Abstract
Relapse to smoking occurs at higher rates in women compared to men, especially when triggered by stress. Studies suggest that sex-specific interactions between nicotine reward and stress contribute to these sex differences. Accordingly, novel treatment options targeting stress pathways, such as guanfacine, an α2-adrenergic receptor agonist, may provide sex-sensitive therapeutic effects. Preclinical studies are critical for elucidating neurobiological mechanisms of stress-induced relapse and potential therapies, but rodent models of nicotine addiction are often hindered by large behavioral variability. In this study, we used nicotine conditioned place preference to investigate stress-induced reinstatement of nicotine preference in male and female mice, and the effects of guanfacine on this behavior. Our results showed that overall, nicotine induced significant place preference acquisition and swim stress-induced reinstatement in both male and female mice, but with different nicotine dose-response patterns. In addition, we explored the variability in nicotine-dependent behaviors with median split analyses and found that initial chamber preference in each sex differentially accounted for variability in stress-induced reinstatement. In groups that showed significant stress-induced reinstatement, pretreatment with guanfacine attenuated this behavior. Finally, we evaluated neuronal activation by Arc immunoreactivity in the infralimbic cortex, prelimbic cortex, anterior insula, basolateral amygdala, lateral central amygdala, and nucleus accumbens core and shell. Guanfacine induced sex-dependent changes in Arc immunoreactivity in the infralimbic cortex and anterior insula. This study demonstrates sex-dependent relationships between initial chamber preference and stress-induced reinstatement of nicotine conditioned place preference, and the effects of guanfacine on both behavior and neurobiological mechanisms.
Keywords: nicotine, conditioned place preference, sex differences, stress, reinstatement, guanfacine
1. INTRODUCTION
Tobacco smoking remains a major public health problem 1. Despite the availability of smoking cessation medications, more than 70% of smokers who make a quit attempt relapse within a year 2. Notably, clinical and epidemiological studies demonstrate significant sex differences in relapse behavior 3. Women showed 31% greater odds of relapse to smoking over a 30-day period compared to men 4, and 44% greater odds over a 3-year period 5.
Nicotine, the primary psychoactive component in tobacco, is a relatively weak primary reinforcer 6,7. However, nicotine can also enhance the rewarding properties of nonpharmacological stimuli 6,8,9, enhance cognitive function 10, and interact with stress and anxiety 11–13 to support addiction-related behaviors. Interactions between these various effects of nicotine may account for the well-documented inter-individual variability in nicotine use and relapse observed in both human studies and rodent models 13–18. Further, individual differences may interact with sex to affect nicotine use. For example, women are less sensitive to the primary reinforcing effects of nicotine than men 19, but are more motivated by nicotine’s effects on stress and negative affect 19–21. Stress is a well-established, sex-divergent factor contributing to relapse to nicotine use 12,22,23. Greater negative affect is reported by women than men during abstinence from nicotine, and this negative affect is a greater predictor of lapse or relapse in women 24–26. Women are also more likely to relapse in response to stressful life events 27. Accordingly, smoking cessation medications also vary in effectiveness between men and women. Nicotine replacement therapies are less effective in women than in men 28, and clonidine, an α2-adrenergic receptor (α2-AR) agonist, is more effective in women than men for achieving smoking cessation 29.
Preclinical studies have demonstrated the role of noradrenaline and α2-ARs in stress-induced drug self-administration 22,30,31 and conditioned place preference (CPP) 32,33. CPP is one useful model to study the rewarding effects of drugs because it involves a short training period relative to self-administration, allows testing in drug-free states, and is adaptable to various species 34. Although stress-induced reinstatement of nicotine seeking has been demonstrated in both self-administration 35,36 and CPP 37–39, only self-administration studies have examined either the effects of α2-AR agonists on stress-induced reinstatement of nicotine seeking 35,36, or sex differences in stress-induced reinstatement 13,40. Further, no preclinical studies using either self-administration or CPP have examined sex differences in the effect of α2-AR agonists on stress-induced reinstatement, despite clinical evidence suggesting its sex-specific mechanisms 29,41,42.
We therefore established a model to investigate sex differences in stress-induced reinstatement of nicotine CPP in male and female mice, and to determine the effects of guanfacine on reinstatement. Other preclinical studies in male rodents have used clonidine, which induces several adverse side effects including sedation and postural hypotension 41. Guanfacine is an FDA-approved α2-AR agonist that is more specific for the α2A subtype and has less sedative and hypotensive effects than clonidine 43. Furthermore, guanfacine attenuates stress-precipitated nicotine craving and ad libitum smoking in abstinent smokers 44. C3H/HeJ mice were selected for this study because they exhibit an increase in locomotion after acute, systemic nicotine injection, unlike most other strains 45, and because mice less sensitive to the hypolocomotor effects of an acute nicotine injection exhibit greater nicotine-induced conditioned place preference 46. We used a counterbalanced CPP design, where nicotine is paired with both initially preferred and non-preferred chambers across animals (as opposed to a biased design, where nicotine is paired only with the initially non-preferred chamber) in order to avoid pitfalls in interpretation and to leverage any variability in the data for more insight into nicotine CPP mechanisms 34,47,48. We used a forced swim stress to induce reinstatement of nicotine CPP, as has been used previously in male mice 38. Finally, we used immunohistochemistry for the immediate-early gene Arc 49 to identify brain regions that are potential substrates for sex differences in the effects of nicotine, stress, and guanfacine.
2. METHODS
2.1. Animals
Male and female C3H/HeJ mice were obtained from The Jackson Laboratory (Bar Harbor, ME, USA) at 6-10 weeks of age, and tested at 10-12 weeks of age, following at least one week of acclimation. Mice were group-housed, maintained on a 12-hour light-dark cycle (lights on at 7:00 AM), and provided standard chow and water ad libitum.
Dose-response, guanfacine, and Arc immunohistochemistry experiments were conducted in independent groups of mice. Sample sizes were determined based on preliminary data, previous studies of sex differences in nicotine CPP 50 and immunohistochemical markers of neuronal activity 51, and a power analysis for behavioral experiments indicating a sample size of approximately 12 mice to detect a moderate effect size (d = 0.3, β = 80%, α = 0.05). All procedures were approved by the Yale University Institutional Animal Care and Use Committee.
2.2. Drugs
Nicotine hydrogen tartrate salt (Sigma-Aldrich, St. Louis, MO, USA) was dissolved in saline and injected subcutaneously (s.c.) at free base concentrations of 0.5, 0.75, or 1.0 mg/kg at 10 ml/kg. Guanfacine (Sigma-Aldrich, St. Louis, MO, USA) was dissolved in saline and injected intraperitoneally (0.15 mg/kg injected at 10 ml/kg).
2.3. Apparatus
CPP training and testing were conducted in a three-chamber CPP apparatus (Med Associates, Inc., Fairfax, VT, USA). The two conditioning chambers are distinguished by tactile cues (flooring consisting of either horizontal or vertical steel rods overlaid by a wire grid) and a single visual cue (opaque tape 1.25 cm x 2.5 cm affixed to the door of one chamber); the interiors of both chambers are black. Manual guillotine doors separate the two conditioning chambers from a neutral, middle, gray chamber with smooth flooring. Med-PC IV software was used to record time spent in each chamber.
2.4. CPP acquisition
All behavioral testing took place in an isolated, dimly lit room with only the experimenter present, between 8 a.m. and 1 p.m. Each day, mice were transferred to the testing room and allowed to acclimate for at least 30 min prior to handling or testing.
On days 1-3, mice were handled as described previously 52, with additional habituation to a syringe (without needle) applied to the back of the neck as if administering a subcutaneous injection. On day 4, the pretest day, each mouse was placed in the middle gray chamber, and after a 5-sec acclimation, the guillotine doors were opened manually. The mice then freely explored all three chambers for 15 min and time spent in each chamber was recorded. Mice that spent > 50% of the time in any of the three chambers were excluded from further evaluation (16/106 males and 14/136 females). Subsequently, conditioning group (control vs. nicotine) and drug-chamber pairings, were pseudo-randomly assigned to achieve a balanced CPP design. On days 5-10, saline or nicotine was injected subcutaneously and the mouse was immediately confined to one conditioning chamber for 30 min. Injection and chamber were alternated between days. Control mice received saline in both conditioning chambers, but one of the saline-paired chambers was arbitrarily labeled as the "drug" chamber solely for data analyses. On day 11, the post-test day, chamber preference was evaluated for 15 min as on the pretest day. Drug preference is expressed as time spent in the drug-paired chamber minus time spent in the saline-paired chamber on test day.
2.5. CPP abstinence, extinction, and reinstatement
Following the post-test, mice were subjected to two weeks of forced abstinence in the home-cage. This abstinence period was first included to reflect the human condition of relapse after abstinence and then empirically tested and found to be necessary for stress-induced reinstatement. Similarly, cue-induced reinstatement of nicotine self-administration has been demonstrated after 7 days of abstinence, but not after 1 day of abstinence 53, and footshock-induced reinstatement of heroin self-administration after 6 and 12 days of abstinence, but not after 1 day of abstinence 54. After the abstinence period, CPP was extinguished by repeated drug-free testing sessions (15-min free exploration). Drug preference on the first day of extinction was considered the “post-abstinence” score, and the last day of extinction was achieved when average drug preference minus the standard error of the mean was below zero. Twenty-four hours after extinction was achieved, mice were tested for stress-induced reinstatement of nicotine CPP by exposure to a 6-min forced swim stress in 21-22°C water, followed by 30 min of recovery in a paper towel-lined cage, then a 15-min test in the CPP apparatus as above. For the dose-response experiment, no injections preceded the swim stress. In guanfacine experiments, mice in each conditioning group were split into two sub-groups with roughly equivalent pretest, acquisition, post-abstinence, and extinction scores, and each group received an injection of either guanfacine (0.15 mg/kg) or saline i.p. 30 min prior to the stressor. The forced swim session was recorded, and videos were subsequently blinded and scored for time spent immobile during the 6-min swim.
2.6. Estrous phase by visualization
Vaginal openings were visually inspected for width of the opening, swelling of the tissue, tissue color and moistness 55. Phase was recorded as “proestrous,” including proestrous and estrous phases, or “non-proestrous,” including diestrous and metestrous phases. We observed a greater proportion of females in non-proestrous phases compared to proestrous phases; this distribution may reflect the Lee-Boot effect, wherein group-housed female mice have been observed to exhibit more irregular estrous cycles with a prolonged diestrous phase 56,57.
2.7. Behavioral conditioning and tissue collection for Arc immunohistochemistry
Separate cohorts of mice were subjected to a parallel behavioral protocol for immunohistochemistry measurements. Briefly, mice were handled for 3 days, then administered alternating injections of nicotine or saline for 6 days, followed immediately by 30 min of isolation in alternating contexts with distinct tactile cues (the smooth floor of an empty mouse cage or a layer of clean bedding). Following conditioning, mice were subjected to 2 weeks of forced abstinence, after which they were administered an injection of guanfacine or saline followed 30 min later by a 6-min forced swim stress, as described above. 90 min after the forced swim stressor, mice were transcardially perfused with ice-cold 1X PBS, then ice-cold 4% paraformaldehyde. Brains were collected and post-fixed in 4% paraformaldehyde overnight, then transferred to 30% sucrose in 1X PBS. Brains were sectioned into 40 µm-thick coronal sections on a freezing sliding microtome (Leica SM 200R) and collected in 1X PBS with 0.02% sodium azide.
2.8. Arc immunohistochemistry
Sections containing the anatomical region of interest were selected from every 6th section of the entire brain for stereological counting and were stained using a standard protocol 58. Regions selected include the anterior insula (AI), infralimbic cortex (IL), prelimbic cortex (PL), nucleus accumbens (NAc) core and shell, basolateral amygdala (BLA), and lateral nucleus of the central amygdala (CeL) (Figure S3). Primary antibody incubation occurred overnight at room temperature in 1:1000 guinea pig anti-Arc (Synaptic Systems, Goettingen, Germany). Secondary antibody incubation occurred for 2 hr at room temperature in 1:1000 donkey anti-guinea pig AlexaFluor647 (MilliporeSigma, St. Louis, MO) and was followed by a 5 min incubation in 1:1000 DAPI (Thermo Scientific, Germany). Sections were then mounted on SuperFrost Plus (Fisher Scientific) glass slides. Coverslips were applied with Fluoromount-G mounting solution (Electron Microscopy Sciences, Hatfield, PA) and sealed with clear nail polish. Tissue was processed in batches, with all experimental groups represented in each batch, and results were pooled for final analysis.
2.9. Arc cell counting
Immunohistochemically stained brain sections were imaged with an Olympus FluoView FV10i confocal microscope with a 10X objective. Image files were blinded prior to analysis using the Fiji image processing package 59. A threshold was applied to create a binary image in which the top 2.5% of the signal intensity histogram was considered foreground and the rest background. The Watershed function was applied, separating putatively overlapping cells with a 1 pixel-wide line. Within each ROI, cells were then counted using the Analyze Particles function with a size restriction of 60-3000 μm2. The area of each ROI was also measured in order to calculate Arc-immunoreactive cells per mm2. See Table S1 for n’s of each group.
2.10. Statistical Analysis
GraphPad Prism 8.0 software was used for data analysis and graph production. For all data, outlier analyses were performed using GraphPad’s ROUT function with the standard Q = 1% 60. No outliers were identified in any behavioral dataset. For Arc data, one outlier was removed from the CeL dataset (female, nicotine conditioning group, saline pre-treatment) and one from the IL dataset (female, control conditioning group, guanfacine pre-treatment). Subsequently, data were analyzed within sex by mixed 2-way Repeated Measures ANOVAs. For the dose-response experiment, ANOVAs were followed up with Dunnet's tests within nicotine dose in order to compare drug preference on acquisition, post-abstinence, extinction, and reinstatement days to the pretest preference as a baseline. For guanfacine and Arc experiments, significant (p < 0.05) interactions were followed by Sidak's multiple comparison tests. For median split analyses, 2-way Repeated Measures ANOVAs were conducted within sex with split subgroup as between-subjects factors and test day as within-subjects factors. Significant interactions were followed by Dunnett’s tests to compare test days to the pretest day within split group, and Tukey’s post-hoc test to compare the same test days across split groups. Brown-Forsythe tests were also conducted to test for significant differences in variance using the lawstat package in R (https://CRAN.R-project.org/package=lawstat). All graphs show means with standard error of the mean.
3. RESULTS
3.1. Stress-induced reinstatement of nicotine CPP is demonstrated in male and female mice administered 0.5 mg/kg nicotine, but only in female mice administered 0.75 mg/kg nicotine
Male and female C3H/HeJ mice were administered 0, 0.5, 0.75, or 1.0 mg/kg nicotine in the drug-paired chamber to determine the dose response curves for stress-induced reinstatement of nicotine CPP (Figure 1). In males, a two-way mixed ANOVA revealed a significant effect of test day (F(4, 152) = 4.75, p = 0.001), but no effect of dose (F(3, 38) = 1.51, p = 0.227) and no interaction (F(12, 152) = 1.53, p = 0.119; Figure 1b). Planned post-hoc analyses with Dunnett’s tests comparing preference scores to the pretest preference within dose showed significant stress-induced reinstatement of nicotine CPP only in the 0.5 mg/kg nicotine group (p = 0.0002). At this dose, there was a trend for a positive increase in drug preference between pretest and acquisition (Mdiff = 101.1, 95% CI [5.76, 196.5]). A follow-up analysis of the multiple cohorts of male mice tested in Figures 1, 3, and 4 confirmed that the 0.5 mg/kg dose of nicotine produces significant CPP acquisition and post-abstinence nicotine-chamber preference, followed by successful extinction (Figure 2a,b). A two-way mixed ANOVA with conditioning group (saline control vs. 0.5 mg/kg nicotine) as a between-subjects factor and test day as a within-subjects factor showed a significant interaction (F(3, 213) = 3.58, p = 0.015) and significant main effects of conditioning group (F(1,71) = 9.02, p = 0.0037) and test day (F(3, 213) = 6.14, p = 0.0005). Dunnett’s post-hoc test showed significant differences between pretest and acquisition preference scores (p = 0.0020) and between pretest and post-abstinence (p < 0.001) in animals administered 0.5 mg/kg nicotine, but no significant differences in the control group (pretest vs. acquisition: p = 0.75; pretest vs. post-abstinence: p = 0.96; Figure 2a). Acquisition scores were also examined by cohort to further demonstrate significant CPP acquisition across cohorts, and there was a main effect of conditioning drug (F(1,67) = 7.53, p = 0.0078) but no effect of cohort (F(2,67) = 0.30, p = 0.74) or an interaction (F(2,67) = 1.0, p = 0.37; Figure 2b). Reinstatement data was not pooled across cohorts due to the experimental variable of treatment prior to the forced swim stressor (none for dose-response experiments, i.e. cohort 1, and saline or guanfacine pre-treatment in cohorts 2 and 3).
Figure 1. Dose-response relationships for stress-induced reinstatement of nicotine CPP in male and female mice.
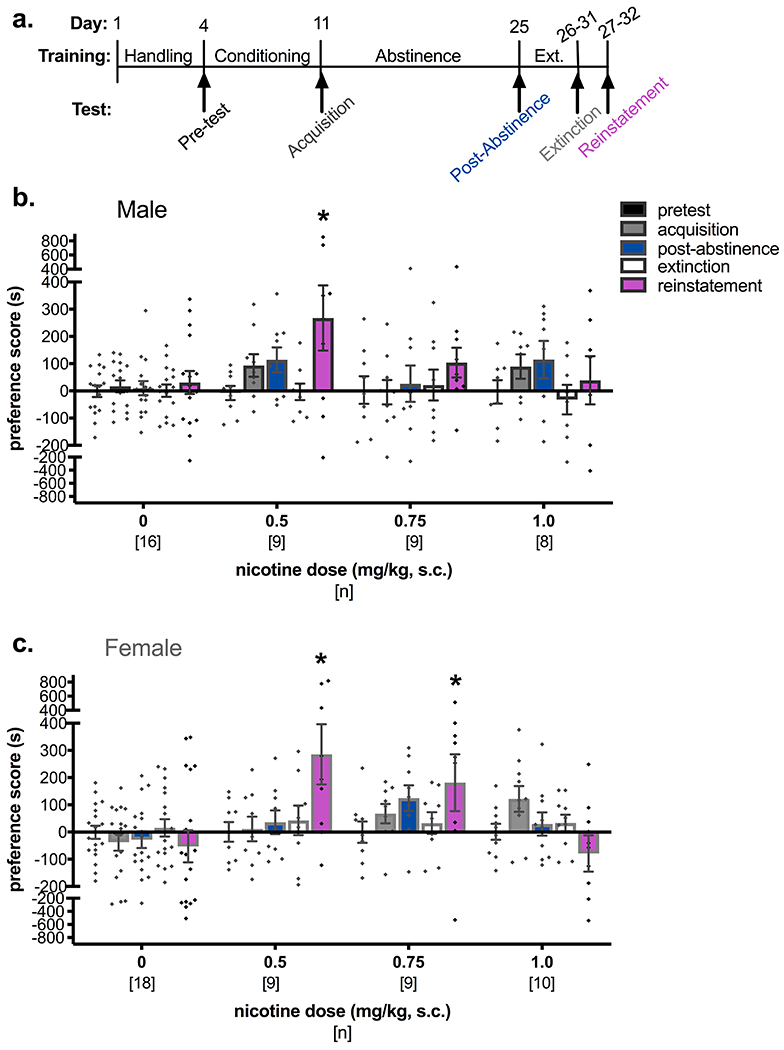
(a) Timeline for CPP conditioning, acquisition, post-abstinence, extinction, and stress-induced reinstatement. Arrows indicate test days for which data are represented in (b) and (c) for male and female mice, respectively. Significant stress-induced reinstatement of nicotine CPP is seen for male mice trained with 0.5 mg/kg nicotine and for female mice trained with 0.5 mg/kg and 0.75 mg/kg nicotine. *p < 0.05 compared to pretest day within nicotine dose using Dunnett’s tests.
Figure 3. Guanfacine produced variable responses to stress-induced reinstatement in male and female mice.
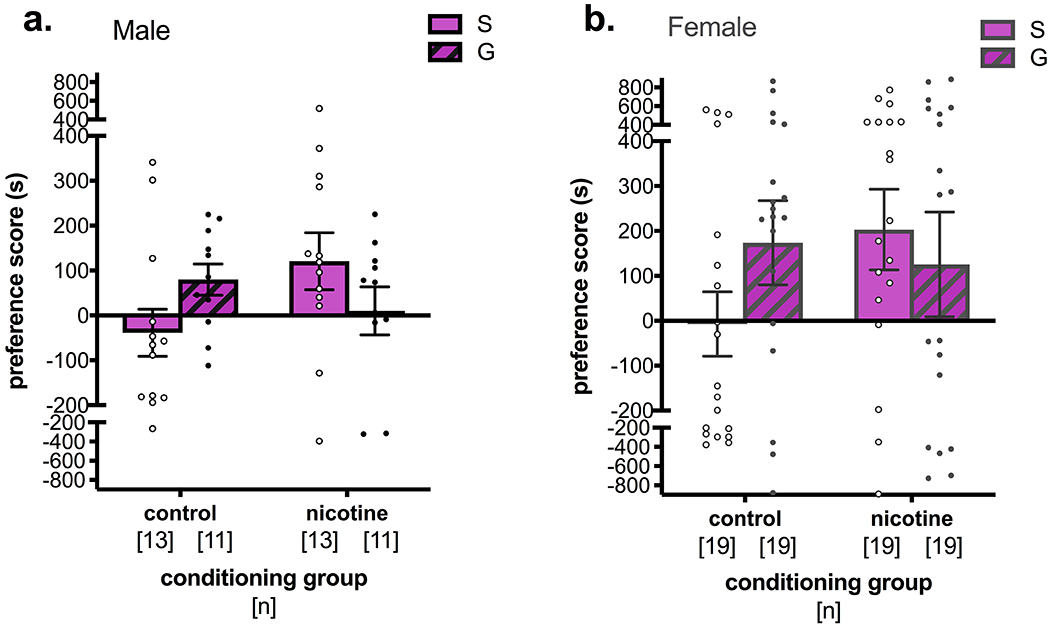
Separate two-way ANOVAs within sex showed (a) a significant interaction of conditioning group and pre-stress treatment in male mice, and a near-significant difference between control- and nicotine-conditioned mice receiving saline pre-treatment. (b) No significant main effects or interactions were observed in female mice. S = saline, G = guanfacine
Figure 4. Median split analyses demonstrate significant interactions between initial chamber preference and subsequent CPP acquisition and stress-induced reinstatement, and significant effects of guanfacine.
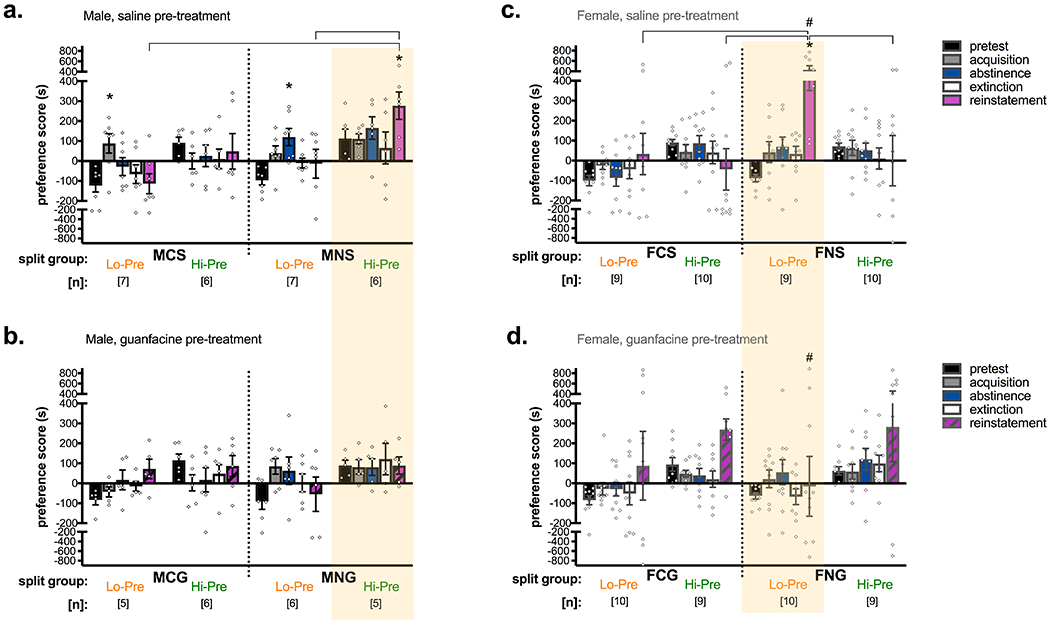
Two-way ANOVAs conducted within sex between split subgroup and test day found significant interactions in both male and female mice. Data were analyzed by split subgroups across pre-treatment conditions, but are presented for male (a,b) and female (c,d) mice separately for saline (a,c) and guanfacine (b,d) pre-treatment for ease of viewing. Further, the yellow box highlights the significant findings regarding stress-induced reinstatement. (a) In male mice, only nicotine treatment in the initially preferred chamber subsequently leads to significant stress-induced reinstatement of nicotine CPP when the swim stress is preceded by a control saline injection. (b) This reinstatement is not significant when male mice in the same split subgroup (i.e. nicotine treatment in the initially preferred chamber) receive guanfacine (0.15 mg/kg, i.p.) prior to the swim stress. (c) In contrast, only female mice with nicotine treatment in the initially non-preferred chamber demonstrate significant stress-induced reinstatement of nicotine CPP with a saline pre-swim treatment, (d) and not with a guanfacine pre-swim treatment. Additionally, these two reinstatement preference scores (FNS Lo-Pre vs. FNG Lo-Pre) are significantly different from each other. *p < 0.05 compared to pretest day within nicotine dose using Dunnett’s tests. Brackets indicate p < 0.05 compared to same test day in different split group using Tukey’s multiple comparison test. #p < 0.05 for comparison to corresponding day, also marked by #, across saline and guanfacine pre-treatment groups by Tukey’s test. MCS = male, control conditioning group, saline pre-treatment prior to stress. MNS = male, nicotine (0.5 mg/kg, s.c.) conditioning group, saline pre-treatment. MCG = male, control conditioning group, guanfacine (0.15 mg/kg, i.p.) pre-treatment. MNG = male, nicotine (0.5 mg/kg, s.c.) conditioning group, guanfacine pre-treatment. FCS = female, control conditioning group, saline pre-treatment. FNS = female, nicotine (0.75 mg/kg, s.c.) conditioning group, saline pre-treatment. FCG = female, control conditioning group, guanfacine (0.15 mg/kg, i.p.) pre-treatment. FNG = female, nicotine (0.75 mg/kg, s.c.) conditioning group, guanfacine pre-treatment. Lo-Pre = initial non-preference for future drug-chamber, Hi-Pre = initial preference for future drug-chamber.
Figure 2. Male and female mice demonstrate significant acquisition of nicotine CPP.
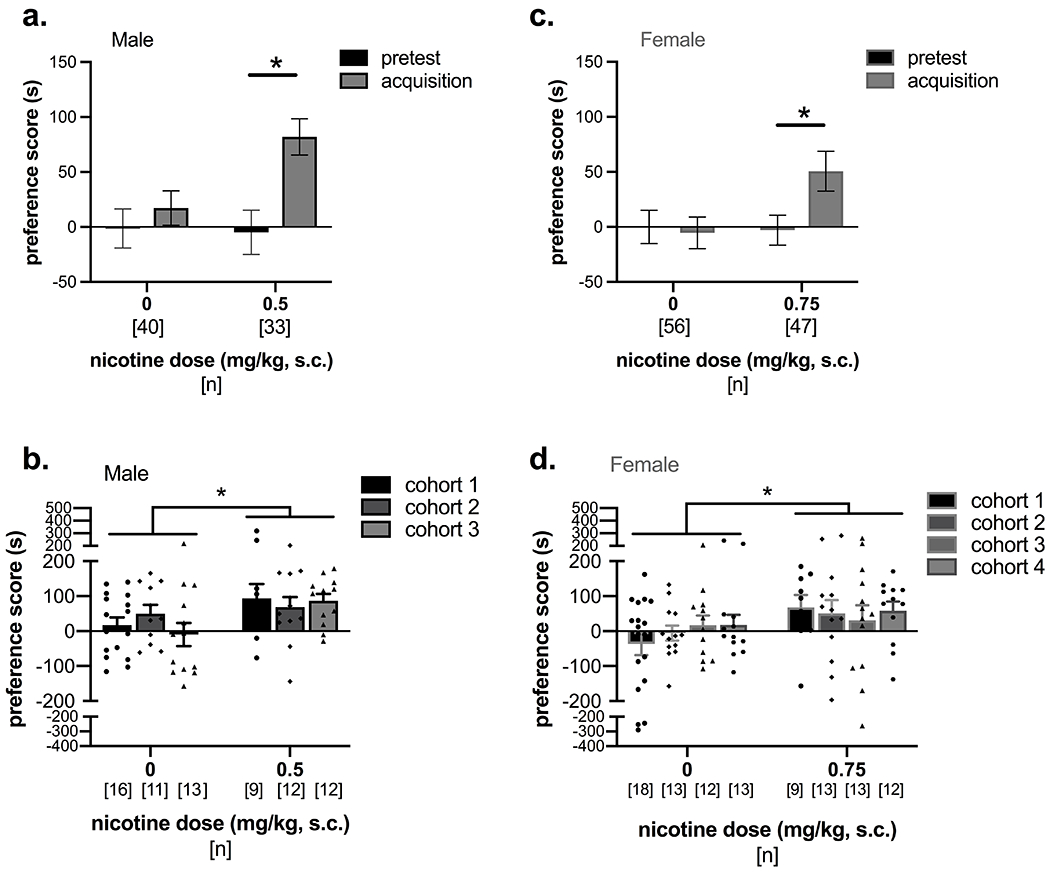
(a) Male mice from all experimental cohorts are combined to demonstrate significant nicotine CPP acquisition and post-abstinence preference, followed by extinction when administered 0.5 mg/kg nicotine. (b) Acquisition scores are shown separately for the control and nicotine conditioning groups of the three independent cohorts of male mice that were combined in (a). Cohort 1 of males is the same as the 0.5 mg/kg group and control mice in Figure 1b, and cohorts 2 and 3 are the same male mice in Figures 3a and 4a-b. There was a significant effect of conditioning group on acquisition score, but no differences by cohort. (c) Female mice from all experimental cohorts are combined to demonstrate significant nicotine CPP acquisition when administered 0.75 mg/kg nicotine. (d) Acquisition scores for control and nicotine groups of the four independent cohorts of female mice combined in (c) show a significant effect of conditioning group, but no differences by cohort. Cohort 1 of females is the same as the 0.75 mg/kg group and control mice in Figure 1c, and cohorts 2-4 are the same mice represented in Figures 3b, 4c-d, and 5. *p < 0.05 compared to pretest day within conditioning group using Dunnett’s tests for (a) and (b), *p < 0.05 main effect of nicotine dose by two-way ANOVA for (c) and (d)
In female mice, a two-way ANOVA across test days and dose revealed a significant interaction (F(12, 168) = 3.23, p = 0.0003) and a significant main effect of dose (F(3, 42) = 3.20, p = 0.033), but no significant main effect of test day (F(4, 168) = 1.66, p = 0.162; Figure 1c). Dunnett’s tests showed significant stress-induced reinstatement of nicotine place preference compared to pretest preference in mice administered 0.5 mg/kg (p = 0.0004) and 0.75 mg/kg (p = 0.041) nicotine. However, the difference in means between pretest and acquisition was greater in the 0.75 mg/kg cohort (Mdiff = 68.5 [95% CI: -35.82, 172.9]) than in the 0.5 mg/kg cohort (Mdiff = 11.04 [95% CI: -113.3, 135.4]). Subsequent cohorts were tested with 0.75 mg/kg nicotine, and follow-up analyses of all cohorts used in our experiments confirmed that treatment with this nicotine dose produced a significant CPP acquisition and post-abstinence preference compared to pretest scores in female mice (Figure 2c,d). A two-way mixed ANOVA showed a significant interaction of test day and conditioning drug (F(3,303) = 3.70, p = 0.012), and Dunnett’s post-hoc test showed a significant increase in preference between pretest and acquisition (p = 0.024) and pretest and post-abstinence tests (p = 0.0007) in the 0.75 mg/kg nicotine group but not in the control conditioning group (pretest vs. acquisition: p = 0.99; pretest vs. post-abstinence: p = 0.97; Figure 2c). Extinction preference scores were not different from pretest preference in either 0.75 mg/kg nicotine or control conditioning groups. Further comparison of acquisition scores by the various cohorts showed a main effect of conditioning group (F(1,95) = 5.30, p = 0.024) but no main effect of cohort (F(3,95) = 0.16, p = 0.92) or an interaction (F(3,95) = 0.65, p = 0.56; Figure 2d).
Previous studies have shown that female rats and mice display a right-shifted dose-response curve for nicotine CPP 50,61,62. Thus testing for the effects of guanfacine on stress-induced reinstatement proceeded with the use of 0.5 mg/kg nicotine to train male mice and 0.75 mg/kg nicotine to train female mice.
3.2. The effect of guanfacine is not significant in whole-cohort analyses
Male and female mice were tested for stress-induced reinstatement of nicotine CPP as above, with the addition of saline or guanfacine (0.15 mg/kg, i.p.) treatment 30 min prior to the forced swim stressor. There was a sex difference in the degree of variance in reinstatement preference scores, as shown by the Brown-Forsythe test (male vs. female: test statistic = 23.39, p < 0.0001). There were no differences in variance between conditioning groups (control vs. nicotine: test statistic = 1.015, p = 0.316) or pre-stress treatments (saline vs. guanfacine: test statistic = 0.469, p = 0.495).
Due to the significant difference in variance and the different nicotine doses used in male (0.5 mg/kg) and female (0.75 mg/kg) mice, separate two-way ANOVAs were conducted within sex. In males, there was a significant interaction between conditioning group and pre-stress treatment (F(1, 44) = 4.57, p = 0.038), but no main effects of conditioning group (F(1, 44) = 0.703, p = 0.406) or pre-stress treatment (F(1, 44) = 0.0056, p = 0.941; Figure 3a). Post-hoc analyses with Sidak’s multiple comparisons tests showed an almost significant stress-induced reinstatement of nicotine CPP in nicotine vs. control male mice receiving saline pre-treatment (t(44) = 2.20, p = 0.066), but not in male mice receiving guanfacine pre-treatment (t(44) = 0.882, p = 0.619). In females, a two-way ANOVA showed no significant main effects of conditioning group (F(1, 44) = 0.738, p = 0.393) or pre-stress treatment (F(1, 44) = 0.301, p = 0.585) or an interaction (F(1, 44) = 1.88, p = 0.175; Figure 3b).
Immobility during the forced swim stressor was analyzed with two-way ANOVAs within sex. Results showed that guanfacine treatment did not change time spent immobile during the forced swim in either males (Figure S1a) or females (Figure S1b), although there was a main effect of nicotine treatment in lowering immobility in males (F(1, 44) = 17.93, p = 0.0001) but not females.
This initial analysis of the data suggests that guanfacine did not significantly attenuate stress-induced reinstatement in either male or female mice, although there was a significant interaction and a trending post-hoc test in male mice. However, there was a notable degree of variability in individuals’ preference scores in the reinstatement test. Subsequently, we further examined this variability to determine whether results might vary based on specific subgroups of mice.
3.3. Initial chamber preference interacts with stress and guanfacine on nicotine CPP reinstatement: male mice
To explore the variability in CPP reinstatement in both male and female mice, we conducted median split analyses, where subjects within each treatment group, comprised of the same sex (M = male, F = female), conditioning group (C = control [saline], N = nicotine), and pre-swim treatment (S = saline, G = guanfacine) were split into two subgroups across the median of preference scores on the pretest day. The “low” subgroup spent less time in, or avoided, the future drug-paired chamber on pretest day (Lo-Pre), and the “high” subgroup demonstrated an initial preference for the future drug-paired chamber (Hi-Pre). A two-way ANOVA was then conducted with median split subgroups across conditioning groups and pre-swim treatments within sex as a factor, and all test days as the second factor. Although the analyses were conducted across all split subgroups within sex, data are presented in Figure 4 as separate for saline and guanfacine pre-treatment subgroups for ease of viewing.
In males, there was a significant interaction between initial chamber preference subgroup and test day (F(28,160) = 1.93, p = 0.0062) and a significant main effect of subgroup (F(7,40) = 5.15, p = 0.0003) but not test day (F(4,160) = 1.92, p = 0.11; Figure 4a,b). The significant interaction was followed by Dunnett’s tests to compare the pretest day to each subsequent test day within subgroup, and Tukey’s tests to compare the same test days across subgroups. In the Lo-Pre nicotine-treated subgroups, preference for the nicotine-paired chamber over the saline-paired chamber (preference score) after acquisition was significantly different from pretest in the subgroup that would subsequently receive guanfacine prior to stress-induced reinstatement (MNG Lo-Pre; p = 0.032; Figure 4b), and nearly significant in the subgroup that would subsequently receive saline prior to stress-induced reinstatement (MNS Lo-Pre; p = 0.094; Figure 4a). Similar, or even greater, average preference score for the nicotine-paired chamber was measured after acquisition in the Hi-Pre MNG and MNS subgroups, but these were not significantly different from pretest, as was expected given the high pretest preference scores. In contrast, Hi-Pre control conditioning subgroups (MCS Hi-Pre and MCG Hi-Pre) show acquisition test preference scores that trend towards zero (Figure 4a,b). Further, the MCG Lo-Pre control group also shows an average preference score on acquisition testing that trends towards zero, and MCS Lo-Pre shows a positive preference score on acquisition testing that is significantly different from pretest (p = 0.0028; Figure 4a). This contrast between control and nicotine conditioning groups supports the idea that nicotine-specific processes drive chamber preference. Further, preference for the nicotine-paired chamber is maintained after two weeks of abstinence; in MNS Lo-Pre, post-abstinence preference is even enhanced over the acquisition score and is significantly different from pretest (p = 0.002; Figure 4a).
Finally, stress-induced reinstatement was significantly different from pretest only for MNS Hi-Pre (p = 0.049; Figure 4a), and this reinstatement was significantly different from reinstatement in MCS Lo-Pre (p < 0.0001), MNS Lo-Pre (p = 0.0046), and MNG Lo-Pre (p = 0.0007). These data show that only male mice that received nicotine in the initially preferred chamber subsequently demonstrate stress-induced reinstatement of nicotine CPP, despite evidence of initial CPP acquisition and post-abstinence maintenance of nicotine-chamber preference in all nicotine-treated mice. Importantly, guanfacine attenuates this stress-induced reinstatement in Hi-Pre nicotine-treated animals (Figure 4a,b; yellow highlight). The raw data for time spent in the saline-paired chamber and the drug-paired chamber are shown in Figure S2 and were divided into the same Lo-Pre and Hi-Pre groups as in Figure 4a,b (i.e. based on pretest difference score). Differences between time spent in the saline-paired chamber and the drug-paired chamber were analyzed by paired two-tailed t-tests with Holm-Sidak corrections for multiple tests across all test days and split subgroups within sex. Analyzing raw chamber times, similar to analyzing difference scores, showed that there was significant reinstatement only in MNS Hi-Pre, and not in MNG Hi-Pre, MNS and MNG Lo-Pre, or any control group (Figure S2a,b).
3.4. Initial chamber preference interacts with stress and guanfacine on nicotine CPP reinstatement: female mice
In contrast to median split analyses by pretest preference in male mice, female mice show an effect of nicotine treatment in the initially non-preferred chamber. Overall, there was a significant interaction between split subgroup and test day (F(28,272) = 1.59, p = 0.033) and significant main effects of split subgroup (F(7,68) = 3.45, p = 0.0032) and test day (F(4,272) = 5.22, p = 0.0005; Figure 4c,d). Post-hoc analyses within and across split subgroups show that the effects of initial chamber preference on acquisition and post-abstinence scores were significant in male but not female mice; there is a similar pattern between pretest, acquisition, and post-abstinence preference scores between female nicotine-treated (FNS and FNG) and male nicotine-treated (MNS and MNG) groups, but no significant post-hoc comparisons in the FNS and FNG groups. However, significant stress-induced reinstatement compared to pretest preference was only observed in the FNS Lo-Pre group, i.e. female mice administered nicotine in the initially non-preferred chamber and treated with saline prior to the swim stressor (p < 0.0001; Figure 4c). Reinstatement in the FNS Lo-Pre group was also significantly different from reinstatement in FCS Lo-Pre (p = 0.0088), FCS Hi-Pre (p = 0.0002), and FNS Hi-Pre (p = 0.0012) groups. Further, guanfacine significantly attenuated stress-induced reinstatement in the parallel Lo-Pre group (FNG Lo-Pre; p = 0.0006 vs. FNS Lo-Pre; Figure 4c,d yellow highlight). Interestingly, there was also trend for guanfacine treatment to increase preferred-chamber preference in both FCG Hi-Pre and FNG Hi-Pre (p = 0.073 vs. pretest) groups (Figure 4d). As for males, analyzing raw chamber times for female mice produced similar findings as did analyses by difference scores, i.e. significant reinstatement in FNS Lo-Pre and no significant reinstatement in FNG Lo-Pre groups, and an increase in reinstated drug-chamber preference that was significant in the FCG Hi-Pre group and observable, though not significant, in the FNG Hi-Pre group (Figure S2c,d).
3.5. Estrous cycle did not affect preference scores in female mice
To determine whether estrous phase also contributed to the variability in preference scores observed in female mice, data were reorganized by estrous phase as measured at several points during the CPP procedure. Estrous phase was dichotomized to proestrous and estrous (p&e) or diestrous and metestrous (d&m). Preference scores were then analyzed by two-way ANOVA, with estrous phase and conditioning group as between-subjects factors. There were no significant effects of estrous phase on acquisition scores, based on estrous phase during conditioning or on acquisition test day (Figure 5a,b). There were also no effects of estrous phase during post-abstinence testing or reinstatement testing on post-abstinence or reinstatement preference scores, respectively (Figure 5c,d).
Figure 5. Estrous phase did not affect acquisition, post-abstinence, or reinstatement preference scores.
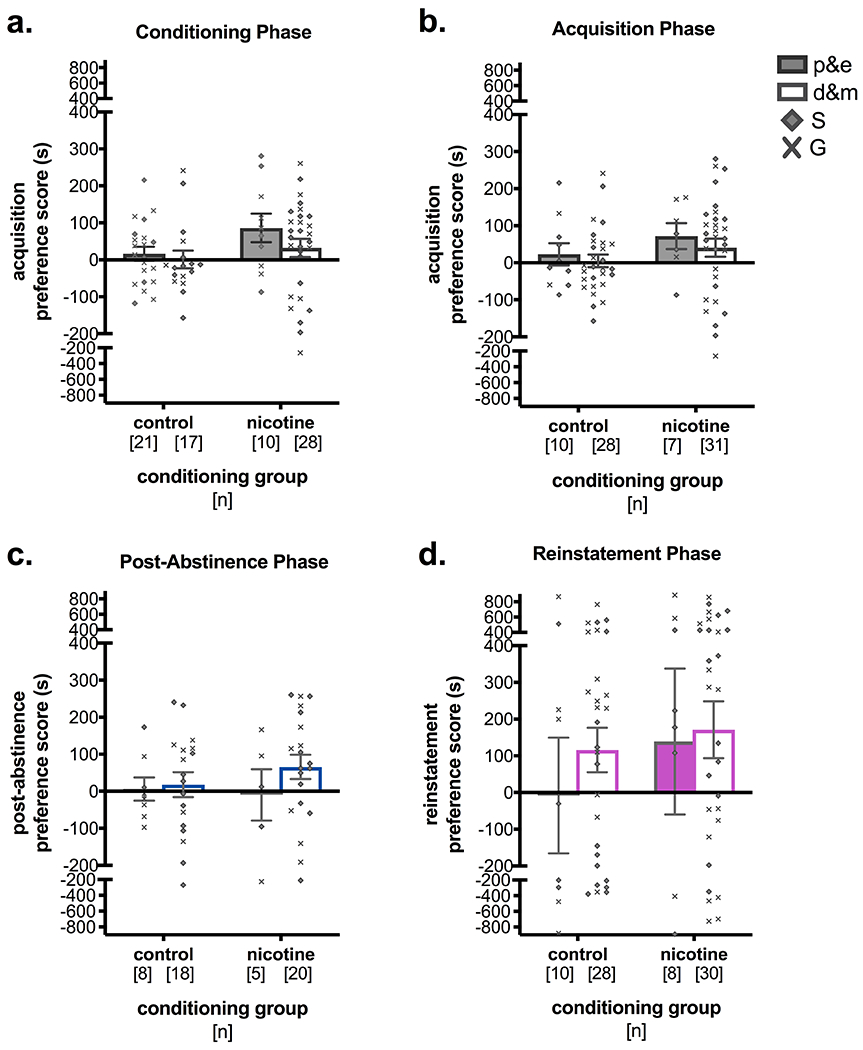
Estrous phase in female mice was measured at key points in the CPP procedure and its relationship to CPP behaviors was examined. (a) Estrous phase was measured at the midpoint of conditioning, the morning prior to conditioning day 4 of 6, and was found to have no influence on CPP acquisition in either control or nicotine (0.75 mg/kg, s.c.) conditioning groups. (b) Similarly, estrous phase on acquisition test day also did not affect CPP preference scores. (c) Estrous phase was also measured after 2 weeks of abstinence, and did not have a significant effect on post-abstinence CPP preference scores. (d) Finally, estrous phase as measured on reinstatement test day did not have a significant effect on stress-induced reinstatement of nicotine CPP. There was also no effect of saline vs. guanfacine pre-treatment on the relationship between estrous phase and reinstatement scores. p&e = proestrous and estrous, d&m = diestrous and metestrous, S = saline, G = guanfacine. *p < 0.05
3.6. Guanfacine had varying effects on Arc expression across brain regions in male and female mice
To gain insight into brain areas potentially involved in sex-specific responses to guanfacine in the context of nicotine treatment and stress, we examined Arc expression in separate cohorts of male and female mice. These mice were subjected to a parallel schedule of nicotine and saline injections in distinct contexts as in CPP behavioral cohorts, followed by two weeks of abstinence and a forced swim stress that was preceded by saline or guanfacine pre-treatment. In subcortical areas, guanfacine had similar effects on Arc expression in male and female mice (see Table S2 for all statistical results, and Figure S3 for representative images). In the BLA, 2-way ANOVAs within sex revealed no significant interactions or main effects of conditioning group, although there were trending main effects of pre-stress treatment in the same direction in both sexes (males: F(1, 20) = 3.86, p = 0.064; females: F(1, 20) = 2.48, p = 0.131; Figure 6a,b). In the CeL, there were significant main effects of conditioning group (male: F(1,20) = 5.17, p = 0.034; female: F(1,19) = 22.11, p = 0.0002) and pre-stress treatment (male: F(1,20) = 9.14, p = 0.007; female: F(1,19) = 8.59, p = 0.008) in both male and female mice, such that CeL Arc immunoreactivity was lower in nicotine-treated compared to control subjects, and higher in guanfacine- vs. saline-treated subjects (Figure 6c,d). There were no significant interactions. In the NAc core, as in the BLA, there were trends toward main effects of conditioning group in the same direction in male and female mice, but neither reached significance (male: F(1,20) = 2.73, p = 0.114; female: F(1,20) = 4.26, p = 0.052; Figure 6e,f). In the NAc shell, there were no significant main effects of conditioning group or pre-stress treatment, and no significant interactions in either male or female mice (Figure 6g,h).
Figure 6. Arc immunoreactivity in subcortical areas of nicotine- or saline-treated mice with or without guanfacine administration before a swim stress.
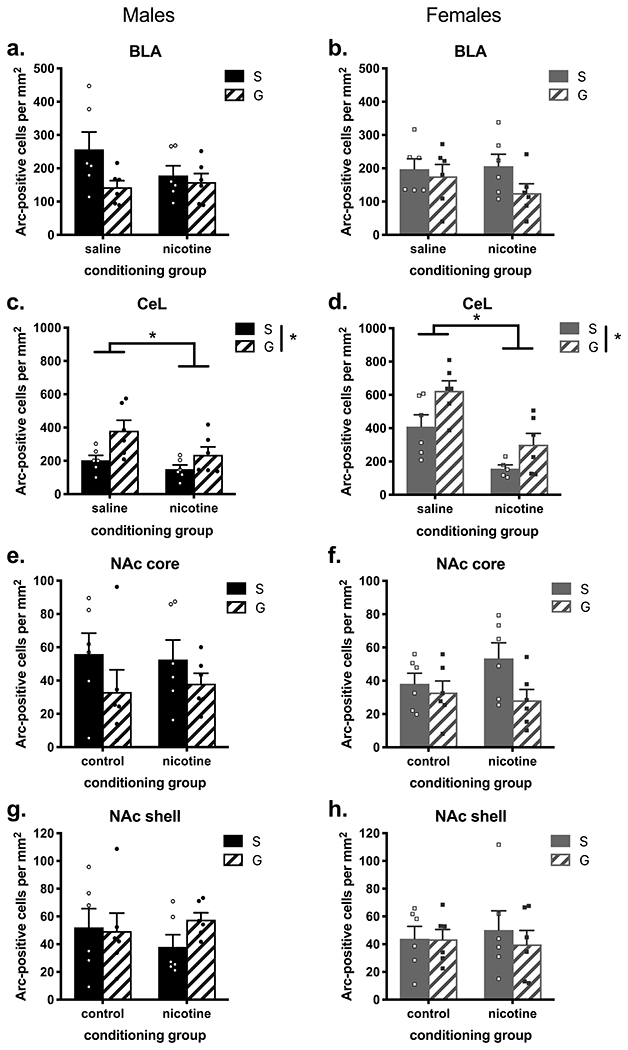
Guanfacine appeared to reduce Arc-immunoreactivity in the BLA of (a) male mice and (b) female mice overall, but neither effect was significant. In the CeL of both (c) male and (d) female mice, nicotine significantly decreased Arc-immunoreactivity, and guanfacine significantly increased Arc-immunoreactivity. In the NAc core, similar to the BLA, guanfacine appeared to decrease mean Arc-immunoreactivity in (e) male mice and (f) female mice, but neither effect was significant. (g, h) There were no main effects of conditioning group or pre-stress treatment and no interaction effects in the NAc shell of male and female mice. n = 5-6 per group. S = saline, G = guanfacine, *p < 0.05
In cortical areas, there were sex-convergent as well as sex-divergent effects of guanfacine on Arc-immunoreactivity. In the IL, there was a significant main effect of pre-stress treatment in male mice (F(1,20) = 6.43, p = 0.020) where guanfacine increased Arc-immunoreactivity (Figure 7a). In contrast, there was a near-significant main effect of pre-stress treatment in female mice in IL (F(1,19) = 3.69, p = 0.070), where guanfacine decreased Arc-immunoreactivity (Figure 7b). In the PL, there were no significant main effects of conditioning group or pre-stress treatment, and no significant interactions in either male or female mice (Figure 7c,d). In the AI, guanfacine decreased Arc-immunoreactivity in male mice with a near-significant effect (F(1,20) = 4.27, p = 0.052; Figure 7e), but no effect was seen in female mice (F(1,20) = 0.26, p = 0.614; Figure 7f).
Figure 7. Arc immunoreactivity in cortical areas of nicotine- or saline-treated mice with or without guanfacine administration before a swim stress.
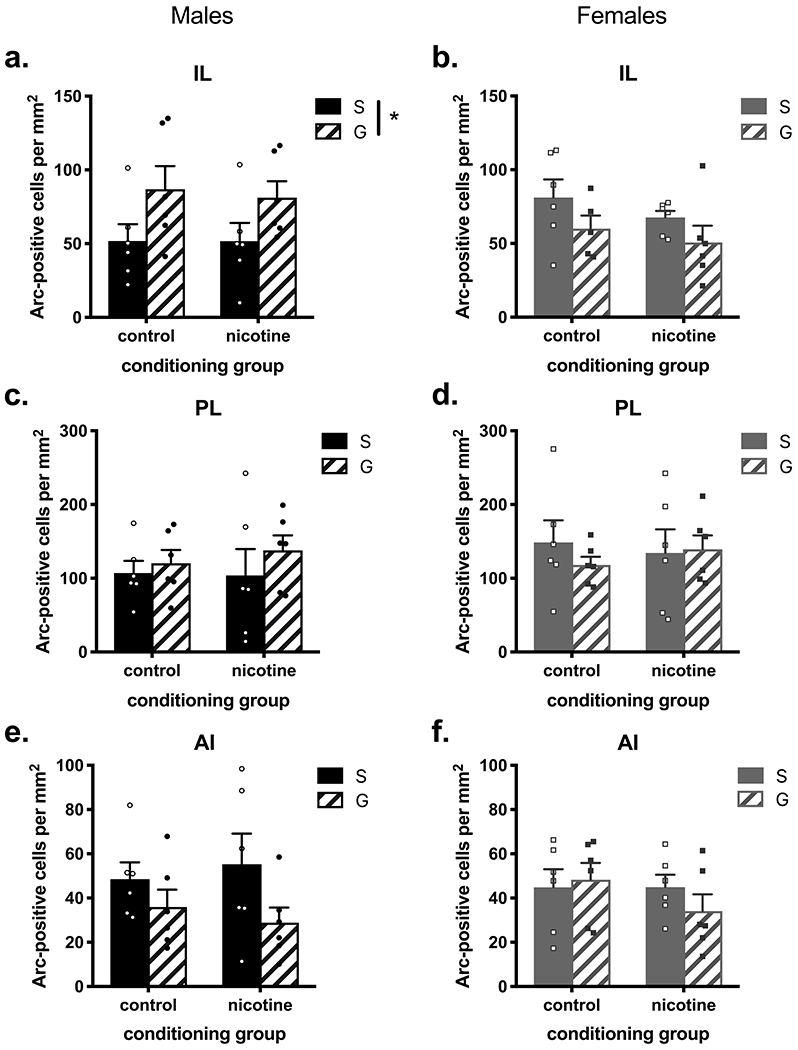
(a) Arc-immunoreactivity in the IL showed a main effect of pre-stress treatment in male mice, and a near-significant effect in (b) female mice but in the opposite direction. (c, d) In the PL, no significant main effects or interactions of conditioning group and pre-stress treatment were found in either sex. (e) In the AI, there was a near-significant main effect of pre-stress treatment in male mice, (f) but no main effect of either conditioning group or pre-stress treatment and no interaction effect in female mice. n = 5-6 per group. S = saline, G = guanfacine, *p < 0.05
4. DISCUSSION
In the current study, we examined stress-induced reinstatement of nicotine CPP in male and female mice and found sex-divergent CPP behaviors in nicotine dose response, especially in interaction with initial chamber preference, and sex-divergent patterns of Arc immunoreactivity in the AI and IL areas of frontal cortex.
First, we found that female mice demonstrate CPP acquisition and stress-induced reinstatement when administered a higher nicotine dose (0.75 mg/kg) than male mice (0.5 mg/kg), consistent with previous studies showing right-shifted dose-response curves for CPP acquisition in female mice and rats compared to males 50,61,62. In our study, CPP acquisition was statistically significant only when cohorts were pooled for analysis (Figure 2). However, the consistent acquisition scores across independently tested cohorts strongly suggests the effect of nicotine is real despite being small (Figure 2b,d). Nicotine is known to be a relatively weak primary reinforcer 6,7 with well-documented variability in the expression of nicotine reward-related behaviors 13,15–18,52,63,64. While previous studies have reported significant nicotine CPP acquisition with n < 12, the small effect size in our study compared to others is likely due to a combination of strain differences 45,52 and the use of counterbalanced vs. biased procedures 65, as discussed further below. Other factors that may contribute are the use of two- vs. three-chamber CPP apparatuses 61, visual vs. textural cues to distinguish the two conditioning compartments 37, and schedule of conditioning sessions 66. Although female mice also showed significant reinstatement when administered 0.5 mg/kg nicotine, there was no indication of CPP acquisition with this dose (Figure 1c), and male mice did not show significant reinstatement when administered 0.75 mg/kg nicotine (Figure 1b). Furthermore, the degree of CPP acquisition and reinstatement at 0.5 mg/kg for males and 0.75 mg/kg for females was not quantitatively different. Only nicotine self-administration studies have compared male and female rats in stress-induced reinstatement 13,40, and these studies found no sex differences when footshock 13 or yohimbine 40, an α2-AR antagonist, was used as a stressor.
Our study design produced a rich behavioral dataset in the various days on which CPP was measured and in the variability in preference scores. This variability in response to nicotine has been well-documented 13,15–18,52,63,64. Our study further explored a potential source of variability with median split analyses based on pretest preference scores. Our balanced CPP design, where nicotine was paired with initially preferred (Hi-Pre) or non-preferred chambers (Lo-Pre) between mice, allowed a virtually even split between two behaviorally meaningful categories. Initial chamber preference is thought to reflect unconditioned anxiogenic responses to the initially non-preferred chamber, such that post-conditioning acquisition of preference for an initially non-preferred chamber may reflect a combination of unconditioned habituation, drug-mediated non-avoidance, and drug-dependent reward or approach 34,48,67. First, our split analyses showed that habituation does factor into post-conditioning preferences (Figure 4, control Lo-Pre). However, nicotine-specific conditioning effects, beyond habituation alone, were observed in the positive acquisition scores in both nicotine Lo-Pre and Hi-Pre groups vs. in control Lo-Pre groups only, as well as in positive post-abstinence scores for nicotine but not control groups (Figure 4). The difference between acquisition and pretest scores in nicotine Hi-Pre groups are not significant, however, likely due to a ceiling effect 68. These patterns were similar between males and females, but more robust in males. Overall, these analyses show that a biased CPP design produces stronger CPP acquisition, as previously demonstrated 63,64, and that post-abstinence preference scores can help distinguish specific nicotine effects from habituation.
The split analyses further present novel and striking findings on sex-dependent interactions between initial chamber preference and subsequent stress-induced reinstatement. These data can be interpreted within the framework of previous hypotheses about sex differences in the effect of nicotine on various behavioral processes. For example, regulation of negative affect seems to be a more prominent motivation for smoking and relapse in women 20,21,42. Our data show that stress-induced reinstatement of nicotine CPP is only observed in female mice when nicotine is paired with the initially non-preferred or anxiogenic chamber, suggesting that nicotine’s ability to support addiction-related behaviors in female mice may depend to some extent on its anxiolytic properties. However, studies on nicotine’s anxiolytic effects are mixed, showing either increased 69–71 or decreased anxiolysis 72,73 in female rodents compared to males. On the other hand, male mice only show stress-induced reinstatement when nicotine is paired with the initially preferred chamber. These data suggest that nicotine’s role as a reinforcement enhancer may be more significant for males 6,8. In other words, nicotine enhances the rewarding effects that drove initial chamber preference in male mice, such that stress reinstates CPP only to the initially preferred chamber. However, sex differences were not observed in nicotine’s reward-enhancing effects in a self-administration paradigm 74, suggesting that this sex difference may specifically interact with stress-induced reinstatement.
Another interesting finding from the split analyses was the dissociation between initial CPP acquisition and subsequent stress-induced reinstatement. In males, nicotine Lo-Pre groups exhibited significant CPP acquisition or post-abstinence nicotine preference scores, but there was no significant stress-induced reinstatement (Figure 4a, b). On the other hand, CPP acquisition and post-abstinence scores were not statistically significant in MNS Hi-Pre mice, due to the high pretest preference, but there was significant CPP reinstatement. Similarly in female mice, CPP acquisition and post-abstinence scores were not significant in nicotine Lo-Pre groups, despite significant reinstatement. These data not only illustrate considerations related to CPP design, as discussed above, but also suggest there may be distinct neurobiological mechanisms mediating initial reward, maintenance of craving, and stress-induced reinstatement 75,76. For example, the noradrenergic system is a potential substrate for these distinct mechanisms, and studies with morphine and heroin have shown that the locus coeruleus and medullary noradrenergic nuclei are differentially involved in withdrawal and stress-induced reinstatement 33,75,77. Also of interest are how these mechanisms interact with sex. Even something as seemingly fundamental as dopamine release in response to nicotine has been shown to differ in nuanced ways between male and female rats 78, and men and women who smoke 79.
When examining responses to guanfacine overall, there was a significant interaction but no significant post-hoc findings in males, and no significant interaction or post-hoc comparisons in females (Figure 3). In contrast to these whole-cohort analyses, our split analyses showed that guanfacine blocked stress-induced reinstatement in both sexes, but only in the sex-specific conditions under which significant reinstatement was observed with saline pre-treatment (i.e. male Hi-Pre, female Lo-Pre; Figure 4 yellow highlights). These results are, to our knowledge, the first demonstration that guanfacine attenuates stress-induced reinstatement of nicotine CPP in male and female mice, however these effects seem to be limited to specific nicotine pairing parameters. Moreover, our results illustrate how a balanced CPP design can mask significant effects of stress and guanfacine on nicotine CPP. Future studies might leverage the sex-specific influence of initial chamber preference to study interactions between stress and nicotine reward.
Surprisingly, guanfacine also seemed to increase preference for the initially preferred chamber in male and female mice. The whole-cohort analyses suggest that this effect occurs in male and female control mice only (Figure 3), yet the split analyses reveal that guanfacine most strikingly increases preference for the initially preferred chamber in female control and nicotine-treated Hi-Pre mice (Figure 4d), and to a lesser extent in male control mice (Figure 4b). The similarity of this effect in male and female control mice, and more notably female control and nicotine Hi-Pre groups, suggests it is independent of nicotine. However, it is unclear why guanfacine would have this effect. Guanfacine also has anxiolytic- and antidepressant-like effects 51,80, and enhances working memory 43. Perhaps guanfacine enhanced spatial working memory for the initially preferred context, thereby increasing time spent in that chamber 81,82.
There are some limitations to our use of guanfacine. For example, clinical studies use chronic guanfacine treatment (2-3 weeks minimum, including a dose escalation period 44,83), whereas we tested an acute injection. Further, the dose 0.15 mg/kg was chosen because it reduced immobility on the second day of a two-day forced swim test in male and female mice 51, but different doses may reveal sex differences in guanfacine sensitivity. In the present study, guanfacine did not decrease immobility in either male or female mice (Figure S1), likely because we used a single 6-min swim vs. a two-day procedure, and C3H/HeJ vs. C57BL/6J mice. Finally, although the focus of this study was to demonstrate stress-induced reinstatement of nicotine CPP and modulate it with guanfacine, further investigations without a stressor might provide more information on the effect of guanfacine itself.
We found no significant effect of estrous phase on CPP (Figure 5), which is consistent with previous studies of intact, freely-cycling female rats 40,84. However, other studies associate higher estradiol levels with greater nicotine reward in rats 85,86, and higher progesterone levels with protective effects against nicotine reward 87 and relapse 88,89 in human subjects. Our study was insufficiently powered to examine estrous phase within the split subgroups, but it is possible that in the Lo-Pre subgroups where significant stress-induced reinstatement was found, there may be significant effects of estrous phase.
Neuronal activation was measured by the immediate early gene Arc 49 following a swim stress (which was preceded by saline or guanfacine treatment) but without subsequent exposure to the nicotine-paired context in order to reflect how the brain is primed by the stressor with or without guanfacine, without being confounded by drug-associated contextual cues or drug-seeking behavior. The Arc experiments are conducted in independent cohorts of mice from the behavioral studies, and thus rather than correlating with behavior, these findings represent an initial survey of brain regions with potential sex-specific responses to guanfacine in the context of nicotine CPP and stress following nicotine abstinence. These experiments did not test the effects of nicotine, stress, or abstinence per se on the expression of immediate early genes, which have been examined in previous studies 90–96.
In the BLA we did not find a significant effect of nicotine on Arc immunoreactivity (Figure 6a,b), although chemogenetic inactivation of BLA CaMKIIα neurons has been shown to induce reinstatement of nicotine CPP in mice 37. There was a near-significant decrease in BLA Arc immunoreactivity after guanfacine, which is consistent with previous research using in vivo electrophysiology and clonidine in the BLA 97. The CeA has a well-established role in stress-induced behaviors 22,36, and the CeL but not the CeM mediates relapse to methamphetamine self-administration 98. Guanfacine increased CeL Arc immunoreactivity (Figure 6c,d), as seen previously 99, but nicotine-treated groups showed decreased CeL activity overall. Previous studies have shown enhanced c-fos immunoreactivity in the CeA after incubation of nicotine self-administration 93 and after footshock which followed nicotine self-administration training 96. However, this enhancement may depend on exposure to drug-paired contexts following incubation or in conjunction with footshock 100. In contrast, our mice were not re-exposed to nicotine-paired contexts following stress and prior to Arc immunohistochemistry. The NAc shell is activated in response to nicotine 101 and nicotine-associated cues 102, while the NAc core has been implicated in nicotine aversion 103 and drug-stress interactions for alcohol 104 and cocaine 105. We found low NAc Arc expression overall, likely because mice were not re-exposed to nicotine or nicotine-paired contexts prior to perfusion. In all of the above regions, males and females showed similar Arc expression patterns, suggesting that these brain regions did not contribute to the observed sex differences in behavior.
There were sex-dependent effects of guanfacine in the IL and AI cortical regions. In the IL, guanfacine increased Arc immunoreactivity in male mice, and decreased Arc immunoreactivity in female mice, although the latter effect did not reach significance (Figure 7a,b). The IL is an important node for both stress-106,107 and drug-related behaviors 108,109, with demonstrated sex differences in stress response 107. Although the PL and not the IL is implicated in footshock-induced reinstatement of cocaine self-administration 110, we found no significant differences in PL Arc immunoreactivity (Figure 7c, d). Lack of re-exposure to the drug-paired context, and/or different neurobiological mechanisms between stress-induced reinstatement of self-administration vs. CPP 34,75, may account for this discrepancy. In the AI, guanfacine decreased Arc immunoreactivity in male mice, but had no effect in female mice (Figure 7e,f). The insula has been associated with increased smoking lapse in humans 111 as well as cue-induced reinstatement of nicotine seeking in rats 53. Future investigations could determine if modulating the activity of the IL or AI drives sex-dependent responses to stress-induced reinstatement and guanfacine.
In conclusion, the current study presents a novel application of nicotine CPP to study sex differences in stress-induced reinstatement. Using this procedure, we demonstrate that initial chamber preference interacts with stress-induced reinstatement but in opposite directions for male and female mice, implying sex-dependent contributions of nicotine’s anxiolytic and reward-enhancing effects to nicotine addiction. Further, acutely-administered guanfacine attenuates reinstatement in both male and female mice, but only in the nicotine-pairing conditions under which significant stress-induced reinstatement was observed for that sex. Finally, Arc immunohistochemical experiments suggest guanfacine has sex-dependent effects on neuronal activity in AI and IL, identifying these brain regions for future investigations into their potential roles in stress-induced reinstatement of nicotine CPP in male vs. female subjects. Overall, this study establishes a preclinical model for studying sex differences in stress-induced nicotine CPP and furthers our knowledge of sex-dependent effects of guanfacine on behavior and patterns of neural activation.
Supplementary Material
Acknowledgements
We would like to thank Drs. Jane Taylor, Ralph DiLeone, Christopher Pittenger, Michael Nitabach, and Stephanie Groman for helpful discussions. This work was supported by National Institutes of Health grants DA033945 (ORWH, NIDA, FDA), DA14241, MH077681, T32 GM007205, and F30DA043943.
Footnotes
Conflict of Interest
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.World Health Organization. WHO Report on the Global Tobacco Epidemic,2017.; 2017. doi:Licence: CC BY-NC-SA 3.0 IGO.
- 2.Jorenby DE, Hays JT, Rigotti NA, et al. Efficacy of Varenicline, an a4b2 Nicotinic Acetylcholine Receptor Partial Agonist, vs Placebo or Sustained-Release Bupropion for Smoking Cessation. JAMA. 2006;296(1):56–63. doi: 10.1001/jama.296.1.56 [DOI] [PubMed] [Google Scholar]
- 3.Smith PH, Bessette AJ, Weinberger AH, Sheffer CE, McKee SA. Sex/gender differences in smoking cessation: A review. Prev Med (Baltim). 2016;92:135–140. doi: 10.1016/j.ypmed.2016.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith PH, Kasza KA, Hyland A, et al. Gender Differences in Medication Use and Cigarette Smoking Cessation: Results From the International Tobacco Control Four Country Survey. Nicotine Tob Res. 2015;17(4):463–472. doi: 10.1093/ntr/ntu212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weinberger AH, Pilver CE, Mazure CM, McKee SA. Stability of smoking status in the US population: a longitudinal investigation. Addiction. 2014;109(9):1541–1553. doi: 10.1111/add.12647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chaudhri N, Caggiula AR, Donny EC, Palmatier MI, Liu X, Sved AF. Complex interactions between nicotine and nonpharmacological stimuli reveal multiple roles for nicotine in reinforcement. Psychopharmacology (Berl). 2006;184(3–4):353–366. doi: 10.1007/s00213-005-0178-1 [DOI] [PubMed] [Google Scholar]
- 7.Risinger FO, Oakes RA. Nicotine-induced conditioned place preference and conditioned place aversion in mice. Pharmacol Biochem Behav. 1995;51(2–3):457–461. doi: 10.1016/0091-3057(95)00007-J [DOI] [PubMed] [Google Scholar]
- 8.Perkins KA, Karelitz JL, Boldry MC. Nicotine acutely enhances reinforcement from non-drug rewards in humans. Front Psychiatry. 2017;8(MAY). doi: 10.3389/fpsyt.2017.00065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Olausson P, Jentsch JD, Taylor JR. Nicotine enhances responding with conditioned reinforcem. Psychopharmacology (Berl). 2004;173(1):98–104. doi: 10.1007/s00213-003-1702-9 [DOI] [PubMed] [Google Scholar]
- 10.Valentine G, Sofuoglu M. Cognitive Effects of Nicotine: Recent Progress. Curr Neuropharmacol. 2018;16(4):403–414. doi: 10.2174/1570159X15666171103152136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shiffman S, Paty J a, Gnys M, Kassel J a, Hickcox M. First lapses to smoking: Within-subjects analysis of real-time reports. J Consult Clin Psychol. 1996;64(2):366–379. doi: 10.1037/0022-006X.64.2.366 [DOI] [PubMed] [Google Scholar]
- 12.Bruijnzeel AW. Tobacco addiction and the dysregulation of brain stress systems. Neurosci Biobehav Rev. 2012;36(5):1418–1441. doi: 10.1016/j.neubiorev.2012.02.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bilkei-Gorzo A, Racz I, Michel K, Darvas M, Maldonado R, Zimmer A. A Common Genetic Predisposition to Stress Sensitivity and Stress-Induced Nicotine Craving. Biol Psychiatry. 2008;63(2):164–171. doi: 10.1016/j.biopsych.2007.02.010 [DOI] [PubMed] [Google Scholar]
- 14.Picciotto MR. Nicotine as a modulator of behavior: Beyond the inverted U. Trends Pharmacol Sci. 2003;24(9):493–499. doi: 10.1016/S0165-6147(03)00230-X [DOI] [PubMed] [Google Scholar]
- 15.Hall FS, Der-Avakian A, Gould TJ, Markou A, Shoaib M, Young JW. Negative affective states and cognitive impairments in nicotine dependence. Neurosci Biobehav Rev. 2015;58:168–185. doi: 10.1016/j.neubiorev.2015.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Falco AM, Bevins RA. Individual differences in the behavioral effects of nicotine: A review of the preclinical animal literature. Pharmacol Biochem Behav. 2015;138:80–90. doi: 10.1016/j.pbb.2015.09.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pogun S, Yararbas G, Nesil T, Kanit L. Sex differences in nicotine preference. J Neurosci Res. 2017;95(1–2):148–162. doi: 10.1002/jnr.23858 [DOI] [PubMed] [Google Scholar]
- 18.Nesil T, Kanit L, Collins AC, Pogun S. Individual differences in oral nicotine intake in rats. Neuropharmacology. 2011;61(1–2):189–201. doi: 10.1016/j.neuropharm.2011.03.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perkins KA, Karelitz JL. Sex Differences in Acute Relief of Abstinence-Induced Withdrawal and Negative Affect due to Nicotine Content in Cigarettes. Nicotine Tob Res. 2015;17(4):443–448. doi: 10.1093/ntr/ntu150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O’Dell LE, Torres O V. A mechanistic hypothesis of the factors that enhance vulnerability to nicotine use in females. Neuropharmacology. 2014;76(PART B):566–580. doi: 10.1016/j.neuropharm.2013.04.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Torres O V, O’Dell LE. Stress is a principal factor that promotes tobacco use in females. Prog Neuro-Psychopharmacology Biol Psychiatry. 2016;65:260–268. doi: 10.1016/j.pnpbp.2015.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mantsch JR, Baker DA, Funk D, Lê AD, Shaham Y. Stress-Induced Reinstatement of Drug Seeking: 20 Years of Progress. Neuropsychopharmacology. 2015:1–22. doi: 10.1038/npp.2015.142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Picciotto MR, Kenny PJ. Molecular Mechanisms Underlying Behaviors Related to Nicotine Addiction. Cold Spring Harb Perspect Med. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pang RD, Leventhal AM. Sex Differences in Negative Affect and Lapse Behavior During Acute Tobacco Abstinence: A Laboratory Study. Exp Clin Psychopharmacol. 2013;21(4):269–276. doi: 10.1037/a0030561.Striving [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Messer S, Siegel A, Bertin L, Erblich J. Sex differences in affect-triggered lapses during smoking cessation: A daily diary study. Addict Behav. 2018;87(November 2017):82–85. doi: 10.1016/j.addbeh.2018.06.019 [DOI] [PubMed] [Google Scholar]
- 26.Xu J, Azizian A, Monterosso J, et al. Gender effects on mood and cigarette craving during early abstinence and resumption of smoking. Nicotine Tob Res. 2008;10(11):1653–1661. doi: 10.1080/14622200802412929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McKee SA, Maciejewski PK, Falba T, Mazure CM. Sex differences in the effects of stressful life events on changes in smoking status. Addiction. 2003;98(6):847–855. [DOI] [PubMed] [Google Scholar]
- 28.Smith PH, Weinberger AH, Zhang J, Emme E, Mazure CM, McKee SA. Sex Differences in Smoking Cessation Pharmacotherapy Comparative Efficacy: A Network Meta-analysis. Nicotine Tob Res. 2016:1–9. doi: 10.1093/ntr/ntw144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Covey LS, Glassman AH. A meta-analysis of double-blind placebo-controlled trials of clonidine for smoking cessation. Br J Addict. 1991;86:991–998. [DOI] [PubMed] [Google Scholar]
- 30.Aston-Jones G, Kalivas PW. Brain norepinephrine rediscovered in addiction research. Biol Psychiatry. 2008;63(11):1005–1006. doi: 10.1016/j.biopsych.2008.03.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Erb S, Hitchcott PK, Rajabi H, Mueller D, Shaham Y, Stewart J. Alpha-2 Adrenergic Receptor Agonists Block Stress-Induced Reinstatement of Cocaine Seeking. Neuropsychopharmacology. 2000;23(2):138–150. [DOI] [PubMed] [Google Scholar]
- 32.Mantsch JR, Weyer A, Vranjkovic O, Beyer CE, Baker DA, Caretta H. Involvement of Noradrenergic Neurotransmission in the Stress but not Cocaine-Induced Reinstatement of Extinguished Cocaine-Induced Conditioned Place Preference in Mice: Role for b-2 Adrenergic Receptors. Neuropsychopharmacology. 2010;35:2165–2178. doi: 10.1038/npp.2010.86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang X, Cen X, Lu L. Noradrenaline in the bed nucleus of the stria terminalis is critical for stress-induced reactivation of morphine-conditioned place preference in rats. Eur J Pharmacol. 2001;432(2–3):153–161. doi: 10.1016/S0014-2999(01)01487-X [DOI] [PubMed] [Google Scholar]
- 34.Bardo MT, Bevins RA. Conditioned place preference: What does it add to our preclinical understanding of drug reward? Psychopharmacology (Berl). 2000;153(1):31–43. doi: 10.1007/s002130000569 [DOI] [PubMed] [Google Scholar]
- 35.Zislis G, Desai T V., Prado M, Shah HP, Bruijnzeel AW. Effects of the CRF receptor antagonist d-Phe CRF(12–41) and the a2-adrenergic receptor agonist clonidine on stress-induced reinstatement of nicotine-seeking behavior in rats. Neuropharmacology. 2007;53(8):958–966. doi: 10.1016/j.neuropharm.2007.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yamada H, Bruijnzeel AW. Stimulation of α2-adrenergic receptors in the central nucleus of the amygdala attenuates stress-induced reinstatement of nicotine seeking in rats. Neuropharmacology. 2011;60(2–3):303–311. doi: 10.1016/j.neuropharm.2010.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nygard SK, Hourguettes NJ, Sobczak GG, Carlezon WA, Bruchas MR. Stress-Induced Reinstatement of Nicotine Preference Requires Dynorphin/Kappa Opioid Activity in the Basolateral Amygdala. J Neurosci. 2016;36(38):9937–9948. doi: 10.1523/JNEUROSCI.0953-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jackson KJ, McLaughlin JP, Carroll FI, Damaj MI. Effects of the kappa opioid receptor antagonist, norbinaltorphimine, on stress and drug-induced reinstatement of nicotine-conditioned place preference in mice. Psychopharmacology (Berl). 2013;226(4):763–768. doi: 10.1007/s00213-012-2716-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Leão RM, Cruz FC, Planeta CS. Exposure to acute restraint stress reinstates nicotine-induced place preference in rats. Behav Pharmacol. 2009;20(1):109–113. doi: 10.1097/FBP.0b013e3283242f41 [DOI] [PubMed] [Google Scholar]
- 40.Feltenstein MW, Ghee SM, See RE. Nicotine self-administration and reinstatement of nicotine-seeking in male and female rats. Drug Alcohol Depend. 2012;121(3):240–246. doi: 10.1016/j.drugalcdep.2011.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cahill K, Stevens S, Perera R, Lancaster T. Pharmacological interventions for smoking cessation: an overview and network meta-analysis. Cochrane Database Syst Rev. 2013;(5):1–50. doi: 10.1002/14651858.CD009329.pub2.www.cochranelibrary.com [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Verplaetse TL, Weinberger AH, Smith PH, et al. Targeting the Noradrenergic System for Gender-Sensitive Medication Development for Tobacco Dependence. Nicotine Tob Res. 2015;17(4):486–495. doi: 10.1093/ntr/ntu280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Arnsten AFT, Cai JX, Goldman-Rakic PS. The alpha-2 adrenergic agonist guanfacine improves memory in aged monkeys without sedative or hypotensive side effects: evidence for alpha-2 receptor subtypes. J Neurosci. 1988;8(11):4287–4298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McKee SA, Potenza MN, Kober H, et al. A translational investigation targeting stress-reactivity and prefrontal cognitive control with guanfacine for smoking cessation. J Psychopharmacol. 2015;29(3):300–311. doi: 10.1177/0269881114562091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marks MJ, Burch JB, Collins AC. Genetics of nicotine response in four inbred strains of mice. J Pharmacol Exp Ther. 1983;226(1):291–302. [PubMed] [Google Scholar]
- 46.Schechter MD, Meehan SM, Schechter JB. Genetic selection for nicotine activity in mice correlates with conditioned place preference. Eur J Pharmacol. 1995;279(1):59–64. doi: 10.1016/0014-2999(95)00139-C [DOI] [PubMed] [Google Scholar]
- 47.Roma PG, Riley AL. Apparatus bias and the use of light and texture in place conditioning. Pharmacol Biochem Behav. 2005;82(1):163–169. doi: 10.1016/j.pbb.2005.08.004 [DOI] [PubMed] [Google Scholar]
- 48.Brielmaier JM, McDonald CG, Smith RF. Nicotine place preference in a biased conditioned place preference design. Pharmacol Biochem Behav. 2008;89(1):94–100. doi: 10.1016/j.pbb.2007.11.005 [DOI] [PubMed] [Google Scholar]
- 49.Lyford GL, Yamagata K, Kaufmann WE, et al. Arc, a growth factor and activity-regulated gene, encodes a novel cytoskeleton-associated protein that is enriched in neuronal dendrites. Neuron. 1995;14(2):433–445. doi: 10.1016/0896-6273(95)90299-6 [DOI] [PubMed] [Google Scholar]
- 50.Lenoir M, Starosciak AK, Ledon J, et al. Sex differences in conditioned nicotine reward are age-specific. Pharmacol Biochem Behav. 2015;132:56–62. doi: 10.1016/j.pbb.2015.02.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mineur YS, Bentham MP, Zhou WL, Plantenga ME, McKee SA, Picciotto MR. Antidepressant-like effects of guanfacine and sex-specific differences in effects on c-fos immunoreactivity and paired-pulse ratio in male and female mice. Psychopharmacology (Berl). 2015;232(19):3539–3549. doi: 10.1007/s00213-015-4001-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Grabus SD, Martin BR, Brown SE, Damaj MI. Nicotine place preference in the mouse: Influences of prior handling, dose and strain and attenuation by nicotinic receptor antagonists. Psychopharmacology (Berl). 2006;184(3–4):456–463. doi: 10.1007/s00213-006-0305-7 [DOI] [PubMed] [Google Scholar]
- 53.Abdolahi A, Acosta G, Breslin FJ, Hemby SE, Lynch WJ. Incubation of nicotine seeking is associated with enhanced protein kinase A-regulated signaling of dopamine- and cAMP- regulated phosphoprotein of 32 kDa in the insular cortex. Eur J Neurosci. 2010;31(4):733–741. doi: 10.1016/j.biotechadv.2011.08.021.Secreted [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shalev U, Morales M, Hope B, Yap J, Shaham Y. Time-dependent changes in extinction behavior and stress-induced reinstatement of drug seeking following withdrawal from heroin in rats. Psychopharmacology (Berl). 2001;156(1):98–107. doi: 10.1007/s002130100748 [DOI] [PubMed] [Google Scholar]
- 55.Byers SL, Wiles M V., Dunn SL, Taft RA. Mouse estrous cycle identification tool and images. PLoS One. 2012;7(4):2–6. doi: 10.1371/journal.pone.0035538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yeadon J 6 Steps for Setting up Timed Pregnant Mice. JAX Blog. https://www.jax.org/news-and-insights/jax-blog/2014/september/six-steps-for-setting-up-timed-pregnant-mice. Published 2014. Accessed March 1, 2018.
- 57.Van der Lee S, Boot LM. Spontaneous pseudopregnancy in mice. Acta Physiol Pharmacol Neerl. 1955;4(3):442–444. [PubMed] [Google Scholar]
- 58.Lewis AS, Pittenger ST, Mineur YS, Stout D, Smith PH, Picciotto MR. Bidirectional Regulation of Aggression in Mice by Hippocampal Alpha-7 Nicotinic Acetylcholine Receptors. Neuropsychopharmacology. 2018;43(6):1267–1275. doi: 10.1038/npp.2017.276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schindelin J, Arganda-Carreras I, Frise E, et al. Fiji: An open-source platform for biological-image analysis. Nat Methods. 2012;9(7):676–682. doi: 10.1038/nmeth.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Motulsky HJ, Brown RE. Detecting outliers when fitting data with nonlinear regression - A new method based on robust nonlinear regression and the false discovery rate. BMC Bioinformatics. 2006;7:1–20. doi: 10.1186/1471-2105-7-123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kota D, Martin BR, Robinson SE, Damaj MI. Nicotine Dependence and Reward Differ between Adolescent and Adult Male Mice. J Pharmacol Exp Ther. 2007;322(1):399–407. doi: 10.1124/jpet.107.121616.initiate [DOI] [PubMed] [Google Scholar]
- 62.Kota D, Martin BR, Damaj MI. Age-dependent differences in nicotine reward and withdrawal in female mice. Psychopharmacology (Berl). 2008;198(2):201–210. doi: 10.1007/s00213-008-1117-8 [DOI] [PubMed] [Google Scholar]
- 63.Yararbas G, Keser A, Kanit L, Pogun S. Nicotine-induced conditioned place preference in rats: Sex differences and the role of mGluR5 receptors. Neuropharmacology. 2010;58(2):374–382. doi: 10.1016/j.neuropharm.2009.10.001 [DOI] [PubMed] [Google Scholar]
- 64.Le Foll B, Goldberg SR. Nicotine induces conditioned place preferences over a large range of doses in rats. Psychopharmacology (Berl). 2005;178(4):481–492. doi: 10.1007/s00213-004-2021-5 [DOI] [PubMed] [Google Scholar]
- 65.Bernardi RE, Spanagel R. Basal activity level in mice predicts the initial and sensitized locomotor response to nicotine only in high responders. Behav Brain Res. 2014;264:143–150. doi: 10.1016/j.bbr.2014.01.046 [DOI] [PubMed] [Google Scholar]
- 66.Brunzell DH, Mineur YS, Neve RL, Picciotto MR. Nucleus accumbens CREB activity is necessary for nicotine conditioned place preference. Neuropsychopharmacology. 2009;34:1993–2001. doi: 10.1038/npp.2009.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tzschentke TM. Measuring reward with the conditioned place preference (CPP) paradigm: Update of the last decade. Addict Biol. 2007;12(3–4):227–462. doi: 10.1111/j.1369-1600.2007.00070.x [DOI] [PubMed] [Google Scholar]
- 68.Cunningham CL, Ferree NK, Howard MA. Apparatus bias and place conditioning with ethanol in mice. Psychopharmacology (Berl). 2003;170(4):409–422. doi: 10.1007/s00213-003-1559-y [DOI] [PubMed] [Google Scholar]
- 69.Pogun S, Yararbas G. Sex differences in nicotine action. Handb Exp Pharmacol. 2009;192:261–291. doi: 10.1007/978-3-540-69248-5_10 [DOI] [PubMed] [Google Scholar]
- 70.Cheeta S, Irvine EE, Tucci S, Sandhu J, File SE. In adolescence, female rats are more sensitive to the anxiolytic effect of nicotine than are male rats. Neuropsychopharmacology. 2001;25(4):601–607. doi: 10.1016/S0893-133X(01)00258-5 [DOI] [PubMed] [Google Scholar]
- 71.Abreu-Villaça Y, Cavina CC, Ribeiro-Carvalho A, et al. Combined exposure to tobacco smoke and ethanol during adolescence leads to short- and long-term modulation of anxiety-like behavior. Drug Alcohol Depend. 2013;133(1):52–60. doi: 10.1016/j.drugalcdep.2013.05.033 [DOI] [PubMed] [Google Scholar]
- 72.Elliott BM, Faraday MM, Phillips JM, Grunberg NE. Effects of nicotine on elevated plus maze and locomotor activity in male and female adolescent and adult rats. Pharmacol Biochem Behav. 2004;77(1):21–28. doi: 10.1016/j.pbb.2003.09.016 [DOI] [PubMed] [Google Scholar]
- 73.Damaj MI. Influence of gender and sex hormones on nicotine acute pharmacological effects in mice. J Pharmacol Exp Ther. 2001;296(1):132–140. [PubMed] [Google Scholar]
- 74.Barrett ST, Geary TN, Steiner AN, Bevins RA. Sex differences and the role of dopamine receptors in the reward-enhancing effects of nicotine and bupropion. Psychopharmacology (Berl). 2017;234(2):187–198. doi: 10.1007/s00213-016-4448-x [DOI] [PubMed] [Google Scholar]
- 75.Aguilar MA, Rodríguez-Arias M, Miñarro J. Neurobiological mechanisms of the reinstatement of drug-conditioned place preference. Brain Res Rev. 2009;59(2):253–277. doi: 10.1016/j.brainresrev.2008.08.002 [DOI] [PubMed] [Google Scholar]
- 76.Pickens CL, Airavaara M, Theberge F, Fanous S, Hope BT, Shaham Y. Neurobiology of the incubation of drug craving. Trends Neurosci. 2011;34(8):411–420. doi: 10.1016/j.tins.2011.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shaham Y, Highfield D, Delfs J, Leung S, Stewart J. Clonidine blocks stress-induced reinstatement of heroin seeking in rats: An effect independent of locus coeruleus noradrenergic neurons. Eur J Neurosci. 2000;12(1):292–302. doi: 10.1046/j.1460-9568.2000.00899.x [DOI] [PubMed] [Google Scholar]
- 78.Dluzen DE, Anderson LI. Estrogen differentially modulates nicotine-evoked dopamine release from the striatum of male and female rats. Neurosci Lett. 1997;230(2):140–142. doi: 10.1016/S0304-3940(97)00487-4 [DOI] [PubMed] [Google Scholar]
- 79.Cosgrove KP, Wang S, Kim S-J, et al. Sex Differences in the Brain’s Dopamine Signature of Cigarette Smoking. J Neurosci. 2014;34(50):16851–16855. doi: 10.1523/JNEUROSCI.3661-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mineur YS, Cahuzac EL, Mose TN, et al. Interaction between noradrenergic and cholinergic signaling in amygdala regulates anxiety- and depression-related behaviors in mice. Neuropsychopharmacology. 2018;(October 2017):1–8. doi: 10.1038/s41386-018-0024-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jin X-C, Ma C-L, Li B-M. The α 2A -adrenoceptor agonist guanfacine improves spatial learning but not fear conditioning in rats. Acta Physiol Sin. 2007;59(2006):739–744. [PubMed] [Google Scholar]
- 82.Kauser H, Sahu S, Kumar S, Panjwani U. Guanfacine ameliorates hypobaric hypoxia induced spatial working memory deficits. Physiol Behav. 2014;123:187–192. doi: 10.1016/j.physbeh.2013.10.028 [DOI] [PubMed] [Google Scholar]
- 83.Milivojevic V, Fox HC, Jayaram-Lindstrom N, Hermes G, Sinha R. Sex differences in guanfacine effects on stress-induced stroop performance in cocaine dependence. Drug Alcohol Depend. 2017;179(July):275–279. doi: 10.1016/j.drugalcdep.2017.07.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Torres O V, Natividad LA, Tejeda HA, Van Weelden SA, O’Dell LE. Female rats display dose-dependent differences to the rewarding and aversive effects of nicotine in an age-, hormone-, and sex-dependent manner. Psychopharmacology (Berl). 2009;206(2):303–312. doi: 10.1007/s00213-009-1607-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Flores RJ, Pipkin JA, Uribe KP, Perez A, O’Dell LE. Estradiol promotes the rewarding effects of nicotine in female rats. Behav Brain Res. 2016;307:258–263. doi: 10.1016/j.bbr.2016.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lynch WJ. Sex and ovarian hormones influence vulnerability and motivation for nicotine during adolescence in rats. Pharmacol Biochem Behav. 2009;94(1):43–50. doi: 10.1016/j.pbb.2009.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sofuoglu M, Mitchell E, Mooney M. Progesterone effects on subjective and physiological responses to intravenous nicotine in male and female smokers. Hum Psychopharmacol Clin Exp. 2009;24:559–564. doi: 10.1002/hup.1055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Allen SS, Bade T, Center B, Finstad D, Hatsukami D. Menstrual phase effects on smoking relapse. Addiction. 2008;103(5):809–821. doi: 10.1111/j.1360-0443.2008.02146.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lynch WJ, Sofuoglu M. Role of Progesterone in Nicotine Addiction: Evidence From Initiation to Relapse. Exp Clin Psychopharmacol. 2010;18(6):451–461. doi: 10.1037/a0021265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Knapska E, Radwanska K, Werka T, Kaczmarek L. Functional Internal Complexity of Amygdala: Focus on Gene Activity Mapping After Behavioral Training and Drugs of Abuse. Physiol Rev. 2007;87(4):1113–1173. doi: 10.1152/physrev.00037.2006 [DOI] [PubMed] [Google Scholar]
- 91.McReynolds JR, Christianson JP, Blacktop JM, Mantsch JR. What does the Fos say? Using Fos-based approaches to understand the contribution of stress to substance use disorders. Neurobiol Stress. 2018;9(June):271–285. doi: 10.1016/j.ynstr.2018.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bland ST, Schmid MJ, Der-Avakian A, Watkins LR, Spencer RL, Maier SF. Expression of c-fos and BDNF mRNA in subregions of the prefrontal cortex of male and female rats after acute uncontrollable stress. Brain Res. 2005;1051(1–2):90–99. doi: 10.1016/j.brainres.2005.05.065 [DOI] [PubMed] [Google Scholar]
- 93.Funk D, Coen K, Tamadon S, Hope BT, Shaham Y, Lê AD. Role of Central Amygdala Neuronal Ensembles in Incubation of Nicotine Craving. J Neurosci. 2016;36(33):8612–8623. doi: 10.1523/JNEUROSCI.1505-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Schiltz CA, Kelley AE, Landry CF. Contextual cues associated with nicotine administration increase arc mRNA expression in corticolimbic areas of the rat brain. Eur J Neurosci. 2005;21(6):1703–1711. doi: 10.1111/j.1460-9568.2005.04001.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Schiltz CA, Kelley AE, Landry CF. Acute Stress and Nicotine Cues Interact to Unveil Locomotor Arousal and Activity-Dependent Gene Expression in the Prefrontal Cortex. Biol Psychiatry. 2007;61(1):127–135. doi: 10.1016/j.biopsych.2006.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yu G, Sharp BM. Nicotine modulates multiple regions in the limbic stress network regulating activation of hypophysiotrophic neurons in hypothalamic paraventricular nucleus. J Neurochem. 2012;122(3):628–640. doi: 10.1111/j.1471-4159.2012.07785.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Buffalari DM, Grace AA. Noradrenergic Modulation of Basolateral Amygdala Neuronal Activity: Opposing Influences of alpha-2 and beta Receptor Activation. J Neurosci. 2007;27(45):12358–12366. doi: 10.1523/JNEUROSCI.2007-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Venniro M, Caprioli D, Zhang M, et al. The anterior insular cortex→central amygdala glutamatergic pathway is critical to relapse after contingency management. Neuron. 2017;96(2):414–427.e8. doi: 10.1016/j.neuron.2017.09.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Savchenko VL, Boughter JD. Regulation of neuronal activation by alpha2A adrenergic receptor agonist. Neurotox Res. 2011;20(3):226–239. doi: 10.1007/s12640-010-9236-5 [DOI] [PubMed] [Google Scholar]
- 100.Erb S, Lopak V, Smith C. Cocaine pre-exposure produces a sensitized and context-specific c-fos mRNA response to footshock stress in the central nucleus of the amygdala. Neuroscience. 2004;129(3):719–725. doi: 10.1016/j.neuroscience.2004.08.012 [DOI] [PubMed] [Google Scholar]
- 101.Sun N, Laviolette SR. Dopamine receptor blockade modulates the rewarding and aversive properties of nicotine via dissociable neuronal activity patterns in the nucleus accumbens. Neuropsychopharmacology. 2014;39(12):2799–2815. doi: 10.1038/npp.2014.130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Schroeder BE, Binzak JM, Kelley AE. A common profile of prefrontal cortical activation following exposure to nicotine- or chocolate-associated contextual cues. Neuroscience. 2001;105(3):535–545. doi: 10.1016/S0306-4522(01)00221-4 [DOI] [PubMed] [Google Scholar]
- 103.Sellings LHL, Baharnouri G, McQuade LE, Clarke PBS. Rewarding and aversive effects of nicotine are segregated within the nucleus accumbens. Eur J Neurosci. 2008;28(2):342–352. doi: 10.1111/j.1460-9568.2008.06341.x [DOI] [PubMed] [Google Scholar]
- 104.Besheer J, Fisher KR, Jaramillo AA, Frisbee S, Cannady R. Stress hormone exposure reduces mglur5 expression in the nucleus accumbens: Functional implications for interoceptive sensitivity to alcohol. Neuropsychopharmacology. 2014;39(10):2376–2386. doi: 10.1038/npp.2014.85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.De Giovanni LN, Guzman AS, Virgolini MB, Cancela LM. NMDA antagonist MK 801 in nucleus accumbens core but not shell disrupts the restraint stress-induced reinstatement of extinguished cocaine-conditioned place preference in rats. Behav Brain Res. 2016;315:150–159. doi: 10.1016/j.bbr.2016.08.011 [DOI] [PubMed] [Google Scholar]
- 106.Shansky RM, Hamo C, Hof PR, McEwen BS, Morrison JH. Stress-induced dendritic remodeling in the prefrontal cortex is circuit specific. Cereb Cortex. 2009;19(10):2479–2484. doi: 10.1093/cercor/bhp003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Shansky RM, Hamo C, Hof PR, Lou W, McEwen BS, Morrison JH. Estrogen promotes stress sensitivity in a prefrontal cortex-amygdala pathway. Cereb Cortex. 2010;20(11):2560–2567. doi: 10.1093/cercor/bhq003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gourley SL, Taylor JR. Going and stopping: Dichotomies in behavioral control by the prefrontal cortex. Nat Neurosci. 2016;19(6):656–664. doi: 10.1038/nn.4275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Pittenger ST, Swalve N, Chou S, et al. Sex Differences in Neurotensin and Substance P Following Nicotine Self-Administration in Rats. Synapse. 2016;70(April):336–346. doi: 10.1002/syn.21907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.McFarland K, Davidge SB, Lapish CC, Kalivas PW. Limbic and Motor Circuitry Underlying Footshock-Induced Reinstatement of Cocaine-Seeking Behavior. J Neurosci. 2004;24(7):1551–1560. doi: 10.1523/JNEUROSCI.4177-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Janes AC, Pizzagalli DA, Richardt S, et al. Brain Reactivity to Smoking Cues Prior to Smoking Cessation Predicts Ability to Maintain Tobacco Abstinence. Biol Psychiatry. 2010;67(8):722–729. doi: 10.1016/j.biopsych.2009.12.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


