Abstract
Summary
Background
Acute diarrhoeal disease management often requires rehydration alone without antibiotics. However, non-indicated antibiotics are frequently ordered and this is an important driver of antimicrobial resistance. The mHealth Diarrhoea Management (mHDM) trial aimed to establish whether electronic decision support improves rehydration and antibiotic guideline adherence in resource-limited settings.
Methods
A cluster randomised controlled trial was done at ten district hospitals in Bangladesh. Inclusion criteria were patients aged 2 months or older with uncomplicated acute diarrhoea. Admission orders were observed without intervention in the pre-intervention period, followed by randomisation to electronic (rehydration calculator) or paper formatted WHO guidelines for the intervention period. The primary outcome was rate of intravenous fluid ordered as a binary variable. Generalised linear mixed-effect models, accounting for hospital clustering, served as the analytical framework; the analysis was intention to treat. The trial is registered with ClinicalTrials.gov (NCT03154229) and is completed.
Findings
From March 11 to Sept 10, 2018, 4975 patients (75·6%) of 6577 screened patients were enrolled. The intervention effect for the primary outcome showed no significant differences in rates of intravenous fluids ordered as a function of decision-support type. Intravenous fluid orders decreased by 0·9 percentage points for paper electronic decision support and 4·2 percentage points for electronic decision support, with a 4·2-point difference between decision-support types in the intervention period (paper 98·7% [95% CI 91·8–99·8] vs electronic 94·5% [72·2–99·1]; pinteraction=0·31). Adverse events such as complications and mortality events were uncommon and could not be statistically estimated.
Interpretation
Although intravenous fluid orders did not change, electronic decision support was associated with increases in the volume of intravenous fluid ordered and decreases in antibiotics ordered, which are consistent with WHO guidelines.
Introduction
Acute diarrhoeal disease management often requires rehydration alone without antibiotics. However, antibiotics are frequently used, which is likely to be an important driver of antimicrobial resistance. A medical provider’s willingness to adhere to guidelines is influenced by a localised clinical approach and nonclinical factors (ward hygiene and sanitation, oral rehydration solution [ORS] made with clean water) within the medical ecosystem (human resources, physical infrastructure, sociological phenomena). The objective of the mHealth Diarrhoea Management (mHDM) trial was to establish whether electronic decision support can improve adherence to the WHO rehydration and antibiotic guidelines. The intention is that increased adherence will enable safe and effective patient care while conserving resources and combating antimicrobial resistance.1,2
Diarrhoeal disease is one of the most common causes of morbidity and mortality globally. In 2016, there were 4·5 billion episodes and 1·7 million deaths across all age groups attributed to diarrhoeal disease.3 It is the second leading cause of death in children between 1 month and 5 years of age.4,5 Although broad multi system interventions have led to reduced mortality, diarrhoeal diseases continue to affect patients of all ages, especially those of lower socioeconomic status.6–8 Effective public health management of diarrhoeal diseases requires multifaceted community and hospital hygiene and sanitation interventions, vaccination campaigns, epidemiological monitoring, advocacy, and standardised (yet accommodating) clinical approaches.2
Assessment of dehydration is the first step in the WHO Integrated Management of Childhood Illness diarrhoeal disease guidelines.9–12 General condition, sunken eyes, thirst, and skin pinch are scored and categorised as no, some, or severe dehydration. These categories approximate 0–4%, 5–9%, and ≥10% total bodyweight lost. Although there are alternative methods,13,14 the WHO Integrated Management of Childhood Illness guidelines remain the international standard of care, balancing practicality with precision. In general, management prioritises rehydration over antibiotics. Patients with no dehydration are managed with oral fluids (eg, ORS) to avert dehydration. ORS is used to correct some dehydration, with intravenous fluids reserved for patients with severe emesis or ileus.1 Severe dehydration is corrected with intravenous fluids with a goal of 0·1 L/kg. Antibiotics are indicated for severe dehydration from acute watery diarrhoea due to cholera15 and invasive diarrhoea (eg, shigellosis).9
The WHO Global Task Force for Cholera Control makes clinical training for large-scale diarrhoeal disease management a high priority, especially for cholera. The electronic adaptation of the WHO guidelines (eg, rehydration calculator) was piloted in an interrupted time-series study during a cholera outbreak in Bangladesh; an approach similarly taken in Afghanistan.16 We found the electronic intervention was associated with improved dehydration assessment, decreased intravenous fluid ordered, and antibiotic class switching to the recommended antibiotic,17 despite a study design that lacked a concurrent reference group and evaluation at only two hospitals. The mHDM trial was designed to address these limitations in the pilot study as well as evaluate how modes of clinical decision support affect guideline adherence at scale in resource-limited settings.
Methods
Study design and participants
In this cluster randomised controlled trial, participants with acute diarrhoeal disease were enrolled at ten district hospitals geographically distributed across Bangladesh (appendix 1 p 5). The study sought to establish whether decision support (electronic or paper) improves guideline adherence. Inclusion criteria were patients 2 months of age or older with uncomplicated acute diarrhoea. Patients were eligible for participation if they presented to the hospital emergency room with acute diarrhoea defined as three or more episodes of loose stools in the 24 h before admission and the duration of disease was less than 7 days. Patients were excluded if they had severe malnutrition, as assessed by a mid-upper arm circumference of less than 110 mm for patients 2 months to less than 6 months old and 115 mm for patients 6 months to less than 5 years old; these patients were excluded because of a lack of capacity to manage malnutrition in the diarrhoea wards and were referred to a paediatric specialist. Patients with a comorbidity (eg, pneumonia), uncontrolled chronic disease (eg, diabetes), or life-threatening illness other than dehydration (eg, sepsis) were excluded. The clinical assessment, and fluid and medication orders, for participants admitted during the pre-intervention period were observed for approximately 6 weeks. Subsequently, hospitals were randomly assigned electronic or paper decision support during the intervention period for approximately 16 weeks.
Adult participants, and parents or guardians of children less than 11 years old, provided informed written consent for themselves or their children. Children aged 11 to less than 18 years provided informed written consent.
Research ethics boards at the Institute of Epidemiology, Disease Control and Research, International Centre for Diarrhoeal Disease Research, Bangladesh and the University of Florida approved this study.
Randomisation
Pre-trial analysis found significant heterogeneity between study hospitals. Therefore, hospitals were paired by means of hierarchical clustering according to similar profiles defined by geographical location, median number of patients per month younger or and older than 5 years, and cholera incidence. Electronic randomisation assigned hospitals in each pair to electronic or paper decision support. Randomisation occurred immediately before the start of the intervention. Participants, research personnel, and analysts were aware of the intervention assigned.
Procedures
Before the pre-intervention, clinicians and nurses were oriented in a 2-h session to the trial objectives without disclosing the type of intervention, which was yet to be assigned. Admitting medical providers (physicians and physician assistants) assessed the dehydration status and provided treatment based on localised clinical approach. Before the intervention, additional training was provided in a 2-h session on the WHO guidelines; training materials and decision-support tools were provided (appendix 1 pp 6–7). For patients with acute watery diarrhoea suggestive of cholera, azithromycin (20 mg/kg once or 1 g once for adults) was indicated for patients younger than 2 years of age with severe dehydration and above 2 years of age with some or severe dehydration;18 this was a localised adaptation because of low cholera incidence among children younger than 2 years of age.6 Patients with bloody diarrhoea were treated with azithromycin (10 mg/kg for children or 500 mg for adults once daily for 5 days) independent of dehydration status. Zinc was recommended for children younger than 5 years of age (10 mg for children aged 2–5 months or 20 mg for those aged 6–59 months, once daily for 10 days).
Research assistants screened patients for enrolment at the emergency room. Those who met inclusion criteria were offered the opportunity to enrol and informed written consent or assent was obtained. Procedures were designed to minimise disruption of existing workflow; one exception was the placement of a scale in the emergency room during the intervention period given that the guidelines recommend weight-based dosing. Health-care providers at hospitals randomised to electronic decision support entered age, gender, a measured weight, clinical signs of dehydration, known medication allergies, and danger signs on the input page (appendix 1 p 6). The output page provided recommen dations for rehydration, danger signs, and medications. Health-care providers at hospitals randomised to paper decision support used a pocket card formatted to reflect the electronic tool (appendix 1 p 7). Patients were transferred from the emergency room to the diarrhoea ward (or general inpatient ward) where the orders were implemented. Clinical signs of dehydration were independently established by a ward nurse. Participants were followed up for 10 days after discharge. Data were recorded electronically by means of software (Outbreak Responder version 0.9) developed for this study. The software is a data collection tool for public health and research professionals. It is built specifically for outbreaks in resource-limited settings with limited connectivity. The development was funded by the National Institutes of Health (DP5OD019893), Stanford University, and the University of Florida. There is a patent pending at the United States Patent and Trademark Office (2020/0082921).
Microbiological observations were done as part of a parallel national cholera surveillance study.19 Four stool samples were collected per study site per day. Sampling was not random and was stratified such that two patients were younger than 5 years old and two were at least 5 years old. If the target of four total samples per day was not reached, over-enrolment in one group was done so that four total patients per day were sampled.
Field samples were placed in transport media (Cary-Blair) and cultured by standard methods at the International Centre for Diarrhoeal Disease Research in Dhaka, Bangladesh.19,20 Qualitative observations were made by two ethnographers during the pre-intervention and intervention periods. The approach included nonparticipant observation (including systematic observations for 76 clinician–patient interactions) and informal conversations (n=138) with clinicians, staff nurses, and patients by means of methods previously described.21
Outcomes
The primary outcome was rate of intravenous fluid ordered, as a binary variable. Secondary outcomes specified a priori were intravenous fluid volumes, antibiotics, and zinc ordered. Non-indicated intravenous fluid orders were defined as intravenous fluids ordered for a patient with an objective assessment of no dehydration. Non-indicated antibiotic orders were defined as antibiotics ordered for patients with non-bloody watery diarrhoea with no dehydration. Clinical course and adverse events were followed for 10 days after discharge. Frequency of diarrhoea among household contacts in the 10-day follow-up period was enumerated. For quantitative outcomes, it was decided a priori to focus on individual-level outcomes for direct clinical applicability. The mixed models chosen to analyse the data allowed for inference on the patient level while accounting for the clustered design of the trial. For qualitative outcomes, the approach prioritised identifying barriers to intervention uptake.
Statistical analysis
To evaluate the hypothesis that intravenous fluid use will decrease with the electronic versus paper decision support, the mean number of patients per study hospital was estimated from pre-study surveillance data from each hospital while recognising that enrolment was census-based and that the exact number of patients enrolled might be above or below these estimates. Logistical limitations restricted the hospital sample size to ten. To calculate power to detect differences between the intervention groups, it was assumed variation of intracluster correlations ranged from 0·01 to 0·075 and power was based on a two-sided pooled Z test comparing the overall difference between the intervention groups in intravenous fluid rate during the post-intervention period (assuming balance between the randomised hospitals in the two arms pre-intervention). For each intervention arm, we calculated an average of 526 patients per hospital, leveraging pre-study governmental data (2631 patients per intervention group). On the basis of data from the pilot study,17 we assumed that the proportion of patients receiving intravenous fluids in the electronic intervention group would be 0·4 (with the paper-based intervention group at 0·45 or greater). With an intracluster correlation of 0·01, there would be 80% power to detect a difference between the electronic and paper intervention groups of 0·10 and 95% power to detect a difference of 0·12 at the post-intervention period. If the intracluster correlation were 0·075, there would be 80% power to detect a difference between the electronic and intervention arms of 0·24 and 95% power to detect a difference of 0·31. Hospitals were matched to address intercluster variability (see randomisation). However, post-study analysis revealed the hospital pairing was not effective at achieving balance across the two interventions during the pre-intervention phase. Therefore, the analyses were ultimately done independently of pairing.22 The primary outcome was binary and, given the sample size (ten hospitals), a logit model was used for convergence. Generalised linear mixed-effect models, including a random effect for hospital, served as the framework for the models. Owing to imbalance between the groups during the pre-intervention period, the primary focus was on tests of the fixed effect of the group (electronic vs paper decision support) by intervention period interaction (preintervention vs intervention). We used the between–within method for specifying test degrees of freedom because of the small number of clusters.22–24
Significance was defined as α = 0·05. Fixed-effect adjustments for patient age, sex, and dehydration status were made in all adjusted models. Effect modification was assessed by examining additional interaction variables, including categorised age, and dehydration status was tested. For continuous outcomes (eg, intravenous fluid volume), unadjusted means and frequencies are reported with 95% CIs and for categorical outcomes (intravenous fluid, antibiotics, zinc), adjusted means and frequencies are reported with 95% CIs. Intravenous fluid volume was log-transformed when modelling; estimates were back-transformed and presented on the original scale and thus represent geometric means (by intervention and period). Adjusted odds ratios from the mixed-effect modelling are reported by comparing decision-support groups within each intervention period. Difference rates were calculated as the percentage-point difference between pre-intervention and post-intervention rates. When inter vention × period interactions were significant, effect sizes (and 95% CIs) of the interactions were computed as the difference in difference rates (ie, between the electronic and paper decision-support groups); an effect size of 0 represents no intervention × period interaction. Effect sizes for interactions involving intravenous fluids are represented as the ratio of ratios of the geometric mean given the log-transformation that we used in the modelling; an effect size of one represents no intervention × period interaction. Statistical analyses were completed in Statistical Analysis Software version 9.4 (SAS Institute, Cary, NC, USA). All data except for restricted items are provided (appendix 2). Missingness, which was not excluded, was for patients for whom intravenous fluids were ordered but did not have a measured weight or intravenous fluid volume (236 [5·1%] patients).
A data and safety monitoring board was assembled at the International Centre for Diarrhoeal Disease Research, Bangladesh. The protocol (appendix 1 pp 30–45) received independent evaluation by three international experts. The US Department of Health and Human Services human experimentation guidelines were followed during this research. The trial is registered at ClinicalTrials.gov, NCT03154229.
Role of the funding source
This work was supported by the National Institutes of Health. Internal support was provided by the University of Florida and Stanford University (Center for Innovation in Global Health). These funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
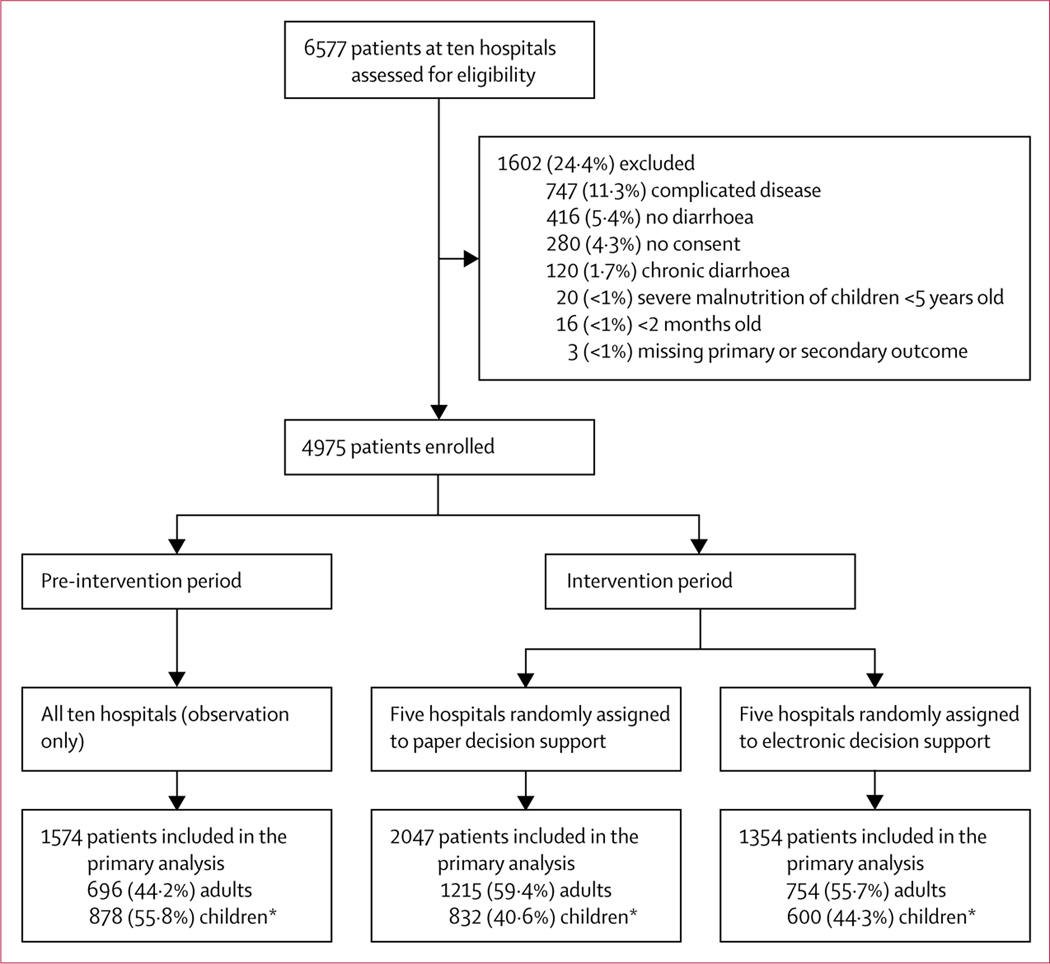
From March 11 to Sept 10, 2018, 4975 patients (75·6%) were enrolled out of 6577 screened (figure 1). 1574 patients were enrolled during the pre-intervention period (March 11 to May 9, 2018) and 3401 were enrolled during the intervention period (April 25 to Sept 10, 2018); 1354 were enrolled at hospitals randomly assigned to electronic decision support and 2047 at hospitals assigned to paper decision support. Although the adjusted model was run on demographic characteristics, a higher percentage of participants younger than 5 years in the pre-intervention period (51·0% electronic and 42·6% paper) compared with the intervention period (31·2% electronic and 32·2% paper; table 1) was observed. These differences are likely to be related to seasonality; viral agents predominate in the winter among paediatric patients and bacterial agents are more common among patients of all ages in the summer.6 During the study period (March–September, 2018), the incidence of Vibrio cholerae was 8·2% and of Shigella spp was 2·1%. V cholerae peaked at 16·0% in May, 2018, and Shigella spp peaked at 5·1% in July, 2018.
Figure 1: Trial profile.
*Younger than 18 years.
Table 1:
Baseline characteristics
| All patients (n=4975) | Pre-intervention (n=1574) |
Intervention (n=3401) |
|||
|---|---|---|---|---|---|
| Paper (n=909) | Electronic (n=665) | Paper (n=2047) | Electronic (n=1354) | ||
| Age, years | |||||
| 0–4 | 1808 (36·3%) | 387 (42·6%) | 339 (51·0%) | 659 (32·2%) | 423 (31·2%) |
| 5–9 | 248 (5·0%) | 37 (4·1%) | 45 (6·8%) | 80 (3·9%) | 86 (6·4%) |
| 10–14 | 167 (3·4%) | 24 (2·6%) | 25 (3·8%) | 55 (2·7%) | 63 (4·7%) |
| 15–19 | 265 (5·3%) | 43 (4·7%) | 25 (3·8%) | 130 (6·4%) | 67 (4·9%) |
| ≥20 | 2487 (50·0%) | 418 (46·0%) | 231 (34·7%) | 1123 (54·9%) | 715 (52·8%) |
| Sex | |||||
| Female | 2518 (50·6%) | 419 (46·1%) | 351 (52·8%) | 1020 (49·8%) | 728 (53·8%) |
| Male | 2457 (49·4%) | 490 (53·9%) | 314 (47·2%) | 1027 (50·2%) | 626 (46·2%) |
| Watery stool | 4960 (99·7%) | 901 (99·1%) | 665 (100·0%) | 2040 (99·7%) | 1354 (100·0%) |
| Bloody stool | 94 (1·9%) | 25 (2.8%) | 12 (1·8%) | 43 (2·1%) | 14 (1·0%) |
| Stools in 24 h | |||||
| 3–6 | 1010 (20·3%) | 185 (20.4%) | 208 (31·3%) | 304 (14·9%) | 313 (23·1%) |
| 7–12 | 1733 (34·8%) | 240 (26·4%) | 330 (49·6%) | 585 (28.6%) | 578 (427%) |
| >12 | 2232 (44·9%) | 484 (53·2%) | 127 (19·1%) | 1158 (56·6%) | 463 (34·2%) |
| Data are n (%). | |||||
The intervention effect for the primary outcome showed no significant differences in rates of intravenous fluids ordered as a function of decision-support type, despite improved dehydration assessments (appendix 1 pp 8, 14). Intravenous fluid orders decreased by 0·9 percentage points for paper and 4·2 percentage points for electronic decision support, with a 4·2-point difference between decision-support types in the intervention period (paper 98·7% [95% CI 91·8–99·8] vs electronic 94·5% [72·2–99·1]; pinteraction=0·31).
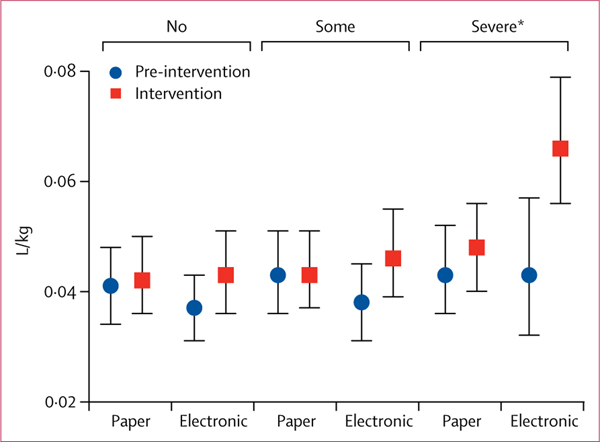
The intervention effect for secondary outcomes showed significant differences. Absolute and weight-adjusted intravenous fluid volumes increased, with higher volumes ordered with electronic decision support in the intervention period (pinteraction=0·0001; appendix 1 p 26). Among patients with severe dehydration, electronic decision support had a greater increase in weight-adjusted intravenous fluid volume compared with paper decision support (pinteraction=0·015; figure 2; appendix 1 p 27). The geometric mean intravenous fluid volume increased from 0·0381 L/kg re-intervention to 0·0620 L/kg during the intervention for the electronic intervention (ratio 1·63, 95% CI 1·23–2·15), where no corresponding increase was observed in the paper intervention (0·0437–0·0475 L/kg; ratio 1·09, 1·00–1·21). The ratio of ratios between electronic and paper was 1·50 (1·11–2·01).
Figure 2: Effect of the intervention on the secondary outcome of the volume of intravenous fluid ordered.
Distribution of weight-adjusted intravenous fluid volume for all ages by dehydration status: no, some, severe (see appendix 1 p 27). *Significant analysis (p<0·05).
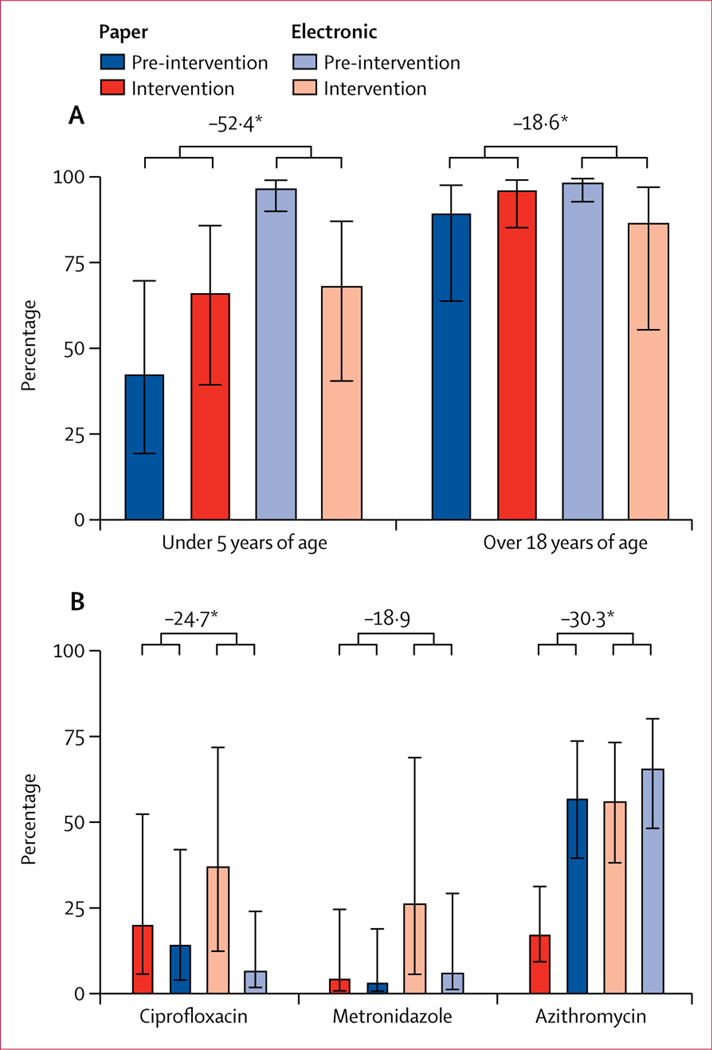
Antibiotic orders significantly decreased by 10·0 percentage points for electronic decision support (pre-intervention rate 98·9% [95% CI 96·8 to 99·7] vs intervention rate 88·9% [74·1 to 95·7]), but increased by 7·8 percentage points for paper decision support (79·8% [58·9 to 91·6] vs 87·6% [72·1 to 95·1]). The intervention-by-period interaction was significant (p<0·0001; appendix 1 p 18), with a −17·8 point (−27·3 to −8·4) difference in difference rates between the electronic and paper intervention effect. Non-indicated antibiotic orders also changed in significantly different ways by intervention type and period. When provided with electronic decision support, non-indicated antibiotic orders decreased by 28·5 percentage points for patients younger than 5 years of age (97·2% [90·3 to 99·3] to 68·7% [40·9 to 87·4]; p<0·0001; appendix 1 p 19). By contrast, there was a 23·8-point increase in non-indicated antibiotic orders for paper decision support (43·1% [19·8 to 70·0] to 66·9% [39·8 to 86·1]; pinteraction<0·0001; −52·4 point [−73·9 to −30·9] difference in difference rates; figure 3A;; appendix 1 p 19). For patients aged 18 years and older, nonindicated antibiotic orders decreased by 11·8 percentage points for electronic decision support (pinteraction<0·0001; 98·9% [93·0 to 99·8] to 87·1% [55·8 to 97·3]) and increased by 6·8 percentage points for paper decision support (90·1% [64·1 to 97·9] to 96·9% [85·5 to 99·4]); pinteraction<0·0001; −18·6 point [−35·5 to −1·7] difference in difference rates; figure 3A; appendix 1 p 20).
Figure 3: Effect of the intervention on the secondary outcome of antibiotics ordered.
(A) Non-indicated antibiotic orders defined as antibiotics ordered for patients with an objective classification of no dehydration and non-bloody watery stools. (B) Comparison of specific antibiotic orders and decision-support type. Error bars are 95% CIs. Difference in difference rates shown above the bars are percentage-point differences. *Significant difference (p<0·05).
For non-bloody acute diarrhoea, antibiotic class switching occurred from non-indicated antibiotics (eg, ciprofloxacin) to the indicated antibiotic (azithromycin; figure 3B; appendix 1 p 21). Ciprofloxacin (appendix 1 p 23) decreased by 30·5 percentage points between the pre-intervention and intervention periods for electronic decision support (37·4% [95% CI 12·4 to 71·8] vs 6·9% [1·7 to 23·9]; pinteraction<0·0001; −24·7 point [−46·6 to −2·7] difference in difference rates). Metronidazole decreased by 20·2 percentage points between the pre-intervention and intervention periods for electronic decision support (26·5% [5·6 to 68·8] vs 6·3% [1·1 to 29·1]); the interaction was significant (p=0·0004; appendix 1 p 22) but the difference in difference rates was not (−18·9 point [−40·2 to 2·4] difference). Azithromycin increased by 9·4 percentage points between the pre-intervention and intervention periods for electronic decision support (56·4% [38·1 to 73·2] vs 65·8% [48·1 to 80·0]) and by 39·6 percentage points for paper decision support (17·7% [9·3 to 31·2] vs 57·3% [39·4 to 73·5]; pinteraction<0·0001; appendix 1 p 21; −30·3 point [−38·8 to −21·7] difference in difference rates). This difference in difference rates is negative because the increase with paper decision support was greater than the increase in digital decision support. Orders for zinc among children younger than 5 years were not significantly different (pinteraction=0·11).
Follow-up rates at discharge and 10 days after discharge between study groups ranged from 71·4% to 83·1% (table 2). There were significant differences in rates for the interactions between decision-support type and intervention period in duration of admission, discharge type, days for diarrhoea to resolve, frequency of diarrhoea among household contacts, and readmission. Adverse events (eg, complications from over-hydration) and severe adverse events (eg, death, significant disability, incapacity) were uncommon and could not be statistically estimated (table 2; appendix 1 pp 11–13). The cause of the one death was acute myocardial infarction in an adult participant.
Table 2:
Hospital and post-discharge course
| Pre-intervention period (n=1574) |
Intervention period (n=3401) |
p values | |||||
|---|---|---|---|---|---|---|---|
| Paper (n=909) | Electronic (n=665) | Paper (n=2047) | Electronic (n=1354) | Intervention × period | Intervention* | Period* | |
| Hospital course† | |||||||
| Duration of admission | |||||||
| ≥72 h | 5·8% (0·8–30·9) | 5·8% (0·8–31·1) | 3·6% (0·6–24·9) | 7·3% (1·1–35·8) | 0·050 | .. | .. |
| Electronic vs paper | 1·01% (0·06–16·59) | 1·01% (0·06–16·59) | 1·70% (0·11–27·48) | 1·70% (0·11–27·48) | .. | .. | .. |
| Complications | 0 | 2 (0·3%) | 2 (0·1%) | 0 | .. | .. | .. |
| Discharge type | |||||||
| With advice | 81·5% (33·3– 97·5) | 79·9% (31·5–97·2) | 71·95 (22·7–95·7) | 65·9% (18·4–94·3) | 0·27 | 0 87 | <0∙0001 |
| Electronic vs paper | 0·90 (0·04–19·25) | 0·90 (0·04–19·25) | 0·76 (0·04–15·95) | 0·76 (0·04–15·95) | .. | .. | .. |
| Discharge type (among patients with intravenous fluids and antibiotics ordered) | |||||||
| Number | 626 | 584 | 1657 | 992 | .. | .. | .. |
| With advice | 83·3% (38·2–97·6) | 81·2% (35·3–97·2) | 70·9% (23·4–95·1) | 66·6% (20·2–94·0) | 0∙75 | 0∙89 | <0∙0001 |
| Electronic vs paper | 0·87 (0·05–16·33) | 0·87 (0·05–16·33) | 0·82 (0·04–15·22) | 0·82 (0·04–15·22) | .. | .. | .. |
| Post-discharge course | |||||||
| Number | 740 (81·4%) | 475 (71·4%) | 1701 (83·1%) | 967 (71·4%) | .. | .. | .. |
| Days for diarrhoea to resolve | |||||||
| ≥3 days | 21·7% (11·2–38·0) | 24·2% (12·6–41·6) | 15·0% (7·4–27·8) | 20·0% (10·3–35·4) | 0∙049 | .. | .. |
| Electronic vs paper | 1·15 (0·38–3·54) | 1·15 (0·38–3·54) | 1·43 (0·47–4·31) | 1·43 (0·47–4·31) | .. | .. | .. |
| Diarrhoea among household contacts‡ | |||||||
| ≥0 | 8·3% (4·6–14·7) | 14·8% (8·3– 24·8) | 7·7% (4·4–13·3) | 7·4% (4·1–13·0) | 0∙016 | .. | .. |
| Electronic vs paper | 1·91 (0·78–4·69) | 1·91 (0·78–4·69) | 0·96 (0·40–2·27) | 0·96 (0·40–2·27) | .. | .. | .. |
| Readmitted | 7·0% (3·1– 15·2) | 3·6% (1·3–9·1) | 4·1% (1·8–9·0) | 0·5% (0·1–1·9) | 0∙031 | .. | .. |
| Electronic vs paper | 0·49 (0·13–1·81) | 0·49 (0·13–1·81) | 0·12 (0·03–0·58) | 0·12 (0·03–0·58) | .. | .. | .. |
| Mortality | 0 | 0 | 1 (0·1%)§ | 0 | .. | .. | .. |
Data are % (95% CI), adjusted odds ratio (95% CI), or n (%).
Main effect p values are reported if the intervention type by period interaction variable is not significant.
The adjusted linear mixed-effects models account for clustering at the hospital level, adjusting for age, sex, and dehydration status of patient.
Frequency of diarrhoea among household contacts is the number of people affected by diarrhoea after discharge divided by the number of people that share the same cooking pot in the household.
The cause of mortality was acute myocardial infarction in an adult participant; after review by the Institutional Review Board, the event was determined to not have a causal relationship with the intervention.
Discussion
This cluster randomised controlled trial found that electronic and paper decision support was not associated with a decrease in total intravenous fluid orders but was associated with improvements in secondary measures of guideline adherence. Improvements included an increase in weight-adjusted intravenous fluid volumes for patients with severe dehydration assessed with electronic decision support, a decrease of non-indicated antibiotic orders with electronic decision support, and class switching to the recommended antibiotic for both methods of decision support. These findings suggest that in resource-limited settings improved guideline adherence for rehydration and antibiotic stewardship is feasible with decision-support interventions.
The study location was chosen because of the large case volumes, resource limitations, and recurrent seasonal diarrhoeal disease outbreaks. This environment was crucial to evaluate the desirability, feasibility, and viability of electronic and paper decision support.25 Ethnographers explored barriers to reducing non-indicated intravenous fluid and antibiotic orders. Most health-care providers were concerned that the nurses lacked the training and capacity to rehydrate with ORS alone, there was a shortage of clean water to make ORS, and patients expected intravenous fluids. They also expressed a reluctance to not order antibiotics out of concern for hospital-acquired infections given poor sanitation, hygiene, and overcrowding. Most health-care providers interviewed also explained that they had insufficient time to do physical examinations let alone time to use electronic (2 min per use) or paper (1 min per use) decision-support tools for all patients. Although this study increased external validity from the pilot study, future studies in multiple countries are needed to further increase generalisability for both the qualitative and quantitative findings.
The implications of this study are both clinical and economic. Despite the failure to reduce intravenous orders, the study revealed opportunities that might precipitate intravenous fluid reduction in future endeavours. Electronic decision support was also positively associated with significant increases in weight-adjusted intravenous fluid volumes ordered for patients with severe dehydration. In addition, there was a significant decrease of non-indicated antibiotic use with electronic decision support. These improvements probably had positive clinical and economic effects that this study was not designed to test (appendix 1 p 4).
The results should be viewed within the context of the study limitations, primarily with respect to lack of adequate statistical power. First, we calculated the sample size to detect a difference between the groups at the intervention period of 7 percentage points or greater of intravenous fluid order rate (our primary outcome), and we observed a difference of 4·2 percentage points. The cluster sample size was small (ten hospitals) because of logistical constraints. Second, although care was taken to pair similar hospitals before randomisation, these pairings were constrained by location and there was significant heterogeneity between clusters, both with respect to the matched pairs as well as between the intervention groups. These factors negatively affected statistical power. Third, there were differences in the participant characteristics between pre-intervention and intervention periods. The differences were probably due to seasonality of aetiological agents. An additional limitation included the placement of a scale in the emergency room that was restricted to the intervention period because the pre-intervention period was obser vational. A measured weight compared with an estimated weight might have altered the thought process of clinicians in weight-based dosing in the intervention period, leading to improved performance. Lastly, orders for fluids and antibiotics were written in the emergency room. The extent to which orders were administered at the ward was not enumerated. This is a limitation because the ultimate objective is to improve the care that the patient actually receives.
In conclusion, decision support (either paper or electronic) improved adherence to WHO clinical guidelines, which has important relevance to diarrhoeal disease management for both patients and institutions in resource-limited settings. For the primary outcome measure of intravenous fluid ordered among all participants, significant differences were not observed between electronic and paper decision support. For the secondary outcomes, significant differences were observed in intravenous fluid volumes ordered among patients with severe dehydration and non-indicated antibiotics ordered in the electronic decision-support group compared with patients in the paper decision-support group. Although both decision-support methods provided benefit, the accessibility of the electronic medium might offer better scalability to improve guideline adherence for acute diarrhoeal disease.
Supplementary Material
Research in context.
Evidence before this study
We searched Pubmed for reports published after Jan 1, 2009, in all languages with the search terms [decision-support OR mHealth OR cell phone] AND [diarrhea OR diarrhoea]. The primary search criteria identified 79 publications. A secondary criterion removed non-interventional studies, qualitative studies, non-human studies, and studies on chronic diseases (eg, Clostridium difficile infection, gastrointestinal cancer, inflammatory bowel disease). Among the eight articles that met these criteria, two were correlative studies between health professionals and non-professionals at the community level, two were pre-studies or post-studies at the hospital level, and four studies had randomised designs. These four studies were: a cluster randomised controlled trial on the assessment of dehydration and danger signs with and without electronic decision support at the level of community health-care workers in Niger; a cluster randomised control trial on guideline adherence with and without text messages sent to community health-care workers in Malawi; a clinical study on the assessment of dehydration with paper or electronic decision support at the level of a hospital in Bangladesh; and a randomised controlled trial on guideline adherence with and without decision support (non-electronic) at the level of a hospital in the Netherlands. Excluding two cited author-affiliated studies in Bangladesh, the most relevant study was a pre-study and post-study in Afghanistan that reported that electronic decision support for the assessment and management of paediatric patients (all-cause) was associated with a total antibiotic decrease of 21·8%; data were not stratified for diarrhoeal disease. Despite this body of literature, we did not identify a cluster randomised control trial that addressed the clinical scope (assessment and treatment of diarrhoeal disease), intent, and scale of the study presented herein.
Added value of this study
To our knowledge, this is the first study to develop and test an electronic adaptation of the WHO diarrhoeal disease guidelines in a cluster randomised controlled trial in resource-limited emergency rooms. The value added is a demonstration of feasibility as well as clinically relevant findings on increasing the volume of fluid used for resuscitation and decreasing antibiotic usage. The lack of decrease of total intravenous fluids reveals valuable barriers to behaviour change that should be addressed in future studies and interventions. Lastly, the data support the public release of this digital adaptation of the WHO guidelines and integration into like-minded software, such as the WHO Global Task Force on Cholera Control mobile application.
Implications of all the available evidence
When approaching the management of a complex clinical challenge, the ideal approach is one that is both life saving and cost saving. The mHDM trial focused on diarrhoeal disease as a model system to identify effective approaches to improving guideline adherence and promote the prudent use of antibiotics. The study found that electronic decision support, and paper decision support to a lesser extent, were associated with increased intravenous fluid volumes needed to resuscitate patients in hypovolemic shock and reduced non-indicated antibiotic use predominantly for paediatric patients. We anticipate the electronic decision-support approach will manifest in life-saving and cost-saving outcomes, and possibly represent a generalisable approach for other diseases in resource-limited settings.
Acknowledgments
This study was funded by the National Institutes of Health (DP5OD019893; R21TW010182), granted to EJN, with internal support provided by the University of Florida and Stanford University (Center for Innovation and Global Health). We thank the participants in this study as well as the compassionate and diligent clinical and research teams that made this study possible. The leadership from our governmental partners (Civil Surgeons) and the determination of the hospital teams (resident medical officers, nurses, support staff) were vital to the success of this trial. We are grateful to Terry Winograd at Stanford University for his kind support and advice. We thank our technology partners at BeeHyv Software Solutions (Hyderabad, India). We also thank Myron Levine (University of Maryland), Rupa Nurra (US Centers for Disease Control and Prevention), and Andrew Azman (Johns Hopkins University) for their external review of the protocol.
Funding US National Institutes of Health.
Footnotes
Declaration of interests
SM, DP, and EJN are associated with a pending patent on the data collector (Outbreak Responder) used in this study (Patent Publication Number 2020/0082921). This pending patent does not apply to the rehydration calculator studied in this trial and there is no intellectual property claimed on the rehydration calculator. All other authors declare no competing interests.
Data sharing
De-identified individual participant data are available in appendix 2, where a datakey is provided. The data are available immediately following publication with no end date. These data are available to all people and for any purpose. The Institutional Review Board-approved study protocol is provided in appendix 1 (pp 30–46).
References
- 1.Rolla CM, Kache S, Smith J. Basic paediatric intensive care in resource limited countries. In: Homer R, Walker I, Bell G, eds. Update in anaesthesia. Special edn. Singapore: COS Printers, 2015: 221–23. [Google Scholar]
- 2.Farmer P, Almazor CP, Bahnsen ET, et al. Meeting cholera’s challenge to Haiti and the world: a joint statement on cholera prevention and care. PLoS Negl Trop Dis 2011; 5: e1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.GBD 2016 Diarrhoeal Disease Collaborators. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of diarrhoea in 195 countries: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect Dis 2018; 18: 1211–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu L, Oza S, Hogan D, et al. Global, regional, and national causes of child mortality in 2000–13, with projections to inform post-2015 priorities: an updated systematic analysis. Lancet 2015; 385: 430–40. [DOI] [PubMed] [Google Scholar]
- 5.Liu L, Oza S, Hogan D, et al. Global, regional, and national causes of under-5 mortality in 2000–15: an updated systematic analysis with implications for the Sustainable Development Goals. Lancet 2016; 388: 3027–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andrews JR, Leung DT, Ahmed S, et al. Determinants of severe dehydration from diarrheal disease at hospital presentation: evidence from 22 years of admissions in Bangladesh. PLoS Negl Trop Dis 2017; 11: e0005512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murray CJ, Barber RM, Foreman KJ, et al. Global, regional, and national disability-adjusted life years (DALYs) for 306 diseases and injuries and healthy life expectancy (HALE) for 188 countries, 1990–2013: quantifying the epidemiological transition. Lancet 2015; 386: 2145–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sarker AR, Islam Z, Khan IA, et al. Cost of illness for cholera in a high risk urban area in Bangladesh: an analysis from household perspective. BMC Infect Dis 2013; 13: 518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.WHO. Pocket book of hospital care for children: guidelines for the management of common childhood illnesses, 2nd edn. Geneva: World Heath Organization, 2013. [PubMed] [Google Scholar]
- 10.WHO. The treatment of diarrhoea: a manual for physicians and other senior health workers. 4th rev. Geneva: World Health Organization, 2005. [Google Scholar]
- 11.WHO. Handbook: IMCI integrated management of childhood illnesses, 4th edn. Geneva: World Health Organization, 2005. [Google Scholar]
- 12.WHO. Integrated management of adolescent and adult illness. WHO/CDS/IMAI/2004.1 rev 3. Geneva: World Health Organization, 2009. [PubMed] [Google Scholar]
- 13.Levine AC, Glavis-Bloom J, Modi P, et al. External validation of the DHAKA score and comparison with the current IMCI algorithm for the assessment of dehydration in children with diarrhoe a prospective cohort study. Lancet Glob Health 2016; 4: e744–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alam NH, Islam S, Sattar S, Monira S, Desjeux JF. Safety of rapid intravenous rehydration and comparative efficacy of 3 oral rehydration solutions in the treatment of severely malnourished children with dehydrating cholera. J Pediatr Gastroenterol Nutr 2009; 48: 318–27. [DOI] [PubMed] [Google Scholar]
- 15.Leibovici-Weissman Y, Neuberger A, Bitterman R, Sinclair D, Salam MA, Paul M. Antimicrobial drugs for treating cholera. Cochrane Database Syst Rev 2014; 6: CD008625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bernasconi A, Crabbé F, Rossi R, et al. The ALMANACH project: preliminary results and potentiality from Afghanistan. Int J Med Inf 2018; 114: 130–35. [DOI] [PubMed] [Google Scholar]
- 17.Haque F, Ball RL, Khatun S, et al. Evaluation of a smartphone decision-support tool for diarrheal disease management in a resource-limited setting. PLoS Negl Trop Dis 2017; 11: e0005290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saha D, Karim MM, Khan WA, Ahmed S, Salam MA, Bennish ML. Single-dose azithromycin for the treatment of cholera in adults. N Engl J Med 2006; 354: 2452–62. [DOI] [PubMed] [Google Scholar]
- 19.Khan AI, Rashid MM, Islam MT, et al. Epidemiology of cholera in Bangladesh: findings from nationwide hospital-based surveillance, 2014–2018. Clin Infect Dis 2019; published online Dec 31. DOI: 10.1093/cid/ciz1075. [DOI] [PubMed] [Google Scholar]
- 20.Islam MT, Khan AI, Sayeed MA, et al. Field evaluation of a locally produced rapid diagnostic test for early detection of cholera in Bangladesh. PLoS Negl Trop Dis 2019; 13: e0007124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pelto PJ. Applied ethnography: guidelines for field research. New York, NY: Taylor and Francis, 2016. [Google Scholar]
- 22.Diehr P, Martin DC, Koepsell T, Cheadle A. Breaking the matches in a paired t-test for community interventions when the number of pairs is small. Stat Med 1995; 14: 1491–504. [DOI] [PubMed] [Google Scholar]
- 23.Turner EL, Prague M, Gallis JA, Li F, Murray DM. Review of recent methodological developments in group-randomized trials: part 2-analysis. Am J Public Health 2017; 107: 1078–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martin DC, Diehr P, Perrin EB, Koepsell TD. The effect of matching on the power of randomized community intervention studies. Stat Med 1993; 12: 329–38. [DOI] [PubMed] [Google Scholar]
- 25.Agarwal S, LeFevre AE, Lee J, et al. Guidelines for reporting of health interventions using mobile phones: mobile health (mHealth) evidence reporting and assessment (mERA) checklist. BMJ 2016; 352: i1174. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.