Abstract
Background/Aim: Neoadjuvant chemotherapy (NAC) using 5-FU (5-fluorouracil)/CDDP (cisplatin) is a standard therapy for stage II/III thoracic esophageal squamous cell carcinoma (ESCC) in Japan. The aim of this study was to investigate whether 5-FU/CDDP could induce immunogenic cell death in ESCC cell lines. Materials and Methods: Tumor samples for immunohistochemistry were obtained from 50 patients (mean age=63.1 years) with pathological stage 0-IVa ESCC who underwent NAC followed by surgery. Cell lines T.T and KYSE30 were used for the in vitro experiments. Results: The concentrations of HMGB1 were elevated in the cell line supernatants treated with 5-FU/CDDP. 5-FU/CDDP treated dendritic cells (DCs) showed a mature phenotype, and enhanced T cell proliferation capacity. In addition, mature DCs were observed in surgical specimens with a histological response after treatment with 5-FU/CDDP chemotherapy. Conclusion: 5-FU/CDDP could induce immunogenic cell death in the tumor microenvironment of ESCC.
Keywords: Immunogenic cell death, dendritic cell, LAMP-3, HMGB1, neoadjuvant chemotherapy
5-FU (5-fluorouracil)/CDDP (cisplatin) has been used as neoadjuvant chemotherapy (NAC) for the treatment of stage II/III thoracic esophageal squamous cell carcinoma (ESCC) in Japan (1). NAC for esophageal cancer has the advantage of suppressing micrometastasis due to the achievement of tumor downstaging before surgery (2).
Immunotherapy is expected to be one of the most important treatments for ESCC; however, the interaction between immunotherapy and chemotherapy is still unknown. In general, because cytotoxic anticancer drugs damage normal bone marrow cells as well as cancer cells, they have been thought to induce immunosuppression due to lymphopenia. On the other hand, it is considered that chemotherapy can induce immunogenic cell death (ICD) of tumor cells, which triggers the T cell immunity mediated by danger-associated molecular patterns (DAMPs), which can activate immune cells, including dendritic cells (DCs) (3). One of the DAMPs is high-mobility group box 1 protein (HMGB1). Extracellular HMGB1 enhances dendritic cell antigen presentation and promotes the so-called maturation of DCs (4).
DC maturation includes the enhanced expression of MHCs, costimulatory molecules such as CD80, CD86 and lysosome-associated membrane glycoprotein 3 (LAMP-3) (5). LAMP-3, which is normally scattered within lysosomes on the surface of dendritic cells, is known to appear as a component of the MHC-II molecule during antigen phagocytosis and maturation of dendritic cells (6,7). Thus, LAMP-3 has been considered as a marker of DC maturation.
The local immune status is an important prognostic factor for various types of tumor, including ESCC (8). We expect that in addition to their antitumor cytotoxicity effect, chemical drugs may induce ICD in ESCC. However, little information is available regarding ICD in ESCC. The aim of this study was to explore the influence of chemotherapy on local infiltrating immune cells in ESCC.
Materials and Methods
Clinical samples for immunohistochemistry. Tumor samples for immunohistochemistry were obtained from 50 patients (mean age=63.1 years) with pathological stage 0-IVa ESCC who underwent NAC (5-FU+CDDP or 5-FU+nedaplatin) followed by surgical treatment at the Department of Surgical Oncology, Osaka City University Hospital, between 2000 and 2015. The median follow-up time was 25 months.
Cell line. The “T.T” and KYSE 30 ESCC cell lines were used in this study. T.T human esophageal squamous cell carcinoma cells were obtained from the Health Science Research Resources Bank (Osaka, Japan). KYSE30 cells were obtained from JCRB Cell bank (Japan) (9). Cells were cultured at 37˚C and in 5% CO2. The medium used was Dulbecco’s modified Eagle’s medium (DMEM; Bioproducts, Walkersville, MD, USA) supplemented with 10% fetal bovine serum (FBS; Gibco, Grand Island, NY, USA), 100 IU/ml of penicillin (ICN Biomedicals, Costa Mesa, CA, USA), 100 mg/ml of streptomycin (ICN Biomedicals, Aurora, OH, USA) and 0.5 mM sodium pyruvate (Bioproducts).
The concentrations of 5-FU and cisplatin used in the in vitro experiments were evaluated for apoptosis by using Annexin V/7-AAD staining and 30 μmol/l for 48 h was determined to be optimal for both drugs.
Isolation of DCs from surgical specimens. Surgical specimens of nine patients with histologically diagnosed primary ESCC were subjected to flow cytometry. The patients who received neoadjuvant chemotherapy (5-FU/CDDP-based chemotherapy) included 4 patients with stage II disease and 2 patients with stage III disease. None of the patients had received any treatment before this study. Tumors were diagnosed histologically based on the 11th Edition of the Japanese Classification of Esophageal Cancer. Primary ESCC cells were obtained from ESCC patients undergoing surgery. The resected tumors were weighed, minced into small pieces (1-3 mm), and then mechanically minced into smaller pieces in PBS + 2 nmol/l EDTA. The tumor portions were then placed in an enzyme solution (Collagenase D), incubated (37˚C) for 30 min and then, mixed again twice. HLA-DR+ cells were enriched by positive magnetic bead selection (MACS; Miltenyi-Biotec, San Diego, CA, USA) using PE-labeled primary monoclonal antibodies and anti-PE microbeads together with LS separation columns and a Vario MACS® magnet.
Immunohistochemistry. Tumor specimens in paraffin-embedded blocks were cut into 4-μm-thick sections. Nonspecific binding was blocked using nonspecific staining blocking reagent (DAKO, Kyoto, Japan). The sections were then reacted with mouse monoclonal anti-LAMP-3 antibody (clone: 16H11.2; Merck, Darmstadt, Germany) and rabbit monoclonal anti-CD8 antibody (clone: EP1150Y; Abcam, Cambridge, UK) at 4˚C overnight. Sections were incubated with secondary antibodies for 10 min at room temperature. After washing in phosphate-buffered saline (PBS), the sections were visualized using 3-3’-diamino-benzidine (DAB) for 5 min and counterstained with hematoxylin. We counted the average number of LAMP-3-positive and CD8-positive cells in 5 randomly selected fields under a light microscope at ×400 magnification.
Treatment with cancer cells. T.T cells (5×105) were plated in 1 ml of full medium treated with 5-FU and/or CDDP (30 μM). Supernatants were collected after 48 h, dying tumor cells were removed by centrifugation, and the supernatants were isolated and immediately frozen. The quantification of HMGB1 in the supernatants was assessed by ELISA, which was performed according to the manufacturer’s instructions (R&D systems, Minneapolis, MN, USA). DCs were generated by culturing purified CD14+ cells isolated from buffy coats in the presence of granulocyte-macrophage colony-stimulating factor (GM-CSF: Miltenyi- Biotec, San Diego, CA, USA) and interleukin-4 (IL-4: Miltenyi- Biotec). The phenotypical assessment of DC maturation after 24 h of co-culture with supernatants of chemical-treated T.T cells was performed by using flow cytometry. Briefly, cells were washed in PBA, incubated with Fc-receptor blocking buffer (2% HS in PBS) for 15 min at 4˚C and subsequently stained with primary antibodies in PBA for 30 min at 4˚C. The following monoclonal directly labeled anti-human antibodies were used: anti-CD11c-APC (clone: B-ly-6; BD Pharmingen, Franklin Lakes, NJ, USA), anti-CD208-PE (clone: I10-1112; BD Pharmingen), anti-CD274-APC (clone: MI-H1; BD Pharmingen), anti-CD80-BB515 (clone: L307.4; BD Pharmingen), anti-CD86-BV421 (clone: 2331 (FUN-1); BD Pharmingen), and anti-HLA-DR-FITC (clone: G46-6; BD Pharmingen). The geometric mean fluorescence intensity (Geo MFI) of maturation markers was assessed in CD11c+ populations. As a positive control, DCs were stimulated with 10 μg/ml lipopolysaccharide (LPS: Sigma-Aldrich, St. Louis, MO, USA).
Assays of cytokine production. Monocyte-derived DCs were cultured for 5 days and adjusted to a final concentration of 1×106 cells/ml in 24-well plates. Next, an equivalent amount of untreated control, 5-FU and/or CDDP supernatant and 10 μg/ml LPS was added to separate wells of the 24-well plates. Supernatants from the designated wells were harvested after 24 h for the quantification of cytokines (e.g., IL-1β, IL-6, IL-10) using an ELISA kit (R&D Systems, Minneapolis, MN, USA).
Mixed lymphocyte reaction (MLR). Allogenic PBLs were stained with 5 μM CFSE (Invitrogen, Carlsbad, CA, USA), according to the manufacturer’s instructions and added to the DCs at a ratio of 10:1 (Lymphocytes: DCs), for an additional 5-day period. After 5 days, co-cultures were stained with a primary anti-CD3-APC antibody (clone: G155-178; BD Pharmingen, Franklin Lakes, NJ, USA) and analyzed by flow cytometry. The percentage of proliferating T cells (CD3+) was determined by assessing the CFSE dilution in the fraction of CD3+ cells.
FITC dextran. After 24 h of incubation with supernatants, monocyte-derived DCs were incubated for 1 h with 1 mg/ml of fluorescein isothiocyanate (FITC)-conjugated dextran (DX) (70,000 Dalton molecular weight, Sigma-Aldrich) at 37˚C, washed extensively, and then examined to detect FITC signaling by CD11c+ cells.
Ethical approval and informed consent. This study's retrospective protocol was approved by the Osaka City University Ethics Committee (Osaka, Japan), and a written informed consent was obtained from all patients. All volunteers provided oral and written informed consent and agreed to the use of their samples for scientific research.
Statistical analysis. The Mann–Whitney test was used to assess the associations between the expression of LAMP-3 and the clinicopathological features. The Kaplan–Meier method was used to produce overall survival (OS) curves, and a log-lank test was used to assess the significance of differences in survival. The day of surgery was used as the starting point for measuring OS. A Cox proportional regression model was used for the univariate and multivariate analyses of prognostic factors. p-Values of <0.05 were considered to indicate statistical significance. The degree of infiltration between the number of LAMP-3 DCs and CD8 T cells was compared by Spearman’s correlation coefficient. All statistical analyses were performed using the JMP software program (SAS Institute, Cary, NC, USA).
Results
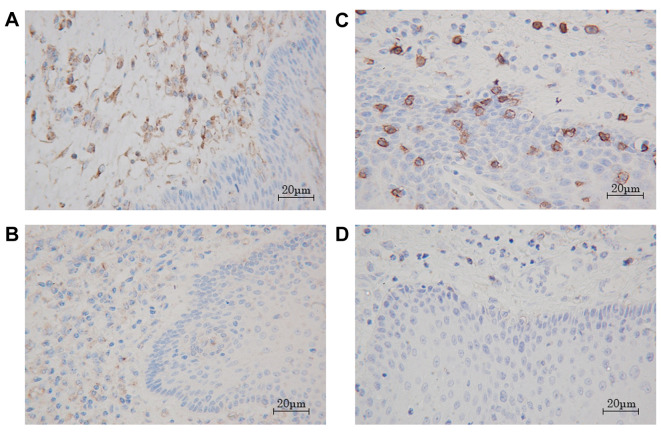
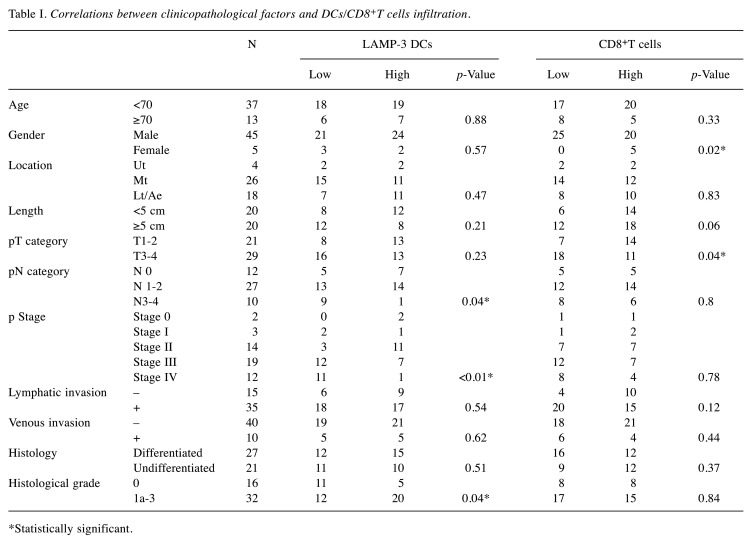
Correlation of LAMP-3 expression with prognosis. In the immunohistochemical analysis, LAMP-3-positive cells were predominantly observed in the peritumoral area and CD8-T cells were in the intratumoral area. These cells appear morphologically as dendritic cells because of their dendrites (Figure 1A, B). We divided the entire cohort into two groups according to the median number of tumor-infiltrating LAMP-3 DCs and CD8-T cells. The correlation between the number of tumor-infiltrating LAMP-3 DCs/CD8-T cells and the clinicopathological features is shown in Table I. The tumor infiltration by LAMP-3 DCs in patients with pN3 and advanced stage was significantly decreased in comparison to those with pN0 and early stage. We found that infiltration of LAMP-3 DCs was increased in 32 cases with any histological changes.
Figure 1. Immunohistochemical staining of LAMP-3 and CD8 T cell in esophagus squamous cell carcinoma. LAMP-3 dendritic cells were mainly distributed in the peritumoral area, but were sparsely present in the intratumoral area (A: high infiltration, B: low infiltration ×200). CD8 T cells were found in both the peritumoral and intratumoral areas (C: high infiltration, D: low infiltration ×200).
Table I. Correlations between clinicopathological factors and DCs/CD8+T cells infiltration.
*Statistically significant.
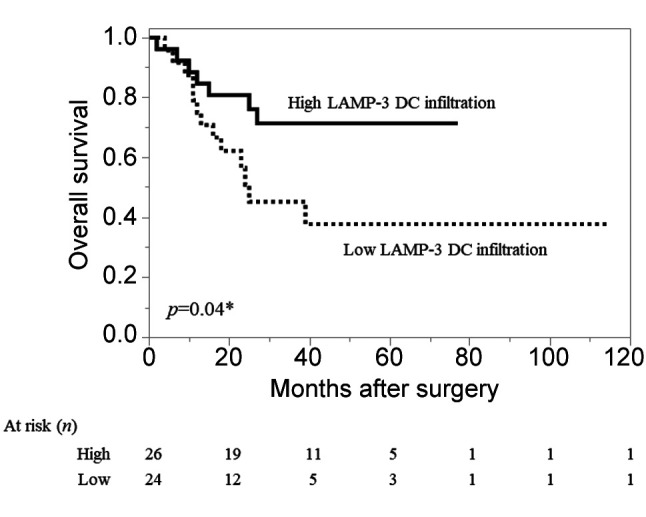
Patients with high infiltration of peritumoral-LAMP-3 DCs had a significantly better prognosis in comparison to those with low infiltration (Figure 2). The 5-year survival rates in patients with high and low LAMP-3 DC infiltration were 71% and 38%, respectively. We observed that the number of infiltrating LAMP-3 DCs and CD8-T cells had a weak positive correlation (r=0.20, p=0.038). Eighteen of the 25 patients with high DC infiltration belonged to the CD8-high infiltration group.
Figure 2. Kaplan–Meier curves of overall survival in patients with ESCC. The survival rate of patients in the high peritumoral-DC group was significantly higher than that of patients in the low peritumoralLAMP-3 DC group (p=0.04, log-rank test).
With regard to the pathological response to NAC, thirteen patients showed downstaging. There was no correlation between downstaging by NAC and the degree of infiltration of LAMP-3 DCs.
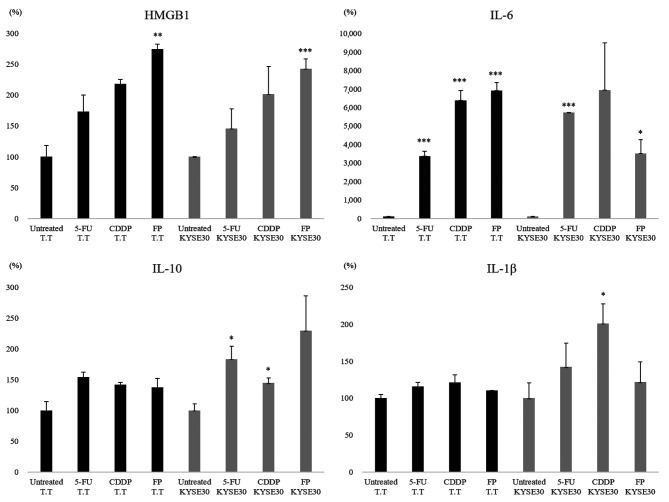
Induction of immunogenic cell death of cancer cells by 5-FU/CDDP treatment. We used two esophageal squamous cell carcinoma cell lines, T.T and KYSE 30, to investigate the effect of 5-FU/CDDP chemotherapy on cancer cells. In both TT and KYSE 30, HMBG1 production was increased by chemotherapy treatment. The combination of 5-FU and CDDP was more effective than either alone (Figure 3). In both T.T and KYSE 30, IL-6 was increased by the chemotherapy treatment (Figure 3). For IL-1B and IL-10, there were statistically significant increases in KYSE (p0.05), and no significant differences were observed in T.T. Despite these differences in cell lines, it was suggested that 5-FU/CDDP treatment affected the production of inflammatory cytokines.
Figure 3. The extracellular release of HMGB1 induced by chemical drugs and cytokine production by dendritic cells (DCs) stimulated with supernatants of chemically stressed T.T cells. The release of HMGB1 into the cell supernatants at 48 h after treatment with 5-FU and/or CDDP was significantly increased in comparison to the release in the untreated control group (p<0.05). The production of pro-inflammatory cytokines (IL-1β, IL-6) was increased among DCs co-cultured with chemical drug-treated tumor cells. **p<0.01, ***p<0.001, in comparison to control (untreated) cells.
We found that the release of HMGB1 into the cell supernatants of T.T and KYSE at 48 h after treatment with 5-FU and/or CDDP was significantly increased in comparison to the release in the untreated control group (p<0.05). The production of pro-inflammatory cytokines (IL-1β, IL-6) was increased among DCs co-cultured with chemotherapy-treated T.T cells. There was no significant change in the production of IL-10 by DCs stimulated with 5-FU and/or CDDP (Figure 3).
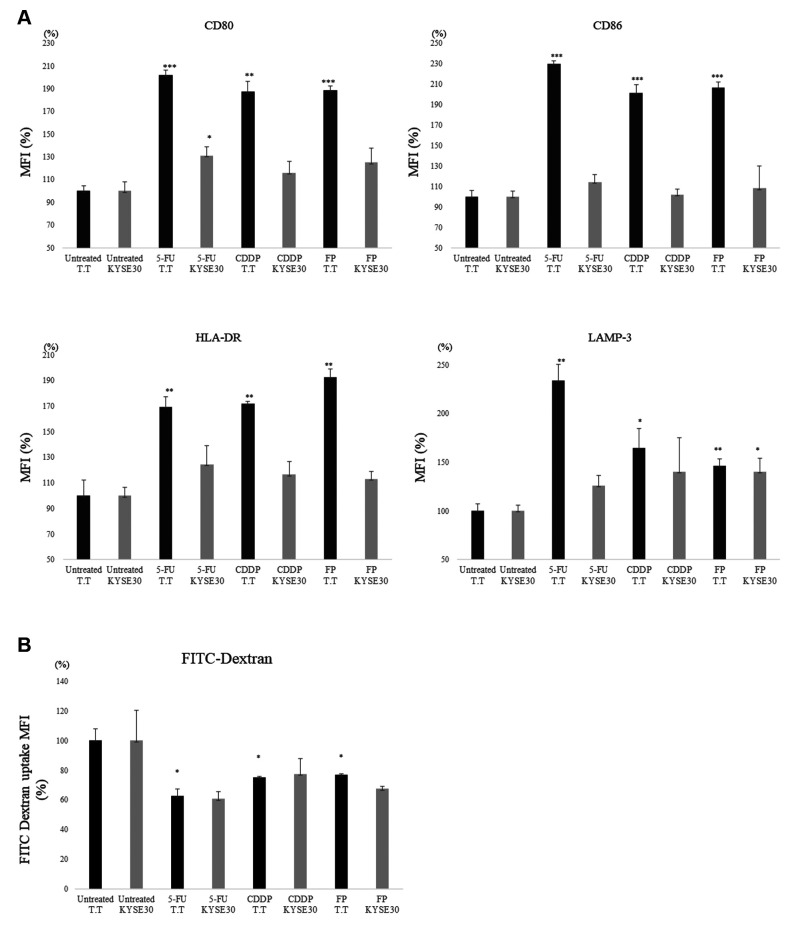
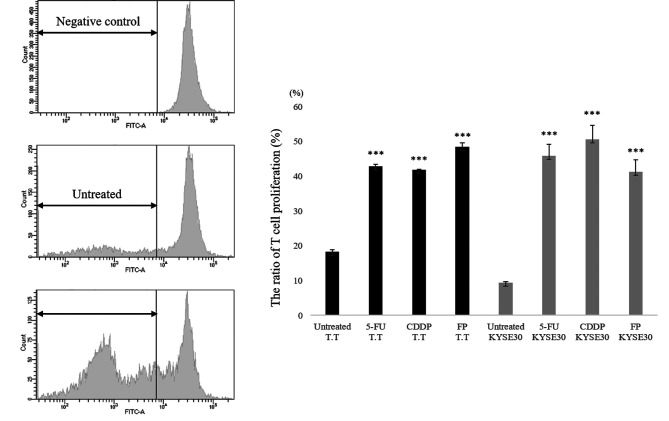
To investigate the role of HMGB1 in mediating DC maturation by supernatants from chemotherapy-treated T.T and KYSE30 cells, we tested the phenotypic changes in DCs stimulated with supernatants from chemically stressed cancer cells. We found that stimulation with supernatants of 5-FU/CDDP-treated cancer cell lines induced the up-regulation of HLA-DR, CD80, CD86, and the expression of LAMP-3 on monocyte-derived DCs in comparison to stimulation with untreated supernatants (Figure 4A). In addition, DCs co-cultured with supernatants of 5-FU/CDDP-treated T.T cells and KYSE30 cells showed a decreased uptake of FITC-Dextran in comparison to the untreated control group (p<0.05) (Figure 4B). Next, we investigated the impact of chemotherapeutic treatment on the T cell proliferation capacity of DCs. As shown in Figure 5, monocyte-derived DCs co-cultured with supernatants of 5-FU and/or CDDP-treated tumor cells showed a significant increase in T cell proliferation in comparison to untreated cells. The results were similar in both T.T and KYSE 30 cell lines.
Figure 4. The phenotype of DCs after interaction with cytostatic-killed T.T cells. A. Stimulation with supernatants of 5-FU and/or CDDP-treated tumor cells induced the up-regulation of HLA-DR, CD80, CD86, and the expression of LAMP-3 on monocyte-derived DCs in comparison to stimulation with untreated supernatants. *p<0.05, **p<0.01, ***p<0.001, in comparison to control (untreated) cells. B. DCs co-cultured with chemical drug-treated tumor cells showed a modest uptake of FITC-Dextran in comparison to the untreated control group (p<0.05). *p<0.05,**p<0.01, ***p<0.001, in comparison to control (untreated) cells.
Figure 5. Evaluation of functional up-regulation of DCs by Allo-MLR. Monocyte-derived DCs co-cultured with 5-FU and/or CDDP-treated tumor cells showed a significant increase in T cell proliferation in comparison to untreated cells (p<0.001). *p<0.05, ***p<0.001, in comparison to control (untreated) cells.
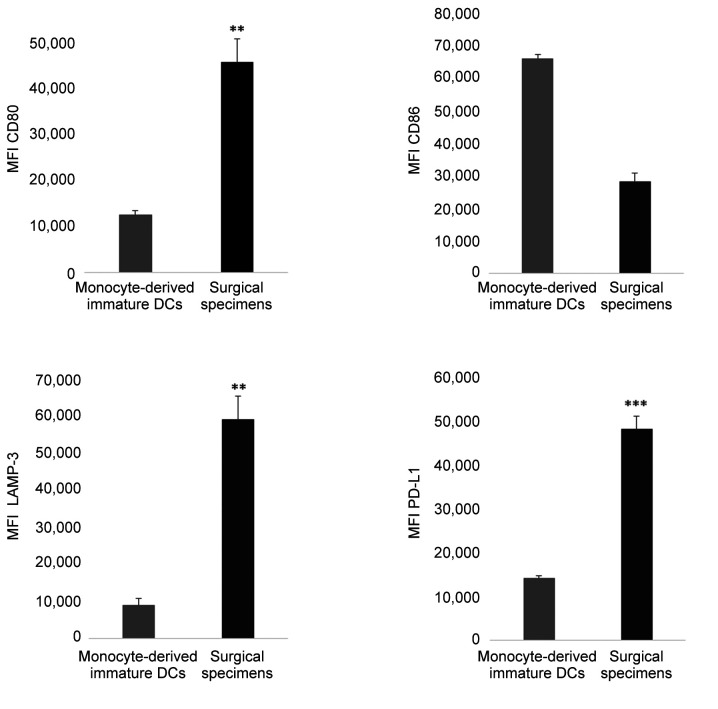
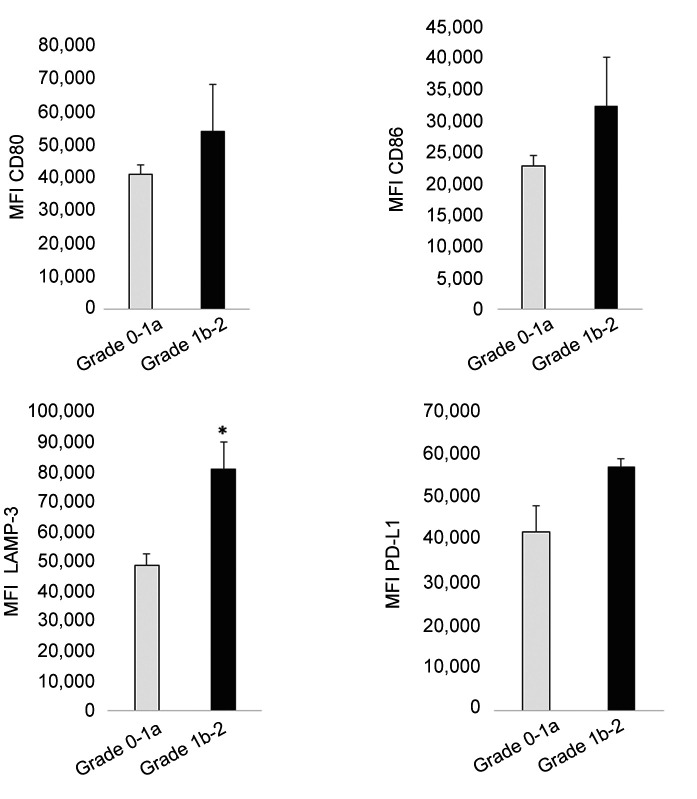
The phenotype of tumor infiltrating DCs of surgical specimens and the relationship with the chemotherapeutic effect. Next, we isolated DCs from tissues and investigated the relationship between the phenotype, including LAMP-3 expression, and the histological changes after chemotherapy. CD11c+ DCs isolated from surgical specimens showed a more activated phenotype, reflecting the increased expression of CD80 (p=0.001), and LAMP-3 (p<0.001) and PD-L1 expression in comparison to peripheral monocyte-derived immature DCs (Figure 6). Focusing on the effect of NAC, the expression of LAMP-3 was significantly elevated in cases in which the effect of NAC was Grade ≥1b in comparison to Grade ≤1a cases (Figure 7).
Figure 6. The phenotype and expression of PD-L1 of tumor-infiltrating dendritic cells (DCs) of surgical specimens in comparison to monocytederived immature DCs. DCs isolated from ESCC specimens showed a more activated phenotype in ESCC CD11c+ DCs, reflecting the increased expression of CD80 (p=0.001), and LAMP-3 (p<0.001) in comparison to peripheral monocyte-derived immature DCs. The mean fluorescence intensity (MFI) is shown. *p<0.05, **p<0.01, ***p<0.001, in comparison to control (monocyte-derived immature DCs).
Figure 7. The phenotype and expression of PD-L1 of tumor-infiltrating DCs of surgical specimens based on the pathological effect of NAC. Patients were classified into two groups based on the pathological effect of NAC (Grade0-1a or Grade1b-2). The expression of LAMP-3 was significantly elevated in cases in which the effect of NAC was Grade ≥1b in comparison to Grade ≤1a cases. The mean fluorescence intensity (MFI) is shown. *p<0.05, **p<0.01, ***p<0.001, in comparison to control (Grade 0-1a).
Discussion
In this study, we investigated the induction of ICD by 5-FU or CDDP, which are key drugs used as neoadjuvant chemotherapy for ESCC. We found the release of HMGB1 into cancer cell supernatants and DC maturation in vitro. Our study suggested that chemotherapy could alter the local immune environment.
Tumor infiltrating mature DCs have been reported to be correlated with a good prognosis in many different types of cancer, including ESCC (10). We previously reported that infiltrating DCs expressing LAMP-3, which is up-regulated on the surface of mature DCs, is an important prognostic factor in ESCC (11). Briefly, we showed that the number of infiltrating LAMP-3 DCs was correlated with the number of intratumoral CD8-T cells. Our report indicated that LAMP-3+ mature DCs may present tumor-antigens to T cells and activate T cells in the tumor microenvironment of ESCC.
Regarding the effect of NAC on local immunity, it has been reported that there was no decrease in TILs (tumor infiltrating lymphocytes) and LAMP-3 DCs after NAC in lung cancer, and that there was an increase in TILs and the tissue expression of PD-L1 after NAC in ovarian cancer (12,13). We showed that the increase in tumor infiltrating mature LAMP-3 DCs was associated with a histological effect by NAC and a favorable prognosis in ESCC. Thus, we found that DCs were both phenotypically and functionally activated by cancer cells treated with 5-FU/CDDP. Some reports have indicated that 5-FU or CDDP induce the release of HMGB1 from dying cells (14-21). It was reported that colorectal cancer cells treated with 5-FU released high levels of HMGB1, and that through these DCs induced the proliferation of IFN-γ producing Th1 cells (22). Additionally, platinum drugs, including CDDP, enhanced the phenotypic maturation of blood myeloid DCs upon interaction with platinum-treated tumor cells, and CD1c+DCs treated with these platinum drugs efficiently stimulated the allogeneic production of T cells in experiments using human melanoma and testicular carcinoma cells (23). Our results imply that NAC using 5-FU/CDDP for ESCC could induce ICD in vitro. However, there is still little information about ICD for ESCC. It has been reported that tumor antigen–specific T-cell responses were induced in patients with ESCC following chemoradiation, along with the elevation of HMGB1 in patients’ serum (24). The frequency of FOXP3+ Tregs was negatively correlated with the therapeutic response (meaning that few FOXP3+ T cells were found in patients that responded to chemoradiation) as well as with cancer-specific survival (25). In the present study, we confirmed that LAMP-3 was highly up-regulated in DCs derived from ESCC with a pathological response to NAC using surgical specimens. Our data also showed that DCs from the surgical specimens showed significantly higher expression of PD-L1 compared to monocyte-derived immature DCs. These results suggest increased local production of IFN-γ by T cells, but significant expression of PD-L1 may lead to immunosuppression. We showed, for the first time in a human clinical study, that ICD was induced in ESCC by proving the maturation of DCs. Furthermore, in contrast to the previous reports, our study also suggested that chemotherapy alone could induce ICD in ESCC. Our data suggest that the effect of NAC on the immune microenvironment is not negative and may enhance the local immune response.
The present study is associated with several limitations. Firstly, the doses of the anticancer drugs used in vitro differed from the clinical doses. The exact dose of the anti-cancer drug locally at the tumor site is unclear. However, the plasma concentrations of 5-FU when using the standard dose for ESCC was 0.53-1.14 μM (26). Furthermore, the Cmax concentrations for CDDP in the plasma of patients are 19-22 μM (27,28). These doses were less than those used in our experiments. Secondly, activation of DCs in the tumor local site was observed, but the detailed mechanism underlying how it promoted invasion of T cells and induced anti-tumor immunity was not revealed. Further investigations will be required to elucidate the precise mechanism through which NAC affects the maturation of DCs in ESCC.
Conflicts of Interest
The Authors have no conflicts of interest to declare in relation to this study.
Authors’ Contributions
J.N. and S.D. performed the experiments. M.Y., Y.M., T.T., T.T., L.S. and K.M. contributed to the collection of samples, analysis, and data management. H.T. contributed to the interpretation of data and review of the article. M.O. provided supervision of experiments. All Authors read and approved the manuscript.
References
- 1.Ando N, Kato H, Igaki H, Shinoda M, Ozawa S, Shimizu H, Nakamura T, Yabusaki H, Aoyama N, Kurita A, Ikeda K, Kanda T, Tsujinaka T, Nakamura K, Fukuda H. A randomized trial comparing postoperative adjuvant chemotherapy with cisplatin and 5-fluorouracil versus preoperative chemotherapy for localized advanced squamous cell carcinoma of the thoracic esophagus (jcog9907) Ann Surg Oncol. 2012;19(1):68–74. doi: 10.1245/s10434-011-2049-9. [DOI] [PubMed] [Google Scholar]
- 2.Kato H, Nakajima M. Treatments for esophageal cancer: A review. Gen Thorac Cardiovasc Surg. 2013;61(6):330–335. doi: 10.1007/s11748-013-0246-0. [DOI] [PubMed] [Google Scholar]
- 3.Garg AD, Dudek-Peric AM, Romano E, Agostinis P. Immunogenic cell death. Int J Dev Biol. 2015;59(1-3):131–140. doi: 10.1387/ijdb.150061pa. [DOI] [PubMed] [Google Scholar]
- 4.Kono K, Mimura K, Kiessling R. Immunogenic tumor cell death induced by chemoradiotherapy: Molecular mechanisms and a clinical translation. Cell Death Dis. 2013;4:e688. doi: 10.1038/cddis.2013.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gardner A, Ruffell B. Dendritic cells and cancer immunity. Trends Immunol. 2016;37(12):855–865. doi: 10.1016/j.it.2016.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Saint-Vis B, Vincent J, Vandenabeele S, Vanbervliet B, Pin JJ, Ait-Yahia S, Patel S, Mattei MG, Banchereau J, Zurawski S, Davoust J, Caux C, Lebecque S. A novel lysosome-associated membrane glycoprotein, dc-lamp, induced upon dc maturation, is transiently expressed in mhc class ii compartment. Immunity. 1998;9(3):325–336. doi: 10.1016/s1074-7613(00)80615-9. [DOI] [PubMed] [Google Scholar]
- 7.Barois N, de Saint-Vis B, Lebecque S, Geuze HJ, Kleijmeer MJ. Mhc class ii compartments in human dendritic cells undergo profound structural changes upon activation. Traffic. 2002;3(12):894–905. doi: 10.1034/j.1600-0854.2002.31205.x. [DOI] [PubMed] [Google Scholar]
- 8.Lin EW, Karakasheva TA, Hicks PD, Bass AJ, Rustgi AK. The tumor microenvironment in esophageal cancer. Oncogene. 2016;35(41):5337–5349. doi: 10.1038/onc.2016.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shimada Y, Imamura M, Wagata T, Yamaguchi N, Tobe T. Characterization of 21 newly established esophageal cancer cell lines. Cancer. 1992;69(2):277–284. doi: 10.1002/1097-0142(19920115)69:2<277::aid-cncr2820690202>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 10.Karthaus N, Torensma R, Tel J. Deciphering the message broadcast by tumor-infiltrating dendritic cells. Am J Pathol. 2012;181(3):733–742. doi: 10.1016/j.ajpath.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 11.Nishimura J, Tanaka H, Yamakoshi Y, Hiramatsu S, Tamura T, Toyokawa T, Muguruma K, Maeda K, Hirakawa K, Ohira M. Impact of tumor-infiltrating lamp-3 dendritic cells on the prognosis of esophageal squamous cell carcinoma. Esophagus. 2019;16(4):333–344. doi: 10.1007/s10388-019-00669-w. [DOI] [PubMed] [Google Scholar]
- 12.Khairallah AS, Genestie C, Auguste A, Leary A. Impact of neoadjuvant chemotherapy on the immune microenvironment in advanced epithelial ovarian cancer: Prognostic and therapeutic implications. Int J Cancer. 2018;143(1):8–15. doi: 10.1002/ijc.31200. [DOI] [PubMed] [Google Scholar]
- 13.Remark R, Lupo A, Alifano M, Biton J, Ouakrim H, Stefani A, Cremer I, Goc J, Regnard JF, Dieu-Nosjean MC, Damotte D. Immune contexture and histological response after neoadjuvant chemotherapy predict clinical outcome of lung cancer patients. Oncoimmunology. 2016;5(12):e1255394. doi: 10.1080/2162402X.2016.1255394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mihailidou C, Chatzistamou I, Papavassiliou AG, Kiaris H. Improvement of chemotherapeutic drug efficacy by endoplasmic reticulum stress. Endocr Relat Cancer. 2015;22(2):229–238. doi: 10.1530/ERC-15-0019. [DOI] [PubMed] [Google Scholar]
- 15.Cirone M, Garufi A, Di Renzo L, Granato M, Faggioni A, D'Orazi G. Zinc supplementation is required for the cytotoxic and immunogenic effects of chemotherapy in chemoresistant p53-functionally deficient cells. Oncoimmunology. 2013;2(9):e26198. doi: 10.4161/onci.26198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aranda F, Bloy N, Galluzzi L, Kroemer G, Senovilla L. Vitamin b6 improves the immunogenicity of cisplatin-induced cell death. Oncoimmunology. 2014;3(9):e955685. doi: 10.4161/21624011.2014.955685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martins I, Kepp O, Schlemmer F, Adjemian S, Tailler M, Shen S, Michaud M, Menger L, Gdoura A, Tajeddine N, Tesniere A, Zitvogel L, Kroemer G. Restoration of the immunogenicity of cisplatin-induced cancer cell death by endoplasmic reticulum stress. Oncogene. 2011;30(10):1147–1158. doi: 10.1038/onc.2010.500. [DOI] [PubMed] [Google Scholar]
- 18.Golden EB, Frances D, Pellicciotta I, Demaria S, Helen Barcellos-Hoff M, Formenti SC. Radiation fosters dose-dependent and chemotherapy-induced immunogenic cell death. Oncoimmunology. 2014;3:e28518. doi: 10.4161/onci.28518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Obeid M, Tesniere A, Ghiringhelli F, Fimia GM, Apetoh L, Perfettini JL, Castedo M, Mignot G, Panaretakis T, Casares N, Metivier D, Larochette N, van Endert P, Ciccosanti F, Piacentini M, Zitvogel L, Kroemer G. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med. 2007;13(1):54–61. doi: 10.1038/nm1523. [DOI] [PubMed] [Google Scholar]
- 20.Aranda F, Bloy N, Pesquet J, Petit B, Chaba K, Sauvat A, Kepp O, Khadra N, Enot D, Pfirschke C, Pittet M, Zitvogel L, Kroemer G, Senovilla L. Immune-dependent antineoplastic effects of cisplatin plus pyridoxine in non-small-cell lung cancer. Oncogene. 2015;34(23):3053–3062. doi: 10.1038/onc.2014.234. [DOI] [PubMed] [Google Scholar]
- 21.Yamamura Y, Tsuchikawa T, Miyauchi K, Takeuchi S, Wada M, Kuwatani T, Kyogoku N, Kuroda A, Maki T, Shichinohe T, Hirano S. The key role of calreticulin in immunomodulation induced by chemotherapeutic agents. Int J Clin Oncol. 2015;20(2):386–394. doi: 10.1007/s10147-014-0719-x. [DOI] [PubMed] [Google Scholar]
- 22.Fang H, Ang B, Xu X, Huang X, Wu Y, Sun Y, Wang W, Li N, Cao X, Wan T. Tlr4 is essential for dendritic cell activation and anti-tumor t-cell response enhancement by damps released from chemically stressed cancer cells. Cell Mol Immunol. 2014;11(2):150–159. doi: 10.1038/cmi.2013.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Di Blasio S, Wortel IM, van Bladel DA, de Vries LE, Duiveman-de Boer T, Worah K, de Haas N, Buschow SI, de Vries IJ, Figdor CG, Hato SV. Human cd1c(+) dcs are critical cellular mediators of immune responses induced by immunogenic cell death. Oncoimmunology. 2016;5(8):e1192739. doi: 10.1080/2162402X.2016.1192739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Suzuki Y, Mimura K, Yoshimoto Y, Watanabe M, Ohkubo Y, Izawa S, Murata K, Fujii H, Nakano T, Kono K. Immunogenic tumor cell death induced by chemoradiotherapy in patients with esophageal squamous cell carcinoma. Cancer Res. 2012;72(16):3967–3976. doi: 10.1158/0008-5472.CAN-12-0851. [DOI] [PubMed] [Google Scholar]
- 25.Vacchelli E, Semeraro M, Enot DP, Chaba K, Poirier Colame V, Dartigues P, Perier A, Villa I, Rusakiewicz S, Gronnier C, Goere D, Mariette C, Zitvogel L, Kroemer G. Negative prognostic impact of regulatory t cell infiltration in surgically resected esophageal cancer post-radiochemotherapy. Oncotarget. 2015;6(25):20840–20850. doi: 10.18632/oncotarget.4428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuwahara A, Yamamori M, Nishiguchi K, Okuno T, Chayahara N, Miki I, Tamura T, Kadoyama K, Inokuma T, Takemoto Y, Nakamura T, Kataoka K, Sakaeda T. Effect of dose-escalation of 5-fluorouracil on circadian variability of its pharmacokinetics in japanese patients with stage iii/iva esophageal squamous cell carcinoma. Int J Med Sci. 2010;7(1):48–54. doi: 10.7150/ijms.7.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dijkgraaf EM, Heusinkveld M, Tummers B, Vogelpoel LT, Goedemans R, Jha V, Nortier JW, Welters MJ, Kroep JR, van der Burg SH. Chemotherapy alters monocyte differentiation to favor generation of cancer-supporting m2 macrophages in the tumor microenvironment. Cancer Res. 2013;73(8):2480–2492. doi: 10.1158/0008-5472.CAN-12-3542. [DOI] [PubMed] [Google Scholar]
- 28.Kroep JR, Smit EF, Giaccone G, Van der Born K, Beijnen JH, Van Groeningen CJ, Van der Vijgh WJ, Postmus PE, Pinedo HM, Peters GJ. Pharmacology of the paclitaxel-cisplatin, gemcitabine-cisplatin, and paclitaxel-gemcitabine combinations in patients with advanced non-small cell lung cancer. Cancer Chemother Pharmacol. 2006;58(4):509–516. doi: 10.1007/s00280-006-0191-z. [DOI] [PubMed] [Google Scholar]