Abstract
Background/Aim: For women who have undergone a mastectomy, breast reconstruction provides psychological as well as aesthetic benefits. Thus, many patients ask for an immediate breast reconstruction (IBR). The present study focuses on risk factors assiociated with complications after IBR. Patients and Methods: A national prospective study (2007-2009) was conducted on 404 patients who underwent an unilateral IBR: 205 implants alone (IA) including 46 tissue expanders, 91 latissimus dorsi musculocutaneous flaps with implant (LDI), 78 autologous latissimus dorsi musculo-cutaneous flaps (LD), and 30 autologous transverse rectus abdominis musculocutaneous flaps (TRAM). Outcomes concerned major and minor complications, as well as early and late complications. Results: Related risks of complications were different according to the IBR technique. Major complications rate remained moderate and concerned 15% of patients. Obesity and diabetes significantly increased the incidence of major complications. Conclusion: To reduce complication rate, the risk factors associated with each type of IBR should be taken into account.
Keywords: Breast cancer, breast reconstruction, complications, morbidity, mastectomy, risk factors
With the development of breast screening programs, oncoplastic surgery and associated conservative treatment, the rate of mastectomy has stabilized at around 30% of breast cancer surgery during the last decade (1). Nowadays, immediate breast reconstruction (IBR) after mastectomy appears as an acceptable choice for patients. Thus, patients ask for an IBR to reduce the psychological and aesthetic impact of the surgery (2).
Therefore, IBR after mastectomy is increasingly proposed at the time of breast cancer treatment (3-6). A large variety of complication rates after IBR have been described in the literature (7-14). These variations could be in part explained by the various definitions of the complications considered and by the type of complication. However, authors mainly agree about the definition of major complications - defined as complications that require re-hospitalization and/or re-intervention. Literature is also heterogeneous on several criteria such as the IBR procedures taken into account, the prospective or retrospective collection of data, sample sizes, and indications for IBR. In a prospective analysis of 404 women treated by mastectomy plus unilateral IBR, the present article focuses on surgical complications risk factors.
Patients and Methods
Patients. Between July 2007 and August 2009, 404 were treated by mastectomy plus unilateral IBR and were prospectively included in STIC-RMI trial (15). STIC-RMI study was an observational, non-randomized, prospective trial, conducted in 22 French public and private hospitals and approved by the local ethic committee (n˚ID-RCB 2007-A00633-50).
Inclusion criteria corresponded to standard indications for mastectomy. Patients with inflammatory breast cancers, bilateral cancers, metastatic cancers, another concurrent cancer and risk-reducing prophylactic mastectomies were excluded.
Methods. Complications were recorded prospectively for each patient over a one-year follow-up period and were classified into two groups: early complications and late complications. Early complications were described as complications that occurred during hospitalization and 8 weeks after leaving the hospital. Late (or delayed) complications corresponded to complications that occurred between 3 and 6 months and then at 12 months after surgery. Complications reporting concerned the intervention sites (breast/axilla operated on and flap donor site) and included: hematoma, seroma/lymphocele, wound infection, wound dehiscence, skin-edge necrosis, flap necrosis, implant excision, re-hospitalization for surgical re-intervention or medical complication, pain and functional sequelae. Using a visual analogic scale, pain was assessed on the third-day post-surgery, on the day of departure and during the follow-up consultations (8 weeks, 3-6 months and 12 months).
We also distinguished minor and major complications. As suggested in literature, major complications were defined as complications causing a re-hospitalization or a re-operation during hospitalization.
Statistical analysis. Statistical analyses were mostly descriptive. Relationships between pairs of variables (complications by IBR type, risk factors) were tested. Chi2 tests were used for categorical data (or Fisher’s exact test if class sizes were too small), and Student t-test or one-way ANOVA were performed when one of the two variables was numerical (or non-parametric tests – Mann-Whitney U-test or Kruskal-Wallis H tests if distributions were not Gaussian or homoscedastic). Logistic regression was used for multivariate analysis of risk factors associated with major complications. Tests were two-sided and standard p-values ≤0.05 were considered significant. SEM statistical software was used to perform statistical calculations and to manage data (16).
Results
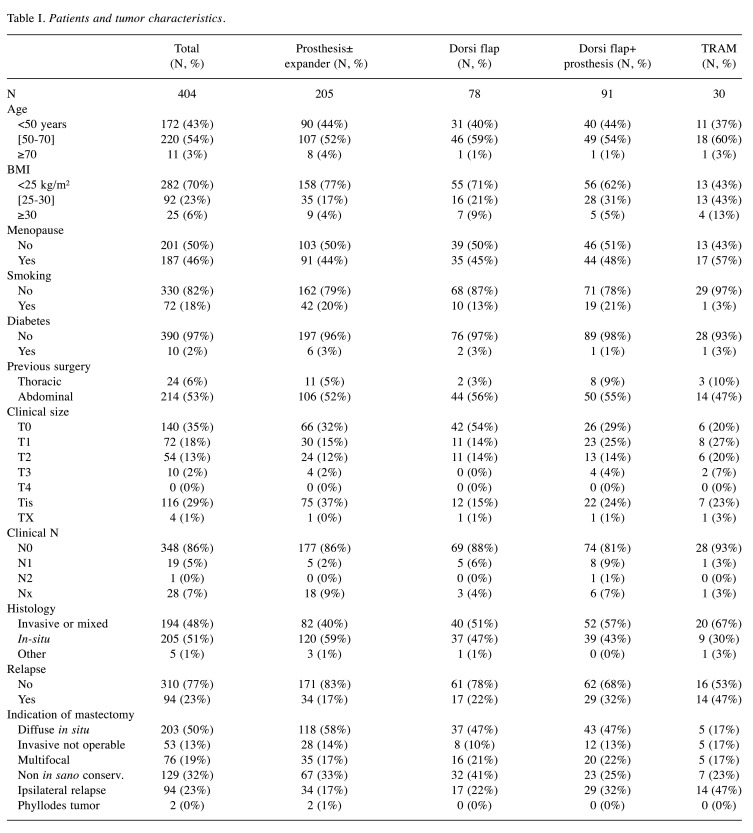
Patients’ characteristics. Patients and tumors characteristics are described in Table I. Mean patient age was 53.9±9.5 years [28-83]. 28% of patients were overweight and 18% were smokers. Approximately, 35% of patients presented with non-palpable tumors (T0) and 86% with clinical negative axilla. IBR indications following mastectomy included diffuse carcinoma in-situ in 44% (n=176), invasive carcinoma not accessible to conservative surgery in 33% (n=132), ipsilateral recurrence after conservative treatment in 23% (n=94) and phyllodes tumors in 0.5% (n=2).
Table I. Patients and tumor characteristics.
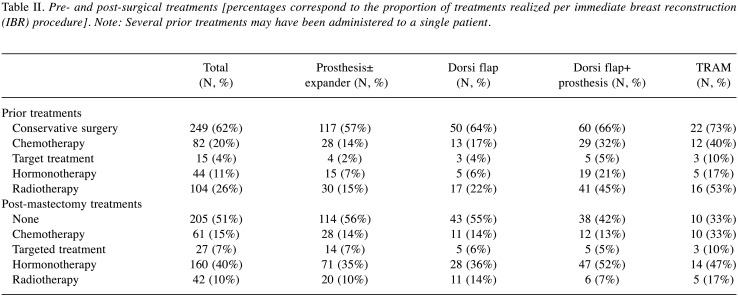
Treatments and techniques. Immediate breast reconstruction was performed in 404 patients. Implant alone (IA) were performed in 205 patients (51%) (46 with tissue expander), latissimus dorsi musculocutaneous flaps with implant (LDI) in 91 patients (23%), autologous latissimus dorsi musculocutaneous flaps (LD) in 78 patients (19%), and autologous transverse rectus abdominis musculocutaneous (TRAM) flaps or deep inferior epigastric perforators (DIEP) flaps in 30 patients (7%). A skin-sparing mastectomy was performed in 184 patients (46%) whereas 15 (4%) underwent nipple sparing surgery (4%). Concurrent axillary surgery consisted of a sentinel lymph node biopsy in 67 patients (17%) and axillary lymph node dissection in 134 patients (33%). Table II exhibits pre- and post-surgery treatments performed according to IBR type.
Table II. Pre- and post-surgical treatments [percentages correspond to the proportion of treatments realized per immediate breast reconstruction (IBR) procedure]. Note: Several prior treatments may have been administered to a single patient.
Risk factors associated with complications. During the 1-year follow-up period, major complication rate reached 18.6% and concerned 60 patients, i.e. 15% of patients (patients re-hospitalized/re-operated at least once), i.e. 75 re-admissions. Most of these complications (10%) occurred within the first two months after surgery compared to the remaining period; major complications represented only 8% of reconstructions. Our analysis focused on the following risk factors for complications: patient’s age at surgery, personal history (diabetes, smoking, and overweight/obesity), previous relapse, adjuvant treatments, and the type of reconstruction.
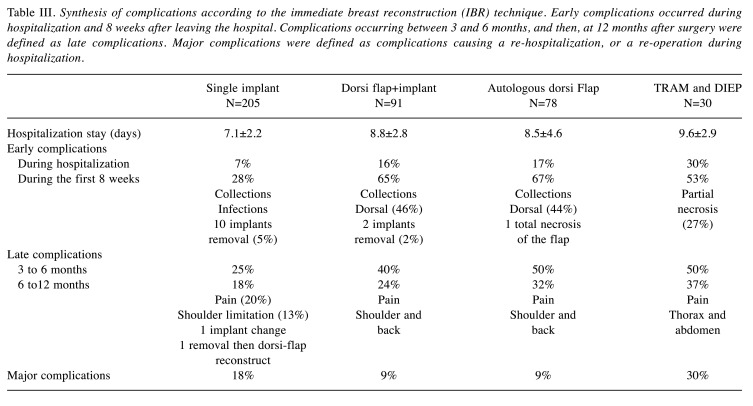
Univariate analysis of risk factors associated with complications. In the univariate analysis, we showed that rates and types of complications differed according to the surgical techniques performed for IBR (Table III). Each IBR type induces specific complications. Thus, early complications occurred in 28% of IA, 65% of LD, 67% of LD and 53% of TRAM. Implant removal was necessary in 5% of IA and 2% of LDI. Necrosis was total in 1 LD and partial in 9 TRAM (27%). Late complications occurred, respectively, at 6 and 12 months in 25% and 18% for IA, 40% and 24% for LDI, 50% and 32% for LD, 50% and 37% for TRAM. Major complications occurred in 15% of patients: 18% in IA, 9% in LDI and LD, and 30% in TRAM. Overall, 13 IBR failures occurred (3%).
Table III. Synthesis of complications according to the immediate breast reconstruction (IBR) technique. Early complications occurred during hospitalization and 8 weeks after leaving the hospital. Complications occurring between 3 and 6 months, and then, at 12 months after surgery were defined as late complications. Major complications were defined as complications causing a re-hospitalization, or a re-operation during hospitalization.
Patients older than 55 years presented an increased risk of early complications during the first 8 weeks (p=0.022), especially for complications such as lymphocele/seroma (at the breast: p=0.049, at the donor site: p=0.017).
During hospitalization, overweight [body mass index (BMI) ≥25 kg/m2 and <30 kg/m 2]/obese (BMI ≥30 kg/m2) women had a higher rate of complications, notably in the breast (p=0.001) and locally, including axilla (p=0.05). This finding was also observed during the first 8 weeks [p=0.00007, overweight: RR=1.4 (1.1-1.7), obese: RR=1.6 (1.1-2.3)], particularly considering wound dehiscence [p=0.019, RR=2.7 (1.2-6.0)] and local pain [p=0.006, overweight: RR=2.3 (1.0-5.4), obese: RR=3.9 (1.3-12.0)]. Surgical site infections were more frequent in obese patients [p=0.0012, RR=9.0 (2.8-28.5)], but not significantly for overweight patients. At 3-6 months and 1 year, obesity appeared to be related to lymphedema occurrence [3-6 months: p=0.001, RR=11.4 (3.2-39.7); 1 year: p=0.005, RR=12.4 (2.7-56.2)]. Overall, surgical revisions and re-admissions for medical complications were more frequent when BMI was >25 (p=0.01). In the obese women group, the revision risk was multiplied by 2.6 (1.5-4.3) (p=0.00042), and the risk for re-hospitalization by 4.3 (1.7-11.2) (p=0.012).
Moreover, pain sequelae frequency was higher in smokers (p=0.029 at 3-6 months, and p=0.015 at 12 months), and appeared mainly at the breast and thorax. No association was evidenced between smoking and the flap or between skin edges necrosis and healing difficulties.
During the 12-months follow-up, the frequency of re-interventions and/or readmission for medical or surgical complications was higher in diabetic than in non-diabetic patients (50% vs. 14%, p=0.006). However, the infection risk was not increased by diabetes. During the first 8 weeks, wound dehiscence tended to be higher in diabetics (40% vs. 7%, p=0.07). Interestingly, patients who underwent an IBR after a post-conservative relapse exhibited a higher rate of breast complications during hospitalization (14% vs. 7%, p=0.05).
Axillary dissection increased lymphedema development at 3-6 months (7% vs. 1%, p=0.025) and 12 months (6% vs. 1%, p=0.043) after intervention. Moreover, lymphedema development was accompanied by painful sequelae in the arm at 3-6 months (17% vs. 5%, p=0.004), in the shoulder at 1 year (15% vs. 4%, p=0.008), and functional sequelae in the same place at 1 year (25% vs. 7%, p=0.036). These complications cannot be attributed to the IBR. However, the assessment of exclusive sentinel lymph node biopsy did not increase the frequency of complications, compared with the population without axillary dissection.
During hospitalization, we also observed higher rates of local breast complications (16% vs. 7%, p=0.01) and hematoma (12% vs. 4%, p=0.01) in relapsing patients previously treated by chemotherapy than in naïve patients. Prior chemotherapy was also associated with more frequent pain sequelae in the arm at 3-6 months (16% vs. 6%, p=0.04). For patients treated by preoperative (neo-adjuvant) chemotherapy, arm pain sequelae occurred more frequently at 3-6 months (16% vs. 6%, p=0.04) as well as functional sequelae at the shoulder (15% vs. 4%, p=0.032) after 12 months.
A higher frequency of lymphedema at 3-6 months after surgery was associated with the administration of adjuvant chemotherapy. This result was likely due to the greater frequency of axillary dissection (31% vs. 14%, p=0.001) when adjuvant chemotherapy is indicated.
Finally, complications were not affected by previous radiotherapy. These results seem to contradict the increase of local complications among patients with recurrent disease, who were already treated by radiotherapy in 75% of cases. Postoperative radiotherapy was related to a higher incidence of lymphedema at 3-6 months (8% vs. 2%, p=0.044). But this relation disappeared at 12 months. No significant association was observed between postoperative radiotherapy and necrosis (flap or skin edges) or delayed healing.
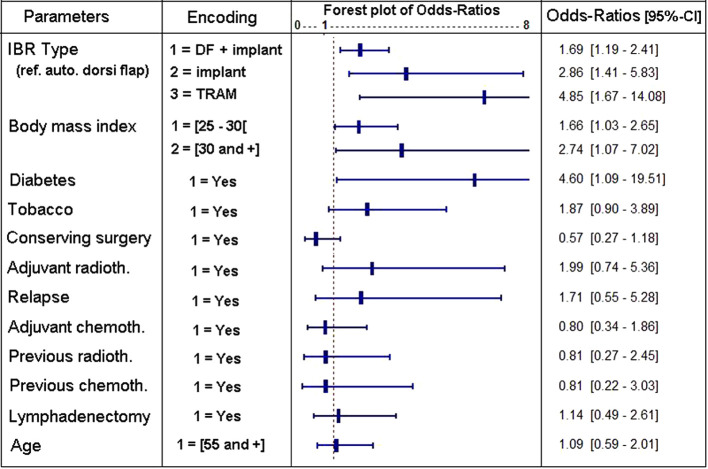
Multivariate analysis of risk factors associated with major complications. In multivariate analysis, only some factors affected independently the risk associated with major complications. We used a statistical model including only significant covariates (Figure 1). The reconstruction procedure (p=0.0037) remains an important factor. Thus, IBR with TRAM corresponded to the highest risk, followed by IA and LDI. Interestingly, autologous LD were associated with the lowest risk. We also demonstrated that a prior attempt to achieve a conservative treatment reduced the risk of major complications (p=0.018). Unsurprisingly, overweight and obesity (p=0.023), just like diabetes (p=0.04), increased the risk associated with major complications. Smoking or adjuvant radiotherapy tended to increase risks but not significantly. Neither prior radiotherapy nor prior or adjuvant chemotherapy affected the risk for major complications.
Figure 1. Results of the logistic regression testing the presence/absence of major complications occurring until 1 year of follow-up [DF+implant= dorsi flap+implant, transverse rectus abdominis musculocutaneous (TRAM)=TRAM or deep inferior epigastric perforators (DIEP)].
Discussion
Complication rates after IBR vary among studies in the literature. This prospective study of 404 IBR is one of the largest studies conducted on unilateral reconstructions. Our 18.6% major complications rate is one of the lowest reported in the literature (between 12% and 37%) (14,17,18). Risk factors for complications have often been analyzed in the literature, but most often within retrospective series and with contradictory conclusions. In our prospective sample, which analyzes the risk for re-intervention/re-hospitalization in a multivariate way, only the following parameters appeared significant: the type of IBR, obesity and diabetes, and a prior attempt at conservative surgery. The latter may suggest that if conservative surgery is mandatory, patients’ clinical characteristics are more favorable.
The impact of IBR procedure type on major complications has never been studied prospectively. In retrospective setting, Berry et al. (8) and Pinsolle et al. (7) observed no difference. In our study, the type of reconstruction predicted major complications, as well as early complications. These latter (during hospitalization or during the eight following weeks) are rarer with implants alone when compared with more complex reconstruction such as dorsi flap or TRAM. Monrigal et al. (9) have also found a higher risk for early complications, especially for TRAM. A retrospective analysis of a 12,129-patients database made-up from 2005 to 2011 has shown that flap IBR was associated with a higher risk of early complications than was single prosthesis IBR (adjusted OR=1.41) (19).
Post-surgical infections (with or without collections) represent the major complication risk after a single-implant IBR: it induced prosthesis removal, and thus, reconstruction failure (5%). The use of expanders seemed to increase this risk since it was multiplied by seven in our study.
Moreover, infection rates vary largely according to IBR procedures. Indeed, Alderman et al. (14) have described a 35% maximal rate for implants or expanders alone versus 13.4% for TRAM. As Pinsolle et al. (7) have shown, implant reconstruction also induces a significantly higher infection rate.
The high percentage of post-surgical complications found with dorsi flaps is due to the collections of seroma, particularly in the dorsal region. Some surgeons try in particular to prevent seroma by quilting sutures using the Chippendale technique (20).
The postoperative risk of TRAM/DIEP concerns the necrosis of the flap and/or the skin edges (26% in our series). It can be limited by the high technical skill of surgeon and respect of contra-indications (obesity and smoking).
Delayed complications mainly gather shoulder, arm, and donor sites pain sequelae, which can induce movement limitations. The occurrence of these phenomena is shared with axillary lymphadenectomy. They are more frequent after flap IBR (40% to 50%) than after implant IBR (25%), and they tend to get better with time (25% to 35% with flap, and 20% with implants).
Several authors have described obesity as a risk factor associated with complications after breast reconstruction (7-9,13,17,19,21-27). However, the BMI cut-offs used to define overweight and obesity are rather disparate. Using a multivariate analysis, overweight (25≤BMI<30 kg/m2) and obesity (BMI≥30 kg/m2) were associated with more frequent re-hospitalizations (×2.6 by overweight and ×4.3 by obesity) and early complications (×1.4 and ×1.6 resp.). The increased risk of major complications in relation to BMI has been evidenced in four other studies. In obese patients, McCarthy et al. (26) have found a twice higher risk for global complications after single prosthesis/expander IBR, and a 7 times-higher risk for reconstruction failure. Ducic et al. (21) observed a flap complication rates of 2.6 times-higher in obese patients after a TRAM reconstruction. We noted a higher frequency of wound dehiscence, lymphedema and implant removal in overweight or obese patients. Higher rate of infection (×9) was also found in obese patients. Similarly, Pinsolle et al. (7) reported an infection risk multiplied by 8 for overweight or obese patients. In obese and overweight patients, IBR should be considered with much caution, and even more so when obesity coexists with other identified risk factors. In this line, Berry et al. (8) have proposed a flowchart for obese patients to pre-operatively evaluate the risk for complications.
In the present study, diabetics presented a 4.6 times-higher risk for major complications than other patients. On the opposite, several authors reported that diabetes did not increase this risk (8,24-26,28). Only Berry et al. (8) reported an overall complication rate multiplied by 3 when diabetic patients are reconstructed using an expander. Infection of surgical site, caused by mammary intervention, was frequently associated with diabetes (22,23), but we could not confirm this conclusion in our series (p=0.45). These discrepancies are presumably related to the limited number of diabetic patients in the cited articles (4% of IA, i.e. 10 patients in our population, from 1% to 4.4% in the literature) (8,24-26). The only series containing an important proportion of diabetics (15%) is reported by Angarita et al. (23). Cohen et al. demonstrated that diabetic patients with IBR may delay adjuvant chemotherapy initiation (29).
Our adjusted (multivariate) analysis did not evidence an increase of complications related to radiotherapy, especially if it had been carried out previously. A posterior radiotherapy seemed to double the risk, but the difference was not statistically significant. In other studies, the risk for complication with radiotherapy prescribed after IBR varies a lot (8,14,17,30-32). Krueger et al. reported a higher complication rate in patients treated by prosthesis/expander IBR plus radiotherapy than in patients who didn’t undergo radiotherapy (68% vs. 31%; p=0.006) (32). Berry et al. (8) found an increased risk for major complications [OR=5.2 (2.4-11.2)] with adjuvant radiotherapy, but also with prior radiotherapy [OR=2.7 (1.5-4.9)]. However, women who had reconstruction using a flap underwent fewer complications than those having reconstruction by prosthesis/expander, if radiotherapy has been received prior to IBR (p=0.005; OR=0.22), as well as post-IBR (p=0.05; OR=0.35). These authors suggested that breast reconstruction using a musculocutaneous flap could be the best strategy for patients previously treated by radiotherapy.
In our series, chemotherapies prior to and/or post mastectomy+IBR were associated with local breast complications: hematoma, pain, arm or shoulder functional sequelae and lymphedema. But using adjusted analysis, these associations were not still observed because chemotherapy was often associated with lymphadenectomy. In a large series of patients who received neo-adjuvant or adjuvant chemotherapy, McCarthy et al. (26) noted no significant difference for local complications. Recently, a meta-analysis confirmed chemotherapy did not increase the complication risk after IBR (33). Monrigal et al. (9) studied a retrospective series of 210 mastectomies+IBR carried out after neo-adjuvant chemotherapy plus radiotherapy: morbidity was not increased by pre-surgical treatments. Thus, for invasive breast cancers treated with a conservative surgery, they recommend such a therapeutic sequence if there is an indication for chemotherapy and radiotherapy, and if patients are eligible for an IBR after the mastectomy to reduce onset-delays of adjuvant treatments.
We showed that smoking was not associated with a higher risk for major complications. Only two studies have reported an IBR-failure rate that was significantly superior among smokers (26,34). Considering all types of complications, smoking has been described as a major risk factor in many studies (7,12,17,19,21,24,26,31,34-36). In a large series of 718 TRAM reconstructions, Chang et al. (35) have found smokers had a significantly increased risk for skin-edge necrosis of mastectomy compared with non-smokers (19% vs. 9%, p=0.005). They demonstrated that smoking confered an increased risk for musculocutaneous flap necrosis (4.4% vs. 0.8%; p=0.025) and hernia (6.7% vs. 2.1%, p=0.016). Vega et al. (24) and Albino et al. (12) also observed this increase of necrosis risk. The frequency of infections was also augmented in several studies (21,24,34).
In line with the literature, no association between age at diagnosis and risk for major complications could be evidenced in our study. Very few studies have reported that age favors complications (17,26,37). However, early complications appeared to be more frequent among older patients (≥55 years) in particular regarding lymphoceles/seroma. Pinsolle et al. (7) and Xue et al. (7,22) observed a higher rate of hematoma after 50 years and a higher rate of infections, respectively. Surprisingly, a study has demonstrated a better outcome in patients over 70 years old after IBR (38).
Conclusion
IBR is a technical challenge with oncologic and esthetic issues for surgeons since morphology, patients’ wishes, therapeutic sequence and risk factors for complications differ from one patient to another. The rate of major complications (re-intervention/re-hospitalization) remains moderate and concerns 15% of patients. With flap-reconstruction, the incidence of major complications was significantly increased by obesity and diabetes. The consideration of these two latter risk factors, before the surgeon decides to perform an IBR, could probably reduce the post-surgical complication rate.
Conflicts of Interest
The Authors declare that they have no conflicts of interest in relation to this study.
Authors’ Contributions
Conception and design: J. Dauplat, C. Pomel ; Investigation: P. Rouanet, E. Delay, K. Clough, J-L Verhaeghe, I. Raoust, M. Bannier, P. Lemasurier, J. Dauplat, C. Pomel ; Writing-original draft preparation: E. Thivat ; All Authors made pertinent contributions to the article, and proofread and approved the final article before submission.
Acknowledgements
STIC-RMI was entirely financed by INCa (The French National Institute of Cancer) by the STIC program (support of innovative and expensive techniques) (agreement: November 2005, 17th). The funding source did not participate in study design, collection, analysis, or interpretation of the data, or writing of the report.
References
- 1.Rouanet P. [Current position of breast reconstruction in oncology] Gynécologie Obstétrique Fertil. 2002;30:985–993. doi: 10.1016/s1297-9589(02)00493-9. [DOI] [PubMed] [Google Scholar]
- 2.Retrouvey H, Kerrebijn I, Metcalfe KA, O’Neill AC, McCready DR, Hofer SOP, Zhong T. Psychosocial functioning in women with early breast cancer treated with breast surgery with or without immediate breast reconstruction. Ann Surg Oncol. 2019;26:2444–2451. doi: 10.1245/s10434-019-07251-9. [DOI] [PubMed] [Google Scholar]
- 3.Malata CM, McIntosh SA, Purushotham AD. Immediate breast reconstruction after mastectomy for cancer. Br J Surg. 2000;87:1455–1472. doi: 10.1046/j.1365-2168.2000.01593.x. [DOI] [PubMed] [Google Scholar]
- 4.Sandelin K, Wickman M, Billgren AM. Oncological outcome after immediate breast reconstruction for invasive breast cancer: a long-term study. Breast Edinb Scotl. 2004;13:210–218. doi: 10.1016/j.breast.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 5.Mustonen P, Lepistö J, Papp A, Berg M, Pietiläinen T, Kataja V, Härmä M. The surgical and oncological safety of immediate breast reconstruction. Eur J Surg Oncol J Eur Soc Surg Oncol Br Assoc Surg Oncol. 2004;30:817–823. doi: 10.1016/j.ejso.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 6.Susini T, Renda I, Giani M, Vallario A, Nori J, Vanzi E, Innocenti A, Lo Russo G, Bianchi S. Changing trends in mastectomy and breast reconstruction. analysis of a single-institution experience between 2004-2016. Anticancer Res. 2019;39:5709–5714. doi: 10.21873/anticanres.13770. [DOI] [PubMed] [Google Scholar]
- 7.Pinsolle V, Grinfeder C, Mathoulin-Pelissier S, Faucher A. Complications analysis of 266 immediate breast reconstructions. J Plast Reconstr Aesthetic Surg JPRAS. 2006;59:1017–1024. doi: 10.1016/j.bjps.2006.03.057. [DOI] [PubMed] [Google Scholar]
- 8.Berry T, Brooks S, Sydow N, Djohan R, Nutter B, Lyons J, Dietz J. Complication rates of radiation on tissue expander and autologous tissue breast reconstruction. Ann Surg Oncol. 2010;17:202–210. doi: 10.1245/s10434-010-1261-3. [DOI] [PubMed] [Google Scholar]
- 9.Monrigal E, Dauplat J, Gimbergues P, Le Bouedec G, Peyronie M, Achard JL, Chollet P, Mouret-Reynier MA, Nabholtz JM, Pomel C. Mastectomy with immediate breast reconstruction after neoadjuvant chemotherapy and radiation therapy. A new option for patients with operable invasive breast cancer. Results of a 20 years single institution study. Eur J Surg Oncol J Eur Soc Surg Oncol Br Assoc Surg Oncol. 2011;37:864–870. doi: 10.1016/j.ejso.2011.07.009. [DOI] [PubMed] [Google Scholar]
- 10.Michy T, Gimbergues P, Le Bouëdec G, Dauplat J. [What surgical procedure for immediate breast reconstruction after preoperative radiotherapy and chemotherapy?] J Chir (Paris) 2007;144:511–515. doi: 10.1016/s0021-7697(07)79777-6. [DOI] [PubMed] [Google Scholar]
- 11.Whitfield GA, Horan G, Irwin MS, Malata CM, Wishart GC, Wilson CB. Incidence of severe capsular contracture following implant-based immediate breast reconstruction with or without postoperative chest wall radiotherapy using 40 Gray in 15 fractions. Radiother Oncol J Eur Soc Ther Radiol Oncol. 2009;90:141–147. doi: 10.1016/j.radonc.2008.09.023. [DOI] [PubMed] [Google Scholar]
- 12.Albino FP, Koltz PF, Ling MN, Langstein HN. Irradiated autologous breast reconstructions: effects of patient factors and treatment variables. Plast Reconstr Surg. 2010;126:12–16. doi: 10.1097/PRS.0b013e3181da878f.. [DOI] [PubMed] [Google Scholar]
- 13.Gimbergues P, Le Bouedec G, Pomel C, Janny-Peyronie M, Dauplat J. [Morbidity of the trans rectus abdominis musculocutaneous flap in breast reconstruction. Retrospective study about 125 patients] Ann Chir. 2003;128:310–315. doi: 10.1016/s0003-3944(03)00091-9. [DOI] [PubMed] [Google Scholar]
- 14.Alderman AK, Wilkins EG, Kim HM, Lowery JC. Complications in postmastectomy breast reconstruction: two-year results of the Michigan Breast Reconstruction Outcome Study. Plast Reconstr Surg. 2002;109:2265–2274. doi: 10.1097/00006534-200206000-00015. [DOI] [PubMed] [Google Scholar]
- 15.Dauplat J, Kwiatkowski F, Rouanet P, Delay E, Clough K, Verhaeghe JL, Raoust I, Houvenaeghel G, Lemasurier P, Thivat E, Pomel C, The STIC-RMI working group Quality of life after mastectomy with or without immediate breast reconstruction. Br J Surg. 2017;104:1197–1206. doi: 10.1002/bjs.10537. [DOI] [PubMed] [Google Scholar]
- 16.Kwiatkowski F, Girard M, Hacene K, Berlie J. [Sem: a suitable statistical software adaptated for research in oncology] Bull Cancer (Paris) 2000;87:715–721. [PubMed] [Google Scholar]
- 17.Seth AK, Hirsch EM, Fine NA, Dumanian GA, Mustoe TA, Galiano RD, Hansen NM, Kim JYS. Additive risk of tumescent technique in patients undergoing mastectomy with immediate reconstruction. Ann Surg Oncol. 2011;18:3041–3046. doi: 10.1245/s10434-011-1913-y. [DOI] [PubMed] [Google Scholar]
- 18.Rusby JE, Waters RA, Nightingale PG, England DW. Immediate breast reconstruction after mastectomy: what are the long-term prospects. Ann R Coll Surg Engl. 2010;92:193–197. doi: 10.1308/003588410X12628812458770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fischer JP, Wes AM, Tuggle CT, Serletti JM, Wu LC. Risk Analysis and Stratification of Surgical Morbidity after Immediate Breast Reconstruction. J Am Coll Surg. 2013;217:780–787. doi: 10.1016/j.jamcollsurg.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 20.Gisquet H, Delay E, Paradol P-O, Toussoun G, Delaporte T, Perol D. [Prevention of seroma by quilting suture after harvesting latissimus dorsi flap. The “Chippendale” technic] Ann Chir Plast Esthét. 2010;55:97–103. doi: 10.1016/j.anplas.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 21.Ducic I, Spear SL, Cuoco F, Hannan C. Safety and risk factors for breast reconstruction with pedicled transverse rectus abdominis musculocutaneous flaps: a 10-year analysis. Ann Plast Surg. 2005;55:559–564. doi: 10.1097/01.sap.0000184463.90172.04. [DOI] [PubMed] [Google Scholar]
- 22.Xue DQ, Qian C, Yang L, Wang XF. Risk factors for surgical site infections after breast surgery: a systematic review and meta-analysis. Eur J Surg Oncol J Eur Soc Surg Oncol Br Assoc Surg Oncol. 2012;38:375–381. doi: 10.1016/j.ejso.2012.02.179. [DOI] [PubMed] [Google Scholar]
- 23.Angarita FA, Acuna SA, Torregrosa L, Tawil M, Escallon J, Ruíz Á. Perioperative variables associated with surgical site infection in breast cancer surgery. J Hosp Infect. 2011;79:328–332. doi: 10.1016/j.jhin.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 24.Vega S, Smartt JM, Jiang S, Selber JC, Brooks CJM, Herrera HR, Serletti JM. 500 Consecutive patients with free TRAM flap breast reconstruction: a single surgeon’s experience. Plast Reconstr Surg. 2008;122:329–339. doi: 10.1097/PRS.0b013e31817f45cb. [DOI] [PubMed] [Google Scholar]
- 25.Mehrara BJ, Santoro TD, Arcilla E, Watson JP, Shaw WW, Da Lio AL. Complications after microvascular breast reconstruction: experience with 1195 flaps. Plast Reconstr Surg. 2006;118:1100–1109. doi: 10.1097/01.prs.0000236898.87398.d6. discussion 1110-1111. [DOI] [PubMed] [Google Scholar]
- 26.McCarthy CM, Mehrara BJ, Riedel E, Davidge K, Hinson A, Disa JJ, Cordeiro PG, Pusic AL. Predicting complications following expander/implant breast reconstruction: an outcomes analysis based on preoperative clinical risk. Plast Reconstr Surg. 2008;121:1886–1892. doi: 10.1097/PRS.0b013e31817151c4. [DOI] [PubMed] [Google Scholar]
- 27.Yazar S, Altinkaya A, Bengur FB, Karadag EC, Kara H, Uras C. Factors associated with complications in immediate breast reconstruction in 1 stage with completely submuscular implants. Ann Plast Surg. 2019;83:264–270. doi: 10.1097/SAP.0000000000001808. [DOI] [PubMed] [Google Scholar]
- 28.Aristei C, Falcinelli L, Bini V, Palumbo I, Farneti A, Petitto RP, Gori S, Perrucci E. Expander/implant breast reconstruction before radiotherapy. Strahlenther Onkol. 2012;188:1074–1079. doi: 10.1007/s00066-012-0231-z. [DOI] [PubMed] [Google Scholar]
- 29.Cohen O, Lam G, Choi M, Ceradini D, Karp N. Risk factors for delays in adjuvant chemotherapy following immediate breast reconstruction. Plast Reconstr Surg. 2018;142:299–305. doi: 10.1097/PRS.0000000000004547. [DOI] [PubMed] [Google Scholar]
- 30.Contant CM, van Geel AN, van der Holt B, Griep C, Tjong Joe Wai R, Wiggers T. Morbidity of immediate breast reconstruction (IBR) after mastectomy by a subpectorally placed silicone prosthesis: the adverse effect of radiotherapy. Eur J Surg Oncol J Eur Soc Surg Oncol Br Assoc Surg Oncol. 2000;26:344–350. doi: 10.1053/ejso.1999.0896. [DOI] [PubMed] [Google Scholar]
- 31.Lin KY, Johns FR, Gibson J, Long M, Drake DB, Moore MM. An outcome study of breast reconstruction: presurgical identification of risk factors for complications. Ann Surg Oncol. 2001;8:586–591. doi: 10.1007/s10434-001-0586-3. [DOI] [PubMed] [Google Scholar]
- 32.Krueger EA, Wilkins EG, Strawderman M, Cederna P, Goldfarb S, Vicini FA, Pierce LJ. Complications and patient satisfaction following expander/implant breast reconstruction with and without radiotherapy. Int J Radiat Oncol. 2001;49:713–721. doi: 10.1016/s0360-3016(00)01402-4. [DOI] [PubMed] [Google Scholar]
- 33.Song J, Zhang X, Liu Q, Peng J, Liang X, Shen Y, Liu H, Li H. Impact of neoadjuvant chemotherapy on immediate breast reconstruction: a meta-analysis. PLoS One. 2014;9:e98225. doi: 10.1371/journal.pone.0098225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Petersen A, Eftekhari ALB, Damsgaard TE. Immediate breast reconstruction: A retrospective study with emphasis on complications and risk factors. J Plast Surg Hand Surg. 2012;46:344–348. doi: 10.3109/2000656X.2012.700025. [DOI] [PubMed] [Google Scholar]
- 35.Chang DW, Reece GP, Wang B, Robb GL, Miller MJ, Evans GR, Langstein HN, Kroll SS. Effect of smoking on complications in patients undergoing free TRAM flap breast reconstruction. Plast Reconstr Surg. 2000;105:2374–2380. doi: 10.1097/00006534-200006000-00010. [DOI] [PubMed] [Google Scholar]
- 36.Padubidri AN, Yetman R, Browne E, Lucas A, Papay F, Larive B, Zins J. Complications of postmastectomy breast reconstructions in smokers, ex-smokers, and nonsmokers. Plast Reconstr Surg. 2001;107:342–349. doi: 10.1097/00006534-200102000-00007. discussion 350-351. [DOI] [PubMed] [Google Scholar]
- 37.Paprottka FJ, Schlett CL, Luketina R, Paprottka K, Klimas D, Radtke C, Hebebrand D. Risk factors for complications after skin-sparing and nipple-sparing mastectomy. Breast Care Basel Switz. 2019;14:289–296. doi: 10.1159/000503218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vanni G, Materazzo M, Pellicciaro M, Morando L, Portarena I, Anemona L, D’angelillo Mr, Barbarino R, Chiaravalloti A, Meucci R, Perretta T, Deiana C, Orsaria P, Caspi J, Pistolese CA, Buonomo OC. Does age matter? estimating risks of locoregional recurrence after breast-conservative surgery. In Vivo. 2020;34:1125–1132. doi: 10.21873/invivo.11884. [DOI] [PMC free article] [PubMed] [Google Scholar]