Abstract
The impact of stressful events is especially important during early life, because certain cortical regions, especially the prefrontal cortex (PFC), are still developing. Consequently, aversive experiences that occur during the peripubertal period can cause long-term alterations in neural connectivity, physiology and related behaviors. Although sex influences the stress response and women are more likely to develop stress-related psychiatric disorders, knowledge about the effects of stress on females is still limited. In order to analyze the long-term effects of peripubertal stress (PPS) on the excitatory and inhibitory circuitry of the adult PFC, and whether these effects are sex-dependent, we applied an unpredictable chronic PPS protocol based on psychogenic stressors. Using two strains of transgenic mice with specific fluorescent cell reporters, we studied male and diestrus females to know how PPS affects the structure and connectivity of parvalbumin expressing (PV+) interneurons and pyramidal neurons. We also studied the expression of molecules related to excitatory and inhibitory neurotransmission, as well as alterations in the expression of plasticity-related molecules. The structure of pyramidal neurons was differentially affected by PPS in male and female mice: while the former had a decreased dendritic spine density, the latter displayed an increase in this parameter. PPS affected the density of puncta expressing excitatory and inhibitory synaptic markers exclusively in the female mPFC. Similarly, only in female mice we observed an increased complexity of the dendritic tree of PV+ neurons. Regarding the perisomatic innervation on pyramidal and PV + neurons by basket cells, we found a significant increase in the density of puncta in stressed animals, with interesting differences between the sexes and the type of basket cell analyzed. Finally, the PPS protocol also altered the total number of somata expressing the polysialylated form of the neural cell adhesion molecule (PSA-NCAM) when we analyzed both sexes together. These results highlight the strong programming effects of aversive experiences during early life for the establishment of cortical circuitry and the special impact of these stressful events on females.
1. Introduction
Although acute stress can trigger the adaptation of individuals to changing environments, chronic stress may lead to alterations in the physiology and connectivity of certain neural circuits (McEwen, 2000). These alterations are strongly influenced by the duration and type of the stressor and have different effects on distinct brain regions (McEwen et al., 2016). The exposure to chronic stress can lead to an altered balance between excitatory and inhibitory neurotransmission (Page and Coutellier, 2019) and constitutes a predisposing factor for the development of different psychiatric disorders (McEwen, 2000). Interestingly, the brain response to chronic stress is also influenced by the age of the individuals and their sex (Hodes and Epperson, 2019; Spear, 2000; Mañas-Ojeda et al., 2020).
The impact of stressful events is especially important during early life, when certain cortical regions, specially the prefrontal cortex (PFC), are still developing (Shaw et al., 2020a). Late stages of neural development extend well into adolescence (in mice from postnatal day (P) 28 until P42 (Spear, 2000; Rice and Barone, 2000), herewith termed peripubertal period). Consequently, aversive experiences occurring during the peripubertal period can modify the construction of neural circuits and elicit long-term disturbances in physiology and behavior (Page and Coutellier, 2018; Page et al., 2018; Papilloud et al., 2018). In fact, several studies have reported that individuals exposed to stressful experiences during the peripubertal period have an increased risk of developing a wide range of psychopathologies (Heim and Nemeroff, 2001; Paus et al., 2008). Most of these stress-related psychiatric disorders are accompanied by alterations in the structure and physiology of the PFC (Arnsten, 2015). In fact, the PFC is especially sensitive to aversive experiences and recent studies have shown how peripubertal stress can induce deficits on some PFC-dependent cognitive function (Watt et al., 2017; Liston et al., 2006; Tzanoulinou et al., 2016).
Many studies have demonstrated that chronic stress can affect the structure and connectivity of different cell types of the adult PFC, including pyramidal neurons (Radley et al., 2008; McEwen and Morrison, 2013), somatostatin expressing Martinotti cells (Gilabert-Juan et al., 2013) and parvalbumin (PV) expressing cells (Page et al., 2018; Czéh et al., 2018; Pesarico et al., 2019). PV + interneurons are the most abundant subtype of cortical interneurons and play a key role in the cortical circuitry, controlling firing synchronization and spike timing of excitatory pyramidal neurons (Ferguson and Gao, 2018). Previous work from our laboratory and others have observed alterations in these inhibitory neurons in the PFC after early life stress (Page et al., 2018; Ueno et al., 2018; Castillo-Gómez et al., 2017). There are also studies describing alterations in these PFC interneurons in psychiatric disorders for which adverse experiences in early life are a risk factor, such as schizophrenia (Gonzalez-Burgos et al., 2015). This control of pyramidal neurons is exerted partially through the perisomatic input coming from PV+ and cholecystokinin (CCK)+ basket cells. Recent work has found that this input is also regulated by chronic stress in adult animals (Pesarico et al., 2019). The synapses coming from CCK + basket cells express high levels of the cannabinoid receptor 1 (CB1R) and their study is particularly interesting because the endocannabinoid (eCB) system is involved in the stress response (Hill and McEwen, 2010; Hill et al., 2008). In particular, it is known that eCB activation of CB1R decreases GABA release within the mPFC, likely increasing the outflow of the principal neurons of this region to contribute to termination of the stress response (Hill et al., 2011). Interestingly, during development, specialized regions of the extracellular matrix, known as perineuronal nets (PNN), appear around the somata and proximal segments of the dendrites of PV + interneurons to exert a neuroprotective effect and control their plasticity (see (Wang and Fawcett, 2012; Testa et al., 2019) for review). Chronic stress alters the expression of some components of the PNN (Pesarico et al., 2019; Ueno et al., 2018), as well as that of other plasticity-related molecules, such as the polysialylated form of the neural cell adhesion molecule (PSA-NCAM) (Gilabert-Juan et al., 2013). PSA-NCAM is highly expressed during development and, as the PNN, has a critical role on the maturation of the connectivity of cortical PV + cells (Di Cristo et al., 2007) and its subsequent plasticity during adulthood (Gilabert-Juan et al., 2011).
Peripubertal animals subjected to chronic stress display in the short-term behavioral changes and structural alterations in excitatory and inhibitory neurons (Sala-Catala et al., 2005; Wommack et al., 2004), although the consequences of peripubertal stress on adult cortical circuitry have been less studied. It is important to note that most of the studies have used predictable stressors and have evaluated the effects of stress immediately after the aversive experience (Potrebić et al., 2020; Toledo-Rodriguez et al., 2012). Juvenile chronic stress reduces the dendritic length and spine density of pyramidal neurons in the PFC and these alterations persist in young adult animals (Pinzón-Parra et al., 2019). However, the effects of peripubertal stress are not limited to principal neurons; this aversive experience can also trigger dysregulations in the prefrontocortical excitatory-inhibitory balance, specifically it can promote alterations in PV + neurons and PNN (Page and Coutellier, 2018). At the behavioral level, sustained peripubertal stress promotes a decrease in locomotor activity (Pinzón-Parra et al., 2019; Platt and Stone, 1982) and an increase of anxiety-like behaviors (Page et al., 2018; Avital and Richter-Levin, 2005).
Women are more susceptible than men to develop psychiatric disorders in which stress is considered a precipitating factor, such as major depression or anxiety (Weissman and Klerman, 1977; Bangasser and Valentino, 2014). However, our knowledge on the effects of chronic stress in females is still very limited. In the adult PFC, the impact of chronic stress on different subpopulations of neurons appears to be sex-dependent, particularly on pyramidal neurons (Garrett and Wellman, 2009) and PV expressing interneurons (Shepard et al., 2016; Page and Coutellier, 2018). Although there is still scarce information available on sex differences in the effects of peripubertal stress (PPS), a recent report using physical stressors has shown that it can induce alterations on depression and anxiety-related behaviors, as well as in the number of PV + cells and PNN in a sex-specific way (Page and Coutellier, 2018).
To address these questions, we have analyzed in adult male and diestrus female mice the impact of an unpredictable chronic PPS paradigm based on psychogenic stressors. In male rats, peripubertal stress was shown to elicit abnormal aggressive behaviors and increased anxiety (Papilloud et al., 2018; Márquez et al., 2013; Walker et al., 2018), as well as alterations in the expression of molecules involved in inhibitory neurotransmission in the medial prefrontal cortex (mPFC) (Tzanoulinou et al., 2016). Here, we have used two different transgenic mouse strains with cell specific fluorescent reporters to study how PPS affects: i) the structure of PV + interneurons and pyramidal neurons of the mPFC; ii) the perisomatic innervation of pyramidal neurons by basket cells (from PV + interneurons and CCK + cells); iii) the expression of molecules related to inhibitory and excitatory synapses in the neuropil of the mPFC; and iv) alterations in PNN and PSA-NCAM expression given their role in interneuronal plasticity.
2. Material and methods
2.1. Animals and experimental procedures
Two different transgenic mouse strains (Jackson Laboratories; Bar Harbor, Maine, USA) were used in our experiments: the THY1 mice line H (C57BL/6- Tg(Thy1-YFP)HJrs/J), which expresses yellow fluorescent protein (YFP) in a subset of pyramidal neurons (Feng et al., 2000), and the PV-tdT line (C57BL/6-Tg(Pvalb-tdTomato)15Gfng/J), which expresses tdTomato red fluorescent protein in PV expressing interneurons (Kaiser et al., 2016). The experimental procedures were performed in both strains and in both sexes. Mice were maintained under standard housing conditions on a 12 h light-dark cycle. Food and water were available ad libitum.
All animal experimentation was conducted in accordance with the Directive 2010/63/EU of the European Parliament and of the Council of September 22, 2010 on the protection of animals used for scientific purposes and was approved by the Committee on Bioethics of the Universitat de València. Every effort was made to minimize the number of animals used and their suffering.
At weaning, male and female mice were randomly assigned to control (CONT) and peripubertal stress (PPS) conditions. A total number of 39 THY1 mice were used, distributed in 4 experimental groups (CONT male = 11, CONT female = 12, PPS male = 8 and PPS female = 8). Likewise, 37 PV-tdT mice were distributed following the same protocol as THY1 mice (CONT male = 11, CONT female = 9, PPS male = 8 and PPS female = 9). On postnatal day 28 (P28) the PPS protocol began, and it lasted until P42 (Fig. 1). A test for anxiety-like behavior (open field test) was performed when the animals reached adulthood (P85). Male mice were perfused at P90, but female mice perfusion was performed, starting at this age, when they reached the diestrus phase to avoid differences due to oscillating sex hormones. All female mice were perfused before P95. The determination of the phase of the estrous cycle was achieved after the microscopic examination of vaginal smears, flushing three to five times the vagina with phosphate buffered saline (PBS). The final flush was placed on a glass slide and stained with toluidine blue. Then we calculated the proportion of leukocytes, nucleated epithelial cells and anucleated cornified cells, as described before (Caligioni, 2009).
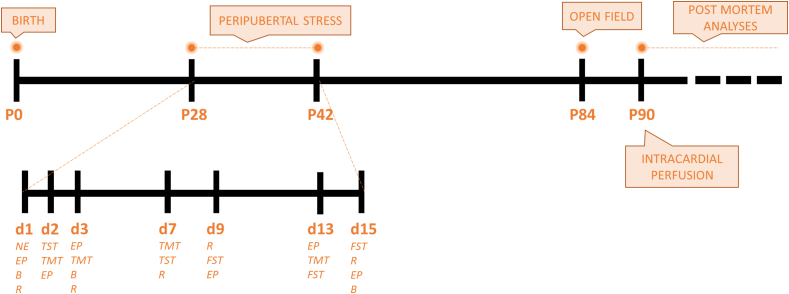
Fig. 1.
Timeline of the experiment.
2.2. Peripubertal stress
The PPS protocol was based on the exposure to different stressors and was a modification of a previously described protocol in rats (Papilloud et al., 2018; Márquez et al., 2013). Briefly, following exposure to an open-field for 5 min on P28, the stress protocol consisted of presenting seven different stressors distributed in 7 non-following days, between P28 and P42, during the light phase, following an unpredictable schedule (Fig. 1). Stressors were applied in two different sessions -morning and afternoon-separated by a resting period of minimum 3 h. The order and timing of the stressors were changed on the different days.
The stressors were: (a) 5 min exposure to an novel environment (NE), a rectangular arena of transparent plexiglass of 40 × 40 cm without cover (Stoelting Europe); (b) 25 min exposure to the synthetic fox odor trimethylthiazoline (TMT) (9 μl) (Phero Tech Inc., Canada), which was placed in a gauze inside a vial situated in the middle of a clean plastic cage (38 × 27.5 × 31 cm); (c) tail puncture simulating the blood collection, which consisted in a small incision made on the distal part of the tail with the help of a blade at 0 min (t0’) and 30 min later (t30’) (animals were held manually for this procedure); (d) 25 min exposure to an elevated platform (EP) measuring 12 × 12 cm and located 95 cm from the ground; (e) 30 min of restrain in a PVC conical tube with multiple holes; (f) 10 min exposure to the tail suspension test (TST); or (g) placement for 10 min in the forced swim test (FST), where animals are placed inside a glass beaker (25 cm tall x 14 cm Ø) half filled with water at 23–25 °C.
Control animals were handled on the days that their experimental counterparts were exposed to stress. In addition, all animals were weighted twice prior (P28–P30), during (P34–P36) and after (P40–P42) the PPS protocol to detect differences in their weight gain.
2.3. Behavioral studies
The open field (OF) was performed at P84, prior to the perfusion. In this test, each mouse was placed in the center of a black opaque plexiglass covered arena (40 × 40 cm) and was left to freely explore the chamber for 10 min. Animals were video-tracked by ANY-maze software (ANY-maze video tracking system v4.98; Stoelting Europe) and we measured the following parameters: (1) total distance travelled and immobile episodes (to study locomotor activity) and (2) time in the center zone/time periphery zone (for the measure of anxiety) (Perez-Rando et al., 2018; Simon et al., 1994). For this last measure we only took into account the first 5 min of the OF test, which refers to the novelty phase. The periphery zone was defined as the area located between 0 and 6 cm from the walls of the apparatus. Ethanol 70% was used to wipe the chamber each time prior to its use and before subsequent tests to remove any scent clues left by the previous subjects.
2.4. Sacrifice and perfusion
The intracardial perfusion was performed under deep pentobarbital anesthesia, first with 0.9% NaCl solution and then with 4% paraformaldehyde in sodium phosphate buffer (PB 0.1 M, pH 7.4). After perfusion, the brains were extracted from the skull and stored in PB 0.1 M. The two hemispheres were separated, and the right hemisphere was cut into 100 μm thick sections with a vibratome (Leica VT 1000E, Leica). The sections were collected in three subseries and stored at 4 °C in PB 0.1M and sodium azide 0.05% until used.
2.5. Immunohistochemistry
After cutting, sections were washed in PBS and then incubated for 1 h in 10% normal donkey serum (NDS; Abcys) in PBS with 0.2% Triton X-100 (PBST; Sigma-Aldrich). Afterward, they were incubated for 48 h at 4 °C with the appropriate primary antibody or antibody cocktail, diluted in PBST and 5% NDS (Table 1). The detection of PNN was carried out using a 1:400 diluted biotinylated Wisteria floribunda agglutinin (WFA, Sigma-Aldrich) (Härtig et al., 1992), which recognizes the N-acetyl-D-galactosamine component of the extracellular matrix. After washing, sections were incubated for 2 h at room temperature with matching secondary antibodies (Table 1) or A647-conjugated streptavidin (1:400, Life Technologies), which were also diluted in PBST. Finally, sections were washed in PB 0.1 M, mounted on slides, and coverslipped using fluorescence mounting medium (Dako North America Inc.).
Table 1.
Primary and secondary antibodies.
| Host | Dilution | incubation | Company | |
|---|---|---|---|---|
| Anti-PV | Guinea Pig | 1:2000 | 48 h, 4 °C | Synaptic Systems |
| Anti-CB1R | Rabbit | 1:1000 | 48 h, 4 °C | Synaptic Systems |
| Anti-VGLUT1 | Goat | 1:2000 | 48 h, 4 °C | Millipore |
| Anti-VGAT | Rabbit | 1:1000 | 48 h, 4 °C | Synaptic Systems |
| Anti-PSA-NCAM | Mouse | 1:1400 | 48 h, 4 °C | Millipore |
| Anti-Guinea Pig A555 | Goat | 1:400 | 2 h, room temp. | Life Technologies |
| Anti-Mouse IgG | Donkey | 1:400 | 2 h, room temp. | Invitrogen |
| Anti-Rabbit A555 | Donkey | 1:400 | 2 h, room temp. | Life Technologies |
| Anti-Goat A555 | Donkey | 1:400 | 2 h, room temp. | Life Technologies |
| Anti-Rabbit A647 | Donkey | 1:400 | 2 h, room temp. | Life Technologies |
| Anti-Mouse IgG A555 | Donkey | 1:400 | 2 h, room temp. | Invitrogen |
2.6. Analysis of the structure of pyramidal neurons
We analyzed the dendritic spine density of YFP expressing pyramidal neurons in THY1 mice. This structural parameter of the YFP + pyramidal neurons was studied using a laser scanning confocal microscope (Leica TCS SPE). We have chosen the IL cortex because it is a prefrontocortical region particularly involved in anxiety behaviors (Berg et al., 2019) and because of the tight control that it specifically exerts over the activity of monoaminergic neurons via descending afferents (Fullana et al., 2020). Moreover, previous results from our laboratories have found alterations in the expression of molecules related to inhibitory neurotransmission in this region after PPS in rats (Tzanoulinou et al., 2016).
For the analysis of the dendritic spine density 6 YFP expressing pyramidal neurons per animal were randomly selected on the IL region layer V. A 63 × oil immersion objective and a 3.5 × additional digital zoom were used to obtain confocal stacks covering the first 200 μm of the dendrite (Z-step size of 0.3 μm). The YFP expressing pyramidal neurons were randomly selected, making sure that they were only analyzed once, but the analyzed dendrites had to meet the following criteria to be included in the study: (a) their length should be at least 200 μm, and (b) no other dendrites should be found crossing their trajectory. Four segments were then established, namely the proximal (0–50 μm), proximal-medial (50–100 μm), medial-distal (100–150 μm), and distal (150–200 μm). The total number of spines in the entire 200 μm segment was also analyzed.
2.7. Analysis of neuropil puncta density and calculation of the E/I ratio
The density of puncta expressing the vesicular GABA transporter (VGAT, a marker of inhibitory synapses) and of the vesicular glutamate transporter 1 (VGLUT1, a marker of excitatory synapses) was studied in the neuropil of layer V in the IL subregion of the mPFC, as described before (Guirado et al., 2014, 2018). This is, from each immunostaining, slices from the same rostral-caudal level were examined under a confocal microscope (Leica TCS SPE) using a 63 × oil objective and 2 × digital zoom magnification. Confocal z-stacks covering the whole depth of the slice were taken with a 0.3 μm step size, making sure that the same field was not imaged twice, and only subsets of confocal planes with the optimal penetration level for each antibody were selected. On these planes, five small regions of neuropil (300 μm2) were selected for analysis, in order to avoid blood vessels and cell somata. Images were processed using FIJI-ImageJ Software (Schindelin et al., 2012) as described before (Guirado et al., 2018). Puncta density means from these five squared areas were determined by a custom-made macro. Briefly, images were first processed applying a Gaussian blur followed by a binary mask representing the expression profile of the molecules of interest (VGAT and VGLUT1). Then the macro measured the fluorescence intensity of the binarized mask and the density of puncta. The E/I ratio in the neuropil was defined as the density of excitatory puncta divided by the density of inhibitory puncta.
2.8. Analysis of the structure of parvalbumin expressing interneurons
We analyzed the complexity of the dendritic arborization of PV expressing interneurons in tdT-PV mice. The structure of the tdT + PV expressing interneurons was studied using a laser scanning confocal microscope (Leica TCS SPE). It was mostly focused on deep layers (V and VI) of the IL region.
For the study of the dendritic arborization of PV expressing interneurons, we performed an immunohistochemistry against PV to boost the tdTomato fluorescent signal. Then, we randomly selected 6 tdT expressing interneurons per animal, making sure that they were only selected once, following a previously described methodology (Gilabert-Juan et al., 2013). Z-series of optical sections (0.8 μm step size; 40 × objective) covering the dendritic tree of selected interneurons were obtained using the sequential scanning mode. To be suitable for analysis, these interneurons had to fulfill the following features: (1) the dendritic arbor of the cell must show at least a process with a length >120 μm, and (2) the soma must be located at least 30 μm deep from the surface of the tissue. The stacks obtained were then processed using FIJI software (Schindelin et al., 2012) to render 3D reconstructions. Neurons were traced using the “Simple neurite tracer” plugin, which also allowed us to analyze their Sholl profile in 3D (Longair et al., 2011) consists on the measure of the number of intersections of the dendrites with spheres of increasing radius centered in the soma (Sholl, 1953). The separation among the spheres of the analysis was set at 20 μm. Finally, we grouped the values in a proximal segment (the 40 μm closest to the soma), a medial segment (between 60 and 120 μm) and a distal segment (the distal 40 μm).
2.9. Analysis of perisomatic puncta on parvalbumin expressing interneurons and YFP expressing pyramidal neurons
We analyzed the density of puncta expressing different synaptic markers in the perisomatic region of PV expressing interneurons and YFP expressing pyramidal neurons, following a previously described protocol (Guirado et al., 2018; Castillo-Gómez et al., 2011). Concretely, we studied the density of puncta expressing the cannabinoid receptor 1 (CB1R) surrounding the somata of PV expressing interneurons and that of CB1R and of PV expressing puncta surrounding the somata of YFP + pyramidal neurons. In brief, between 20 and 30 pyramidal neurons and PV expressing interneurons on the layer V of the IL region were imaged per animal, making sure that they were only captured once, in three different sections. Confocal z-stacks covering the whole depth of the neuronal somata were taken with 0.3 μm step size using a 63 × oil objective with 2 × digital zoom magnification and images were processed using FIJI (Schindelin et al., 2012). The profile of the soma of these neurons was drawn and puncta localized within an area 0.5 μm distal from the edge of this profile were analyzed. A punctum was defined as a structure displaying an area between 0.15 μm2 and 2.5 μm2 32. The density of puncta (number of puncta per μm of soma perimeter) was analyzed on a single confocal plane from each selected neuron, in which the penetration of each antibody was optimal. The images were converted to 8-bit-deep and binarized using a determined threshold value, which depended on the marker, but it was the same for all images with the same marker. The images were then processed with a blur filter to reduce noise and separate closely apposed puncta. Finally, the density of puncta surrounding the soma was calculated (Guirado et al., 2018).
2.10. Study of the number of parvalbumin expressing cells and perineuronal nets
The total number of PV + neurons, PNN and PV + cells surrounded by PNN from the deep layers (V and VI) of the IL cortex was estimated using a modified version of the fractionator method (West, 1993; Nacher et al., 2002). We counted cells covering 100% of the sample area, this is, within each section, all labeled were counted. The fractionator sampling scheme refers to the methodology of examining 1 out of every 3 brain sections. Thus, our modification of the optical dissector combined with a 1:3 fractionator sampling is truly a modification of the optical fractionator method. 1:3 systematic and random series of sections covering the whole rostral to caudal extension of the IL were imaged using an Olympus FV-10 confocal microscope with a 10x magnification objective. The counting analysis was performed using FIJI/ImageJ imaging software (NIH (Schindelin et al., 2012),), making sure that the cells were only counted once. Cells appearing in the upper focal plane were omitted to prevent counting cell caps.
2.11. Study of the cell number and fluorescence intensity of PSA-NCAM expressing neurons
The total number of cells expressing the plasticity-related molecule PSA-NCAM was also estimated using the modified version of the fractionator method, as described above. Using the 1:3 fractionator, we then calculated the total number of PSA-NCAM immunoreactive neurons in the IL cortex per animal, being this number the sampling unit for statistical analyses.
To study the fluorescence intensity of PSA-NCAM + cells, all labeled neurons were imaged using a confocal microscope (Leica TCS SPE), keeping exactly the same settings. Afterward, using FIJI, we performed a dynamic binarization, setting the threshold in the top 10% of the histogram, of the PSA-NCAM signal to create a mask to calculate the fluorescence intensity.
2.12. Statistics
The experimental design and statistical analysis were based on the indications of Diester and collaborators (Diester et al., 2019). We first analyzed pooled data from both sexes and then data were segregated by sex and analyzed separately. However, we decided not to analyze sex as a between-subjects factor since the determination of differences between sexes was not an objective of our study. All slides were coded prior to quantitative analysis, and the code was not broken until the quantification was completed. For all the statistical analyses, the mean ± SEM was determined, and the resulting values were then subjected to Student's t-test analysis, except for the longitudinal weight study, which was analyzed using a repeated measures ANOVA (time x group) followed by a Bonferroni post hoc test to detect pairwise differences. All statistical analyses were performed using the IBM SPSS statistics software (v24.0). Outliers were detected and removed from analysis using Grubbs test. Probability values less than 0.05 (p < 0.05) were considered as statically significant. All graphs were drawn using GraphPad Prism v6 (GraphPad software Inc., USA).
3. Results
3.1. Peripubertal stress modifies locomotor activity particularly in adult females
We monitored the weight gain of the animals throughout 2-day intervals during PPS (P30–P28, P36–P34, P42–P40) and found a significantly lower weight gain in stressed male animals from P34 to P36 [Bonferroni post hoc test (P36–P34) p = 0.014; F2,18 (time) = 60.02, p < 0.0001; F1,9(group) = 0.664, p = 0.436; F2,18(time x group) = 7.853, p = 0.004]. There were no significant changes in this parameter when analyzing female mice or both sexes together.
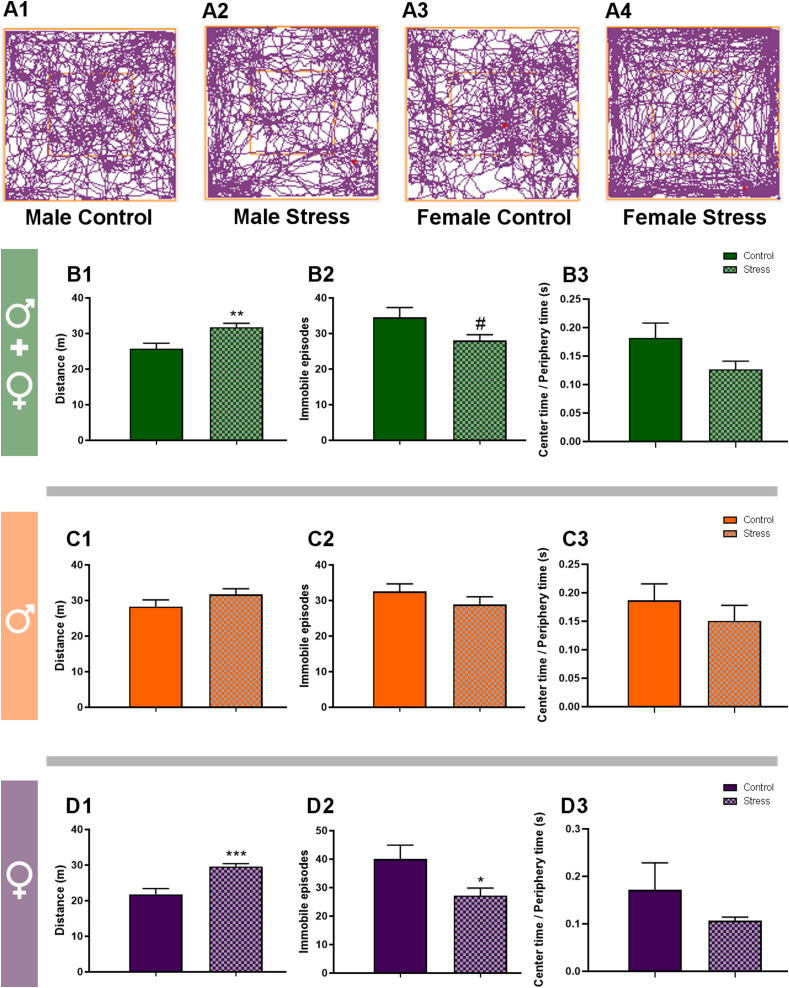
We also evaluated whether mice exposed to stress during puberty showed differential behavioral responses in the OF when compared to their respective control groups (Fig. 2). Animals were tested in the OF at P84 to evaluate long-term effects of PPS on locomotor activity. We found increases that were statistically significant or show a tendency towards significance when both sexes were considered together (Fig. 2B1-2; distance travelled: p = 0.003, t = 0.134; immobile episodes: p = 0.059, t = 1.951), but not when males were analyzed separately (Fig. 2C1-2; p > 1.000 in all parameters). By contrast, stressed females showed significant increases in these locomotion related parameters (Fig. 2D1-2; distance travelled: p = 0.0007, t = −4.486; immobile episodes: p = 0.032, t = 1.989). When studying anxiety-related behaviors, we calculated the center time/periphery time ratio and did not find any significant differences in any of the experimental groups (Fig. 2B3, C3, D3).
Fig. 2.
Behavioral effects of peripubertal stress in the Open Field Test at P84.
Behavioral results of PV-tdT mice in the open field, 42 days (P84) after the PPS protocol or corresponding unstressed controls. A: Representative track-plot reports recorded during the OF test session to test the locomotion and anxiety-related behavior (A1-A4). B-D: Graphs showing changes in behavioral parameters related to locomotor activity (B1–B2, C1–C2, D1-D2) and anxiety-related (B3, C3, D3) in the OF test. Graphs in green include data from all experimental animals (not separated by sex, B; N: controls = 27, PPS = 17). Graphs in orange include data from males (C: N: controls = 16, PPS = 8). Graphs in purple include data from females (D; N: controls = 8, PPS = 9). Values represent mean ± S.E.M. Asterisks in graphs indicate statistically significant effects between groups (Control x Stress) after unpaired Student's t-test. Symbols: # 0.1 > p > 0.05; *p < 0.05; **p < 0.01; ***p < 0.001.
3.2. Peripubertal stress alters dendritic spine density of pyramidal neurons differentially in males and females
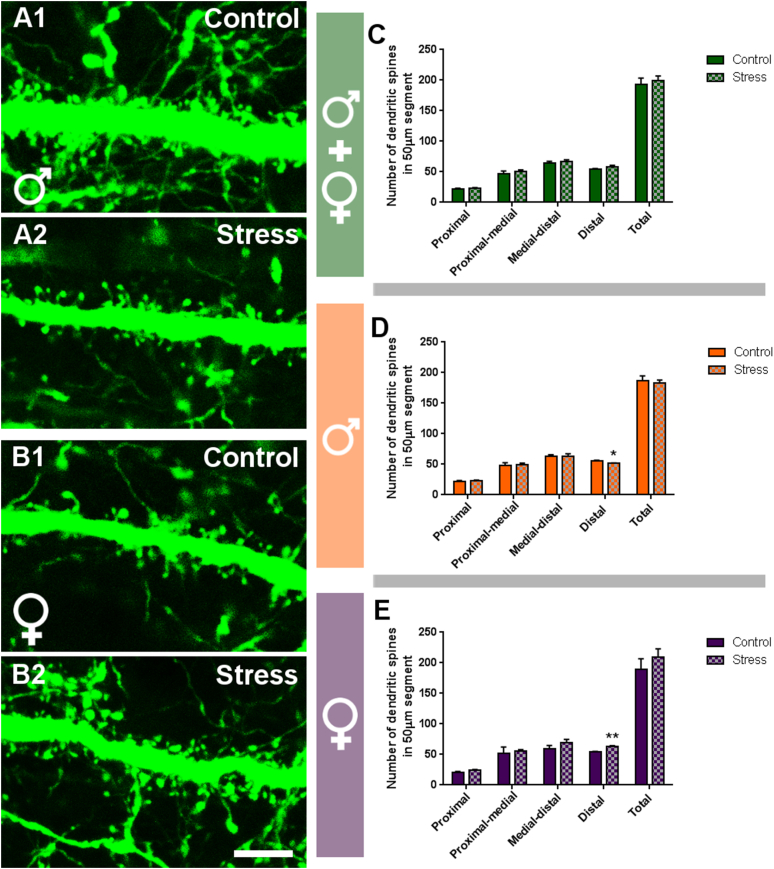
Males submitted to PPS had a significantly lower density of spines in the distal 50 μm subsegment of their dendrites when compared to control mice (Fig. 3D; p = 0.045, t = 2.371), while this parameter was significantly higher in stressed females (Fig. 3E; p = 0.002, t = -4.584). Consequently, these differences were not significant when both sexes were analyzed together (Fig. 3C). No significant differences were detected when analyzing the other 50 μm subsegments of the dendrites or the whole extension of the dendritic segment considered (data not shown).
Fig. 3.
Analysis of the density of dendritic spines in pyramidal neurons of the infralimbic cortex.
A & B: Representative 2D projections of distal segments of spiny apical dendrites of pyramidal neurons from control and stressed males (A) and females (B) at P84. C-E: Graphs showing the results of the analysis of dendritic spine density. Spine density was determined in four 50 μm-length segments located 0–50 μm (proximal), 50–100 μm (proximal-medial), 100–150 μm (medial-distal) and 150–200 μm (distal) from the soma and in the total length (total). The graph in green includes data from all experimental animals (not separated by sex) (C; N: controls = 13, PPS = 11). The graph in orange includes data from males (D; N: controls = 6, PPS = 6). The graph in purple includes data from females (E; N: controls = 6, PPS = 6). Values represent mean ± S.E.M. Asterisks in graphs indicate statistically significant effects between groups (Control x Stress) after unpaired Student's t-test. Symbols: # 0.1 > p > 0.05; *p < 0.05; **p < 0.01; ***p < 0.001. Scale bar: 5 μm.
3.3. Peripubertal stress increases the density of puncta expressing inhibitory synaptic markers and alters the E/I ratio
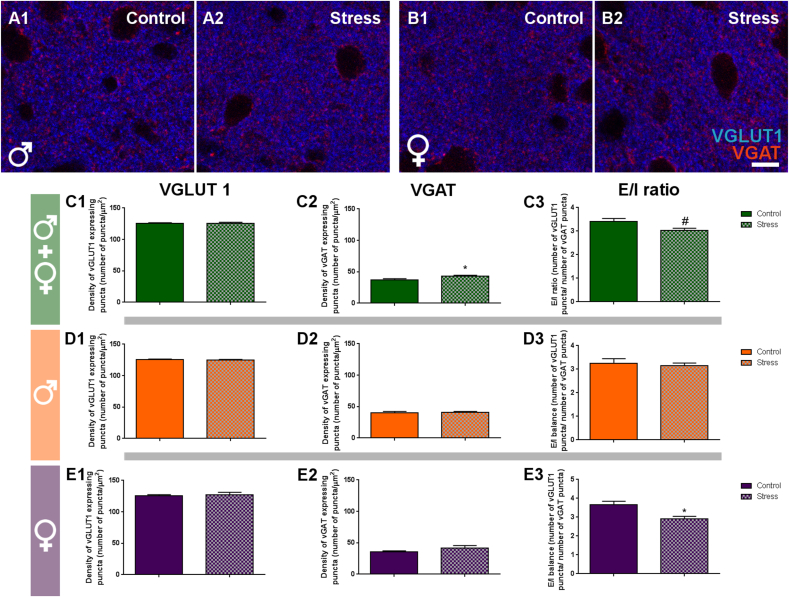
To have a readout of the putative alterations in the ratio of excitatory and inhibitory synapses induced by PPS in adult animals, we studied with immunohistochemistry the density of puncta expressing GABA and glutamate vesicular 1 transporters (VGAT and VGLUT1) in the neuropil of the IL (Fig. 4A and B). Regarding VGLUT1+ puncta, we did not find significant differences in any of the same sex comparisons (Fig. 4C1-E1). However, the density of VGAT + puncta in stressed animals was significantly affected by PPS when both all animals were analyzed together (p = 0.041, t = -2.127) (Fig. 4C2-E2). The E/I ratio [(number of VGLUT1+ puncta/μm2)/(number of VGAT + puncta/μm2); Fig. 4C3-E3] was significantly reduced in stressed females (p = 0.018, t = 2.622) and there was also a trend towards a decrease when both sexes were analyzed together (p = 0.053, t = 2.011).
Fig. 4.
Analysis of the density of inhibitory and excitatory puncta in the infralimbic cortex.
A & B: Representative single confocal planes of puncta expressing excitatory (blue, VGLUT1) and inhibitory (red, VGAT) synaptic markers in the neuropil of stressed and control mice in males (A) and females (B) at P84. C-D: Graphs representing the effects of PPS on the density of VGLUT1+ (C1, D1, E1) and VGAT + puncta (C2, D2, E2) in the IL subregion of the mPFC. The effects on the E/I ratio (density of VGLUT1+ puncta/density of VGAT + puncta) were also plotted (C3, D3, E3). Graphs in the first row (green) include data from all experimental animals (not separated by sex) (C; N: controls = 23, PPS = 13). Graphs in the second row (orange) include data from males (D; N: controls = 11, PPS = 6). Graphs in the third row (purple) include data from females (E; N: controls = 12, PPS = 7). Values represent mean ± S.E.M. Asterisks in graphs indicate statistically significant effects between groups (Control x Stress) after unpaired Student's t-test. Symbols: # 0.1 > p > 0.05; *p < 0.05; **p < 0.01; ***p < 0.001. Scale bar 20 μm. VGLUT1: Vesicular Glutamate Transporter-1. VGAT: Vesicular GABA Transporter.
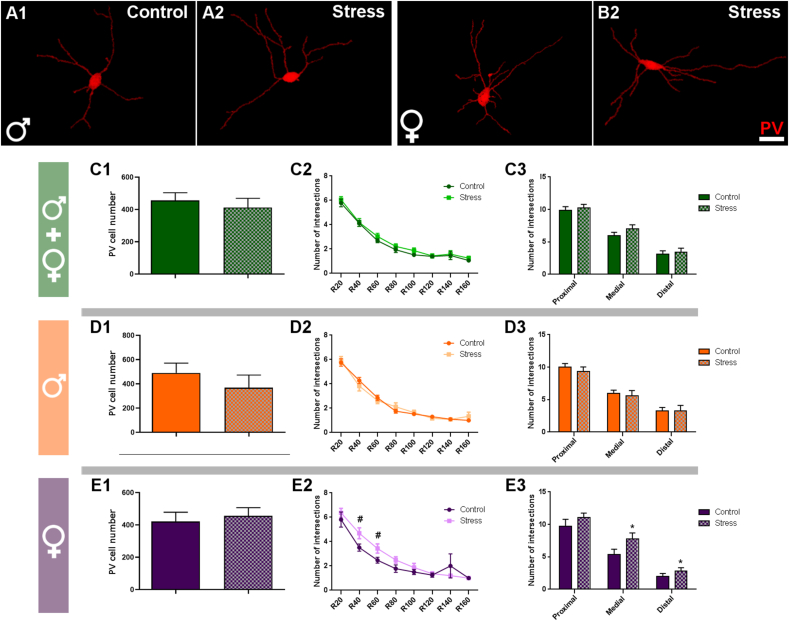
3.4. Peripubertal stress induces an increase in dendritic complexity of parvalbumin expressing interneurons only in females
In order to analyze whether PV expressing neurons were affected by PPS, we estimated the number of PV + somata in the IL and the complexity of the dendritic arbor of these cells using Sholl analysis (Sholl, 1953) (Fig. 5A and B). We did not observe any statistically significant difference induced by PPS in the density number of PV expressing somata when analyzing both sexes together or separately (Fig. 5C1-E1). In the Sholl analysis, we found that the female stressed group displayed a trend towards an increase in the number of intersections with Sholl spheres in the 40 μm (p = 0.055, t = -2.103) and 60 μm radii (p = 0.053, t = -2.113) (Fig. 5C2-E2). There were no significant differences in males or when we analyzed both sexes together. However, when we grouped the intersections in 3 different regions, taking in consideration the distance to the soma (Fig. 5C3-E3), we observed that in the medial (p = 0.041, t = -2.408) and distal (p = 0.049, t = 1.202) regions, the stressed females had a significantly higher number when compared to female control mice.
Fig. 5.
Analysis of the number of somata and complexity of the dendritic arbor of parvalbumin expressing interneurons in the infralimbic cortex.
A & B: Representative 3D reconstructions of the dendritic arbor of PV expressing interneurons in control and stressed animals, both in males (A) and females (B) at P84. C1- E1: Graphs representing the total number of TdTomato expressing somata in IL cortex. C2- E2: graphs showing the results of Sholl analysis of these PV-expressing interneurons, indicating the number of intersections per Sholl sphere (see material and methods for details). C3- E3: Graphs showing the results of Sholl analysis grouping the data in proximal (spheres in 0–60 μm), medial (spheres in 60–100 μm) and distal (spheres in 100–160 μm) regions. Graphs in green include data from all experimental animals (not separated by sex) (C; N: controls = 18, PPS = 15). Graphs in orange include data from males (D; N: controls = 10, PPS = 7). Graphs in purple include data from females (E; N: controls = 8, PPS = 8). Values represent mean ± S.E.M. Asterisks in graphs indicate statistically significant effects between groups (Control x Stress) after unpaired Student's t-test. Symbols: # 0.1 > p > 0.05; *p < 0.05; **p < 0.01; ***p < 0.001. Scale bar 25 μm.
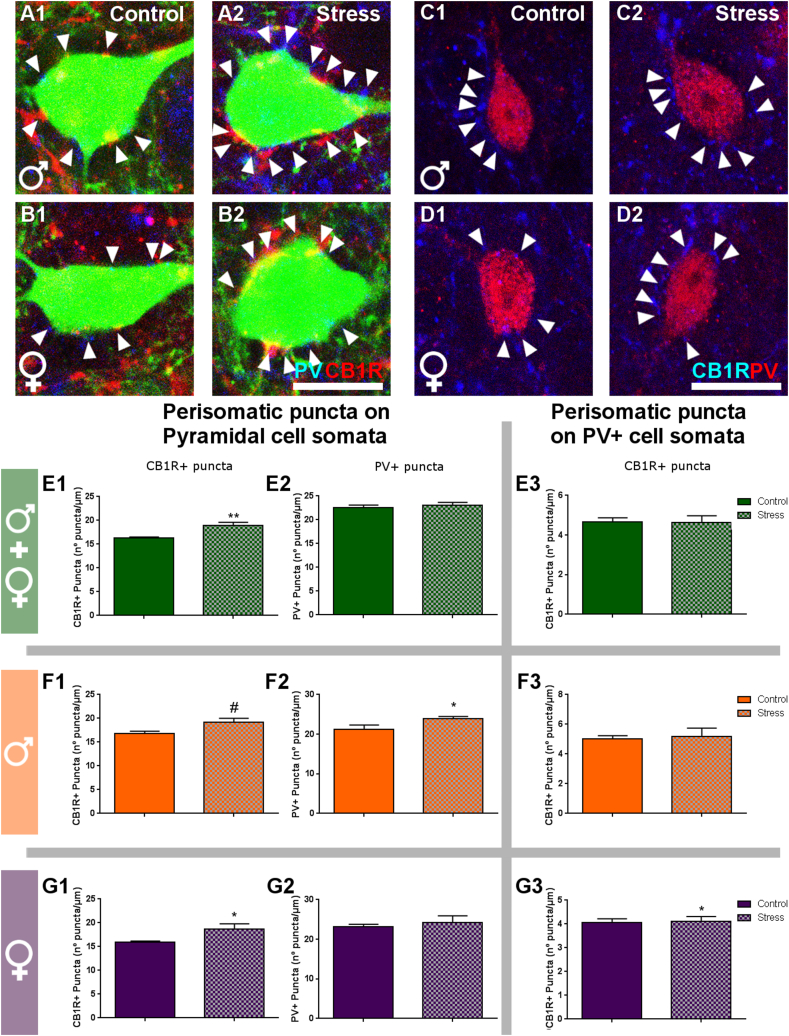
3.5. Peripubertal stress alters the density of puncta expressing excitatory and inhibitory synaptic markers in the perisomatic region of parvalbumin expressing interneurons and pyramidal neurons
To have an estimation of the synaptic input that PV or cholecystokinin (CCK) expressing basket cells establish onto the somata of YFP + pyramidal neurons (Fig. 6A and B) and PV + interneurons (Fig. 6C and D) in layer V of the IL, we quantified the density of perisomatic puncta expressing PV or the CB1R, which is highly expressed in the synapses of cortical CCK + basket cells. In PPS animals, pyramidal neuron somata were surrounded by a higher density of CB1R + puncta when both all animals were analyzed together (p = 0.002, t = -3.660, Fig. 6E1). We also observed a trend towards an increase in this density in male stressed mice (p = 0.085, t = -1.936, Fig. 6F1) and a significantly higher density in female stressed mice (p = 0.035, t = -2.378, Fig. 6G1) when compared with their respective controls. When analyzing PV + perisomatic puncta on pyramidal neurons, we observed a significantly higher density in the male stressed group when compared to the male control group (p = 0.046, t = -2.318). No significant differences were found when considering both sexes together or only females (Fig. 6E2-G2). The analysis of CB1R + puncta on the perisomatic region of PV expressing interneurons revealed a significantly higher density in the female stressed group compared to the female control group (p = 0.046, t = -2.421), but not in males or when grouping all animals together (Fig. 6E3-F3).
Fig. 6.
Density of endocannabinoid receptor 1 and parvalbumin immunoreactive puncta in the perisomatic region of pyramidal neurons and parvalbumin expressing interneurons in the infralimbic cortex.
A-D: Confocal images (single confocal planes) comparing the density of CB1R and PV expressing puncta (indicated by arrows) in pyramidal (A, B) and PV + cells (C, D) between control and stressed mice at P84. E-G: Histograms representing changes induced by stress on the density (number of puncta/μm of soma perimeter) of CB1R (E1-G1) and PV (E2-G2) expressing puncta in the perisomatic region of pyramidal neurons. Histograms in E3-G3 represent the density of CB1R positive puncta surrounding PV expressing cell somata. Graphs in green include data from all experimental animals (not separated by sex) (E; N: controls = 23, PPS = 13). Graphs in orange include the data from males (F; N: controls = 11, PPS = 6). Graphs in purple include the data from females (G; N: controls = 12, PPS = 7). Values represent mean ± S.E.M. Asterisks in graphs indicate statistically significant effects between groups (Control x Stress) after unpaired Student's t-test. Symbols: # 0.1 > p > 0.05; *p < 0.05; **p < 0.01; ***p < 0.001. Scale bar 10 μm CB1R: endocannabinoid receptor 1. PV: parvalbumin.
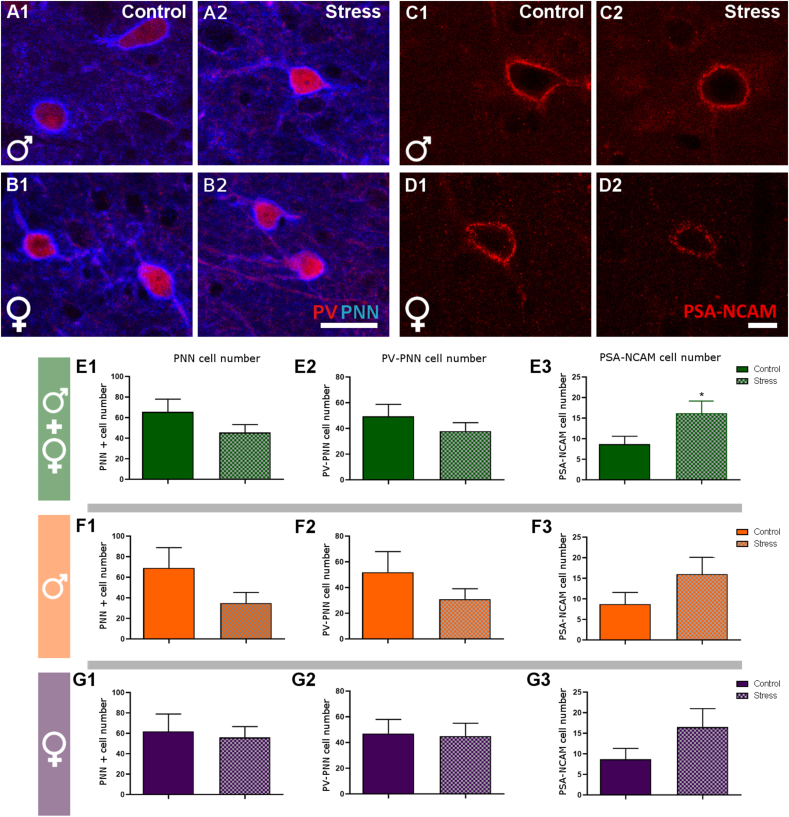
3.6. Peripubertal stress alters the number of PSA-NCAM expressing somata when analyzing all animals together but not the number of PNN or PV + cells surrounded by PNN
Since the expression of molecules related to interneuronal plasticity, such as components of the PNN or PSA-NCAM, is altered after chronic exposure to different stressors (Ueno et al., 2018; Sandi, 2004), we analyzed these parameters in layer V of the IL in the present experiment (Fig. 7A-D). PPS did not induce significant changes either in the total number of PNN (Fig. 7E1-G1) or in the number of PV + cell surrounded by PNN in stressed animals (Fig. 7E2-G2). The number of PSA-NCAM expressing somata, was increased in stressed animals considering both sexes together (p = 0.034, t = 2.203), but not separately (Fig. 7E3-G3). We also analyzed the fluorescence intensity of PSA-NCAM immunoreactive neurons and did not find significant changes between the experimental groups.
Fig. 7.
Analysis of plasticity-related molecules in the infralimbic cortex.
A & B: Representative single confocal planes showing PV-immunoreactive somata surrounded by PNN in control and stressed males (A) and females (B) at P84. C & D: Representative single confocal planes showing PSA-NCAM-expressing somata in control and stressed males (C) and females (D). E-G: Graphs representing changes in the number of PNN (E1, F1, G1), PV + somata surrounded by PNN (E2, F2, G2), and the PSA-NCAM (E3, F3, G3). Graphs in the first line (green color) include data from all experimental animals (not separated by sex) (E; N: controls = 12, PPS = 12). Graphs in the second line (orange color) only include the data from male animals (F; N: controls = 6, PPS = 6). Graphs in the third line (purple color) only include the data from female animals (G; N: controls = 6, PPS = 6). Values represent mean ± S.E.M. Asterisks in graphs indicate statistically significant effects between groups (Control x Stress) after unpaired Student's t-test. Symbols: # 0.1 > p > 0.05; *p < 0.05; **p < 0.01; ***p < 0.001. Scale bar: 30 μm. PV: parvalbumin. PNN: perineuronal nets. PSA-NCAM: polysialylated form of the neural cell adhesion molecule.
4. Discussion
The development of the PFC begins in utero and continues well into postnatal life, during infancy, adolescence and early adulthood (Fuster, 2002). During this postnatal period, both excitatory (Petanjek et al., 2011) and inhibitory neurons (Caballero and Tseng, 2016; McKlveen et al., 2016) undergo the final stages of maturation, remodeling their structure and connectivity. This protracted development of the PFC makes this cortical region especially vulnerable to aversive experiences. Stress suffered during adolescence has an important impact on the structure of PFC pyramidal neurons and on the development of PFC-dependent cognitive functions (Tzanoulinou et al., 2014, 2016). In this study, we have used a murine model of peripubertal stress to explore long-term alterations in the structure and connectivity of PV expressing interneurons and pyramidal neurons in the mPFC of males and diestrus females.
The PPS protocol that we have used is based on a previously developed and repeatedly validated protocol in rats, which has also been related to long-term alterations in social and affective behavior during adulthood (Papilloud et al., 2018; Wommack et al., 2004; Garrett and Wellman, 2009; Sandi, 2004). Stressors are applied from P28 to P42, a transitional period covering late childhood and adolescence in rodents, which is associated with different neuroanatomical changes in key brain regions like the PFC that parallel some behavioral changes (Brenhouse and Andersen, 2011). Several studies have explored the impact of stress during adolescence, but most of them are based on physical stressors (Ueno et al., 2018; Carneiro De Oliveira et al., 2016). Our model is based on exposure to unpredictable stressors with a potent psychogenic effect and, therefore, may better mimic some of the aversive experiences that occur during human childhood and adolescence.
A wealth of observations in animals (Brenhouse and Andersen, 2011; Shaw et al., 2020b) and humans (Eid et al., 2019) show that females are more vulnerable than male to develop stress-related disorders, such as major depression and anxiety. Here, we found important sex-dependent differences following peripubertal stress; in fact, most effects were only observed in diestrus females or when considering both sexes together, but rarely only in males. Interestingly, this occurs not only at the behavioral level, but also at cellular and molecular levels.
At the behavioral level, we found increased locomotion in adult mice when considering both sexes together and only in diestrus females, in agreement with previous reports (Castillo-Gómez et al., 2017; Ishikawa et al., 2014; Eiland et al., 2012). A recent study in male mice using chronic physical stress also found increases in locomotor activity, although the animals were tested immediately after the stress and not in adulthood (Ueno et al., 2018). However, some other studies in male mice, using isolation and forced swimming, observed decreases in the distance travelled (Page and Coutellier, 2018). In the open field test, we did not observe significant changes in anxiety-related parameters, although decreases in the stressed animals can be observed in every experimental group. Similar results in anxiety-related behavior have been found in recent studies, in which male mice were chronically exposed to physical stressors during the same peripubertal period of our study (Page and Coutellier, 2018) or when male rats were exposed to a similar PPS paradigm as used in our study (Tzanoulinou et al., 2020). Future studies expanding the behavioral characterization to measure anxiety social behaviors and cognition, in the context of PFC neuroarchitecture analyses, are warranted. To date, those analyses have only been performed in rats (Márquez et al., 2013; Tzanoulinou et al., 2020; Veenit et al., 2013).
Importantly, we found differential effects of stress on spine density of pyramidal neurons in the IL depending on the sex analyzed: it increased in diestrus females, while it decreased in males. These changes were restricted to the most distal segment of dendrite analyzed, which is a common feature in this type of analyses. Our results in males are consistent with previous reports describing decreased spine density in the mPFC after chronic stress, both during early life and during adulthood. Although most studies in adult mice have been focused on the prelimbic cortex (PrL), chronic corticosterone treatment reduces dendritic spine density in pyramidal neurons of the adult IL (Gourley et al., 2013). Studies on the effects of chronic stress during early life are scarce, but chronic restraint (Eiland et al., 2012) or social stress (Urban et al., 2019) during adolescence and maternal separation (Chocyk et al., 2013) in male rats induce similar structural changes to those observed by us. However, in these studies, samples from animals were analyzed immediately after the stressors. In addition, another study using chronic restraint stress in male and female mice together did not find alterations in spine density in the mPFC (Chohan et al., 2014). Male rats subjected to foot-shock stress during adolescence and analyzed during adulthood, show decreases in spine density in IL pyramidal neurons (Lyttle et al., 2015), which is consistent with our results. In this line, a previous study from our laboratory showed that postweaning social isolation also decreased the density of spines in medial/distal segments of pyramidal neurons in the mPFC of male mice (Castillo-Gómez et al., 2017). However, recent studies using social instability stress during adolescence in male mice, have rendered conflicting results: while one study found significant decreases of spine density in the mPFC and specifically in the IL (Wang et al., 2020), other did not find changes in this parameter (Breach et al., 2019). Unfortunately, studies on the effects of stress on females are still very scarce but they generally show that adult female rodents subjected to stress exhibit either no dendritic changes or even dendritic hypertrophy in the mPFC (Wellman et al., 2020; Wohleb et al., 2018; Iqbal and Ma, 2020). However, a recent study that focused in the PrL found that chronic stress and corticosterone treatment at adulthood had similar impact (i.e., decrease) on the dendritic spine density of pyramidal neurons in both sexes (Anderson et al., 2020). Future studies should aim at determining the differential impact of stress depending on the sex, brain region, age, and species.
The observed changes in pyramidal neuron structure may be linked to alterations in the excitatory/inhibitory balance, which is crucial for PFC function and is also altered in chronic stress models and stress-related psychiatric disorders (Page and Coutellier, 2019). To this end, we analyzed the density of puncta expressing specific inhibitory or excitatory synaptic proteins to explore this balance. We did not find changes in the density of puncta expressing the excitatory marker VGLUT1, consistent with our previous results following postweaning social isolation in adult mice (Castillo-Gómez et al., 2017). By contrast, we observed a significant increase in the density of VGAT immunoreactive puncta, which leads to a decrease in the ratio between excitatory and inhibitory puncta. These results can be observed when considering both sexes together and in diestrus females, suggesting again a higher susceptibility of females to PPS. This increase in inhibitory puncta suggests an enhancement of inhibitory neurotransmission and, consequently, a hypoactivity of the mPFC. This finding agrees with previous results in young rats subjected to different chronic stress paradigms. Specifically, chronic variable stress was shown to increase miniature inhibitory postsynaptic currents, suggesting elevated GABA release, accompanied by increased inhibitory appositions and terminals onto glutamatergic cells (McKlveen et al., 2016). However, previous work from our laboratories showed a reduced expression of GAD6 protein in the PrL and IL in PPS adult male rats (Tzanoulinou et al., 2016). Other studies in mice, using as stressors a combination of social isolation and forced swim during adolescence, found a trend towards a reduction in VGAT mRNA, but not of GAD67 or gephyrin in adult individuals (Page et al., 2018). Similar negative results were found in our lab when analyzing the impact of postweaning social isolation (Castillo-Gómez et al., 2017).
Stress-induced hypoactivity of the PFC has been hypothesized to be due to increased activation of PV expressing cells (Page and Coutellier, 2019). Here, we explored this possibility by analyzing the structure and connectivity of PV + interneurons in the mPFC, as well as the presence of PNN surrounding these inhibitory cells. The final steps of the maturation of the PFC involve increases in the glutamatergic input to PV + cells and in the expression of PV, which, interestingly, occur sooner in females, at least in the hippocampus (Wu et al., 2014). These last stages of the development of PV expressing cells are also governed by the apparition of PNN surrounding these interneurons (Caballero and Tseng, 2016; Ueno et al., 2017). Chronic stress has an important impact on prefrontocortical PV-expressing interneurons during adulthood. Different studies employing different stress paradigms in rats and mice have reported increases in the number or density of PV immunoreactive cells and the expression of PV mRNA (Pesarico et al., 2019; Shepard et al., 2016; Shepard and Coutellier, 2018). However, other studies did not find changes (Zadrozna et al., 2011) or found decreases in these parameters (Czéh et al., 2018; Banasr et al., 2017; Todorović et al., 2019). Importantly, these later studies used longer stress protocols or found their effects exclusively in anhedonic/vulnerable animals. The data from animals stressed during the peripubertal period is also heterogenous. Our data indicating a lack of difference in the number of PV expressing interneurons in the mPFC of male mice is in agreement with studies using a combination of social isolation and forced swimming (Page et al., 2018) or chronic restraint stress (Clarke et al., 2019). However, another study, using a combination of physical stressors during the adolescence found a reduction in the density of PV + cells in the PrL, although only in male mice (Page and Coutellier, 2018); no differences were detected in the IL cortex or when analyzing females (although the estrous cycle phase was not controlled at sacrifice). Ueno et al. (2018) also found no differences in the density of PV + interneurons in the mPFC after applying physical stressors to male mice during early adolescence; although these results were obtained from juvenile animals. Our results here suggest that, although there are no stress-induced differences in the number of PV + interneurons, the effects of PPS on these cells appear to be stronger in diestrus females, because they display an increased dendritic arborization. This increase may reflect the consequences of the changes in the excitatory/inhibitory ratio discussed above.
In order to study alterations in the inhibitory connectivity of pyramidal neurons in the PFC of PPS animals, we analyzed the density of PV + puncta on their perisomatic region. Surprisingly, and contrasting with the majority of the results from our study, a significant increase in this parameter was only found in males. These results are suggestive of an increased inhibition on pyramidal neurons. Interestingly, and in line with these results, a recent study has found an increase in the density of excitatory puncta on PV + cells after chronic unpredictable stress in adult mice (both sexes) (Shepard and Coutellier, 2018). Similarly, adult rats submitted to 2 weeks of chronic unpredictable stress showed increased inhibition on mPFC pyramidal neurons and in the density of perisomatic inhibitory puncta, which is mainly coming from PV basket cells (McKlveen et al., 2016). However, no changes in these parameters were observed in other study in which adult rats were subjected to restraint stress during 10 days (Pesarico et al., 2019). A possible explanation for these opposing results is that the activity of PV + neurons might be different in different phases of stress, since longer stress protocols seem to alter the inhibitory input from PV + interneurons.
In the cerebral cortex, the CB1R is mostly expressed in the presynaptic terminals of CCK expressing basket cells and particularly in the PFC is expressed mainly by inhibitory neurons (Wȩdzony and Chocyk, 2009; Katona and Freund, 2012). To further analyze the effects of PPS on the perisomatic inhibition of pyramidal neurons, but also to analyze this type of inhibition on PV expressing neurons, we studied the density of perisomatic puncta expressing CB1R on these two neuronal populations. PPS induced an important increase in CB1R density on both neuronal populations, but only in diestrus females and when all animals were analyzed together. Similar alterations in the cannabinoid system have been frequently described following stress exposure. For instance, chronic restraint stress in adult rats increases CB1R receptor binding in the mPFC and the same effect can be observed in adolescent rats (Lee and Hill, 2013). Chronic unpredictable stress during adulthood (Hill et al., 2008) and maternal deprivation also lead to increased CB1R expression in the PFC (Marco et al., 2014). Interestingly, in line with these results and our own here, a study has described that the dorsolateral PFC of major depression patients has an increased density of CB1R (Choi et al., 2012).
Regarding the percentage of PV expressing cells covered by PNN in the adult PFC of rodents subjected to PPS, contradicting results have been reported. While one study found an increase in this percentage when rats were exposed to social isolation and forced swim during adolescence (Page et al., 2018), another study did not find differences in this parameter when analyzing mice subjected to peripubertal physical stressors (Page and Coutellier, 2018), which is consistent with our present results. Similar negative results were obtained in other experiment using peripubertal physical stressors (Ueno et al., 2018). By contrast, postweaning social isolation increased the number of PNN and that of PV + interneurons covered by PNN in the mPFC (Castillo-Gómez et al., 2017).
One limitation of our study is the lack of physiological measurements, specially related to alterations in the HPA axis allowing us to contrast our behavioral and neurobiological findings with stress reactivity However, former evidence using similar stressors at the same developmental age supports their high capacity to elicit HPA axis activation and long-term changes in corticosterone reactivity (Walker et al., 2018; Tzanoulinou et al., 2020). In addition, our results suggest that the alterations that PPS is producing in the IL cortex circuitry may render animals, particularly females, more susceptible to stress during adult life. This hypothesis should be tested, analyzing the response of PPS animals to stressors during adulthood.
Finally, when testing for potential changes in plasticity molecules, we found that PPS led to an increase in the number of PSA-NCAM immunoreactive cells in the IL when all animals were analyzed together. These results are similar to those recently obtained by (Tzanoulinou et al., 2020) in the hippocampus of adult rats previously submitted to a similar PPS protocol. However, other early life adverse experiences, such as social isolation rearing, appear to have no effect on PSA-NCAM expression (Castillo-Gómez et al., 2017). There are also contradicting results regarding the effects of different chronic stress paradigms on PSA-NCAM expression in the adult PFC: while some reports failed to find changes in this parameter after restrain stress (Gilabert-Juan et al., 2013), others using social isolation found decreases (Djordjevic et al., 2010, 2012). Notably, previous studies from our lab showed that the manipulation of PSA-NCAM expression has an important impact on the structure and connectivity of prefrontocortical PV + basket cells, particularly modifying their perisomatic contacts on pyramidal neurons (Castillo-Gómez et al., 2011).
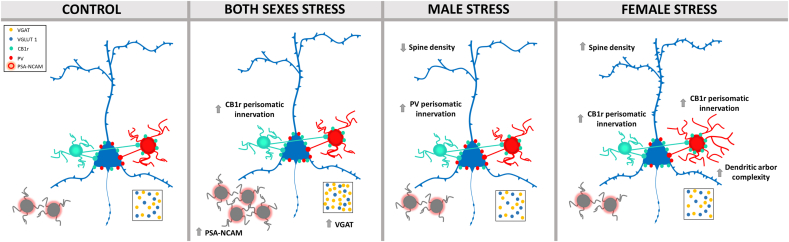
5. Conclusion
In summary, we present evidence for long-term alterations of excitatory and particularly inhibitory circuitry in the PFC and increased anxiety-related behavior as a consequence of PPS exposure, which is specially visible in diestrus females (Fig. 8). These results highlight the peripubertal period, comprising childhood and early adolescence, as a window of higher vulnerability to aversive experiences in female than in male mice. Our results additionally remark the importance of conducting stress studies in both sexes, particularly given the higher incidence of stress-related disorders in women.
Fig. 8.
Summary of the stress effects on prefrontocortical networks.
Funding
This work was supported by the Spanish Ministry of Science and Innovation (RTI2018-098269-B-I00 and RTI2018-095698-B-I00), the Generalitat Valenciana (PROMETEU/2020/024 and GV/2019/088), the Universitat Jaume I (UJI-A2020-20), Swiss National Science Foundation (SNSF) [176206; NCCR Synapsy: 51NF40-158776 and -185897] and ERA-NET (Biostress; SNSF No 31NE30_189061). CB-F and SC were supported by predoctoral fellowships from the Generalitat Valenciana (ACIF/2016/376 and GRISOLIAP/2017/087), JA was supported by a FPI fellowship from the Spanish Ministry of Science and Innovation (PRE2019-088458) and MP-R held a “Atracció de talent” postdoctoral fellowship from University of Valencia.
CRediT authorship contribution statement
Clara Bueno-Fernandez: Formal analysis, Investigation. Marta Perez-Rando: Formal analysis, Investigation. Julia Alcaide: Investigation. Simona Coviello: Investigation. Carmen Sandi: Conceptualization, Writing – review & editing. Esther Castillo-Gómez: Conceptualization, Writing – review & editing. Juan Nacher: Conceptualization, Writing – review & editing, Supervision, Project administration, Funding acquisition.
Contributor Information
Esther Castillo-Gómez, Email: escastil@uji.es.
Juan Nacher, Email: nacher@uv.es.
References
- Anderson R.M. Evidence for similar prefrontal structural and functional alterations in male and female rats following chronic stress or glucocorticoid exposure. Cerebr. Cortex. 2020;30:353–370. doi: 10.1093/cercor/bhz092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten A.F.T. Stress weakens prefrontal networks: molecular insults to higher cognition. Nat. Neurosci. 2015;18:1376–1385. doi: 10.1038/nn.4087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avital A., Richter-Levin G. Exposure to juvenile stress exacerbates the behavioural consequences of exposure to stress in the adult rat. Int. J. Neuropsychopharmacol. 2005;8:163–173. doi: 10.1017/S1461145704004808. [DOI] [PubMed] [Google Scholar]
- Banasr M. Characterization of GABAergic marker expression in the chronic unpredictable stress model of depression. Chronic Stress. 2017;1 doi: 10.1177/2470547017720459. 247054701772045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangasser D.A., Valentino R.J. Sex differences in stress-related psychiatric disorders: neurobiological perspectives. Front. Neuroendocrinol. 2014;35:303–319. doi: 10.1016/j.yfrne.2014.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg L., Eckardt J., Masseck O.A. Enhanced activity of pyramidal neurons in the infralimbic cortex drives anxiety behavior. PloS One. 2019;14 doi: 10.1371/journal.pone.0210949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breach M.R., Moench K.M., Wellman C.L. Social instability in adolescence differentially alters dendritic morphology in the medial prefrontal cortex and its response to stress in adult male and female rats. Dev. Neurobiol. 2019;79:839–856. doi: 10.1002/dneu.22723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenhouse H.C., Andersen S.L. Developmental trajectories during adolescence in males and females: a cross-species understanding of underlying brain changes. Neurosci. Biobehav. Rev. 2011;35:1687–1703. doi: 10.1016/j.neubiorev.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caballero A., Tseng K.Y. GABAergic function as a limiting factor for prefrontal maturation during adolescence. Trends Neurosci. 2016;39:441–448. doi: 10.1016/j.tins.2016.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caligioni C.S. Assessing reproductive status/stages in mice. Curr. Protoc. Neurosci. 2009 doi: 10.1002/0471142301.nsa04is48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carneiro De Oliveira P.E. Stress-induced locomotor sensitization to amphetamine in adult, but not in adolescent rats, is associated with increased expression of ΔFosB in the nucleus accumbens. Front. Behav. Neurosci. 2016;10 doi: 10.3389/fnbeh.2016.00173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo-Gómez E., Varea E., Blasco-Ibáñez J.M., Crespo C., Nacher J. Polysialic acid is required for dopamine D2 receptor-mediated plasticity involving inhibitory circuits of the rat medial prefrontal cortex. PloS One. 2011;6 doi: 10.1371/journal.pone.0029516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo-Gómez E. Early social isolation stress and perinatal nmda receptor antagonist treatment induce changes in the structure and neurochemistry of inhibitory neurons of the adult amygdala and prefrontal cortex. eNeuro. 2017;4 doi: 10.1523/ENEURO.0034-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chocyk A., Majcher-Maoelanka I., Dudys D., Przyborowska A., Wêdzony K. Impact of early-life stress on the medial prefrontal cortex functions-a search for the pathomechanisms of anxiety and mood disorders. Pharmacol. Rep. 2013;65:1462–1470. doi: 10.1016/s1734-1140(13)71506-8. [DOI] [PubMed] [Google Scholar]
- Chohan T.W. Partial genetic deletion of neuregulin 1 and adolescent stress interact to alter NMDA receptor binding in the medial prefrontal cortex. Front. Behav. Neurosci. 2014;8 doi: 10.3389/fnbeh.2014.00298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi K. Expression pattern of the cannabinoid receptor genes in the frontal cortex of mood disorder patients and mice selectively bred for high and low fear. J. Psychiatr. Res. 2012;46:882–889. doi: 10.1016/j.jpsychires.2012.03.021. [DOI] [PubMed] [Google Scholar]
- Clarke D.J. Neuregulin 1 deficiency modulates adolescent stress-induced dendritic spine loss in a brain region-specific manner and increases complement 4 expression in the Hippocampus. Schizophr. Bull. 2019;45:339–349. doi: 10.1093/schbul/sby029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czéh B. Long-term stress disrupts the structural and functional integrity of GABAergic neuronal networks in the medial prefrontal cortex of rats. Front. Cell. Neurosci. 2018;12 doi: 10.3389/fncel.2018.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Cristo G. Activity-dependent PSA expression regulates inhibitory maturation and onset of critical period plasticity. Nat. Neurosci. 2007;10:1569–1577. doi: 10.1038/nn2008. [DOI] [PubMed] [Google Scholar]
- Diester C.M., Banks M.L., Neigh G.N., Negus S.S. Experimental design and analysis for consideration of sex as a biological variable. Neuropsychopharmacology. 2019;44:2159–2162. doi: 10.1038/s41386-019-0458-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djordjevic A., Adzic M., Djordjevic J., Radojcic M.B. Chronic social isolation suppresses proplastic response and promotes proapoptotic signalling in prefrontal cortex of Wistar rats. J. Neurosci. Res. 2010;88:2524–2533. doi: 10.1002/jnr.22403. [DOI] [PubMed] [Google Scholar]
- Djordjevic J., Djordjevic A., Adzic M., Radojcic M.B. Effects of chronic social isolation on wistar rat behavior and brain plasticity markers. Neuropsychobiology. 2012;66:112–119. doi: 10.1159/000338605. [DOI] [PubMed] [Google Scholar]
- Eid R.S., Gobinath A.R., Galea L.A.M. Sex differences in depression: insights from clinical and preclinical studies. Prog. Neurobiol. 2019;176:86–102. doi: 10.1016/j.pneurobio.2019.01.006. [DOI] [PubMed] [Google Scholar]
- Eiland L., Ramroop J., Hill M.N., Manley J., McEwen B.S. Chronic juvenile stress produces corticolimbic dendritic architectural remodeling and modulates emotional behavior in male and female rats. Psychoneuroendocrinology. 2012;37:39–47. doi: 10.1016/j.psyneuen.2011.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng G. Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP. Neuron. 2000;28:41–51. doi: 10.1016/s0896-6273(00)00084-2. [DOI] [PubMed] [Google Scholar]
- Ferguson B.R., Gao W.J. Pv interneurons: critical regulators of E/I balance for prefrontal cortex-dependent behavior and psychiatric disorders. Front. Neural Circ. 2018;12 doi: 10.3389/fncir.2018.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fullana N. Astrocyte control of glutamatergic activity: downstream effects on serotonergic function and emotional behavior. Neuropharmacology. 2020;166 doi: 10.1016/j.neuropharm.2019.107914. [DOI] [PubMed] [Google Scholar]
- Fuster J.M. Frontal lobe and cognitive development. J. Neurocytol. 2002;31:373–385. doi: 10.1023/a:1024190429920. [DOI] [PubMed] [Google Scholar]
- Garrett J.E., Wellman C.L. Chronic stress effects on dendritic morphology in medial prefrontal cortex: sex differences and estrogen dependence. Neuroscience. 2009;162:195–207. doi: 10.1016/j.neuroscience.2009.04.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilabert-Juan J., Castillo-Gomez E., Pérez-Rando M., Moltó M.D., Nacher J. Chronic stress induces changes in the structure of interneurons and in the expression of molecules related to neuronal structural plasticity and inhibitory neurotransmission in the amygdala of adult mice. Exp. Neurol. 2011;232:33–40. doi: 10.1016/j.expneurol.2011.07.009. [DOI] [PubMed] [Google Scholar]
- Gilabert-Juan J., Castillo-Gomez E., Guirado R., Moltó M.D., Nacher J. Chronic stress alters inhibitory networks in the medial prefrontal cortex of adult mice. Brain Struct. Funct. 2013;218 doi: 10.1007/s00429-012-0479-1. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Burgos G., Cho R.Y., Lewis D.A. Alterations in cortical network oscillations and parvalbumin neurons in schizophrenia. Biol. Psychiatr. 2015;77:1031–1040. doi: 10.1016/j.biopsych.2015.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourley S.L., Swanson A.M., Koleske A.J. Corticosteroid-induced neural remodeling predicts behavioral vulnerability and resilience. J. Neurosci. 2013;33:3107–3112. doi: 10.1523/JNEUROSCI.2138-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guirado R., Perez-Rando M., Sanchez-Matarredona D., Castrén E., Nacher J. Chronic fluoxetine treatment alters the structure, connectivity and plasticity of cortical interneurons. Int. J. Neuropsychopharmacol. 2014;17:1635–1646. doi: 10.1017/S1461145714000406. [DOI] [PubMed] [Google Scholar]
- Guirado R., Carceller H., Castillo-Gómez E., Castrén E., Nacher J. Automated analysis of images for molecular quantification in immunohistochemistry. Heliyon. 2018;4 doi: 10.1016/j.heliyon.2018.e00669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Härtig W., Brauer K., Brückner G. Wisteria floribunda agglutinin-labelled nets surround parvalbumin-containing neurons. Neuroreport. 1992;3:869–872. doi: 10.1097/00001756-199210000-00012. [DOI] [PubMed] [Google Scholar]
- Heim C., Nemeroff C.B. The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biol. Psychiatr. 2001;49:1023–1039. doi: 10.1016/s0006-3223(01)01157-x. [DOI] [PubMed] [Google Scholar]
- Hill M.N., McEwen B.S. Involvement of the endocannabinoid system in the neurobehavioural effects of stress and glucocorticoids. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2010;34:791–797. doi: 10.1016/j.pnpbp.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill M.N. Regional alterations in the endocannabinoid system in an animal model of depression: effects of concurrent antidepressant treatment. J. Neurochem. 2008;106:2322–2336. doi: 10.1111/j.1471-4159.2008.05567.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill M.N. Recruitment of prefrontal cortical endocannabinoid signaling by glucocorticoids contributes to termination of the stress response. J. Neurosci. 2011;31:10506–10515. doi: 10.1523/JNEUROSCI.0496-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodes G.E., Epperson C.N. Sex differences in vulnerability and resilience to stress across the life span. Biol. Psychiatr. 2019;86:421–432. doi: 10.1016/j.biopsych.2019.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iqbal J., Ma X.M. Impact of subchronic variable stress on ovariectomy and dendritic spine density in prefrontal cortex in mice. Neuroreport. 2020;31:213–219. doi: 10.1097/WNR.0000000000001384. [DOI] [PubMed] [Google Scholar]
- Ishikawa J., Ogawa Y., Owada Y., Ishikawa A. Hyperlocomotor activity and stress vulnerability during adulthood induced by social isolation after early weaning are prevented by voluntary running exercise before normal weaning period. Behav. Brain Res. 2014;264:197–206. doi: 10.1016/j.bbr.2014.02.007. [DOI] [PubMed] [Google Scholar]
- Kaiser T., Ting J.T., Monteiro P., Feng G. Transgenic labeling of parvalbumin-expressing neurons with tdTomato. Neuroscience. 2016;321:236–245. doi: 10.1016/j.neuroscience.2015.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katona I., Freund T.F. Multiple functions of endocannabinoid signaling in the brain. Annu. Rev. Neurosci. 2012;35:529–558. doi: 10.1146/annurev-neuro-062111-150420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T.T.Y., Hill M.N. Age of stress exposure modulates the immediate and sustained effects of repeated stress on corticolimbic cannabinoid CB1 receptor binding in male rats. Neuroscience. 2013;249:106–114. doi: 10.1016/j.neuroscience.2012.11.017. [DOI] [PubMed] [Google Scholar]
- Liston Conor, Miller Melinda M., Goldwater Deena S., Radley Jason J., Rocher Anne B., Hof Patrick R., Morrison John H., McEwen Bruce S. Stress-induced alterations in prefrontal cortical dendritic morphology predict selective impairments in perceptual attentional set-shifting. J. Neurosci. 2006;26(30):7870–7874. doi: 10.1523/JNEUROSCI.1184-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longair M.H., Baker D.A., Armstrong J.D. Simple neurite tracer: open source software for reconstruction, visualization and analysis of neuronal processes. Bioinformatics. 2011;27:2453–2454. doi: 10.1093/bioinformatics/btr390. [DOI] [PubMed] [Google Scholar]
- Lyttle K. Repeated fluvoxamine treatment recovers juvenile stress-induced morphological changes and depressive-like behavior in rats. Brain Res. 2015;1616:88–100. doi: 10.1016/j.brainres.2015.04.058. [DOI] [PubMed] [Google Scholar]
- Mañas-Ojeda A., Ros-Bernal F., Olucha-Bordonau F.E., Castillo-Gómez E. Becoming stressed: does the age matter? Reviewing the neurobiological and socio-affective effects of stress throughout the lifespan. Int. J. Mol. Sci. 2020;21:1–23. doi: 10.3390/ijms21165819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marco E.M. Consequences of early life stress on the expression of endocannabinoid- related genes in the rat brain. Behav. Pharmacol. 2014;25:547–556. doi: 10.1097/FBP.0000000000000068. [DOI] [PubMed] [Google Scholar]
- Márquez C. Peripuberty stress leads to abnormal aggression, altered amygdala and orbitofrontal reactivity and increased prefrontal MAOA gene expression. Transl. Psychiatry. 2013;3:e216. doi: 10.1038/tp.2012.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen B.S. The neurobiology of stress: from serendipity to clinical relevance. Brain Res. 2000;886:172–189. doi: 10.1016/s0006-8993(00)02950-4. [DOI] [PubMed] [Google Scholar]
- McEwen B.S., Morrison J.H. The brain on stress: vulnerability and plasticity of the prefrontal cortex over the life course. Neuron. 2013;79:16–29. doi: 10.1016/j.neuron.2013.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen B.S., Nasca C., Gray J.D. Stress effects on neuronal structure: Hippocampus, amygdala, and prefrontal cortex. Neuropsychopharmacology. 2016;41:3–23. doi: 10.1038/npp.2015.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKlveen J.M. Chronic stress increases prefrontal inhibition: a mechanism for stress-induced prefrontal dysfunction. Biol. Psychiatr. 2016;80:754–764. doi: 10.1016/j.biopsych.2016.03.2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nacher J., Alonso-Llosa G., Rosell D., McEwen B. PSA-NCAM expression in the piriform cortex of the adult rat. Modulation by NMDA receptor antagonist administration. Brain Res. 2002;927:111–121. doi: 10.1016/s0006-8993(01)03241-3. [DOI] [PubMed] [Google Scholar]
- Page C.E., Coutellier L. Adolescent stress disrupts the maturation of anxiety-related behaviors and alters the developmental trajectory of the prefrontal cortex in a sex- and age-specific manner. Neuroscience. 2018;390:265–277. doi: 10.1016/j.neuroscience.2018.08.030. [DOI] [PubMed] [Google Scholar]
- Page C.E., Coutellier L. Prefrontal excitatory/inhibitory balance in stress and emotional disorders: evidence for over-inhibition. Neurosci. Biobehav. Rev. 2019;105:39–51. doi: 10.1016/j.neubiorev.2019.07.024. [DOI] [PubMed] [Google Scholar]
- Page C.E., Alexander J., Shepard R., Coutellier L. Npas4 deficiency interacts with adolescent stress to disrupt prefrontal GABAergic maturation and adult cognitive flexibility. Gene Brain Behav. 2018;17:e12459. doi: 10.1111/gbb.12459. [DOI] [PubMed] [Google Scholar]
- Papilloud A., Guillot de Suduiraut I., Zanoletti O., Grosse J., Sandi C. Peripubertal stress increases play fighting at adolescence and modulates nucleus accumbens CB1 receptor expression and mitochondrial function in the amugdala. Transl. Psychiatry. 2018;8:156. doi: 10.1038/s41398-018-0215-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T., Keshavan M., Giedd J.N. Why do many psychiatric disorders emerge during adolescence? Nat. Rev. Neurosci. 2008;9:947–957. doi: 10.1038/nrn2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Rando M., Castillo-Gomez E., Bueno-Fernandez C., Nacher J. The TrkB agonist 7,8-dihydroxyflavone changes the structural dynamics of neocortical pyramidal neurons and improves object recognition in mice. Brain Struct. Funct. 2018 doi: 10.1007/s00429-018-1637-x. [DOI] [PubMed] [Google Scholar]
- Pesarico A.P. Chronic stress modulates interneuronal plasticity: effects on PSA-NCAM and perineuronal nets in cortical and extracortical regions. Front. Cell. Neurosci. 2019;13:197. doi: 10.3389/fncel.2019.00197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petanjek Z. Extraordinary neoteny of synaptic spines in the human prefrontal cortex. Proc. Natl. Acad. Sci. U. S. A. 2011;108:13281–13286. doi: 10.1073/pnas.1105108108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinzón-Parra C. Juvenile stress causes reduced locomotor behavior and dendritic spine density in the prefrontal cortex and basolateral amygdala in Sprague-Dawley rats. Synapse. 2019;73 doi: 10.1002/syn.22066. [DOI] [PubMed] [Google Scholar]
- Platt J.E., Stone E.A. Chronic restraint stress elicits a positive antidepressant response on the forced swim test. Eur. J. Pharmacol. 1982;82:179–181. doi: 10.1016/0014-2999(82)90508-8. [DOI] [PubMed] [Google Scholar]
- Potrebić M., Pavković Ž., Lončarević-Vasiljković N., Kanazir S., Pešić V. Altered hedonic, novelty-, stress- and D-amphetamine-induced response to due to social isolation in peripuberty. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2020;110186 doi: 10.1016/j.pnpbp.2020.110186. [DOI] [PubMed] [Google Scholar]
- Radley J.J. Repeated stress alters dendritic spine morphology in the rat medial prefrontal cortex. J. Comp. Neurol. 2008;507:1141–1150. doi: 10.1002/cne.21588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice D., Barone S., Jr. Critical periods of vulnerability for the developing nervous System : evidence from humans and animal models critical periods of vulnerabilityfor the developing nervous System : evidence from humans and animal models development of the brain in utero. Environ. Health Perspect. 2000;108:511–533. doi: 10.1289/ehp.00108s3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sala-Catala J., Torrero C., Regalado M., Salas M., Ruiz-Marcos A. Movements restriction and alterations of the number of spines distributed along the apical shafts of layer V pyramids in motor and primary sensory cortices of the peripubertal and adult rat. Neuroscience. 2005;133:137–145. doi: 10.1016/j.neuroscience.2005.01.009. [DOI] [PubMed] [Google Scholar]
- Sandi C. Stress, cognitive impairment and cell adhesion molecules. Nat. Rev. Neurosci. 2004;5:917–930. doi: 10.1038/nrn1555. [DOI] [PubMed] [Google Scholar]
- Schindelin J. Fiji: an open-source platform for biological-image analysis. Nat. Methods. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw G.A., Dupree J.L., Neigh G.N. Adolescent maturation of the prefrontal cortex: role of stress and sex in shaping adult risk for compromise. Gene Brain Behav. 2020;19 doi: 10.1111/gbb.12626. [DOI] [PubMed] [Google Scholar]
- Shaw G.A., Dupree J.L., Neigh G.N. Adolescent maturation of the prefrontal cortex: role of stress and sex in shaping adult risk for compromise. 2020;19 doi: 10.1111/gbb.12626. [DOI] [PubMed] [Google Scholar]
- Shepard R., Coutellier L. Changes in the prefrontala glutamatergic and parvalbumin systems of mice exposed to unpredictable chronic stress. Mol. Neurobiol. 2018;55:2591–2602. doi: 10.1007/s12035-017-0528-0. [DOI] [PubMed] [Google Scholar]
- Shepard R., Page C.E., Coutellier L. Sensitivity of the prefrontal GABAergic system to chronic stress in male and female mice: relevance for sex differences in stress-related disorders. Neuroscience. 2016;332:1–12. doi: 10.1016/j.neuroscience.2016.06.038. [DOI] [PubMed] [Google Scholar]
- Sholl D.A. Dendritic organization in the neurons of the visual and motor cortices of the cat. J. Anat. 1953;87:387–406. [PMC free article] [PubMed] [Google Scholar]
- Simon P., Dupuis R., Costentin J. Thigmotaxis as an index of anxiety in mice. Influence of dopaminergic transmissions. Behav. Brain Res. 1994;61:59–64. doi: 10.1016/0166-4328(94)90008-6. [DOI] [PubMed] [Google Scholar]
- Spear L.P. The adolescent brain and age-related behavioral manifestations. Neurosci. Biobehav. Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Testa D., Prochiantz A., Di Nardo A.A. Perineuronal nets in brain physiology and disease. Semin. Cell Dev. Biol. 2019;89:125–135. doi: 10.1016/j.semcdb.2018.09.011. [DOI] [PubMed] [Google Scholar]
- Todorović N., Mićić B., Schwirtlich M., Stevanović M., Filipović D. Subregion-specific protective effects of fluoxetine and clozapine on parvalbumin expression in medial prefrontal cortex of chronically isolated rats. Neuroscience. 2019;396:24–35. doi: 10.1016/j.neuroscience.2018.11.008. [DOI] [PubMed] [Google Scholar]
- Toledo-Rodriguez M., Pitiot A., Paus T., Sandi C. Stress during puberty boosts metabolic activation associated with fear-extinction learning in hippocampus, basal amygdala and cingulate cortex. Neurobiol. Learn. Mem. 2012;98:93–101. doi: 10.1016/j.nlm.2012.05.006. [DOI] [PubMed] [Google Scholar]
- Tzanoulinou S. Long-term behavioral programming induced by peripuberty stress in rats is accompanied by gabaergic- related alterations in the amygdala. PloS One. 2014;9 doi: 10.1371/journal.pone.0094666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzanoulinou S. Neuroligin-2 expression in the prefrontal cortex is involved in attention deficits induced by peripubertal stress. Neuropsychopharmacology. 2016;41:751–761. doi: 10.1038/npp.2015.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzanoulinou S., Gantelet E., Sandi C., Márquez C. Programming effects of peripubertal stress on spatial learning. Neurobiol. Stress. 2020;13 doi: 10.1016/j.ynstr.2020.100282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno H. Region-specific impairments in parvalbumin interneurons in social isolation-reared mice. Neuroscience. 2017;359:196–208. doi: 10.1016/j.neuroscience.2017.07.016. [DOI] [PubMed] [Google Scholar]
- Ueno H. Juvenile stress induces behavioral change and affects perineuronal net formation in juvenile mice. BMC Neurosci. 2018;19:1–21. doi: 10.1186/s12868-018-0442-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban K.R., Geng E., Bhatnagar S., Valentino R.J. Age- and sex-dependent impact of repeated social stress on morphology of rat prefrontal cortex pyramidal neurons. Neurobiol. Stress. 2019;10 doi: 10.1016/j.ynstr.2019.100165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veenit V., Cordero M.I., Tzanoulinou S., Sandi C. Increased corticosterone in peripubertal rats leads to long-lasting alterations in social exploration and aggression. Front. Behav. Neurosci. 2013;7 doi: 10.3389/fnbeh.2013.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker S.E. Alterations in brain microstructure in rats that develop abnormal aggression following peripubertal stress. Eur. J. Neurosci. 2018;48:1818–1832. doi: 10.1111/ejn.14061. [DOI] [PubMed] [Google Scholar]
- Wang D., Fawcett J. The perineuronal net and the control of cns plasticity. Cell Tissue Res. 2012;349:147–160. doi: 10.1007/s00441-012-1375-y. [DOI] [PubMed] [Google Scholar]
- Wang H., Xiao L., Wang H., Wang G. Involvement of chronic unpredictable mild stress-induced hippocampal LRP1 up-regulation in microtubule instability and depressive-like behavior in a depressive-like adult male rat model. Physiol. Behav. 2020;215 doi: 10.1016/j.physbeh.2019.112749. [DOI] [PubMed] [Google Scholar]
- Watt M.J., Weber M.A., Davies S.R., Forster G.L. Impact of juvenile chronic stress on adult cortico-accumbal function: implications for cognition and addiction. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2017;79:136–154. doi: 10.1016/j.pnpbp.2017.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman M.M., Klerman G.L. Sex differences and the epidemiology of depression. Arch. Gen. Psychiatr. 1977;34:98–111. doi: 10.1001/archpsyc.1977.01770130100011. [DOI] [PubMed] [Google Scholar]
- Wellman C.L., Bollinger J.L., Moench K.M. International Review of Neurobiology. Academic Press Inc.; 2020. Effects of stress on the structure and function of the medial prefrontal cortex: insights from animal models; pp. 150 129–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West M.J. New stereological methods for counting neurons. Neurobiol. Aging. 1993;14:275–285. doi: 10.1016/0197-4580(93)90112-o. [DOI] [PubMed] [Google Scholar]
- Wohleb E.S., Terwilliger R., Duman C.H., Duman R.S. Stress-induced neuronal colony stimulating factor 1 provokes microglia-mediated neuronal remodeling and depressive-like behavior. Biol. Psychiatr. 2018;83:38–49. doi: 10.1016/j.biopsych.2017.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wommack J.C., Salinas A., Melloni R.H., Delville Y. Behavioural and neuroendocrine adaptations to repeated stress during puberty in male golden hamsters. J. Neuroendocrinol. 2004;16:767–775. doi: 10.1111/j.1365-2826.2004.01233.x. [DOI] [PubMed] [Google Scholar]
- Wu Y.W.C., Du X., Van den Buuse M., Hill R.A. Sex differences in the adolescent developmental trajectory of parvalbumin interneurons in the hippocampus: a role for estradiol. Psychoneuroendocrinology. 2014;45:167–178. doi: 10.1016/j.psyneuen.2014.03.016. [DOI] [PubMed] [Google Scholar]
- Wȩdzony K., Chocyk A. Cannabinoid CB1 receptors in rat medial prefrontal cortex are colocalized with calbindin- but not parvalbumin- and calretinin-positive GABA-ergic neurons. Pharmacol. Rep. 2009;61:1000–1007. doi: 10.1016/s1734-1140(09)70161-6. [DOI] [PubMed] [Google Scholar]
- Zadrozna M. Different pattern of changes in calcium binding proteins immunoreactivity in the medial prefrontal cortex of rats exposed to stress models of depression. Pharmacol. Rep. 2011;63:1539–1546. doi: 10.1016/s1734-1140(11)70718-6. [DOI] [PubMed] [Google Scholar]