Abstract
Background
New gene therapy approaches have emerged as promising treatment options for rare congenital disorders and certain tumor entities for which previously only procedures of limited curative potential had been available, if at all.
Methods
Based on a selective literature search, the principles of gene therapy, the current status of clinical application, and the methods and results of gene therapy approaches are discussed.
Results
In vivo gene therapy relies mostly on the use of vectors based on modified adeno-associated viruses to introduce a functioning copy of the missing or defective genetic information into the target cells. In ex vivo gene therapy, the target cells are extracted, genetically modified using a viral vector, and then returned to the patient. Predominantly lentiviral vectors are used for this purpose. With regard to monogenic disorders, gene therapies are available for the treatment of patients with severe combined immunodeficiency (ADA-SCID), congenital retinal dystrophy (RPE65 mutations), transfusion-dependent ß-thalassemia, and spinal muscular atrophy. In spinal muscular atrophy, for example, single-dose in vivo gene therapy leads to progress in motor development that could not be expected to occur in the natural course. These effects are particularly pronounced when the gene therapy is administered before the onset of symptoms.
Conclusion
The first gene treatments have now been approved and bring hope of long-term therapeutic benefit after a single administration. The numbers of patients who come into question for specific therapies are often low, so that many different aspects—generation of evidence on efficacy and safety, determining indications, performance of the treatment, pricing—bring new challenges.
Somatic gene therapy is defined as the addition, removal, or modification of the genetic information in a patient’s somatic cells for the purpose of treating or preventing disease. After decades of research and some setbacks, the number of successful and approved gene therapeutics has increased continually in recent years (figure 1) (1). To date, gene therapy is used primarily in the therapy of monogenic, mostly rare diseases, and in malignancies.
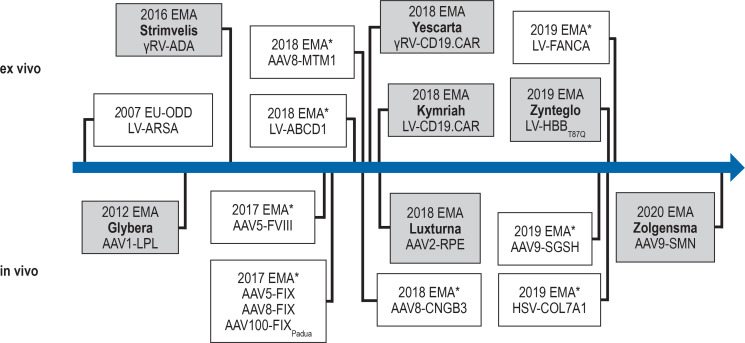
Figure 1.
Gene therapeutics in the EU
The time axis lists all gene therapies (on a grey background) approved by the European Medicines Agency (EMA), as well as selected drugs for novel therapies to treat monogenetic disorders that have recognized Priority Medicine (PRIME) status in the EU (38). The PRIME kitemark (*) enables the priority development of medical drugs that are considered promising and for which no alternative preparations exist. The figure displays the applied vector types and the relevant therapeutic genes. The disorder that can be treated with this preparation is given in parentheses below. The license for Glybera expired in 2017. LV-ARSA was recommended for approval in October 2020.
AAV, adeno-associated virus; AAVx, AAV serotype x vector; ABCD1, ATP-binding cassette D1 (cerebral adrenoleukodystrophy); ADA, adenosine monophosphate desaminase (ADA deficiency); ARSA, arylsulfatase A (metachromatic leukodystrophy); CD19.CAR, chimeric antigen receptor directed against CD19 (CAR-T-cell therapy); CNGB3, cGMP-gated cation channel B3 (achromatopsia); COL7A1; collagen type-7-A1-chain (dystrophic epidermolysis bullosa); FANCA, Fanconi Anemia-Complementation Group A (Fanconi-anemia typ A); FVIII, factor VIII (hemophilia A); FIX, factor IX (hemophilia B); γRV, gamma-retroviral vector; HBB, ß-globin (ß thalassemia); HSV, herpes simplex virus-based vector; LPL, lipoprotein lipase (LPL deficiency); LV, lentiviral vector; MTM1, myotubularin 1 (myotubular myopathy); ODD, orphan drug designation; RPE, retinoid-isomerohydrolase (retinal dystrophy); SGSH, heparan sulfamidase (Sanfilippo disease type A); SMN, survival motor neuron (spinal muscular atrophy)
cme plus
This article has been certified by the North Rhine Academy for Continuing Medical Education. Participation in the CME certification program is possible only over the internet: cme.aerzteblatt.de. The deadline for submissions is 20 December 2021.
Viral vectors are the most commonly used method for inserting the genetic information into the target cells. This so called transduction can be done ex vivo or in vivo (Figure 2, Box 1). In contrast to germ cell therapy, which is prohibited in Germany for ethical reasons, in somatic gene therapy the genetic material is inserted into differentiated somatic cells or their precursors. It is therefore not passed on to the subsequent generation. These therapies are mostly given as a single treatment and can occasionally affect the disease course in the long term and in a sustained way. Because of the novelty and the expense of up to 2 million Euro, gene therapies are associated with particular challenges for the healthcare system. Furthermore, at the time they gain approval, data on long term safety and efficacy are often still limited.
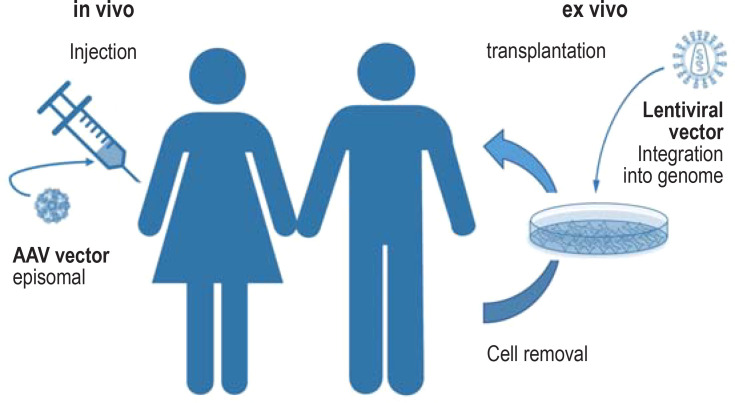
Figure 2.
Principles of gene addition therapy
In ex vivo gene therapy, the target cells are isolated from the body first, in order to modify them genetically by applying a viral vector. When using a lentiviral vector, the therapeutic gene is integrated into the host genome in a stable way, which means it is passed to the daughter cells. Subsequently the cells are implanted back into the patient. The cases of leukemia triggered by retroviral insertional mutagenesis in early gene therapy studies have not occurred since second generation retroviral and lentiviral vectors have been used.
In in vivo gene therapy, viral vectors that code for the therapeutic gene are transferred directly into the body of the patient. The vectors used for these treatments are mostly derived from adeno-associated viruses (AAV) (39). In contrast to lentiviral vectors, AAV vectors do not integrate their genome into the host genome. It remains in the cell nucleus as an episome but is not multiplied during cell division. AAV vectors are therefore particularly suitable for gene transfer in de facto post-mitotic tissue, such as retina, liver, or muscle.
BOX 1. Important terms relating to gene therapy.
Antisense oligonucleotide Synthetically produced fragment of ribonucleic acid (RNA), which can affect splicing of pre-RNA. Nusinersen (Spinraza) is an antisense oligonucleotide that is approved for the treatment of spinal muscular atrophy. The treatment does not change the genetic information directly, only the way in which it is read.
CAR T-cell therapy The so called chimeric antigen receptor (CAR) T-cell therapy is a novel therapeutic approach, which was developed primarily to treat cancer. Lymphocytes are taken from the patient and gene transfer is used to furnish these cells with specific CARs. Upon infusion, the CAR T-cells recognize tumor-associated antigens in a targeted manner and destroy the tumor cells by activating the immune system.
CRISPR-Cas9 Clustered regularly interspaced short palindromic repeats-Cas9 is an instrument used in genome editing consisting of enzyme (Cas9) and a short guide RNA. The complex is referred to in vernacular language as “gene scissors.”
Episome Circular extrachromosomal DNA molecule that is not integrated into the genome.
Expression cassette DNA segment that is responsible for the synthesis of RNA. In addition to the protein coding sequence, it contains regulatory sequences that control transcription (for example, promotor) and translation.
Insertional mutagenesis Genotoxic effects as a result of the insertion of genetic information into the cellular genome, especially in the genetic modification of stem cells by using retroviral vectors.
Transduction In genetics, this describes the delivery of genetic material into target cells by using viral vectors.
Viral vectors Intentionally modified virus particles that are used in gene technology to deliver genetic material into target cells. They are in vernacular language referred to as gene ferries.
In contrast to gene transfer as explained above, genome editing aims to correct a disease causing mutation directly in the genome of the affected cells in an organ. Since the initial description of the CRISPR-Cas technology in 2012, genome editing with programmable nucleases as evolved at great speed. In addition to CRISPR-Cas, two further platforms—zinc finger nucleases (ZFNs) and transcription activator-like effector nucleases (TALENs)—have reached the clinical trial stage (2).
This review article describes recent developments in gene therapy. First, we identified the gene therapeutics approved in Europe and Germany from the websites of the European Medicines Agency (EMA) and the Paul-Ehrlich-Institute (table). We then conducted a selective literature search in PubMed with the substances and higher-level topics as search terms.
Table. Gene therapies licensed for use in Europe.
|
Product Year of approval |
Indication*1 | Prevalence*2 | Viral vector or cell therapy | Application |
| Alipogentiparvovec(Glybera) 2012 (approval expired in 2017) |
Adults with familial lipoprotein lipase deficiency (LPLD) with severe or multiple episodes of pancreatitis in spite of a low-fat diet | Target population in Germany: 17–35 | AAV1 vector coding for human LPL gene | Several intramuscular injections of the vector in one session |
| Talimogen laherparepvec (Imlygic) 2015 |
Adults with non-resectable, local or distant metastatic melanoma (stages IIIB, IIIC, and IVM1a) | Target population in Germany: 75–225 | Vector coding for human GM-CSF, which is based on attenuated HSV-1 | Repeated injection of the vector into cutaneous, subcutaneous, and/or nodal lesions |
| Strimvelis 2016 |
Patients with severe combined immunodeficiency caused by adenosine deaminase deficiency (ADA-SCID), for which no suitable human leukocyte antigen (HLA) compatible stem cell donor from the family is available | Prevalence 0.02 : 10 000*3 means a prevalence for all of Germany of about 160 | Ex vivo therapy of autologous CD34+-cells transduced by using retroviral vector carrying the ADA coding sequence | Intravenous administration of the genetically modified CD34+ –cells |
| Tisagenlecleucel (Kymriah) 2018 |
Patients aged up to 25 years with refractory or recurrent B-cell acute lymphoblastic leukemia (ALL); adults with diffuse large B cell lymphoma (DLBCL) after a minimum of two systemic treatments | Target population in Germany: 438–702 | Ex vivo therapy of lentivirally transduced autologous T-cells that express a chimeric antigen receptor (CAR) directed against CD19 | Intravenous administration of the genetically modified T-cells |
| Axicabtagene ciloleucel (Yescarta) 2018 |
Adults with recurrent or refractory diffuse large B cell lymphoma (DLBCL) and primary mediastinal large B cell lymphoma (PMBCL) after a minimum of two systemic treatments | Target population in Germany: 871–1 064 | Ex vivo therapy of retrovirally transduced autologous T-cells, that express a chimeric antigen receptor (CAR) directed against CD19 | Intravenous administration of the genetically modified T-cells |
| Voretigen neparvovec (Luxturna) 2018 |
Patients with vision loss owing to hereditary retinal dystrophy, as a result of confirmed biallelic RPE65 mutations | Target population in Germany: 189–290 | AAV2 vector coding for the human RPE65 gene | Subretinal injection of the vector |
| Betibeglogene autotemcel (Zynteglo) 2019 |
Patients > 12 years with transfusion dependent β-thalassemia (TDT), who do not have a β0/β0 genotype, and for whom no stem cell donor is available | Target population in Germany: 49–53 | Ex vivo therapy of CD34+-cells transduced with a lentiviral vector coding for β-Globin (T87Q) | Intravenous administration of the genetically modified CD34+ -cells |
| Onasemnogene abeparvovec (Zolgensma) 2020 |
Patients with spinal muscular atrophy (SMA) owing to a biallelic mutation in the SMN1 gene and the clinical phenotype of SMA type 1 or up to 3 SMN2 copies | Target population in Germany: 71–120 patients with SMA type 1, 770 bis 941 patients with later disease onset, SMA incidence at birth about 100 per year | AAV9 vector coding for the human SMN1- gene | Intravenous infusion of the vector |
In contrast to the usual practice of the Deutsches Ärzteblatt we list the medications used for the therapy under their proprietary names in order to facilitate easier recognition, as even experts are often unfamiliar with the vector names. See Box 2 regarding the terminology of gene therapeutics. *1 Details on the precise area of use should be taken from the product leaflets/information. *2 The reported prevalence rates of patients suitable for the relevant therapy are often subject to great uncertainties. Unless reported otherwise the data are from the reports of the Institute for Quality and Efficiency in Healthcare (IQWiG) in the context of benefit assessments by the Federal Joint Committee (www.g-ba.de). Some prevalence rates refer to patients in the statutory health insurance scheme (GKV), this proportion accounts for 90 % of the total population. *3 vgl. www.ema.europa.eu/en/medicines/human/orphan-designations/eu305313
Examples of gene therapies in monogenic disorders
The advantage of ex vivo gene therapy lies in the fact that the product can and must be characterized in detail before the transplantation. The production of genetically modified cells is therefore subject to extensive laws, regulations, and guidelines. For targeted in vivo treatment, the logistical hurdles are not quite as steep, but suitable methods are required to achieve sufficient efficacy in the target organ while simultaneously minimizing the extent of immune reactions and the risk of undesirable modifications to the germline. Since in most cases, only very few patients are suitable candidates for a specific gene therapy, the numbers of patients treated in clinical studies are often small. Furthermore, the fact that the disorders are severe and progressive, without any therapeutic alternative, often prevents the carrying out of randomized or placebo controlled trials, for methodological and ethical reasons. In the following paragraphs we will explain important developmental steps relating to individual organs.
Liver (hemophilia)
Hemophilia is a bleeding disorder, in which—because of genetic defects—Factor VIII (hemophilia A) or IX (hemophilia B) is not produced in sufficient amounts. Current therapy consists of lifelong substitution of the relevant clotting factors. Initial studies of gene transfer date back as far as the 1990s, but the expression of Factor IX was only short-lived, owing to a strong immune response against the adeno-associated virus (AAV) based vector (3). Subsequently the viral vectors and expression cassettes were improved, and several studies of AAV based gene therapy of hemophilia A and B were initiated (4). In an open label treatment study, 15 adult patients with hemophilia A were treated in a single session with different dosages of AAV5 based gene therapy. Over the three year follow-up period it was observed that the 13 patients who had received a higher dose did not need any further factor substitutions and the bleeding rate was reduced by more than 90%. Almost all patients experienced temporarily higher transaminase levels after the treatment, which were reversed by giving temporary cortisone treatment and did not trigger clinical symptoms. One patient developed a fever, headache, and aching limbs after the infusion, which ceased after symptomatic therapy (5, 6). Further potentially limiting factors are pre-existing antibodies against the AAV capsid used or against the respective clotting factor.
Eye (retinopathies)
Ophthalmology is the specialty in which gene transfer has made the greatest progress, in the treatment of retinopathies. In contrast to the often used systemic application, the treatment is administered by means of direct subretinal injection of the vector after vitrectomy. The effective substance Voretigene neparvovec is an AAV2 based gene addition for the treatment of Leber congenital amaurosis (type 2) and retinitis pigmentosa (type 20), which are both caused by mutations of the RPE65 gene. If the disorders remain untreated they will lead to complete blindness. An open label randomized trial in a wait-list control group design, 21 of a total of 31 patients were randomized to direct treatment. The primary endpoint, a standardized mobility test to measure functional vision in environments of different levels of brightness (multi-luminance mobility testing, MLMT) showed significant improvements in eyesight and orientation in poorly lit environments after the treatment compared with the control group. This positive effect was sustained in one study over a period of four years. Improvement of visual acuity in well lit environments did not reach significance (7, 8). The therapy was approved by the EMA in 2018 for the treatment of children and adults with a retinopathy owing to RPE65 mutations whose retinal tissues was still functional. Both eyes were treated with a time interval. Currently, clinical trials are being conducted to evaluate AAV based gene therapy in numerous additional gene related retinopathies.
Neuromuscular disorders
Potential gene therapeutic approaches for the treatment of different, mostly rapidly progressive neuromuscular disorders have been the subject of research for decades. A first breakthrough was achieved in the treatment of spinal muscular atrophy (SMA). This is an autosomal-recessive disorder and is due to mutations of the SMN1 gene. The resultant deficiency in SMN protein leads to the degeneration of the motor neurons in the spinal cord and associated progressive muscular weakness. Clinically, a wide disease spectrum has been observed, with onset ranging from infancy to young adulthood. Most common is SMA onset within the first six months of life, which—if left untreated—will lead to death within the first two years of life because of the involvement of the respiratory muscles.
Nusinersen is a causal treatment that has been available since 2017. It’s a splicing modifier, which after an initial saturation phase is administered intrathecally every four months via lumbar puncture (9, 10).
Gene therapy for SMA is done by using an AAV9 vector, which transports the SMN1 gene into the motor neurons of the spinal cord after s single intravenous application. Sufficient transduction after intravenous administration is successful especially in infancy. A study of the substance onasemnogene abeparvovec showed in 12 infants, who received a higher dose of the gene therapy, motor progress that was not observed in the natural course. 24 months after the treatment, all treated children were event-free—that is, they survived without long-term ventilation—compared with less than 8% over the natural course (11). The most common adverse effects were asymptomatic increase in transaminase levels, thrombocytopenia, and temporary fever and vomiting. Recently, individual cases have been described of subacute liver failure after the therapy had been given (12).
Onasemnogene abeparvovec was approved by the US Food and Drug Administration (FDA) for the treatment of children with SMA up to the age of 2 years in 2019. The demand of affected families to be given a treatment, which incurs costs of up to 2 million Euro, before this has been approved for use in Europe led to great media interest and public discussions in Germany, too (13, 14).
The approval of onasemnogene abeparvovec issued by the EMA in 2020 is notably wider than the populations investigated in clinical trials and has prompted debates about the safety and efficacy in this extended patient population (15, 16). Clinical studies that directly compare nNusinersen and onasemnogene abeparvovec regarding safety and efficacy do not exist.
For the most common hereditary muscle disorder in children—the X-linked inherited Duchenne muscular dystrophy—the size of the defective dystrophin gene is a problem as it exceeds the capacity of the viral vectors. For this reason, the so called microdystrophin genes have been developed, which contain the most important elements of the dystrophin gene but are notably smaller (17). The clinical efficacy of AAV based gene therapy using microdystrophins is currently being investigated in clinical trials.
Clear clinical improvements have recently been observed for X-linked inherited myotubular myopathy, whose estimated incidence is about 1 : 50 000 male newborns (18). It is mostly associated with severe congenital muscle weakness, which necessitates artificial respiration. An open label treatment study in 12 patients described improvements in motor and respiratory function after AAV8 based gene therapy to the point where artificial respiration was no longer required (18).
However, in 2020, two children who had received a higher dose of this gene therapy died from sepsis, which was probably due to liver injury. The use of the therapy in further patients has therefore been stopped (20).
Immune system
For ADA-SCID, an extremely rare immune defect (severe combined immunodeficiency [SCID]) with depletion of the T, B, and NK lymphocytes and consequently lacking adaptive humoral and cellular immune response, gene therapy was approved in Europe as early as 2016. Untreated, the affected infants will die within their first few years of life. Therapeutic approaches to date consist of bone marrow transplantation or enzyme substitution therapy. Gene therapy in ADA-SCID is administered ex vivo. To this end, CD34+-cells are extracted from a bone marrow biopsy specimen or peripheral blood, corrected via a retroviral vector with an intact ADA gene, and then infused intravenously.
Gene therapy is approved for patients who do not have a suitable donor for bone marrow transplantation. Because the disease is so rare and the therapy so complex, the procedure is currently provided in Europe in only one center in Milan (Italy). For the 12 participants in the pivotal studies, follow-up data with a median observation period of 12 years are now available, which showed a survival rate of 100%, of which 92% were event-free (21). For other hereditary immune defects, gene therapies are already in advanced clinical development (22, 23).
Disorders of hematopoiesis
In 2019 the first gene therapy was approved for the treatment of hereditary hemoglobinopathy. The preparation Betibeglogene autotemcel can be used in adolescent and adult patients with transfusion dependent ß-thalassemia (non-ß0/ß0 genotype) if no suitable donor for a stem cell transplant is available (24).
Gene therapy is done ex vivo, by adding to the autologous hematopoietic stem cells (HSC) functional copies of the ß-globin gene (ßA-T87Q globin gene variant) by means of a lentivirus vector. The primary outcome measure of the studies that was relevant for approval was a minimum period of 1 year without transplantation in the follow-up period of 24 months. This was achieved in 11 of the 14 patients with a non-ß0/ß0 genotype. The only serious adverse effect associated with to Betibeglogene autotemcel was thrombocytopenia in one of 45 patients (25).
In this context it is worth mentioning that phase 1/2 trials of CRISPR edited HSC in ß-thalassemia and sickle cell disease seem to have been successful in their first patients. For instance,a 20 year old transfusion dependent thalassemia patient had presented with “normal” blood counts for 18 months and had not required any more transfusions (26).
Mucoviscidosis
Mucoviscidosis, also known as cystic fibrosis, is an autosomal recessive metabolic disorder with an incidence of about 1:4 000 neonates. Mutations in the CFTR gene disrupt the function of the chloride channel, which affects in particular the respiratory tract, the pancreas, the small bowel, and the male sexual organs (27). Especially the pulmonary manifestation is the target of gene therapeutic interventions.
In 2015 the results of a double blind placebo controlled phase 2b trial were published, which investigated the monthly inhalation of a CFTR gene/liposome complex. Altogether after 12 months a small, statistically significant therapeutic success was seen with regard to the primary outcome measure (forces expiratory volume at 1 second [FEV1]), of 3.7% (95% confidence interval [0.1; 7.3] compared with placebo. The individual response was, however, so heterogeneous that the clinical efficacy overall was rated as non-satisfactory (28). Since then research has focused on the development of new vectors that enable better transduction of the target cells, especially in the respiratory tract (29).
Particularities and challenges of gene therapies
In contrast to conventional drug treatments, gene therapeutics (box 2) are mostly administered in a single session. Using these innovative therapies requires relevant expertise, both regarding the application (for example, ex vivo preparation, subretinal injection) as well as regarding monitoring and treatment of potential adverse effects (immune reaction, off-target effects).
BOX 2. Terminology for gene therapeutics.
The World Health Organization published requirements for the proprietary names of gene therapeutics (40). The names as a rule consist of two words, with the first word relating to the gene product used and the second word to the vector used.
Example: Onasemnogene abeparvovec (Zolgensma) for the treatment of spinal muscular atrophy. In this preparation, the word component “semnogene” refers to the included SMN gene, whereas “parvo” refers to the vector, an adeno-associated virus (Parvoviridae). The final syllable “vec” refers to the fact that this is a non-replicating vector.
Since the context so far has mostly been one of rare diseases, approval of gene transfer therapies is mostly based on studies with small patient numbers and follow-up periods of very few years. This seems sensible in order not to withhold effective treatments from affected patients, but it is also associated with a risk of as yet unknown adverse effects (box 3).
BOX 3. Ethical, regulatory, and economic characteristics/particularities of somatic gene therapies.
To date, somatic gene therapies have been used mainly in monogenic, mostly rare disorders, which have a severe and progressive course and for which hardly any therapeutic alternatives exist. As in- vivo gene therapies can be used only once because of immunization, the difficult question in clinical research already is that of adequate dosage. On the one hand, the dose should lead to a sufficient cell transduction and, on the other hand, toxic effects owing to an excessively high viral load should be avoided.
For methodological and ethical reasons, the case numbers and observation/follow-up periods are often limited in clinical studies, and randomized placebo controlled trials are more likely to be the exception. The available evidence is therefore limited. The regulatory authorities are then faced with the difficult task of balancing the remaining uncertainties and risks with the potentially sustained benefit of the therapy for the affected patients.
Finally, it needs to be discussed how the financial value of these innovative therapies can be determined while considering the development costs, but also the capacity of the solidarity community. A “learning system” would make sense in this context. It would enable even after “early” approval, a continuous and timely new evaluation of efficacy and safety, and therefore the validity/value of gene therapies. A comprehensive follow-up and documentation of all treated patients will be the main crucial element in this. The indication and the reimbursement could then be adjusted dynamically to the growing evidence.
Often the effect size of a gene therapeutic depends on the stage of the disease at the time of the treatment. In spinal muscular atrophy, initial studies showed that a presymptomatic start of treatment is particularly promising and often enables age-appropriate learning/development of the ability to walk independently (3). In an open label study of presymptomatic gene therapy for SMA, seven of 29 patients gained the ability to walk independently. All others had not reached the age threshold at the time of interim analysis (31). Currently, efforts are under way to include genetic testing for spinal muscular atrophy in the general newborn screening (32).
On the other hand, the therapeutic benefit of gene therapy in advanced disease stages can be very limited. Which patients benefit to which degree from gene therapy can be determined conclusively only in the context of long term studies. Depending on the question, product or disease specific registries are suitable, with the latter also enabling comparative analysis of different products (24, 33).
Registries have to meet certain quality criteria regarding data collection and analysis (34, 35). Ideally, long term, if possible industry independent, follow-up should be ensured for all treated patients. This requires relevant regulatory specifications (36) and is a laborious and complex undertaking for treatment centers. In view of the, in some cases, extremely high medication costs, relevant resources will have to be made available for documenting efficacy and safety. This may be the only way to allow the appropriate and economical use of these therapies in the mid term.
From a health economic perspective too, the introduction of gene therapies entails certain challenges. In some cases, costs of up to 2 million Euro for a single-dose treatment contrasts with the long term costs of conventional therapy. For this reason, new reimbursement methods are under discussion that are guided by the therapeutic success. For CAR T-cell therapy (box 1) using Tisagenlecleucel, for example, a partial reimbursement of the price by the pharmaceutical manufacturer was agreed if a patient were to die from cancer within a defined time period. Other models prefer price adjustments that are guided by the total evidence for a product and not the individual case (37).
Outlook—the importance of gene therapy
The first gene therapies for monogenic disorders are already in use in clinical practice, and it is to be expected that the number of approved gene therapies will increase further over the coming years. In addition to the classic virus vector based gene transfer, targeted genome editing is increasingly drawing attention. The potential of somatic gene therapy has become vastly more than just replacing defective or lacking genes and can also be used to insert particular enzymes or other effector proteins, such as CRISPR-Cas genetic scissors. This means that disorders with a dominant inheritance, non-monogenic entities, such as cardiovascular disorders, inflammatory disorders, and Parkinson’s disease, could also be treated with gene therapy in the future.
Key Messages.
Gene therapies replace or repair “malfunctioning” genes and open up new therapeutic options.
Owing to genetic, tissue specific, and immunological particularities, not all hereditary disorders are equally suitable for a gene therapeutic approach.
A single treatment session often leads to long-term therapeutic effects, in particular for certain cases of monogenic disorders.
For methodological and ethical reasons, approval is often not based on double blinded randomized trials but on open label studies with limited case numbers. The systematic collection of data on clinical effects and adverse effects therefore should be continued after a drug has been approved.
The high costs of therapy and the effort involved in applying and monitoring these novel therapies present new challenges for the healthcare system (among others, ensuring access, quality assurance, and economic viability).
Questions for the article in issue 51–52/2020: Gene Therapy for Monogenic Inherited Disorders—Opportunities and Challenges.
CME credits for this unit can be obtained via cme.aerzteblatt.de until 20 December 2021
Only one answer is possible per question. Please select the answer that is most appropriate.
Question 1
Which disorder is treated with the effective substance onasemnogene abeparvovec, approved by the European Medicines Agency (EMA) in 2020?
Glycogen storage disease type 2 (Pompe disease)
Myasthenia gravis
Becker muscular dystrophy (BMD)
Spinal muscular atrophy (SMA)
Duchenne muscular dystrophy
Question 2
Which term describes the addition of genetic information into a target cell by using a viral vector?
Induction
Transduction
Imputation
Transcription
Translation
Question 3
Which of the following disorders is treated by using ex vivo gene therapy?
Hereditary retinal dystrophy owing to RPE65 mutations
Spinal muscular atrophy (SMA)
Familiar lipoprotein lipase deficiency
Non-resectable metastatic melanoma
Severe combined immune deficiency (ADA-SCID)
Question 4
Which restrictions exist for the selection of a beta-thalassemia patient regarding therapy using Betibeglogene autotemcel?
Patients have to be of age
A suitable stem cell donor has to be available
Patients must be transfusion-dependent
Patients must have a ß0/ß0 genotype
Patients should be younger than 12 years
Question 5
Voretigen neparvovec is used to treat retinal dystrophy. Which genotype is required for the treatment to be successful?
A biallelic RPE65 mutation
A biallelic mutation in the SMN1 gene
A monoallelic RPE65 mutation
A monoallelic mutation in the SMN1 gene
A biallelic mutation in the RPE65 gene and in the SMN1 gene
Question 6
What are the general advantages of somatic gene therapy?
The genetic information is passed down to offspring
The treatment can usually be given in a single session
No viral vectors are used
To date no known adverse effects have been observed
The more advances the disorder the more effective the therapy
Question 7
For which disorders is the so called CAR T-cell therapy (chimeric antigen receptor) approved to date?
Retinal disorders
Hemophilia a
Autoimmune diseases
B-cell lymphoma/leukemia
Type 1 diabetes
Question 8
Gene addition treatment comprises two different principles—in vivo therapy and ex vivo therapy. What are the advantages of ex vivo therapy?
The production of the modified cells is not subject to any legislation
The product can be characterized before transplantation
No vector is needed to insert the genetic information
The added genetic information is not integrated into the cell genome
No cells/tissues have to be removed from the patient
Question 9
The license of the first gene therapy originally approved by the EMA expired in 2017. The therapy is used for which disorder?
Lipoprotein lipase deficiency (LPLD)
Retinopathy
Duchenne muscular dyustrophy
B cell lymphoma
Severe combined immunodeficiency (ADA-SCID)
Question 10
Which pathophysiological mechanism is underlying muscle weakness in spinal muscular atrophy with SMN protein deficiency?
Degenerative processes in the motor cortex
Pyramidal tract lesions
Malformation of muscle fibers
Degeneration of motor neurons in the spinal cord
Malformation of the neuromuscular junction
Acknowledgments
Translated from the original German by Birte Twisselmann, PhD.
Footnotes
Conflict of interest statement
Prof. Kirschner received consultancy fees and travel expenses from Avexis, Biogen, Pfizer, Roche, Sarepta, and Scholar Rock. He received lecture honoraria from Avexis, Biogen, and Roche. Study support (third party funding) was made available to him by Biogen and Roche. He is the director of the SMArtCARE registry for the long term follow up of SMA and a member of the board of the European Reference Network for rare neuromuscular diseases (EURO-NMD).
Prof. Cathomen holds shares in CRISPR Therapeutics and patents with relation to the subject from Freiburg University Medical Center. He received lecture honoraria from the Initiative Gesundheitsindustrie Hessen [IGH, an alliance formed of four partnering groups: Hesse’s state government, companies active in Hesse’s healthcare industries, the Hesse-Thuringia area of the IG BCE [the trade union for mining, chemicals, and energy], and Hesse’s institutions of higher education], and Medscape Oncology. Study support (third party funding) was made available to him by Cellectis SA and Miltenyi Biotec.
References
- 1.Paul Ehrlich Institut. Gentherapeutika. www.pei.de/DE/arzneimittel/atmp/gentherapeutika/gentherapeutika-node.html (last accessed on 26 February 2020) [Google Scholar]
- 2.Cornu TI, Mussolino C, Cathomen T. Refining strategies to translate genome editing to the clinic. Nat Med. 2017;23:415–423. doi: 10.1038/nm.4313. [DOI] [PubMed] [Google Scholar]
- 3.Mingozzi F, High KA. Immune responses to AAV in clinical trials. Curr Gene Ther. 2007;7:316–324. doi: 10.2174/156652307782151425. [DOI] [PubMed] [Google Scholar]
- 4.Miesbach W, Schwäble J, Müller MM, Seifried E. Treatment options in hemophilia. Dtsch Arztebl Int. 2019;116:791–798. doi: 10.3238/arztebl.2019.0791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pasi KJ, Rangarajan S, Mitchell N, et al. Multiyear follow-up of AAV5-hFVIII-SQ gene therapy for hemophilia A. N Engl J Med. 2020;382:29–40. doi: 10.1056/NEJMoa1908490. [DOI] [PubMed] [Google Scholar]
- 6.Perrin GQ, Herzog RW, Markusic DM. Update on clinical gene therapy for hemophilia. Blood. 2019;133:407–414. doi: 10.1182/blood-2018-07-820720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maguire AM, Russell S, Wellman JA, et al. Efficacy, safety, and durability of voretigene neparvovec-rzyl in RPE65 mutation-associated inherited retinal dystrophy: results of phase 1 and 3 trials. Ophthalmology. 2019;126:1273–1285. doi: 10.1016/j.ophtha.2019.06.017. [DOI] [PubMed] [Google Scholar]
- 8.Russell S, Bennett J, Wellman JA, et al. Efficacy and safety of voretigene neparvovec (AAV2-hRPE65v2) in patients with RPE65-mediated inherited retinal dystrophy: a randomised, controlled, open-label, phase 3 trial. Lancet. 2017;390:849–860. doi: 10.1016/S0140-6736(17)31868-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Finkel RS, Mercuri E, Darras BT, et al. Nusinersen versus sham control in infantile-onset spinal muscular atrophy. N Engl J Med. 2017;377:1723–1732. doi: 10.1056/NEJMoa1702752. [DOI] [PubMed] [Google Scholar]
- 10.Mercuri E, Darras BT, Chiriboga CA, et al. Nusinersen versus sham control in later-onset spinal muscular atrophy. N Engl J Med. 2018;378:625–635. doi: 10.1056/NEJMoa1710504. [DOI] [PubMed] [Google Scholar]
- 11.Mendell JR, Al-Zaidy S, Shell R, et al. Single-dose gene-replacement therapy for spinal muscular atrophy. N Engl J Med. 2017;377:1713–1722. doi: 10.1056/NEJMoa1706198. [DOI] [PubMed] [Google Scholar]
- 12.Feldman AG, Parsons JA, Dutmer CM, et al. Subacute liver failure following gene replacement therapy for spinal muscular atrophy Type I. J Pediatr. 2020 doi: 10.1016/j.jpeds.2020.05.044. S0022 3476(20)30682-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Servais L, Kirschner J, Muntoni F. Randomisation versus prioritisation in a managed access programme: lessons from spinal muscular atrophy. Neuromuscul Disord. 2020;30:267–269. doi: 10.1016/j.nmd.2020.03.006. [DOI] [PubMed] [Google Scholar]
- 14.Ziegler A, Müller-Felber W, Hahn A, von Moers A, Schara U, Kirschner J. Spinale Muskelatrophie: Gentherapie ohne Zulassung. Dtsch Arztebl. 2019;116 A-2232. [Google Scholar]
- 15.Kirschner J, Bernert G, v. der Hagen M, et al. Zur Gentherapie der Spinalen Muskelatrophie mit Onasemnogene Abeparvovec. Stellungnahme der Gesellschaft für Neuropädiatrie. Monatsschr Kinderheilkd. 2020;168:938–941. [Google Scholar]
- 16.Kirschner J, Butoianu N, Goemans N, et al. European ad-hoc consensus statement on gene replacement therapy for spinal muscular atrophy. Eur J Paediatr Neurol. 2020 doi: 10.1016/j.ejpn.2020.07.001. S1090 3798(20)30142-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Duan D. Systemic AAV micro-dystrophin gene therapy for duchenne muscular dystrophy. Mol Ther. 2018;26:2337–2356. doi: 10.1016/j.ymthe.2018.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vandersmissen I, Biancalana V, Servais L, et al. An integrated modelling methodology for estimating the prevalence of centronuclear myopathy. Neuromuscul Disord. 2018;28:766–777. doi: 10.1016/j.nmd.2018.06.012. [DOI] [PubMed] [Google Scholar]
- 19.Audentes. Audentes therapeutics tresents new positive data from ASPIRO, the clinical trial evaluating AT132 in patients with X-linked myotubular myopathy (XLMTM), at the 24th International Annual Congress of the World Muscle Society. www.audentestx.com/press_release/audentes-therapeutics-presents-new-positive-data-aspiro-clinical/ (last accessed on 26 February 2020) [Google Scholar]
- 20.Paulk N. Gene therapy: it’s time to talk about high-dose AAV. www.genengnews.com/topics/genome-editing/gene-therapy-its-time-to-talk-about-high-dose-aav/ (last accessed on 20 July 2020) [Google Scholar]
- 21.European Medicine Agency. Zusammenfassung der Merkmale des Arzneimittels Strimvelis. www.ema.europa.eu/en/documents/product-information/strimvelis-epar-product-information_de.pdf (last accessed on 20 July 2020) [Google Scholar]
- 22.Cavazzana M, Bushman FD, Miccio A, Andre-Schmutz I, Six E. Gene therapy targeting haematopoietic stem cells for inherited diseases: progress and challenges. Nat Rev Drug Discov. 2019;18:447–462. doi: 10.1038/s41573-019-0020-9. [DOI] [PubMed] [Google Scholar]
- 23.Booth C, Romano R, Roncarolo MG, Thrasher AJ. Gene therapy for primary immunodeficiency. Hum Mol Genet. 2019;28:R15–R23. doi: 10.1093/hmg/ddz170. [DOI] [PubMed] [Google Scholar]
- 24.Schuessler-Lenz M, Enzmann H, Vamvakas S. Regulators‘ advice can make a difference: European Medicines Agency approval of zynteglo for beta thalassemia. Clin Pharmacol Ther. 2020;107:492–494. doi: 10.1002/cpt.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.European Medicine Agency. Zusammenfassung der Merkmale des Arzneimittels Zynteglo. www.ema.europa.eu/en/documents/product-information/zynteglo-epar-product-information_de.pdf (last accessed on 20 July 2020) [Google Scholar]
- 26.Frangoul H, Altshuler D, Cappellini MD, et al. CRISPR-Cas9 Gene Editing for Sickle Cell Disease and beta-Thalassemia. N Engl J Med. 2021;384:252–260. doi: 10.1056/NEJMoa2031054. [DOI] [PubMed] [Google Scholar]
- 27.Naehrig S, Chao CM, Naehrlich L. Cystic fibrosis—diagnosis and treatment. Dtsch Arztebl Int. 2017;114:564–574. doi: 10.3238/arztebl.2017.0564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alton E, Armstrong DK, Ashby D, et al. Repeated nebulisation of non-viral CFTR gene therapy in patients with cystic fibrosis: a randomised, double-blind, placebo-controlled, phase 2b trial. Lancet Respir Med. 2015;3:684–691. doi: 10.1016/S2213-2600(15)00245-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yan Z, McCray PB Jr, Engelhardt JF. Advances in gene therapy for cystic fibrosis lung disease. Hum Mol Genet. 2019;28:R88–R94. doi: 10.1093/hmg/ddz139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schorling DC, Pechmann A, Kirschner J. Advances in treatment of spinal muscular atrophy—new phenotypes, new challenges, new implications for care. J Neuromuscul Dis. 2020;7:1–13. doi: 10.3233/JND-190424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Novartis. Zolgensma® data shows rapid, significant, clinically meaningful benefit in SMA including prolonged event- free survival, motor milestone achievement and durability now up to 5 years post-dosing. www.avexis.com/Content/pdf/newsarticle-IVasset-20200323.pdf (last accessed on 8 August 2020) [Google Scholar]
- 32.Vill K, Kolbel H, Schwartz O, et al. One year of newborn screening for SMA—results of a german pilot project. J Neuromuscul Dis. 2019;6:503–515. doi: 10.3233/JND-190428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pechmann A, Konig K, Bernert G, et al. SMArtCARE—a platform to collect real-life outcome data of patients with spinal muscular atrophy. Orphanet J Rare Dis. 2019;14 doi: 10.1186/s13023-019-0998-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McGettigan P, Alonso Olmo C, Plueschke K, et al. Patient registries: an underused resource for medicines evaluation: operational proposals for increasing the use of patient registries in regulatory assessments. Drug Saf. 2019;42:1343–1351. doi: 10.1007/s40264-019-00848-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stausberg J, Maier B, Bestehorn K, et al. Memorandum Register für die Versorgungsforschung: Update 2019. Gesundheitswesen. 2020;82:e39–e66. doi: 10.1055/a-1083-6417. [DOI] [PubMed] [Google Scholar]
- 36.Gemeinsamer Bundesausschuss. G-BA konkretisiert Verfahren zu anwendungsbegleitender Datenerhebung - Gentherapie Zolgensma erster Fall. www.g-ba.de/presse/pressemitteilungen/874/ (last accessed on 20 July 2020) [Google Scholar]
- 37.Korzilius H. Arzneimittel: Modelle für gerechtere Preise. Dtsch Arztebl. 2019;116 A-503. [Google Scholar]
- 38.European Medicine Agency. PRIME: priority medicines. www.ema.europa.eu/en/human-regulatory/research-development/prime-priority-medicines (last accessed on 26 February 2020) [Google Scholar]
- 39.Buning H, Srivastava A. Capsid modifications for targeting and improving the efficacy of AAV vectors. Mol Ther Methods Clin Dev. 2019;12:248–265. doi: 10.1016/j.omtm.2019.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.World Health Organization. Nomenclature schemes for advanced therapies. www.who.int/medicines/services/inn/Nomenclature_schemes_advanced_therapies_201707.pdf (last accessed on 20 July 2020) [Google Scholar]