Abstract
Brain tumors are challenging to handle and cause severe mortality and morbidity. The primary therapy for brain tumors, a combination of radiotherapy, chemotherapy (i.e temozolomide), and corticosteroids, is considered inadequate to improve patients' clinical conditions and associated with many adverse effects. There is an urgent need for new compounds or repurposing of existing therapies, which could improve brain tumor patients' prognosis. Metformin, commonly used for type 2 diabetes medication, has been examined for its protective action in cancer, reducing cancer risk and cancer-related mortality. However, its effect on cancer is still in rigorous debate. This study examines recent studies on the effects of metformin in primary brain tumor patients through systematic reviews. The literature search was performed on PubMed, ScienceDirect, and SpringerLink databases for articles published between 2013 and 2020. We selected clinical studies comparing the therapeutic outcomes of brain tumor therapy with and without metformin. The clinical benefits of the drug were assessed through the overall survival (OS) and progression-free survival (PFS) of brain tumor patients. Those studies demonstrated that the combination of metformin with temozolomide given post-radiotherapy resulted in better OS and PFS. Nonetheless, the efficacy and safety of metformin need further clinical testing in the wider population.
Keywords: Antidiabetic, Antineoplastic, Primary brain tumor, Metformin, Survival
antidiabetic, antineoplastic, primary brain tumor, metformin, survival
1. Introduction
Tumors in the central nervous system (CNS) had an incidence rate of 23.41 cases per 100,000 people in the United States in 2012–2016 [1]. In 2016, at the global level, there were 330,000 incident cases of CNS cancer, with an age-standardized incidence rate of 4.63 per 100,000 person-years, which significantly increased by 17.3% between 1990 and 2016 [2]. A total of 85–90% of CNS tumors are brain tumors [3]. Primary brain tumors have a high mortality rate and are ranked first in cancers in terms of mortality in the 0–14 year old (31%) and 12–24 year old (22%) age groups in the UK, ahead of leukemia [4, 5]. Glioma is the largest primary tumor in the brain (60.9%), three-quarters of which are glioblastoma and astrocytoma [6].
Glioblastoma was the most malignant tumor (14.6% of all tumors and 48.3% of malignant tumors) and meningioma the most benign tumor (37.6% of all tumors and 53.3% of benign tumors) in the United States population in the period 2012–2016 [1]. In the Department of Neurology, Dr. Cipto Mangunkusumo Hospital, Indonesia, it was recorded that between 2011 and 2015, the majority of primary tumors were astrocytoma (47%), followed by meningioma (26%). Data from Dharmais Cancer Hospital, Indonesia, from 1993-2012 showed an incidence of brain tumors of 1% of all malignancies, with the majority being glioma (67%) and meningioma (16.3%) [6].
Although the prevalence of brain tumors compared to other types of cancer is small, they are very difficult to treat and cause serious mortality and morbidity [7, 8]. Therapeutic options used during the last few years were a joint treatment regimen, including temozolomide and radiotherapy. Even so, the prognosis remains very poor [9]. Tumor size can interfere with the respiratory center and severely affect patient survival, as brain tumors do not undergo metastasis. Therefore, the only reliable indicator in assessing brain tumors' clinical response is patient survival [10, 11]. High morbidity and mortality rates, as well as a poor choice of therapy, demand further research related to the clinical development of brain tumor sufferers [8]. One of the efforts being made in this area is research into the possibility of drug repurposing, namely the use of existing therapies known to be safe for new indications.
Metformin has been used as an antidiabetic due to its effectiveness and safety. Metformin is a biguanide group that can reportedly decrease the incidence of cancer in diabetes mellitus patients. For 40 years, biguanide has been used in oncological therapy to attempt at “metabolic rehabilitation” of patients with breast cancer, colorectal cancer, and stomach cancer. Currently, more than 100 clinical studies assessing the role of metformin as an anti neoplasm treatment and prevention of cancer [12]. However, there is not much research on brain tumors, and there has been no review article research into the effectiveness of metformin for brain tumors. On a molecular level, metformin's main effect as an anti neoplasm is through inhibition of phosphorylation oxidative in mitochondria and activation of AMPK (adenosine monophosphate activated protein kinase) [13].
A study from Aljofan & Riethmacher [14], which reviewed 12 studies on the mechanism of metformin as antineoplastic, revealed that metformin is adequate for inhibit various types of cancer in vitro and in vivo by a direct or indirect route. The studies reported several exciting potential mechanisms and external factors that may explain the metformin's antineoplastic effect. These mechanisms, including a direct act of metformin by targeting the AMPK pathway in tumor cells that control metabolism, angiogenesis, inflammation, and cancer stem cells or by inhibiting cancer growth and proliferation via a glucose related mechanism, which includes decreasing insulinemia and glycemia. Both the cancer cell sensitivity to metformin and the antineoplastic mechanism of actions of metformin stated were declared cell dependent. So, the different and sometimes conflicting mechanisms shown it could be due to the physiological differences between different cells. Nevertheless, all of the stated antineoplastic effect theories revolve around or are linked to AMPK activation, and the external factors are mainly glucose related. The antidiabetic activity of metformin shows an essential way of its antineoplastic activity and that it is likely to exert its antineoplastic action by using AMPK in cancer cells [14]. AMPK regulates expression and phosphorylation of p53, which is known plays a role in promoting apoptosis, autophagy, and antiproliferative effect [15]. Some cancer types have gene mutations p53, and primary brain tumors become tumors with the highest p53 mutations [13].
Research on repurposing metformin as an antineoplastic in brain tumors in recent years create an interesting area of study. Therefore a clinical review of the effect of metformin as an antineoplastic agent on brain tumors is highly necessitated. This systematic review of recent primary literatures was conducted to examine its role.
2. Method
2.1. Data collection
A literature search was conducted through PubMed, ScienceDirect, and SpringerLink databases to identify relevant articles, which were limited to ones published between 2013 and 2020. The keywords used were 'metformin', 'diabetes mellitus', 'brain tumor', 'glioma' and 'glioblastoma'. On the basis of the data, a systematic review of metformin as an antineoplastic in brain tumors was conducted.
2.2. Inclusion and exclusion criteria
The literature search focused on clinical studies of metformin for brain tumors. Studies were selected based on inclusion criteria defined using the PICO framework, namely Population, Intervention, Comparison, and Outcome, to determine relevant articles. In more detail:
Population: Patients aged 17 and above diagnosed with a brain tumor according to 2016 WHO classification, namely is a primary brain tumor, not a metastatic tumor.
Intervention: Use of metformin with or without a history of diabetes mellitus.
Comparison: Patients without metformin use.
Outcomes: 1. To establish the potential of metformin as an antineoplastic in brain tumor patients; and 2. To assess the clinical outcome of metformin use in brain tumor patients.
Exclusion criteria for article selection included, among others:
-
1.
Research articles published outside the period 2013–2020.
-
2.
Articles that were not original research.
-
3.
The research methods adopted were non-clinical studies.
-
4.
Articles were not full text, or the full text could not be accessed.
2.3. Search results and schemes
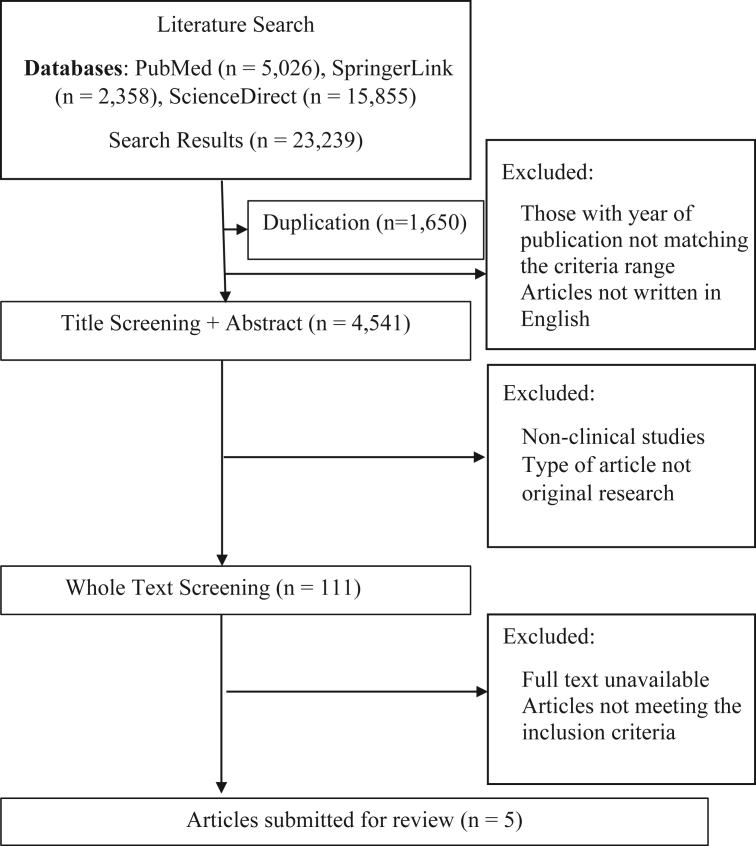
Based on the online databases' search results using predefined keywords, 23,239 research articles were identified, consisting of 5,026 from PubMed, 15,855 from ScienceDirect, and 2,358 from SpringerLink. After the screening process of these articles, five were judged to have met the specified criteria and were included in the subsequent review process. The search results are briefly described in Figure 1.
Figure 1.
Literature search scheme.
3. Results and discussion
3.1. Results
Brain tumors are very difficult to treat and cause serious mortality and morbidity [7, 8]. The p53 pathway plays a dominant role in their development; in addition, primary brain tumors have the highest p53 mutations. The regulation of p53 expression and phosphorylation is regulated by AMPK [15]. Unlike other tumors, brain tumors have a blood-brain barrier system that blocks the entry of some chemotherapy agents. In in vivo studies, metformin has been shown to be able to cross the blood-brain barrier [16]. The combination of prognosis, poor therapeutic choice, the dominance of the p53 pathway, and the potential for metformin as an antineoplastic prompt further studies regarding the repurposing of metformin as an antineoplastic in brain tumors.
AMPK activation induces p53 phosphorylation in Ser15 and promotes human MDMX phosphorylation on ser342, inhibiting p53 ubiquitination and p53 stabilization [17]. p53 plays a role in promoting apoptosis, autophagy, and antiproliferation [13]. Metformin is easily found on the market and is the first line for treating type 2 diabetes mellitus. However, there has been no systematic review of related clinical studies.
This review article has been compiled systematically to examine recent studies' development (2013–2020) regarding the effects of metformin in brain tumor patients through a clinical review. Systematic methods have been used to make it easier for readers to obtain information and to avoid bias. An article search through an online database resulted in 111 articles, five of which were selected according to the inclusion criteria set. A cohort study design was employed, which is commonly used to investigate the causes of disease and the relationship between risk factors and clinical outcomes. In such a study on repurposing metformin as an antineoplastic in brain tumors, cohort design is useful in determining the effects of metformin use and the clinical outcomes in the form of patient survival.
In this review, a population group is examined prospectively or retrospectively. The total population was 4,173 patients, with 278 receiving metformin, who came from several regions, including Germany and the USA. Their age was in the range of 17–90 years old. The clinical benefit of metformin was expressed in terms of overall survival (OS) and progression-free survival (PFS) values, which reflect patient survival. The OS has become a gold standard endpoint for clinical assessment of tumor treatment. Improvement in it clearly shows a significant clinical benefit for patients; PFS, depending on its magnitude, may also have a high value [18]. Moreover, through the articles selected, the value of dose-limiting toxicities (DLT) and maximum tolerated dose (MTD) of repurposing metformin as an antineoplastic was also established [19]. The studies on the development of repurposing metformin as an antineoplastic for brain tumors discussed are summarized in Table 1.
Table 1.
Studies on metformin as an antineoplastic for brain tumors.
| No. | Authors and year | Type of Study | Region | Subject Characteristics | Types of Brain Tumors | OS and PFS Values | Results |
|---|---|---|---|---|---|---|---|
| 1 | Maraka et al. [19] | Cohort | Austin, USA |
Total: 85 - Male: 54 - Female: 31 Ages: 21-77 |
A new diagnosis of glioblastoma | OS: 2 years; PFS: 6 months | Metformin can be safely combined with TMZ for newly-diagnosed glioblastoma patients. MTD of metformin in combination therapy with temozolomide: 850 mg twice daily. Effective dose of temozolomide used: 150–200 mg/m2. The most common DLTs with metformin are gastrointestinal disorders. |
| 2 | Seliger et al. [20] | Retrospective cohort | Three randomized prospective multicenter clinical trials (m CENTRIC, CORE, AVAglio) |
Total: 1,731 - Male: 1,026 - Female: 705 Ages: 18-84 |
A new diagnosis of glioblastoma | 1. At baseline, HR OS = 0.87; HR PFS = 0.84. 2. In the TMZ / RT period, HR OS = 0.97; HR PFS = 1.02. |
The use of metformin at baseline and metformin during the TMZ / RT period was not statistically associated with OS or PFS. Metformin has been indicated to produce better survival in patients on metformin monotherapy at baseline, but not in TMZ/RT. Other antidiabetics tend to result in worse survival Issues to consider when taking metformin: glioma condition and diabetes severity. |
| 3 | Seliger et al. [21] | Retrospective cohort | Population-based clinical cancer registry, Regensburg (patients from Lower Bavaria and Upper Palatinate, Germany) |
Total: 1,093 - Male: 619 - Female: 474 Ages: ≥ 18 |
HGG WHO grade III and HGG WHO grade IV | 1. OS patients HGG = 1.2 years: WHO grade III = 2.3 years: WHO grade IV = 1.0 year. 2. PFS patients HGG = 0.8 years: WHO grade III = 2.5 years: WHO grade IV = 0.7 years. |
OS and PFS of WHO HGG grade III patients taking metformin significantly longer, but not grade IV. |
| 4 | Adeberg et al. [9] | Retrospective cohort | Heidelberg University Hospital and German Cancer Research Center (DKFZ), Heidelberg, Germany |
Total: 276 - Male: 169 - Female: 107 Ages: 17.2–86.6 years |
Glioblastoma multiforme | 1. PFS of all patients = 6.77 months OS of all patients = 14.85 months. 2. PFS of patients on metformin therapy = 10.13 months: without metformin therapy = 4.67 months: reference group without diabetes = 6.7 months. |
PFS was significantly higher in diabetic patients on metformin therapy. The increase in PFS was significantly associated with concomitant use of temozolomide therapy. PFS decreased on corticosteroid therapy. |
| 5 | Welch and Grommes [22] | Retrospective cohort | Memorial Sloan Kettering Cancer Center, New York, USA |
Total: 988 (DM: 124) - Male: 86 - Female: 37 Ages: 29-90 |
Glioblastoma | 1. OS diabetic patients = 10 months: non diabetes = 13.4 months. 2. OS of steroid therapy patients = 9 months: without steroid therapy = 17 months. 3. OS metformin therapy patients = 10 months: other antidiabetic monotherapy = 6 months. 4. OS patients with glioblastoma-diabetes metformin therapy = 14 months: OS patients with other antidiabetic therapy = 8 months |
Metformin has the potential to improve the survival (OS) of glioblastoma patients. |
Data are shown in median (minimum-maximum) values. Note: OS = median overall survival; PFS = median progression free survival; TMZ = temozolomide; RT = radiotherapy; MTD = maximum tolerated dose; DLT = dose-limiting toxicities; HR = hazard ratio; HGG = high grade glioma; WHO = World Health Organization.
3.1.1. Patient survival as an indicator in assessing the clinical response to brain tumors
In recent years, research on metformin effect on brain tumors has been conducted. The size of the tumor can interfere with the respiratory center because intracranial pressure can aggravate the patient's symptoms, consequently impacting survival [10, 11]. Therefore, the aim of antineoplastic therapy, including metformin, is to reduce the tumor size in order to reduce symptoms and improve patient survival [8]. The only reliable indicator in assessing brain tumors' clinical response is patient survival [10, 11].
Clinical studies have been conducted to analyze patient survival based on progression-free survival (PFS) and overall survival (OS). PFS is the time from the initiation treatment until progress occurs, with either an improvement or worsening of the disease. This value can be used to measure direct clinical benefit based on the disease and observed response. Furthermore, OS represents the duration of patient survival from the time of the initiation of treatment. OS is universally accepted as a measure of clinical benefit. Improvement in OS shows a significant clinical benefit for patients, whereas PFS depends on its extent [18].
3.1.2. Glioblastoma as a most malignant brain tumor, and alternative therapies
The types of brain tumors that are widely targeted for testing are glioblastoma and high-grade glioma (HGG), which include primary brain tumors. The 2016 WHO classification indicates that glioblastoma is WHO grade IV. The population involved in this systematic review was patients with glioblastoma (WHO grade IV) and WHO grade III. A total of four articles used a glioblastoma population, and one used WHO grade III and IV HGG populations, with or without IDH mutations.
Glioblastoma is the most malignant brain tumor in adults and cannot be cured [6].The treatment option used in recent years has been a combined regimen, including temozolomide and radiotherapy. Despite this, the prognosis remains very poor [9]. The growth nature of gliomas makes surgery ineffective. Therefore, emphasizes the need for new therapies as alternative options [16].
3.1.3. Confirmation of antineoplastic activity of metformin
Preclinical studies report that metformin has potential as an antineoplastic. Its mechanism works through antiproliferative effects, the introduction of apoptosis, and reduction of angiogenesis through AMPK activation, mTOR inhibition, and reduction of inflammatory cytokine production [13, 14, 17]. Out of the various metformin mechanisms as an antineoplastic, the most discussed downstream effector is AMPK [13, 15].
External factors glucose-related also influence the effects of metformin as antineoplastic. More glucose needs of tumor cells are met in hyperglycemia conditions. The characteristic of tumor cells is to produce energy through aerobic glycolysis or known as Warburg effect [23].
3.1.4. Antineoplastic action of metformin through AMPK activation
In many tumor cells, AMPK activation is known to induce antiproliferative effects. Glucose deprivation may temporarily inhibit this activity but is very unlikely to be of a significant impact for some reasons. Earliest, the Warburg effect was suggested in some instances to be a temporary effect. Later on, prolonged glucose deprivation can induce an inflammatory response and increase ROS production, thus eventually damaging cell membrane and nucleic acids. Possibly, a low glucose-induced rise in ROS will rather increase cellular apoptosis, thus contributing further to metformin's antitumor activity. This theory has been backed and supported by some studies that confirmed that the combination of metformin and glucose withdrawal were quite lethal to tumor cells [14].
AMPK activation promotes phosphorylation of human MDMX on ser342, which inhibits p53 ubiquitination and stabilization. p53 is a core component in regulating growth and survival under various stresses and is the dominant pathway in brain tumors. The molecular markers in most glioblastomas are mutations in three main pathways: the p53 pathway, the PI3K pathway, and the retinoblastoma pathway [6]. p53 plays a role in promoting apoptosis, autophagy, and inhibition of the Akt and mTOR pathways [17]. Furthermore, mTOR inhibition can interfere with protein synthesis and suppress tumor cell proliferation [17]. P53 will detect DNA damage, which then activates p21 to inhibit cyclin B, further interfering with the start of the tumor cell cycle. P53 also inhibits progression from the S-phase to the M-phase, which means tumor cell mitosis is prevented [6].
3.1.5. Metformin has the potential to improve glioblastoma patients survival
A study of the effects of metformin on a population of 276 German primary and secondary glioblastoma patients showed progress in prolonging the survival of those with diabetes. The increase in PFS was significantly associated with the methylated MGMT gene promoter status and simultaneously with temozolomide therapy. Comparing the PFS of diabetic patients with metformin therapy and without metformin therapy and those without diabetes was 10.13 months: 4.57 months: 6.7 months. However, lower PFS rates were observed with continued therapy with corticosteroid use [9].
The same benefit was shown in a study involving 988 primary glioblastoma patients in the USA. Research by Welch and Grommes [22] showed that metformin can improve the survival (OS) of glioblastoma patients. The OS ratio of patients with metformin therapy and antidiabetic monotherapy was 10 months: 6 months. Comparing metformin administration to diabetic-glioblastoma patients who had received surgery, radiotherapy, and chemotherapy and those with other antidiabetic administration was 14 months: 8 months [22].
3.1.6. Corticosteroid therapy and hyperglycemia are negative factors
The two studies conducted on glioblastoma patients discussed above show that metformin therapy in primary and secondary glioblastoma patients in both the German and US populations provided the benefit of the prolonged OS. That is, metformin has the potential to improve patient survival. Patients with diabetes got benefit even more. The condition is specifically associated with hyperglycemia, which can affect tumor progression. Corticosteroid therapy and hyperglycemia are negative factors relevant to the survival of glioblastoma patients [9]. Steroid use should be minimized to improve glycemic control and improve survival [22]. To achieve the expected therapeutic goals, namely reducing tumor size to reduce symptoms and improve the survival of brain tumor patients, metformin can be used in conjunction with temozolomide. Moreover, the effectiveness of therapy can be supported by minimizing the use of corticosteroids.
In addition, a study of 85 newly diagnosed glioblastoma patients in Austin, USA, showed that metformin could be safely combined with temozolomide, with a median survival of 2 years. Common side effects of using metformin include gastrointestinal disturbances [17].
3.1.7. High glucose levels may also affect the action of metformin
On the other hand, a study of 1,731 patients with newly diagnosed glioblastoma forming a population derived from three randomized prospective multicenter clinical trials (m CENTRIC, CORE, and AVAglio) obtained different results, that metformin was not significantly associated with OS or PFS in these patients. In other words, metformin did not prolong the survival of patients with newly diagnosed glioblastoma [20]. The different results of this study were possibly due to several disparate conditions:the population used, the clinical trial population, primary and secondary glioblastoma, and including the type of oncologic treatment regimen [9, 20, 22]. Furthermore, differences in outcome may have also occurred due to different timing and doses of metformin administration (together with or after radiotherapy).
From the studies conducted on the effect of metformin on glioblastoma, either with a new diagnosis or not stated, there is good potential for metformin to prolong patient survival. However, a study from Seliger et al. [20] obtained different results because the study included large population which only 7% of the used metformin. It makes the study results less representative and not comparable in assessing metformin as medication for glioblastoma [20].
Another parameter that distinguishes this study is the patient's glucose levels. The population had a median glucose level of 105 mg/dL (including fasting and non-fasting glucose levels) [20], lower than the median glucose level of patients in the other studies 198.5 mg/dL [9, 21, 22]. High glucose levels may also affect the action of metformin, which is an antidiabetic and antineoplastic, both of which are known to affect AMPK signaling activation. The activation of AMPK by metformin may result in better survival rates among brain tumor patients.
3.1.8. IDH mutation factors influence metformin therapy in tumors
A study of 1,093 WHO HGG grade III and grade IV patients in Germany demonstrated that OS and PFS were longer in grade III patients with the use of metformin but not in grade IV patients [21]. Important prognostic factors in HGG are age, status based on KPS, tumor grade, IDH status, and several genetic, molecular factors such as telomerase reverse transcriptase (TERT) and the extent of surgery [6]. From these prognostic factors, this study shows that the use of metformin in tumors is influenced by IDH mutation factors [21].
IDH 1 and 2 enzyme mutations occur in approximately 50% of WHO grade III patients and 5–10% of glioblastoma patients (WHO grade IV), and is a significant predictive factor for OS. The presence of IDH 1 and IDH 2 mutations in HGG showed a 2-3x increase in life expectancy compared to tumors with IDH-wildtype. The effect of metformin on OS prolongation in WHO grade III patients was associated with IDH mutations. It is known that IDH 1 and 2 mutations occurred in 54 patients, five of whom were using metformin. Patients with IDH mutations who use metformin have better survival rates. Nonetheless, these results suggest that OS and PFS are low in WHO grade IV (glioblastoma) patients. This result is different from previous studies. It may be because the multicenter population of the previous studies began in 2013, and no standard therapy for HGG had been established. Therefore, standard therapy in the form of resection, radiotherapy, or chemotherapy was not discussed in this study [21].
All the selected studies show that there is potential for metformin as an antineoplastic in brain tumor patients included malignant brain tumors (glioblastoma and WHO grade III). Apart from hyperglycemia, metformin in conditions of IDH mutation is also considered to produce more favorable results. IDH mutation is common in secondary glioblastoma and WHO grade III cases. In one study, there was an increase in the median life expectancy of glioma patients with IDH 1 mutations compared to those without [6]. It used metformin as an adjuvant to standard therapies of temozolomide. Therefore, repurposing metformin as an antineoplastic is considered to help improve the survival of brain tumor patients, especially those with malignant brain tumors,glioblastoma and HGG.
3.2. Discussion
Metformin is known to be safe for wide use wide by the public, especially diabetes mellitus patients, with affordable price [24]. Therefore, repurposing it as an antineoplastic in brain tumors is more economical than using existing therapies with good safety records. Research has also shown that metformin can cross the blood-brain barrier, which increases the potential for it to be used as a new antineoplastic in brain tumors [25].
The action mechanism of metformin as an antineoplastic provides apoptotic, autophagic, and antiproliferative effects through the p53 pathway with AMPK activation. It activation will phosphorylate p53 and promote the phosphorylation of human MDMX, which inhibits p53 ubiquitination and p53 stabilization. p53 plays a role in promoting apoptosis, autophagy, and inhibition of the Akt and mTOR pathways. Inhibition of mTOR interfereprotein synthesis and suppress tumor cell proliferation [17].
The metformin antineoplastic activity is also influenced by glucose levels on the extracellular environment. AMPK activation in normal glucose levels be able to induce antiproliferative effects. The viewed survival in low-level glucose may be caused by the Warburg effect where tumor cells reprogram their metabolism. Some studies have shown the metformin with glucose deprivation is deadly for tumor cells. Glucose deprivation improve the antitumor effect of metformin in inducing AMPK activation [14, 23].
The studies that have been conducted have assessed the clinical outcome of the benefits of using metformin via OS and PFS [9, 19, 20, 21, 22]. These assessments, especially OS, adequately illustrate the clinical benefit of prolonging survival in primary or secondary glioblastoma patients. Nonetheless, one recent study from Seliger, et al. [20] obtain that the use of metformin at baseline and metformin during the TMZ / RT period was not statistically associated with OS or PFS. Differences in metformin dosage, length of metformin use (after, or in conjunction with, radiotherapy), and differences in the type of population studied could be caused in the result variation.
Metformin dosage can affect the mechanism of metformin. As an antineoplastic, at high doses (~mM), metformin inhibits complex I of the mitochondrial electron transport chain, while low dose metformin (~μM) can activate AMPK through the lysosomal pathway, independent of the AMP/ATP ratio [26]. Not all the studies indicate the metformin dosage used. However, in experimental studies, the dose of metformin as an antineoplastic was much higher than as an antidiabetic [20]. There were no case reports of hypoglycemia on the use of metformin as an antineoplastic [19, 26]. However, further research is needed to determine how effective the metformin dose is as adjuvant therapy for brain tumors.
Apart from the therapeutic dose, the period of use of metformin is also a determining factor. Metformin is considered to have a synergistic effect with temozolomide, but not with the use of corticosteroids [9, 22]. Concomitant use with temozolomide therapy provides better survival, while corticosteroid use worsens survival. The use of corticosteroids should be minimized because they can affect the patient's blood glucose levels, impacting tumor progression, and inhibiting metformin activity. The maximum recommended dose for metformin in combination with temozolomide is 850 mg twice a day. The recommended dose of temozolomide is 150–200 mg/m2 [19]. In the articles reviewed, this combination of metformin and temozolomide was given post-radiotherapy. However, it is known that side effects may occur with the use of metformin as an antineoplastic, such as disorders of the gastrointestinal system.
In recent years, research on metformin as an antineoplastic agent in brain tumors has been conducted. However, from the many types of brain tumors that exist, the scope of study is still fairly limited to certain types of tumors, including glioblastoma (WHO grade IV) and WHO grade III. Although these types of tumors have high prevalence and malignancy, research concerning other types of tumors is also necessary. Research is yet to be conducted for brain tumors that are commonly found in Indonesia, astrocytoma and meningioma. However, there are many cases of brain tumors and p53 mutations in Indonesia. Different types of brain tumors, as well as racial differences, may imply differences in the therapeutic dose of metformin for patients in Indonesia compared to the brain tumor patients studied in Germany and the USA. Therefore, further research needs to be conducted on astrocytoma, meningioma, and other types of brain tumors.
Apart from the type of tumor, the populations studied are also quite limited. The study does not cover all continents and countries. Thus, further research is needed with wider populations to better assess metformin's efficacy and safety for brain tumors.
3.3. Limitations
This review article provides systematic information about metformin as an antidiabetic and its role as an antineoplastic in brain tumors, which was previously unavailable to clinical studies. However, there are also some limitations in the study, including the fact that the population involved is homogeneous (it only comes from a few regions and does not cover all continents and countries); the types of brain tumor studied are limited, and the percentage of subjects receiving metformin is relatively low.
4. Conclusion
In writing this systematic review, we conclude that metformin has a prolonged OS and PFS survival effect on brain tumor patients, exerting a synergistic effect combined with temozolomide. This combination therapy was given after radiotherapy. The mechanism of metformin as an antineoplastic involves AMPK activation, which induces p53 phosphorylation and human MDMX phosphorylation, which inhibit p53 ubiquitination and p53 stabilization, resulting in apoptosis, autophagy, and antiproliferation processes.
Declarations
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data included in article/supplementary material/referenced in article.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
This study was supported by PDUPT Grant, Ministry of Research and Higher Education, Republic of Indonesia, Indonesia No. NKB-94/UN2.RST/HKP.05.00/2020. We also would like to acknowledge Apriliana Cahya Khayrani for helping us in editing the manuscript.
References
- 1.Ostrom Q.T. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2012-2016. Neuro Oncol. 2019;21:V1–V100. doi: 10.1093/neuonc/noz150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patel A.P. Global, regional, and national burden of brain and other CNS cancer, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18(4):376–393. doi: 10.1016/S1474-4422(18)30468-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnson D.R., Guerin J.B., Giannini C., Morris J.M., Eckel L.J., Kaufmann T.J. 2016 updates to the WHO brain tumor classification system: what the radiologist needs to know’. Radiographics. 2017;37(7):2164–2180. doi: 10.1148/rg.2017170037. [DOI] [PubMed] [Google Scholar]
- 4.McNeill K.A. Epidemiology of brain tumors. Neurol. Clin. 2016 doi: 10.1016/j.ncl.2016.06.014. [DOI] [PubMed] [Google Scholar]
- 5.Gittleman H.R. Trends in central nervous system tumor incidence relative to other common cancers in adults, adolescents, and children in the United States, 2000 to 2010. Cancer. 2015 doi: 10.1002/cncr.29015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Neuro-Oncology Study Group . Indonesian Sociey of Neurologist (PERDOSSI); Jakarta: 2019. Book of Teaching Neuro-Oncology. [Google Scholar]
- 7.Chun A.L. Breaking down brain cancer. Nature. 2018 [Google Scholar]
- 8.J Strong M., Garces J. Brain tumors: epidemiology and current trends in treatment. J. Brain Tumors Neurooncol. 2016;1(1):1–21. [Google Scholar]
- 9.Adeberg S. Metforminbeeinflusst die Progression bei diabetischen Glioblastompatienten. Strahlentherapie und Onkol. 2015;191(12):928–935. doi: 10.1007/s00066-015-0884-5. [DOI] [PubMed] [Google Scholar]
- 10.Deanna Glass-Macenka E.W., Hays Lora, Varner Ashley. Frankly Speak. About Brain Tumors; 2013. Understanding Brain Tumors. [Google Scholar]
- 11.McKinney P.A. 2004. Brain Tumours: Incidence, Survival, and Aetiology; pp. 12–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Biswas A.K., Khan M.A., Alam S., Choudhary A.K. ‘Metformin – the newer role of the old medicine - in cancer prevention and treatment! 2017;6(6):130–134. [Google Scholar]
- 13.Saini N., Yang X. Metformin as an anti-cancer agent: actions and mechanisms targeting cancer stem cells. Acta Biochim. Biophys. Sin. 2018;50(2):133–143. doi: 10.1093/abbs/gmx106. [DOI] [PubMed] [Google Scholar]
- 14.Aljofan M., Riethmacher D. Anticancer activity of metformin: a systematic review of the literature. Futur. Sci. OA. 2019;5(8) doi: 10.2144/fsoa-2019-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kasznicki J., Sliwinska A., Drzewoski J. Metformin in cancer prevention and therapy. Ann. Transl. Med. 2014;2(6):1–11. doi: 10.3978/j.issn.2305-5839.2014.06.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Molenaar R.J. Study protocol of a phase IB/II clinical trial of metformin and chloroquine in patients with IDH1-mutated or IDH2-mutated solid tumours. BMJ Open. 2017;7(6):1–12. doi: 10.1136/bmjopen-2016-014961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li M., Li X., Zhang H., Lu Y. Molecular mechanisms of metformin for diabetes and cancer treatment. Front. Physiol. 2018;9(July):1–7. doi: 10.3389/fphys.2018.01039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hess L.M., Brnabic A., Mason O., Lee P., Barker S. Relationship between progression-free survival and overall survival in randomized clinical trials of targeted and biologic agents in oncology. J. Cancer. 2019;10(16):3717–3727. doi: 10.7150/jca.32205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maraka S., Groves M.D., Mammoser A.G., Melguizo-Gavilanes I., Conrad C.A., Tremont-lukats I.W.…Penas-Prado M. Phase 1 lead-in to a phase 2 factorial study of temozolomide plus memantine, mefloquine, and metformin as postradiation adjuvant therapy for newly diagnosed glioblastoma. HHS Publ. Access. 2020;125(3):424–433. doi: 10.1002/cncr.31811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seliger C. Use of metformin and outcome of patients with newly diagnosed glioblastoma: pooled analysis. Int. J. Cancer. 2020;146(3):803–809. doi: 10.1002/ijc.32337. [DOI] [PubMed] [Google Scholar]
- 21.Seliger C. Use of metformin and survival of patients with high-grade glioma. Int. J. Cancer. 2019;144(2):273–280. doi: 10.1002/ijc.31783. [DOI] [PubMed] [Google Scholar]
- 22.Welch M.R., Grommes C. Retrospective analysis of the effects of steroid therapy and antidiabetic medication on survival in diabetic glioblastoma patients. 2013;2:237–246. doi: 10.2217/cns.13.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Devic S. Warburg effect - a consequence or the cause of carcinogenesis? J. Canc. 2016 doi: 10.7150/jca.14274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang W., Castelino R.L., Peterson G.M. Metformin usage in type 2 diabetes mellitus: are safety guidelines adhered to? Intern. Med. J. 2014 doi: 10.1111/imj.12369. [DOI] [PubMed] [Google Scholar]
- 25.Łabuzek K., Suchy D., Gabryel B., Bielecka A., Liber S., Okopień B. Quantification of metformin by the HPLC method in brain regions, cerebrospinal fluid and plasma of rats treated with lipopolysaccharide. Pharmacol. Rep. 2010;62(5):956–965. doi: 10.1016/s1734-1140(10)70357-1. [DOI] [PubMed] [Google Scholar]
- 26.Yi Y., Zhang W., Yi J., Xiao Z. Role of p53 family proteins in metformin anti-cancer activities. J. Cancer. 2019;10 doi: 10.7150/jca.30659. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supplementary material/referenced in article.