Abstract
Background
This systematic review aimed to investigate whether diabetes mellitus is a risk factor for low bone density, as this might be important and necessary for doctors specialized in treating patients with low bone density.
Methods
PubMed, Embase, CINAHL, and SciELO were searched for cohort, case-control, and cross-sectional studies that investigated the effects of diabetes mellitus on bone mineral density till January 2020. Data screening and extraction are done independently, whereas the methodological quality of the studies was assessed according to the Newcastle-Ottawa Scale (NOS).
Results
A total of 14 studies that met the eligibility criteria including 24,340 participants were enrolled. The overall quality of the studies had a scale of over 6 points. The overall odds ratio (OR) regarding the risk of diabetes mellitus in low bone density patients was 1.20 [95% confidence interval (CI)0.80–1.79, P = 0.30], and type 2 diabetes mellitus (T2DM) (OR = 0.69 [0.11, 4.55], P = 0.70). Subgroup analysis revealed that whether females or males, developed or developing countries, T2DM, studies after 2015, and quality over 7 points (all P values > 0.05) showed no significant differences with the risk of low bone density, except type 1 diabetes mellitus (T1DM) (OR = 3.83 [1.64, 8.96], P = 0.002), and studies before 2015 (OR = 1.76 [1.06, 2.92], P = 0.03), and quality below 7 points (OR = 2.27 [1.50, 3.43], P = 0.0001). Funnel plot showed no significant asymmetry.
Conclusions
These findings revealed no relationship between T2DM and low bone density, and also, the evidence between T1DM and low bone density is inadequate, requiring further analysis of well-designed cohort studies.
Keywords: Osteoporosis, Diabetes mellitus, Systematic review, Meta-analysis, Risk factors
Background
Osteoporosis is a chronic metabolic bone disease that is characterized by decreased bone mass and deterioration of microarchitectural bone tissue, which leads to fractures in individuals [1]. It has been reported that 50% of women and 20% of men over 50 years of age experience osteoporotic-related fracture, causing morbidity and mortality [2]. The imbalance in the activities of osteoclasts and osteoblasts resulted in decreased bone mineral density and irreversible bone mass loss by accelerating bone resorption and/or slow bone formation [3, 4]. Clinically, low bone density most often occurs through estrogen reduction in postmenopausal women and age-related bone loss in females [5, 6]. Other risk factors of osteoporosis included gene, cigarette smoking, alcohol consumption, abnormally high plasma serum parathyroid hormone (PTH) levels, physical inactivity, and chronic use of some medications, for example, corticosteroids [7–9]. Inadequate physical activity always leads to a sedentary lifestyle in the elderly, paralyzed, or limited activity due to accelerated bone loss [10–12].
Diabetes mellitus is a chronic metabolic disease, especially type 2 diabetes mellitus (T2DM), in which insulin resistance might lead to hyperglycemia. There are a total of 383 million people around the world (8.3%) who suffer from T2DM, and it is estimated that the number of patients will reach 592 million by 2035, with a prevalence of 10% [13]. Poor diabetes management is associated with heart disease, stroke, blindness, renal failure, foot amputation, and even death [14]. Diabetes and osteoporosis are both common disease conditions, especially in older patients, and might occur together at times. More than 50 years ago, Albright and Reifenstein proved that diabetes mellitus might possibly show association with bone mass loss, resulting in osteoporosis [14]. Since then, a lot of attention has been paid by several researchers [14–16]. The pathogenesis of low bone density in type 1 diabetes mellitus (T1DM) is related to decreased peak bone mass because of deficiency in insulin and insulin-like growth factors, leading to slow osteoblast growth and poor collagen synthesis [17]. The interaction, in turn, exists between T2DM and bone health due to several factors, including the direct effects of T2DM on bone metabolism and strength and indirect effects of antidiabetic medication-induced altered bone metabolism [18].
However, the research results on the effects of T2DM on bone mineral density (BMD) in clinical epidemiology still remained controversial. Some authors have reported that T2DM is associated with low bone density, few others reported normal bone density, and then few others showed increased BMD [19–21]. Two systematic reviews were conducted in China and Iran, and the pooled prevalence of osteoporosis in T2DM patients in China was 37.8% [22], while the prevalence of lumbar and femoral neck osteoporosis in postmenopausal Iranian women with T2DM was 25.26 and 17.45%, respectively [23]. Although these two reviews claimed that osteoporosis had affected quite a large number of patients with T2DM in China mainland and Iran, these two studies could not still answer the question of whether diabetes mellitus is a risk factor of low bone density?
Hence, this systematic review was conducted to investigate whether diabetes mellitus is a risk factor for low bone density based on a large sample size to provide evidence for physicians as well as the health supervision department.
Methods
Evidence acquisition
The guidelines of the Meta-analysis of Observational Studies in Epidemiology Group [24] were followed for the present meta-analysis.
Data sources and searches
The studies were systematically searched from the PubMed, Embase, CINAHL, and SciELO databases from their inception to January 2020. The search strategy included different combinations of search terms related to risk factors, diabetes, osteoporosis, and low bone density. The search terms used in the present meta-analysis were diabetes mellitus, diabetes, DM, T2DM, type 2 diabetes mellitus, type 2 DM, T1DM, type 1 diabetes mellitus, type 1 DM, osteoporosis, and low bone density in all fields. There was no restriction regarding the country. The reference lists of the retrieved articles and relevant review articles were searched manually for any new studies. In the present meta-analysis, only the data of published articles were included to ensure the quality of studies and results. The search was limited to the English language.
Inclusion and exclusion criteria
Type of studies
Although there are potential limitations for meta-analysis of observational studies, no evidence could be obtained with regard to some areas of health policy from randomized controlled trials. One such example is regarding the association of diabetes mellitus with osteoporosis or low bone density. Thus, cohort studies, cross-sectional studies, and case-control studies were included. If a published study had more than one publication, then the most recent publication or publication with the most complete dataset was selected.
Type of participants
Low bone density was defined as BMD values of 2.5 standard deviations below the mean value for young adults (T score ≤ 2.5) based on the lowest T score at the skeletal site according to the International Society for Clinical Densitometry (one diagnostic category) [25]. To ensure the quality of studies and results, the present review included studies with a sample size of over 100 patients.
Exposure factors
The factors related to low bone density included diabetes mellitus, irrespective of type 1 diabetes mellitus (T1DM) or T2DM.
Outcomes
The outcomes reported were adjusted or non-adjusted odds ratios (ORs) with a corresponding measure of variance or original categorical data related to the risk factors of diabetes mellitus to low bone density.
Exclusion criteria
The exclusion criteria were (1) reviews, comments, and lectures, (2) repeated studies, (3) the results in the studies could not be transformed into relative risks (RRs) and their 95% CIs, (4) animal or cell studies, and (5) studies that explored only the mechanism of diabetes mellitus.
Data extraction
One reviewer (Jingying Qiu) gathered all the papers that presented the risk factors associated with low bone density, including T1DM and T2DM. The studies were selected for inclusion by two reviewers (Jingying Qiu and Zhichun Dong) independently. After deleting the duplications, the titles and abstracts of all identified potential studies were screened. The full texts of all possibly relevant articles were retrieved for comprehensive assessment based on the inclusion criteria. Any disagreements between the two reviewers were resolved by discussion or by reaching a consensus with a third reviewer (Chengjiang Li).
The required data such as the first author, publication year, country, design, sample size, source, sex, and diabetes type, and diagnosis were entered into a pre-designed form. To minimize study selection bias, data extraction and quality evaluation were done by two reviewers (Jingying Qiu and Zhichun Dong) independently. Any disagreements were resolved by consulting a third reviewer (Chengjiang Li).
Assessment of quality
The quality of included studies was assessed using the Newcastle Ottawa Scale (NOS) for case-controlled studies [24] by two reviewers (Jingying Qiu and Zhichun Dong) independently. Any disagreements were resolved by consulting a third reviewer (Chengjiang Li). NOS for case-controlled studies were assessed based on the following items: (1) 1 point for the adequacy of case definition; (2) 1 point for the representativeness of the cases; (3) 1 point for controls’ selection; (4) 1 point for controls’ definition; (5) 2 points for comparability of the cases and controls according to the design or analysis; (6) 1 point for exposure ascertainment; (7) 1 point for the same method of ascertainment for the cases and controls; and (8) 1 point for the non-response rate) [24]. The studies that scored 5 or more NOS criteria were considered as high quality [26, 27]. As only case-controlled studies were included in this systematic review, only the items of NOS case-controlled studies were listed.
Statistical analysis
The RevMan analysis software (RevMan 5.3) from the Cochrane Collaboration was used in this meta-analysis. The OR with 95% CI was used to estimate the strength of the association for dichotomous variables. Heterogeneity was quantified using the I2 index. If the I2 test indicated significant heterogeneity, i.e., a value > 50%, then a random-effects model was conducted; otherwise, a fixed-effects model was used. Subgroup analysis was conducted based on sex (male or female), economic level (developed or developing countries), and type of diabetes (T1DM or T2DM). A funnel plot was conducted to detect publication bias.
Results
Characteristics of studies included
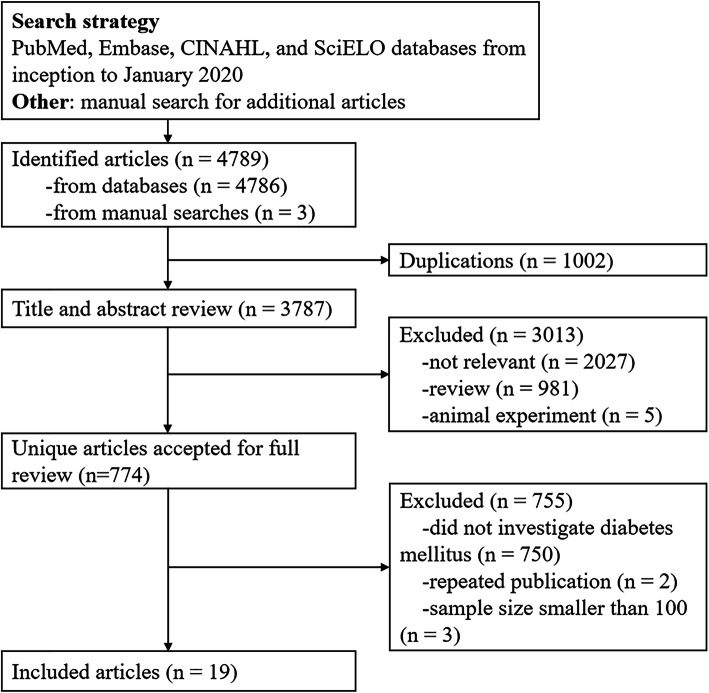
A total of 4789 publications were identified through multiple search engines, and 1002 of these were excluded due to duplications. After examining the titles and abstracts, 3013 articles were excluded. After reviewing the full-texts of the remaining 774 articles, 3 studies were excluded due to sample size smaller than 100, 2 studies were excluded due to repeated publications, 750 studies were excluded as diabetes mellitus was not investigated, and 5 studies were excluded because no information is given about BMD [28–32]. Finally, 14 relevant studies were included in this systematic review [33–46] (Fig. 1).
Fig. 1.
Summary of study identification and selection process
These 14 case-controlled studies included 24,340 participants, and the sample size ranged from 255 to 6267. Five studies were conducted in China [33, 34, 39, 44, 46], two in Korea [43, 45], two in Jordan [35, 41], and the remaining in other countries. Most of the low bone density patients underwent dual-energy X-ray absorptiometry. Four studies were conducted only in females [35, 38, 40, 41], one study only in males [45], one study reported the data on both females and males separately [33], and the remaining included both females and male participants. Two studies only checked T2DM patients [37, 41], and one study checked T1DM and T2DM separately [40]. The main characteristics of the included studies are presented in Table 1.
Table 1.
The characteristics of studies included in the meta-analysis
| Study | Country | Design | Participant | Diagnose | Sex | Sample size | Diabetes type |
|---|---|---|---|---|---|---|---|
| Shaw CK 1993 [33] | China-Taiwan | Case-controlled study | Volunteers (15 to 83 years old) living in Lin-Kou Township | BMD of the lumbar spine (L2 to L4) measured by dual-photon absorptiometry (DP4, Lunar Radiation Corp, Madison, WI) | Female and male | 404 | Both |
| Chumnumnawin M 2008 [34] | Thailand | Case-controlled study | Priests older than 20 years old to the out-patients Department of Priest Hospital with other complaints | BMD of calcaneum measured by ultrasonography | Both | 659 | Both |
| El-Heis MA 2013 [35] | Jordan | Case-controlled study | Women referred to the Radiology Department at King Abdullah University Hospital | BMD of the lumbar spine (L1 to L4) and femoral hip (neck, trochanter) measured by DXA | Female | 384 | Both |
| Zhou R 2013 [36] | China | Case-controlled study | Sampled from three randomly selected communities in Chongqing, aged 60 and over | BMD of the femoral neck measured by DXA (Prodigy fan beam densitometer; Lunar, GE Medical System, Madison, WI) | Both | 1729 | Both |
| Daisuke A 2015 [37] | Japan | Case-controlled study | Consecutive outpatients aged 50 years at our hospital | BMD of the lumbar spine (L2 to L4) measured by DXA | Both | 255 | T2DM |
| Saei GNM 2015 [38] | Iran | Case-controlled study | 360 non-pregnant women over the age of 15 who referred for bone density testing to the Urmia Imam Khomeini Academic Hospital | BMD of the femoral neck and the lumbar spine L1-L4 measured by DXA | Female | 360 | Both |
| Liu D 2016 [39] | China | Case-controlled study | Local elderly people in 8 communities Chongqing city | BMD of vertebral and femoral neck measured by DXA (GE Medical Systems, Madison, WI) | Both | 1802 | Both |
| Neglia C 2016 [40] | Italy | Case-controlled study | Postmenopausal subjects from the Ionian and Salento Osteoporosis Registry/Euro Mediterranean Registry of Osteoporosis | AD-SoS T-score of distal metaphysis of the first phalanges by ultrasound measurements performed by DBM Sonic Bone Profiler 1200 | Female | 4909 | T1/2DM |
| Dana H 2017 [41] | Jordan | Case-controlled study | All Jordanian women aged over 45 years, of menopausal duration of more than 1 year | BMD of the lumbar spine L1–L4 and left femoral neck measured by DXA | Female | 1079 | T2DM |
| Heidari B 2017 [42] | Iran | Case-controlled study | Aged 60 years and older from the Amirkola health and ageing project cohort | BMD of the lumbar spine (L2 to L4) and femoral neck measured by DXA | Both | 553 | Both |
| Lee SH 2017 [43] | Korea | Representative case-controlled study | Adults older than 50 years of age from the Korea National Health and Nutrition Examination Survey | BMD of the lumbar spine, femoral neck, and total proximal femur measured by DXA (Hologic Inc., Bedford, MA, USA) | Both | 1081 | Both |
| Lin HH 2018 [44] | China-Taiwan | Case-controlled study | Participants older than 50 years, underwent a health examination at a preventive examination agency in urban Taiwan | BMD of the lumbar spine, total hip, and femoral neck measured by DXA (LunarProdigy Advance; GE Healthcare, Madison, WI, USA) | Both | 2007 | Both |
| Yoo JE 2018 [45] | Korea | Case-controlled study | Men aged ≥30 years and who underwent dual-energy X-ray absorptiometry in Korean National Health and Nutrition Survey | BMD of the femoral neck, totalfemur, and lumbar spine (L1–L4) measured by DXA (DISCOVERY-W fan-beam densitometer;Hologic, Bedford, MA, USA) | Male | 6104 | Both |
| Wang Y 2019 [46] | China | Case-controlled study | General middle-aged and older population in China | Mean BMD measured by DXA (Alara Inc., Fremont, CA, US) | Both | 6267 | Both |
BMD bone mineral density, DXA dual-energy X-ray absorptiometry
The quality of the studies
Most of the studies reported the definition of cases. Four studies demonstrated potential selection bias or did not demonstrate the representativeness of the cases [33, 35, 40, 44]. Only one study did not report the details regarding the selection of controls [35]. The comparability was done in all studies. All studies used a structured interview for blinding to case/control status in order to identify the exposure factors. The response rates were reported in all the studies. The overall quality of the studies had a scale of 6 points and was presented in Table 2.
Table 2.
Quality assessment of studies included in this meta-analysis by Newcastle-Ottawa Scale
| Study | Selection | Comparability | Exposure | Quality evaluation | |||||
|---|---|---|---|---|---|---|---|---|---|
| Cases definition | Cases representativeness | Control selection | Controls definition | Comparability | Ascertainment of exposure | Same ascertainment | Non-response rate | ||
| Shaw CK 1993 [33] | a | b | a | a | b | b | a | a | 7 |
| Chumnumnawin M 2008 [34] | a | a | b | a | b | b | a | a | 7 |
| El-Heis MA 2013 [35] | a | b | b | a | b | b | a | a | 6 |
| Zhou R 2013 [36] | a | a | a | a | a + b | b | a | a | 8 |
| Daisuke A 2015 [37] | a | a | b | a | b | b | a | a | 7 |
| Saei GNM 2015 [38] | a | a | b | a | b | b | a | a | 7 |
| Liu D 2016 [39] | a | a | a | a | a + b | b | a | a | 9 |
| Neglia C 2016 [40] | a | b | c | a | b | b | a | a | 6 |
| Dana H 2017 [41] | a | a | a | a | b | b | a | a | 8 |
| Heidari B 2017 [42] | a | a | a | a | b | b | a | a | 8 |
| Lee SH 2017 [43] | a | a | a | a | b | b | a | a | 8 |
| Lin HH 2018 [44] | a | b | b | a | b | b | a | a | 6 |
| Yoo JE 2018 [45] | a | a | b | a | a + b | b | a | a | 8 |
| Wang Y 2019 [46] | a | a | b | a | b | b | a | a | 7 |
Total
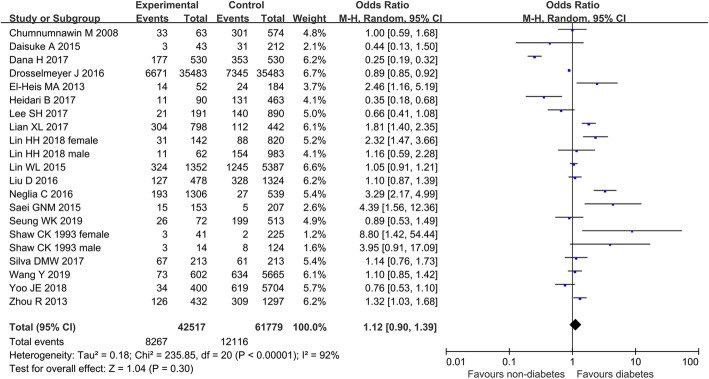
Fourteen studies combined low bone density rates for 4599 diabetic and 19,741 non-diabetic patients. The results showed a statistically significant heterogeneity among the studies (I2 = 93%, P < 0.00001), and no difference was observed with regard to the prevalence of diabetes between osteoporotic and normal patients (14 studies, n = 24,340, OR = 1.20, 95% CI = [0.80, 1.79], P = 0.38). Forest plot of diabetes-related to low bone density was presented in Fig. 2.
Fig. 2.
Forest plot on the relationship of diabetes mellitus and osteoporosis
Subgroup analysis
Subgroup analysis was conducted based on different populations, economic level, sex, diabetes type, and quality. The results revealed no significant differences regarding the prevalence of diabetes between low bone density and normal patients in Asians (14 studies, n = 22,495, OR = 1.10, 95% CI = [0.74, 1.63], P = 0.64), but not in Europeans (1 study, n = 1845, OR = 3.29, 95% CI = [2.17, 4.99], P < 0.00001). The results showed differences with regard to diabetes rate between Asian and European populations (I2 = 92.8%, P = 0.0002).
The studies before 2015 revealed positive correlation between diabetes and low bone density (6 studies, n = 3621, OR = 1.76, 95% CI = [1.06, 2.92], P = 0.03), while the studies after 2015 showed no significant differences (8 studies, n = 20,719, OR = 0.92, 95% CI = [0.53, 1.59], P = 0.77).
Four studies were conducted in developed countries, and 10 studies in developing countries. There was no difference regarding the prevalence of diabetes between low bone density and normal patients both in developed (4 studies, n = 9285, OR = 0.99, 95% CI = [0.409, 2.41], P = 0.97) and developing countries (10 studies, n = 15,055, OR = 1.28, 95% CI = [0.80, 2.07], P = 0.31). The results showed no significant differences in diabetes rate between developed and developing countries (I2 = 0%, P = 0.61).
Seven studies have reported either in female or male patients only, or separately. There was no difference in the prevalence rate of diabetes between low bone density and normal patients both in females (6 studies, n = 4729, OR = 2.21, 95% CI = [0.62, 7.83], P = 0.22) and males (3 studies, n = 1736, OR = 1.02, 95% CI = [0.31, 3.39], P = 0.97). The results showed no differences with regard to diabetes rate between female and male patients (I2 = 0%, P = 0.39).
Three studies reported T1DM or T2DM only, or separately. The results showed differences in the rate of diabetes between low bone density and normal patients both in T1DM (1 study, n = 1845, OR = 3.83, 95% CI = [1.64, 8.96], P = 0.002), while no difference in T2DM patients (3 studies, n = 3160, OR = 0.69, 95% CI = [0.11, 4.55], P = 0.70). These results suggested significant differences in the diabetic rate between T1DM and T2DM patients (I2 = 62.1%, P = 0.10).
There was no difference in the rate of diabetes between low bone density and normal patients both in studies with quality of over 7 points (12 studies, n = 20,837, OR = 0.94, 95% CI = [0.63, 1.41], P = 0.76), and below 7 points, showing significant differences (3 studies, n = 4088, OR = 2.27, 95% CI = [1.50, 3.43], P = 0.0001). These results suggested significant differences in diabetic rate between studies with quality of over and below 7 points (I2 = 88.7%, P = 0.0001). The results of subgroup analysis were presented in Table 3.
Table 3.
The results of subgroup analysis
| Subgroup | Studies | Heterogeneity analysis | OR | P value |
|---|---|---|---|---|
| Population | ||||
| Asian | 14 | 91%, P < 0.00001 | 1.08 [0.74, 1.57] | 0.69 |
| European | 1 | / | 3.29 [2.17, 4.99] | P < 0.00001 |
| Year | ||||
| Before 2015 | 6 | 67%, P = 0.006 | 1.76 [1.06, 2.92] | 0.03 |
| After 2015 | 9 | 95%, P < 0.00001 | 0.92 [0.56, 1.52] | 0.75 |
| Sex | ||||
| Female | 6 | 97%, P < 0.00001 | 2.21 [0.62, 7.83] | 0.22 |
| Male | 3 | 83%, P = 0.003 | 1.02 [0.31, 3.39] | 0.97 |
| Economic Level | ||||
| Developed countries | 5 | 89%, P < 0.00001 | 0.98 [0.49, 1.96] | 0.95 |
| Developing countries | 10 | 93%, P < 0.00001 | 1.28 [0.80, 2.07] | 0.31 |
| Diabetes type | ||||
| T1DM | 1 | /, P = 0.002 | 3.83 [1.64, 8.96] | 0.002 |
| T2DM | 3 | 98%, P < 0.00001 | 0.69 [0.11, 4.55] | 0.70 |
| Quality | ||||
| Over 7 points | 13 | 91%, P < 0.00001 | 0.89 [0.67, 1.17] | 0.40 |
| Below 7 points | 6 | 86%, P < 0.00001 | 1.71 [1.19, 2.45] | 0.004 |
OR odds ratio, T2DM type 2 diabetes mellitus, T1DM type 1 diabetes mellitus
Sensitivity analysis
Sensitivity analysis revealed that after excluding 8 studies [26, 32, 33, 35–37, 39, 41], the results still remained the same (11 studies, n = 25,861, OR = 1.05, 95% CI = [0.91, 1.20], P = 0.52).
Publication Bias
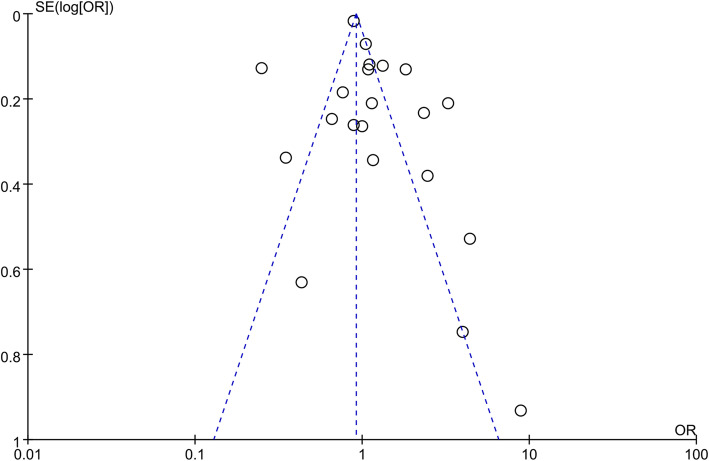
No significant asymmetry was observed in the funnel plot (Fig. 3).
Fig. 3.
Funnel plot for publication bias
Discussion
This is the first report based on the epidemiological data to study whether diabetes mellitus is a risk factor for low bone density. A total of 14 articles with 24,340 participants were included in this review, and the results suggested that the evidence regarding the relationship between diabetes mellitus and low bone density still remains to be inadequate. The overall quality of each study was over 6 points, and so publication bias was ruled out. Subgroup analysis revealed that females or males, developed or developing countries and T2DM patients showed no significant difference with regard to the risk of low bone density, and also, the evidence between T1DM and low bone density remains to be inadequate. The damage of T1DM and T2DM on bone health was different.
Low bone density with type 1 diabetes mellitus
T1DM, which is also considered insulin-dependent diabetes, occurs when insulin is insufficient and causes hyperglycemia in young patients [47, 48]. It is usually diagnosed in childhood or early adulthood. Only one study explored the risk factor of T1DM to low bone density, and so this systematic review could not provide evidence of whether T1DM acts as a risk factor for osteoporosis. Previous studies have shown that the risk of hip fracture is increased in both T1DM and T2DM patients, whereas low bone density based on bone scans occurs more frequently in T1DM than expected, while the incidence in T2DM patients is lower [49]. The latter might be due to the differences in body size, i.e., the T2DM patients were more obese or had a higher body mass index than the general population, and BMD increases with increasing body size [50]. T1DM occurs due to overall poor bone health and lower peak bone mass in adolescents and is mainly manifested due to the following aspects: (1) abnormal growth hormone (GH)-insulin-like growth factor-1 (IGF-1) axis, which leads to bone loss before the bone reaches to its peak mass in a decreased adolescent growth potential [51, 52]; (2) disobeying planned medical management, making metabolic disorder worse [53, 54]; (3) dietary control leads to insufficient dietary calcium intake [55]; and (4) increased urinary calcium excretion [56]. In addition, individuals with T1DM were associated with an increased risk of celiac disease, leading to intestinal malabsorption, poor growth, and low bone density [57]. While the insulin to control glucose in T1DM patients was reported to improve bone health [21].
Low bone density with type 2 diabetes mellitus
Several studies indicated that despite good BMD results, T2DM patients showed an exact association with a higher risk of fragility fractures. However, some other studies have reported that diabetes mellitus patients are associated with fractures due to lower BMD than those who did not. In this study, only three studies have reported the risk factors of T2DM to low bone density, showing a negative association. In addition, there are bone structural abnormalities, such as obvious loss of trabecular bone loss but decreased cortical BMD and increased cortical porosity, which in turn decreases the bone strength that is associated with lower strength stress index [58]. Lower strength stress index is an important predictor of fracture [59, 60]. Also, the pathogenesis might vary in different T2DM patient populations, which might be due to obesity, old age, diabetic complications, duration, and medication. T2DM is a variable disease condition, and similar is the condition with osteoporosis. The best example is the effect of peroxisome proliferator-activated receptor-γ (PPAR-γ) agents such as thiazolidinediones (TZDs), rosiglitazone, and pioglitazone [61]. These agents might not be so commonly used at the moment now as the former has been removed from the market, and the latter was reduced in application due to side effects, such as increased fracture risk. In bone, PPAR-γ controls the differentiation of mesenchymal and hematopoietic cells. The activation of PPAR-γ by TZDs causes an imbalance in bone remodeling, increases bone resorption, and decreases bone formation. Laboratory studies suggested that the use of selective PPAR-γ modulators can separate the harmful effects of PPAR-γ on the bone from its beneficial antidiabetic effects [61]. It was found that women with T2DM who initiated insulin intake experienced more rapid BMD loss at the femoral neck when compared to those women who did not use insulin [62].
Limitation of this review
This meta-analysis is conducted based on several case-controlled studies in many different countries, and included a relatively large sample size, and ruled out publication bias. However, the evidence collected is still limited. Firstly, the case-controlled studies were less expensive and time-consuming and required the collection of a larger mass of data with a low evidence level. Also, most of the studies were single-center studies, and so the conclusions still need further confirmation. In addition, due to the different characteristics of the studies, heterogeneity was considered inevitable, although sensitivity analysis could not change the results. The potential sources of heterogeneity mainly occur due to different types of diabetes mellitus or other reasons. What’s more, the included studies evaluated BMD at different bone sites with different techniques (DXA and ultrasonography or radiographic absorptiometry) and so are not adequately comparable.
Conclusions
This systematic review and meta-analysis confirmed that the evidence regarding the relationship between diabetes mellitus and low bone density is inadequate. Subgroup analysis revealed that whether females or males, developed or developing countries and T2DM patients showed no significant differences with regard to the risk of low bone density, and also between T1DM and low bone density. Due to differences in T1DM and T2DM, and due to close relation to female menopause, our study suggested that the reporting of the details separately in females and males, and it should be clear whether it is T1DM or T2DM. So, well-designed cohort studies are expected in the future for further confirmation of our study results.
Acknowledgements
Not applicable.
Abbreviations
- NOS
Newcastle-Ottawa Scale
- OR
Odds ratio
- CI
Confidence interval
- PTH
Parathyroid hormone
- T2DM
Type 2 diabetes mellitus
- BMD
Bone mineral density
- DXA
Dual-energy X-ray absorptiometry
- TZDs
Thiazolidinediones
Authors’ contributions
JYQ carried out the studies, participated in collecting data, and drafted the manuscript. CJL performed the statistical analysis and participated in its design. ZCD and JW helped to draft the manuscript. All authors read and approved the final manuscript.
Funding
Not applicable.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The meta-analysis based on public literature is not applicable for ethical approval.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jingying Qiu, Email: 41539377@qq.com.
Chengjiang Li, Email: hzcjli03@163.com.
Zhichun Dong, Email: lao-dong-de@163.com.
Jing Wang, Email: wjing0525@163.com.
References
- 1.Zarowitz BJ, Cheng LI, Allen C, O’Shea T, Stolshek B. Osteoporosis prevalence and characteristics of treated and untreated nursing home residents with osteoporosis. J Am Med Dir Assoc. 2015;16(4):341–348. doi: 10.1016/j.jamda.2015.01.073. [DOI] [PubMed] [Google Scholar]
- 2.Cosman F, de Beur SJ, LeBoff MS, Lewiecki EM, Tanner B, Randall S, et al. Clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int. 2014;25(10):2359–2381. doi: 10.1007/s00198-014-2794-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zaidi M. Skeletal remodeling in health and disease. Nat Med. 2007;13(7):791–801. doi: 10.1038/nm1593. [DOI] [PubMed] [Google Scholar]
- 4.Sealand R, Razavi C, Adler RA. Diabetes mellitus and osteoporosis. Curr Diab Rep. 2013;13(3):411–418. doi: 10.1007/s11892-013-0376-x. [DOI] [PubMed] [Google Scholar]
- 5.Li H, Xiao Z, Quarles LD, Li W. Osteoporosis: Mechanism, Molecular Target, and Current Status on Drug Development. Curr Med Chem. 2020. 10.2174/0929867327666200330142432. [DOI] [PMC free article] [PubMed]
- 6.Fistarol M, Rezende CR, Figueiredo Campos AL, Kakehasi AM, Geber S. Time since menopause, but not age, is associated with increased risk of osteoporosis. Climacteric. 2019;22(5):523–526. doi: 10.1080/13697137.2019.1634046. [DOI] [PubMed] [Google Scholar]
- 7.Gao ST, Lv ZT, Zhou CK, Mao C, Sheng WB. Association between IGF-1 polymorphisms and risk of osteoporosis in Chinese population: a meta-analysis. BMC Musculoskelet Disord. 2018;19(1):141. doi: 10.1186/s12891-018-2066-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fu SC, Wang P, Qi MX, Peng JP, Lin XQ, Zhang CY, Zhao GX, He GH. The associations of TNF-alpha gene polymorphisms with bone mineral density and risk of osteoporosis: a meta-analysis. Int J Rheum Dis. 2019;22(9):1619–1629. doi: 10.1111/1756-185X.13647. [DOI] [PubMed] [Google Scholar]
- 9.Chen YW, Ramsook AH, Coxson HO, Bon J, Reid WD. Prevalence and risk factors for osteoporosis in individuals with COPD: a systematic review and meta-analysis. Chest. 2019;156(6):1092–1110. doi: 10.1016/j.chest.2019.06.036. [DOI] [PubMed] [Google Scholar]
- 10.Schacter GI, Leslie WD. Diabetes and bone disease. Endocrinol Metab Clin N Am. 2017;46(1):63–85. doi: 10.1016/j.ecl.2016.09.010. [DOI] [PubMed] [Google Scholar]
- 11.Chau DL, Edelman SV, Chandran M. Osteoporosis and diabetes. Curr Diab Rep. 2003;3(1):37–42. doi: 10.1007/s11892-003-0051-8. [DOI] [PubMed] [Google Scholar]
- 12.Merlotti D, Gennari L, Dotta F, Lauro D, Nuti R. Mechanisms of impaired bone strength in type 1 and 2 diabetes. Nutr Metab Cardiovasc Dis. 2010;20(9):683–690. doi: 10.1016/j.numecd.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 13.Domingueti CP, Dusse LM, Carvalho M, de Sousa LP, Gomes KB, Fernandes AP. Diabetes mellitus: the linkage between oxidative stress, inflammation, hypercoagulability and vascular complications. J Diabetes Complicat. 2016;30(4):738–745. doi: 10.1016/j.jdiacomp.2015.12.018. [DOI] [PubMed] [Google Scholar]
- 14.Albright FRE. Bone development in diabetic children: A roentgen study. Am J Med Sci. 1948;12:313–319. [Google Scholar]
- 15.Strotmeyer ES, Cauley JA, Schwartz AV, Nevitt MC, Resnick HE, Zmuda JM, Bauer DC, Tylavsky FA, de Rekeneire N, Harris TB, Newman AB, Health ABC Study Diabetes is associated independently of body composition with BMD and bone volume in older white and black men and women: the health, aging, and body composition study. J Bone Miner Res. 2004;19(7):1084–1091. doi: 10.1359/JBMR.040311. [DOI] [PubMed] [Google Scholar]
- 16.van Daele PL, Stolk RP, Burger H, Algra D, Grobbee DE, Hofman A, Birkenhäger JC, Pols HA. Bone density in non-insulin-dependent diabetes mellitus. The Rotterdam Study. Ann Intern Med. 1995;122(6):409–414. doi: 10.7326/0003-4819-122-6-199503150-00002. [DOI] [PubMed] [Google Scholar]
- 17.Nyman JS, Even JL, Jo CH, Herbert EG, Murry MR, Cockrell GE, Wahl EC, Bunn RC, Lumpkin CK, Jr, Fowlkes JL, Thrailkill KM. Increasing duration of type 1 diabetes perturbs the strength-structure relationship and increases brittleness of bone. Bone. 2011;48(4):733–740. doi: 10.1016/j.bone.2010.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goswami R, Nair A. Diabetes mellitus, vitamin D & osteoporosis: Insights. Indian J Med Res. 2019;150(5):425–428. doi: 10.4103/ijmr.IJMR_1920_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leidig-Bruckner G, Ziegler R. Diabetes mellitus a risk for osteoporosis? Exp Clin Endocrinol Diabetes. 2001;109(Suppl 2):S493–S514. doi: 10.1055/s-2001-18605. [DOI] [PubMed] [Google Scholar]
- 20.Schwartz AV. Diabetes mellitus: does it affect bone? Calcif Tissue Int. 2003;73(6):515–519. doi: 10.1007/s00223-003-0023-7. [DOI] [PubMed] [Google Scholar]
- 21.Thrailkill KM, Lumpkin CK, Jr, Bunn RC, Kemp SF, Fowlkes JL. Is insulin an anabolic agent in bone? Dissecting the diabetic bone for clues. Am J Physiol Endocrinol Metab. 2005;289(5):E735–E745. doi: 10.1152/ajpendo.00159.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Si Y, Wang C, Guo Y, Xu G, Ma Y. Prevalence of osteoporosis in patients with type 2 diabetes mellitus in the Chinese mainland: a systematic review and meta-analysis. Iran J Public Health. 2019;48(7):1203–1214. [PMC free article] [PubMed] [Google Scholar]
- 23.Parizad N, Baghi V, Karimi EB, Ghanei GR. The prevalence of osteoporosis among Iranian postmenopausal women with type 2 diabetes: a systematic review and meta-analysis. Diabetes Metab Syndr. 2019;13(4):2607–2612. doi: 10.1016/j.dsx.2019.07.036. [DOI] [PubMed] [Google Scholar]
- 24.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of observational studies in epidemiology (MOOSE) group. JAMA. 2000;283(15):2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 25.Hans D, Downs RW, Jr, Duboeuf F, Greenspan S, Jankowski LG, Kiebzak GM, Petak SM. Skeletal sites for osteoporosis diagnosis: the 2005 ISCD official positions. J Clin Densitom. 2006;9(1):15–21. doi: 10.1016/j.jocd.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 26.Dodwell ER, Latorre JG, Parisini E, Zwettler E, Chandra D, Mulpuri K, Snyder B. NSAID exposure and risk of nonunion: a meta-analysis of case-control and cohort studies. Calcif Tissue Int. 2010;87(3):193–202. doi: 10.1007/s00223-010-9379-7. [DOI] [PubMed] [Google Scholar]
- 27.Athanasiou T, Aziz O, Mangoush O, Weerasinghe A, Al-Ruzzeh S, Purkayastha S, et al. Do off-pump techniques reduce the incidence of postoperative atrial fibrillation in elderly patients undergoing coronary artery bypass grafting? Ann Thorac Surg. 2004;77(5):1567–1574. doi: 10.1016/j.athoracsur.2003.10.040. [DOI] [PubMed] [Google Scholar]
- 28.Lin WL, Hsieh YW, Lin CL, Sung FC, Wu CH, Kao CH. A population-based nested case-control study: the use of 5-alpha-reductase inhibitors and the increased risk of osteoporosis diagnosis in patients with benign prostate hyperplasia. Clin Endocrinol (Oxf) 2015;82:503–508. doi: 10.1111/cen.12599. [DOI] [PubMed] [Google Scholar]
- 29.Drosselmeyer J, Rapp MA, Hadji P, Kostev K. Depression risk in female patients with osteoporosis in primary care practices in Germany. Osteoporos Int. 2016;27(9):2739–2744. doi: 10.1007/s00198-016-3584-9. [DOI] [PubMed] [Google Scholar]
- 30.Lian XL, Zhang YP, Li X, Jing LD, Cairang ZM, Gou JQ. Exploration on the relationship between the elderly osteoporosis and cardiovascular disease risk factors. Eur Rev Med Pharmacol Sci. 2017;21(19):4386–4390. [PubMed] [Google Scholar]
- 31.Silva DMW, Borba VZC, Kanis JA. Evaluation of clinical risk factors for osteoporosis and applicability of the FRAX tool in Joinville City, Southern Brazil. Arch Osteoporos. 2017;12(1):111. doi: 10.1007/s11657-017-0405-5. [DOI] [PubMed] [Google Scholar]
- 32.Kim SW, Kim EH, Lee J, Choi YC, Kim SM, Shin HY. Risk of osteoporosis in patients with chronic inflammatory neuropathy- a population-based cohort study. Sci Rep. 2019;9(1):9131. doi: 10.1038/s41598-019-45591-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shaw CK. An epidemiologic study of osteoporosis in Taiwan. Ann Epidemiol. 1993;3(3):264–271. doi: 10.1016/1047-2797(93)90029-4. [DOI] [PubMed] [Google Scholar]
- 34.Chumnumnawin M, Sawetchaikul S, Sresuriyasawad V. Prevalence of osteoporosis of the priests. J Med Assoc Thail. 2008;91(Suppl 1):S57–S62. [PubMed] [Google Scholar]
- 35.El-Heis MA, Al-Kamil EA, Kheirallah KA, Al-Shatnawi TN, Gharaibia M, Al-Mnayyis A. Factors associated with osteoporosis among a sample of Jordanian women referred for investigation for osteoporosis. East Mediterr Health J. 2013;19(5):459–464. doi: 10.26719/2013.19.5.459. [DOI] [PubMed] [Google Scholar]
- 36.Zhou R, Zhou H, Cui M, Wang Y, Tan J, Sawmiller D, Xu J. Association between aortic calcification and the risk of osteoporosis in a chinese cohort: the Chongqing osteoporosis study. Calcif Tissue Int. 2013;93(5):419–425. doi: 10.1007/s00223-013-9776-9. [DOI] [PubMed] [Google Scholar]
- 37.Asaoka D, Nagahara A, Shimada Y, Matsumoto K, Ueyama H, Matsumoto K, Nakagawa Y, Takeda T, Tanaka I, Sasaki H, Osada T, Hojo M, Watanabe S. Risk factors for osteoporosis in Japan: is it associated with helicobacter pylori? Ther Clin Risk Manag. 2015;11:381–391. doi: 10.2147/TCRM.S80647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saei Ghare Naz M, Ozgoli G, Aghdashi MA, Salmani F. Prevalence and Risk Factors of Osteoporosis in Women Referring to the Bone Densitometry Academic Center in Urmia, Iran. Glob J Health Sci. 2015;8:135–145. doi: 10.5539/gjhs.v8n7p135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu D, Zhou H, Tao Y, Tan J, Chen L, Huang H, Chen Y, Li Y, Zhou R. Alzheimer’s disease is associated with increased risk of osteoporosis: the Chongqing aging study. Curr Alzheimer Res. 2016;13(10):1165–1172. doi: 10.2174/15672050113109990149. [DOI] [PubMed] [Google Scholar]
- 40.Neglia C, Argentiero A, Chitano G, Agnello N, Ciccarese R, Vigilanza A, et al. Diabetes and obesity as independent risk factors for osteoporosis: updated results from the ROIS/EMEROS registry in a population of five thousand post-menopausal women living in a region characterized by heavy environmental pressure. Int J Environ Res Public Health. 2016;13(11). 10.3390/ijerph13111067. [DOI] [PMC free article] [PubMed]
- 41.Hyassat D, Alyan T, Jaddou H, Ajlouni KM. Prevalence and risk factors of osteoporosis among Jordanian postmenopausal women attending the National Center for diabetes, endocrinology and genetics in Jordan. Biores Open Access. 2017;6(1):85–93. doi: 10.1089/biores.2016.0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Heidari B, Muhammadi A, Javadian Y, Bijani A, Hosseini R, Babaei M. Associated factors of bone mineral density and osteoporosis in elderly males. Int J Endocrinol Metab. 2017;15:e39662. doi: 10.5812/ijem.39662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee SH, Kwon HY. Prevalence of osteoporosis in Korean patients with chronic obstructive pulmonary disease and their health-related quality of life according to the Korea National Health and nutrition examination survey 2008-2011. J Bone Metab. 2017;24(4):241–248. doi: 10.11005/jbm.2017.24.4.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lin HH, Huang CY, Hwang LC. Association between metabolic syndrome and osteoporosis in Taiwanese middle-aged and elderly participants. Arch Osteoporos. 2018;13(1):48. doi: 10.1007/s11657-018-0467-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yoo JE, Park HS. Prevalence and associated risk factors for osteoporosis in Korean men. Arch Osteoporos. 2018;13(1):88. doi: 10.1007/s11657-018-0506-9. [DOI] [PubMed] [Google Scholar]
- 46.Wang Y, Xie D, Li J, Long H, Wu J, Wu Z, He H, Wang H, Yang T, Wang Y. Association between dietary selenium intake and the prevalence of osteoporosis: a cross-sectional study. BMC Musculoskelet Disord. 2019;20(1):585. doi: 10.1186/s12891-019-2958-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wongdee K, Charoenphandhu N. Osteoporosis in diabetes mellitus: possible cellular and molecular mechanisms. World J Diabetes. 2011;2(3):41–48. doi: 10.4239/wjd.v2.i3.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014;37(Suppl 1):S81–90. 10.2337/dc14-S081. [DOI] [PubMed]
- 49.Vestergaard P. Discrepancies in bone mineral density and fracture risk in patients with type 1 and type 2 diabetes--a meta-analysis. Osteoporos Int. 2007;18(4):427–444. doi: 10.1007/s00198-006-0253-4. [DOI] [PubMed] [Google Scholar]
- 50.De Laet C, Kanis JA, Odén A, Johanson H, Johnell O, Delmas P, et al. Body mass index as a predictor of fracture risk: a meta-analysis. Osteoporos Int. 2005;16(11):1330–1338. doi: 10.1007/s00198-005-1863-y. [DOI] [PubMed] [Google Scholar]
- 51.DeShields SC, Cunningham TD. Comparison of osteoporosis in US adults with type 1 and type 2 diabetes mellitus. J Endocrinol Investig. 2018;41(9):1051–1060. doi: 10.1007/s40618-018-0828-x. [DOI] [PubMed] [Google Scholar]
- 52.Dunger DB, Acerini CL. IGF-I and diabetes in adolescence. Diabetes Metab. 1998;24(2):101–107. [PubMed] [Google Scholar]
- 53.Johnson SB, Silverstein J, Rosenbloom A, Carter R, Cunningham W. Assessing daily management in childhood diabetes. Health Psychol. 1986;5(6):545–564. doi: 10.1037/0278-6133.5.6.545. [DOI] [PubMed] [Google Scholar]
- 54.Weissberg-Benchell J, Glasgow AM, Tynan WD, Wirtz P, Turek J, Ward J. Adolescent diabetes management and mismanagement. Diabetes Care. 1995;18(1):77–82. doi: 10.2337/diacare.18.1.77. [DOI] [PubMed] [Google Scholar]
- 55.Albertson AM, Tobelmann RC, Marquart L. Estimated dietary calcium intake and food sources for adolescent females: 1980-92. J Adolesc Health. 1997;20(1):20–26. doi: 10.1016/S1054-139X(96)00179-6. [DOI] [PubMed] [Google Scholar]
- 56.Brown IR, McBain AM, Chalmers J, Campbell IW, Brown ER, Lewis MJ. Sex difference in the relationship of calcium and magnesium excretion to glycaemic control in type 1 diabetes mellitus. Clin Chim Acta. 1999;283(1-2):119–128. doi: 10.1016/S0009-8981(99)00040-6. [DOI] [PubMed] [Google Scholar]
- 57.Kaur N, Bhadada SK, Minz RW, Dayal D, Kochhar R. Interplay between type 1 diabetes mellitus and celiac disease: implications in treatment. Dig Dis. 2018;36(6):399–408. doi: 10.1159/000488670. [DOI] [PubMed] [Google Scholar]
- 58.Ho-Pham LT, Chau PMN, Do AT, Nguyen HC, Nguyen TV. Type 2 diabetes is associated with higher trabecular bone density but lower cortical bone density: the Vietnam osteoporosis study. Osteoporos Int. 2018;29(9):2059–2067. doi: 10.1007/s00198-018-4579-5. [DOI] [PubMed] [Google Scholar]
- 59.Dennison EM, Jameson KA, Edwards MH, Denison HJ, Aihie Sayer A, Cooper C. Peripheral quantitative computed tomography measures are associated with adult fracture risk: the Hertfordshire cohort study. Bone. 2014;64:13–17. doi: 10.1016/j.bone.2014.03.040. [DOI] [PubMed] [Google Scholar]
- 60.Sheu Y, Zmuda JM, Boudreau RM, Petit MA, Ensrud KE, Bauer DC, Gordon CL, Orwoll ES, Cauley JA, for the Osteoporotic Fractures in Men (MrOS) Research Group Bone strength measured by peripheral quantitative computed tomography and the risk of nonvertebral fractures: the osteoporotic fractures in men (MrOS) study. J Bone Miner Res. 2011;26(1):63–71. doi: 10.1002/jbmr.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lecka-Czernik B. Bone loss in diabetes: use of antidiabetic thiazolidinediones and secondary osteoporosis. Curr Osteoporos Rep. 2010;8(4):178–184. doi: 10.1007/s11914-010-0027-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ruppert K, Cauley J, Lian Y, Zgibor JC, Derby C, Solomon DH. The effect of insulin on bone mineral density among women with type 2 diabetes: a SWAN Pharmacoepidemiology study. Osteoporos Int. 2018;29(2):347–354. doi: 10.1007/s00198-017-4276-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.