Abstract
Background/Aim: The prognosis of colorectal cancer is reported to differ depending on the tumor site, and clinical differences depending on the site of occurrence have gained attention. The aim was to compare nutrition index and inflammatory markers according to the site of colon cancer. Patients and Methods: We retrospectively analyzed 272 cases of stage I-III colon cancer (55% males, 45% females). The clinical characteristics, nutrition index and inflammatory markers were compared between patients with right colon cancer (RCC, n=119) and those with left colon cancer (LCC, n=153), and the relapse-free survival was then compared. Results: RCC was associated with older age (p=0.03), female gender (p=0.003), higher T stage (p=0.01), elevated platelet/lymphocyte ratio (PLR) (p=0.009), and elevated CONUT score (p=0.028). The prognostic values differed between RCC and LCC (RCC: CONUT score, p=0.04, LCC: PLR, p=0.02). Conclusion: RCC was associated with an elevated CONUT score and PLR. In RCC, the CONUT score was an independent recurrence factor, and in LCC, the PLR was an independent recurrence factor.
Keywords: CONUT score, mGPS, NLR, PLR, CAR, colorectal cancer
There are several reports of a relationship between the laterality of the primary site of colon cancer (right side; splenic flexure/left side: including descending colon, sigmoid colon, rectal sigmoid part) and the prognosis and therapeutic effect (1). It is reported that the prognosis of RCC (right colon cancer) is significantly worse than that of LCC (left colon cancer), and that the therapeutic effect of the anti-epidermal growth factor receptor (EGFR) antibody drug cetuximab is significantly better in LCC compared to RCC (2). In addition to the location of colon cancer, due to differences in biological characteristics, mismatch repair gene defect on the right side, KRAS/BRAF mutation, microRNA-31, left is associated with CIN, p53, NRAS, microRNA-146a, microRNA-147b, microRNA-1288 (3). The clinical symptoms are also different in right- and left-side colon cancer, with iron deficiency anemia due to subclinical bleeding on the right side and bloody stools and defecation disorder on the left side (4).
Clinicians need a simple prognostic tool for colorectal cancer (CRC). Simple prognostic tools have been developed using inflammatory markers and nutrition indices. The CONtrolling NUTritional status [CONUT, which consists of the albumin (ALB) level, the total lymphocyte count (TLC), and serum total cholesterol level], the modified Glasgow prognosis score (mGPS), and the Prognostic Nutritional Index (PNI) are widely established predictors used for cancer patients) (5-7). However, to the best of our knowledge, there are no reports of differences in a nutrition index or inflammatory markers between RCC and LCC in CRC patients after curative surgery. We conducted the present study to determine the usefulness of predictive indicators, and we investigated whether there are differences in the nutritional index and inflammatory markers in CRC patients after curative surgery depending on the location of the CRC.
Patients and Methods
Patient selection. We retrospectively analyzed the cases of patients with stage I-III CRC diagnosed based on the 8th edition (8) of the U.S. Joint Commission on Cancer (AJCC) staging system who underwent a radical resection at Teikyo University Hospital in Japan between 2012 and 2017. We enrolled 272 consecutive patients. The surgery for all patients was elective. This retrospective study was approved by Teikyo University Ethics (Registration no. 16-032) and complies with the STROBE guidelines (9).
The variables evaluated included patient age, gender, tumor location, histological grade, TNM (tumor, lymph node, metastasis) stage, individual medical history, preoperative test data, and follow-up. The CONUT scores, mGPS, neutrophil/lymphocyte ratio (NLR), platelet/lymphocyte ratio (PLR), and C-reactive protein/albumin ratio (CAR) were calculated based on preoperative laboratory data. Patients were excluded if they had received adjuvant chemotherapy, had multiple primary malignancies, or had familial adenomatous polyposis or Lynch syndrome. The histopathological, clinical, and laboratory data were obtained from the patients, and blood sampling was conducted within 1-2 weeks prior to the surgery.
CONUT score. The CONUT score for each patient was calculated using the data on serum ALB, TLC, and total cholesterol levels, based on a prior study that used preoperative serum samples (10). The patients’ ALB concentrations were scored as follows: ≥3.5 g/dl=0 points; <3.5 and >3.0 g/dl=2 points; ≤2.99 and ≥2.5 g/dl=4 points; and <2.5 g/dl=6 points. The TLC was scored as follows: ≥1,600/mm3=0 points; 1,599-1,200/mm3=1 point; 1,199-800/mm3=2 points; <800/mm3=3 points. The total cholesterol concentrations were scored as: ≥180 mg/dl=0 points; 140-179 mg/dl=1 point; 100-139 mg/dl=2 points; <100 mg/dl=3 points. The CONUT score is defined as the sum of items (1), (2), and (3). The CONUT score thus ranges from 0 to 12, with lower scores indicating a good nutritional status.
mGPS score. The GPS score is as follows: a score of 2 is given for both hypoalbuminemia (<3.5 g/dl) and elevated C-reactive protein (CRP) (>1.0 mg/dl). Hypoalbuminemia (<3.5 g/dl) or CRP >1.0 mg/dl is given the score of 1. The both ALB ≥3.5g/dl and CRP ≤1.0 mg/dl is given the score of 0 (11). However, hypoalbuminemia alone was not associated with reduced survival, and thus the modified (m)GPS was created. The mGPS score 2 is given for hypoalbuminemia (<3.5 g/dl) plus elevated CRP (>1.0 mg/dl), score 1 is for elevated CRP (>10 mg/l), and score 0 is given for hypoalbuminemia (<3.5 g/dl) or a serum ALB level >3.5 g/dl and a serum CRP level <1.0 mg/dl (12).
Indices of patients’ general conditions: The NLR, PLR, and CAR values. The patients’ NLR, PLR and CAR values as indicators of their general condition were obtained by taking blood within 1-2 weeks preoperatively.
Follow-up. Surgical resection was defined as curative when there was no evidence of tumor recurrence and the distant metastases were histologically and macroscopically complete. The patients were followed up every 3 months for the first 3 years, every 6 months for the next 2 years, and once a year thereafter. At each follow-up, all patients underwent a physical examination and measurements of serum carcinoembryonic antigen (CEA) and CA19-9 (carbohydrate antigen 19-9). They also underwent a colonoscopy examination 1-2 years after their surgery (for patients with rectal cancer, the examination was conducted every year after surgery). Thoracoabdominal computed tomography (CT) scans were usually taken every 6 months. Recurrence was defined as the appearance of a radiological, clinical, and/or pathological diagnosis of cancer cells that were local or distant from their original location.
Statistical analysis. Relapse-free survival (RFS) was calculated from the date that the patient underwent surgery to that of recurrence or death, using the Kaplan-Meier method. A Cox regression analysis was performed to identify factors that are significantly associated with RFS. Probability (p)-values ≤0.05 were considered significant. The Pearson product-moment correlation coefficient was used for the bivariate correlation. All statistical analyses were performed using JMP 15 software (SAS, Cary, NC, USA).
We conducted a receiver operating characteristic (ROC) curve analysis to determine the cutoff values for the CONUT score, NLR, PLR, and CAR. For each value, an ROC curve was created by plotting the sensitivity and specificity of each result under investigation. The score closest to the point with both maximum sensitivity and specificity was selected as the cutoff value, and the largest number of tumors were correctly classified for clinical outcome.
The clinicopathological factors examined were as follows: sex, age, cancer location site (right side vs. left side), histology [tub 1 and tub 2 vs. others (por and pap, etc.)], preoperative CONUT score, mGPS score, presence/absence of vascular invasion, presence/absence of lymph invasion pT category (T1,T2 vs. ≥T3), N category, tumor size, preoperative CEA level, preoperative CA19-9 level, NLR, PLR, and CAR.
Compliance with ethical standards. The present study was conducted in accord with the Declarations of Helsinki and was approved by the Ethics Committee of the Teikyo University (approval date, 23 August 2016, registration no. 16-032).
Results
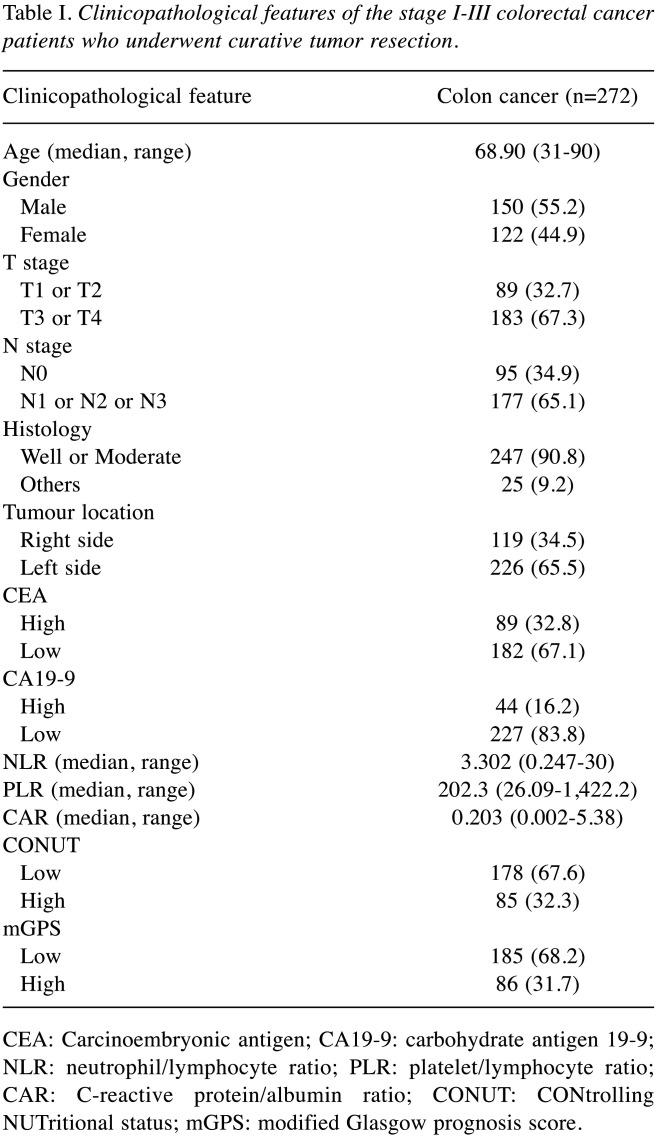
Patient characteristics. The study included a total of 272 patients. There were 119 patients diagnosed with RCC and 153 patients diagnosed with LCC. The median age was 68.9 (range=31-90 years); 150 (55.1%) patients were males and 122 (44.9%) were females. The depth of tumor invasion was 89 (32.7%) for T1 and T2, 183 (67.3%) for T3 and T4.
There were 177 (65.1%) patients with lymph node metastases and 95 (34.9%) patients without lymph node metastasis, 89 (32.8%) patients with high preoperative CEA levels, and 44 (16.2%) patients with high preoperative CA19-9 levels. The median NLR was 3.302 (range=0.247-30); the PLR was 202.3 (range=26.09-1,422.2), and the CAR was 0.203 (range=0.002-5.38) (Table I).
Table I. Clinicopathological features of the stage I-III colorectal cancer patients who underwent curative tumor resection.
CEA: Carcinoembryonic antigen; CA19-9: carbohydrate antigen 19-9; NLR: neutrophil/lymphocyte ratio; PLR: platelet/lymphocyte ratio; CAR: C-reactive protein/albumin ratio; CONUT: CONtrolling NUTritional status; mGPS: modified Glasgow prognosis score.
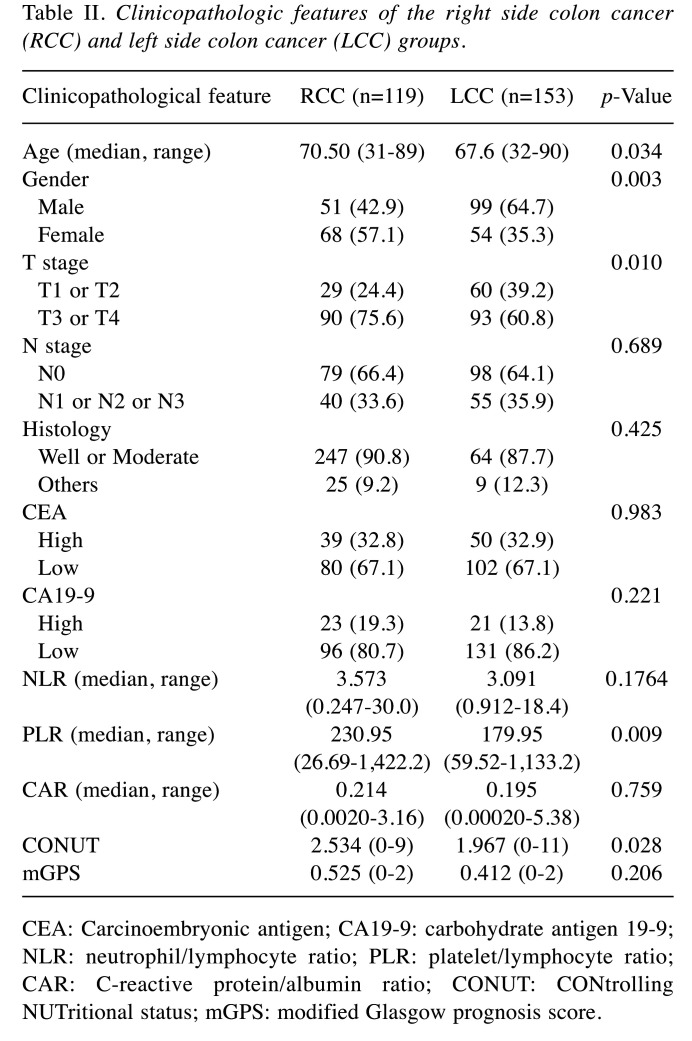
Comparison of clinicopathological features in the RCC and LCC groups. The gender distribution, PLR, and CONUT score were significantly different between the RCC and LCC groups. RCC was more common in women, and it exhibited less invasion. The PLR, which is an index of the immune system, was high and the CONUT score was high in the RCC patients (Table II). This indicates that there is a possibility of poor immunonutrition. Table I summarizes these and other clinical features of the cohort classified according to the primary site of the tumor.
Table II. Clinicopathologic features of the right side colon cancer (RCC) and left side colon cancer (LCC) groups.
CEA: Carcinoembryonic antigen; CA19-9: carbohydrate antigen 19-9; NLR: neutrophil/lymphocyte ratio; PLR: platelet/lymphocyte ratio; CAR: C-reactive protein/albumin ratio; CONUT: CONtrolling NUTritional status; mGPS: modified Glasgow prognosis score
Determination of cut-off values. The ROC curve analysis results indicated that the most appropriate cutoff value for the NLR was 3.51. We categorized the patients into the high NLR group (NLR ≥3.51; n=67, 24.8%) and low NLR group (NLR <3.51; n=203, 75.1%), the high PLR group (PLR ≥194.6; n=94, 34.8%) and low PLR group (PLR <194.6; n=176, 65.1%), and the high CAR group (CAR ≥0.023; n=129, 47.4%) and low CAR group (CAR <0.023; n=143, 52.6%).
The CONUT score cutoff was 3. All patients were categorized into the high CONUT score group (score ≥3; n=85, 32.3%) or low CONUT score group (score=0, 1, or 2; n=178, 67.6%). The mGPS score cutoff was 2. The patients were categorized into the high CONUT score group (score 2; n=86, 31.7%) or low CONUT score group (score=0 or 1; n=185, 68.2%) (Table II).
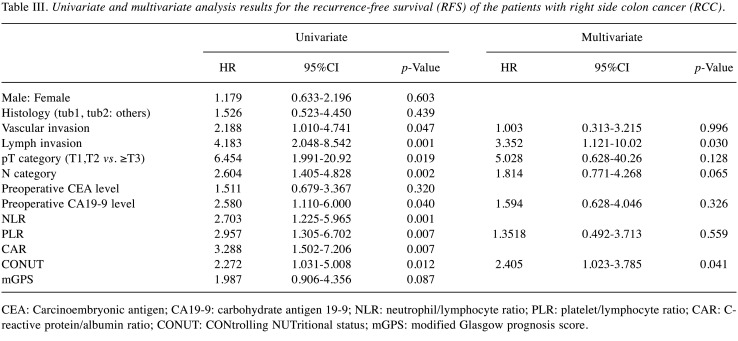
Prognostic factors in the RCC patients. We used a univariate analysis and the Cox regression model to identify risk factors for recurrence after surgery in the RCC group. We examined each nutritional index and clinicopathological factor and found that lymph invasion, vascular invasion, pT category, N category, preoperative CA19-9 level, NLR, PLR, and CONUT score were significantly associated with poor RFS in the univariate survival analyses (Table Ⅱ). Other factors (including gender, age, preoperative CEA level, histology, CAR, and mGPS) were not significantly associated with RFS.
Since the values of the inflammatory markers NLR, PLR, and CAR were all significantly different between the RCC group and LCC group we performed a multivariate analysis using the PLR, which is the lowest value, by measuring the Akaike’s information criterion (AIC) value. The multivariate analysis identified lymph invasion and the CONUT score as independent prognostic factors associated with RFS in the patients with RCC (Table Ⅲ). In the RCC group, the CONUT score was an independent predictor of recurrence, but inflammatory markers were not recurrence factors.
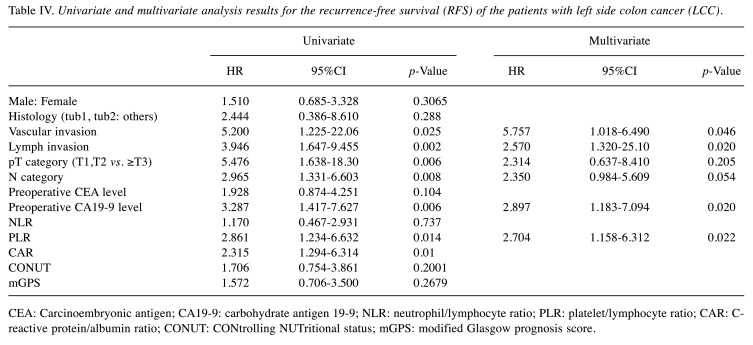
Prognostic factors in the LCC patients. We also examined the presence/absence of recurrence in terms of nutritional factors and clinical factors for the LCC group, and we observed that lymph invasion, vascular invasion, the pT category, the N category, the preoperative CA19-9 level, and the PLR were significantly associated with poor RFS in the univariate survival analyses (Table IV). Since the inflammatory markers PLR and CAR were both significantly different in the RCC group and the LCC group, the multivariate analysis was performed using the PLR, which is the lowest value, by measuring the AIC value. The multivariate analysis identified vascular invasion, lymph invasion, the preoperative CA19-9 level, and the PLR as independent prognostic factors associated with RFS in the patients with LCC (Table IV).
Table IV. Univariate and multivariate analysis results for the recurrence-free survival (RFS) of the patients with left side colon cancer (LCC).
CEA: Carcinoembryonic antigen; CA19-9: carbohydrate antigen 19-9; NLR: neutrophil/lymphocyte ratio; PLR: platelet/lymphocyte ratio; CAR: Creactive protein/albumin ratio; CONUT: CONtrolling NUTritional status; mGPS: modified Glasgow prognosis score.
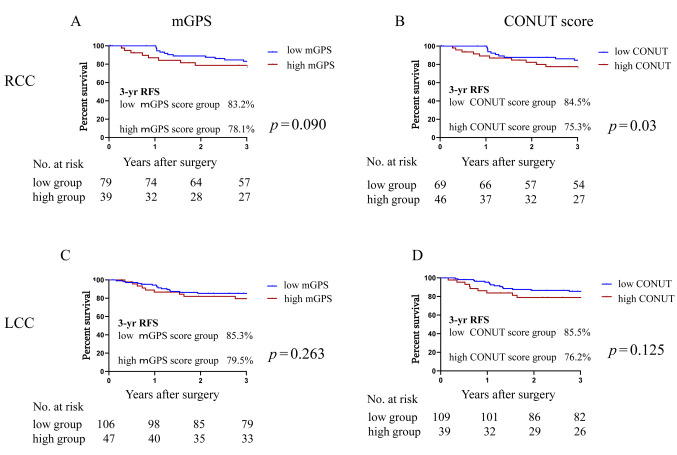
RFS and nutrition factors examined with Kaplan-Meier survival curves. We used the nutritional indicators mGPS and CONUT score as recurrence predictors in this study, and our analyses revealed that these nutritional indicators were not significant recurrence predictors in the RCC or LCC patients. The patients with high nutrition scores in the RCC group were more likely to experience recurrence (Figure 1).
Figure 1. Kaplan-Meier curves of the 3-year recurrence-free survival (RFS) rate based on the patients’ nutrition scores. (A) modified Glasgow Prognostic Score (mGPS) in the right side colon cancer (RCC) group. (B) controlling nutritional status (CONUT) score in RCC. (C) mGPS in the left side colon cancer (LCC) group. (D) CONUT score in LCC.
As shown in Figure 1A, the 3-year RFS rate of the RCC group was 83.2% in the low-mGPS group and 78.1% in the high-mGPS group, with no significant between-group difference [hazard ratio (HR)=1.93, 95% confidence interval (CI)=0.83-4.46, p=0.09]. Similarly, in the LCC group, the 3-year RFS rate of the low-mGPS group was 85.3% and that of the high-mGPS group was 79.5%, with no significant difference (HR=1.57, 95%CI=0.66-3.70, p=0.26) (Figure 1C).
As shown in Figure 1B, the 3-year RFS rate of the RCC group was 84.5% in the low-CONUT score group, which was a significantly higher rate than the 75.3% in the high-CONUT score group (HR=1.93, 95%CI=1.05-4.46, p=0.04). Similarly, in the LCC group, the 3-year RFS rate of the low-CONUT score group was 85.5% and that of the high-CONUT score group was 76.2%, with no significant between-group difference (HR=1.49, 95%CI=0.62-3.60, p=0.23) (Figure 1D).
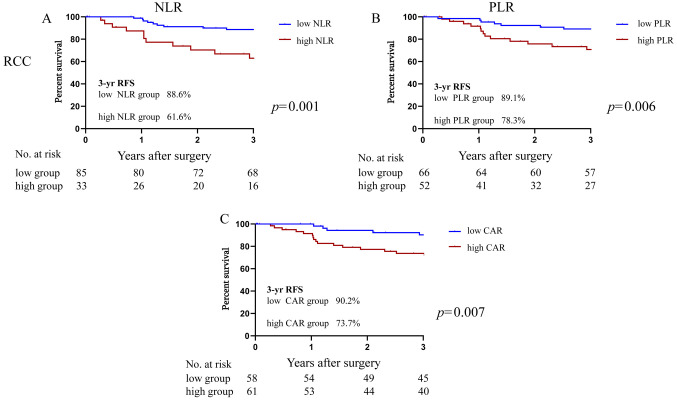
RFS and inflammatory markers. We used the NLR, PLR, and CAR, which can be easily obtained as inflammatory markers. As shown in Figure 2, among the patients with RCC, the 3-year RFS rate was significantly higher at 88.6% in the low-NLR group compared to the 61.6% in the high-NLR group (HR=2.56, 95%CI=1.02-6.45, p=0.02). Similar significant differences were observed in the RCC patients: the 3-year RFS rate of the low-PLR group was 89.1% and that of the high-PLR group was 78.3% (HR=2.87, 95%CI=1.28-6.44, p=0.008). The 3-year RFS rate of the low-CAR group of RCC patients was 90.2% and that of the high-CAR group was 73.7% (HR=3.29, 95%CI=1.50-7.20, p=0.007).
Figure 2. Kaplan-Meier curves of the 3-year recurrence-free survival (RFS) rate based on the inflammatory makers in right side colon cancer (RCC). (A) neutrophil/lymphocyte ratio (NLR). (B) platelet/lymphocyte ratio (PLR). (C) C-reactive protein/albumin ratio (CAR).
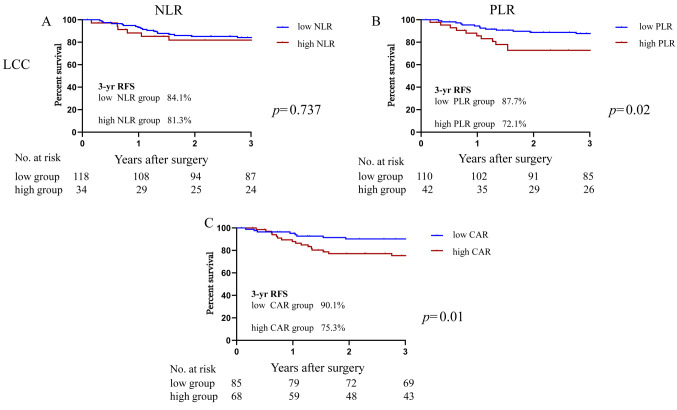
As shown in Figure 3, the 3-year RFS rate of the LCC group was 84.1% in the low-NLR group and not significantly different at 81.3% in the high-NLR group (HR=1.13, 95%CI=0.44-2.93, p=0.79). In the LCC patients, the low-PLR group’s 3-year RFS rate was 85.3%, which was significantly higher than that of the high-PLR group at 79.5% (HR=2.25, 95%CI=1.01-5.50, p=0.039). Similarly, the 3-year RFS rate of the low-CAR group was significantly higher at 87.7% compared to the high-CAR group’s rate at 72.1% (HR=2.86, 95%CI=1.29-6.31, p=0.01).
Figure 3. Kaplan-Meier curves of the 3-year recurrence-free survival (RFS) rate based on the inflammatory makers in left side colon cancer (LCC). (A) neutrophil/lymphocyte ratio (NLR). (B) platelet/lymphocyte ratio (PLR). (C) C-reactive protein/albumin ratio (CAR).
Discussion
Clinical differences have recently been described in colorectal cancer, suggesting that the mechanism underlying the development of this cancer differs between RCC and LCC. We have reported various differences depending on the site of origin of colorectal cancer. Proximal Por/Muc/Sig colon cancers were suggested to be a distinct subpopulation with a favorable oncologic outcome. Using the tumor location and the patient’s gender might be helpful in the risk stratification after curative surgery for Por/Muc/Sig cancers (13). Second, patients receiving angiotensin-converting-enzyme inhibitor/angiotensin II receptor blocker treatment for early-stage colorectal cancer and LCC have reported lower recurrence rates (14).
Our present analyses revealed two important findings regarding the differences between nutritional indicators and inflammatory markers in cases of right and left CRC. First, we observed that the RCC group had higher PLR values and CONUT scores than the LCC group. Second, the inflammatory markers were not independent predictors of recurrence in RCC, but the PLR was an independent predictor of recurrence in the LCC group. The nutrition index (i.e., the CONUT score) was an independent predictor of recurrence in patients with RCC.
Comparing the clinical factors of RCC and LCC patients, the RCC group included more elderly people and more women. It was also shown that RCC tends to show deeper cancer infiltration. There were many cases of RCC with a high PLR and high CONUT score. Charlton et al. reported that patients with RCC (vs. LCC) tended to be older, female, and have mucinous adenocarcinoma (15). There are similar reports on clinical factors, and those reports support our present findings (13,15). Herein, the patients with RCC tended to have deeper cancer invasion compared to LCC patients. The diagnosis of colon cancer suggests that the diagnosis of RCC may be delayed compared to LCC (16). In addition, CRC screening using the fecal occult blood test has been reported to have lower sensitivity and specificity for the diagnosis of RCC compared to that for LCC and rectal cancers (17).
We investigated the differences between nutritional indicators and inflammatory markers based on the site of CRC. There is no prior report that the PLR or the CONUT score to be high in RCC, and this is a new finding. It may be related to the fact that more RCC cancers are infiltrative compared to LCC. The CONUT score as a nutritional index is composed of three common factors as noted above: the serum ALB level, the peripheral lymphocyte count, and the total cholesterol concentration. As the tumor progresses, the intestinal tract narrows and food intake decreases. This is likely to explain why the CONUT score increases. We also observed that the PLR was high in patients with RCC. Platelets are associated with the clinical stage, with increased platelets reported in stage III and stage IV patients. Patients with RCC tend to be more infiltrated with cancer compared to LCC, and the stage is advanced. As a result, platelets may be high and PLR may be high in RCC patients (18). RCC tend to be asymptomatic compared to LCC. As a result, RCC are found at a higher stage than LCC. As the stage progresses, platelets increase and nutritional status worsens. RCC has a higher PLR and a higher CONUT score. As to patients with RCC, elevated PLR indicated poorer survival rate as well. It has been reported that elevated platelets may protect cancer cells from being detected or attacked by the autoimmune system. Moreover, platelets could promote tumor cell adhesion to the vascular endothelium, or interact with cancer cells through its ligands (19,20).
We examined recurrence prediction and clinical factors, and inflammatory and nutritional indicators. The results of our analyses revealed that in the RCC group, lymph invasion and the CONUT score were independent predictors of recurrence, and the inflammatory markers was not predictors of recurrence. Although many studies have reported that a nutrition index such as the CONUT score and the mGPS predict the prognosis of patients with CRC (21,22), there has been no report regarding the different prognostic values of these parameters according to the tumor location in stage I-III CRC.
The serum ALB level is one of the components of the CONUT score. Serum ALB has been reported to correlate with tumor necrosis because inflammatory cytokines reduce the synthesis of ALB (12). The present RCC group had more advanced tumors than the LCC group. It is thus understandable that the CONUT score was a recurrence factor only in RCC.
According to the present results, venous invasion, lymph invasion, the CA19-9 level, and the PLR were independent predictors of recurrence in the LCC group, and the nutritional index was not a predictor of recurrence. It has been reported that the inflammatory markers NLR, PLR, and CAR predict the prognosis of CRC patients, but there is little information about the different prognostic values of these parameters according to the tumor location in stage I-III CRC (22-24). Guo et al. analyzed the cases of R0 resected stage I-III CRC patients and observed that the PLR was an independent predictor in the patients with LCC (24).
Although cancer-related inflammation is known to adversely affect survival, the mechanisms underlying the significant differences in inflammatory markers between RCC and LCC remain unclear. Anatomically, the right side of the colon is connected to the ileum (which contains many lymphoid follicles), and the right side of the colon mesentery may contain more of a lymphatic system compared to the left side of the colon, and this disparity may more easily cause cancer-related inflammation in RCC. Regarding nutritional indicators, we speculate that the cause of the difference between the LCC and RCC patient values is that the diagnosis of RCC is delayed compared to LCC.
Our findings demonstrate that recurrence and the values of a nutritional index and inflammatory markers were significantly different between patients with RCC and those with LCC. This result indicates that the recurrence rate varies depending on the site of cancer, and one of the potential mechanisms involves systemic inflammation and nutrition. To the best of our knowledge, this study is the first to investigate the different prognostic values of a nutrition index and inflammatory biomarkers for RCC and LCC, and our findings suggest that exploring differences in inflammatory mechanisms may be helpful for designing tailor-made medical treatments for CRC according to the tumor location. Further research on inflammatory mechanisms and trophic factors that contribute to the prognosis of CRC is needed.
This study has limitations to address. It was retrospective in design and included patients from a single institution. The sample size was relatively small (n=272). In addition, there is no consensus regarding cut-off values of the CONUT score and inflammatory markers. We selected the CONUT score and inflammatory marker cut-off values by performing an ROC analysis. Our findings require further review and validation in a greater number of CRC patients drawn from more facilities and countries. Lastly, the results of this study do not apply to stage IV patients, as we included only stage I-III CRC patients who underwent curative surgery.
Conclusion
The prognostic value of inflammatory markers and a nutritional index were significantly different between RCC and LCC. Compared to patients with LCC, those with RCC had elevated CONUT scores and elevated PLRs. In the RCC group, the CONUT score was an independent recurrence factor, and in the LCC group, the PLR was an independent recurrence factor.
Conflicts of Interest
The Authors declare that they have no conflicts of interest.
Authors’ Contributions
TH made substantial contribution to conception, conducted a literature search and drafted the manuscript. TH, TO, YH, RS, KN, YF, SF and KM made the contribution for acquisition of data. TH, YH, KA, RK, KO, KN, YO, MT, YO, TY, TF and KO reviewed the manuscript and gave final approval for publication. All Authors read and approved the final manuscript.
Acknowledgements
This study was supported in part by the 21st Fujii Tomoko Academic Encouragement Award.
Table III. Univariate and multivariate analysis results for the recurrence-free survival (RFS) of the patients with right side colon cancer (RCC).
CEA: Carcinoembryonic antigen; CA19-9: carbohydrate antigen 19-9; NLR: neutrophil/lymphocyte ratio; PLR: platelet/lymphocyte ratio; CAR: Creactive protein/albumin ratio; CONUT: CONtrolling NUTritional status; mGPS: modified Glasgow prognosis score.
References
- 1.Loupakis F, Yang D, Yau L, Feng S, Cremolini C, Zhang W, Maus MK, Antoniotti C, Langer C, Scherer SJ, Müller T, Hurwitz HI, Saltz L, Falcone A, Lenz HJ. Primary tumor location as a prognostic factor in metastatic colorectal cancer. J Natl Cancer Inst. 2015;107(3):dju427. doi: 10.1093/jnci/dju427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brulé SY, Jonker DJ, Karapetis CS, O’Callaghan CJ, Moore MJ, Wong R, Tebbutt NC, Underhill C, Yip D, Zalcberg JR, Tu D, Goodwin RA. Location of colon cancer (right-sided versus left-sided) as a prognostic factor and a predictor of benefit from cetuximab in ncic co.17. Eur J Cancer. 2015;51(11):1405–1414. doi: 10.1016/j.ejca.2015.03.015. [DOI] [PubMed] [Google Scholar]
- 3.Shen H, Yang J, Huang Q, Jiang MJ, Tan YN, Fu JF, Zhu LZ, Fang XF, Yuan Y. Different treatment strategies and molecular features between right-sided and left-sided colon cancers. World J Gastroenterol. 2015;21(21):6470–6478. doi: 10.3748/wjg.v21.i21.6470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saidi HS, Karuri D, Nyaim EO. Correlation of clinical data, anatomical site and disease stage in colorectal cancer. East Afr Med J. 2008;85(6):259–262. doi: 10.4314/eamj.v85i6.9622. [DOI] [PubMed] [Google Scholar]
- 5.Minami S, Ogata Y, Ihara S, Yamamoto S, Komuta K. Pretreatment glasgow prognostic score and prognostic nutritional index predict overall survival of patients with advanced small cell lung cancer. Lung Cancer (Auckl) 2017;8:249–257. doi: 10.2147/lctt.s142880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miyake M, Morizawa Y, Hori S, Marugami N, Iida K, Ohnishi K, Gotoh D, Tatsumi Y, Nakai Y, Inoue T, Anai S, Torimoto K, Aoki K, Tanaka N, Shimada K, Konishi N, Fujimoto K. Integrative assessment of pretreatment inflammation-, nutrition-, and muscle-based prognostic markers in patients with muscle-invasive bladder cancer undergoing radical cystectomy. Oncology. 2017;93(4):259–269. doi: 10.1159/000477405. [DOI] [PubMed] [Google Scholar]
- 7.Watanabe J, Otani S, Sakamoto T, Arai Y, Hanaki T, Amisaki M, Tokuyasu N, Honjo S, Ikeguchi M. Prognostic indicators based on inflammatory and nutritional factors after pancreaticoduodenectomy for pancreatic cancer. Surg Today. 2016;46(11):1258–1267. doi: 10.1007/s00595-016-1308-6. [DOI] [PubMed] [Google Scholar]
- 8.Riedl J, Pabinger I, Ay C. Platelets in cancer and thrombosis. Hamostaseologie. 2014;34(1):54–62. doi: 10.5482/hamo-13-10-0054. [DOI] [PubMed] [Google Scholar]
- 9.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The strengthening the reporting of observational studies in epidemiology (strobe) statement: Guidelines for reporting observational studies. Int J Surg. 2014;12(12):1495–1499. doi: 10.1016/j.ijsu.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 10.Ignacio de Ulibarri J, Gonzalez-Madrono A, de Villar NG, Gonzalez P, Gonzalez B, Mancha A, Rodriguez F, Fernandez G. Conut: A tool for controlling nutritional status. First validation in a hospital population. Nutr Hosp. 2005;20(1):38–45. [PubMed] [Google Scholar]
- 11.McMillan DC, Elahi MM, Sattar N, Angerson WJ, Johnstone J, McArdle CS. Measurement of the systemic inflammatory response predicts cancer-specific and non-cancer survival in patients with cancer. Nutr Cancer. 2001;41(1-2):64–69. doi: 10.1080/01635581.2001.9680613. [DOI] [PubMed] [Google Scholar]
- 12.McMillan DC, Crozier JE, Canna K, Angerson WJ, McArdle CS. Evaluation of an inflammation-based prognostic score (gps) in patients undergoing resection for colon and rectal cancer. Int J Colorectal Dis. 2007;22(8):881–886. doi: 10.1007/s00384-006-0259-6. [DOI] [PubMed] [Google Scholar]
- 13.Ishihara S, Watanabe T, Akahane T, Shimada R, Horiuchi A, Shibuya H, Hayama T, Yamada H, Nozawa K, Matsuda K, Maeda K, Sugihara K. Tumor location is a prognostic factor in poorly differentiated adenocarcinoma, mucinous adenocarcinoma, and signet-ring cell carcinoma of the colon. Int J Colorectal Dis. 2012;27(3):371–379. doi: 10.1007/s00384-011-1343-0. [DOI] [PubMed] [Google Scholar]
- 14.Ozawa T, Hashiguchi Y, Yagi T, Fukushima Y, Shimada R, Hayama T, Tsuchiya T, Nozawa K, Iinuma H, Ishihara S, Matsuda K. Angiotensin i-converting enzyme inhibitors/angiotensin ii receptor blockers may reduce tumor recurrence in left-sided and early colorectal cancers. Int J Colorectal Dis. 2019;34(10):1731–1739. doi: 10.1007/s00384-019-03379-y. [DOI] [PubMed] [Google Scholar]
- 15.Charlton ME, Kahl AR, Greenbaum AA, Karlitz JJ, Lin C, Lynch CF, Chen VW. Kras testing, tumor location, and survival in patients with stage iv colorectal cancer: Seer 2010-2013. J Natl Compr Canc Netw. 2017;15(12):1484–1493. doi: 10.6004/jnccn.2017.7011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ratcliffe R, Kiff RS, Kingston RD, Walsh SH, Jeacock J. Early diagnosis in colorectal cancer. Still no benefit. J R Coll Surg Edinb. 1989;34(3):152–155. [PubMed] [Google Scholar]
- 17.Logan RF, Patnick J, Nickerson C, Coleman L, Rutter MD, von Wagner C. Outcomes of the bowel cancer screening programme (bcsp) in england after the first 1 million tests. Gut. 2012;61(10):1439–1446. doi: 10.1136/gutjnl-2011-300843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guo T, Krzystanek M, Szallasi Z, Szallasi A. Thrombocytosis portends adverse prognostic significance in patients with stage ii colorectal carcinoma. F1000Res. 2014;3:180. doi: 10.12688/f1000research.4856.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guo YH, Sun HF, Zhang YB, Liao ZJ, Zhao L, Cui J, Wu T, Lu JR, Nan KJ, Wang SH. The clinical use of the platelet/lymphocyte ratio and lymphocyte/monocyte ratio as prognostic predictors in colorectal cancer: A meta-analysis. Oncotarget. 2017;8(12):20011–20024. doi: 10.18632/oncotarget.15311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goubran HA, Stakiw J, Radosevic M, Burnouf T. Platelet-cancer interactions. Semin Thromb Hemost. 2014;40(3):296–305. doi: 10.1055/s-0034-1370767. [DOI] [PubMed] [Google Scholar]
- 21.Hayama T, Ozawa T, Okada Y, Tsukamoto M, Fukushima Y, Shimada R, Nozawa K, Matsuda K, Fujii S, Hashiguchi Y. The pretreatment controlling nutritional status (conut) score is an independent prognostic factor in patients undergoing resection for colorectal cancer. Sci Rep. 2020;10(1):13239. doi: 10.1038/s41598-020-70252-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takagi K, Buettner S, Ijzermans JNM. Prognostic significance of the controlling nutritional status (conut) score in patients with colorectal cancer: A systematic review and meta-analysis. Int J Surg. 2020;78:91–96. doi: 10.1016/j.ijsu.2020.04.046. [DOI] [PubMed] [Google Scholar]
- 23.Pedrazzani C, Mantovani G, Fernandes E, Bagante F, Luca Salvagno G, Surci N, Campagnaro T, Ruzzenente A, Danese E, Lippi G, Guglielmi A. Assessment of neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio and platelet count as predictors of long-term outcome after r0 resection for colorectal cancer. Sci Rep. 2017;7(1):1494. doi: 10.1038/s41598-017-01652-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guo D, Li X, Xie A, Cao Q, Zhang J, Zhang F, Li W, Chen J. Differences in oncological outcomes and inflammatory biomarkers between right-sided and left-sided stage i-iii colorectal adenocarcinoma. J Clin Lab Anal. 2020;34(4):e23132. doi: 10.1002/jcla.23132. [DOI] [PMC free article] [PubMed] [Google Scholar]