Abstract
Gastric cancer (GC) is characterized by high morbidity and mortality rates worldwide. Helicobacter pylori infection, high salt intake, smoking, alcohol, low fiber intake, family history of GC, obesity and precancerous lesions, including chronic atrophic gastritis and intestinal metaplasia, are considered general risk factors for GC. Image enhancement endoscopy methods, which improve the visualization of mucosal structures and vascularity, may be used for the early diagnosis of GC, such as narrow band imaging, which can reveal fine details of subtle superficial abnormalities of early gastric cancer (EGC). Mitochondria are well-known for their role in producing ATP via the tricarboxylic acid cycle. In cancer cells, the energetic metabolism can be reprogrammed as anaerobic glycolysis for energy production and anabolic growth. In addition to their dominant metabolic functions, mitochondria participate in several central signaling pathways, such as the apoptotic pathway and NLRP3 inflammasome activation. Conversely, mitochondrial dynamics, including fission/fusion and mitophagy, can also contribute to the pathogenesis of cancer. The dysfunction and dysregulation of mitochondria have been associated with several ageing and degenerative diseases, as well as cancer. The present review focuses on energy metabolism and mitochondrial dynamics, and summarizes the changes in gastric carcinogenesis, the diagnosis of EGC and indicates potential targeted treatments.
Keywords: diagnosis of early gastric cancer, mitochondrial dynamics, reprogrammed energy metabolism
1. Introduction
Chronic atrophic gastritis (CAG) is considered a common risk factor for the development of gastric cancer (GC). Endoscopic imaging and biopsy are crucial for early detection and diagnosis of GC (1). Image-enhanced endoscopy combined with biopsy, according to the Sydney protocol and regular endoscopic surveillance, are recommended for patients with extensive CAG or intestinal metaplasia (2). A visible lesion may be treated by endoscopic mucosal resection or endoscopic submucosal dissection. However, when a lesion is invisible, regular endoscopic surveillance is required for high-risk patients. The interval between Helicobacter pylori eradication and cancer occurrence may vary from several months to >10 years (3). Surveillance endoscopy is one of the methods enabling the early diagnosis of GC (4). Once an existing lesion is identified, it can be treated in a timely manner. Interval cancer can occur due to missed lesions or to a newly developed lesion during surveillance (5). Thus, it is essential to identify a molecular biological marker for the detection of invisible lesions at the organelle level.
Recently, diverse pathophysiological functions of mitochondria have been reported, including mitochondrial dynamics (6), metabolic reprogramming (7), mitochondria-released damage-associated molecular patterns and NLRP3 inflammasome activation (8), mitochondrial DNA (mtDNA), autophagy and mitophagy (9), mitochondrial outer membrane permeabilization (10) and mitochondrial aging (11). In addition, mitochondrial (mt)DNA mutations, deletions and impaired DNA replication are the most common causes of mitochondrial dysfunction (12). mtDNA sensing via STING signaling participates in inflammation and cancer (12,13). The effects of mitochondrial dynamics on carcinogenesis and cancer progression have also been reported, highlighting the potential use of mitochondrial biomarkers in cancer detection and prognosis, as well as the potential targeting of mitochondrial dynamics for treating cancer (14). However, there is still a paucity of research associated with GC.
The present review summarizes the role of mitochondrial dynamics and energy metabolism reprogramming in GC to identify potential indicators for biologically complemented endoscopy and further promote translating discoveries of molecular biology. Thus, fission and glycolysis from mitochondria may be useful in detecting GC. If an electron microscope can be installed on the endoscopy system, the mitochondrial dynamics may be observable during the early stages of GC. Furthermore, when fission is increased and fusion is decreased, further precision biopsy of the targeted tissue should be performed to detect metabolic activity. The combination of both approaches may enable early diagnosis and provide a novel treatment strategy. However, further investigation is required.
2. Mitochondrial dynamics: Fission and fusion
Mitochondria are responsible for energy supply and are involved in several biological processes, including cell death and proliferation (6). Mitochondria constantly maintain a dynamic shape, which may change in response to cellular bioenergetic demands, such as nutrient status, which is defined as mitochondrial dynamics (12). The mitochondrial morphology is a result of the interplay between rapid fusion and fission events (15). The key components mediating these processes belong to the dynamin family of GTPases that utilize GTP hydrolysis to drive mechanical work on biological membranes (16). Mitofusin proteins, Mfn1 and Mfn2, are involved in the fusion of the outer mitochondrial membrane, while GTPase optic atrophy 1 mediates the fusion of the inner mitochondrial membrane (17). Mitochondrial fission is mediated by the GTPase dynamin-related protein 1 (Drp1) following its recruitment by the membrane-anchored proteins, namely mitochondrial fission factor and fission protein 1 (Fis1) (18). Commonly, the mitochondrial fission/fusion machinery is involved in generating new mitochondria, and eliminating old, damaged and non-repairable mitochondria (6). Mitochondrial fission plays an important role in mitochondrial proliferation, mitochondrial distribution during cell division and the removal of damaged mitochondria via mitophagy (19). Unopposed mitochondrial fission causes mitochondrial fragmentation, which is generally associated with metabolic dysfunction and several diseases, such as degenerative diseases and cancer (20). It has been reported that impaired mitochondrial fission is associated with mitochondrial elongation (21). In addition, unopposed fusion results in a hyperfused network and serves to counteract metabolic insults, preserve cellular integrity and protect against autophagy (20). It was previously reported that impaired mitochondrial fusion may promote fission-induced mitochondrial fragmentation (21). Thus, the maintenance of mitochondrial fission/fusion balance plays a key role in cell cycle progression (6). The dynamics is critical for the effects of fission/fusion on morphology regulation, content exchange, and the maintenance of mtDNA and mitochondrial oxidative phosphorylation (OXPHOS) activity (22,23).
3. Fission, mitophagy and GC
Fission isolates depolarized mitochondria, while it coordinates the downregulation of fusion mediators to prevent network reintegration, thereby facilitating mitophagy, mainly via interactions between Parkin, Bcl-2/adenovirus E1B 19 kDa protein interacting protein 3 (BNIP3) and Drp1 (24). Increasing Drp1 results in excessive mitochondrial fragmentation and deficiencies, decreases mitochondrial motility and shortens mitochondrial length (25), which may be further enhanced in hypoxia (26). Fission can also be triggered by stress stimuli, such as nutrient deprivation, DNA damage, inflammation and mitochondrial membrane depolarization (27). Given that mitochondria-associated membranes related to the endoplasmic reticulum at specific regions can facilitate calcium (Ca2+) flux into the mitochondria and further control the homeostasis and metabolism of Ca2+, close coupling of these organelles increases mitochondrial Ca2+ levels, thus initiating apoptosis (28). It has also been reported that enhanced fission attenuates adherence to inhibit Ca2+ overload in mitochondria and apoptosis (29). In terms of mitophagy, this process maintains cellular health by selectively enclosing damaged and depolarized mitochondria in autophagic vacuoles for lysosome-mediated elimination (30). Mitophagy degrades dysfunctional mitochondria and further attenuates reactive oxygen species (ROS) generation, which in turn promotes cell survival and protects against cell death (31). Increasing evidence suggest that several modulators of mitophagy are deregulated in human cancer, including Parkinson protein 2 E3 ubiquitin protein ligase, FUN14 domain containing 1, BNIP3 and BNIP3L (32,33). In addition, a study revealed that impaired mitophagy can enhance the aggressiveness in GC cells under hypoxia by activating the mtROS/hypoxia-inducible factor (HIF)-1α interplay (34). Mitophagy may also be enhanced by overexpression of Opa-interacting protein 5, thus plays an important role in cell survival and death in docetaxel-treated GC cells (35). Another study demonstrated that Drp1 expression is upregulated, and the expression levels of the mitophagy-related regulators, PTEN-induced putative kinase 1 and Parkin, are downregulated in patients with GC (36). Given that mitophagy can clear the damaged part of mitochondria and mtDNA, it protects healthy cells from malignant transformation and tumor cells from apoptosis (31). It has been suggested that, in the early stages of GC, mitophagy is associated with tumor suppression, whereby it can promote tumor growth at the advanced stages of GC. For example, mitophagy was increased in advanced-stage GC to sustain the viability and migration of GC cells (37), since mitophagy in solids tumor may be activated by two common factors, namely hypoxia and low nutrient supply (38) (Fig. 1).
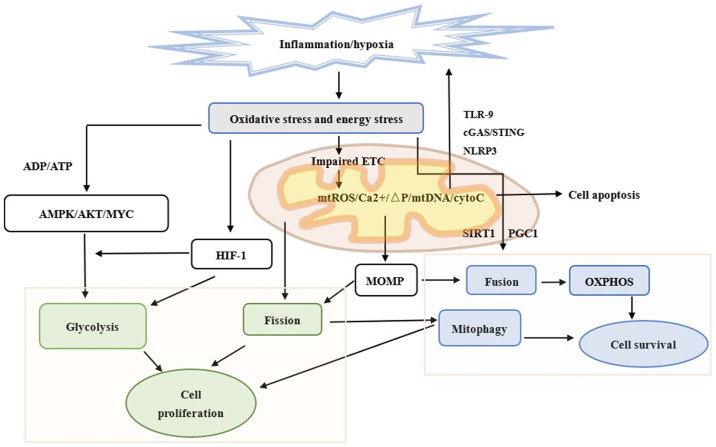
Figure 1.
Different mitochondrial dynamics and energy metabolism in an epithelial cell subjected to chronic inflammation. Chronic inflammation causes the injury of epithelial cells. Mitochondria is involved in further innate immune responses, including cGAS-STING signaling, TLR-9 and NLRP3 inflammasome formation following the release of mtDNA. Mitochondria is also associated with apoptosis. When atrophic epithelial cells preserve their programed cell death ability and surrounding inflammation is sufficiently severe, cells undergo apoptosis instead of necrosis. Chronic inflammation can also damage mitochondria and lead to changes in mitochondrial metabolism and dynamics via HIF-1, AMPK and MOMP. Fission and glycolysis promote cell proliferation and invasion. Fusion and OXPHOS are compatible with cell survival. Mitophagy protects both normal and cancer cells by selectively eliminating damaged mitochondria. Green outline represents proliferation, blue outline represents survival and the text without boxes represent apoptosis. cGAS, cyclic GMP–AMP synthase; STING, stimulator of interferon genes; TLR-9, Toll-like receptor-9; NLRP3, NOD-like receptor family pyrin domain-containing 3; mtDNA, mitochondrial DNA; HIF-1, hypoxia-inducible factor-1; AMPK, AMP-activated protein kinase; MOMP, mitochondrial outer membrane permeabilization; OXPHOS, oxidative phosphorylation; ETC, electron transport chain; mtROS, mitochondrial ROS; ΔP, increased potential; cytoC, cytochrome c; PGC1, proliferator-activated receptor-γ coactivator.
4. Fusion and GC
Mitochondrial fusion results in a more interconnected mitochondrial network and enhances the communication with the endoplasmic reticulum (39). Fusion allows the diffusion of matrix content among mitochondria, diluting the accumulated mtDNA mutations and oxidized proteins (40). Fusion is commonly enhanced by starvation by triggering the protein kinase A-mediated phosphorylation of Drp1 (at Ser637) to blunt fission (41). In addition, mitochondrial fusion is required for mtDNA maintenance (22). Thus, impaired mitochondrial fusion is often accompanied by bioenergetic defects due to loss of mtDNA (42). Furthermore, mitochondrial fusion is also associated with increased OXPHOS and ATP generation via remodeling of the cristae (43,44), and downregulation of OPA1, which is responsible for fusion, resulted in mitochondrial dysfunction and mtDNA stress (45). The number of mitochondria is regulated by mitochondrial biogenesis to meet the energy demands of the cells and compensate for their damage (46). A study demonstrated that peroxisome proliferator-activated receptor gamma coactivator (PGC-1) and the protein deacetylase sirtuin 1 (SIRT1) can regulate fusion and OXPHOS (14). Thus, activation of PGC-1α by SIRT1 induces mitochondrial biogenesis and confers metabolic advantages (14). Another study revealed that PGC-1β can induce mitochondrial fusion by upregulating Mfn2 expression via estrogen-related receptor α coactivation (47). Mfn2 expression is downregulated in GC tissues compared with normal gastric mucosal tissues, and is negatively associated with tumor size, indicating an antitumor role of Mfn2 (48). In vitro experiments have demonstrated that overexpression of Mfn2 can suppress gastric cancer cell proliferation and colony formation (48). SIRT1 is an enzyme that mediates NAD+-dependent deacetylation of target substrates (49). Given that the cellular redox balance of NAD+ and NADH is highly associated with catabolic fluxes, SIRT1 can act as a sensor, directly connecting metabolic perturbations with transcriptional output (49). SIRT1 expression is significantly downregulated in GC tissues, which is associated with poor prognosis (50). It has also been reported that SIRT1 exerts inhibitory effects on chemoresistance and cancer stem cell properties via Forkhead box O3 and AMP-activated protein kinase (AMPK) (51). AMPK, another key energy metabolic sensor, plays a key role in maintaining cellular energy homeostasis and is activated upon alterations in the cellular AMP/ATP ratio (52). Previous studies have demonstrated that, upon energy deficiency, AMPK activation may result in increased PGC-1α expression and phosphorylation to modulate the expression of several key players in mitochondrial biogenesis and OXPHOS of fatty acids (53,54) (Fig. 1).
5. Reprogrammed energy metabolism and GC
Energy metabolism is essential for maintaining cellular homeostasis and biological functions, and includes ATP production in the cytosol (glycolysis) and mitochondria (OXPHOS) (55), which can be reprogrammed during carcinogenesis (56). Cancer cells undergo metabolic reprogramming, including enhanced glycolysis, mutations in genes encoding tricarboxylic acid (TCA) cycle enzymes, upregulation of de novo lipid synthesis and glutaminolysis (57). Glycolysis is characterized by an increased rate of glucose uptake and its glycolytic conversion to lactate, even under oxygen-rich conditions (55). There are several pathways and transcriptional regulators involved in the regulation of metabolic reprogramming, such as PI3K/AKT pathway and HIF-1 (58,59). The PI3K/AKT pathway can regulate several aspects of this metabolic program (58). A previous study demonstrated that AKT activation was sufficient to induce glycolysis by promoting glucose transporter 1 and phosphorylating pyruvate dehydrogenase kinase to inhibit pyruvate dehydrogenase and favor lactate dehydrogenase (LDH) activity (60). It has been reported that HIF-1 is overexpressed in human cancers as a result of intratumoral hypoxia, as well as genetic alterations, such as gain-of-function mutations in oncogenes and loss-of-function mutations in tumor suppressor genes (61). HIF-1 may also be triggered by the accumulation of TCA substrates (62), while its degradation is regulated by O2-dependent prolyl hydroxylation (PHs) (61). HIF-1α maintains its stability by avoiding the hydroxylation of PHs in cancer cells, since PHs can be inhibited by the increased levels of cytosolic pyruvate, lactate, succinate, fumarate and ROS (59). Most genes encoding glycolytic enzymes and transporters are the targets of HIF-1α, and its overexpression in cancer cells is associated with increased levels of glycolytic proteins (63). A study revealed that HIF-1α levels were high in certain tumors, even under oxygen-rich conditions, indicating that hormones or growth factors can cause the stabilization of HIF-1α expression, which may serve important roles in carcinogenesis (64). A previous study suggested that HIF-1α can act as a negative regulator of mitochondrial biogenesis and oxidative phosphorylation to inhibit the conversion of pyruvate to acetyl-CoA and mitochondrial respiration and to promote LDH expression (65). HIF-1α activation can also inhibit MYC transcription to further downregulate PGC-1α and PGC-1β expression, which in turn regulates mitochondrial biogenesis and OXPHOS (54). In GC, inhibiting HIF-1α signaling attenuates the migratory and invasive abilities of GC cells, and epithelial-to-mesenchymal transition (66), whereas activation of HIF-1α signaling promotes cell metastasis and glucose metabolism (67).
The tumor microenvironment favors the growth and expansion of cancer and inflammatory cells, which in turn directly or indirectly promotes gastric tumorigenesis by secreting soluble factors or modulating immune responses (68). It has been reported that NF-κB is activated in chronic inflammation, thus promoting the further activation of tumor-promoting genes, such as IL-6 and cyclooxygenase (COX)-2 (69). NF-κB and HIF-1 can link inflammatory signaling to hypoxia and coordinate the activation of both COX-2 and IL-6, and the Janus kinase/STAT3 pathway (70). It has been reported that STAT3 cooperates with NF-κB and HIF-1 in the regulation of both genes (71). NF-κB can be strongly induced by hypoxia and chronic inflammation, and is involved in the reprogramming of tumor glycolysis by interacting with HIF-1α (70). Given that inflammation can induce cells lacking oxygen and upregulate HIF-1α, glycolysis gradually becomes the main energy source instead of OXPHOS (55) (Fig. 1)
6. Association between mitochondrial dynamics and energy metabolism: Fission and glycolysis
Mitochondrial morphological changes are a type of primary signal to shape metabolic reprogramming during cellular quiescence or activation (14,72). Recent studies have demonstrated that increased mitochondrial fission promotes a pro-tumorigenic phenotype (12,73,74). Several studies have been performed in different cell types that alter their mitochondrial morphology to meet their energy demands, functions and behaviors. Conversely, certain cells, such as T cells and stem cells, have higher energy demands to perform their metabolic and cell-specific functions (75,76). When T cells recognize major histocompatibility complexes presented by antigen-presenting cells in response to infection or tumors, they proliferate and differentiate into different T-cell subsets (23). Effector T cells display looser cristae remodeling via fission with reduced electron transport chain (ETC) complexes, thus attenuating ETC efficiency and promoting aerobic glycolysis (23). Conversely, in memory T cells, tight cristae remodeling via fusion with enhanced ETC complex activity is observed, thus enhancing ETC efficiency and OXPHOS (23). Endothelial progenitor cells (EPCs) accelerate glycolysis to produce lactate during angiogenesis by upregulating the expression levels of HIF1α and vascular endothelial growth factor (77). In human EPCs, downregulation of Fis1 expression is associated with mitochondrial dysfunction and may contribute to the impaired activity of EPCs during the senescence process (73). However, upregulation of Fis1 expression in senescent EPCs restores the younger phenotype (73). Another study investigated the function of mitochondrial fission genes in embryonic stem cells (ESCs). Transmission electron microscopy revealed a significant increase in the cytoplasm-to-nucleus ratio and mitochondrial elongation in dynamin-1-like protein (−/−) ESCs caused by incomplete fission. In addition, increased OXPHOS and intracellular ATP concentration and reduced glycolysis was observed, which were associated with mitochondrial elongation (78). The proliferation and invasion of tumor cells also require faster and increased energy supply (79). Thus, Drp1 expression is upregulated in several types of cancer cells, including liver (80), breast (81) and lung cancers (82), and may be considered as a biomarker for predicting poor survival in patients with these types of cancer. A study on ovarian cancer demonstrated that glycolysis is promoted by activating PI3K/AKT/HIF-1α signaling, while mitochondrial fission is enhanced by phosphorylation of Drp1 at Ser616 (83). As a member of the AMPK family, salt-inducible kinase 2 was demonstrated to be involved in both pathways (83). In addition, Drp1 expression was significantly upregulated in pancreatic cancer (PC) cells and tissues via downregulation of microRNA-29a expression (74). High Drp1 expression was associated with poor survival of patients with PC, while Drp1 promoted both the proliferation and metastasis of PC cells, mainly through facilitating aerobic glycolysis (74). Another study revealed that Drp1 may promote KRAS-driven tumor growth by supporting both glycolysis and mitochondrial function (84). Taken together, these findings suggest a mutual association between Drp1 and glycolysis, and the promoting effect of Drp1 and glycolysis on cancer cell proliferation and invasion.
7. Conclusions
GC is the fifth most common type of cancer and the third most common cause of cancer-associated mortality, with 784,000 mortalities reported in 2018 worldwide (85). Early detection and treatment can improve the outcome of patients with GC. Innovative endoscopic techniques may be more accurate in achieving cytological or even biological diagnosis. Mitochondria are strongly associated with carcinogenesis. The present review summarized the role of mitochondria dynamics, reprogramming of energy metabolism and their changes in GC. Based on current literature, it can be concluded that mitochondria in GC are characterized by fission and enhanced glycolysis to meet the increased energy requirements of cancer cells, and decrease necrosis via mitophagy. Upregulated expression levels of Drp1 and HIF-1α are associated with fission and glycolysis, respectively. The balance of mitochondrial fission and fusion and the ratio of glycolysis to OXPHOS are positively associated with different stages of carcinogenesis. When increased fission and glycolysis and decreased apoptosis and fusion are detected in high-risk patients, they may indicate that cells are in the process of malignant transformation. Thus, treatment is required to inhibit this process, which may be a promising approach to the detection of early gastric cancer via organelle- and molecular-level endoscopy in the future.
Acknowledgements
Not applicable.
Funding Statement
The present study was supported by the 1·3·5 project for disciplines of excellence Clinical Research Incubation Project, West China Hospital, Sichuan University, China (grant no. 20HXFH016).
Funding
The present study was supported by the 1·3·5 project for disciplines of excellence Clinical Research Incubation Project, West China Hospital, Sichuan University, China (grant no. 20HXFH016).
Availability of data and materials
Not applicable.
Authors' contributions
HY designed the present review and drafted the initial manuscript. BH contributed to designing and reviewing the manuscript. HY, YL and BH contributed to revising the manuscript for important intellectual content. Data authentication is not applicable. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Pimentel-Nunes P, Libânio D, Marcos-Pinto R, Areia M, Leja M, Esposito G, Garrido M, Kikuste I, Megraud F, Matysiak-Budnik T, et al. Management of epithelial precancerous conditions and lesions in the stomach (MAPS II): European Society of Gastrointestinal Endoscopy (ESGE), European Helicobacter and Microbiota Study Group (EHMSG), European Society of Pathology (ESP), and Sociedade Portuguesa de Endoscopia Digestiva (SPED) guideline update 2019. Endoscopy. 2019;51:365–388. doi: 10.1055/a-0859-1883. [DOI] [PubMed] [Google Scholar]
- 2.Banks M, Graham D, Jansen M, Gotoda T, Coda S, di Pietro M, Uedo N, Bhandari P, Pritchard DM, Kuipers EJ, et al. British Society of Gastroenterology guidelines on the diagnosis and management of patients at risk of gastric adenocarcinoma. Gut. 2019;68:1545–1575. doi: 10.1136/gutjnl-2018-318126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Take S, Mizuno M, Ishiki K, Kusumoto C, Imada T, Hamada F, Yoshida T, Yokota K, Mitsuhashi T, Okada H. Risk of gastric cancer in the second decade of follow-up after Helicobacter pylori eradication. J Gastroenterol. 2020;55:281–288. doi: 10.1007/s00535-019-01654-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shichijo S, Hirata Y. Characteristics and predictors of gastric cancer after Helicobacter pylori eradication. World J Gastroenterol. 2018;24:2163–2172. doi: 10.3748/wjg.v24.i20.2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choi SI, Park B, Joo J, Kim YI, Lee JY, Kim CG, Choi IJ, Kook MC, Cho SJ. Three-year interval for endoscopic screening may reduce the mortality in patients with gastric cancer. Surg Endosc. 2019;33:861–869. doi: 10.1007/s00464-018-6353-3. [DOI] [PubMed] [Google Scholar]
- 6.Horbay R, Bilyy R. Mitochondrial dynamics during cell cycling. Apoptosis. 2016;21:1327–1335. doi: 10.1007/s10495-016-1295-5. [DOI] [PubMed] [Google Scholar]
- 7.Kim SY. Cancer Energy Metabolism: Shutting Power off Cancer Factory. Biomol Ther (Seoul) 2018;26:39–44. doi: 10.4062/biomolther.2017.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou R, Yazdi AS, Menu P, Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011;469:221–225. doi: 10.1038/nature09663. [DOI] [PubMed] [Google Scholar]
- 9.Jung S, Jeong H, Yu SW. Autophagy as a decisive process for cell death. Exp Mol Med. 2020;52:921–930. doi: 10.1038/s12276-020-0455-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bock FJ, Tait SWG. Mitochondria as multifaceted regulators of cell death. Nat Rev Mol Cell Biol. 2020;21:85–100. doi: 10.1038/s41580-019-0173-8. [DOI] [PubMed] [Google Scholar]
- 11.Sun N, Youle RJ, Finkel T. The Mitochondrial Basis of Aging. Mol Cell. 2016;61:654–666. doi: 10.1016/j.molcel.2016.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Srinivasan S, Guha M, Kashina A, Avadhani NG. Mitochondrial dysfunction and mitochondrial dynamics-The cancer connection. Biochim Biophys Acta Bioenerg. 2017;1858:602–614. doi: 10.1016/j.bbabio.2017.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu S, Feng M, Guan W. Mitochondrial DNA sensing by STING signaling participates in inflammation, cancer and beyond. Int J Cancer. 2016;139:736–741. doi: 10.1002/ijc.30074. [DOI] [PubMed] [Google Scholar]
- 14.Maycotte P, Marín-Hernández A, Goyri-Aguirre M, Anaya-Ruiz M, Reyes-Leyva J, Cortés-Hernández P. Mitochondrial dynamics and cancer. Tumour Biol. 2017 May 4; doi: 10.1177/1010428317698391. (Epub ahead of print). doi: 10.1177/1010428317698391. [DOI] [PubMed] [Google Scholar]
- 15.Liu X, Weaver D, Shirihai O, Hajnóczky G. Mitochondrial ‘kiss-and-run’: Interplay between mitochondrial motility and fusion-fission dynamics. EMBO J. 2009;28:3074–3089. doi: 10.1038/emboj.2009.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chan DC. Mitochondrial dynamics and its involvement in disease. Annu Rev Pathol. 2020;15:235–259. doi: 10.1146/annurev-pathmechdis-012419-032711. [DOI] [PubMed] [Google Scholar]
- 17.Schrepfer E, Scorrano L. Mitofusins, from mitochondria to metabolism. Mol Cell. 2016;61:683–694. doi: 10.1016/j.molcel.2016.02.022. [DOI] [PubMed] [Google Scholar]
- 18.Cantó C. Mitochondrial dynamics: Shaping metabolic adaptation. Int Rev Cell Mol Biol. 2018;340:129–167. doi: 10.1016/bs.ircmb.2018.05.004. [DOI] [PubMed] [Google Scholar]
- 19.Ni HM, Williams JA, Ding WX. Mitochondrial dynamics and mitochondrial quality control. Redox Biol. 2015;4:6–13. doi: 10.1016/j.redox.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wai T, Langer T. Mitochondrial dynamics and metabolic regulation. Trends Endocrinol Metab. 2016;27:105–117. doi: 10.1016/j.tem.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 21.Bhatia D, Capili A, Choi ME. Mitochondrial dysfunction in kidney injury, inflammation, and disease: Potential therapeutic approaches. Kidney Res Clin Pract. 2020;39:244–258. doi: 10.23876/j.krcp.20.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yan C, Duanmu X, Zeng L, Liu B, Song Z. Mitochondrial DNA: Distribution, mutations, and elimination. Cells. 2019;8:379. doi: 10.3390/cells8040379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Buck MD, O'Sullivan D, Klein Geltink RI, Curtis JD, Chang CH, Sanin DE, Qiu J, Kretz O, Braas D, van der Windt GJ, et al. Mitochondrial dynamics controls T cell fate through metabolic programming. Cell. 2016;166:63–76. doi: 10.1016/j.cell.2016.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Friedman JR, Nunnari J. Mitochondrial form and function. Nature. 2014;505:335–343. doi: 10.1038/nature12985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Campello S, Lacalle RA, Bettella M, Mañes S, Scorrano L, Viola A. Orchestration of lymphocyte chemotaxis by mitochondrial dynamics. J Exp Med. 2006;203:2879–2886. doi: 10.1084/jem.20061877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu W, Li W, Chen H, Jiang L, Zhu R, Feng D. FUNDC1 is a novel mitochondrial-associated-membrane (MAM) protein required for hypoxia-induced mitochondrial fission and mitophagy. Autophagy. 2016;12:1675–1676. doi: 10.1080/15548627.2016.1193656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Twig G, Elorza A, Molina AJ, Mohamed H, Wikstrom JD, Walzer G, Stiles L, Haigh SE, Katz S, Las G, et al. Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. EMBO J. 2008;27:433–446. doi: 10.1038/sj.emboj.7601963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kumar V, Maity S. ER Stress-sensor proteins and ER-mitochondrial crosstalk-signaling beyond (ER) stress response. Biomolecules. 2021;11:173. doi: 10.3390/biom11020173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Szabadkai G, Simoni AM, Chami M, Wieckowski MR, Youle RJ, Rizzuto R. Drp-1-dependent division of the mitochondrial network blocks intraorganellar Ca2+ waves and protects against Ca2+-mediated apoptosis. Mol Cell. 2004;16:59–68. doi: 10.1016/j.molcel.2004.09.026. [DOI] [PubMed] [Google Scholar]
- 30.Zhang J, Ney PA. Reticulocyte mitophagy: Monitoring mitochondrial clearance in a mammalian model. Autophagy. 2010;6:405–408. doi: 10.4161/auto.6.3.11245. [DOI] [PubMed] [Google Scholar]
- 31.Palikaras K, Lionaki E, Tavernarakis N. Mechanisms of mitophagy in cellular homeostasis, physiology and pathology. Nat Cell Biol. 2018;20:1013–1022. doi: 10.1038/s41556-018-0176-2. [DOI] [PubMed] [Google Scholar]
- 32.Xu Y, Shen J, Ran Z. Emerging views of mitophagy in immunity and autoimmune diseases. Autophagy. 2020;16:3–17. doi: 10.1080/15548627.2019.1603547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu HM, Hu F. The role of autophagy and mitophagy in cancers. Arch Physiol Biochem. 2019 Oct 9; doi: 10.1080/13813455.2019.1675714. (Epub ahead of print). doi: 10.1080/13813455.2019.1675714. [DOI] [PubMed] [Google Scholar]
- 34.Shida M, Kitajima Y, Nakamura J, Yanagihara K, Baba K, Wakiyama K, Noshiro H. Impaired mitophagy activates mtROS/HIF-1α interplay and increases cancer aggressiveness in gastric cancer cells under hypoxia. Int J Oncol. 2016;48:1379–1390. doi: 10.3892/ijo.2016.3359. [DOI] [PubMed] [Google Scholar]
- 35.Kim TW, Lee SJ, Park YJ, Park SY, Oh BM, Park YS, Kim BY, Lee YH, Cho HJ, Yoon SR, et al. Opa-interacting protein 5 modulates docetaxel-induced cell death via regulation of mitophagy in gastric cancer. Tumour Biol. 2017 Oct 15; doi: 10.1177/1010428317733985. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 36.Marzetti E, Lorenzi M, Landi F, Picca A, Rosa F, Tanganelli F, Galli M, Doglietto GB, Pacelli F, Cesari M, et al. Altered mitochondrial quality control signaling in muscle of old gastric cancer patients with cachexia. Exp Gerontol. 2017;87:92–99. doi: 10.1016/j.exger.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 37.Yan H, Qiu C, Sun W, Gu M, Xiao F, Zou J, Zhang L. Yap regulates gastric cancer survival and migration via SIRT1/Mfn2/mitophagy. Oncol Rep. 2018;39:1671–1681. doi: 10.3892/or.2018.6252. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 38.Ferro F, Servais S, Besson P, Roger S, Dumas JF, Brisson L. Autophagy and mitophagy in cancer metabolic remodelling. Semin Cell Dev Biol. 2020;98:129–138. doi: 10.1016/j.semcdb.2019.05.029. [DOI] [PubMed] [Google Scholar]
- 39.de Brito OM, Scorrano L. An intimate liaison: Spatial organization of the endoplasmic reticulum-mitochondria relationship. EMBO J. 2010;29:2715–2723. doi: 10.1038/emboj.2010.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Santel A, Frank S, Gaume B, Herrler M, Youle RJ, Fuller MT. Mitofusin-1 protein is a generally expressed mediator of mitochondrial fusion in mammalian cells. J Cell Sci. 2003;116:2763–2774. doi: 10.1242/jcs.00479. [DOI] [PubMed] [Google Scholar]
- 41.Yu R, Liu T, Ning C, Tan F, Jin SB, Lendahl U, Zhao J, Nistér M. The phosphorylation status of Ser-637 in dynamin-related protein 1 (Drp1) does not determine Drp1 recruitment to mitochondria. J Biol Chem. 2019;294:17262–17277. doi: 10.1074/jbc.RA119.008202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Amati-Bonneau P, Valentino ML, Reynier P, Gallardo ME, Bornstein B, Boissière A, Campos Y, Rivera H, de la Aleja JG, Carroccia R, et al. OPA1 mutations induce mitochondrial DNA instability and optic atrophy ‘plus’ phenotypes. Brain. 2008;131:338–351. doi: 10.1093/brain/awm298. [DOI] [PubMed] [Google Scholar]
- 43.Elezaby A, Sverdlov AL, Tu VH, Soni K, Luptak I, Qin F, Liesa M, Shirihai OS, Rimer J, Schaffer JE, et al. Mitochondrial remodeling in mice with cardiomyocyte-specific lipid overload. J Mol Cell Cardiol. 2015;79:275–283. doi: 10.1016/j.yjmcc.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yao CH, Wang R, Wang Y, Kung CP, Weber JD, Patti GJ. Mitochondrial fusion supports increased oxidative phosphorylation during cell proliferation. Elife. 2019;8:e41351. doi: 10.7554/eLife.41351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rodríguez-Nuevo A, Díaz-Ramos A, Noguera E, Díaz-Sáez F, Duran X, Muñoz JP, Romero M, Plana N, Sebastián D, Tezze C, et al. Mitochondrial DNA and TLR9 drive muscle inflammation upon Opa1 deficiency. EMBO J. 2018;37:e96553. doi: 10.15252/embj.201796553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang H, Zhang Z. Sepsis-induced myocardial dysfunction: the role of mitochondrial dysfunction. Inflamm Res. 2021 Mar 8; doi: 10.1007/s00011-021-01447-0. (Epub ahead of print). doi: 10.1007/s00011-021-01447-0. [DOI] [PubMed] [Google Scholar]
- 47.Liesa M, Borda-d'Agua B, Medina-Gómez G, Lelliott CJ, Paz JC, Rojo M, Palacín M, Vidal-Puig A, Zorzano A. Mitochondrial fusion is increased by the nuclear coactivator PGC-1beta. PLoS One. 2008;3:e3613. doi: 10.1371/journal.pone.0003613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang GE, Jin HL, Lin XK, Chen C, Liu XS, Zhang Q, Yu JR. Anti-tumor effects of Mfn2 in gastric cancer. Int J Mol Sci. 2013;14:13005–13021. doi: 10.3390/ijms140713005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tang BL. Sirt1 and the mitochondria. Mol Cells. 2016;39:87–95. doi: 10.14348/molcells.2016.2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li H, He C, Wang X, Wang H, Nan G, Fang L. MicroRNA-183 affects the development of gastric cancer by regulating autophagy via MALAT1-miR-183-SIRT1 axis and PI3K/AKT/mTOR signals. Artif Cells Nanomed Biotechnol. 2019;47:3163–3171. doi: 10.1080/21691401.2019.1642903. [DOI] [PubMed] [Google Scholar]
- 51.An Y, Wang B, Wang X, Dong G, Jia J, Yang Q. SIRT1 inhibits chemoresistance and cancer stemness of gastric cancer by initiating an AMPK/FOXO3 positive feedback loop. Cell Death Dis. 2020;11:115. doi: 10.1038/s41419-020-2308-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gowans GJ, Hardie DG. AMPK: A cellular energy sensor primarily regulated by AMP. Biochem Soc Trans. 2014;42:71–75. doi: 10.1042/BST20130244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shi HJ, Xu C, Liu MY, Wang BK, Liu WB, Chen DH, Zhang L, Xu CY, Li XF. Resveratrol improves the energy sensing and glycolipid metabolism of blunt snout bream megalobrama amblycephala fed high-carbohydrate diets by activating the AMPK-SIRT1-PGC-1α network. Front Physiol. 2018;9:1258. doi: 10.3389/fphys.2018.01258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Scarpulla RC. Metabolic control of mitochondrial biogenesis through the PGC-1 family regulatory network. Biochim Biophys Acta. 2011;1813:1269–1278. doi: 10.1016/j.bbamcr.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yang H, Du L, Zhang Z. Potential biomarkers in septic shock besides lactate. Exp Biol Med (Maywood) 2020;245:1066–1072. doi: 10.1177/1535370220919076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bose S, Le A. Glucose metabolism in cancer. Adv Exp Med Biol. 2018;1063:3–12. doi: 10.1007/978-3-319-77736-8_1. [DOI] [PubMed] [Google Scholar]
- 57.Pavlova NN, Thompson CB. The emerging hallmarks of cancer metabolism. Cell Metab. 2016;23:27–47. doi: 10.1016/j.cmet.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Alzahrani AS. PI3K/Akt/mTOR inhibitors in cancer: At the bench and bedside. Semin Cancer Biol. 2019;59:125–132. doi: 10.1016/j.semcancer.2019.07.009. [DOI] [PubMed] [Google Scholar]
- 59.Rodríguez-Enríquez S, Marín-Hernández Á, Gallardo-Pérez JC, et al. Transcriptional regulation of energy metabolism in cancer cells. Cells. 2019;8:1225. doi: 10.3390/cells8101225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gonnella R, Santarelli R, Farina A, Granato M, D'Orazi G, Faggioni A, Cirone M. Kaposi sarcoma associated herpesvirus (KSHV) induces AKT hyperphosphorylation, bortezomib-resistance and GLUT-1 plasma membrane exposure in THP-1 monocytic cell line. J Exp Clin Cancer Res. 2013;32:79. doi: 10.1186/1756-9966-32-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3:721–732. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- 62.Gottlieb E, Tomlinson IP. Mitochondrial tumour suppressors: A genetic and biochemical update. Nat Rev Cancer. 2005;5:857–866. doi: 10.1038/nrc1737. [DOI] [PubMed] [Google Scholar]
- 63.Koch A, Ebert EV, Seitz T, Dietrich P, Berneburg M, Bosserhoff A, Hellerbrand C. Characterization of glycolysis-related gene expression in malignant melanoma. Pathol Res Pract. 2020;216:152752. doi: 10.1016/j.prp.2019.152752. [DOI] [PubMed] [Google Scholar]
- 64.Hägg M, Wennström S. Activation of hypoxia-induced transcription in normoxia. Exp Cell Res. 2005;306:180–191. doi: 10.1016/j.yexcr.2005.01.017. [DOI] [PubMed] [Google Scholar]
- 65.Gogvadze V, Orrenius S, Zhivotovsky B. Mitochondria in cancer cells: What is so special about them? Trends Cell Biol. 2008;18:165–173. doi: 10.1016/j.tcb.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 66.Zhou Y, Xu Q, Shang J, Lu L, Chen G. Crocin inhibits the migration, invasion, and epithelial-mesenchymal transition of gastric cancer cells via miR-320/KLF5/HIF-1α signaling. J Cell Physiol. 2019;234:17876–17885. doi: 10.1002/jcp.28418. [DOI] [PubMed] [Google Scholar]
- 67.Gan L, Meng J, Xu M, Liu M, Qi Y, Tan C, Wang Y, Zhang P, Weng W, Sheng W, et al. Extracellular matrix protein 1 promotes cell metastasis and glucose metabolism by inducing integrin β4/FAK/SOX2/HIF-1α signaling pathway in gastric cancer. Oncogene. 2018;37:744–755. doi: 10.1038/onc.2017.363. [DOI] [PubMed] [Google Scholar]
- 68.Oya Y, Hayakawa Y, Koike K. Tumor microenvironment in gastric cancers. Cancer Sci. 2020;111:2696–2707. doi: 10.1111/cas.14521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bruning U, Fitzpatrick SF, Frank T, Birtwistle M, Taylor CT, Cheong A. NFκB and HIF display synergistic behaviour during hypoxic inflammation. Cell Mol Life Sci. 2012;69:1319–1329. doi: 10.1007/s00018-011-0876-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.D'Ignazio L, Bandarra D, Rocha S. NF-κB and HIF crosstalk in immune responses. FEBS J. 2016;283:413–424. doi: 10.1111/febs.13578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lavecchia A, Di Giovanni C, Cerchia C. Novel inhibitors of signal transducer and activator of transcription 3 signaling pathway: An update on the recent patent literature. Expert Opin Ther Pat. 2014;24:383–400. doi: 10.1517/13543776.2014.877443. [DOI] [PubMed] [Google Scholar]
- 72.Mishra P, Chan DC. Metabolic regulation of mitochondrial dynamics. J Cell Biol. 2016;212:379–387. doi: 10.1083/jcb.201511036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang HH, Wu YJ, Tseng YM, Su CH, Hsieh CL, Yeh HI. Mitochondrial fission protein 1 up-regulation ameliorates senescence-related endothelial dysfunction of human endothelial progenitor cells. Angiogenesis. 2019;22:569–582. doi: 10.1007/s10456-019-09680-2. [DOI] [PubMed] [Google Scholar]
- 74.Liang J, Yang Y, Bai L, Li F, Li E. DRP1 upregulation promotes pancreatic cancer growth and metastasis through increased aerobic glycolysis. J Gastroenterol Hepatol. 2020;35:885–895. doi: 10.1111/jgh.14912. [DOI] [PubMed] [Google Scholar]
- 75.Almeida L, Lochner M, Berod L, Sparwasser T. Metabolic pathways in T cell activation and lineage differentiation. Semin Immunol. 2016;28:514–524. doi: 10.1016/j.smim.2016.10.009. [DOI] [PubMed] [Google Scholar]
- 76.Van Wyngene L, Vandewalle J, Libert C. Reprogramming of basic metabolic pathways in microbial sepsis: therapeutic targets at last? EMBO Mol Med. 2018;10:e8712. doi: 10.15252/emmm.201708712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ren R, Guo J, Shi J, Tian Y, Li M, Kang H. PKM2 regulates angiogenesis of VR-EPCs through modulating glycolysis, mitochondrial fission, and fusion. J Cell Physiol. 2020;235:6204–6217. doi: 10.1002/jcp.29549. [DOI] [PubMed] [Google Scholar]
- 78.Seo BJ, Choi J, La H, Habib O, Choi Y, Hong K, Do JT. Role of mitochondrial fission-related genes in mitochondrial morphology and energy metabolism in mouse embryonic stem cells. Redox Biol. 2020;36:101599. doi: 10.1016/j.redox.2020.101599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hsu PP, Sabatini DM. Cancer cell metabolism: Warburg and beyond. Cell. 2008;134:703–707. doi: 10.1016/j.cell.2008.08.021. [DOI] [PubMed] [Google Scholar]
- 80.Lin XH, Qiu BQ, Ma M, Zhang R, Hsu SJ, Liu HH, Chen J, Gao DM, Cui JF, Ren ZG, et al. Suppressing DRP1-mediated mitochondrial fission and mitophagy increases mitochondrial apoptosis of hepatocellular carcinoma cells in the setting of hypoxia. Oncogenesis. 2020;9:67. doi: 10.1038/s41389-020-00251-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Liu B, Fan Y, Song Z, Han B, Meng Y, Cao P, Tan K. Identification of DRP1 as a prognostic factor correlated with immune infiltration in breast cancer. Int Immunopharmacol. 2020;89:107078. doi: 10.1016/j.intimp.2020.107078. [DOI] [PubMed] [Google Scholar]
- 82.Rehman J, Zhang HJ, Toth PT, Zhang Y, Marsboom G, Hong Z, Salgia R, Husain AN, Wietholt C, Archer SL. Inhibition of mitochondrial fission prevents cell cycle progression in lung cancer. FASEB J. 2012;26:2175–2186. doi: 10.1096/fj.11-196543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gao T, Zhang X, Zhao J, Zhou F, Wang Y, Zhao Z, Xing J, Chen B, Li J, Liu S. SIK2 promotes reprogramming of glucose metabolism through PI3K/AKT/HIF-1α pathway and Drp1-mediated mitochondrial fission in ovarian cancer. Cancer Lett. 2020;469:89–101. doi: 10.1016/j.canlet.2019.10.029. [DOI] [PubMed] [Google Scholar]
- 84.Nagdas S, Kashatus JA, Nascimento A, Hussain SS, Trainor RE, Pollock SR, Adair SJ, Michaels AD, Sesaki H, Stelow EB, et al. Drp1 promotes KRas-driven metabolic changes to drive pancreatic tumor growth. Cell Rep. 2019;28:1845–1859.e5. doi: 10.1016/j.celrep.2019.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.