Abstract
Acute or degenerative meniscus tears are the most common knee lesions. Meniscectomy provides symptomatic relief and functional recovery only in the short- to mid-term follow-up but significantly increases the risk of osteoarthritis. For this reason, preserving the meniscus is key, although it remains a challenge. Allograft transplants present many disadvantages, so during the last 20 years preclinical and clinical research focused on developing and investigating meniscal scaffolds. The aim of this systematic review was to collect and evaluate all the available evidence on biosynthetic scaffolds for meniscus regeneration both in vivo and in clinical studies. Three databases were searched: 46 in vivo preclinical studies and 30 clinical ones were found. Sixteen natural, 15 synthetic, and 15 hybrid scaffolds were studied in vivo. Among them, only 2 were translated into clinic: the Collagen Meniscus Implant, used in 11 studies, and the polyurethane-based scaffold Actifit®, applied in 19 studies. Although positive outcomes were described in the short- to mid-term, the number of concurrent procedures and the lack of randomized trials are the major limitations of the available clinical literature. Few in vivo studies also combined the use of cells or growth factors, but these augmentation strategies have not been applied in the clinical practice yet. Current solutions offer a significant but incomplete clinical improvement, and the regeneration potential is still unsatisfactory. Building upon the overall positive results of these “old” technologies to address partial meniscal loss, further innovation is urgently needed in this field to provide patients better joint sparing treatment options.
Keywords: Meniscal scaffold, Meniscectomy, Osteoarthritis, Regenerative medicine, Collagen, Polyurethane
Graphical abstract
Highlights
-
•
Animal studies employed natural, synthetic and hybrid natural/synthetic scaffolds.
-
•
Only in a few animal studies scaffold augmentation with cells or GFs was tested.
-
•
Only two meniscal scaffolds have reached clinical application: CMI and Actifit.
-
•
Clinical results are promising, but complete meniscus regeneration has not been achieved.
-
•
There is urgent need for technological innovation in the field of meniscal regeneration.
1. Introduction
Meniscal preservation is one the pillars of “joint preserving surgery” which aims at decreasing the need for total knee replacement [1,2]. In fact, even though meniscectomy is shown to provide symptomatic relief and functional recovery in the short- to mid-term follow-up, its long-term consequences are well-known and it could be considered as one of the “heaviest” risk factors for the onset of osteoarthritis (OA) even in young and middle-aged patients [3,4]. The management of meniscal damage has its own chapter in the history of orthopaedics [5] and, surprisingly, the very first surgical treatment, described in 1874, was a meniscal reinsertion rather than a meniscectomy [6]. Also, current guidelines suggest saving meniscal tissue whenever possible, but not every meniscal tear can be sutured: as a result, meniscectomy still represents the most common arthroscopic procedure performed worldwide [7]. Meniscectomized knees undergo relevant biomechanical and kinematic impairment, whose entity depends on the amount of meniscal resection: first, there is a variable loss of knee stability, both in antero-posterior and rotational translations, associated to a reduction of the congruency between the articular surfaces of the tibia and femur. Subsequently, an increase in local peak contact pressure and a reduction of joint lubrication occur, leading to a gradual cartilage damage in the affected compartment, ultimately resulting in the onset of OA often associated to extensive subchondral bone damage, especially in knees presenting with varus-valgus malalignment.
Based on these premises, to address massive meniscus loss, allograft transplantation has been introduced into clinical practice more than 30 years ago [[8], [9], [10], [11], [12]]. However, despite positive findings, the use of allografts is affected by some drawbacks, mainly related to the limited availability of musculoskeletal tissue banks and the strict regulations on the use of allograft existing in some countries. Furthermore, meniscal allograft is best indicated in total or sub-total meniscectomized patients, whereas, in the majority of cases, clinicians deal with partial meniscal defects.
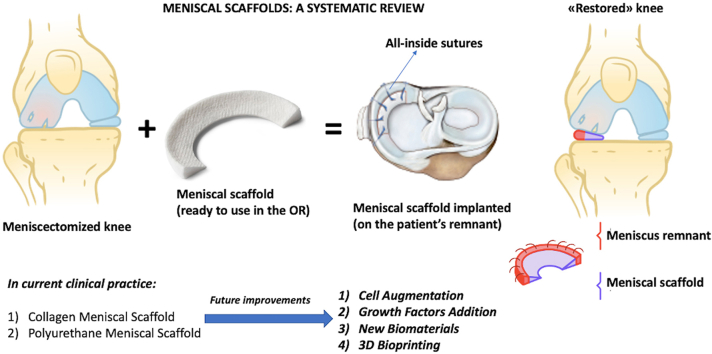
Meniscal scaffolds have been developed to address these limitations [13]: scaffolds are biomimetic materials able to stimulate tissue repair and regeneration by recruiting autologous stromal cells that can populate the 3-dimensional architecture of the scaffold, and restore the meniscal fibro-cartilage.
The rationale behind the development of meniscal scaffolds was therefore to offer a treatment option for partial meniscal loss, in order to restore the native biomechanics of the knee and provide a substratum for meniscal regeneration, thus preventing (or delaying) the onset of irreversible joint degenerative changes over time.
In the last two decades several different biomaterials underwent pre-clinical testing [14,15] aiming at stimulating joint tissues regeneration. Among these, meniscal scaffolds reached the clinical application based on experimental evidence that meniscal tissue healing can be boosted by proper biologic stimuli even in the presence of a mild degenerative joint environment [16]. The advantage of meniscal scaffolds is that they represent an on-the-shelf technology with the possibility of being modelled to match the actual size of the meniscal defect, without the risk of impairing their biologic and biomechanical properties, which are strictly linked to the particular biomaterial used [17]. Still, despite numerous in vitro and in vivo studies, only few scaffolds were adopted in humans and [18] research is still intense in this field.
The aim of the present systematic review was to summarize the current state of the art in meniscal scaffolds’ application, from preclinical in vivo to clinical studies, to understand the evolution occurred over time and identify the new trends of research and areas deserving further investigation.
2. Materials and methods
The search of articles of this systematic review was conducted on February 2021, according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) principles. The search was conducted on 3 databases: www.pubmed.com, www.webofknowledge.com and www.scopus.com. In the first database the MeSHes were as follows: (“Meniscus"[Mesh] OR “Tibial Meniscus Injuries"[Mesh] OR “Menisci, Tibial"[Mesh]) AND “Tissue Scaffolds"[Mesh]) and in the second and third databases the keywords were: “((Meniscus OR Tibial Meniscus Injuries OR Menisci, Tibial) AND (Tissue Scaffolds))”. The limits were English language for www.pubmed.com and English language and article type for www.scopus.com and www.webofknowledge.com. For all the 3 searches, the date of publication was set from 2000 up to today. The following inclusion criteria were used for article selection: 1) animal trials describing the radiologic, histologic, and immuno-histochemical results of meniscal scaffolds; 2) clinical trials of any level of evidence including more than 10 patients treated by meniscal scaffold implantation.
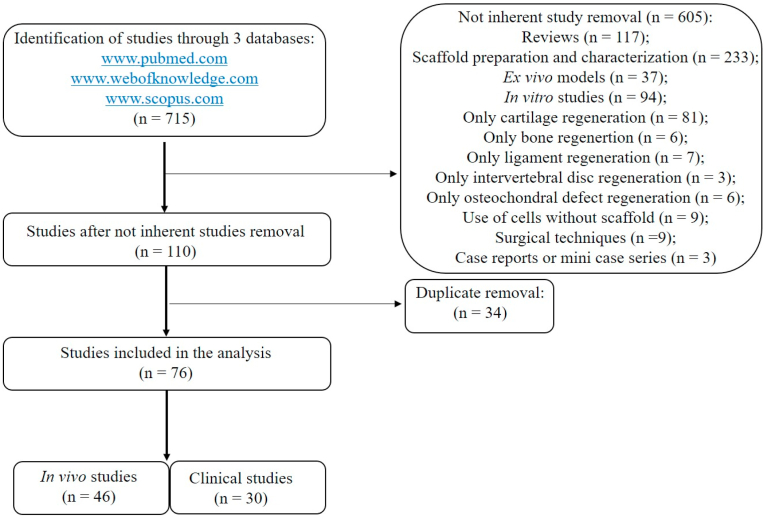
A total of 715 articles were found. The articles were screened by reading titles and abstracts by two authors (FV, NDV). A total of 605/715 articles were excluded because: they were reviews (117/605); investigated scaffold preparation and characterization (233/605); ex vivo models (37/605); in vitro studies (94/605); only cartilage (81/605), bone (6/605), ligament (7/605), intervertebral disc (3/605) or osteochondral defect (6/605) regeneration; use of cells without scaffold (9/605); description of mere surgical techniques (9/605); case reports or mini case series (3/605). A total of 110 studies were screened and 34 duplicate articles were excluded (34/110). Finally, 76 studies were included in the present review: 46 were in vivo studies and 30 were clinical ones (Fig. 1).
Fig. 1.
Flowchart of the papers' selection process.
All data were extracted and reviewed from article texts, tables, and figures by the same two independent investigators (FV and NDV). Discrepancies between the two reviewers were resolved by discussion and consensus. The final results were reviewed by the senior investigator (MF). Risk of bias assessment of the included articles was done following the Coleman methodology score modified by Kon et al. [19] for clinical papers and SYRCLE for in vivo preclinical studies [20]. The assessment was independently performed by two authors (FV and NDV). Any divergence was discussed with the senior investigator, who made the final judgement.
3. Results
3.1. In vivo results
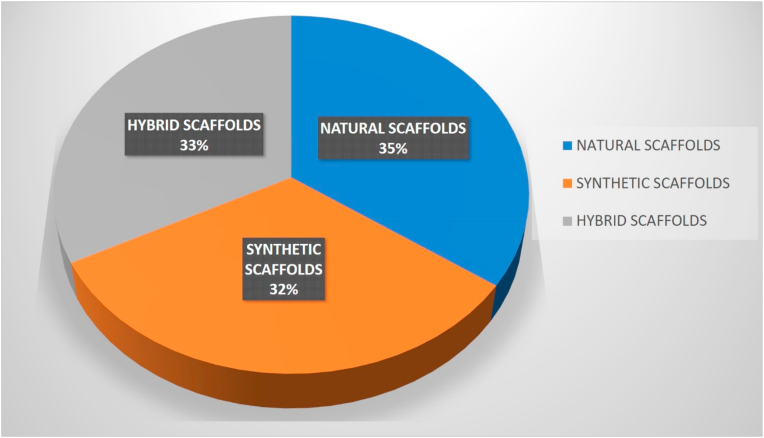
Sixteen studies investigated natural scaffolds [[21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36]], 15 synthetic [[37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51]] and 15 hybrid natural/synthetic [[52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63], [64], [65], [66]] ones (Fig. 2). Some of them were implanted without cells [[21], [22], [23], [24], [25], [26], [27], [28], [29],[37], [38], [39], [40], [41], [42], [43], [44],[52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62]] and some with cells [[30], [31], [32], [33], [34], [35], [36],[45], [46], [47], [48], [49], [50], [51],[63], [64], [65], [66]]. Twenty-one in vivo studies were conducted in rabbits [21,[23], [24], [25],28,29,[33], [34], [35], [36],39,41,45,46,[50], [51], [52],[61], [62], [63], [64]], 15 in sheep [27,31,32,37,38,[53], [54], [55], [56], [57], [58], [59], [60],65,66], 6 in dogs [22,26,[42], [43], [44],47] and 4 in pigs or miniature pigs [30,40,48,49] (Table 1).
Fig. 2.
Scaffolds' use in animal studies.
Table 1.
Summary of in vivo preclinical studies.
| Ref. | Animals | CTR | Scaffold | Evaluations | Results: Study group Vs CTR |
|---|---|---|---|---|---|
| Natural scaffolds: cell-free | |||||
| Li C et al. Sci Rep 2017 | 15 skeletally mature Japanese big-ear rabbits (3.0–3.5 kg) | Empty defect | Decellularized autologous ST | 6 wks, 3 and 6 mo: Macroscopy; Histology; Histomorphometry; Biomechanics |
↑ covering ratio, Ishida score, Toluidine blue, COLL I; ↓ COLL III, elastic modulus, hardness |
| Hansen R et al. J Orthop Res 2013 | 9 skeletally mature mixed breed dogs (25.5 kg) | / | CMI | 3 and 6 wks, 12, 13, 17 mo: Histology |
Organized and integrated tissue, scaffold degeneration |
| Kawaguchi Y et al. Orthop Traumatol Surg Res 2019 | 75 mature Japanese White rabbits (3.8 ± 0.3 kg) | Empty defect | Autologous meniscal fragments, decellularized or not | 3 and 6 wks, 3 mo: Macroscopy; Histology; Histomorphometry; Biomechanics |
↑ gross evaluation score, cross-sectional area, histological score, maximal load, linear stiffness; Not decellularized scaffold: ↑ histological score, maximal load, linear stiffness than decellularized scaffold |
| Kobayashi Y et al. Am J Sports Med 2010 | 75 mature Japanese White rabbits (3.5 ± 0.2 kg) | Empty defect | Autologous meniscal fragments | 3 and 6 wks, 3 mo: Macroscopy; Histology; Histomorphometry; Biomechanics |
↑ gross and histological score, cross sectional area, maximal load, linear stiffness |
| Gastel JA et al. Arthroscopy 2001 | 12 NZW rabbits (3.5–4 kg) | / | SIS | 1, 3, 6 mo: Histology |
No incorporation at 1 mo; More organized and fibrous tissue at 3 mo; More organized tissue resembling native meniscus, integration at 6 mo |
| Cook JL et al. Am J Sports Med 2006 | 51 adult conditioned dogs (2–5 yrs, 22.0–34.4 kg) | Empty defect | SIS | Every mo: Clinical lameness; 3, 6, 12 mo: Macroscopy; Histology; Ultrasound; Biomechanics |
Animals survived, no surgical complications. ↓ lameness; ↑ mature appearance, attachment, CSA%, TSA%, tissue regeneration, organized tissue, cell density, integration |
| Gruchenberg K et al. Knee Surg Sports Traumatol Arthrosc 2015 | 27 skeletally mature Merino sheep (4.2 ± 0.9 yrs) | / | Silk fibroin | 3, 6 mo: Macroscopy; Biomechanics; Histology |
No inflammation, scaffold in place = Eeq than native meniscus, scaffold not always fixed |
| Yuan Z et al. Biomaterials 2016 | 32 NZW rabbits (3 mo) | Empty defect | AMECM and/or DCB | 3, 6 mo: Histology; Histomorphometry; RT-PCR; Biomechanics |
DCB and AMECM + DCB ↑ regenerated meniscal tissue than AMECM and empty defect; AMECM + DCB ↑ regenerated meniscus than DCB; AMECM + DCB ↑ chondrocyte-like cells, PGs, Col1, Col2, Acan, Sox9, well-organized collagen, Ishida score, tensile modulus than AMECM, DCB and empty defect |
| Natural scaffolds: cell-free with GFs | |||||
| Pan Z et al. Acta Biomaterialia 2017 | 20 male NZW rabbits (5 mo) | Empty defect | Multi-layer acellular rabbit Achilles tendon with or without i.a. gefitinib (30 mM) injection | 2, 4 mo: Macroscopy; Histology; Histomorphometry; Biochemics |
Scaffolds with and without gefitinib ↑ meniscus covering ratio; Scaffold with gefitinib ↑ Safranin-O, Pauli score, collagens than empty defect and scaffold without gefitinib |
| Natural scaffolds: with cells | |||||
| Peretti GM et al. Am J Sports Med 2004 | 16 Yorkshire pigs (3 mo) | Scaffold or empty defect | Decellularized allogenic meniscal slides + autologous chondrocytes | 9 wks: Macroscopy; Histology; Histomorphometry |
↑ lesion repair, fibro-cartilaginous matrix, GAGs |
| Martinek V et al. Arch Orthop Trauma Surg 2006 | 25 adult Merino sheep (80.0 ± 10.6 kg) | Scaffold or empty defect | CMI + autologous fibrochondrocytes (10 × 106 cells/3.25 cm scaffold) | 3 mo: Macroscopy; Histology |
No intra- or post-operative complications, stable joint. ↑ regeneration, vascularization; ↓ fibrochondrocytes, scar tissue; ↑ matrix, scaffold remodeling than scaffold |
| Whitehouse MR et al. Stem Cells Transl Med 2017 | 30 skeletally mature sheep (>2 yrs) | Scaffold or empty defect | Collagen sponge + BMSCs (1 × 106 cells/cm2) | 13 wks, 6 mo: Macroscopy; Histology |
↑ meniscus healing at 13 wks; No healing at 6 mo |
| Angele P et al. J Biomed Mater Res 2008 | 24 NZW rabbits (5 mo) | Scaffold or empty defect | HA/gelatin + autologous BMSCs (105 cells/μl) cultured in CM | 3 mo: Macroscopy; Histology; Histomorphometry; IHC |
No infection, chronic inflammation. ↑ integration, fibrocartilage with hyaline cartilage-like areas, cross-sectional width, COLL II |
| Zellner J et al. J Biomed Mater Res Part A 2010 | 60 NZW rabbits (5 mo) | Scaffold or empty defect | HA/gelatin + autologous BMSCs (1,5 × 106 cells/scaffold) cultured or not in CM or BM | 6 wks, 3 mo: Macroscopy; Histology; IHC |
Scaffold + BM ↑ PG; Scaffold + BMSCs ↑ PG, moderate COLL II, integration than scaffold; Scaffold + precultured BMSCs ↑fibroblastic and meniscus-like cells, PG, COLL II |
| Zellner J et al. J Biomed Mater Res Part B Appl Biomater 2013 | 30 NZW rabbits (5 mo) | Scaffold or empty defect | HA/gelatin + autologous BMSCs (1 × 106 cells/scaffold) precultured or not in CM | 6 wks, 3 mo: Macroscopy; Histology; Histomorphometry; IHC; Biomechanics |
Scaffold + BMSCs ↑ healing, scar strength than scaffold; Scaffold + precultured BMSCs ↑healing, matrix, COLL II, scar strength than scaffold |
| Natural scaffolds: with cells and GFs | |||||
| Koh RH et al. Acta Biomaterialia 2017 | 6 NZW rabbits (3–3.3 kg) | Scaffold or medium with TGF-β3 | COL-RF-HA + human T-MSCs (1 × 106 cells/construct) cultured with conditioned medium from meniscal fibrochondrocytes, cultured with TGF-β3 | 10 wks: Histology; IHP; Histomorphometry |
↑ cellularity, PGs, GAGs, collagens, COLL II |
| Synthetic scaffolds: cell-free | |||||
| Zhang ZZ et al. Acta Biomaterialia 2016 | 24 NZW rabbits (3 mo) | Empty defect | PCL (pore of 215, 320 or 515 μm) | 3 mo: Macroscopy; Histology; IHC |
No complications, weight change, inflammation. ↑ macroscopical aspect; Scaffold (pore of 215 μm) ↑ macroscopical aspect, COLL I, COLL II than scaffolds (pore of 320, 515 μm) |
| Otsuki S et al. Am J Sports Med 2019 | 16 minipigs (42.6 mo, 51.6 kg) | Empty defect | PGA coated with PLLA/PCL sponge | 1, 2, 6 mo: Macroscopy; Histology; IHC |
↑ meniscus size, Ishida score, collagen structure, COLL I, COLL II; ↓ Pauli score |
| Testa Pezzin AP et al. Artif Org 2003 | 34 adult NZW rabbits (5–7 mo, 2–4 kg) | / | PLLA/PPD | 3, 6, 12, 14 wks: Histology |
Degradation at 6 wks and tissue infiltration; Fibrocartilage at 12 wks; Aligned fibers at 14 wks |
| Tienen TG et al. Biomaterials 2003 | 12 adult Beagles (13.1 ± 1.6 Kg) | Empty defect | PUEs based on PLLA/PCL | 3, 6 mo: Histology; IHC; Macroscopy; Histomorphometry |
Normal gait, no infections. ↑ intact menisci, COLL II; chondrocyte-like cells, COLL II and PGs, almost complete integration, starting of scaffold degradation, macrophages, giant cells, no inflammation |
| Galley NK et al. Clin Orthop Relat Res 2011 | 13 healthy, skeletally mature Columbia X Rambouillet sheep |
/ | Actifit® | 3, 6, 12 mo: Histology; Biomechanics |
tissue infiltration, stained matrix; ↓ compressive modulus; = permeability than native meniscus |
| Maher SA et al. Arthroscopy 2010 | 42 skeletally mature Columbia X Rambouillet ewes | Empty defect | Actifit® | 3, 6, 12 mo: Macroscopy; Histology |
Normal gait. regenerated and dense tissue filling the defect, cell infiltration, matrix deposition, PGs, collagen |
| Welsing RTC et al. Am J Sports Med 2008 | 13 adult Beagle dogs (12.5 ± 0.2 Kg) | Empty defect | PCL-PU | 6, 24 mo: Histology; Histomorphometry; IHC |
Well connected, cartilage-like tissue with chondrocyte-like cells, COLL I, COLL II, collagen filled the pores, no inflammation, polymer fragments = compression-stress curves than native meniscus at 6 mo |
| Heijkants RGJC et al. J Mater Sci Mater Med 2004 | 12 adult Beagles (13.1 ± 1.6 Kg) | Empty defect | PCL-PU | 6 mo: Biomechanics; Histology; IHC |
No infections. Tissue infiltration; = compression curve, PG, COLL I, COLL II than native meniscus |
| Synthetic scaffolds: with cells | |||||
| Zhang ZZ et al. Am J Sports Med 2017 | 72 skeletally mature NZW rabbits (3.0 kg) | Scaffold or empty defect | PCL + allogenic BMSCs (5 × 106 cells/scaffold) | 3, 6 mo: Macroscopy; Histology; IHC; Biomechanics |
No weight changes, complications. ↑ scaffold integration, shape, total macroscopic score, COLL I, COLL II, tensile modulus, aggregate modulus, ultimate tensile strength, GAGs |
| Gu Y et al. Exp Ther Med 2012 | 24 Beagle dogs (6 mo) | Scaffold or empty defect | PLGA + chondrogenically induced myoblasts (1.5 × 107/0.3 ml) | 3 mo: Macroscopy; Histology; IHC; Histomorphometry |
↑ defect filling, Th, integration, meniscus-like fibrocartilage with hyaline cartilage-like areas, COLL II; Scaffold ↑ Th than empty defect |
| Weinand C et al. Arch Orthop Trauma Surg 2006 | 15 swines | Scaffold or empty defect | Woven PLGA mesh + chondrocytes (1 × 106 cells/ml) | 3 mo: Macroscopy; Histology |
Well ambulation, no infections, no swelling, no redness. Closed lesion, complete healing, fibrous tissue, some histiocytes |
| Weinand C et al. Am J Sports Med 2006 | 28 swines | Scaffold or empty defect | Woven PLGA mesh + autologous or allogenic chondrocytes (1 × 106 cells/ml) | 3 mo: Macroscopy; Histology; Histomorphometry |
No swelling. Partial of full closure of lesion, new scar-like tissue, few histiocytes, ↑ healed lesion%,; Scaffold or empty defect no closure, no healing |
| Koch M et al. Stem Cell Int 2018 | 14 NZW rabbits (12–14 wks, 2.8–3.2 kg) | Scaffold | Actifit®+BMSC suspension (200 μl, 2 × 104 nucleated cells/μl) | 6 wks, 3 mo: Macroscopy; Histology; IHC |
↑ meniscus score, integration, healing, PGs |
| Kang SW et al. J Biomed Mater Res 2006 | 9 NZW rabbits (6 wks, 3.5 ± 0.4 kg) | Scaffold | PGA/PLGA + allogenic meniscal chondrocytes (2 × 106 cells/scaffold) | 6, 10 wks, 9 mo: Histology; IHC; Water content and total collagen content; Cytoindentation |
Meniscus tissue formation with original scaffold shape, slightly larger than the original scaffold, structure similar to native meniscus, fibrocartilage, fibrochondrocytes, collagens, organized structure, PGs, COLL I, COLL II, water content, Young's modulus similar to native meniscus. Scaffold no maintenance of the shape and size of the original scaffold |
| Esposito AR et al. Biores Open Access 2013 | 24 NZW rabbits (5 mo) | Scaffold or empty defect | PLDLA/PCL-T + fibrochondrocytes | 12, 24 wks: Macroscopy; Histology |
↑ Fibrocartilaginous tissue Scaffold + fibrochondrocytes and scaffold complete healing of the incision, with no external scar, tissue with features similar to those of native meniscus |
| Hybrid Natural/Synthetic scaffolds: cell-free | |||||
| Gao S et al. Acta Biomater 2018 | 20 NZW rabbits (3 kg) | Empty defect | DMECM sponge + DMECM/PCL fiber films | 3, 6 mo: Macroscopy; Histology; IHC; Biomechanics; Biochemics |
↑ meniscus-like cells, meniscus covering area, regeneration, GAGs, COLL I, COLL II, Ishida score |
| Ghodbane SA et al. Tissue Eng Part A 2019 | 24 skeletally mature Dorset Finn Cross Sheep (2–3 yrs) | Empty defect | COLL reinforced with polymer fiber (pDTDDD) | 3, 6 mo: Histology; Histomorphometry; IHP; Biochemics |
normal cell morphology; ↑ vascularization, collagenous bundles, COLL I, GAGs, Collagen; ↓ lymphocytes and multinucleated giant cells |
| Merriam AR et al. Am J Sports Med 2015 | 20sheep | Empty defect | COLL reinforced with polymer fiber (pDTDDD) | 4, 8 mo: Biomechanics; Histology; IHP |
Neomeniscus tissue, no scaffold, organized tissue, COLL I, COLL II; ↓ multinucleated giant cells, inflammatory cells |
| Patel JM et al. Am J Sports Med 2016 | 10 skeletally mature Dorset Finn Cross sheep (2–3 yrs) | Empty defect | COLL reinforced with polymer fiber (pDTDDD) | 13 mo: Biomechanics; Histology; IHC; Biochemics |
↑ cell infiltration, areas of dense ECM deposition with aligned collagen fibers, blood vessels, COLL I, COLL II; ↓ inflammatory cells |
| Patel JM et al. Am J Sports Med 2018 | 12 skeletally mature Dorset Finn cross sheep (2–3 yrs) | / | COLL reinforced with polymer fiber (pDTDDD) | 24 mo: Macroscopy; Histology; IHC; Biomechanics |
↓ coverage%, Eeq, Ultimate tensile load, tensile stiffness than native meniscus; Dense tissue deposition and organization, no inflammation, vascular ingrowth, bundles of collagen, no calcification, chondrocyte-like cells, COLL I, COLL II |
| Patel JM et al. Tissue Eng Part A 2016 | 9 skeletally mature Dorset Finn cross sheep (2–3 yrs) | / | COLL–HA reinforced with PLLA fibers | 2, 4, 6, 8 mo: Biomechanics; Histology; Biochemics |
↓ Aggregate modulus; = collagen, GAGs than native meniscus; Polymer fibers observed, cell infiltration, new collagen deposition and organization at 4 mo, reduced during time, areas of aligned ECM; ↓ macrophages |
| Chiari C et al. Osteoarthritis Cartilage 2006 | 8 skeletally mature Austrian stone sheep (50–64 kg) | Total MMx or PMx empty defect | HA/PCL | 6 wks: Histology |
No degeneration, stable joints. Total MMx or PMx with or without scaffold excellent bonding between the implant and the capsule, tissue formation, vessels, giant cells; PMx with or without scaffold fibrous tissue |
| Cojocaru DG et al. J Biomed Mater Res 2020 | 12sheep | Empty defect | 3D meniscus-shaped PGA-HA | 6 mo: Histology; IHC; Histomorphometry |
No inflammation. repair tissue rich in PGs and COLL I, not completely covering the whole defect area |
| Demirkiran ND et al. Acta Orthop Traumatol Turc 2019 | 16 adult NZW rabbits (12 mo) | Empty defect | Multilayer meniscal scaffold or Actifit® | 2 mo: Macroscopy; Histology; Biomechanics |
No animals died, no redness, swelling, heat, disruption or abscess, inflammation. Multilayer meniscal scaffold ↑ maniscus areas, Th defect area, Hayes scores, compression tests than empty defect |
| Shimomura K et al. Biomaterials 2019 | 18 skeletally mature NZW rabbits (3.3–4.0 kg) | Scaffold or empty defect | Electrospun nanofibrous PCL/PEO scaffold + TEC | 1, 2, 3 mo: Histology; IHC; Biomechanics |
No inflammation or immunological rejection. ↑ GAGs, COLL II, cross-sectional areas |
| Hybrid Natural/Synthetic scaffolds: cell-free with GFs | |||||
| Nakagawa Y et al. Am J Sports Med 2019 | 34 skeletally mature sheep (2.4 yrs, 55.3 kg) | Scaffold | GF–laden PCL + CTGF (5 or 10 μg)+TGF-b3 (5 or 10 μg) | 6, 12 mo: MRI; Macroscopy; Histology; Histomorphometry |
↑ regenerated tissue, fibrous tissue; Scaffold + CTGF (5 μg)+TGF-β3 (5 μg) ↑ matrix organization, PG, fibrocartilage; ↓ MRI score than scaffold + CTGF (10 μg)+TGF-β3 (10 μg) and scaffold; Scaffold + CTGF (10 μg)+TGF-β3 (10 μg) ↑ PG than scaffold |
| Hybrid Natural/Synthetic scaffolds: with cells | |||||
| Moradi L et al. Biomaterials 2017 | 29 NZW rabbits (5 mo) | Scaffold | PVA/Ch cross-linked by PPU chains + autologous chondrocytes and/or allogenic ADSCs (5 × 104 cells/cm2) | 7 mo: Macroscopy; Histology; IHP |
No infection, no chronic inflammation. Scaffold + chondrocytes or chondrocytes and ADSCs ↑ regenerated tissue, GAGs, parallel COLL I and COLL II fibers than scaffold or scaffold + ADSCs |
| Kon E et al. Tissue Eng Part A 2008 | 26 skeletally mature adult sheep (3.1 ± 1.8 yrs) | Scaffold or empty defect | HA/PCL with transtibial fixation of the horns or sutured to the capsule and meniscal ligament and transtibial fixation of the horns + autologous chondrocytes (25 × 106 cells/cm3) | 17 wks: Macroscopy; Histology |
All animals tolerated surgery well and survived, normal gait. All joints stable. Residual scaffold, giant cells, mononuclear histiocytes, vascularization, cellular tissue, few lymphocytes and plasma cells; ↑ small foci of cartilage areas |
| Kon E et al. Tissue Eng Part A 2012 | 18 skeletally mature adult Bergamasca– Massese sheep (70 ± 5 kg) |
Scaffold or empty defect | HA/PCL + autologous chondrocytes (25 × 106 cells/cm3) | 12 mo: Macroscopy; Histology |
All animals tolerated surgery well and survived, normal gait. ↑ macroscopic score, cartilage metaplasia; ↓ fibrosis than scaffold; Scaffold and scaffold + chondrocytes scaffold residuals, foreign body reaction, well-organization and tight integration, vessels, inflammatory response than empty defect |
| Hybrid Natural/Synthetic scaffolds: with cells and GFs | |||||
| Chen C et al. Am J Sports Med 2020 | 30 mature NZW rabbits (16–18 wks; 2.5–3.0 kg) | Scaffold or empty defect | GC/4-Arm PEG-CHO Hydrogel + autologous BMSCs (2 × 107 cells/ml)+TGFβ (1 mg/ml) |
2 mo: Macroscopy; Hystology; Histomorphometry; IHC |
Standard C-shape, defect region invisible; ↑ GAGs, Ishida score, COLL II; ↓ COLL I than scaffold + BMSCs and empty defect; Caffold + BMSCs ↑ Ishida score than empty defect |
3.1.1. Natural scaffolds
3.1.1.1. Cell-free scaffolds
Some scaffolds were made by only one tissue: decellularized autologous supraspinatus tendon (ST) [21]; collagen meniscus implant (CMI) [22]; autologous meniscal fragments [23,24]; porcine small intestine submucosa (SIS) [25,26]; silk fibroin [27]. Other scaffolds were instead made by a combination of 2 biomaterials: an acellular swine meniscus extracellular matrix (AMECM) with demineralized bovine cancellous bone (DCB) [28]; a 2-layered 3D structure, composed of decellularized allogenic flexor digitorum superficialis tendons (FDST) and acellular Achilles tendon fragments [29].
-
•One biomaterial: Positive results were observed in all cases.
-
1)Decellularized autologous ST significantly increased the covering ratio, proteoglycans (PGs) and Collagen I (COLL I) content and reduced COLL III, even if elastic modulus and hardness decreased [21];
-
2)CMI showed an increase in organization and integration of the regenerated tissue [22];
-
3)meniscus fragments (decellularized or not) revealed macroscopic and microscopic improvements in the regenerated meniscus and also biomechanical test confirmed these findings (cross-sectional area, maximal load and linear stiffness), with better results reached by non-decellularized fragments [23,24];
- 4)
-
5)Silk fibroin scaffold remained in place until 6 months from its implantation. It induced a regenerated tissue with an equilibrium modulus (Eeq) similar to the native meniscus [27].
-
1)
-
•More biomaterials:
-
1)AMECM, combined with DCB, significantly increased the regeneration of a meniscal tissue, containing higher chondrocyte-like cells, PGs and collagens, gene expression of COLL I, COLL II, Aggrecan and Sox9 and tensile modulus than AMECM or DCB separately [28];
-
2)a multi-layered scaffold composed of decellularized allogenic FDST and Achilles tendon fragments [29] regenerated meniscal tissue with a high integration rate and collagen content following the intra-articular injection of gefitinib, a tyrosine kinase inhibitor present on the intracellular side of epidermal growth factor receptor (EGFR).
-
1)
3.1.1.2. Scaffolds with cells
Decellularized allogenic meniscus fragments [30], CMI [31] and collagen type I sponge [32] were implanted, after the seeding of autologous chondrocytes [30], autologous fibrochondrocytes [31] or BMSCs [32] and all the studies have as comparison groups the scaffold without cells and the empty defect. Three are composite scaffolds, hyaluronan ester (HA)/gelatin (70/30) seeded with autologous BMSCs or BM [[33], [34], [35]] and 1 was made by riboflavin-induced photocrosslinked collagen hydrogel and crosslinked HA (COL-RF-HA) scaffold, loaded with tonsillar MSCs (T-MSCs) and cultured in conditioned medium of meniscal fibrochondrocytes, previously cultured with TGF-β3 [36].
-
•One biomaterial:
-
1)Decellularized allogenic meniscus slides increased lesion repair, GAGs, fibro-cartilage matrix formation [30];
-
2)CMI, that regenerated a tissue with higher vascularization, matrix remodeling and lower scar formation [31];
-
3)Collagen type I sponge significantly increased meniscus healing, but, after 6 months, no healing was observed [32].
-
1)
-
•More biomaterials:
-
1)HA/gelatin scaffold with autologous BMSCs, induced the regeneration of a tissue with higher integration, fibrocartilage aspect with hyaline-cartilage areas, PGs, cross-sectional area and COLL II in comparison to scaffold alone [33,34], but when loaded with BM in toto, only PG increased in the regenerated tissue [34]. However, the major effects were observed when scaffold was loaded with BMSCs, pre-cultured in chondrogenic medium, showing the highest tissue healing and strength, fibroblasts, meniscus-like cells, PGs and COLL II [34,35];
-
2)the COL-RF-HA scaffold, which was cultured in conditioned medium from meniscal fibro-chondrocytes, previously expanded in presence of TGF-β3. It induced the formation of a new tissue with higher cellularity, collagens, PG and GAGs in comparison to scaffold cultured in medium containing TGFβ3 or acellular scaffold [36].
-
1)
3.1.2. Synthetic scaffolds
3.1.2.1. Cell-free scaffolds
One synthetic scaffold was composed by one component: Polycaprolactone (PCL) [37]. Other scaffolds were composed by polyglycolic-Acid (PGA) felt layer coated with a PLLA/PCL (50:50) sponge [38], poly(p-dioxanone) (PPD)/PLLA [39], polyester urethanes (PUEs) based on PLLA/PCL [40], porous aliphatic polyurethane (PU) and PCL (Actifit®) [41,42], or PCL-PU [43,44].
-
•One biomaterial:
-
1)PCL alone didn't increase complications or inflammation and the macroscopic appearance of new regenerated tissue improved in comparison to empty defects. Best results were obtained with pore size of 215 μm than with the other two types of pore sizes, 320 and 515 μm [37].
-
1)
-
•More biomaterials:
-
1)PGA coated with PLLA/PCL sponge significantly increased meniscus size, collagens and structure than empty defect. An increase in PCNA-positive cells and TNFα was observed only at 2 months, disappearing at 6 months [38];
-
2)PUEs based on PLLA/PCL scaffold increased the formation of intact menisci with COLL II, chondrocyte-like cells, PGs, and initial scaffold degradation was also observed [40];
-
3)PPD/PLLA blend degraded, generating fibro-cartilaginous tissue and aligned fibers [39];
- 4)
- 5)
-
1)
3.1.2.2. Scaffolds with cells
The single-material scaffolds seeded with cells were: PCL [45]; Poly(lactide-co-glycolide) (PLGA) (90/10) [[46], [47], [48]]. They were seeded with autologous BMSCs [45], chondrogenically induced myoblasts [46] or chondrocytes [47,48].
The scaffolds composed by more biomaterials and seeded with cells were instead: Actifit® [49]; PGA/PLGA [50]; and poly(L-co-D,l-lactic acid) (PLDLA)/poly(caprolactone-triol) (PCL-T) (70/30) [51]. These were seeded with BMSCs [45], chondrocytes [50] or fibro-chondrocytes [51].
-
•One biomaterial:
-
1)PCL scaffolds significantly increased tissue integration, GAGs, COLL I, COLL II and biomechanics (tensile modulus, aggregate modulus, ultimate tensile strength) [45];
-
2)non-woven PLGA scaffold induced a higher defect filling, scaffold integration, meniscus-like fibrocartilage formation and COLL II and tissue thickness in comparison to unseeded scaffold or empty defects [46]. The woven PLGA meshes closed the lesion, inducing a complete healing with fibrous tissue and some histiocytes. However, scaffold without cells was not able to regenerate new tissue. No differences were observed between autologous or allogenic cells [47,48].
-
1)
-
•More biomaterials:
-
1)Actifit® significantly increased meniscus regeneration, integration, healing, PG content [49];
-
2)PGA/PLGA induced the formation of a meniscus-like tissue within the original scaffold shape, slightly larger than the original scaffold and with structure, fibrocartilage, fibrochondrocytes, collagens, PGs, COLL I, COLL II, water content, and Young's modulus similar to native meniscus [50];
-
3)PLDLA/PCL-T induced a complete tissue healing, with no scar tissue, and features similar to the native meniscus [51].
-
1)
3.1.3. Hybrid natural-synthetic scaffolds
3.1.3.1. Cell-free scaffolds
The following cell-free scaffolds were investigated: decellularized meniscus extracellular matrix (DMECM) sponge and DMECM/PCL fiber [52]; Type I bovine Achilles tendon collagen sponge reinforced with p(DTDDD) fibers [[53], [54], [55], [56]]; COLL–HA reinforced with PLLA fibers [57]; PCL/HA (70/30), augmented with PLA fibers or PET nets [58]; PGA/HA [59]; multi layered scaffold, composed by PHBV, HA and strontium ranelate, and durable cellulose fibers from Luffa cylindrical [60]; PCL (MW = 80 kDa) and PEO (50/50) electrospun nanofibrous and TEC [61]; PCL (MW = 65000) in which TGFβ3 and CTGF were encapsulated in PLGA μS [62].
-
1)
The first scaffold of the aforementioned list induced the formation of a meniscus tissue with higher meniscus-like cells, covering area, regeneration, GAGs, COLL I and COLL II than empty defects. In addition, the regenerated tissue showed water, collagen and GAG content similar to native meniscus, but with lower tensile modulus [52];
-
2)
COLL reinforced with p(DTDDD) fibers, regenerated a tissue with better cell morphology, higher GAGs, vascularization, collagenous bundles, chondrocyte-like cells and no calcification and lower lymphocytes and multinucleated giant cells than empty defects [[53], [54], [55], [56]]. The biomechanical features (Aggregate modulus, Ultimate tensile load, Tensile stiffness and Eeq) of the regenerated tissue were inferior, while permeability was higher than native meniscus [[54], [55], [56]]. Collagen and GAG content were similar to native meniscus [56];
-
3)
COLL-HA reinforced with PLLA fibers induced the regeneration of a tissue with lower aggregate modulus, but similar collagen and GAG than native meniscus; scaffold fibers were always present, with concomitant cell infiltration, new collagen deposition and organized areas of aligned ECM and macrophages [57];
-
4)
PCL mixed with HA increased tissue formation and vascularization [58];
-
5)
PGA/HA scaffold regenerated a tissue rich in PGs and COLL I [59];
-
6)
The multi-layered scaffold composed of PHBV, HA, strontium ranelate and durable cellulose fibers increased meniscus areas, tissue thickness and presented also better compression tests in comparison to empty defect [60];
-
7)
PCL/PEO scaffold, combined with TEC, increased GAG, COLL II and cross-sectional areas more than scaffold alone or empty defect [61];
-
8)
PCL mixed with HA scaffold was loaded with two GFs (TGFβ3 and CTGF), showing higher meniscus regeneration with higher matrix and PG when GFs were loaded at a concentration of 5 μg rather than 10 μg [62].
3.1.3.2. Scaffolds with cells
PVA/Ch cross-linked with PPU (PVA/Ch/PPU 1:4:1) [63]; HA/PCL (70/30) [64, 65]; and GC/4-Arm PEG-CHO hydrogel (1:1) [66] scaffolds were implanted with: chondrocytes and/or ADSCs [63], chondrocytes alone [64, 65] or BMSCs [66]. Here follows a more detailed description of the outcomes of each of the aforementioned scaffolds:
-
1)
PVA/Ch/PPU scaffold, seeded with autologous chondrocytes alone or chondrocytes and allogenic ADSCs, significantly induced the formation of a regenerated meniscus similar to the native one, containing higher GAGs, COLL I and COLL II than scaffold alone or scaffold seeded with ADSCs [63];
-
2)
HA/PCL significantly increased macroscopic score, cartilage metaplasia and significantly reduced fibrosis than scaffold alone [64] and, when the scaffold was fixed with two sutures, increased giant cells, mononuclear histiocytes, vascularization, and cellularized tissue were seen [65];
-
3)
GC/4-Arm PEG-CHO hydrogel created a standard C-shaped tissue with higher GAGs and COLL II when seeded with autologous BMSCs, cultured with the GF TGFβ, in comparison to the scaffold cultured in growth medium or empty defects [66].
3.2. Clinical results
3.2.1. CMI scaffold
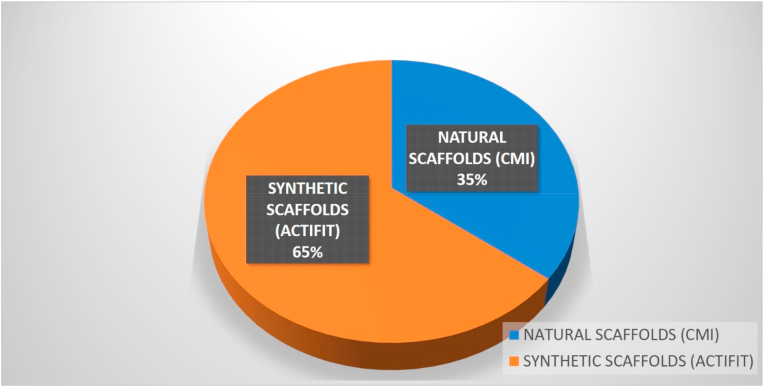
The analysis of the available literature yielded 10 trials, all prospective, that included 593 patients in total [[67], [68], [69], [70], [71], [72], [73], [74], [75], [76]] (Fig. 3). A detailed synopsis of the main features of the included studies is available in Table 2.
Fig. 3.
Scaffolds' use in clinical studies.
Table 2.
Summary of clinical studies (MM=medial meniscus; LM= lateral meniscus; MRI= magnetic resonance imaging; mo=months).
| Ref. | COLEMAN SCORE | PZ | INCLUSION CRITERIA | SCAFFOLD | EVALUATIONS | RESULTS |
|---|---|---|---|---|---|---|
| Zaffagnini S et al. Am J Sports Med 2011 [67] | 63 | Prospective controlled study: 36 pz (40 yrs, 24–60 yrs). A) Scaffold (18 pz); B) No Scaffold (18 pz) |
Irreparable acute meniscal tears requiring partial MMx or chronic prior loss of MM > 25% | CMI | 3, 6, 12, 24, 60, 120 mo: VAS, IKDC, Lysholm score, Tegner scale; SF-36; MRI |
Group A): ↓ VAS; ↑ IKDC, Tegner scale, SF-36, than group B) at 120 mo. Group A): myxoid degeneration signal in 11 pz |
| Zaffagnini S et al. Am J Sports Med 2012 [68] | 55 | Case series: 25 pz (36.3 ± 11.5 yrs, 16.2–53.4 yrs) | Irreparable acute LM tears requiring partial LMx or prior loss of LM > 25% | CMI | 6, 24 mo: subjective Lysholm score, VAS, Tegner scale, IKDC; EQ-5D; MRI (Genovese score) |
No complications. 6, 24 mo: ↑ Lysholm score, IKDC; ↓ VAS than presurgery. 24 mo: ↑ Tegner activity level, EQ-5D than presurgery and 6 mo. Scaffold with identical shape and size than native meniscus in 3 pz, smaller in 18 pz, totally reasorbed in 3 pz, full maturation in 37.5% cases |
| Bulgheroni E et al. Knee Surg Sports Traumatol Arthrosc 2015 [69] | 34 | Comparative trial: 34 pz (33.6 yrs). A) Scaffold (17 pz); B) No scaffold (17 pz) |
Partial MMx or chronic prior loss of MM > 25%, intact anterior and posterior meniscus attachments, intact rim (≥1 mm) | CMI | 9.6 ± 2.5 yrs: Lysholm score, Tegner Scale, IKDC; EQ-5D; VAS |
Groups A), B): ↑ Lysholm score, Tegner scale, IKDC than presurgery. Group B): ↓ VAS than presurgery |
| Schenk L et al. Knee Surg Sports Traumatol Arthrosc 2020 [70] | 61 | Prospective study: 39 pz (34 ± 10 yrs) | CMI performed due to prophylactic (n = 25) or therapeutic indication (n = 14) after subtotal meniscectomy | CMI | 36–84 mo: VAS, Tegner Scale, Lysholm score, IKDC |
↓ VAS, IKDC; ↑ Tegner scale, Lysholm score. Meniscus implant resorbed in 21% of patients and partially resorbed in 79% of patients. Meniscus graft isointense in 21% of patients, slightly hypertense in 74% of patients and highly hyperintense in 5%. |
| Zaffagnini S et al. Arthroscopy 2015 [71] | 63 | Prospective, single-arm, multicenter study: 43 pz (30.1 ± 12.0 yrs) | Irreparable, acute lateral meniscal lesion requiring partial meniscectomy or degenerative loss of lateral meniscal tissue greater than 25% | CMI | 6, 12, 24 mo: Lysholm score, VAS (strenuous activity, routine and at rest), Tegner activity scale, functional evaluation and satisfactional questionnaire |
↓ VAS; ↑ Lysholm score, Tegner scale |
| Hirschmann MT et al. Knee Surg Sports Traumatol Arthrosc 2013 [72] | 60 | Prospective study: 67 pz (36 ± 10 yrs) | Subtotal medial or lateral meniscectomy | CMI | 12 mo: IKDC, Tegner scale, Lysholm score, VAS and satisfactional questionnaire |
↓ Tegner scale, VAS; ↑ Lysholm score, IKDC |
| Bulgheroni P et al. Knee 2010 [73] | 62 | Prospective study: 34 pz (Mean 39 yrs) | Irreparable medial meniscus tears with meniscus removal greater than 25% of total meniscus or presence of persistent pain after meniscectomy | CMI | 24, 60 mo: Lysholm score, Tegner scale, MR arthrography |
↑ MR, Lysholm score, Tegner scale |
| Rodkey WG et al. J Bone Joint Surg Am 2008 [74] | 58 | Prospective randomized study, two study arms: 311 pz A) no previous surgery (157 pz; mean age 40 yy); B) previous surgery (154 pz; mean age 39 yy) |
Irreparable injury of the medial meniscus or a previous partial medial meniscectomy | CMI | 59 mo mean (16–92): Lysholm score, Tegner scale, VAS, second-look arthroscopy, biopsy and histological evaluation, patient self assestment |
Groups A), B): ↑ Tegner scale, Lysholm score. VAS and self assessment not significantly different. At second-look arthroscopy ↑ total tissue surface area |
| Zaffagnini S et al. Knee Surg Sports Traumatol Arthrosc 2007 [75] | 57 | Prospective study: 8 pz (Mean 25 yrs) | Irreparable meniscal tear or a previous meniscectomy involving the medial meniscus | CMI | 3, 6, 12, 24, and from 72 to 96 mo: X-ray, MRI, CKRS, IKDC, VAS, subjective evaluation, second look artroscopy |
↓ VAS, CKRS, IKDC. At second look arthroscopy presence of new tissue in some cases |
| Kovacs BK et al. Knee Surg Sports Traumatol Arthrosc 2019 [76] | 54 | Prospective study: 57 pz (43.6 ± 11 yrs) | Meniscectomy involving medial or lateral meniscus | CMI | 12, 24, and 36–96 mo: VAS, Lysholm score, MRI, CMI morphology |
↓ VAS; ↑ Lysholm score. Abnormal and inhomogeneous signal intensity and irregular margins of the CMI which tend to decrease over time |
| Bouyarmane H et al. Orthop Traumatol Surg Res 2014 [77] | 48 | Prospective, single-arm, multicentric study: 54 pz (16–50 yrs) | Postmeniscectomy syndrome: painful LM tear or partial lateral meniscectomy (32–60 mm) | Actifit® | 6, 12, 24 mo: VAS, IKDC, KOOS |
↓ VAS; ↑ IKDC, KOOS than presurgery. 24 mo: ↓ VAS than 12 mo |
| De Conick T et al. Am J Sports Med 2013 [78] | 51 | Prospective case-series study: 26 pz (17–50 yrs) | Irreparable symptomatic meniscal tear or partial meniscectomy (8 LM, 18 MM) | Actifit® | 3, 12, 24 mo: KOOS, IKDC, Lysholm score, VAS; MRI (RD, rim Th) |
↑ Medial scaffold RD; ↓ Medial rim Th than presurgery During time: ↓ Rim Th. 24 mo: ↓ VAS; ↑ Lysholm score, KOOS, IKDC than presurgery |
| Dhollander A et al. Am J Sports Med 2016 [79] | 56 | Case series: 44 pz (17–50 yrs) | Partial meniscectomy or irreparable meniscus tears (29–70 mm) (29 MM, 15 LM) | Actifit® | 24, 60 mo: KOOS, IKDC, VAS; MRI (Genovese sore, signal, size) |
↓ VAS; ↑ KOOS, IKDC than presurgery. Markedly hyperintense (60% of pz), slightly hyperintense (40% of pz). ↓ size than native meniscus |
| Efe T et al. Knee Surg Sports Traumatol Arthrosc 2012 [80] | 55 | Case series: 10 pz (18–45 yrs) | A chronic, symptomatic, irreparable MM lesion or partial MM loss | Actifit® | 2, 6 wks: Complications. 6, 12 mo: KSS, KOOS, VAS, UCLA activity score; MRI (size, signal) |
No complications. ↑ KOOS, KSS than presurgery. Stable scaffold, no reabsorbed, hyperintensity, no variation during time |
| Faivre B et al. Orthop Traumatol Surg Res 2015 [81] | 40 | Prospective study: 20 pz (Mean 28.7 yrs) | Post-meniscectomy syndrome, partial meniscectomy (12 LM, 8 MM) | Actifit® | 12, 24 mo: KOOS, IKDC; MRI (aME, rME, cCCI, antME, postME, sCCI) |
24 mo: ↑ IKDC, KOOS sports and recreational activities. postME than presurgery. 12 mo: ↑ aME; ↓ sCCI than presurgery |
| Filardo G et al. Knee Surg Sports Traumatol Arthrosc 2017 [82] | 57 | Prospective study: 16 pz (45 ± 13 yrs) | Partial meniscectomy for acute meniscal tear or previous traumatic or degenerative meniscal damage (>25%) (12 MM, 4 LM) | Actifit® | 24, 36, 48, 60, 72 mo: IKDC, Tegner scale; MRI (aME, rME, cCCI, Genovese score) |
No major implant-related AEs. ↑ IKDC, Tegner scale than presurgery. Prevalently morphology type II. Significant meniscal extrusion. aME = 3.8 ± 1.5 mm and mean cCCI = 21.1% |
| Gelber PE et al. Knee Surg Sports Traumtaol Arthrosc 2015 [83] | 50 | Prospective study: 60 pz (27–62 yrs). A) Scaffold (30 pz); B) No scaffold (30 pz) |
Symptomatic varus knees treated with open-wedge high tibial osteotomies, MM defect > 25 mm | Actifit® | Median 31.2 mo: WOMET, IKDC, Kujala scores, VAS |
Complications in 3 pz: deep vein thrombosis in the post-operative period, 2 local infection in the wound. Group A): ↓ WOMET and Kujala improvement, VAS dropped than group B) |
| Kon E et al. Knee Surg Sports Traumtaol Arthrosc 2014 [84] | 49 | Prospective study: 18 pz (45 ± 12.9 yrs) | Irreparable acute meniscal tears requiring partial meniscectomy or chronic prior loss of meniscal tissue > 25% (13 MM, 5 LM) | Actifit® | 6, 12, 24 mo: ICRS, IKDC, Tegner scale; MRI (Genovese score) |
No major AEs. 12, 24 mo: ↑ IKDC objective score, Tegner scale than presurgery. 6, 12, 24 mo: ↑ IKDC subjective score than presurgery. Prevalently morphology type II and I. |
| Leroy A et al. Traumatol Surg Res 2014 [85] | 45 | Prospective, non-comparative study: 15 pz (30 ± 8 yrs, 19–47 yrs) | Partial meniscectomy (9 LM, 6 MM) | Actifit® | 12, 24, 60 mo: VAS, IKDC, KOOS; MRI (Genovese score) |
3 pz reoperated. ↓ VAS; ↑ IKDC, KOOS pain, KOOS ADL. Morphology type II, involution of the implant volume |
| Monllau JC et al. Arthroscopy 2018 [86] | 49 | Retrospective study: 32 pz (41.3 ± 11.1 yrs, 23–60 yrs) | Partial meniscectomy (21 medial, 11 lateral) | Actifit® | Mean 70.2 ± 7.5 mo: KOOS, IKDC, Lysholm score, Tegner scale; pz satisfaction; MRI (scaffold extrusion, signal, Genovese score, meniscus volume) |
↑ KOOS, IKDC, Lysholm score than pre-surgery. Scaffold extrusion = 2.4 mm, morphology type IIb, slight hyperintensity |
| Schuttler KF et al. Knee Surg Sports Traumtaol Arthrosc 2015 [87] | 48 | Case series study: 18 pz (Mean 32.5 yrs, 17–49 yrs) | Chronic, symptomatic, irreparable MM lesion or partial MM loss (mean length: 45 mm) | Actifit® | 6, 12, 24 mo: KOOS, KSS, UCLA scale, VAS; MRI (scaffold extrusion, reabsorbtion, signal intensity) |
↑ KOOS pain, Symptom, ADL, Sport/Rec, QOL, KSS function score, KSS knee score; ↓ VAS than presurgery. 24 mo: ↑ UCLA scale than presurgery. Prevalently no scaffold extrusion, no reabsorbtion |
| Schuttler KF et al. Knee Surg Sports Traumtaol Arthrosc 2016 [88] | 51 | Case series study: 18 pz (Mean 32.5 yrs, 17–49 yrs) | Chronic, symptomatic, irreparable MM lesion or partial MM loss (44.5 mm) | Actifit® | 6, 12, 24, 48 mo: KOOS, KSS, UCLA Scale, VAS; MRI (Genovese score) |
↑ KOOS, KSS function and knee; ↓ VAS than presurgery. 24 mo: ↑UCLA scale than presurgery. Prevalently morphology type IIb, signal intensity type I |
| Verdonk R et al. Am J Sports Med 2011 [89] | 67 | Prospective, single-arm multicenter proof-of-principle study: 52 pz (30.8 ± 9.4 yrs, 16–50 yrs) | Irreparable meniscus tear or partial meniscus loss (34 MM, 18 LM) (47.1 ± 10 mm) | Actifit® | 3 mo: DCE-MRI; 12 mo: Histology; 1 wk, 3 and 12 mo: MRI (scaffold position and integration) |
SAEs in 2 pz: postoperative infection, severe cartilage damage. No scaffold tears, normal position, integration. Tissue ingrowth in 81.4% after 3 mo. Vital material, no necrosis, cell death, reactions or fibrous tissue |
| Verdonk P et al. Am J Sports Med 2012 [90] | 56 | Prospective, single-arm, multicenter proof-of-principle study: 52 pz (30.8 ± 9.4 yrs) | Irreparable meniscus tear or partial meniscus loss (34 MM, 18 LM) | Actifit® | 1 wk, 3, 6, 12, 24 mo: VAS. 3, 6, 12, 24 mo: IKDC subjective, KOOS, Lysholm score; AEs |
1 pz with postoperative infection due to procedure at 3 mo. 1 pz with unicompartmental knee arthroplasty at 6 mo. 6, 12, 24 mo: ↓ VAS; ↑ IKDC, Lysholm score, KOOS. Treatment failure in 17.3% pz, 9 SAEs |
| Baynat C et al. Orthop Traumatol Surg Res 2014 [91] | 49 | Prospective study: 18 pz (20–46 yrs) | Partial meniscus defect (13 MM, 5 LM) with VAS score >6/10 everyday for more than 6 mo | Actifit® | 3, 6, 12, 24 mo: Lysholm score; MRI (signal, extrusion); Histology |
1 pz with residual knee pain due to median saphenous nerve injury. 1 pz with quadriceps weakness due to a femoral nerve conduction block. ↑ Lysholm score than presurgery, intermediate signal intensity, 1 meniscus extrusion, normal chondrocytes and fibro-chondrocytes |
| Gelber PE et al. Knee 2015 [92] | 47 | Case series: 54 pz (40.2 yrs, 17–58 yrs) | Joint line pain due to a meniscus resection or a large irreparable meniscus tear (40 MM, 14 LM) | Actifit® | Median 39 mo: WOMET, subjective IKDC, Kujala score, VAS; Pz satisfaction; MRI (Genovese score) |
↑ WOMET, IKDC, Kujala, VAS than presurgery; worse morphology, size than native meniscus, morphology type II. Pz satisfaction = 3.5 ± 0.7 |
| Toanen C et al. Am J Sports Med 2020 [93] | 60 | Case series, European Multicentric Study: 155 pz in 6 centers (33.7 ± 10.4 yrs) | Irreparable meniscal tear or partial meniscus loss (101 MM, 54 LM) | Actifit® | Mean 60 mo: KOOS, Lysholm score, IKDC subjective, VAS; MRI (Genovese score, signal intensity, size, extrusion) |
↓VAS; ↑ Lysholm score, KOOS, IKDC than presurgery. Scaffold type II, slightly hyperintense signal, extrusion 3 ± 1.2 mm;↓ size than native meniscus |
| Akkaya M et al. J Knee Surg 2020 [94] | 51 | Retrospective study; 23 pz | Post-meniscectomy syndrome in partially removed MM. Meniscectomy performed 12.7 months (10-23) before scaffold implantation. | Actifit® | 6, 12, 24, 36 mo: VAS, KOOS, IKDC, Tegner scale 1, 12, 24, 36 mo: MRI (Genovese score, Outerbridge classification), X-Ray (Ahlbäck-Rydberg classification) |
↓VAS at 36 mo ↑ Lysholm score, KOOS, IKDC at each f-up Mean postoperative scaffold extrusion at 36 mo: 2.39 mm (2.30–2.56 mm) No significant difference in cartilage damage between preoperative and 36 mo f-up period |
| Condello V et al. Knee Surg Sports Traumatol Arthrosc 2019 [95] | 33 | Retrospective study; 67 pz | Unicompartmental femorotibial pain after meniscectomy (54 MM, 13 LM) Cartilage damage ≤ III ICRS grade |
Actifit® | Mean f-up 36 mo (12–75 mo) NRS, KOOS, IKDC, Lysholm score, Tegner Scale |
↓ NRS ↑ KOOS, IKDC, Lysholm score, Tegner Scale than pre-surgery. |
| Bulgheroni E et al. Cartilage 2016 [96] | 64 | Comparative not randomized trial: 53 pz (Mean 36.55 yrs). A) CMI (28 pz); B) Actifit® (25 pz) |
Irreparable MM tear or partial MM loss | CMI; Actifit® |
6, 12, 24 mo: Lysholm score, Tegner scale. 24 mo: MRI (Signal intensity, scaffold size, reasorption). 45 mo: Histology |
Groups A), B): ↑ Lysholm score, Tegner scale at 12 mo than presurgery. Scaffold in situ, with irregularity, high signal intensity, partially reasorbed. Group A): 3 complications (Neuroapraxia of infrapatellar branch of the saphenous nerve, persistent synovitis, superficial Infection). Fibrous tissue rich in spindle and rounded fibroblast-like cells, blood vessels. Group B): 5 complications (joint stiffness, synovitis). Avascular tissue, more cartilaginous-like tissue, chondroblast-like, large and active cells. Group A): ↓ reduced size than Group B) |
The quality of methodology, assessed by the modified Coleman score, revealed overall modest results, with scores ranging from 34 [69] to 63 [67,71]. Only one randomized controlled trial is currently available [74]. Looking at the clinical results, all the trials revealed positive findings in terms of pain reduction and increase in subjective functional scores. Few studies documented stability of results up to a long-term evaluation (in the range of 8–10 years' follow-up) [67,75,76]. Anyway, these encouraging findings should be carefully weighed against the high number of concomitant procedures performed in most of the trials (Table 3), which represents a confounding bias in the assessment of the CMI scaffold contribution to the final outcome. To this regard, only one trial reported results of isolated CMI implantation in a small cohort of patients evaluated up to 8 years’ follow-up [75].
Table 3.
Description of concomitant procedures performed together with meniscal scaffold implantation in clinical trials.
| Reference (same order of papers presented in Table 2) | Concomitant procedures | Osteotomy |
Ligament reconstruction |
Cartilage treatment |
Total |
||||
|---|---|---|---|---|---|---|---|---|---|
| n = | % | n = | % | n = | % | n = | % | ||
| CMI | |||||||||
| 67 | Yes | 0 | 0.0% | 4 | 11.1% | 4 | 11.1% | 8 | 22.2% |
| 68 | Yes | 0 | 0.0% | 4 | 16.0% | 6 | 24.0% | 10 | 40.0% |
| 69 | Yes | 0 | 0.0% | 34 | 100.0% | 0 | 0.0% | 34 | 100.0% |
| 70 | Yes | 0 | 0.0% | 24 | 61.5% | 0 | 0.0% | 24 | 61.5% |
| 71 | Yes | 2 | 5.0% | 6 | 14.0% | 1 | 2.0% | 9 | 21.0% |
| 72 | Yes | 5 | 7.5% | 45 | 67.2% | 0 | 0.0% | 50 | 74.6% |
| 73 | Yes | 2 | 5.9% | 11 | 32.4% | 0 | 0.0% | 13 | 38.2% |
| 74 | Yes | 0 | 0.0% | 85 | 27.3% | 0 | 0.0% | 85 | 27.3% |
| 75 | No | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% |
| 76 | Yes | 4 | 7.0% | 38 | 66.7% | 0 | 0.0% | 42 | 73.7% |
| ACTIFIT® | |||||||||
| 77 | Yes | 4 | 7.4% | 0 | 0.0% | 2 | 3.7% | 6 | 11.1% |
| 78 | Yes | 2 | 7.7% | 6 | 23.1% | 1 | 3.8% | 9 | 34.6% |
| 79 | Yes | 4 | 9.1% | 4 | 9.1% | 0 | 0.0% | 8 | 18.2% |
| 80 | No | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% |
| 81 | Yes | 0 | 0.0% | N/A | N/A | N/A | N/A | N/A | N/A |
| 82 | Yes | 4 | 25.0% | 3 | 18.8% | 7 | 43.8% | 11 | 68.8% |
| 83 | Yes | 60 | 100.0% | 0 | 0.0% | 0 | 0.0% | 60 | 100.0% |
| 84 | Yes | 4 | 22.2% | 3 | 16.7% | 7 | 38.9% | 11 | 61.1% |
| 85 | Yes | 0 | 0.0% | 5 | 33.3% | 1 | 6.7% | 6 | 40.0% |
| 86 | Yes | 13 | 40.6% | 10 | 31.3% | 14 | 43.8% | 25 | 78.1% |
| 87 | No | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% |
| 88 | No | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% |
| 89 | Yes | 0 | 0.0% | 17 | 32.7% | 0 | 0.0% | 17 | 32.7% |
| 90 | Yes | 0 | 0.0% | 17 | 32.7% | 0 | 0.0% | 17 | 32.7% |
| 91 | Yes | 10 | 55.6% | 6 | 33.3% | 0 | 0.0% | 12 | 66.7% |
| 92 | Yes | 14 | 25.9% | 21 | 38.9% | 17 | 31.5% | 40 | 74.1% |
| 93 | Yes | 43 | 27.7% | 29 | 18.7% | 6 | 3.9% | 68 | 43.9% |
| 94 | No | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% |
| 95 | Yes | 23 | 34,3% | 26 | 38.8% | 7 | 10.4% | 56 | 83.6% |
| ACTIFIT® Vs CMI | |||||||||
| 96 | Yes | 14 | 26,40% | 20 | 37.7% | 4 | 7.5% | 34 | 64.2% |
Interestingly, the only randomized trial published [74] revealed that CMI implantation is able to provide more symptomatic relief and restore better knee function compared to meniscectomy alone at mid-term follow-up. Second look arthroscopies documented new tissue regrowth within the scaffold and also histology showed formation of fibro-chondrocyte matrix.
Similar results were found in a controlled study by Zaffagnini et al. [67], who investigated the difference among patients who received meniscectomy alone and those treated by meniscectomy and concurrent CMI implantation: the latter presented lower pain and higher IKDC subj. score at follow-up and also less progression to OA compared to meniscectomized patients, thus supporting the chondroprotective role of the collagen scaffold.
Another study investigated the different outcomes of patients receiving ACL reconstruction and concurrent medial meniscectomy or CMI implantation [69]: even in this case, the scaffold group showed less pain and better arthrometric parameters, thus confirming that meniscal tissue preservation significantly contributes to knee stability.
With regard to MRI evaluations, the most adopted method was the Genovese score: overall, the MRI appearance of the CMI scaffold evolved positively over time, with just a very few cases of complete resorption of the scaffold. Anyway, complete healing and restoration of normal “meniscus-like” tissue occurred in a limited percentage of patients, whereas the majority of them still showed different intensity of signal at the site of CMI implantation, smaller overall size of the treated meniscus and a certain amount of extrusion [67,70,74,76].
3.2.2. ACTIFIT® scaffold
Nineteen studies analyzed the potential of Actifit® as a synthetic meniscal substitute, for a total of 777 patients treated [[77], [78], [79], [80], [81], [82], [83], [84], [85], [86], [87], [88], [89], [90], [91], [92], [93], [94], [95]] (Fig. 3). A detailed synopsis of the included trials is available in Table 2. Almost all these investigations (16 out of 19) have been prospectively performed [[77], [78], [79], [80], [81], [82], [83], [84], [85],[87], [88], [89], [90], [91], [92], [93]] and only 3 retrospective studies [86,94,95] were found. Four studies reported on a multi-centric clinical examination [77,89,90,93].
The methodological quality of the studies was quite modest, with the modified Coleman scores ranging from 40 [81] to 67 [89].
All studies described positive clinical outcomes following Actifit® implantation. Significant relief of pain was found. Clinical improvements in the operated knee have also been documented by different clinical scales (IKDC objective and subjective score, KOOS score, Lysholm scale, Cincinnati rating system, KSS, Tegner scale, WOMET, Kujala, UCLA scale).
No major implant-related adverse events (AEs) and complications have been reported, as described in 3 different clinical examinations [80,82,84]. The total number of patients who suffered AEs or complications was 19, in particular: 3 deep vein thrombosis [83], 2 local wound infections [83], 4 revision surgery for persisting symptoms (including 1 unicondylar knee arthroplasty after 6 months) [85,90], 2 post-operative infections [89,90], 1 progression of cartilage damage [89], 1 residual pain for saphenous nerve injury [91], 1 quadriceps weakness for femoral nerve damage related to anesthesia [91], 5 joint stiffness [90,91].
Radiological outcomes of Actifit® have been described in 15 papers which adopted MRI to assess different size, reabsorption, hyperintensity and extrusion of the meniscal substitute [[78], [79], [80], [81], [82],[84], [85], [86], [87], [88], [89],[91], [92], [93], [94]].
Nine studies employed the Genovese scale for the MRI assessment, resulting, in the majority of cases, in “type II” scaffolds (i.e. smaller and with a hyperintense signal at MRI compared to a normal meniscus) [79,82,[84], [85], [86],88,[92], [93], [94]]. Complete resorption of the scaffold was a rare event in the available literature, whereas reduction in size relative to native meniscus, slightly or marked hyperintensity and implant extrusion have been revealed as common findings in the majority of MRI examinations. The main issue in the evaluation of Actifit® implant is the massive presence of patients who underwent associated surgeries, such as osteotomies and concurrent cartilage treatments (Table 3), that might have biased the interpretation of data.
Only 4 trials included patients treated only by Actifit® implantation [80,87,88,94]. Considering that two of them [87,88] examined the same population at different follow-up times, the total number of patients that can provide for unbiased data about the polyurethane meniscal scaffold is 51.
The first study [80] included 10 patients evaluated at 6 and 12 months: KOOS and KSS scales were significant better at both follow-ups and MRI exams described stable, non-reabsorbed and non-hyperintense scaffolds.
The study by Akkaya M et al. [94] confirmed good clinical results with significantly better scores in Lysholm, KOOS, IKDC and lower pain at 36 months. At the same follow-up time, slight scaffold extrusion was observed with a mean value of 2.4 mm. Cartilage status was assessed through Outerbridge grading scale and no difference was found between baseline and 36 months’ follow-up.
In the other two studies, Schuttler KF et al. [87,88] revealed significant improvements in KOOS, KSS, UCLA scale and VAS at 6, 12, 24 and 48 months from surgery. No scaffold extrusion and reabsorption were observed at MRI. Similar findings were reported also by Condello et al. [95], who also documented that delayed Actifit® implantation (i.e. more than 6 months after meniscectomy) leads to inferior clinical outcomes.
3.2.3. CMI vs Actifit®
Only one prospective, non-randomized trial [96] compared the outcomes of CMI and Actifit® scaffolds. Fifty-three patients in total were included and evaluated up to 24 months: no significant difference was detected in any clinical scores considered. A similar rate of complications was observed between the two groups. Biopsies performed during second look arthroscopies revealed different histologic findings: the CMI was mainly replaced by fibrous tissue, whereas in the case of the Actifit® an avascular cartilaginous-like tissue was present. In all cases, the scaffolds were still visible at macroscopic and microscopic level, thus suggesting an incomplete meniscal healing process after two years from implantation.
4. Discussion
The present systematic review described the current state of the art in the field of meniscal regeneration, starting from the in vivo preclinical evidence and then coming to clinical application, underlying the potential to offer a significant clinical improvement, but also the limitations of the current solutions to provide an effective meniscus regeneration.
The “ideal” scaffold for meniscal reconstruction should have proper biomechanical features, integrity, porosity, degradation rate, and strength. Furthermore, it should induce cell migration, attachment, proliferation, and differentiation, and be biocompatible and non-toxic [13]. Several different natural, synthetic, and hybrid scaffolds have been tested in the 46 in vivo preclinical studies [[21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63]], but only one natural (CMI) and one synthetic (Actifit®) scaffold have been translated into clinical practice [[67], [68], [69], [70], [71], [72], [73], [74], [75], [76], [77], [78], [79], [80], [81], [82], [83], [84], [85], [86], [87], [88], [89], [90], [91], [92], [93], [94], [95], [96]]. In vivo preclinical studies investigated 16 natural [[21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36]], 15 synthetic [[37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51]] and 15 hybrid [[52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63], [64], [65], [66]] scaffolds. The most frequently used natural scaffolds are meniscus fragments [23,24,30], HA/gelatin [[33], [34], [35]] or CMI [22,31], while PCL, alone [39] or in combination with other synthetic materials [40,[42], [43], [44],46], is the most adopted solution among synthetic scaffolds. COLL is the most employed as natural part in hybrid scaffolds [[53], [54], [55], [56], [57]]. Unfortunately, there are no trials comparing two or more scaffolds in the same animal model.
In the field of tissue engineering, combinations of cells or signaling molecules, such as GFs, to scaffolds may improve the formation of a well-organized tissue, with high mechanical properties and features similar to the native meniscus. In all types of scaffolds, only few studies added cells [[30], [31], [32], [33], [34], [35], [36],[45], [46], [47], [48], [49], [50], [51],[63], [64], [65], [66]] compared to those that employed the scaffolds alone [[21], [22], [23], [24], [25], [26], [27], [28], [29],[37], [38], [39], [40], [41], [42], [43], [44],[52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62]]. The most used cells are chondrocytes [30,31,[48], [49], [50], [51],[63], [64], [65]], followed by MSCs [[32], [33], [34], [35], [36],45,46,66] and myoblasts [47]. There were also a few studies that used GFs in conjunction with the scaffold: gefitinib (a tyrosine kinase inhibitor present on the intracellular side of EGFR) was injected around the scaffold [29], BMSCs were cultured in conditioned medium of fibro-chondrocytes, previously cultured in presence of TGFβ3 [36], GF-laden PCL scaffold was loaded with different concentrations of CTGF and TGFβ3 [62], and PEG-CHO scaffold was loaded TGFβ [66]. In all cases when cells or GFs were added, better results were obtained compared to the scaffold alone.
Table 4 shows the risk of bias and quality assessment performed for each study. First of all, with regard to quality assessment, it was observed that the indication for the 3Rs was not included in all in vivo studies and that the relevance of human biology was not included or little discussed. In addition, several studies did not indicate allocation concealment, randomization, blinding, details for animal number calculation. Details of experimental procedure were frequently reported; however, few studies evaluated the welfare related assessment, health status of animals and adverse event records. On the other hand, all studies described precise details for experimental procedures, experimental animals and statistical methods. With regards to the risk of bias, the overall absence of animal randomization should be noted, both during the surgical procedure (i.e. allocation of animals to experimental groups) and in the evaluation phases. Seven studies clearly affirmed that the outcomes were assessed randomly in studies regarding natural [24,25,35], synthetic [39,48] and hybrid [55,61] scaffolds. The outcomes were blindly assessed in 3 studies on hybrid scaffolds [56,57,63]. All studies indicated the uniformity of baseline characteristics among experimental groups and the protocol and outcome measurements were specified, but all studies did not acknowledge the presence of other potential bias. The quality assessment total score did not show significant differences among studies considering the scaffold types (from 20.4 for natural scaffolds to 22.9 for hybrid scaffolds).
Table 4.
SYRCLE's tool for assessing risk of bias of the studies.
| SYRCLE |
QUALITY ASSESSMENT |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ref. | Sequence generation | Baseline characteristics | Allocation concealment | Random housing | Blinding | Random outcome assessment | Blinding | Incomplete outcome data | Selective outcome reporting | Other sources of bias | Title | Abstract | Introduction | Methods | Results | Discussion | Total |
| Natural scaffolds | |||||||||||||||||
| Li C et al. Sci Rep 2017 | NO | YES | NO | NO | UNCLEAR | UNCLEAR | UNCLEAR | NO | YES | NO | 1 | 1 | 3 | 8 | 2 | 2 | 17 |
| Hansen R et al. J Orthop Res 2013 | NO | YES | NO | NO | UNCLEAR | UNCLEAR | UNCLEAR | YES | YES | NO | 1 | 1 | 2 | 12 | 5 | 3 | 24 |
| Kawaguchi Y et al. Orthop Traumatol Surg Res 2019 | NO | YES | NO | NO | UNCLEAR | UNCLEAR | UNCLEAR | NO | YES | NO | 1 | 1 | 1 | 7 | 2 | 2 | 14 |
| Kobayashi Y et al. Am J Sports Med 2010 | NO | YES | NO | NO | UNCLEAR | YES | UNCLEAR | NO | YES | NO | 1 | 2 | 2 | 13 | 3 | 3 | 24 |
| Gastel JA et al. Arthroscopy 2001 | NO | YES | NO | NO | UNCLEAR | YES | UNCLEAR | NO | YES | NO | 1 | 2 | 2 | 13 | 3 | 3 | 24 |
| Cook JL et al. Am J Sports Med 2006 | NO | YES | UNCLEAR | NO | UNCLEAR | UNCLEAR | UNCLEAR | NO | YES | NO | 1 | 1 | 1 | 14 | 3 | 3 | 23 |
| Gruchenberg K et al. Knee Surg Sports Traumatol Arthrosc 2015 | NO | YES | NO | NO | UNCLEAR | UNCLEAR | UNCLEAR | NO | YES | NO | 0 | 2 | 1 | 10 | 0 | 0 | 13 |
| Pan Z et al. Acta Biomaterialia 2017 | NO | YES | UNCLEAR | NO | UNCLEAR | UNCLEAR | UNCLEAR | YES | YES | NO | 1 | 2 | 3 | 14 | 6 | 3 | 29 |
| Yuan Z et al. Biomaterials 2016 | NO | YES | UNCLEAR | NO | UNCLEAR | UNCLEAR | UNCLEAR | YES | YES | NO | 1 | 2 | 1 | 11 | 7 | 2 | 24 |
| Peretti GM et al. Am J Sports Med 2004 | NO | YES | NO | NO | UNCLEAR | UNCLEAR | UNCLEAR | NO | YES | NO | 0 | 2 | 1 | 9 | 3 | 3 | 18 |
| Martinek V et al. Arch Orthop Trauma Surg 2006 | NO | YES | NO | NO | UNCLEAR | UNCLEAR | UNCLEAR | YES | NO | YES | 0 | 2 | 1 | 8 | 6 | 3 | 20 |
| Whitehouse MR et al. Stem Cells Transl Med 2017 | NO | YES | NO | NO | UNCLEAR | UNCLEAR | UNCLEAR | NO | YES | NO | 0 | 1 | 1 | 11 | 2 | 3 | 18 |
| Angele P et al. J Biomed Mater Res 2008 | NO | YES | NO | NO | UNCLEAR | UNCLEAR | UNCLEAR | NO | YES | NO | 0 | 1 | 1 | 10 | 1 | 1 | 14 |
| Zellner J et al. J Biomed Mater Res Part A 2010 | NO | YES | NO | NO | UNCLEAR | UNCLEAR | UNCLEAR | NO | YES | NO | 0 | 1 | 1 | 10 | 3 | 1 | 16 |
| Zellner J et al. J Biomed Mater Res Part B Appl Biomater 2013 | NO | YES | UNCLEAR | NO | UNCLEAR | YES | UNCLEAR | NO | YES | NO | 0 | 2 | 2 | 14 | 4 | 2 | 24 |
| Koh RH et al. Acta Biomaterialia 2017 | NO | YES | NO | NO | UNCLEAR | UNCLEAR | UNCLEAR | NO | YES | NO | 1 | 2 | 2 | 13 | 3 | 3 | 24 |
| Synthetic scaffolds | |||||||||||||||||
| Zhang ZZ et al. Acta Biomaterialia 2016 | NO | YES | NO | NO | UNCLEAR | YES | UNCLEAR | NO | YES | NO | 1 | 2 | 3 | 13 | 5 | 1 | 25 |
| Otsuki S et al. Am J Sports Med 2019 | NO | YES | NO | NO | UNCLEAR | UNCLEAR | UNCLEAR | NO | YES | NO | 0 | 1 | 1 | 11 | 3 | 3 | 19 |
| Testa Pezzin AP et al. Artif Org 2003 | NO | YES | NO | NO | UNCLEAR | UNCLEAR | UNCLEAR | NO | YES | NO | 1 | 2 | 1 | 10 | 2 | 2 | 18 |
| Tienen TG et al. Biomaterials 2003 | NO | YES | NO | NO | UNCLEAR | UNCLEAR | UNCLEAR | NO | YES | NO | 0 | 1 | 1 | 7 | 1 | 2 | 12 |
| Galley NK et al. Clin Orthop Relat Res 2011 | NO | YES | NO | NO | UNCLEAR | UNCLEAR | UNCLEAR | NO | YES | NO | 0 | 2 | 3 | 11 | 3 | 0 | 19 |
| Maher SA et al. Arthroscopy 2010 | NO | YES | NO | NO | UNCLEAR | UNCLEAR | UNCLEAR | YES | YES | NO | 1 | 2 | 2 | 13 | 7 | 1 | 26 |
| Welsing RTC et al. Am J Sports Med 2008 | NO | YES | NO | NO | UNCLEAR | UNCLEAR | UNCLEAR | NO | YES | NO | 0 | 2 | 3 | 11 | 5 | 1 | 22 |
| Heijkants RGJC et al. J Mater Sci Mater Med 2004 | NO | YES | NO | NO | UNCLEAR | UNCLEAR | UNCLEAR | NO | YES | NO | 0 | 1 | 1 | 9 | 3 | 1 | 15 |
| Zhang ZZ et al. Am J Sports Med 2017 | NO | YES | NO | NO | UNCLEAR | UNCLEAR | UNCLEAR | NO | YES | NO | 0 | 1 | 3 | 12 | 6 | 3 | 25 |
| Gu Y et al. Exp Ther Med 2012 | NO | YES | YES | NO | UNCLEAR | UNCLEAR | UNCLEAR | NO | YES | NO | 0 | 1 | 1 | 13 | 3 | 1 | 19 |
| Weinand C et al. Arch Orthop Trauma Surg 2006 | NO | YES | NO | NO | UNCLEAR | YES | UNCLEAR | NO | YES | NO | 1 | 2 | 3 | 12 | 6 | 1 | 25 |
| Weinand C et al. Am J Sports Med 2006 | NO | YES | NO | NO | UNCLEAR | UNCLEAR | UNCLEAR | NO | YES | NO | 1 | 2 | 2 | 12 | 3 | 2 | 22 |
| Koch M et al. Stem Cell Int 2018 | NO | YES | NO | NO | UNCLEAR | UNCLEAR | UNCLEAR | NO | YES | NO | 0 | 1 | 2 | 10 | 5 | 2 | 20 |
| Kang SW et al. J Biomed Mater Res 2006 | NO | YES | NO | NO | UNCLEAR | UNCLEAR | UNCLEAR | NO | YES | NO | 0 | 2 | 1 | 8 | 4 | 2 | 17 |
| Esposito AR et al. Biores Open Access 2013 | NO | YES | NO | NO | UNCLEAR | UNCLEAR | UNCLEAR | NO | YES | NO | 0 | 2 | 1 | 12 | 5 | 3 | 23 |
| Natural/synthetic scaffolds | |||||||||||||||||
| Gao S et al. Acta Biomater 2018 | NO | YES | NO | NO | UNCLEAR | UNCLEAR | UNCLEAR | NO | YES | NO | 1 | 2 | 3 | 10 | 3 | 3 | 22 |
| Ghodbane SA et al. Tissue Eng Part A 2019 | NO | YES | NO | NO | UNCLEAR | UNCLEAR | UNCLEAR | NO | YES | NO | 0 | 2 | 2 | 13 | 6 | 2 | 25 |
| Merriam AR et al. Am J Sports Med 2015 | NO | YES | NO | NO | UNCLEAR | UNCLEAR | UNCLEAR | NO | YES | NO | 1 | 2 | 2 | 11 | 3 | 2 | 21 |
| Patel JM et al. Am J Sports Med 2016 | NO | YES | NO | NO | UNCLEAR | YES | UNCLEAR | NO | YES | NO | 1 | 2 | 2 | 12 | 6 | 2 | 25 |
| Patel JM et al. Am J Sports Med 2018 | NO | YES | NO | NO | UNCLEAR | UNCLEAR | YES | NO | YES | NO | 1 | 1 | 3 | 14 | 7 | 3 | 29 |
| Patel JM et al. Tissue Eng Part A 2016 | NO | YES | NO | NO | UNCLEAR | UNCLEAR | YES | NO | YES | NO | 1 | 2 | 2 | 11 | 5 | 1 | 22 |
| Chiari C et al. Osteoarthritis Cartilage 2006 | NO | YES | NO | NO | UNCLEAR | UNCLEAR | UNCLEAR | NO | YES | NO | 0 | 1 | 2 | 11 | 1 | 3 | 18 |
| Nakagawa Y et al. Am J Sports Med 2019 | NO | YES | NO | YES | UNCLEAR | UNCLEAR | UNCLEAR | NO | YES | NO | 1 | 1 | 3 | 11 | 2 | 0 | 18 |
| Cojocaru DG et al. J Biomed Mater Res 2020 | NO | YES | NO | YES | UNCLEAR | UNCLEAR | UNCLEAR | NO | YES | NO | 1 | 2 | 3 | 13 | 7 | 0 | 26 |
| Demirkiran ND et al. Acta Orthop Traumatol Turc 2019 | NO | YES | NO | YES | UNCLEAR | UNCLEAR | UNCLEAR | NO | YES | NO | 1 | 2 | 3 | 13 | 3 | 3 | 25 |
| Shimomura K et al. Biomaterials 2019 | NO | YES | NO | YES | UNCLEAR | YES | UNCLEAR | NO | YES | NO | 0 | 2 | 2 | 13 | 3 | 2 | 22 |
| Moradi L et al. Biomaterials 2017 | NO | YES | NO | YES | UNCLEAR | UNCLEAR | YES | NO | YES | NO | 1 | 2 | 3 | 13 | 2 | 2 | 23 |
| Chen C et al. Am J Sports Med 2020 | NO | YES | NO | YES | UNCLEAR | UNCLEAR | UNCLEAR | NO | YES | NO | 1 | 2 | 2 | 14 | 1 | 1 | 21 |
| Kon E et al. Tissue Eng Part A 2008 | NO | YES | NO | YES | UNCLEAR | UNCLEAR | UNCLEAR | NO | YES | NO | 1 | 2 | 3 | 13 | 3 | 3 | 25 |
| Kon E et al. Tissue Eng Part A 2012 | NO | YES | NO | YES | UNCLEAR | UNCLEAR | UNCLEAR | NO | YES | NO | 1 | 2 | 2 | 14 | 3 | 3 | 25 |
Out of the variety of scaffolds used in vivo, only 2 of these, CMI [[67], [68], [69], [70], [71], [72], [73], [74], [75], [76], 96] and Actifit® [[77], [78], [79], [80], [81], [82], [83], [84], [85], [86], [87], [88], [89], [90], [91], [92], [93], [94], [95], [96]], were brought into clinical practice. The first one is a collagen-based implant released in the late 90ies, whereas the latter one is a polyurethane-based scaffold which reached the market a few years later [1]. Both of them are cell-free scaffolds which aim at promoting regeneration of meniscal fibrocartilage by stimulating resident stromal cells from adjacent tissues, especially synovium. Despite the relevant differences in biochemical features and composition, both scaffolds proved to be safe [83, 85, [89], [90], [91], 96 and were able to provide a certain amount of meniscal healing and inherent chondroprotection, as revealed by histologic data and by MRI evaluation [67, 68, 75, 76, [78], [79], [80], [81], [82], [84], [85], [86], [87], [88], [89], [91], [92], [93], [94], [95], [96], [99], [100], [101]]. This translated into a clinical benefit and improvement in patients’ subjective score, documented up to mid/long-term evaluations [[67], [68], [69], [70], [71], [72], [73], [74],76,78,82,84,86,90,91,[93], [94], [95], [96]]. However, a complete meniscal healing has been seen only occasionally and we are still far from reaching it with the current technologies. Furthermore, we should acknowledge that the available clinical evidence is affected by some significant flaws: first of all, the modest quality of the majority of trials, with a significant rate of concurrent procedures (realignments, ligament reconstructions and cartilage treatments), and the lack of randomized evaluation (with only one comparative trial published [96]) between the performance of CMI and Actifit®, in spite of the fact that both of them have been available for decades on the market. This is perhaps the most remarkable issue in the field of meniscal regeneration: the lack of “new” products released for clinical use in the last 10–12 years.
Although an intense pre-clinical research and an increasing awareness on the properties of biomimetic materials, clinicians nowadays have limited options and can rely on “old” technologies to treat partial meniscal loss. CMI and Actifit® have paved the way of meniscal regeneration but they need to be upgraded to provide a better quality of the regenerated tissue and, therefore, better and longer lasting outcomes. Still, the experience gained with these scaffolds in the last two decades allowed to gain important insights that should be considered to further develop this field. CMI scaffolds showed poor biomechanical properties, which could led to iatrogenic damage of the scaffold during implant positioning and suture [97]. To overcome these problems, Actifit® has been developed claiming superior mechanical properties. While intra-operatively this was perceived as a sufficient strength by surgeons, this may be not enough to sustain the high joint stresses, with even weight bearing and simple walking causing numerous and critical stress cycles. Due to the dynamic nature of this stress, scaffolds must sustain compression but also shear forces, overall jeopardizing the circumferential structure needed to preserve the joint from abnormal loads. Beside some technical implantation issues that may affect proper implant positioning, the insufficient strength of the available scaffolds likely results in the observed extrusion over time. Accordingly, new scaffolds should be produced with proper biomechanical properties to match what required by the normal joint function while new tissue is formed. This leads to another important point, the need of the implanted biomaterial to be readily replaced by regenerated tissue. As previously described, the presence of scaffold material was documented long after implantation, underlying the slow processes of induced tissue regeneration. Thus, from one side new scaffolds should present stronger biomechanical features, on the other side they should guide tissue regeneration and be readily replaced by a functional meniscus tissue. The balance of these material properties is a challenge to be addressed by future implants, together with another more macroscopic aspect. Available scaffolds present a pre-defined shape, which poorly matches the specific anatomic requirement of each individual patient. Progresses in the field of bioprinting could address this problem, producing tailored implants which could have the correct shape but also optimal internal architecture, and even proper cell distribution [98]. The use of cells to prompt a faster tissue regeneration could be a key aspect, as demonstrated by preclinical findings of better results when cell-based implants were compared with cell-free scaffolds. Moreover, cells or other adjuvant treatments should be considered also in terms of joint environment modulation to favour homeostatic features promoting the healing processes. The importance of the articular environment is increasingly recognized, with new evidence underlying better results when scaffolds are implanted in acute lesions rather than in joints affected by chronic degenerative processes [95]. The fact that augmentation with cells and growth-factors had been tested only in the in vivo preclinical setting could be explained by several reasons: the obvious increase in costs, the stringent regulations limiting cell-based approaches in many countries and also the technical challenges related to loading autologous cells/GFs into biosynthetic scaffolds for human application.
The need for innovation in this field is urgent since meniscectomy is still the most common arthroscopic procedure performed all over the world and is the most significant risk factor for the onset of knee OA. In the next decades we will assist to a drastic increase of post-meniscectomy OA patients, who will require prolonged treatments ultimately leading to metal resurfacing. The social and economic burden on National Health Systems is doomed to increase, and therefore a closer collaboration between Research Centres, Regulatory Bodies, and Industries is desirable, with the goal of developing and offering new “joint sparing strategies” for patients affected by meniscal lesions.
5. Conclusions
Several biosynthetic meniscal scaffolds have been tested in the animal model in the last 20 years, and also augmentation with cells and growth factors has been attempted to improve meniscus regeneration. Anyway, only two acellular scaffolds reached clinical practice: the CMI (11 studies) and the Actifit® (19 studies). Although positive outcomes were documented up to mid-long term, the overall quality of the available evidence is modest and both scaffolds present limited regenerative potential associated to structural flaws that do not qualify them as the “ideal” meniscal replacements. Future research in the field of 3D-bioprinting, the introduction of new biomaterials and the possibility of translating biologic augmentation also in the human setting might open the way to novel devices with superior biomechanical and regenerative properties, able to effectively protect knee joints from the onset of post-meniscectomy OA.
Funding
This project received funding from the European Union's Horizon 2020 research and innovation programme under the grant agreement No. 814444 (MEFISTO).
Availability of data and material
All the data available have been included in the manuscript.
Code availability
Not applicable.
Ethics approval
Not applicable due to the type of paper.
Consent to participate
Not applicable due to the type of paper.
Consent for publication
Not applicable due to the type of paper.
CRediT authorship contribution statement
F. Veronesi: Conceptualization, Methodology, Writing – original draft, preparation. B. Di Matteo: Conceptualization, Methodology, Writing – original draft, preparation. N.D. Vitale: Methodology, Investigation, Data curation, Writing – original draft, preparation. G. Filardo: Writing – review & editing. A. Visani: Conceptualization, Investigation, Data curation. E. Kon: Writing – original draft, preparation, Funding acquisition. M. Fini: Writing – review & editing, Project administration.
Declaration of competing interest
All the authors declare no conflict of interest with regard to the content of the manuscript.
Acknowledgements
The authors would like to thank the Italian Ministry of Health.
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
References
- 1.Verdonk R., Madry H., Shabshin N., Dirisamer F., Peretti G.M., Pujol N., Spalding T., Verdonk P., Seil R., Condello V., Di Matteo B., Zellner J., Angele P. The role of meniscal tissue in joint protection in early osteoarthritis. Knee Surg. Sports Traumatol. Arthrosc. 2016;24:1763–1774. doi: 10.1007/s00167-016-4069-2. [DOI] [PubMed] [Google Scholar]
- 2.Di Matteo B., Perdisa F., Gostynska N., Kon E., Filardo G., Marcacci M. Meniscal scaffolds - preclinical evidence to support their use: a systematic review. Open Orthop. J. 2015;9:143–156. doi: 10.2174/1874325001509010143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feeley B.T., Lau B.C. Biomechanics and clinical outcomes of partial meniscectomy. J. Am. Acad. Orthop. Surg. 2018;26:853–863. doi: 10.5435/JAAOS-D-17-00256. [DOI] [PubMed] [Google Scholar]
- 4.Giuffrida A., Di Bari A., Falzone E., Iacono F., Kon E., Marcacci M., Gatti R., Di Matteo B. Conservative vs. surgical approach for degenerative meniscal injuries: a systematic review of clinical evidence. Eur. Rev. Med. Pharmacol. Sci. 2020;24:2874–2885. doi: 10.26355/eurrev_202003_20651. [DOI] [PubMed] [Google Scholar]
- 5.Di Matteo B., Moran C.J., Tarabella V., Viganò A., Tomba P., Marcacci M., Verdonk R. A history of meniscal surgery: from ancient times to the twenty-first centur. Knee Surg. Sports Traumatol. Arthrosc. 2016;24:1510–1518. doi: 10.1007/s00167-015-3717-2. [DOI] [PubMed] [Google Scholar]
- 6.Di Matteo B., Tarabella V., Filardo G., Viganò A., Tomba P., Marcacci M. Thomas Annandale: the first meniscus repair, Knee. Surg. Sports. Traumatol. Arthrosc. 2013;21:1963–1966. doi: 10.1007/s00167-013-2490-3. [DOI] [PubMed] [Google Scholar]
- 7.Abrams G.D., Frank R.M., Gupta A.K., Harris J.D., McCormick F.M., Cole B.J. Trends in meniscus repair and meniscectomy in the United States, 2005-2011. Am. J. Sports Med. 2013;41:2333–2339. doi: 10.1177/0363546513495641. [DOI] [PubMed] [Google Scholar]
- 8.von Lewinski G., Milachowski K.A., Weismeier K., Kohn D., Wirth C.J. Twenty-year results of combined meniscal allograft transplantation, anterior cruciate ligament reconstruction and advancement of the medial collateral ligament. Knee Surg. Sports Traumatol. Arthrosc. 2007;15:1072–1082. doi: 10.1007/s00167-007-0362-4. [DOI] [PubMed] [Google Scholar]
- 9.Novaretti J.V., Patel N.K., Lian J., Vaswani R., de Sa D., Getgood A., Musahl V. Long-term survival analysis and outcomes of meniscal allograft transplantation with minimum 10-year follow-up: a systematic review. Arthroscopy. 2019;35:659–667. doi: 10.1016/j.arthro.2018.08.031. [DOI] [PubMed] [Google Scholar]
- 10.Waugh N., Mistry H., Metcalfe A., Loveman E., Colquitt J., Royle P., Smith N.A., Spalding T T. Meniscal allograft transplantation after meniscectomy: clinical effectiveness and cost-effectiveness. Knee Surg. Sports Traumatol. Arthrosc. 2019;27:1825–1839. doi: 10.1007/s00167-019-05504-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Southworth T.M., Naveen N.B., Tauro T.M., Chahla J., Cole B.J. Meniscal allograft transplants. Clin. Sports Med. 2020;39:93–123. doi: 10.1016/j.csm.2019.08.013. [DOI] [PubMed] [Google Scholar]
- 12.Smith N.A., Parkinson B., Hutchinson C.E., Costa M.L., Spalding T. Is meniscal allograft transplantation chondroprotective? A systematic review of radiological outcomes. Knee Surg. Sports Traumatol. Arthrosc. 2016;24:2923–2935. doi: 10.1007/s00167-015-3573-0. [DOI] [PubMed] [Google Scholar]
- 13.Sun J., Vijayavenkataraman S., Liu H. An overview of scaffold design and fabrication technology for engineered knee meniscus. Materials (Basel) 2017;10:29. doi: 10.3390/ma10010029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kon E., Robinson D., Shani J., Alves A., Di Matteo B., Ashmore K., De Caro F., Dulic O., Altschuler N. Reconstruction of large osteochondral defects using a hemicondylar aragonite-based implant in a caprine model. Arthroscopy. 2020 doi: 10.1016/j.arthro.2020.02.026. S0749-8063. 30181-X. [DOI] [PubMed] [Google Scholar]
- 15.Schuette H.B., Kraeutler M.J., McCarty E.C. Matrix-Assisted autologous chondrocyte transplantation in the knee: a systematic review of mid- to long-term clinical outcomes. Orthop. J. Sports. Med. 2017;5 doi: 10.1177/2325967117709250. 2325967117709250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Makris E.A., Hadidi P., Athanasiou K.A. The knee meniscus: structure-function, pathophysiology, current repair techniques, and prospects for regeneration. Biomaterials. 2011;32:7411–7431. doi: 10.1016/j.biomaterials.2011.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Houck D.A., Kraeutler M.J., Belk J.W., McCarty E.C., Bravman J.T. Similar clinical outcomes following collagen or polyurethane meniscal scaffold implantation: a systematic review. Knee Surg. Sports Traumatol. Arthrosc. 2018;26:2259–2269. doi: 10.1007/s00167-018-4838-1. [DOI] [PubMed] [Google Scholar]
- 18.Veronesi F., Vandenbulcke F., Ashmore K., Di Matteo B., Nicoli Aldini N., Martini L., Fini M., Kon E. Meniscectomy-induced osteoarthritis in the sheep model for the investigation of therapeutic strategies: a systematic review. Int. Orthop. 2020;44:779–793. doi: 10.1007/s00264-020-04493-1. [DOI] [PubMed] [Google Scholar]
- 19.Kon E., Verdonk P., Condello V., Delcogliano M., Dhollander A., Filardo G., Pignotti E., Marcacci M. Matrix-assisted autologous chondrocyte transplantation for the repair of cartilage defects of the knee: systematic clinical data review and study quality analysis. Am. J. Sports Med. 2009;37:156S–166S. doi: 10.1177/0363546509351649. [DOI] [PubMed] [Google Scholar]
- 20.Hooijmans C.R., Rovers M.M., de Vries R.B., Leenaars M., Ritskes-Hoitinga M., Langendam M.W. SYRCLE's risk of bias tool for animal studies. BMC Med. Res. Methodol. 2014;14:43. doi: 10.1186/1471-2288-14-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li C., Hu X., Meng Q., Zhang X., Zhu J., Dai L., Cheng J., Zhong M., Shi W., Ren B., Zhang J., Fu X., Duan X., Ao Y. The potential of using semitendinosus tendon as autograft in rabbit meniscus reconstruction. Sci. Rep. 2017;7:7033. doi: 10.1038/s41598-017-07166-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hansen R., Bryk E., Vigorita V. Collagen scaffold meniscus implant integration in a canine model: a histological analysis. J. Orthop. Res. 2013;31:1914–1919. doi: 10.1002/jor.22456. [DOI] [PubMed] [Google Scholar]
- 23.Kawaguchi Y., Kondo E., Iwasaki N., Tanaka Y., Yagi T., Yasuda K. Autologous living chondrocytes contained in the meniscal matrix play an important role in in vivo meniscus regeneration induced by in situ meniscus fragment implantation. Orthop. Traumatol. Surg. Res. 2019;105:683–690. doi: 10.1016/j.otsr.2018.12.017. [DOI] [PubMed] [Google Scholar]
- 24.Kobayashi Y., Yasuda K., Kondo E., Katsura T., Tanabe Y., Kimura M., Tohyama H. Implantation of autogenous meniscal fragments wrapped with a fascia sheath enhances fibrocartilage regeneration in vivo in a large harvest site defect. Am. J. Sports Med. 2010;38:740–748. doi: 10.1177/0363546509350749. [DOI] [PubMed] [Google Scholar]
- 25.Gastel J.A., Muirhead W.R., Lifrak J.T., Fadale P.D., Hulstyn M.J., Labrador D.P. Meniscal tissue regeneration using a collagenous biomaterial derived from porcine small intestine submucosa. Arthroscopy. 2001;17:151–159. doi: 10.1053/jars.2001.20959. [DOI] [PubMed] [Google Scholar]
- 26.Cook J.L., Fox D.B., Malaviya P., Tomlinson J.L., Kuroki K., Cook C.R. Long-term outcome for large meniscal defects treated with small intestinal submucosa in a dog model. Am. J. Sports Med. 2006;34:32–42. doi: 10.1177/0363546505278702. [DOI] [PubMed] [Google Scholar]
- 27.Gruchenberg K., Ignatius A., Friemert B., von Lubken F., Skaer N., Gellynck K., Kessler O., Durselen L. In vivo performance of a novel silk fibroin scaffold for partial meniscal replacement in a sheep model. Knee Surg. Sports Traumatol. Arthrosc. 2015;23:2218–2229. doi: 10.1007/s00167-014-3009-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yuan Z., Liu S., Hao C., Guo W., Gao S., Wang M., Chen M., Sun Z., Xu Y., Wang Y., Peng J., Yuan M., Guo Q.Y. AMECM/DCB scaffold prompts successful total meniscus reconstruction in a rabbit total meniscectomy model. Biomaterials. 2016;111:13–26. doi: 10.1016/j.biomaterials.2016.09.017. [DOI] [PubMed] [Google Scholar]
- 29.Pan Z., Wu Y., Zhang X., Fu Q., Li J., Yang Y., Yu D., Xu Y., Lu X., Sun H., Zhang X., Heng B.C., Bunpetch V., Zhang S., Ouyang H. Delivery of epidermal growth factor receptor inhibitor via a customized collagen scaffold promotes meniscal defect regeneration in a rabbit model. Acta Biomater. 2017;62:210–221. doi: 10.1016/j.actbio.2017.07.008. [DOI] [PubMed] [Google Scholar]
- 30.Peretti G.M., Gill T.J., Xu J.W., Randolph M.A., Morse K.R., Zaleske D.J. Cell-based therapy for meniscal repair. A large animal study. Am. J. Sports Med. 2004;32:146–158. doi: 10.1177/0095399703258790. [DOI] [PubMed] [Google Scholar]
- 31.Martinek V., Ueblacker P., Braun K., Nitschke S., Mannhardt R., Specht K., Gansbacher B., Imhoff A.B. Second generation of meniscus transplantation: in-vivo study with tissue engineered meniscus replacement. Arch. Orthop. Trauma. Surg. 2006;126:228–234. doi: 10.1007/s00402-005-0025-1. [DOI] [PubMed] [Google Scholar]
- 32.Whitehouse M.R., Howells N.R., Parry M.C., Austin E., Kafienah W., Brady K., Goodship A.E., Eldridge J.D., Blom A.W., Hollander A.P. Repair of torn avascular meniscal cartilage using undifferentiated autologous mesenchymal stem cells: from in vitro optimization to a first-in-human study, stem. Cells. Transl. Med. 2017;6:1237–1248. doi: 10.1002/sctm.16-0199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Angele P., Johnstone B., Kujat R., Zellner J., Nerlich M., Goldberg V., Yoo J. Stem cell based tissue engineering for meniscus repair. J. Biomed. Mater. Res. 2008;85A:445–455. doi: 10.1002/jbm.a.31480. [DOI] [PubMed] [Google Scholar]
- 34.Zellner J., Mueller M., Berner A., Dienstknecht T., Kujat R., Nerlich M., Hennemann B., Koller M., Prantl L., Angele M., Angele P. Role of mesenchymal stem cells in tissue engineering of meniscus. J. Biomed. Mater. Res. 2010;94A:1150–1161. doi: 10.1002/jbm.a.32796. [DOI] [PubMed] [Google Scholar]
- 35.Zellner J., Hierl K., Mueller M., Pfeifer C., Berner A., Dienstknecht T., Krutsch W., Geis S., Gehmert S., Kujat R., Dendorfer S., Prantl L., Nerlich M., Angele P. Stem cell-based tissue-engineering for treatment of meniscal tears in the avascular zone. J. Biomed. Mater. Res. B Appl. Biomater. 2013;101B:1133–1142. doi: 10.1002/jbm.b.32922. [DOI] [PubMed] [Google Scholar]
- 36.Koh R.H., Jin Y., Kang B.J., Hwang N.S. Chondrogenically primed tonsil-derived mesenchymal stem cells encapsulated in riboflavin-induced photocrosslinking collagen-hyaluronic acid hydrogel for meniscus tissue repairs. Acta Biomater. 2017;53:318–328. doi: 10.1016/j.actbio.2017.01.081. [DOI] [PubMed] [Google Scholar]
- 37.Zhang Z.Z., Jiang D., Ding J.X., Wang S.J., Zhang W., Zhang J.Y., Qi Y.S., Chen X.S., Yu J.K. Role of scaffold mean pore size in meniscus regeneration. Acta Biomater. 2016;43:314–326. doi: 10.1016/j.actbio.2016.07.050. [DOI] [PubMed] [Google Scholar]
- 38.Otsuki S., Nakagawa K., Murakami T., Sezaki S., Sato H., Suzuki M., Okuno N., Wakama H., Kaihatsu K., Neo M. Evaluation of meniscal regeneration in a mini pig model treated with a novel polyglycolic acid meniscal scaffold. Am. J. Sports Med. 2019;47:1804–1815. doi: 10.1177/0363546519850578. [DOI] [PubMed] [Google Scholar]
- 39.Testa Pezzin A.P., Cardoso T.P., do Carmo Alberto Rincón M., de Carvalho Zavaglia C.A., de Rezende Duek E.A. Bioreabsorbable polymer scaffold as temporary meniscal prosthesis. Artif. Organs. 2003;27:428–431. doi: 10.1046/j.1525-1594.2003.07251.x. [DOI] [PubMed] [Google Scholar]
- 40.Tienen T.G., Heijkants R.G.J.C., Buma P., De Groot J.H., Pennings A.J., Veth R.P.H. A porous polymer scaffold for meniscal lesion repair—a study in dogs. Biomaterials. 2003;24:2541–2548. doi: 10.1016/s0142-9612(03)00053-x. [DOI] [PubMed] [Google Scholar]
- 41.Galley N.K., Gleghorn J.P., Rodeo S., Warren R.F., Maher S.A., Bonassar L.J. Frictional properties of the meniscus improve after scaffold augmented repair of partial meniscectomy: a pilot study. Clin. Orthop. Relat. Res. 2011;469:2817–2823. doi: 10.1007/s11999-011-1854-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maher S.A., Rodeo S.A., Doty S.B., Brophy R., Potter H., Foo L.F., Rosenblatt L., Deng X.H., Turner A.S., Wright T.M., Warren R.F. Evaluation of a porous polyurethane scaffold in a partial meniscal defect ovine model. Arthroscopy. 2010;26:1510–1519. doi: 10.1016/j.arthro.2010.02.033. [DOI] [PubMed] [Google Scholar]
- 43.Welsing R.T.C., van Tienen T.G., Ramrattan N., Heijkants R., Schouten A.J., Veth R.P.H., Buma P. Effect on tissue differentiation and articular cartilage degradation of a polymer meniscus implant. A 2-year follow-up study in dogs. Am. J. Sports Med. 2008;36:1978–1989. doi: 10.1177/0363546508319900. [DOI] [PubMed] [Google Scholar]
- 44.Heijkants R.G.J.C., Van Calck R.V., De Groot J.H., Pennings A.J., Schouten A.J. Design, synthesis and properties of a degradable polyurethane scaffold for meniscus regeneration. J. Mater. Sci. Mater. Med. 2004;15:423–427. doi: 10.1023/b:jmsm.0000021114.39595.1e. [DOI] [PubMed] [Google Scholar]
- 45.Zhang Z.Z., Wang S.J., Zhang J.Y., Jiang W.B., Huang A.B., Qi Y.S., Ding J.X., Chen X.S., Jiang D., Yu J.K. 3D-Printed poly(e-caprolactone) scaffold augmented with mesenchymal stem cells for total meniscal substitution. A 12- and 24-week animal study in a rabbit model. Am. J. Sports Med. 2017;45:1497–1511. doi: 10.1177/0363546517691513. [DOI] [PubMed] [Google Scholar]
- 46.Gu Y., Zhu W., Hao Y., Lu L., Chen Y., Wang Y. Repair of meniscal defect using an induced myoblast-loaded polyglycolic acid mesh in a canine model. Exp. Ther. Med. 2012;3:293–298. doi: 10.3892/etm.2011.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weinand C., Peretti G.M., Adams S.B., Jr., Randolph M.A., Savvidis E., Gill T.J. Healing potential of transplanted allogeneic chondrocytes of three different sources in lesions of the avascular zone of the meniscus: a pilot study. Arch. Orthop. Trauma. Surg. 2006;126:599–605. doi: 10.1007/s00402-005-0100-7. [DOI] [PubMed] [Google Scholar]
- 48.Weinand C., Peretti G.M., Adams S.B., Jr., Bonassar L.J., Randolph M.A., Gill T.J. An allogenic cell–based implant for meniscal lesions. Am. J. Sports Med. 2006;34:1779–1789. doi: 10.1177/0363546506290666. [DOI] [PubMed] [Google Scholar]
- 49.Koch M., Achatz F.P., Lang S., Pfeifer C.G., Pattappa G., Kujat R., Nerlich M., Angele P., Zellner J. Tissue engineering of large full-size meniscus defects by a polyurethane scaffold: accelerated regeneration by mesenchymal stromal cells, stem. Cell. Int. 2018;2018:8207071. doi: 10.1155/2018/8207071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kang S.W., Son S.M., Lee J.S., Lee E.S., Lee K.Y., Park S.G., Park J.H., Kim B.S. Regeneration of whole meniscus using meniscal cells and polymer scaffolds in a rabbit total meniscectomy model. J. Biomed. Mater. Res. 2006;77A:659–671. doi: 10.1002/jbm.a.30579. [DOI] [PubMed] [Google Scholar]
- 51.Esposito A.R., Moda M., de Melo Cattani S.M., de Santana G.M., Barbieri J.A., Moron Munhoz M., Cardoso T.P., Peris Barbo M.L., Russo T., D'Amora U., Gloria A., Ambrosio L., de Rezende Duek E.A. PLDLA/PCL-T scaffold for meniscus tissue engineering. Biores. Open. Access. 2013;2:138–147. doi: 10.1089/biores.2012.0293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gao S., Chen M., Wang P., Li Y., Yuan Z., Guo W., Zhang Z., Zhang X., Jing X., Li X., Liu S., Sui X., Xi T., Guo Q. An electrospun fiber reinforced scaffold promotes total meniscus regeneration in rabbit meniscectomy model. Acta Biomater. 2018;73:127–140. doi: 10.1016/j.actbio.2018.04.012. [DOI] [PubMed] [Google Scholar]
- 53.Ghodbane S.A., Brzezinski A., Patel J.M., Plaff W.H., Marzano K.N., Gatt C.J., Dunn M.G. Partial meniscus replacement with a collagen-hyaluronan infused three-dimensional printed polymeric scaffold, tissue. Eng. Part. A. 2019;25:379–389. doi: 10.1089/ten.TEA.2018.0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Merriam A.R., Patel J.M., Culp B.M., Gatt C.J., Dunn M.G. Successful total meniscus reconstruction using a novel fiber-reinforced scaffold. A 16- and 32-week study in an ovine model. Am. J. Sports Med. 2015;43:2528–2537. doi: 10.1177/0363546515595065. [DOI] [PubMed] [Google Scholar]
- 55.Patel J.M., Merriam A.R., Culp B.M., Gatt C.J., Dunn M.G. One-Year outcomes of total meniscus reconstruction using a novel fiber-reinforced scaffold in an ovine model. Am. J. Sports Med. 2016;44:898–907. doi: 10.1177/0363546515624913. [DOI] [PubMed] [Google Scholar]
- 56.Patel J.M., Ghodbane S.A., Brzezinski A., Gatt C.J., Dunn M.G. Tissue-Engineered total meniscus replacement with a fiber-reinforced scaffold in a 2-year ovine model. Am. J. Sports Med. 2018;46:1844–1856. doi: 10.1177/0363546517752668. [DOI] [PubMed] [Google Scholar]
- 57.Patel J.M., Merriam A.R., Kohn J., Gatt C.J., Dunn M.G. Negative outcomes of poly(L-lactic acid) fiber-reinforced scaffolds in an ovine total meniscus replacement model, tissue. Eng. Part. A. 2016;22:1116–1125. doi: 10.1089/ten.TEA.2016.0143. [DOI] [PubMed] [Google Scholar]
- 58.Chiari C., Koller U., Dorotka R., Eder C., Plasenzotti R., Lang S., Ambrosio L., Tognana E., Kon E., Salter D., Nehrer S. A tissue engineering approach to meniscus regeneration in a sheep model. Osteoarthritis Cartilage. 2006;14:1056–1065. doi: 10.1016/j.joca.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 59.Cojocaru D.G., Hondke S., Krüger J.P., Bosch C., Croicu C., Florescu S., Lazarescu A., Patrascu J.M., Dauner M., Gresser G.T., Endres M. Meniscus-shaped cell-free polyglycolic acid scaffold for meniscal repair in a sheep model. J. Biomed. Mater. Res. 2020;108B:809–818. doi: 10.1002/jbm.b.34435. [DOI] [PubMed] [Google Scholar]
- 60.Demirkıran N.D., Havıtçıoglu H., Ziylan A., Cankurt U., Hüsemoglu B. Novel multilayer meniscal scaffold provides biomechanical and histological results comparable to polyurethane scaffolds: an 8 week rabbit study. Acta Orthop. Traumatol. Turcica. 2019;53:120–128. doi: 10.1016/j.aott.2019.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shimomura K., Rothrauff B.B., Hart D.A., Hamamoto S., Kobayashi M., Yoshikawa H., Tuan R.S., Nakamura N. Enhanced repair of meniscal hoop structure injuries using an aligned electrospun nanofibrous scaffold combined with a mesenchymal stem cell derived tissue engineered construct. Biomaterials. 2019;92:346–354. doi: 10.1016/j.biomaterials.2018.11.009. [DOI] [PubMed] [Google Scholar]
- 62.Nakagawa Y., Fortier L.A., Mao J.J., Lee C.H., Goodale M.B., Koff M.F., Uppstrom T.J., Croen B., Wada S., Carballo C.B., Potter H.G., Rodeo S.A. Long-term evaluation of meniscal tissue formation in 3-dimensional–Printed scaffolds with sequential release of connective tissue growth factor and TGF-b3 in an ovine model. Am. J. Sports Med. 2019;47:2596–2607. doi: 10.1177/0363546519865513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Moradi L., Vasei M., Dehghan M.M., Majidi M., Mohajeri S.F., Bonakdar S. Regeneration of meniscus tissue using adipose mesenchymal stem cells-chondrocytes co-culture on a hybrid scaffold: in vivo study. Biomaterials. 2017;126:18–30. doi: 10.1016/j.biomaterials.2017.02.022. [DOI] [PubMed] [Google Scholar]
- 64.Kon E., Chiari C., Marcacci M., Delcogliano M., Salter D.M., Martin I., Ambrosio L., Fini M., Tschon M., Tognana E., Plasenzotti R., Nehrer S. Tissue engineering for total meniscal substitution: animal study in sheep model, tissue. Eng. Part. A. 2008;14:1067–1080. doi: 10.1089/ten.tea.2007.0193. [DOI] [PubMed] [Google Scholar]
- 65.Kon E., Filardo G., Tschon M., Fini M., Giavaresi G., Marchesini Reggiani L., Chiari C., Nehrer S., Martin I., Salter D.M., Path F.R.C., Ambrosio L., Marcacci M. Tissue engineering for total meniscal substitution: animal study in sheep model—results at 12 Months, tissue. Eng. Part. A. 2012;18:1573–1582. doi: 10.1089/ten.TEA.2011.0572. [DOI] [PubMed] [Google Scholar]
- 66.Chen C., Song J., Qiu J., Zhao J. Repair of a meniscal defect in a rabbit model through use of a thermosensitive, injectable, in situ crosslinked hydrogel with encapsulated bone mesenchymal stromal cells and transforming growth factor β1. Am. J. Sports Med. 2020;48:884–894. doi: 10.1177/0363546519898519. [DOI] [PubMed] [Google Scholar]
- 67.Zaffagnini S., Marcheggiani Muccioli G.M., Lopomo N., Bruni D., Giordano G. Prospective long-term outcomes of the medial collagen meniscus implant versus partial medial meniscectomy. A minimum 10-year follow-up study. Am. J. Sports Med. 2011;39:977–985. doi: 10.1177/0363546510391179. [DOI] [PubMed] [Google Scholar]
- 68.Zaffagnini S., Marcheggiani Muccioli G.M., Bulgheroni P., Bulgheroni E., Grassi A., Bonanzinga T., Kon E., Filardo G., Busacca M., Marcacci M. Arthroscopic collagen meniscus implantation for partial lateral meniscal defects. A 2-year minimum follow-up study. Am. J. Sports Med. 2012;40:2281–2288. doi: 10.1177/0363546512456835. [DOI] [PubMed] [Google Scholar]
- 69.Bulgheroni E., Grassi A., Bulgheroni P., Marcheggiani Muccioli G.M., Zaffagnini S., Marcacci M. Long-term outcomes of medial CMI implant versus partial medial meniscectomy in patients with concomitant ACL reconstruction. Knee Surg. Sports Traumatol. Arthrosc. 2015;23:3221–3227. doi: 10.1007/s00167-014-3136-9. [DOI] [PubMed] [Google Scholar]
- 70.Schenk L., Bethge L., Hirschmann A., Berbig R., Lüthi U., Arnold M.P., Hirschmann M.T. Ongoing MRI remodeling 3-7 years after collagen meniscus implantation in stable knees. Knee Surg. Sports Traumatol. Arthrosc. 2020;29:1099–1104. doi: 10.1007/s00167-019-05714-w. [DOI] [PubMed] [Google Scholar]
- 71.Zaffagnini S., Grassi A., Marcheggiani Muccioli G.M., Holsten D., Bulgheroni P., Monllau J.C., Berbig R., Lagae K., Crespo R., Marcacci M. Two-Year clinical results of lateral collagen meniscus implant: a multicenter study. Arthroscopy. 2015;31:1269–1278. doi: 10.1016/j.arthro.2015.01.025. [DOI] [PubMed] [Google Scholar]
- 72.Hirschmann M.T., Keller L., Hirschmann A., Schenk L., Berbig R., Lüthi U., Amsler F., Friederich N.F., Arnold M.P. One-year clinical and MR imaging outcome after partial meniscal replacement in stabilized knees using a collagen meniscus implant. Knee Surg. Sports Traumatol. Arthrosc. 2013;21:740–747. doi: 10.1007/s00167-012-2259-0. [DOI] [PubMed] [Google Scholar]
- 73.Bulgheroni P., Murena L., Ratti C., Bulgheroni E., Ronga M., Cherubino P. Follow-up of collagen meniscus implant patients: clinical, radiological, and magnetic resonance imaging results at 5 years. Knee. 2010;17:224–229. doi: 10.1016/j.knee.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 74.Rodkey W.G., DeHaven K.E., Montgomery W.H., 3rd, Baker C.L., Jr., Hormel S.E., Steadman J.R., Cole B.J., Briggs K.K. Comparison of the collagen meniscus implant with partial meniscectomy. A prospective randomized trial. J. Bone. Joint. Surg. Am. 2008;90:1413–1426. doi: 10.2106/JBJS.G.00656. [DOI] [PubMed] [Google Scholar]
- 75.Zaffagnini S., Giordano G., Vascellari A., Bruni D., Neri M.P., Iacono F., Kon E., Presti M.L., Marcacci M. Arthroscopic collagen meniscus implant results at 6 to 8 years follow up. Knee Surg. Sports Traumatol. Arthrosc. 2007;15:175–183. doi: 10.1007/s00167-006-0144-4. [DOI] [PubMed] [Google Scholar]
- 76.Kovacs B.K., Huegli R., Harder D., Cedro L., Berbig R., Amsler F., Bensler S., Hirschmann M.T., Hirschmann A. MR variability of collagen meniscal implant remodelling in patients with good clinical outcome. Knee Surg. Sports Traumatol. Arthrosc. 2019 doi: 10.1007/s00167-019-05715-9. [DOI] [PubMed] [Google Scholar]
- 77.Bouyarmane H., Beaufils P., Pujola N., Bellemans J., Roberts S., Spalding T., Zaffagnini S., Marcacci M., Verdonk P., Womack M., Verdonk R. Polyurethane scaffold in lateral meniscus segmental defects:Clinical outcomes at 24 months follow-up. Orthop. Traumatol. Surg. Res. 2014;100:153–157. doi: 10.1016/j.otsr.2013.10.011. [DOI] [PubMed] [Google Scholar]
- 78.De Coninck T., Huysse W., Willemot L., Verdonk R., Verstraete K., Verdonk P. Two-Year follow-up study on clinical and radiological outcomes of polyurethane meniscal scaffolds. Am. J. Sports Med. 2013;41:64–72. doi: 10.1177/0363546512463344. [DOI] [PubMed] [Google Scholar]
- 79.Dhollander A., Verdonk P., Verdonk R. Treatment of painful, irreparable partial meniscal defects with a polyurethane scaffold. Midterm clinical outcomes and survival analysis. Am. J. Sports Med. 2016;44:2615–2621. doi: 10.1177/0363546516652601. [DOI] [PubMed] [Google Scholar]
- 80.Efe T., Getgood A., Schofer M.D., Fuchs-Winkelmann S., Mann D., Paletta J.R.J., Heyse T.J. The safety and short-term efficacy of a novel polyurethane meniscal scaffold for the treatment of segmental medial meniscus deficiency. Knee Surg. Sports Traumatol. Arthrosc. 2012;20:1822–1830. doi: 10.1007/s00167-011-1779-3. [DOI] [PubMed] [Google Scholar]
- 81.Faivre B., Bouyarmane H., Lonjon G., Boisrenoult P., Pujol N., Beaufils P. Actifit® scaffold implantation: influence of preoperative meniscal extrusion on morphological and clinical outcomes. Orthop. Traumatol. Surg. Res. 2015;101:703–708. doi: 10.1016/j.otsr.2015.06.016. [DOI] [PubMed] [Google Scholar]
- 82.Filardo G., Kon E., Perdisa F., Sessa A., Di Martino A., Busacca M., Zaffagnini S., Marcacci M. Polyurethane-based cell-free scaffold for the treatment of painful partial meniscus loss. Knee Surg. Sports Traumatol. Arthrosc. 2017;25:459–467. doi: 10.1007/s00167-016-4219-6. [DOI] [PubMed] [Google Scholar]
- 83.Gelber P.E., Isart A., Erquicia J.L., Pelfort X., Tey-Pons M., Monllau J.C. Partial meniscus substitution with a polyurethane scaffold does not improve outcome after an open-wedge high tibial osteotomy. Knee Surg. Sports Traumatol. Arthrosc. 2015;23:334–339. doi: 10.1007/s00167-014-3206-z. [DOI] [PubMed] [Google Scholar]
- 84.Kon E., Filardo G., Zaffagnini S., Di Martino A., Di Matteo B., Marcheggiani Muccioli G.M., Busacca M., Marcacci M. Biodegradable polyurethane meniscal scaffold for isolated partial lesions or as combined procedure for knees with multiple comorbidities: clinical results at 2 years. Knee Surg. Sports Traumatol. Arthrosc. 2014;22:128–134. doi: 10.1007/s00167-012-2328-4. [DOI] [PubMed] [Google Scholar]
- 85.Leroy A., Beaufils P., Faivre B., Steltzlen C., Boisrenoult P., Pujol N. Actifit® polyurethane meniscal scaffold: MRI and functional outcomes after a minimum follow-up of 5 years. Orthop. Traumatol. Surg. Res. 2014;103:609–614. doi: 10.1016/j.otsr.2017.02.012. [DOI] [PubMed] [Google Scholar]
- 86.Monllau J.C., Poggioli F., Erquicia J., Ramírez E., Pelfort X., Gelber P., Torres-Claramunt R. Magnetic resonance imaging and functional outcomes after a polyurethane meniscal scaffold implantation: minimum 5-year follow-up. Arthroscopy. 2018;34:1621–1627. doi: 10.1016/j.arthro.2017.12.019. [DOI] [PubMed] [Google Scholar]
- 87.Schuttler K.F., Pottgen S., Getgood A., Rominger M.B., Fuchs-Winkelmann S., Roessler P.P., Ziring E., Efe T. Improvement in outcomes after implantation of a novel polyurethane meniscal scaffold for the treatment of medial meniscus deficiency. Knee Surg. Sports Traumatol. Arthrosc. 2015;23:1929–1935. doi: 10.1007/s00167-014-2977-6. [DOI] [PubMed] [Google Scholar]
- 88.Schüttler K.F., Haberhauer F., Gesslein M., Heyse T.J., Figiel J., Lorbach O., Efe T., Roessler P.P. Midterm follow-up after implantation of a polyurethane meniscal scaffold for segmental medial meniscus loss: maintenance of good clinical and MRI outcome. Knee Surg. Sports Traumatol. Arthrosc. 2016;24:1478–1484. doi: 10.1007/s00167-015-3759-5. [DOI] [PubMed] [Google Scholar]
- 89.Verdonk R., Verdonk P., Huysse W., Forsyth R., Heinrichs E.L. Tissue ingrowth after implantation of a novel, biodegradable polyurethane scaffold for treatment of partial meniscal lesions. Am. J. Sports Med. 2011;39:774–782. doi: 10.1177/0363546511398040. [DOI] [PubMed] [Google Scholar]
- 90.Verdonk P., Beaufils P., Bellemans J., Djian P., Heinrichs E.L., Huysse W., Laprell H., Siebold R., Verdonk R. Successful treatment of painful irreparable partial meniscal defects with a polyurethane scaffold. Two-year safety and clinical outcomes. Am. J. Sports Med. 2012;40:844–853. doi: 10.1177/0363546511433032. [DOI] [PubMed] [Google Scholar]
- 91.Baynat C., Andro C., Vincent J.P., Schiele P., Buisson P., Dubrana F., Gunepin F.X. Actifit® synthetic meniscal substitute: experience with 18 patients in Brest, France, Orthop. Traumatol. Surg. Res. 2014;100S:S385–S389. doi: 10.1016/j.otsr.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 92.Gelber P.E., Petrica A.M., Isart A., Mari-Molina R., Monllau J.C. The magnetic resonance aspect of a polyurethane meniscal scaffold is worse in advanced cartilage defects without deterioration of clinical outcomes after a minimum two-year follow-up. Knee. 2015;22:389–394. doi: 10.1016/j.knee.2015.01.008. [DOI] [PubMed] [Google Scholar]
- 93.Toanen C., Dhollander A., Bulgheroni P., Filardo G., Zaffagnini S., Spalding T., Monllau J.C., Gelber P., Verdonk R., Beaufils P., Pujol N., Verdonk P. Polyurethane meniscal scaffold for the treatment of partial meniscal deficiency: 5-year follow-up outcomes. A European multicentric study. Am. J. Sports Med. 2020;48:1347–1355. doi: 10.1177/0363546520913528. [DOI] [PubMed] [Google Scholar]
- 94.Akkaya M., Şimşek M.E., Gürsoy S., Çay N., Bozkur M. Medial meniscus scaffold implantation in combination with concentrated bone marrow aspirate injection: minimum 3-year follow-up. J. Knee Surg. 2020;33:838–846. doi: 10.1055/s-0040-1701452. [DOI] [PubMed] [Google Scholar]
- 95.Condello V., Dei Giudici L., Perdisa F., Screpis D.U., Guerriero M., Filardo G., Zorzi C. Polyurethane scaffold implants for partial meniscus lesions: delayed intervention leads to an inferior outcome. Knee Surg. Sports Traumatol. Arthrosc. 2021;29:109–116. doi: 10.1007/s00167-019-05760-4. [DOI] [PubMed] [Google Scholar]
- 96.Bulgheroni E., Grassi A., Campagnolo M., Bulgheroni P., Mudhigere A., Gobbi A. Comparative study of collagen versus synthetic-based meniscal scaffolds in treating meniscal deficiency in young active population. Cartilage. 2016;7:29–38. doi: 10.1177/1947603515600219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rodkey W.G., Steadman J.R., Li S.T. Collagen scaffolds: a new method to preserve and restore the severely injured meniscus, Sports. Med. Arthrosc. Rev. 1999;7:63–73. [Google Scholar]
- 98.Filardo G., Petretta M., Cavallo C., Roseti L., Durante S., Albisinni U., Grigolo B. Patient-specific meniscus prototype based on 3D bioprinting of human cell-laden scaffold. Bone Joint Res. 2019;8:101–106. doi: 10.1302/2046-3758.82.BJR-2018-0134.R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rodkey W.G., Steadman J.R., Li S.T. A clinical study of collagen meniscus implants to restore the injured meniscus. Clin. Orthop. Relat. Res. 1999;367:S281–S292. doi: 10.1097/00003086-199910001-00027. [DOI] [PubMed] [Google Scholar]
- 100.Stone K.R., Steadman J.R., Rodkey W.G., Li S.T. Regeneration of meniscal cartilage with use of a collagen scaffold. Analysis of preliminary data. J. Bone. Joint. Surg. Am. 1997;79:1770–1777. doi: 10.2106/00004623-199712000-00002. [DOI] [PubMed] [Google Scholar]
- 101.Stone K.R., Stoller D.W., Irving S.G., Elmquist C., Gildengorin G. 3D MRI volume sizing of knee meniscus cartilage. Arthroscopy. 1994;10:641–644. doi: 10.1016/s0749-8063(05)80062-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the data available have been included in the manuscript.