Abstract
This study compared the chemical properties of the indigenous Nigerian soaps with the conventional soaps in order to determine whether or not they met acceptable standards. The locally made soaps were obtained from markets in Ile-Ife, Osun State and Okitipupa, Ondo State, Nigeria. The soap samples were acid digested and trace metals (Cd, Pb, Cu, Zn, and Hg) in the digested samples were profiled using Atomic Absorption Spectrometry. Documented techniques were adopted to analyze the soaps for pH, moisture content, free fatty acid, chloride content, free caustic alkali, matters insoluble in water and ethanol. The margin of safety (MoS) and hazard index (HI) associated with the use of the soaps were also evaluated. The locally made soaps had higher physicochemical properties than the conventional soaps. Mercury (Hg) had the highest concentration in the locally made soaps ranging from 106.50 ± 0.23–273.58 ± 0.49 μg/g and 46.35 ± 0.22–55.12 ± 0.65 μg/g in the conventional soaps, while Cd had the least concentration in the locally made soaps ranging from 2.95 ± 0.45–6.05 ± 0.60 μg/g and 2.88 ± 0.11–5.20 ± 0.60 μg/g in the conventional soaps. Although highly mercuric soaps are known to kill bacteria and fungi, the observed MoS (<100) and HI (>1) indicated that the soaps might be safe if only restricted to occasional use by adults and children. A careful preliminary investigation and selection of the raw materials used in the production of indigenous soaps should be considered a necessary step.
Keywords: Cocoa pods, Metals, Palm bunches, Physicochemical properties, Soaps
Cocoa pods; Metals; Palm bunches; Physicochemical properties; Soaps.
1. Introduction
Soaps are surfactants made of natural products and represent a part of the requisite cleansing products used for domestic processes. They are required in the removal of germs, contaminants and dirt. Soaps are produced from the saponification of oil with alkali. Commonly used oils in Nigeria include coconut oil, lard, marine oil, palm kernel oil and so on. Presently, palm kernel oil and palm oil are the most widely used.
Indigenous Nigerian soap has been in use in many African countries for centuries. It has over the time translated to African black soap because of its widespread acceptance and use for bathing and general skin care where treatment of skin rashes, body odours, irritations, acne, oily skin, eczema and dermatitis, among others, are to be taken care of. Women, especially among the teeming rural populations, also use black soap on babies because of its purity and gentleness on sensitive skin and for skin care during and after pregnancy to treat skin conditions caused by hormonal changes (https://www.byrdie.com). The origin of indigenous Nigerian soap could be traced to the Yoruba people and communities in Nigeria, Benin Republic and Togo where the black soap is called “ose dudu” (https://www.byrdie.com).
Indigenous black soap is made from ashes obtained from dried agricultural wastes, such as plantain peels, cocoa pods, palm tree bunches, shea butter tree bark, and a combination of vegetable oils, such as palm oil, palm kernel oil, shea butter, coconut oil, or cocoa butter. The agricultural waste of interest is roasted in an earthen pot to produce ash which contains the alkali. Water is added to the ashes to dissolve the alkali, thoroughly stirred and filtered. A vegetable oil, such as shea butter, coconut oil, palm kernel oil, or cocoa butter is heated to high temperatures and added with vigorous stirring from time to time over a 24-hour period. The soap that is formed through saponification solidifies and moves to the top where it is scooped out, and allowed to cure for about two weeks.
Some cosmetic companies, such as Dudu Osun (in Nigeria), often rely heavily on black soap as their major raw material to which additives (natural ingredients and odoriferous oils) are added to improve the commercial appeal and germicidal effects of the packaged soap. Color cosmetics such as eye shadows and lipsticks, hair products such as sprays and shampoos, and skin care products such as soaps and creams, all belong to the group called “cosmetics”. Cosmetics is defined as “any article intended to be rubbed, poured, sprinkled or sprayed on, or introduced into or otherwise applied to the human body or any part thereof for cleansing, beautifying, promoting attractiveness, or altering the appearance, and includes any article intended for use as a component of cosmetics” (Onojah and Emurotu, 2017). Several harmful effects on the well-being of the users, such as photoreactions, sensitization, skin irritation and allergy, are associated with the use of cosmetics as a result of the different proportions of substances present in them and differences in individual user's response. Examples of such substances include heavy metals, formaldehyde, parabens, p-phenylenediamine, triethanolamine, and phthalates (Borowska and Brzoska, 2015; Janeckova et al., 2019; Swierczek et al., 2019).
Today, cosmetics that contain natural ingredients are considered healthier and ecological, and they seem to be on high demand by consumers. The devastating effects of synthetic surfactants, the need for products that are more environmentally friendly and sustainable are being emphasized; therefore, consumers keep complaining about the use of synthetic chemicals in the production of cosmetics and beauty products. Soaps made from natural substances such as plant extract or essential oils are prepared without the use of a non-natural surfactant (Antignac et al., 2011; Hayati et al., 2020).
Physicochemical properties such as pH, free caustic alkali, moisture content, chloride content, residual glycerol and trace elements composition are important in the assessment of the quality of soap. These parameters are dependent on the kind of oil used, the purity and strength of alkali, the completeness of saponification (Roila et al., 2001; Issa et al., 2020) and the sources of the raw materials used for soap production.
The various chemical substances used in the formulation of soaps and other cosmetics have various levels of toxicity effects on humans and environment ascribed to them (Madsen et al., 2001; Tjandraatmadja et al., 2010; Soylak et al., 2013). Therefore, the use of cosmetics represents a potential source of human exposure to these chemical substances (Squance et al., 2015; Iwegbue et al., 2016; Sani et al., 2016). However, the effect of this incessant exposure and the snowballing interactions on health risk assessment are not properly understood (Squance et al., 2015). The trace metal contents of soaps are of particular health and environmental concerns as a result of various exposure pathways ranging from domestic effluents into the environment to dermal contact with the soaps during bathing to direct exposure during washing (Iwegbue et al., 2019).
This study would highlight areas of competitiveness, deficiencies and possible advantages of the locally made soaps with the generally acceptable ones. It is therefore important to assess the compatibility of Nigerian locally made soaps and the conventional soaps with acceptable standards, hence this study.
2. Materials and methods
2.1. Sample collection
The samples for this study were black soaps popularly called “ose dudu”. They are often made from cocoa pods, palm bunches and banana stems. In this work, two types of black soaps, those made from cocoa pods and palm bunches, were obtained from local markets within Ile-Ife, Osun State and Okitipupa area of Ondo State. Two household conventional soaps, Lux and Joy, were also obtained from shops in Ile-Ife, to serve as standards. Samples A1, A2, and A3 were labelled as soaps made from cocoa pods, B1, B2, and B3 were labelled as soaps made from palm bunches, while C1 and C2 were Lux and Joy soaps respectively.
2.2. Digestion of samples
For each sample, 0.5 g soap was weighed and placed in a quick-fit 50 mL refluxing flask. To this was added 5 mL HNO3 and refluxing was carried out using the quick-fit set of apparatus for approximately 1½ hours. Thereafter, 1 mL HClO4 and 1 mL HF acids were added and further digestion was done for about 30 min. Solution of each of the digested sample was quantitatively transferred into a 25 mL volumetric flask and made up to the mark with doubly distilled water. This was stored in a small pretreated plastic container and labelled in readiness for trace metal profiling. A blank was also prepared. Trace metals in the samples were analysed using Atomic Absorption Spectrophotometry (AAS, Bulk Model 205).
2.3. Determination of physicochemical properties
The physicochemical properties determined in this study include total free caustic alkali, free fatty acid, levels of chloride as AgCl, matters insoluble in water, matters insoluble in ethanol, moisture content, and pH. The methods used in the determination of the physicochemical properties are described below.
2.3.1. Total free caustic alkali
0.25 g of each samples was weighed and dissolved in 25 mL of distilled water. Activated charcoal was added and boiled in thermostated heating mantle for 30 min at 100 °C. The heated mixture was filtered and the beaker rinsed with distilled water. The filtrate was made up to 100 mL mark. 20 cm3 of the filtrate was titrated against 0.25 M HNO3 acid. A blank titration was also carried out by titrating 20 cm3 of distilled water against 0.25 M HNO3. Free caustic alkali content was evaluated from the relationship in Eq. (1) (Panda, 2011):
| [1] |
where V = volume of alkali solution (mL)
N = normality of alkali solution
F = 4.7 for potassium oxide (K2O)
W = sample weight (g)
2.3.2. Free fatty acid
A 1-g soap sample was dissolved in 25 mL of ethanol and filtered. The filtrate was heated to incipient boiling and 0.5 mL of 1% phenolphthalein indicator solution was added and titrated against 0.1 M NaOH solution. Free fatty acid content was evaluated from the relationship in Eq. (2) (Panda, 2011):
| [2] |
where V = volume of acid solution (mL)
N = normality of acid solution
F = 28.3 for oleic acid
W = sample weight (g)
2.3.3. Determination of chloride as AgCl
This involved dissolving 0.5 g of soap sample in 100 mL of water followed by filtration. Thereafter, 0.5 mL of concentrated HNO3 was added to the filtrate and 0.1 N AgNO3 solution was added slowly with constant stirring until no precipitate further forms. The determination was carried out under subdued light. The precipitate was heated to about 85 °C and a few drops of AgNO3 solution were added. The beaker was allowed to stand for 1 h. The precipitate was then filtered using a properly dried filter paper with known mass. Hence, the mass of AgCl was determined using the relationship in Eq. (3):
| [3] |
where A = volume of silver nitrate solution required by the sample (mL)
B = volume of silver nitrate solution required by the blank (mL)
N = normality of silver nitrate
W = weight of sample (g)
2.3.4. Determination of matters insoluble in water
A known amount of soap sample (between 2 and 5 g) was weighed and dissolved in about 50 mL of distilled water. The solution was filtered using a filter paper already dried to a constant weight (W1) at 80–100 °C. The filter paper and the residue were put in the oven and dried again to a constant weight (W2) to determine the percentage matter insoluble in water, using the relationship in Eq. (4) (Onyango et al., 2014):
| [4] |
where W2 = weight of dried filter paper + dried residue
W1 = weight of dried filter paper
W = weight of soap sample used
2.3.5. Determination of matters insoluble in ethanol
Also, a known amount of soap sample (between 2 and 5 g) was weighed and dissolved in about dissolved in about 50 mL of ethanol. The solution was filtered using a filter paper already dried to a constant weight (W1) at 80–100 °C. The filter paper and its content (residue) were put in the oven and dried again to a constant weight (W2) to determine the percentage matter insoluble in ethanol, using the relationship in Eq. (5) (Onyango et al., 2014):
| [5] |
where W2 = weight of dried filter paper + dried residue
W1 = weight of dried filter paper
W = weight of soap sample used
2.3.6. Determination of moisture content
A known mass of each sample of soap was placed in a properly dried paper of known weight, and dried in the oven at 80–100 °C to a constant weight. The moisture content was then determined using the relationship in Eq. (6) (Owoicho 2021):
| [6] |
where W2 = weight of dried paper + soap sample
W1 = weight of dried paper + dried soap sample
W = weight of soap sample used
2.3.7. Determination of pH
For each soap sample, 2 g was dissolved in 20 mL of distilled water and filtered. The filtrate was taken immediately for determination on a pH meter (Model 4330).
2.4. Evaluation of exposure risk to humans
The uncertainty factor termed “margin of safety” (MoS) was used to determine the potential human health risks emanating from exposure to metals in the soaps. The MoS is obtained by normalizing “the lowest no-observed adverse-effect-level (NOAEL) value of the metals” with their corresponding estimated systemic exposure dosage (SED) as shown in Eq. (7) (SCCS, 2012):
| [7] |
The SED was evaluated by using the expression in Eq. (8) (SCCS, 2012):
| [8] |
where Cs means the concentration of metal (μg/g) in the soap; AA represents the daily quantity of soap used (20 g); SSA means the exposed skin surface area with the soap (860 cm2); F represents the application frequency (2); RF means the retention factor (0.01); BF denotes the bioaccessibility factor; the unit conversion factor is 10−3; and BW means the body weight of humans (adults: 70 kg; children: 16 kg). The ages of the adults and children were assumed to be 55 years and 14 years respectively.
The NOAEL values of the metals were obtained by using the expression in Eq. (9) (SCCS, 2012):
| NOAEL = RfD × UF × MF | [9] |
where RfD means the daily exposure amount to the populace (Pb:4 × 10−3, Cu:4 × 10−2, Hg:3 × 10−4, Zn:3 × 10−1, and Cd:1 × 10−3); UF means the uncertainty factor (100); and MF means the modifying factor (1). The minimum acceptably safe level for a human skin product is estimated to have a MoS value of 100. In this study, we assumed 50% (as the midpoint scenario) and 100% (as the worst-case scenario) systemic availability of the investigated metals in evaluating the safety of these soaps (SCCS, 2012).
3. Results and discussion
3.1. Physicochemical properties of the soaps
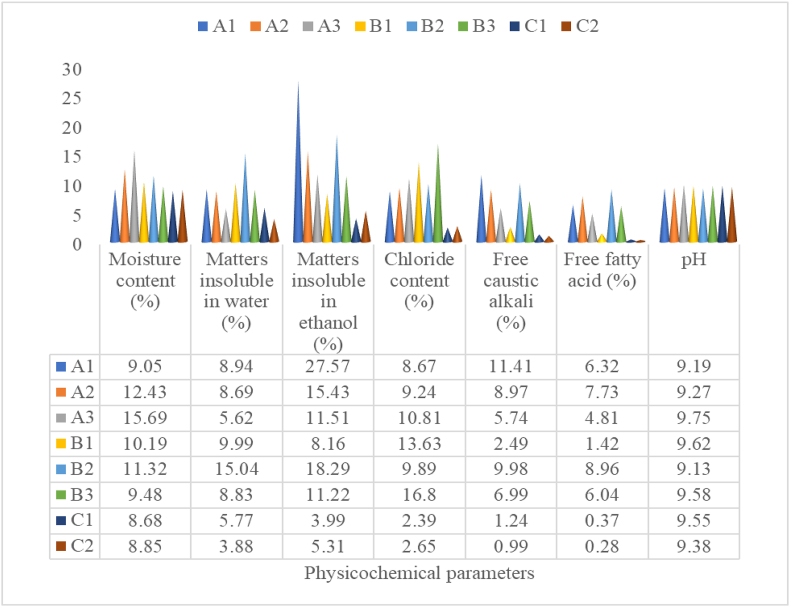
The percentage composition of physicochemical properties of the three categories of soaps is presented in Figure 1. The physicochemical properties of the soaps were determined in triplicates. The coefficient of variation was generally less than 20% indicating that little dispersion existed in the replicate analysis of the physicochemical properties. Minimal variations in the physicochemical properties existed among the same kind of soaps.
Figure 1.
Physicochemical Properties of the Soaps. A1, A2, A3 = soaps made from cocoa pods. B1, B2, B3 = soaps made from palm bunches. C1 = Lux, C2 = JOY.
3.1.1. Moisture content (%)
The shelf-life of a product can be assessed by its moisture content. The moisture content of the locally made soaps from cocoa pods ranged from 9.05 ± 0.22 to 15.69 ± 0.25 % while it ranged from 9.48 ± 0.15 to 11.32 ± 0.61 % in the locally made soaps from palm bunches. The moisture content in the locally made soaps were higher than that of Lux and Joy soaps with moisture contents of 8.68 ± 0.23 and 8.85 ± 0.35 % respectively. This indicated that the soaps from cocoa pods had significantly higher water retention capacity than those made from palm bunches as well as the conventional soaps. However, the moisture content of the soaps reported in this study falls within the limits (10–15%) of Encyclopedia of Industries Chemical analysis. Hence, storage of the soaps over a period of 1–2 years might not cause water induced deteriorations and the soaps are considered safe for bathing and other domestic purposes (Onyango et al., 2014). The % moisture content of the studied soaps were lower than the findings of earlier reports (Ogunsuyi and Akinnawo, 2012; Osuji et al., 2013). A relative upsurge in the moisture content of soaps may translate to a corresponding upsurge in free fatty acid levels, facilitated by the reaction of unsaponified fat with excess water in the soap to produce glycerol and free fatty acid, a phenomenon described as soap hydrolysis (Mahesar et al., 2019).
3.1.2. Matters insoluble in water (%)
The % matters insoluble in water of the soaps made from cocoa pods ranged from 5.62 ± 0.55 to 8.94 ± 1.02 % while it ranged from 8.83 ± 0.62 to 15.04 ± 1.51 % in the soaps made from palm bunches. Conventional Lux and Joy soaps had 5.77 ± 0.29 and 3.88 ± 0.61 % matters insoluble in water respectively. The locally made soaps had relatively higher levels of matters insoluble in water than the conventional soaps. The studied soaps had % matters insoluble in water above the acceptable levels (≤0.50) of the Standard Organization of Nigeria. This indicated that the soaps probably contained some sort of waxes and fats that are insoluble in water. The results presented in this study, however, compared favourably with those reported by Nangbes et al. (2014). To an extent, matter insoluble in water affects the lathering effects of soap as more soaps will be consumed with higher levels of matter insoluble in water.
3.1.3. Matters insoluble in ethanol (%)
The quantity of builders, fillers, whitening agents, bleaches, and fluorescing agents in the finished product is expressed as the matters insoluble in ethanol (Issa et al., 2020). The % matters insoluble in ethanol of the locally made soaps from cocoa pods ranged from 11.51 ± 0.67 to 27.57 ± 1.29 % while it ranged from 8.16 ± 0.24 to 18.29 ± 1.62 % in the locally made soaps from palm bunches. Lux and Joy soaps had 3.99 ± 0.51 and 5.31 ± 0.29 % matters insoluble in ethanol respectively. These levels were higher than the acceptable limits of the Standard Organization of Nigeria (≤2.00) as well as the findings of Nangbes et al. (2014). This indicated that the soaps probably contained ethanol insoluble fats, kaolin, sodium silicate and so on.
3.1.4. Chloride content (%)
Chloride content of the locally made soaps from cocoa pods ranged from 8.67 ± 0.23 to 10.81 ± 1.11 % while it ranged from 9.89 ± 0.62 to 16.80 ± 2.92 % in the soaps made from palm bunches. The soaps used as standards (Lux and Joy soaps) had relatively lower % chloride content (2.39 ± 0.41 and 2.65 ± 0.01 respectively). The % chloride content of these soaps exceeded the acceptable limits of the Standard Organization of Nigeria (≤0.75). This raises doubts on the quality of the soaps as high chloride content has been reported to cause soaps to crack (Taiwo et al., 2008). The chloride content in this study was higher than the findings of Hayati et al. (2020).
3.1.5. Free caustic alkali (%)
The free caustic alkali is a measure of the abrasiveness of soaps (Mahesar et al., 2019). The free caustic alkali in the locally made soaps from cocoa pods ranged from 5.74 ± 0.25 to 11.41 ± 1.21 % while it ranged from 2.49 ± 0.11 to 9.98 ± 0.22 % in the soaps made from palm bunches. Lux and Joy soaps (used as standards) had relatively lower % free caustic alkali (1.24 ± 0.09 and 0.99 ± 0.15 % respectively). However, these levels were higher than the acceptable limits of free caustic alkali set by the Standard Organization of Nigeria (≤0.05). This free caustic alkali levels reported in this study were also higher than the findings of earlier workers (Mak-Mensah and Firempong, 2011; Warra, 2013; Beetseh and Anza, 2013; Idoko et al., 2018). In a bid to increasing the mildness of the soaps, the body effects of the high free caustic alkali of the studied soaps could be reduced by adding humectants such as propylene glycol, glycerol and so on to the soap and/or using inorganic polyprotic acids such as phosphoric acid as a preservative for the finished product (Woollatt 1985; Nangbes et al., 2014).
3.1.6. Free fatty acid (%)
Free fatty acid is an important factor for estimating the transparency of a soap (Hayati et al. 2020). The free fatty acid content of the locally made soaps made from cocoa pods ranged from 4.81 ± 0.32 to 7.73 ± 0.27 % while it ranged from 1.42 ± 0.14 to 8.96 ± 0.61 % in soaps made from palm bunches. The soaps used as standards had relatively lower free fatty acid content (0.37 ± 0.06 and 0.28 ± 0.04 %). Free fatty acid must be less than or equal to 0.30 % in soaps (SON, 1997). Results from this study indicated higher than the acceptable levels and the 4% free fatty acid level indicated as the level that must not be exceeded if a soap sample must maintain its transparency (Tokosh, 1996). The free fatty acid levels of the locally made soaps in this study were also higher than the findings of Hayati et al. (2020).
3.1.7. pH
The pH of the locally made soaps from cocoa pods ranged from 9.19 ± 0.11 to 9.75 ± 0.02 while it ranged from 9.13 ± 0.01 to 9.62 ± 0.03 in locally made soaps from palm bunches. The results of the pH of the locally made soaps compared favourably with that of Lux (9.55 ± 0.02) and Joy (9.38 ± 0.01) soaps respectively. The acceptable limits of pH in soaps set by Standard Organization of Nigeria are 6.5–8.5. Generally, in aqueous solutions, soaps are alkaline, and these alkaline substances neutralize the body's protective acid mantle, acting as barriers against viruses and bacteria in the process. Nevertheless, a healthy human skin has a pH value ranging between 5.4 to 5.9 (Mak-Mensah and Firempong, 2011). pH values higher than the acceptable limits observed for the studied soap samples, due to incomplete hydrolysis of the saponification process, are an indication that the soaps would be corrosive to the skin. This corrosive action can be mitigated by the addition of excess fat and/or oil to reduce the harshness of the soap (Warra et al., 2011).
3.2. Heavy metal composition of the soap samples
A spike recovery method was adopted in a bid to validating the efficiency of the analytical method due to the unavailability of certified reference material for this type of product. As presented in Table 1, the %R values obtained were within the 70–110% recovery range for evaluating the precision and accuracy of a method. The R2 values ranging from 0.9897 in Pb to 0.9991 in Cu showed the high linearity of the AAS used, indicating the reliability of the AAS to give accurate results. Results of the composition (μg/g) of lead (Pb), copper (Cu), mercury (Hg), zinc (Zn), and cadmium (Cd) in the soap samples are presented in Table 2. Generally, the locally made soaps from cocoa pods had higher metal composition than the locally made soaps from palm bunches. The conventional soaps (Lux and Joy) had comparatively lower metal contents. In the locally made soaps, the order of decreasing mean metal concentration was Hg > Zn > Pb > Cu > Cd while in the conventional soaps, it followed the order: Hg > Zn > Cu > Pb > Cd.
Table 1.
Validation Parameters for the investigated Metals using AAS.
| Metals | Current (mA) | Wavelength (nm) | Calibration curve (R2) | %R | LOD | LOQ |
|---|---|---|---|---|---|---|
| Pb | 10 | 282.9 | 0.9897 | 94.66 ± 3.10 | 0.013 | 0.04 |
| Cu | 6 | 325.1 | 0.9991 | 89.56 ± 1.09 | 0.03 | 0.09 |
| Hg | 10 | 253.7 | 0.9986 | 96.35 ± 2.02 | 0.005 | 0.015 |
| Zn | 8 | 214.2 | 0.9924 | 93.39 ± 1.63 | 0.005 | 0.015 |
| Cd | 8 | 228.6 | 0.9935 | 92.33 ± 3.10 | 0.01 | 0.03 |
LOD = limit of detection, LOQ = limit of quantification.
Table 2.
Heavy metals composition (μg/g) of the soap samples.
| Sample | Pb | Cu | Hg | Zn | Cd |
|---|---|---|---|---|---|
| A1 | 27.10 ± 1.05 | 29.00 ± 0.00 | 273.58 ± 0.49 | 50.82 ± 0.12 | 6.05 ± 0.60 |
| A2 | 24.35 ± 0.14 | 17.30 ± 0.10 | 110.10 ± 0.11 | 37.16 ± 0.01 | 2.95 ± 0.45 |
| A3 | 16.25 ± 0.09 | 15.83 ± 0.33 | 129.31 ± 0.40 | 39.75 ± 0.22 | 5.15 ± 0.01 |
| Mean ± SD | 22.57 ± 0.42 | 20.71 ± 0.14 | 171.00 ± 0.33 | 42.58 ± 0.12 | 4.72 ± 0.35 |
| B1 | 15.71 ± 0.04 | 67.00 ± 0.00 | 197.36 ± 0.84 | 26.30 ± 0.00 | 5.09 ± 0.13 |
| B2 | 10.76 ± 0.03 | 701.64 ± 2.85 | 106.5 ± 0.23 | 119.21 ± 0.20 | 3.65 ± 0.10 |
| B3 | 18.63 ± 0.10 | 14.23 ± 1.13 | 125.29 ± 0.15 | 52.53 ± 0.32 | 4.78 ± 0.41 |
| Mean ± SD | 15.03 ± 0.06 | 260.96 ± 1.32 | 143.05 ± 0.41 | 66.01 ± 0.17 | 4.51 ± 0.21 |
| C1 | 3.37 ± 0.12 | 9.82 ± 0.14 | 55.12 ± 0.65 | 17.54 ± 0.55 | 5.20 ± 0.60 |
| C2 | 5.29 ± 0.06 | 3.10 ± 0.22 | 46.35 ± 0.22 | 32.32 ± 0.13 | 2.88 ± 0.11 |
Values are means of triplicate analysis.
3.2.1. Lead (Pb)
Pb had a mean concentration of 22.57 ± 0.42 μg/g and 15.03 ± 0.06 μg/g in the locally made soaps from cocoa pods and palm bunches respectively. These values exceeded the WHO limit (10 μg/g) for Pb in cosmetics (Sukender et al. 2012). Established source of Pb in the environment is vehicular emissions. The relative upsurge in Pb levels of the locally made soaps as opposed to the conventional soaps could be an indication that the cocoa pods and palm bunches were obtained from plants grown on farmlands either close to road sides or farmlands enriched by Pb. Heavy metals can enrich a farmland through a combination of anthropogenic and natural processes. It is very important that Pb risks are considered in the formulation of cosmetics. The Pb levels of the locally made soaps were higher than those reported in previous findings (Abulude et al., 2007; Chauhan et al., 2010; Sani and Shehu, 2018).
3.2.2. Copper (Cu)
Cu had a mean concentration of 20.71 ± 0.14 μg/g and 260.96 ± 1.32 μg/g in the locally made soaps from cocoa pods and palm bunches respectively. The higher levels of Cu observed in the palm bunches could be due to its high retaining ability for Cu. The levels of Cu in the locally made soaps were higher than that of the conventional soaps and also higher than those reported in previous findings (Iwegbue et al., 2019).
3.2.3. Mercury (Hg)
Mercury had a mean concentration of 171.00 ± 0.33 μg/g and 143.05 ± 0.41 μg/g in the locally made soaps from cocoa pods and palm bunches respectively. Levels of Hg were higher in the locally made soaps relative to the conventional soaps. However, Hg levels in the soaps were all above the acceptable limit (1 μg/g) for Hg in cosmetics as stated by WHO (Sukender et al., 2012). The high Hg levels observed in the black soaps probably justified the recurring use of the soaps in the treatment of bacterial and fungal diseases, because mercuric soaps have the potential to kill bacteria and fungi. Mercury is usually added to soaps in its inorganic forms (HgCl2) so as to lighten human skin (Adebajo, 2002). However, excessive exposure to Hg can result in neurological and dermal toxicity (Sin and Tsang, 2003). Mercury levels in these soaps were relatively higher than that of previous studies (Alizadeh et al., 2017; Alam et al., 2019).
3.2.4. Zinc (Zn)
Mean concentrations of 42.58 ± 0.12 μg/g and 66.01 ± 0.17 μg/g in the locally made soaps from cocoa pods and palm bunches respectively were recorded in the present study. These levels were higher than that of the conventional soaps. Levels of zinc above 40 μg/g may be toxic (Al-Weher 2008). Zinc is incorporated into soaps in the form of ZnO as a binder due to its ability to protect the skin against ultraviolet radiation (Swierczek et al., 2019). However, excessive exposure to Zn can cause neurological disorders, fragile hair and nails (Iwegbue et al., 2015).
3.2.5. Cadmium (Cd)
Cd had mean concentrations of 4.72 ± 0.35 μg/g and 4.51 ± 0.21 μg/g in the locally made soaps from cocoa pods and palm bunches respectively. These values were higher than that of the conventional soaps. However, the values recorded were all higher than the WHO acceptable limit (0.3 μg/g) for Cd in cosmetics (Sukender et al., 2012). Cadmium is an extremely toxic element even at low concentrations. Long-term exposure to Cd can result in renal dysfunction (Sani and Shehu, 2018). Nevertheless, Cd is used in cosmetics as a coloured pigment (Godt et al., 2006). Predominant controlling factors for the human skin absorption of Cd are the interaction between free Cd ions and sulfhydryl radicals of cysteine present in epidermal keratins, or complexation and induction with metallothionein (Fasanya-Odewumi et al., 1998). They were also higher than the findings of earlier reports (Szarek et al., 2001; Abulude et al., 2007; Sani and Shehu, 2018).
3.3. Safety evaluation of the use of studied soaps with respect to metal levels
The systemic exposure damage (SED) for metals in the studied soaps upon use by adults and children is presented in Table 3. In this study, 50% and 100% bioaccessibility to the metals were used as the midpoint and worst-case scenario respectively. The SED values were generally higher at 100% bioaccessibility than 50% bioaccessibility, as expected. The results also indicated that the children were the more vulnerable population to the metal levels upon the use of the studied soaps, due to their relatively higher SED values. This is consistent with the assertion that children are more vulnerable to environmental contaminants than adults as reported in previous environmental studies (Chonokhuu et al., 2019; Hanfi and Yarmoshenko, 2020). Any human skin product must have a minimum safe acceptable level called “margin of safety” value of 100 (SCCS, 2012). Varying margin of safety (MoS) values (Table 4) were observed for the studied soaps. Notable among the results was the significantly lower MoS levels of Hg which were less than 1 for both adults and children at both systemic availability levels. Zinc was a notable exception at 50% and 100% systemic availability level for adults as a result of its greater than 100 MoS value. In addition to Hg, the lower MoS levels observed for the other metals suggested that there could be significant non-carcinogenic risks associated with the use of the studied soaps, particularly by the children. The MoS values reported in this study were lower than those reported by Lim et al. (2018) and Iwegbue et al. (2019). The hazard index (HI) values (Table 5) were generally greater than 1 except for Zn at both systemic availability levels for adults. This also suggested that the soaps may be unsafe for use, especially by the children.
Table 3.
Systemic Exposure Damage (SED) for Metals in Studied Soaps upon usage by adults and children.
| 50 % bioaccessibility factor |
100 % bioaccessibility factor |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pb | Cu | Hg | Zn | Cd | Pb | Cu | Hg | Zn | Cd | |
| Adults | ||||||||||
| A1 | 6.65E-02 | 7.12E-02 | 6.72E-01 | 1.24E-01 | 1.48E-02 | 1.33E-01 | 1.42E-01 | 1.34E+00 | 2.49E-01 | 2.97E-02 |
| A2 | 5.98E-02 | 4.25E-02 | 2.70E-01 | 9.13E-02 | 7.24E-03 | 1.19E-01 | 8.50E-02 | 5.41E-01 | 1.82E-01 | 1.44E-02 |
| A3 | 3.99E-02 | 3.88E-02 | 3.17E-01 | 9.76E-02 | 1.26E-02 | 7.98E-02 | 7.77E-02 | 6.35E-01 | 1.95E-01 | 2.53E-02 |
| B1 | 3.86E-02 | 1.64E-01 | 4.84E-01 | 6.46E-02 | 1.25E-02 | 7.72E-02 | 3.29E-01 | 9.69E-01 | 1.29E-01 | 2.50E-02 |
| B2 | 2.64E-02 | 1.72E+00 | 2.61E-01 | 2.92E-01 | 8.96E-03 | 5.28E-02 | 3.44E+00 | 5.23E-01 | 5.85E-01 | 1.79E-02 |
| B3 | 4.57E-02 | 3.49E-02 | 3.07E-01 | 1.29E-01 | 1.17E-02 | 9.15E-02 | 6.99E-02 | 6.15E-01 | 2.58E-01 | 2.34E-02 |
| C1 | 8.28E-03 | 2.41E-02 | 1.35E-01 | 4.30E-02 | 1.27E-02 | 1.65E-02 | 4.82E-02 | 2.70E-01 | 8.61E-02 | 2.55E-02 |
| C2 |
1.29E-02 |
7.61E-03 |
1.13E-01 |
7.94E-02 |
7.07E-03 |
2.59E-02 |
1.52E-02 |
2.27E-01 |
1.58E-01 |
1.41E-02 |
| Children | ||||||||||
| A1 | 2.91E-01 | 3.11E-01 | 2.94E+00 | 5.46E-01 | 6.50E-02 | 5.82E-01 | 6.23E-01 | 5.88E+00 | 1.09E+00 | 1.30E-01 |
| A2 | 2.61E-01 | 1.85E-01 | 1.18E+00 | 3.99E-01 | 3.17E-02 | 5.23E-01 | 3.71E-01 | 2.36E+00 | 7.98E-01 | 6.34E-02 |
| A3 | 1.74E-01 | 1.70E-01 | 1.39E+00 | 4.27E-01 | 5.53E-02 | 3.49E-01 | 3.40E-01 | 2.78E+00 | 8.54E-01 | 1.10E-01 |
| B1 | 1.68E-01 | 7.20E-01 | 2.12E+00 | 2.82E-01 | 5.47E-02 | 3.37E-01 | 1.44E+00 | 4.24E+00 | 5.65E-01 | 1.09E-01 |
| B2 | 1.15E-01 | 7.54E+00 | 1.14E+00 | 1.28E+00 | 3.92E-02 | 2.31E-01 | 15.08E+00 | 2.28E+00 | 2.56E+00 | 7.84E-02 |
| B3 | 2.00E-01 | 1.52E-01 | 1.34E+00 | 5.64E-01 | 5.13E-02 | 4.00E-01 | 3.05E-01 | 2.69E+00 | 1.12E+00 | 1.02E-01 |
| C1 | 3.62E-02 | 1.05E-01 | 5.92E-01 | 1.88E-01 | 5.59E-02 | 7.24E-02 | 2.11E-01 | 1.18E+00 | 3.77E-01 | 1.11E-01 |
| C2 | 5.68E-02 | 3.33E-02 | 4.98E-01 | 3.47E-01 | 3.09E-02 | 1.13E-01 | 6.66E-02 | 9.96E-01 | 6.94E-01 | 6.19E-02 |
Table 4.
Margin of Safety (MoS) for Metals in Studied Soaps upon usage by adults and children.
| 50 % bioaccessibility factor |
100 % bioaccessibility factor |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pb | Cu | Hg | Zn | Cd | Pb | Cu | Hg | Zn | Cd | |
| Adults | ||||||||||
| A1 | 6.00 | 56.13 | 0.04 | 240.24 | 6.72 | 3.00 | 28.06 | 0.02 | 120.12 | 3.36 |
| A2 | 6.68 | 94.09 | 0.11 | 328.56 | 13.79 | 3.34 | 47.04 | 0.05 | 164.28 | 6.89 |
| A3 | 10.01 | 102.83 | 0.09 | 307.15 | 7.90 | 5.00 | 51.41 | 0.04 | 153.57 | 3.95 |
| B1 | 10.36 | 24.29 | 0.06 | 464.23 | 7.99 | 5.18 | 12.14 | 0.03 | 232.11 | 3.99 |
| B2 | 15.12 | 2.32 | 0.11 | 102.41 | 11.15 | 7.56 | 1.16 | 0.05 | 51.20 | 5.57 |
| B3 | 8.73 | 114.39 | 0.09 | 232.42 | 8.51 | 4.36 | 57.19 | 0.04 | 116.21 | 4.25 |
| C1 | 48.30 | 165.77 | 0.22 | 696.08 | 7.82 | 24.15 | 82.88 | 0.11 | 348.04 | 3.91 |
| C2 |
30.77 |
525.13 |
0.26 |
377.76 |
14.13 |
15.38 |
262.56 |
0.13 |
188.88 |
7.06 |
| Children | ||||||||||
| A1 | 1.37 | 12.83 | 0.01 | 54.91 | 1.53 | 0.68 | 6.41 | 0.005 | 27.45 | 0.76 |
| A2 | 1.52 | 21.50 | 0.02 | 75.09 | 3.15 | 0.76 | 10.75 | 0.01 | 37.54 | 1.57 |
| A3 | 2.28 | 23.50 | 0.02 | 70.20 | 1.80 | 1.14 | 11.75 | 0.01 | 35.10 | 0.90 |
| B1 | 2.36 | 5.55 | 0.01 | 106.11 | 1.82 | 1.18 | 2.77 | 0.007 | 53.05 | 0.91 |
| B2 | 3.45 | 0.53 | 0.02 | 23.40 | 2.54 | 1.72 | 0.26 | 0.01 | 11.70 | 1.27 |
| B3 | 1.99 | 26.14 | 0.02 | 53.12 | 1.94 | 0.99 | 13.07 | 0.01 | 26.56 | 0.97 |
| C1 | 11.04 | 37.89 | 0.05 | 159.10 | 1.78 | 5.52 | 18.94 | 0.02 | 79.55 | 0.89 |
| C2 | 7.03 | 120.03 | 0.06 | 86.34 | 3.22 | 3.51 | 60.01 | 0.03 | 43.17 | 1.61 |
Table 5.
Hazard Index (HI) for Metals in Studied Soaps upon usage by adults and children.
| 50 % bioaccessibility factor |
100 % bioaccessibility factor |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pb | Cu | Hg | Zn | Cd | Pb | Cu | Hg | Zn | Cd | |
| Adults | ||||||||||
| A1 | 16.64 | 1.78 | 2240.75 | 0.41 | 14.86 | 33.29 | 3.56 | 4481.50 | 0.83 | 29.73 |
| A2 | 14.95 | 1.06 | 901.77 | 0.30 | 7.24 | 29.91 | 2.12 | 1803.54 | 0.60 | 14.49 |
| A3 | 9.98 | 0.97 | 1059.11 | 0.32 | 12.65 | 19.96 | 1.94 | 2118.22 | 0.65 | 25.30 |
| B1 | 9.65 | 4.11 | 1616.47 | 0.21 | 12.50 | 19.30 | 8.23 | 3232.94 | 0.43 | 25.01 |
| B2 | 6.60 | 43.10 | 872.28 | 0.97 | 8.96 | 13.21 | 86.20 | 1744.57 | 1.95 | 17.93 |
| B3 | 11.44 | 0.87 | 1026.18 | 0.43 | 11.74 | 22.88 | 1.74 | 2052.37 | 0.86 | 23.49 |
| C1 | 2.07 | 0.60 | 451.45 | 0.14 | 12.77 | 4.14 | 1.20 | 902.91 | 0.28 | 25.55 |
| C2 |
3.24 |
0.19 |
379.62 |
0.26 |
7.07 |
6.49 |
0.38 |
759.25 |
0.52 |
14.15 |
| Children | ||||||||||
| A1 | 72.83 | 7.79 | 9803.28 | 1.82 | 65.03 | 145.66 | 15.58 | 19606.57 | 3.64 | 130.07 |
| A2 | 65.44 | 4.64 | 3945.25 | 1.33 | 31.71 | 130.88 | 9.29 | 7890.50 | 2.66 | 63.42 |
| A3 | 43.67 | 4.25 | 4633.60 | 1.42 | 55.36 | 87.34 | 8.50 | 9267.21 | 2.84 | 110.72 |
| B1 | 42.22 | 18.00 | 7072.06 | 0.94 | 54.71 | 84.44 | 36.01 | 14144.13 | 1.88 | 109.43 |
| B2 | 28.91 | 188.56 | 3816.25 | 4.27 | 39.23 | 57.83 | 377.13 | 7632.50 | 8.54 | 78.47 |
| B3 | 50.06 | 3.82 | 4489.55 | 1.88 | 51.38 | 100.13 | 7.64 | 8979.11 | 3.76 | 102.77 |
| C1 | 9.05 | 2.63 | 1975.13 | 0.62 | 55.90 | 18.11 | 5.27 | 3950.26 | 1.25 | 111.80 |
| C2 | 14.21 | 0.83 | 1660.87 | 1.15 | 30.96 | 28.43 | 1.66 | 3321.75 | 2.31 | 61.92 |
4. Conclusion
In this study, locally made soaps in Nigeria were compared with conventional soaps in terms of metal levels, pH, free fatty acids, moisture content, alkali content, matters insoluble in ethanol and water, and chloride content, in a bid to assessing their compatibility with acceptable standards. While the 100% bioaccessibility level is a worst-case scenario, the metal levels were considered unsafe even at 50% systemic bioavailability level. The presence of the metals in the soaps might be related to the use of raw materials obtained from variously contaminated sources and other materials used during the production chain. The investigated physicochemical parameters were present at levels above the tolerable limits set by regulatory agencies, thereby rendering the soaps unsafe for long-term predominant domestic uses. The locally made soaps had relatively higher metal levels and physicochemical properties compared to the conventional soaps. It is therefore recommended that the raw materials used in the local production of the indigenous soaps be carefully selected so as to mitigate the potential risks users may be exposed to as a result of using the soaps as well as mitigate or reduce to the barest minimum the potential risks of the domestic effluents on the ecosystem.
Declarations
Author contribution statement
John A. O. Oyekunle: Conceived and designed the experiments; Wrote the paper.
Odunayo T. Ore: Analyzed and interpreted the data; Wrote the paper.
Oluseyi H. Ogunjumelo: Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Michael S. Akanni: Conceived and designed the experiments.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data included in article/supplementary material/referenced in article.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Contributor Information
John A.O. Oyekunle, Email: oyekunle@oauife.edu.ng.
Odunayo T. Ore, Email: oreodunayo@yahoo.com.
References
- Abulude F.O., Ogunkoya F.O., Ogunleye R.F., Emidun O., Abulude A.I. Assessment of the content of Pb, Cd, Ni and Cr in soaps and detergents from Akure Nigeria. Res.J. Environ. Toxicol. 2007;1(2):102–104. [Google Scholar]
- Adebajo S.B. An epidemiological survey of the use of cosmetic skin lightning cosmetics among traders in Lagos, Nigeria. West African. J. Med. 2002;2191:51–55. [PubMed] [Google Scholar]
- Alam M.F., Akhter M., Mazumder B., Ferdous A., Hossain M.D., Dafader N.C., Ahmed F.T., Kundu S.K., Taheri T., Atique Ullah A.K.M. Assessment of some heavy metals in selected cosmetics commonly used in Bangladesh and human health risk. J. Analyt. Sci. Tech. 2019;10(1) [Google Scholar]
- Alizadeh A., Balali-Mood M., Mahdizadeh A., Zanjani B. Mercury and lead levels in common soaps from local markets in mashhad, Iran.Iran. J. Toxicol. 2017;11(4):1–3. [Google Scholar]
- Al-Weher S.M. Levels of heavy metal Cd, Cu and Zn in three fish species collected from the Northern Jordan Valley, Jordan. Jordan J. Biol. Sci. 2008;1(1):41–46. [Google Scholar]
- Antignac E., Nohynek G.J., Re T., Clouzeau J., Toutain H. Safety of botanical ingredients in personal care products/cosmetics. Food Chem. Toxicol. 2011;49(2):324–341. doi: 10.1016/j.fct.2010.11.022. [DOI] [PubMed] [Google Scholar]
- Beetsch C.I., Anza M.K. Chemical characterization of Local Black Soap made by using cassava peels ashes (alkali base) and palm oil in North Central Zone of Nigeria. Civ. Environ. Res. 2012;3(4) ISSN 2224-5790. [Google Scholar]
- Borowska S., Brzóska M.M. Metals in cosmetics: implications for human health. J. Appl. Toxicol. 2015;35(6):551–572. doi: 10.1002/jat.3129. [DOI] [PubMed] [Google Scholar]
- Chauhan A.S., Bhadauria R., Singh A.K., Lodhi S.S., Chaturvedi D.K., Tomari V.S. Determination of lead and chromium in cosmetics products. J. Chem. Pharmaceut. Res. 2010;2(6):92–97. [Google Scholar]
- Chonokhuu S., Batbold C., Chuluunpurev B., Battsengel E., Dorjsuren B., Byambaa B. Contamination and health risk assessment of heavy metals in the soil of major cities in Mongolia. Int. J. Environ. Res. Publ. Health. 2019;16(14):2552. doi: 10.3390/ijerph16142552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasanya-Odewumi C., Latinwo L.M., Ikediobi C.O., Gilliard L., Sponholtz G., Nwoga J., Stino F., Hamilton N., Erdos G.W. The genotoxicity and cytotoxicity ofdermally-administered cadmium: effects of dermal cadmium administration. Int. J. Mol. Med. 1998;1(1):1001–1006. doi: 10.3892/ijmm.1.6.1001. [DOI] [PubMed] [Google Scholar]
- Godt J., Scheidig F., Grosse-Siestrup C., Esche V., Brandenburg P., Reich A., Groneberg D.A. The toxicity of cadmium and resulting hazards for human health. J. Occup. Med. Toxicol. 2006;1(1):22. doi: 10.1186/1745-6673-1-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanfi M.Y., Yarmoshenko I.V. Health risk assessment quantification from heavy metals contamination in the urban soil and urban surface deposited sediment. J. Taib. Univers. Sci. 2020;14(1):285–293. [Google Scholar]
- Hayati S.N., Rosyida V.T., Darsih C., Nisa K., Indrianingsih A.W., Apriyana W., Ratih D. Physicochemical properties, antimicrobial and antioxidant activity ofganoderma transparent soap. IOP Conf. Ser. Earth Environ. Sci. 2020;462 [Google Scholar]
- Idoko O., Emmanuel S.A., Salau A.A., Obigwa P.A. Quality assessment on some soaps sold in Nigeria. Nigerian J. Techn. 2018;37(4):1137–1140. [Google Scholar]
- Issa M., Isaac I., Matthew O., Shalangwa B., Sunday M. Physicochemical analysis for quality and safety of some selected animal soaps compared to human soaps in plateau state, Nigeria. IOSR J. Appl. Chem. 2020;13(3):25–28. [Google Scholar]
- Iwegbue C.M.A., Bassey F.I., Tesi G.O., Onyeloni S.O., Obi G., Martincigh B.S. Safety evaluation of metal exposure from commonly used moisturizing and skin lightning creams in Nigeria. Regul. Toxicol. Pharmacol. 2015;71:484–490. doi: 10.1016/j.yrtph.2015.01.015. [DOI] [PubMed] [Google Scholar]
- Iwegbue C.M.A., Emakunu O.S., Lari B., Egobueze F.E., Tesi G.O., Nwajei G.E., Martincigh B.S. Risk of human exposure to metals in some household hygienic products in Nigeria. Toxicology Reports. 2019;6:914–923. doi: 10.1016/j.toxrep.2019.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwegbue C.M.A., Emakunu O.S., Obi G., Nwajei G.E., Martincigh B.S. Evaluation of human exposure to metals from commonly used hair care products in Nigeria. Toxicol. Rep. 2016;3:796–803. doi: 10.1016/j.toxrep.2016.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janečková M., Bartoš M., Lenčová J. Isotachophoretic determination of triethanolamine in cosmetic products. MonatshefteFürChemie - Chemical Monthly. 2019;150(3):387–390. [Google Scholar]
- Lim D.S., Roh T.H., Kim M.K., Kwon Y.C., Choi S.M., Kwack S.J. Non-cancer, cancer, and dermal sensitization risk assessment of heavy metals in cosmetics. J. Toxicol. Environ. Health, Part A. 2018;81(11):432–452. doi: 10.1080/15287394.2018.1451191. [DOI] [PubMed] [Google Scholar]
- Madsen T., Boyd H.B., Nylen D., Pedersen A.R., Petersen G.I., Simonsen F. 2001. Environmental and Health Assessment of Substances in Household Detergents and Cosmetic Detergent Products, Environmental Project No. 615, Miljoprojekt Denmark: the Danish EPA. [Google Scholar]
- Mahesar S.A., Chohan R., Tufail S., Sherazi H. Evaluation of physico-chemical properties in SelectedBranded soaps. Pak. J. Anal. Environ. Chem. 2019;20(2):177–183. [Google Scholar]
- Mak-Mensah E.E., Firempong C.K. Chemical characteristics of toilet soap prepared from neem (Azadirachtaindica A. Juss) seed oil. Asian J. Plant Sci. Res. 2011;1(4):1–7. [Google Scholar]
- Nangbes J.E., Zukdimma N.A., Wufem B.M., Lawam L.D. Quality survey and safety of some toilet soaps in the Nigerian market: a case study of B/ladi, bokkos and pankshin, plateau state. IOSR J. Appl. Chem. 2014:29–35. [Google Scholar]
- Ogunsuyi H.O., Akinnawo C.A. Quality assessment of soaps produced from palm bunch ash-derived alkali and coconut oil. J. Appl. Sci. Environ. Manag. 2012;16(4):363–366. [Google Scholar]
- Onojah P.K., Emurotu J.E. Heavy metals in selected skin lighting creams and medicated soap. Int. J. Innov. Sci. Mathem. 2017;5(3):95–99. [Google Scholar]
- Onyango P., Vivian O., Nathan A.O., Linda M., Wesley N.O. Assessment of the physicochemical properties of selected commercial soaps manufactured and sold in Kenya. Open J. Appl. Sci. 2014;4:433–440. [Google Scholar]
- Osuji C.N., Akunna T.O., Ahaotu E.O. Use of palm oil sludge in toilet soap production. Int. J. Appl. Sci. Eng. 2013;1(2):73–78. [Google Scholar]
- Owoicho I. Quality evaluation of soaps produced from neem seed oil and shea-butter oil. World J. Adv. Engin. Techn. Sci. 2021;2(1):45–50. [Google Scholar]
- Panda H. Niir Project Consultancy Services; 2011. Herbal Soaps & Detergents Handbook. [Google Scholar]
- Roila A., Salmiah A., Razmah G. Properties of sodium soap derived from palm-based dihydroxystearic acid. J. Oil Palm Research. 2001;1:33–38. [Google Scholar]
- Sani A., Shehu A. Determination of some heavy metals concentration in selected detergents used in kano metropolis, Nigeria. Environ Toxicol Stud J. 2018;2(1):1–3. [Google Scholar]
- Sani A., Gaya M.B., Abubakar F.A. Determination of some heavy metals in selectedcosmetic products sold in Kano metropolis, Nigeria. Toxicol. Rep. 2016;3:866–869. doi: 10.1016/j.toxrep.2016.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scientific Committee on Consumer Safety (SCCS) 8th Revision, Adopted by the SCCS at its 17th Plenary Meeting of 11. December 2012. (SCCS/1501/12), the SCCS’snotes of guidance for the testing of cosmetic substance and the safety evaluation.http://ec.europa.eu/growth/sectors/cosmetics/assessment/docs/sccs-notes-of-guidance-for-testing-cosmetic-substances_enpdf [Google Scholar]
- Sin K., Tsang H. Large-scale mercury exposure due to a cream cosmetic: community-wide case series. Hong Kong Med. J. 2003;9(5):329–334. [PubMed] [Google Scholar]
- SON Nigerian industrial standard; guidelines for classification and sampling cosmetics and toiletries. Standards organization of Nigeria. Federal Ministry Indus. 1997;9 [Google Scholar]
- Soylak M., Unsal Y.E., Tuzen M. Evaluation of metal contents of household detergentsamples from Turkey by flame atomic absorption spectrometry. Environ. Monit. Assess. 2013;185:9663–9668. doi: 10.1007/s10661-013-3281-5. [DOI] [PubMed] [Google Scholar]
- Squance M.L., Reeves G., Attia J., Bridgman H., Guest M. Self-reported Lupus flare:association with everyday home and personal product exposure. Toxicol. Rep. 2015;2:880–888. doi: 10.1016/j.toxrep.2015.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukender K., Jaspreet S., Sneha D., Munish G. AAS estimation of heavy metalsand trace elements in Indian herbal cosmetic preparations. Res. J. Chem. Sci. 2012;2(3):46–51. [Google Scholar]
- Świerczek L., Cieślik B., Matysiak A., Konieczka P. Determination of heavy metals in eyeshadows from China. MonatsheftefürChemie - Chemical Monthly. 2019;150:1675–1680. [Google Scholar]
- Szarek J., Felsmann M.Z., Kmarkiewicz E., Felsmann M. Cadmium levels and in young soot originating from industrial and agricultural regions of north middle Poland. Pol. J. Environ. Stud. 2001;10:489–491. [Google Scholar]
- Taiwo A., Oluwadare I., Shobo A., Amolegbe S. Physical and chemical characteristics of soap. Sci. Res. Essays. 2008;3:515–517. [Google Scholar]
- Tjandraatmadja G., Pollard C., Sheedy C., Gozukara Y. CSIRO; Australia: 2010. Sources of Contaminants inDomestic Wastewater: Nutrients and Additional Elements from HouseholdProducts, Water for a Healthy Country Research Flagship Report ISSN: 1835-095X. [Google Scholar]
- Tokosh R. Transparent soap formulations and methods of making same. U.S. Patent No. 5,529,714. U.S. Patent and Trademark Office; Washington, DC: 1996. [Google Scholar]
- Warra A.A., Wawata I.G., Gunu S.Y., Atiku F.A. Soap preparation from Soxhlet extracted Nigerian Cotton seed oil. Adv. Appl. Sci. Res. 2011;2(5):617–623. 2011. [Google Scholar]
- Warra A.A. A report on soap making in Nigeria using indigenous technology and raw materials. Afr. J. Pure Appl. Chem. 2013;7(4):139–145. [Google Scholar]
- Woollatt E. first ed. Ellis Hardwood Ltd; West Sessux, England: 1985. The Manufacturer of Soaps Other Detergents and Glycerin. 34-35, 47- 55, 267 and 284. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supplementary material/referenced in article.