Abstract
Background: Situs inversus totalis (SIT) is a rare congenital condition in which the thoracic and abdominal organs are inverted like a mirror image. Case Report: We present a case of synchronous gastric cancer and gastrointestinal stromal tumor (GIST) associated with SIT in a 74-year-old man who was admitted to our department to treat gastric cancer. Esophagogastroduodenoscopy revealed a depressed lesion and a submucosal tumor (SMT) in the middle-third of the stomach. Abdominal contrast-enhanced computed tomography revealed complete inversion of the internal organs, and the common hepatic artery branched from the superior mesenteric artery. The patient underwent laparoscopic distal gastrectomy with regional lymph node dissection and Billroth I reconstruction. The macroscopic observation of the resected specimen revealed a depressed lesion measuring 2.0×1.5 cm in diameter and an SMT measuring 2.2×1.8 cm. Conclusion: Careful preoperative anatomic evaluation is important in SIT because the situs anomalies may be accompanied by major vascular anomalies.
Keywords: Situs inversus, gastric cancer, laparoscopic distal gastrectomy, gastrointestinal stromal tumor, three-dimensional computed tomography
Situs inversus totalis (SIT) is a relatively uncommon congenital condition where the position of the cardiopulmonary and abdominal organs is inverted. Its prevalence ranges from 1 per 8,000 to 1 per 25,000 of the population (1). In patients with SIT, surgical procedures for gastric tumors could be technically difficult and confusing due to the anatomical anomalies, including major perigastric vessels (2). Although laparoscopic surgery is widely selected due to the overall progress of laparoscopic procedures in recent years, laparoscopic gastrectomy in patients with SIT and gastric tumor remains very rare.
We report a case of a patient who had synchronous gastric cancer and gastrointestinal stromal tumor (GIST) with SIT and was treated by laparoscopic distal gastrectomy with regional lymph node dissection. We also discuss how the surgical treatment was managed based on the clinical characteristics of previously reported cases.
Case Report
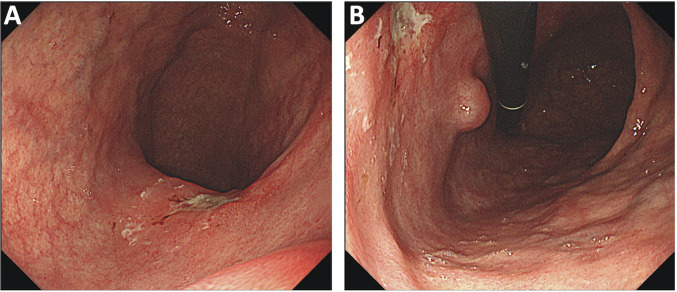
A 74-year-old man was presented to our hospital for the treatment of gastric cancer which was detected by a medical health checkup. He had a medical history of emphysema, and an unremarkable family history. His laboratory data were either within or outside normal limits, including the serum levels of tumor markers. A chest radiograph showed dextrocardia with upper lobe predominant bullae. Esophagogastroduodenoscopy showed a depressed lesion on the posterior wall of the middle third of the stomach (Figure 1), and biopsy samples suggested a moderately differentiated tubular adenocarcinoma. A submucosal tumor (SMT) was also detected in an adjacent area in the oral side of the depressed lesion.
Figure 1. Esophagogastroduodenoscopy shows a superficial depressing-type tumor and a submucosal tumor. A shallow ulcer (A and B) and a submucosal tumor (B) are observed on the posterior wall of the middle-third of the stomach.
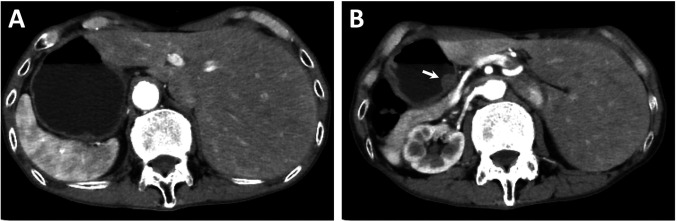
Abdominal contrast-enhanced computed tomography (CT) reveled that all abdominal organs were positioned in complete inversion; however, distant metastases were not detected (Figure 2). A three-dimensional reconstruction image of CT angiography revealed both complete transposition of vessels and the branching of the common hepatic artery (CHA) from the superior mesenteric artery (SMA) (Figure 3A). Double-contrast barium imaging showed a lesion of the deformed wall with gastric fold concentration in the posterior wall and an intragastric protruding lesion in the lesser curvature of the gastric body (Figure 3B).
Figure 2. Abdominal contrast-enhanced computed tomography shows situs inversus totalis. Complete transposition of the abdominal viscera (A and B), with a mass protruding into the gastric lumen (B, arrow).
Figure 3. Three-dimensional reconstruction image of computed tomography angiography (3DCTA) and double-contrast barium imaging. 3DCTA shows complete transposition of vessels: the common hepatic artery is noted to branch from the superior mesenteric artery (A, arrow). Doublecontrast barium imaging shows a lesion of the deformed wall, with gastric fold concentration, in the posterior wall (B, white arrowhead) and an intragastric protruding lesion in the lesser curvature of the middle-third of the stomach (B, black arrowhead).
Based on these findings the patient was diagnosed with gastric cancer and SMT with SIT and underwent laparoscopic distal gastrectomy with standard lymph node dissection, followed by Billroth I reconstruction. We observed right sided stomach and spleen, and left sided gall bladder, liver, and appendix (Figure 4A). The positions of both the surgeon and the assistant were the opposite of their usual positions: the surgeon was positioned on the left side of the patient to perform lymph node dissection in the perigastric and suprapancreatic areas, and the assistant was positioned on the right side to perform dissection of infrapyloric lymph nodes. We started the dissection from the middle portion of the gastrocolic mesentery towards the lower pole of the spleen, and the left gastroepiploic artery and vein which were located on the right side of the patient were cut after clipping (Figure 4B). After the right gastric vein and artery had been cut following clipping, the suprapancreatic lymph nodes were dissected, and the left gastric vein and artery were cut after clipping (Figure 4C). Because the CHA branched from the SMA, the splenic vein was located on the upper edge of the pancreas (Figure 4D). The operating time was 335 min, and the estimated blood loss was 20 ml.
Figure 4. Intraoperative findings during laparoscopic distal gastrectomy. The stomach and spleen are located on the right side of the abdomen (A and B). After lymph node dissection, the splenic artery is noted on the right side of the patient (C, arrow), stumps of the left gastric artery and vein are shown. The splenic vein is located on the upper edge of the pancreas because the common hepatic artery branched from the superior mesenteric artery (D, arrowhead).
Macroscopic observation of the resected specimen revealed a slightly depressed lesion measuring 2.1×1.3 cm in diameter and an SMT measuring 2.2×1.8 cm (Figure 5). Microscopic examination of the depressed lesion confirmed the diagnosis of a moderately differentiated adenocarcinoma invaded to the muscularis propria of the gastric wall. There was no lymphovascular infiltration and lymph node metastasis. The SMT was diagnosed as a GIST, which was classified as low-risk according to Fletcher’s classification (3). The postoperative patient course was favorable, and he was discharged 12 days after the operation. The patient was well without recurrence or symptoms at the 1-month follow-up.
Figure 5. Gross examination of the surgically resected specimen. The resected specimen reveals a slightly depressed lesion measuring 21×13 mm (arrowheads) and a submucosal tumor measuring 22×18 mm (arrow) .

Discussion
We describe a rare case of gastric cancer with GIST in a patient with SIT who underwent laparoscopic distal gastrectomy. Using keywords such as “gastric cancer”, “situs inversus totalis” and “laparoscopic gastrectomy”, we searched the Medline and PubMed databases (English language) for articles published from 2000 to 2020. Data on the patients’ characteristics and treatment outcome were collected from each report. Based on the results of this search, our case is the first report of the synchronous occurrence of gastric cancer and SIT-associated GIST treated via laparoscopic gastrectomy in the English literature.
The clinicopathologic characteristics of 15 previously reported cases (1,4-16) and those of our patient are shown in Table I. This cohort comprised 13 men and 3 women with a median age of 59 years (range=40–80 years). The locations of the gastric cancer were as follows: 2 cases had lesions in the upper one-third of the stomach, 3 were in the middle-third of the stomach, and 10 were in the lower one-third of the stomach. The size of gastric cancer ranged from to 1.5 to 8.0 cm, with a median size of 3.2 cm. The gastric cancers showed varying depths of invasion: lesions were confined to the mucosa in 4 cases, invaded the submucosa in 5 cases, muscularis propria in 2, subserosa in 3, and penetrated the serosa in 1 case. Pathological results showed 7 intestinal-type and 8 diffuse-type carcinomas, and the operative methods were total gastrectomy in 2 patients and distal gastrectomy in 13 patients.
Table I. Characteristics of patients who had situs inversus totalis with gastric cancer patient and underwent laparoscopic gastrectomy.
U: Upper-third of the stomach; M: middle-third of the stomach; L: lower-third of the stomach; m: mucosa; sm: submucosa; mp: muscularis propria; ss: subserosal; se: serosa, Distal: distal gastrectomy; Total: total gastrectomy; ND: not described
In a patient with SIT, the occurrence of abdominal malignancies is not common; however, surgeons should anticipate the complexity to remove cancer with optimal procedure. Further, preoperative familiarity for the anatomical diversity seems necessary when the surgeon operates on a patient with SIT, because SIT patients often have several abnormal variations including the arteries and veins (1). Three-dimensional reconstruction was useful for demonstrating the anatomical variations and for verifying the position of structures and the location of vessels. In the present case, we noted a CHA branched from SMA, which was preoperatively recognizable by detailed interpretation of abdominal contrast-enhanced CT. This variation could also be shown by three-dimensional reconstruction. We could perform a safe and curative resection following preoperative evaluation of the accurate anatomical location of vessels and by using appropriate procedures during the operation.
In our patient, during the laparoscopic surgery, the surgeon stood on the opposite side of the usual position. In the previous reports (including the present case), 7 of the 16 patients were operated while the surgeon was standing in positions opposite to their normal positions. Even in routine laparoscopic gastrectomy, the standing positions of surgeons often change during surgery, which seems to also be effective in patients with SIT. A left-handed surgeon might have a technical advantage during laparoscopic surgery in SIT patients because the right-handed operating surgeon may have difficulties with left hand stress during the operating procedure (1,17).
According to the literature, malignancies in patients with SIT may be sporadic, and synchronous occurrence of multiple primary gastrointestinal malignancies is rare (17,18). The association of Helicobacter pylori (H. pylori) infection with the synchronous occurrence of primary gastric cancer and malignant lymphoma has been well known (19). However, there are no previous reports on the association between H. pylori infection and the development of GIST, while H. pylori has been involved in the development of gastric cancer. Regarding the cause of the synchronous occurrence of GIST and gastric cancer, although the occurrence seems coincidental, the development of these tumors may implicate common carcinogens (20).
In conclusion, gastric cancer and GIST in a patient with SIT is extremely rare, and three-dimensional reconstruction image of CT angiography may be useful to recognize vascular locations preoperatively for a successful laparoscopic surgery. Description of a higher number of related cases is necessary to comprehend the pathology and to formulate the best treatment procedure for this disease entity.
Conflicts of Interest
The Authors have no conflicts of interest to declare regarding this study.
Authors’ Contributions
T. Namikawa, M. Maeda, K. Yokota and Jun Iwabu performed the surgical procedure; T. Namikawa and H. Maeda reviewed literature data; T. Namikawa, N. Tanioka, M. Munekage, S. Uemura, H. Kitagawa and Y. Nagata performed preoperative investigation the patient; T. Namikawa prepared the draft of the manuscript; M. Kobayashi was advisor of the surgical procedures; T. Namikawa and K. Hanazaki reviewed the final version of the manuscript. All Authors read and approved the final version of the manuscript.
References
- 1.Kigasawa Y, Takeuchi H, Kawakubo H, Fukuda K, Nakamura R, Takahashi T, Wada N, Kitagawa Y. Laparoscopy-assisted distal gastrectomy in a case of gastric cancer with situs inversus totalis: a case report. Asian J Endosc Surg. 2017;10(1):47–50. doi: 10.1111/ases.12326. [DOI] [PubMed] [Google Scholar]
- 2.Namikawa T, Tsuda S, Fujisawa K, Iwabu J, Uemura S, Tsujii S, Maeda H, Kitagawa H, Kobayashi M, Hanazaki K. Superficial spreading-type gastric cancer with situs inversus totalis. In Vivo. 2018;32(3):685–689. doi: 10.21873/invivo.11294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fletcher CD, Berman JJ, Corless C, Gorstein F, Lasota J, Longley BJ, Miettinen M, O’Leary TJ, Remotti H, Rubin BP, Shmookler B, Sobin LH, Weiss SW. Diagnosis of gastrointestinal stromal tumors: A consensus approach. Hum Pathol. 2002;33(5):459–465. doi: 10.1053/hupa.2002.123545. [DOI] [PubMed] [Google Scholar]
- 4.Yamaguchi S, Orita H, Yamaoka T, Mii S, Sakata H, Hashizume M. Laparoscope-assisted distal gastrectomy for early gastric cancer in a 76-year-old man with situs inversus totalis. Surg Endosc. 2003;17(2):352–353. doi: 10.1007/s00464-002-4504-y. [DOI] [PubMed] [Google Scholar]
- 5.Futawatari N, Kikuchi S, Moriya H, Katada N, Sakuramoto S, Watanabe M. Laparoscopy-assisted distal gastrectomy for early gastric cancer with complete situs inversus: report of a case. Surg Today. 2010;40(1):64–67. doi: 10.1007/s00595-009-4007-8. [DOI] [PubMed] [Google Scholar]
- 6.Seo KW, Yoon KY. Laparoscopy-assisted distal gastrectomy for early gastric cancer and laparoscopic cholecystectomy for gallstone with situs inversus totalis: a case report. J Korean Surg Soc. 2011;81:S34–38. doi: 10.4174/jkss.2011.81.Suppl1.S34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim HB, Lee JH, Park DJ, Lee HJ, Kim HH, Yang HK. Robot-assisted distal gastrectomy for gastric cancer in a situs inversus totalis patient. J Korean Surg Soc. 2012;82(5):321–324. doi: 10.4174/jkss.2012.82.5.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fujikawa H, Yoshikawa T, Aoyama T, Hayashi T, Cho H, Ogata T, Shirai J, Oshima T, Yukawa N, Rino Y, Masuda M, Tsuburaya A. Laparoscopy-assisted distal gastrectomy for an early gastric cancer patient with situs inversus totalis. Int Surg. 2013;98(3):266–270. doi: 10.9738/INTSURG-D-13-00054.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Min SH, Lee CM, Jung HJ, Lee KG, Suh YS, Shin CI, Kim HH, Yang HK. Laparoscopic distal gastrectomy in a patient with situs inversus totalis: a case report. J Gastric Cancer. 2013;13(4):266–272. doi: 10.5230/jgc.2013.13.4.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sumi Y, Maehara R, Matsuda Y, Yamashita K, Nakamura T, Suzuki S, Kuroda D, Kakeji Y. Laparoscopy-assisted distal gastrectomy in a patient with situs inversus totalis. JSLS. 2014;18(2):314–318. doi: 10.4293/108680813X13693422521953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ye MF, Tao F, Xu GG, Sun AJ. Laparoscopy-assisted distal gastrectomy for advanced gastric cancer with situs inversus totalis: A case report. World J Gastroenterol. 2015;21(35):10246–10250. doi: 10.3748/wjg.v21.i35.10246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morimoto M, Hayakawa T, Kitagami H, Tanaka M, Matsuo Y, Takeyama H. Laparoscopic-assisted total gastrectomy for early gastric cancer with situs inversus totalis: report of a first case. BMC Surg. 2015;15:75. doi: 10.1186/s12893-015-0059-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shibata K, Kawamura H, Ichikawa N, Shibuya K, Yoshida T, Ohno Y, Homma S, Taketomi A. Laparoscopic total gastrectomy for advanced gastric cancer in a patient with situs inversus totalis. Asian J Endosc Surg. 2018;11(1):39–42. doi: 10.1111/ases.12404. [DOI] [PubMed] [Google Scholar]
- 14.Alhossaini R, Hyung WJ. Robotic assisted distal gastrectomy for gastric cancer in a patient with situs inversus totalis: with Video. J Gastrointest Surg. 2017;21(12):2144–2145. doi: 10.1007/s11605-017-3576-x. [DOI] [PubMed] [Google Scholar]
- 15.Dai HB, Wang ZC, Feng XB, Wang G, Li WY, Hang CH, Jiang ZW. Case report about a successful full robotic radical gastric cancer surgery with intracorporeal robot-sewn anastomosis in a patient with situs inversus totalis and a two-and-a-half-year follow-up study. World J Surg Oncol. 2018;16(1):41. doi: 10.1186/s12957-018-1311-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ojima T, Nakamura M, Nakamori M, Yamaue H. Robotic distal gastrectomy with D2 lymphadenectomy for gastric cancer in a patient with situs inversus totalis. Surg Oncol. 2019;30:98–99. doi: 10.1016/j.suronc.2019.06.007. [DOI] [PubMed] [Google Scholar]
- 17.Kim YW, Ryu H, Kim DS, Kim IY. Double primary malignancies associated with colon cancer in patients with situs inversus totalis: two case reports. World J Surg Oncol. 2011;9:109. doi: 10.1186/1477-7819-9-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iwamura T, Shibata N, Haraguchi Y, Hisashi Y, Nishikawa T, Yamada H, Hayashi T, Toyoda K. Synchronous doublecancerof thestomachand rectum withsitusinversus totalisand polysplenia syndrome. J Clin Gastroenterol. 2001;33(2):148–153. doi: 10.1097/00004836-200108000-00012. [DOI] [PubMed] [Google Scholar]
- 19.Namikawa T, Munekage E, Fukudome I, Maeda H, Kitagawa H, Togitani K, Takasaki M, Yokoyama A, Kobayashi M, Hanazaki K. Clinicopathological characteristics and therapeutic outcomes of synchronous gastric adenocarcinoma and gastric lymphoma. Anticancer Res. 2014;34(9):5067–5074. [PubMed] [Google Scholar]
- 20.Namikawa T, Munekage E, Munekage M, Maeda M, Yatabe T, Kitagawa H, Sakamoto K, Obatake M, Kobayashi M, Hanazaki K. Synchronous large gastrointestinal stromal tumor and adenocarcinoma in thestomachtreated with imatinib mesylate followed by total gastrectomy. Anticancer Res. 2016;36(4):1855–1859. [PubMed] [Google Scholar]