclib in combination with endocrine therapy as first-line therapy for metastatic disease, with a primary endpoint of objective response rate [41].
The overall response rates observed in our cohort (28.1%) is aligned with a previous retrospective single institutional study reported by Staudacher et al., which assessed the outcomes of metastatic breast cancer patients treated with platinum-based chemotherapy in a cohort of 143 patients [42]. In this study, 76.9% of the patients had visceral disease and 67.1% had received previous anthracycline and taxane therapy [42]. Importantly, there is no mention regarding visceral crisis status in this previous study. Objective response rates were 33% for patients with triple-negative breast cancer and 22% for non-triple-negative breast cancer, with a median overall survival of 11 months for the entire cohort [42]. The better overall survival observed in this study compared to our results might be justified by a less pre-treated population (median number of prior treatment lines 2.1 vs. 3.7 in our cohort) and the absence of visceral crisis.
Furthermore, the presence of visceral crisis, a life-threatening condition often requires an aggressive and immediate treatment, yet too often the line separating therapeutic obstinacy is crossed, leading to therapeutic futility and unnecessary patient suffering at the end of life. In a systematic review of 38 international studies evaluating 1.2 million patients, Cardona-Morrel et al. demonstrated that non-beneficial administration of drugs occurred on average in 33–38% of dying patients [43]. Similarly, 30.6% of the patients in our cohort received platinum-based chemotherapy during the last 30 days of life, and this was even more frequent for patients with visceral crisis (42.6%). Prognostication in advanced cancer is an unmet need, often relying on subjective and informal clinical intuition/experience. However, prognostication might offer a chance to focus on the quality of patient’s life during his/her last days and might avoid futile aggressive therapies. In fact, a standardized palliative care has been shown to enable patients not only to experience significant improvements in both quality of life and mood, but also to live two months longer on average, according to a randomized clinical trial involving 151 patients with metastatic non-small-cell lung cancer [44]. Similar results have been demonstrated in studies including breast cancer patients [[45], [46], [47]]. Realizing the actual limitations of prognostication methods, efforts have been made to develop more accurate prognostic tools combining clinical and laboratory variables that may predict survival [48,49]. Importantly, it has also been suggested that relative members play an important role during end of life treatment decisions and this needs to also be assessed to ensure that patient’s preferences remain paramount [[50], [51], [52]].
Unfortunately, we were not able to identify baseline factors associated with an increased chance of visceral crisis resolution with this treatment regimen neither in the whole cohort nor according to breast cancer subtypes. Moreover, not all subgroup analysis could be performed within some breast cancer subtypes due to the small number of events. In addition, since only few patients were known to carry BRCA 1/2 mutations, we could not study the impact of germline BRCA mutations in the resolution of visceral crisis upon treatment with platinum-based chemotherapy. On the other hand, our study suggests that three clinical characteristics – namely high bilirubin, poor ECOG Performance Status, and >3 treatment lines – may be considered as markers of futility for platinum-based chemotherapy in metastatic breast cancer patients who develop visceral crisis. Those “futility markers” are easily assessed, non-expensive, and minute-read, thus they might help guiding oncologists and patients decision on when exclusive palliative care should be implemented. Importantly, as these results were not confirmed in the multivariate analysis within breast cancer subtypes this hypotheses needs to be tested in a prospective study with a less heterogeneous population.
To our knowledge, this is the largest study to report the efficacy of platinum-based chemotherapy in patients with metastatic breast cancer and high burden disease and/or visceral crisis in a real-world setting. Nevertheless, given the limitations inherent to a retrospective, single-center study associated with the challenges in identifying patients considered to be in visceral crisis and their resolution, as well as response rates through review of medical records, this manuscript should be considered as an exploratory, hypotheses-generating study. Another important limitation is the heterogeneity of the study population and the long period of data collection, as well as the lack of similar series including patients with visceral crisis in order to compare and discuss our results. Therefore, our results need validation in appropriately designed prospective multicenter prognostic studies and clinical trials comparing different treatment modalities (including target therapies) for patients with this condition, preferentially within each breast cancer subtype.
5. Conclusion
Our results highlight the poor prognosis of metastatic breast cancer patients who develop visceral crisis. Patients with visceral crisis presented a significantly lower overall survival compared to patients without visceral crisis, albeit those who overcame it benefited from platinum-based chemotherapy, with similar overall survival as those patients without visceral crisis. This study highlights the potential prognostic implication of three easily assessable clinical features: hyperbilirubinemia, poor ECOG performance status, and >3 previous treatment lines. Considering the retrospective nature of this study, the long time-interval of collected data, and the heterogeneity of the population, our results need future validation in properly designed, prospective, and preferentially multicenter studies.
Compliance with ethical standards
This study was approved by an internal ethics committee (CE3105), and informed consent was not required as all patient-related data were retrieved from medical records only, and patient identification were kept confidential.
Funding
This study has received no funding.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.This study was approved by an internal ethics committee (EC3105), and informed consent was not required as patient’s information was retrieved from retrospective review of medical records only. The identity of the patients was kept always anonymously.
Author contributions
MA: Conceptualization, data collection, statistical analysis, graphics, manuscript writing and review, RSC: Data collection, manuscript writing and review, SF: Data collection, manuscript writing and review, DE: Data collection, manuscript writing and review, AA: Conceptualization, manuscript writing and review, EA: Conceptualization, Supervision, manuscript writing and review. All authors have read and approved this manuscript and ensure this submission is not under consideration elsewhere.
Declaration of competing interest
MAF: none. RSC, SF: Travel grant from Pfizer for the ESMO conference 2019. DE: Funding for his fellowship (2018–2019: Novartis. Speaker fee: Janssen. AA: Advisory role, speaker fees and research funding for his institute from: Roche, Lilly, Amgen, EISAI, BMS, Pfizer, Novartis, MSD, Genomic Health, Ipsen, AstraZeneca, Bayer, Leo Pharma. EA: honoraria and advisory board: Roche/GNE, Novartis, Seattle Genetics and Zodiacs; travel grants: Roche/GNE, GSK/Novartis; co-principal investigator of the LORELEI trial (NCT02273973). Research grant for his institute: Roche/GNE, Astra-Zeneca, Novartis, and Servier.
Acknowledges
None.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.breast.2021.03.002.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Waks A.G., Winer E.P. Breast cancer treatment: a review. J Am Med Assoc. 2019;321:288–300. doi: 10.1001/jama.2018.19323. [DOI] [PubMed] [Google Scholar]
- 2.Harbeck N., Penault-Llorca F., Cortes J. Breast cancer. Nat Rev Dis Primers. 2019;5(1–31) doi: 10.1038/s41572-019-0111-2. [DOI] [PubMed] [Google Scholar]
- 3.Prat A., Perou C.M. Deconstructing the molecular portraits of breast cancer. Mol Oncol. 2011;5(5–23) doi: 10.1016/j.molonc.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sørlie T., Perou C.M., Tibshirani R. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci Unit States Am. 2001;98:10869–10874. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perou C.M., Sørlie T., Eisen M.B. Molecular portraits of human breast tumours. Nature. 2000;406:747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 6.Swain S.M., Miles D., Kim S.-B. End-of-study analysis from the phase III, randomized, double-blind, placebo (Pla)-controlled CLEOPATRA study of first-line (1L) pertuzumab (P), trastuzumab (H), and docetaxel (D) in patients (pts) with HER2-positive metastatic breast cancer (MBC) J Clin Orthod. 2019;37 1020–1020. [Google Scholar]
- 7.Sledge G.W., Toi M., Neven P. JAMA Oncol; 2019. The effect of abemaciclib plus fulvestrant on overall survival in hormone receptor–positive, ERBB2-negative breast cancer that progressed on endocrine therapy—MONARCH 2: a randomized clinical trial [internet]https://jamanetwork.com/journals/jamaoncology/fullarticle/2752266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Im S.-A., Lu Y.-S., Bardia A. Overall survival with ribociclib plus endocrine therapy in breast cancer. N Engl J Med. 2019;381:307–316. doi: 10.1056/NEJMoa1903765. [DOI] [PubMed] [Google Scholar]
- 9.Robson M., Im S.-A., Senkus E. Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. N Engl J Med. 2017;377:523–533. doi: 10.1056/NEJMoa1706450. [DOI] [PubMed] [Google Scholar]
- 10.Litton J.K., Rugo H.S., Ettl J. Talazoparib in patients with advanced breast cancer and a germline BRCA mutation. N Engl J Med. 2018;379:753–763. doi: 10.1056/NEJMoa1802905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schmid P., Adams S., Rugo H.S. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med. 2018;379:2108–2121. doi: 10.1056/NEJMoa1809615. [DOI] [PubMed] [Google Scholar]
- 12.André F., Ciruelos E., Rubovszky G. Alpelisib for PIK3CA-mutated, hormone receptor–positive advanced breast cancer. N Engl J Med. 2019;380:1929–1940. doi: 10.1056/NEJMoa1813904. [DOI] [PubMed] [Google Scholar]
- 13.Jones R.H., Carucci M., Casbard A.C. Capivasertib (AZD5363) plus fulvestrant versus placebo plus fulvestrant after relapse or progression on an aromatase inhibitor in metastatic ER-positive breast cancer (FAKTION): a randomized, double-blind, placebo-controlled, phase II trial. J Clin Orthod. 2019;37 doi: 10.1016/S1470-2045(19)30817-4. 1005–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Piccart M., Hortobagyi G.N., Campone M. Everolimus plus exemestane for hormone-receptor-positive, human epidermal growth factor receptor-2-negative advanced breast cancer: overall survival results from BOLERO-2†. Ann Oncol. 2014;25:2357–2362. doi: 10.1093/annonc/mdu456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cardoso F., Senkus E., Costa A. 4th ESO–ESMO international consensus guidelines for advanced breast cancer (ABC 4)†. Ann Oncol. 2018;29:1634–1657. doi: 10.1093/annonc/mdy192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cardoso F., Paluch-Shimon S., Senkus E. 5th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 5)† [Internet] Ann Oncol. 2020 doi: 10.1016/j.annonc.2020.09.010. http://www.sciencedirect.com/science/article/pii/S0923753420424603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rugo H.S., Rumble R.B., Macrae E. Endocrine therapy for hormone receptor–positive metastatic breast cancer: American society of clinical Oncology guideline. J Clin Orthod. 2016;34:3069–3103. doi: 10.1200/JCO.2016.67.1487. [DOI] [PubMed] [Google Scholar]
- 18.Hortobagyi G.N. Ribociclib for the first-line treatment of advanced hormone receptor-positive breast cancer: a review of subgroup analyses from the MONALEESA-2 trial [Internet] Breast Cancer Res. 2018;20 doi: 10.1186/s13058-018-1050-7. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6194611/ [cited 2019 Oct 4] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.von Elm E., Altman D.G., Egger M. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61:344–349. doi: 10.1016/j.jclinepi.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 20.Fumet J.-D., Wickre M., Jacquot J.-P. Successfully treatment by eribulin in visceral crisis: a case of lymphangitic carcinomatosis from metastatic breast cancer. BMC Canc. 2018;18:839. doi: 10.1186/s12885-018-4725-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sbitti Y., Slimani K., Debbagh A. Visceral crisis means short survival among patients with luminal A metastatic breast cancer: a retrospective cohort study. World J Oncol. 2017;8(105–109) doi: 10.14740/wjon1043w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Egger S., Willson M., Morgan J. Platinum-containing regimens for metastatic breast cancer [Internet] Cochrane Database Syst Rev. 2017 doi: 10.1002/14651858.CD003374.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grigorian A., O’Brien C.B. Hepatotoxicity secondary to chemotherapy. J Clin Transl Hepatol. 2014;2(95–102) doi: 10.14218/JCTH.2014.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Diamond J.R., Finlayson C.A., Borges V.F. Hepatic complications of breast cancer. Lancet Oncol. 2009;10:615–621. doi: 10.1016/S1470-2045(09)70029-4. [DOI] [PubMed] [Google Scholar]
- 25.Shah N., Mohammad A.S., Saralkar P. Investigational chemotherapy and novel pharmacokinetic mechanisms for the treatment of breast cancer brain metastases. Pharmacol Res. 2018;132:47–68. doi: 10.1016/j.phrs.2018.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tutt A., Tovey H., Cheang M.C.U. Carboplatin in BRCA1/2-mutated and triple-negative breast cancer BRCAness subgroups: the TNT Trial. Nat Med. 2018;24:628–637. doi: 10.1038/s41591-018-0009-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Giordano S.H., Temin S., Chandarlapaty S. Systemic therapy for patients with advanced human epidermal growth factor receptor 2-positive breast cancer: ASCO clinical practice guideline update. J Clin Oncol. 2018;36:2736–2740. doi: 10.1200/JCO.2018.79.2697. [DOI] [PubMed] [Google Scholar]
- 28.González A., Lluch A., Aba E. A definition for aggressive disease in patients with HER-2 negative metastatic breast cancer: an expert consensus of the Spanish Society of Medical Oncology (SEOM) Clin Transl Oncol. 2017;19:616–624. doi: 10.1007/s12094-016-1571-4. [DOI] [PubMed] [Google Scholar]
- 29.Turner N.C., Finn R.S., Martin M. Clinical considerations of the role of palbociclib in the management of advanced breast cancer patients with and without visceral metastases. Ann Oncol. 2018;29:669–680. doi: 10.1093/annonc/mdx797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Finn R.S., Martin M., Rugo H.S. Palbociclib and letrozole in advanced breast cancer. N Engl J Med. 2016;375:1925–1936. doi: 10.1056/NEJMoa1607303. [DOI] [PubMed] [Google Scholar]
- 31.Turner N.C., Slamon D.J., Ro J. Overall survival with palbociclib and fulvestrant in advanced breast cancer. N Engl J Med. 2018;379:1926–1936. doi: 10.1056/NEJMoa1810527. [DOI] [PubMed] [Google Scholar]
- 32.Slamon D.J., Neven P., Chia S. Overall survival with ribociclib plus fulvestrant in advanced breast cancer. N Engl J Med. 2019;382:514–524. doi: 10.1056/NEJMoa1911149. [DOI] [PubMed] [Google Scholar]
- 33.Johnston S., Martin M., Di Leo A. MONARCH 3 final PFS: a randomized study of abemaciclib as initial therapy for advanced breast cancer. npj Breast Canc. 2019;5(5) doi: 10.1038/s41523-018-0097-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hortobagyi G.N., Stemmer S.M., Burris H.A. Ribociclib as first-line therapy for HR-positive, advanced breast cancer. N Engl J Med. 2016;375:1738–1748. doi: 10.1056/NEJMoa1609709. [DOI] [PubMed] [Google Scholar]
- 35.Im S.-A., Lu Y.-S., Bardia A. Overall survival with ribociclib plus endocrine therapy in breast cancer. N Engl J Med. 2019;381:307–316. doi: 10.1056/NEJMoa1903765. [DOI] [PubMed] [Google Scholar]
- 36.Sirohi B., Arnedos M., Popat S. Platinum-based chemotherapy in triple-negative breast cancer. Ann Oncol. 2008;19:1847–1852. doi: 10.1093/annonc/mdn395. [DOI] [PubMed] [Google Scholar]
- 37.Heinemann V., Stemmler H.J., Wohlrab A. High efficacy of gemcitabine and cisplatin in patients with predominantly anthracycline- and taxane-pretreated metastatic breast cancer. Canc Chemother Pharmacol. 2006;57:640–646. doi: 10.1007/s00280-005-0093-5. [DOI] [PubMed] [Google Scholar]
- 38.Tas F., Guney N., Derin D. Biweekly administration of gemcitabine and cisplatin chemotherapy in patients with anthracycline and taxane-pretreated metastatic breast cancer. Invest N Drugs. 2008;26:363–368. doi: 10.1007/s10637-007-9110-3. [DOI] [PubMed] [Google Scholar]
- 39.Burch P.A., Mailliard J.A., Hillman D.W. Phase II study of gemcitabine plus cisplatin in patients with metastatic breast cancer: a north central cancer treatment group trial [internet] Am J Clin Oncol. 2005;28 doi: 10.1097/01.coc.0000144815.54746.d0. https://journals.lww.com/amjclinicaloncology/Fulltext/2005/04000/Phase_II_Study_of_Gemcitabine_Plus_Cisplatin_in.17.aspx [DOI] [PubMed] [Google Scholar]
- 40.Vassilomanolakis M., Koumakis G., Barbounis V. Vinorelbine and cisplatin in metastatic breast cancer patients previously treated with anthracyclines. Ann Oncol. 2000;11:1155–1160. doi: 10.1023/a:1008377724931. [DOI] [PubMed] [Google Scholar]
- 41.Kotzur F., Bidner H., Bronger H. Abstract OT-26-01: ABEMACARE: abemaciclib in combination with endocrine therapy as first line therapy in metastatic breast cancer patients with symptomatic visceral metastases or high tumor burden. Canc Res. 2021;81 OT-26-01. [Google Scholar]
- 42.Staudacher L., Cottu P.H., Diéras V. Platinum-based chemotherapy in metastatic triple-negative breast cancer: the Institut Curie experience. Ann Oncol. 2011;22:848–856. doi: 10.1093/annonc/mdq461. [DOI] [PubMed] [Google Scholar]
- 43.Cardona-Morrell M., Kim J., Turner R. Non-beneficial treatments in hospital at the end of life: a systematic review on extent of the problem. Int J Qual Health Care. 2016;28:456–469. doi: 10.1093/intqhc/mzw060. [DOI] [PubMed] [Google Scholar]
- 44.Temel J.S., Greer J.A., Muzikansky A. Early palliative care for patients with metastatic non–small-cell lung cancer. N Engl J Med. 2010;363:733–742. doi: 10.1056/NEJMoa1000678. [DOI] [PubMed] [Google Scholar]
- 45.Hui D., Kim S.H., Roquemore J. Impact of timing and setting of palliative care referral on quality of end-of-life care in cancer patients. Cancer. 2014;120:1743–1749. doi: 10.1002/cncr.28628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zimmermann C., Swami N., Krzyzanowska M. Early palliative care for patients with advanced cancer: a cluster-randomised controlled trial. Lancet. 2014;383:1721–1730. doi: 10.1016/S0140-6736(13)62416-2. [DOI] [PubMed] [Google Scholar]
- 47.Bakitas M., Lyons K.D., Hegel M.T. Effects of a palliative care intervention on clinical outcomes in patients with advanced cancer: the Project ENABLE II randomized controlled trial. J Am Med Assoc. 2009;302:741–749. doi: 10.1001/jama.2009.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Laird B.J., Kaasa S., McMillan D.C. Prognostic factors in patients with advanced cancer: a comparison of clinicopathological factors and the development of an inflammation-based prognostic system. Clin Canc Res. 2013;19:5456. doi: 10.1158/1078-0432.CCR-13-1066. [DOI] [PubMed] [Google Scholar]
- 49.Gwilliam B., Keeley V., Todd C. Development of Prognosis in Palliative care Study (PiPS) predictor models to improve prognostication in advanced cancer: prospective cohort study. BMJ. 2011;343:d4920. doi: 10.1136/bmj.d4920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Laidsaar-Powell R., Butow P., Bu S. Family involvement in cancer treatment decision-making: a qualitative study of patient, family, and clinician attitudes and experiences. Patient Educ Counsel. 2016;99:1146–1155. doi: 10.1016/j.pec.2016.01.014. [DOI] [PubMed] [Google Scholar]
- 51.Shin D.W., Cho J., Roter D.L. Attitudes toward family involvement in cancer treatment decision making: the perspectives of patients, family caregivers, and their oncologists. Psycho Oncol. 2017;26:770–778. doi: 10.1002/pon.4226. [DOI] [PubMed] [Google Scholar]
- 52.Laryionava K., Pfeil T.A., Dietrich M. The second patient? Family members of cancer patients and their role in end-of-life decision making. BMC Palliat Care. 2018;17(29) doi: 10.1186/s12904-018-0288-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.