Abstract
Background/Aim: High risk Human papillomavirus (hr-HPV) and smoking are independant risk factors for head and neck squamous cell carcinomas (HNSCC). While hr-HPV+ HNSCC has a better prognosis than smoking-associated HNSCC no systematic data are yet available about the combined risk. Patients and Methods: We performed a meta-analysis to assess the overall survival of HNSCC patients relative to the hr-HPV and smoking status. A literature review up to November 2019 was conducted in PubMed and Cochrane Library using the search terms ‘HPV, Smoking and HNSCC’. Results: Nine out of 748 articles were included, 1,436 out of 2,080 patients were hr-HPV+. The prevalence of hr-HPV+ smokers was 36%. The meta-analysis showed a significantly better 5-year overall survival for HPV+ non-smokers compared to smokers with risk ratio of 1.94 (95% confidence intervaI=1.46-2.58). Conclusion: Smoking is a negative prognostic factor for overall survival in patients with hr-HPV+ HNSCC and should thus be an important part of staging and treatment.
Keywords: HNSCC, smoking, HPV, p16, PCR, ISH
Head and neck cancer is the sixth most common cancer worldwide. Approximately 630,000 new cases are diagnosed annually resulting in more than 350,000 deaths per year. Head and neck cancer accounts for approximately 3-4% of all malignancies in Europe and the United States (1,2). More than 90% of these cases are head and neck squamous cell carcinomas (HNSCC) (3). Despite their anatomic proximity, HNSCC are a heterogeneous group of tumor entities with differences in etiology, diagnostic and therapeutic approaches as well as prognosis (3). The risk of developing HNSCC is associated with several factors. It is well accepted that HNSCC is etiologically associated with excessive tobacco and alcohol consumption. Both smoking and drinking are important risk factors and have a synergistic effect (4). Furthermore, infection with high-risk human papilloma viruses (hr-HPV) have been identified as a the second major causative agent (5). Approximately 40% of all HNSCCs are reported to be hr-HPV+, as well as approximately 70% of oropharyngeal squamous cell carcinomas (OPSCCs) (6). HPV-16, HPV-18, HPV-31 and HPV-33 are classified as hr- HPV. HPV-16 is the most common HPV subtype and is associated with 86-92% of all HPV+ HNSCCs (5,6). From an epidemiological perspective, the incidence of smoking-associated HNSCC is declining, while that of hr-HPV-induced cancer is increasing, especially in younger patients (7).Moreover, the underlying cause of HNSCC, i.e. toxins such as tobacco and alcohol, or hr-HPV+, leads to significant differences not only in etiology, but also in prognosis; Hr-HPV+ status being associated with an improved prognosis. Accordingly, several studies suggested a survival difference of up to 26.9% between hr-HPV+ and HPV− HNSCC (8). Because hr-HPV+ tumors differ from and have a more favorable prognosis than HPV− HNSCC, the recently released Eighth Edition of the American Joint Committee on Cancer created a separate staging algorithm for hr-HPV+ OPSCC, distinguishing it from HPV− OPSCC. Ηr-HPV+ OPSCC that used to be designated as ‘advanced stage’ is now categorized as ‘lower stage’, which gives a much more accurate survival prediction (9,10). Smoking, on the other hand, is correlated with poor prognosis. The negative impact of smoking on survival has been well documented (11-13). The 5-year overall survival (OS) rate for never-smokers compared with current-/former smokers is 77.7% vs. 57.5/58.1% respectively (14). Consequently, our goal was to assess how these two independent prognostic factors interact with each other. To our knowledge, no meta-analysis of survival according to combined HPV/smoking status has been performed. The aim of the current study was to perform a meta-analysis on the survival of patients in these distinct groups and to compare smoking and non-smoking hr-HPV+ groups.
Patients and Methods
Literature search. We conducted a comprehensive search looking for published literature that evaluated survival of HNSCC patients according to both hr-HPV and smoking status in the PubMed (http://www.ncbi.nlm.nih.gov/pubmed) and Cochrane Library (https://www.cochranelibrary.com) databases. This search included articles published up to 12/26/2019. The following search terms were used: ‘HPV, smoking, head and neck cancer’. Additionally, we checked references quoted in original or review articles that may not have been found in the database during the initial literature search. Our search strategy was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses and logged in the Prospective Register of Systematic Reviews (15). We screened all search results and included studies of patients with HNSCC, investigating survival rates according to HPV and smoking status. When abstracts met our inclusion criteria, the articles were read in detail. Exclusion criteria were: Patient survival information not reported, evaluation of survival based on only one marker (HPV or smoking) or the survival of HPV and smoking status was not combined (e.g. both parameters were assessed separately), non-HNSCC primary cancer (e.g. pre-cancerous lesions, esophageal cancer, lung cancer, skin cancer), cell culture or animal models, systematic reviews or case reports and articles written in a language other than English or German. Finally, this meta-analysis included studies with the following criteria: (i) Proportion of the HNSCC subgroups smoking versus nonsmoking; (ii) numerical survival data for the subgroups [5-year overall survival (OS) or Kaplan-Meier curves of OS by subgroup].
Study selection and data extraction. Two Authors (A.M. and V.V) independently selected the studies using the inclusion and exclusion criteria. Relevant data from all eligible publications were extracted: First author’s name, study design, study time, sample number, gender, tumor location, HPV detection method, smoking definition, number of patients in the smoking and non-smoking hr-HPV+ groups. The 5-year OS rate was extracted from the Kaplan-Meier curve using GraphClick (Version 3.0.3, Arizona Software 2010, www.arizona-software.ch/graphclick). In case of discrepancies, findings were re-analyzed and discussed until consensus was reached.
Statistical analysis. The 5-year OS of the smoking and non-smoking groups were tested using the risk ratio (RR). The summary RR was estimated including the 95% confidence interval (CI). The heterogeneity was tested using a chi-squared-based Q test; a p-value greater than 0.05 means homogeneity. The existence of heterogeneity was evaluated by using the I2 index in the meta-analysis, which was represented as a percentage value between 0 and 100. We initially applied a fixed-effects model (Mantel-Haenszel method and chi-squared test) to the data. When there was significant heterogeneity, we used the random-effects model (DerSimonian-Liard method). We compared the RR of the 5-year OS of the two groups using a forest plot. The funnel plot examined publication bias. For all statistical analysis, we used R Version 3.5.1: A language and environment for statistical computing [R Foundation for Statistical Computing, R Core Team (2018), Vienna, Austria].
Risk of bias. Publication bias was assessed using a funnel plot. After formulating a review question in a hypothetical randomized trial (target trial), the risk of bias was analyzed for each study using the ROBINS-I tool (16). The tool includes seven domains: Confounding, selection of participants for the study (pre-intervention), classification of intervention (at intervention), deviation from intended interventions, missing data, measurements of outcome, selection of the reported results (post-intervention). The risk of bias analysis was performed independently by two Authors (T.H. and V.V.). All eligible publications were analyzed in detail. Relevant information was extracted to assess the risk of bias in each of the aforementioned domains using the classifications low, moderate, serious or critical risk of bias. Conflicting results were discussed to reach a consensus.
Results
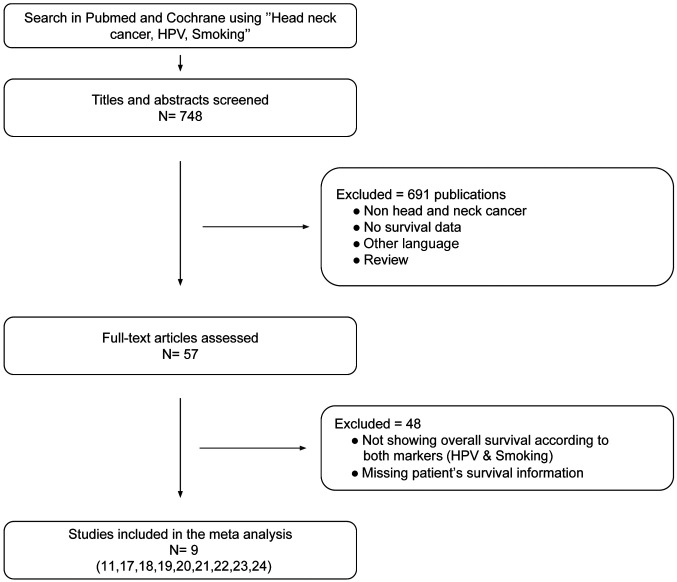
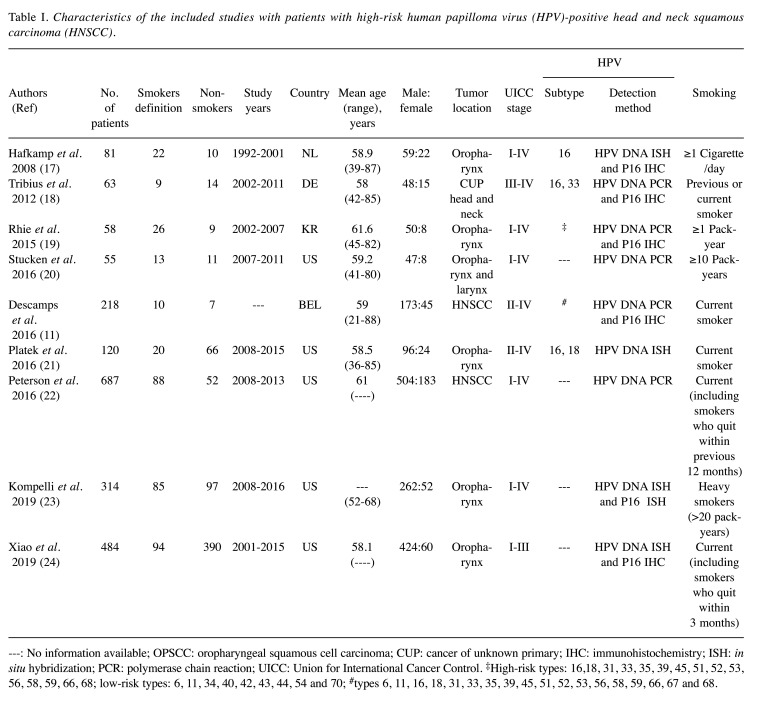
Literature search. A total of 748 publications were identified. After multiple assessment phases only nine publications met our inclusion criteria (Figure 1), with total of 2,080 patients (mean=231), 1,663 (80%) male and 417 (20%) female. However, only HPV+ patients were included in our study, representing 1,436 patients (range per study=46-484 patients). All nine included studies were cohort studies. The main characteristics of the identified studies are summarized in Table I (11,17-24). A total of 367 (36%) were HPV+ smokers and 656 (64%) were HPV+ non-smokers. Five studies investigated only OPSCC (17,19,21,23,24), three studies included patients with HNSCC (11,20,22), and one study reported patients with carcinoma of unknown primary (18). Three studies were performed in Europe (11,17,18), five studies in North America (20-24), and one study in Asia (19). For determination of HPV states, six studies used the hr-HPV surrogate marker p16 followed by HPV detection using HPV polymerase chain reaction (PCR) or in-situ hybridization (ISH) for DNA detection (11,17-19,23,24). In three studies, HPV was detected using HPV PCR or ISH without studying the p16 status (20-22).
Figure 1. Flow diagram for the literature search to investigate the impact of smoking in patients according to PRISMA criteria (15).
Table I. Characteristics of the included studies with patients with high-risk human papilloma virus (HPV)-positive head and neck squamous carcinoma (HNSCC).
---: No information available; OPSCC: oropharyngeal squamous cell carcinoma; CUP: cancer of unknown primary; IHC: immunohistochemistry; ISH: in situ hybridization; PCR: polymerase chain reaction; UICC: Union for International Cancer Control. ‡High-risk types: 16,18, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68; low-risk types: 6, 11, 34, 40, 42, 43, 44, 54 and 70; #types 6, 11, 16, 18, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 67 and 68.
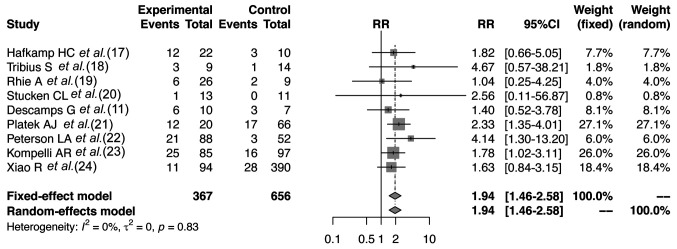
Meta-analysis. All nine studies investigated the 5-year OS of the two hr-HPV+ HNSCC smoking and nonsmoking subgroups (Figure 2). Ηr-HPV+ non-smoking patients had a better prognosis compared with hr-HPV+ smoking patients (no significant heterogeneity, p=0.83, fixed-effect model: RR=1.94, 95% CI=1.46-2.58).
Figure 2. Forest plot of the meta-analysis comparing the 5-year survival of smoking (experimental) and non-smoking (control) patients. CI: Confidence intervaI; RR: risk ratio.
Subgroup analysis. The sub-meta-analyses according to tumor locations included OPSCC and HNSCC other than OPSCC (OPSCC: no significant heterogeneity, p=0.83; fixed effect model, RR=1.86, 95% CI=1.36-2.54, and HNSCC without OPSCC: no significant heterogeneity, p=0.38; fixed effects model: RR=2.23; 95% CI=1.07-4.65). In both sub analyses, RR and CI were essentially unaltered compared with the major meta-analyses of the complete data set.
The sub-meta-analyses for HPV proof showed comparable results to the major meta-analyses (ISH HPV DNA PCR/ISH and p16: no significant heterogeneity, p=0.91; fixed effect model, RR=1.68; 95% CI=1.19-2.38, and HPV DNA PCR or ISH without p16: no significant heterogeneity, p=0.68, fixed effects model, RR=2.58, 95% CI=1.59-4.20).
Sensitivity analysis. The parameters of 50% of the most recent studies (no significant heterogeneity, p=0.59; fixed effects model, RR=1.97, 95% CI=1.45-2.68) (11,21-24) confirmed the results of the whole meta-analysis with the whole data set.
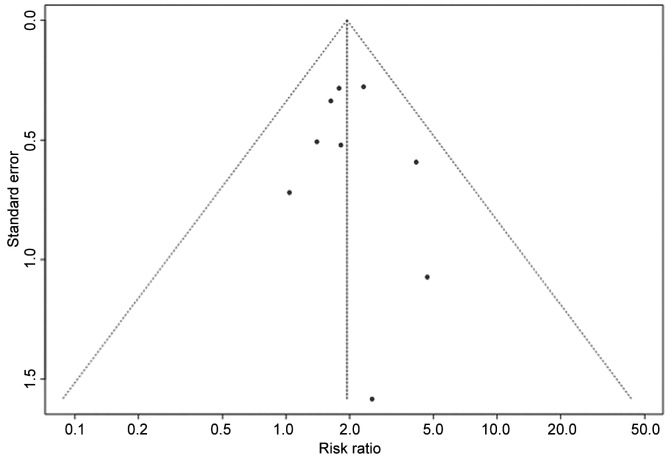
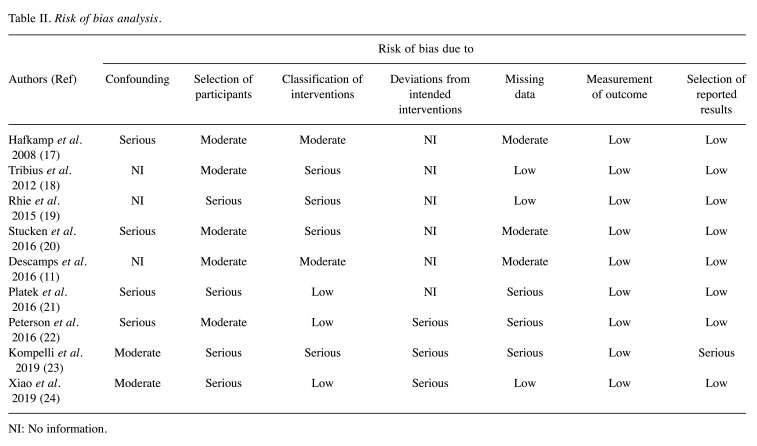
Publication bias and risk of bias assessment. The funnel plot shape did not reveal any obvious evidence of asymmetry (Figure 3). Egger’s test showed no significant publication bias (p=0.68). The risk of bias was assessed using the Risk of Bias in Non-randomized Studies - of Interventions (ROBINS)-I tool, a tool designed specifically to assess the risk of bias in systematic reviews (25). The mapping of domain-level judgements is shown in Table II. Confounding domains included age, gender, co-morbidities and TNM classification. Alcohol consumption, surgery, radiotherapy and chemotherapy were defined as hypothetical co-interventions in the pre-defined target trial. Usually, no controlling method was applied for all criteria which led to the judgement of moderate to high risk of bias in any included study. The wide variety of criteria for patient inclusion in all studies regarding HPV detection method, HPV subtype and tumor sublocalization of HNSCC resulted in moderate to serious risk of bias. p16 testing followed by DNA ISH or PCR was considered the gold standard for HPV detection and all high-risk HPV subtypes. The HNSCC sublocalizations would have been included in the analysis in an ideal trial. Furthermore, there was a lack of consistent definition of the intervention group ‘smoker’. Some authors defined smoking using standardized measures such as pack-years, while others focused on the timely aspect and defined a smoker as a patient who actively/currently smokes. Serious risk of bias was determined in the ‘deviation of intended intervention’ domain if there was evidence of imbalance of co-interventions among the sub-groups, e.g. higher alcohol consumption in connection with smoking. Moreover, missing data can introduce bias if the same HPV detection methods are not applied to all patients. The measurement of the OS outcome was judged as low-risk of bias in all studies and the selection of reported results carried generally low to moderate risk of bias.
Figure 3. Funnel plot of the meta-analysis of smokers and non-smokers with high-risk human papilloma virus-positive head and neck squamous carcinoma.
Table II. Risk of bias analysis.
NI: No information
Discussion
Many studies have investigated HNSCC patient outcomes according to HPV or smoking status. It is known that hr-HPV+ cancers tend to have better outcomes than HPV-negative ones and that smokers usually have a worse prognosis than non-smokers (8-12). Recently Chen et al. published a systematic review showing that all included studies reported that smoking was associated with worse outcomes in hr-HPV+ cancer (26). Variability of the reported studies regarding reported outcomes and smoking metrics prohibited further analyses. Yet to our knowledge, there has never been a meta-analysis of data and results regarding prognosis of patients with hr-HPV+ HNSCC based on smoking status. Our meta-analysis compares the outcome of smokers and non-smokers with hrHPV+ HNSCC.
The analysis showed that smoking plays an important prognostic role in HNSCC, even in the presence of HPV. Our results suggest that non-smokers with hr-HPV+ HNSCC have a better 5-year OS compared to their smoking counterparts. Others showed an increased hazard ratio for hr-HPV+ smokers (27-29). Further studies examined the disease-specific and disease-free survival (30-34). Going back to the pathogenesis of the HNSCC, we can find two different pathways for its carcinogenesis. In HPV-associated HNSCC, infection with hr-HPV-types leads to an increased expression of E6 and E7, which degrade and inactivate p53 and enhance expression of retinoblastoma protein, which causes overexpression of p16 (35,36). On the other hand, HPV− HNSCCs are associated with smoking and alcohol, and exposure to carcinogenic substances such as tobacco-specific nitrosamines that lead to p53 mutation (37,38). However, in hr-HPV+ carcinomas, smoking is an independent risk factor for reduced treatment efficiency and OS (39-41). The significant negative impact of smoking on prognosis might be associated with increased smoking-related comorbidities, such as pulmonary and cardiovascular diseases, therefore contributing to a worse survival chance after treatment and increased mortality. Furthermore, studies have shown that smoking increases tumor aggressiveness in cancer cells by stimulation proliferation, angiogenesis, migration, invasion and reducing the response to cytotoxic cancer agents such as chemo- or radiotherapy (42,43). House et al. recently showed that miR-133a-3p is a target of smoking-induced changes in patients with HPV+ OPSCC and alters the expression (44). Therefore, recording the smoking status should be an important part of staging and treatment even in patients with hr-HPV+ HNSCC to predict individual patient risk (45). This also highlights the importance of smoking cessation programs for patients who are actively undergoing treatment as a smoking habit is a potentially modifiable adverse risk factor that might lead to improved outcomes possibly even after diagnosis. The Eighth Edition of the American Joint Committee on Cancer TNM classification distinguishes p16-associated OPSCC from p16− OPSCC (9). The negative impact of smoking is not taken into account in the present classification (39).
HPV detection methods also play an important role. p16 is used as a surrogate marker for the detection of hr-HPV in HNSCC, and p16 immunohistochemistry (IHC) has a sensitivity of 94% and specificity of 82-83%. HPV DNA PCR has a sensitivity of 97-98% and specificity of 84-87%, whilst HPV DNA ISH has a sensitivity of 85-88% and specificity of 83-88% (46,47). Therefore, the combination of p16 IHC followed by HPV PCR or ISH remains a gold standard for HPV detection in HNSCC to confirm the HPV-related etiology of HNSCC because, as previously demonstrated, p16+ HNSCC with HPV-independent carcinogenesis may exist at a low percentage (10). In the present sub-meta-analysis, six out of nine studies used HPV DNA PCR or ISH to confirm the etiology of HPV-associated HNSCC in patients with p16+ IHC (Table I). Even the sub-meta-analysis including the three studies using only HPV DNA PCR or ISH confirmed the results shown using the whole data set. However, this detection strategy does not confirm p16-associated carcinogenesis and there is the possibility that HPV DNA may also have been an innocent bystander (in cases with p16− HNSCC) (10).
There are some limitations to our study: (a) This meta-analysis is based on data extracted from published literature and not from individual patient data. (b) All included studies were found to carry moderate to serious risk of bias. The vast majority of publications were retrospective cohort studies/non-randomized, controlled trials. These types of studies have greater susceptibility to bias. An important factor to be considered is the role of confounding factors and co-interventions. One problem was the different definitions of smoking (risk of bias analysis). Apart from smoking, there are other factors which also play an important role in prognoses and outcomes of patients with HNSCC such as comorbidities, tumor stage, therapy regimes (e.g. surgery, chemotherapy or radiation). Some studies included different subsites of HNSCC (11,20,22), and others focused on OPSCC only (17,19,21,23,24).
This heterogeneity in patient selection should be noted because in patients with OPSCC, the impact of HPV in tumorigenesis is accepted. In other sub-localizations, there is no difference for HNSCC with confirmed p16/HPV detection, and HPV detection is therefore not required.
Conclusion
Smoking plays a significant role in the prognosis of patients with hr-HPV+ HNSCC. Smokers with hr-HPV+ HNSCC have worse survival outcomes compared to hr-HPV+ nonsmokers. Smoking status should be an important part of staging and be considered when tailoring the treatment strategy even for patients with HPV-associated HNSCC, since these patients are at possible risk of undertreatment.
Conflicts of Interest
The Authors declare that they have no conflicts of interest in regard to this study.
Authors’ Contributions
Moonef Alotaibi conducted the systematic review, collected and analyzed the data, wrote the main article. Valeria Valova collected and analyzed the data, conducted the risk of bias analysis, wrote the main article. Toni Hänsel conducted and supervised the risk of bias analysis, revised the main article. Carmen Stromberger revised the main article. Grzegorz Kofla revised the main article. Heidi Olze revised the main paper. Iris Piwonski revised the main article. Andreas Albers revised the main article. Sebastian Ochsenreither revised the main article. Annekatrin Coordes designed the review, conducted the systematic review and supervised the work. All Authors commented on the article at all stages.
Acknowledgements
The Authors would like to thank Ulrich Gauger from Private Statistical Office, Berlin, Germany.
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55(2):74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 3.Vigneswaran N, Williams MD. Epidemiologic trends in head and neck cancer and aids in diagnosis. Oral Maxillofac Surg Clin North Am. 2014;26(2):123–141. doi: 10.1016/j.coms.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blot WJ, McLaughlin JK, Winn DM, Austin DF, Greenberg RS, Preston-Martin S, Bernstein L, Schoenberg JB, Stemhagen A, Fraumeni JF Jr. Smoking and drinking in relation to oral and pharyngeal cancer. Cancer Res. 1988;48(11):3282–3287. doi: 10.1038/sj.bjc.6603713. [DOI] [PubMed] [Google Scholar]
- 5.Dayyani F, Etzel CJ, Liu M, Ho CH, Lippman SM, Tsao AS. Meta-analysis of the impact of human papillomavirus (hpv) on cancer risk and overall survival in head and neck squamous cell carcinomas (hnscc) Head Neck Oncol. 2010;2:15. doi: 10.1186/1758-3284-2-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abogunrin S, Di Tanna GL, Keeping S, Carroll S, Iheanacho I. Prevalence of human papillomavirus in head and neck cancers in european populations: A meta-analysis. BMC Cancer. 2014;14:968. doi: 10.1186/1471-2407-14-968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tinhofer I, Johrens K, Keilholz U, Kaufmann A, Lehmann A, Weichert W, Stenzinger A, Stromberger C, Klinghammer K, Becker ET, Dommerich S, Stolzel K, Hofmann VM, Hildebrandt B, Moser L, Ervens J, Bottcher A, Albers A, Stabenow R, Reinecke A, Budach V, Hoffmeister B, Raguse JD. Contribution of human papilloma virus to the incidence of squamous cell carcinoma of the head and neck in a european population with high smoking prevalence. Eur J Cancer. 2015;51(4):514–521. doi: 10.1016/j.ejca.2014.12.018. [DOI] [PubMed] [Google Scholar]
- 8.Li H, Torabi SJ, Yarbrough WG, Mehra S, Osborn HA, Judson B. Association of human papillomavirus status at head and neck carcinoma subsites with overall survival. JAMA Otolaryngol Head Neck Surg. 2018;144(6):519–525. doi: 10.1001/jamaoto.2018.0395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lydiatt WM, Patel SG, O’Sullivan B, Brandwein MS, Ridge JA, Migliacci JC, Loomis AM, Shah JP. Head and neck cancers-major changes in the american joint committee on cancer eighth edition cancer staging manual. CA Cancer J Clin. 2017;67(2):122–137. doi: 10.3322/caac.21389. [DOI] [PubMed] [Google Scholar]
- 10.Albers AE, Qian X, Kaufmann AM, Coordes A. Meta analysis: Hpv and p16 pattern determines survival in patients with hnscc and identifies potential new biologic subtype. Sci Rep. 2017;7(1):16715–16715. doi: 10.1038/s41598-017-16918-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Descamps G, Karaca Y, Lechien JR, Kindt N, Decaestecker C, Remmelink M, Larsimont D, Andry G, Hassid S, Rodriguez A, Khalife M, Journe F, Saussez S. Classical risk factors, but not hpv status, predict survival after chemoradiotherapy in advanced head and neck cancer patients. J Cancer Res Clin Oncol. 2016;142(10):2185–2196. doi: 10.1007/s00432-016-2203-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Cicco R, de Melo Menezes R, Nicolau UR, Pinto CAL, Villa LL, Kowalski LP. Impact of human papillomavirus status on survival and recurrence in a geographic region with a low prevalence of hpv-related cancer: A retrospective cohort study. Head Neck. 2020;42(1):93–102. doi: 10.1002/hed.25985. [DOI] [PubMed] [Google Scholar]
- 13.Mirghani H, Leroy C, Chekourry Y, Casiraghi O, Auperin A, Tao Y, Nguyen F, Caroline E, Breuskin I, Plana AM, Hartl D, Janot F, Temam S, Gorphe P, Blanchard P. Smoking impact on hpv driven head and neck cancer’s oncological outcomes. Oral Oncol. 2018;82:131–137. doi: 10.1016/j.oraloncology.2018.05.007. [DOI] [PubMed] [Google Scholar]
- 14.Abrahao R, Anantharaman D, Gaborieau V, Abedi-Ardekani B, Lagiou P, Lagiou A, Ahrens W, Holcatova I, Betka J, Merletti F, Richiardi L, Kjaerheim K, Serraino D, Polesel J, Simonato L, Alemany L, Agudo Trigueros A, Macfarlane TV, Macfarlane GJ, Znaor A, Robinson M, Canova C, Conway DI, Wright S, Healy CM, Toner M, Cadoni G, Boccia S, Gheit T, Tommasino M, Scelo G, Brennan P. The influence of smoking, age and stage at diagnosis on the survival after larynx, hypopharynx and oral cavity cancers in europe: The arcage study. Int J Cancer. 2018;143(1):32–44. doi: 10.1002/ijc.31294. [DOI] [PubMed] [Google Scholar]
- 15.Moher D, Liberati A, Tetzlaff J, Altman DG for the PRISMA group Preferred reporting items for systematic reviews and meta-analyses: The prisma statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sterne JA, Hernan MA, Reeves BC, Savovic J, Berkman ND, Viswanathan M, Henry D, Altman DG, Ansari MT, Boutron I, Carpenter JR, Chan AW, Churchill R, Deeks JJ, Hrobjartsson A, Kirkham J, Juni P, Loke YK, Pigott TD, Ramsay CR, Regidor D, Rothstein HR, Sandhu L, Santaguida PL, Schunemann HJ, Shea B, Shrier I, Tugwell P, Turner L, Valentine JC, Waddington H, Waters E, Wells GA, Whiting PF, Higgins JP. Robins-i: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. doi: 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hafkamp HC, Manni JJ, Haesevoets A, Voogd AC, Schepers M, Bot FJ, Hopman AH, Ramaekers FC, Speel EJ. Marked differences in survival rate between smokers and nonsmokers with hpv 16-associated tonsillar carcinomas. Int J Cancer. 2008;122(12):2656–2664. doi: 10.1002/ijc.23458. [DOI] [PubMed] [Google Scholar]
- 18.Tribius S, Hoffmann AS, Bastrop S, Gorogh T, Haag J, Rocken C, Clauditz T, Grob T, Wilczak W, Tennstedt P, Borcherding A, Petersen C, Hoffmann M. Hpv status in patients with head and neck of carcinoma of unknown primary site: Hpv, tobacco smoking, and outcome. Oral Oncol. 2012;48(11):1178–1184. doi: 10.1016/j.oraloncology.2012.05.022. [DOI] [PubMed] [Google Scholar]
- 19.Rhie A, Park WS, Choi MK, Kim JH, Ryu J, Ryu CH, Kim JI, Jung YS. Genomic copy number variations characterize the prognosis of both p16-positive and p16-negative oropharyngeal squamous cell carcinoma after curative resection. Medicine (Baltimore) 2015;94(50):e2187. doi: 10.1097/md.0000000000002187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stucken CL, de Almeida JR, Sikora AG, Tong CC, Genden EM. Impact of human papillomavirus and smoking on survival outcomes after transoral robotic surgery. Head Neck. 2016;38(3):380–386. doi: 10.1002/hed.23915. [DOI] [PubMed] [Google Scholar]
- 21.Platek AJ, Jayaprakash V, Merzianu M, Platek ME, Cohan DM, Hicks WL Jr., Marimuthu SP, Winslow TB, Gupta V, Arshad H, Kuriakose MA, Dibaj S, Marshall JR, Reid ME, Warren GW, Singh AK. Smoking cessation is associated with improved survival in oropharynx cancer treated by chemoradiation. Laryngoscope. 2016;126(12):2733–2738. doi: 10.1002/lary.26083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peterson LA, Bellile EL, Wolf GT, Virani S, Shuman AG, Taylor JM, Rozek LS. Cigarette use, comorbidities, and prognosis in a prospective head and neck squamous cell carcinoma population. Head Neck. 2016;38(12):1810–1820. doi: 10.1002/hed.24515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kompelli AR, Morgan P, Li H, Harris W, Day TA, Neskey DM. Prognostic impact of high-risk pathologic features in hpv-related oropharyngeal squamous cell carcinoma and tobacco use. Otolaryngol Head Neck Surg. 2019;160(5):855–861. doi: 10.1177/0194599818818446. [DOI] [PubMed] [Google Scholar]
- 24.Xiao R, Pham Y, Ward MC, Houston N, Reddy CA, Joshi NP, Greskovich JF Jr., Woody NM, Chute DJ, Lamarre ED, Prendes BL, Lorenz RR, Scharpf J, Burkey BB, Geiger JL, Adelstein DJ, Koyfman SA. Impact of active smoking on outcomes in hpv+ oropharyngeal cancer. Head Neck. 2020;42(2):269–280. doi: 10.1002/hed.26001. [DOI] [PubMed] [Google Scholar]
- 25.Whiting P, Savovic J, Higgins JP, Caldwell DM, Reeves BC, Shea B, Davies P, Kleijnen J, Churchill R. Robis: A new tool to assess risk of bias in systematic reviews was developed. J Clin Epidemiol. 2016;69:225–234. doi: 10.1016/j.jclinepi.2015.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen SY, Massa S, Mazul AL, Kallogjeri D, Yaeger L, Jackson RS, Zevallos J, Pipkorn P. The association of smoking and outcomes in hpv-positive oropharyngeal cancer: A systematic review. Am J Otolaryngol. 2020;41(5):102592. doi: 10.1016/j.amjoto.2020.102592. [DOI] [PubMed] [Google Scholar]
- 27.Hong AM, Martin A, Chatfield M, Jones D, Zhang M, Armstrong B, Lee CS, Harnett G, Milross C, Clark J, Elliott M, Smee R, Corry J, Liu C, Porceddu S, Rees G, Rose B. Human papillomavirus, smoking status and outcomes in tonsillar squamous cell carcinoma. Int J Cancer. 2013;132(12):2748–2754. doi: 10.1002/ijc.27956. [DOI] [PubMed] [Google Scholar]
- 28.Hawkins PG, Mierzwa ML, Bellile E, Jackson WC, Malloy KM, Chinn SB, Spector ME, Shuman AG, Stucken CL, McLean SA, Bradford CR, Prince ME, Carey TE, Worden FP, Swiecicki PL, Taylor JMG, Wolf GT, Eisbruch A, Casper KA. Impact of american joint committee on cancer eighth edition clinical stage and smoking history on oncologic outcomes in human papillomavirus-associated oropharyngeal squamous cell carcinoma. Head Neck. 2019;41(4):857–864. doi: 10.1002/hed.25336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kumar B, Cordell KG, Lee JS, Worden FP, Prince ME, Tran HH, Wolf GT, Urba SG, Chepeha DB, Teknos TN, Eisbruch A, Tsien CI, Taylor JM, D’Silva NJ, Yang K, Kurnit DM, Bauer JA, Bradford CR, Carey TE. Egfr, p16, hpv titer, bcl-xl and p53, sex, and smoking as indicators of response to therapy and survival in oropharyngeal cancer. J Clin Oncol. 2008;26(19):3128–3137. doi: 10.1200/JCO.2007.12.7662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ljokjel B, Haave H, Lybak S, Aarstad HH, Karlsdottir A, Vintermyr OK, Aarstad HJ. The impact of hpv infection, smoking history, age and operability of the patient on disease-specific survival in a geographically defined cohort of patients with oropharyngeal squamous cell carcinoma. Acta Otolaryngol. 2014;134(9):964–973. doi: 10.3109/00016489.2014.927590. [DOI] [PubMed] [Google Scholar]
- 31.Maxwell JH, Kumar B, Feng FY, Worden FP, Lee JS, Eisbruch A, Wolf GT, Prince ME, Moyer JS, Teknos TN, Chepeha DB, McHugh JB, Urba SG, Stoerker J, Walline HM, Kurnit DM, Cordell KG, Davis SJ, Ward PD, Bradford CR, Carey TE. Tobacco use in human papillomavirus-positive advanced oropharynx cancer patients related to increased risk of distant metastases and tumor recurrence. Clin Cancer Res. 2010;16(4):1226–1235. doi: 10.1158/1078-0432.CCR-09-2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haughey BH, Sinha P. Prognostic factors and survival unique to surgically treated p16+ oropharyngeal cancer. Laryngoscope. 2012;122 Suppl:S13–33. doi: 10.1002/lary.23493. [DOI] [PubMed] [Google Scholar]
- 33.Lassen P, Lacas B, Pignon JP, Trotti A, Zackrisson B, Zhang Q, Overgaard J, Blanchard P, Group MC. Prognostic impact of hpv-associated p16-expression and smoking status on outcomes following radiotherapy for oropharyngeal cancer: The march-hpv project. Radiother Oncol. 2018;126(1):107–115. doi: 10.1016/j.radonc.2017.10.018. [DOI] [PubMed] [Google Scholar]
- 34.Billfalk-Kelly A, Yu E, Su J, O’Sullivan B, Waldron J, Ringash J, Bartlett E, Perez-Ordonez B, Weinreb I, Bayley A, Bratman SV, Cho J, Giuliani M, Hope A, Hosni A, Kim J, Hansen AR, de Almeida J, Tong L, Xu W, Huang SH. Radiologic extranodal extension portends worse outcome in cn+ tnm-8 stage i human papillomavirus-mediated oropharyngeal cancer. Int J Radiat Oncol Biol Phys. 2019;104(5):1017–1027. doi: 10.1016/j.ijrobp.2019.03.047. [DOI] [PubMed] [Google Scholar]
- 35.Tran N, Rose BR, O’Brien CJ. Role of human papillomavirus in the etiology of head and neck cancer. Head Neck. 2007;29(1):64–70. doi: 10.1002/hed.20460. [DOI] [PubMed] [Google Scholar]
- 36.Kobayashi K, Hisamatsu K, Suzui N, Hara A, Tomita H, Miyazaki T. A review of hpv-related head and neck cancer. J Clin Med. 2018;7(9) doi: 10.3390/jcm709024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brennan JA, Boyle JO, Koch WM, Goodman SN, Hruban RH, Eby YJ, Couch MJ, Forastiere AA, Sidransky D. Association between cigarette smoking and mutation of the p53 gene in squamous-cell carcinoma of the head and neck. N Engl J Med. 1995;332(11):712–717. doi: 10.1056/nejm199503163321104. [DOI] [PubMed] [Google Scholar]
- 38.Jethwa AR, Khariwala SS. Tobacco-related carcinogenesis in head and neck cancer. Cancer Metastasis Rev. 2017;36(3):411–423. doi: 10.1007/s10555-017-9689-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DI, Nguyen-Tan PF, Westra WH, Chung CH, Jordan RC, Lu C, Kim H, Axelrod R, Silverman CC, Redmond KP, Gillison ML. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363(1):24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Granata R, Miceli R, Orlandi E, Perrone F, Cortelazzi B, Franceschini M, Locati LD, Bossi P, Bergamini C, Mirabile A, Mariani L, Olmi P, Scaramellini G, Potepan P, Quattrone P, Ang KK, Licitra L. Tumor stage, human papillomavirus and smoking status affect the survival of patients with oropharyngeal cancer: An Italian validation study. Ann Oncol. 2012;23(7):1832–1837. doi: 10.1093/annonc/mdr544. [DOI] [PubMed] [Google Scholar]
- 41.O’Sullivan B, Huang SH, Siu LL, Waldron J, Zhao H, Perez-Ordonez B, Weinreb I, Kim J, Ringash J, Bayley A, Dawson LA, Hope A, Cho J, Irish J, Gilbert R, Gullane P, Hui A, Liu FF, Chen E, Xu W. Deintensification candidate subgroups in human papillomavirus-related oropharyngeal cancer according to minimal risk of distant metastasis. J Clin Oncol. 2013;31(5):543–550. doi: 10.1200/jco.2012.44.0164. [DOI] [PubMed] [Google Scholar]
- 42.Sobus SL, Warren GW. The biologic effects of cigarette smoke on cancer cells. Cancer. 2014;120(23):3617–3626. doi: 10.1002/cncr.28904. [DOI] [PubMed] [Google Scholar]
- 43.Warren GW, Sobus S, Gritz ER. The biological and clinical effects of smoking by patients with cancer and strategies to implement evidence-based tobacco cessation support. Lancet Oncol. 2014;15(12):e568–580. doi: 10.1016/s1470-2045(14)70266-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.House R, Majumder M, Janakiraman H, Ogretmen B, Kato M, Erkul E, Hill E, Atkinson C, Barth J, Day TA, Palanisamy V. Smoking-induced control of mir-133a-3p alters the expression of egfr and hur in hpv-infected oropharyngeal cancer. PLoS One. 2018;13(10):e0205077. doi: 10.1371/journal.pone.0205077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tota JE, Gillison ML, Katki HA, Kahle L, Pickard RK, Xiao W, Jiang B, Graubard BI, Chaturvedi AK. Development and validation of an individualized risk prediction model for oropharynx cancer in the us population. Cancer. 2019;125(24):4407–4416. doi: 10.1002/cncr.32412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Prigge ES, Arbyn M, von Knebel Doeberitz M, Reuschenbach M. Diagnostic accuracy of p16(ink4a) immunohistochemistry in oropharyngeal squamous cell carcinomas: A systematic review and meta-analysis. Int J Cancer. 2017;140(5):1186–1198. doi: 10.1002/ijc.30516. [DOI] [PubMed] [Google Scholar]
- 47.Qureishi A, Winter S. Letter to editor: Current and future techniques for human papilloma virus (hpv) testing in oropharyngeal squamous cell carcinoma. Eur Arch Otorhinolaryngol. 2017;274(12):4259. doi: 10.1007/s00405-017-4675-8. [DOI] [PubMed] [Google Scholar]