Abstract
Background
Prior to the first recorded outbreak of Rift Valley fever (RVF) in Uganda, in March 2016, earlier studies done until the 1970’s indicated the presence of the RVF virus (RVFV) in the country, without any recorded outbreaks in either man or animals. While severe outbreaks of RVF occurred in the neighboring countries, none were reported in Uganda despite forecasts that placed some parts of Uganda at similar risk.
The Ministry of Agriculture, Animal Industry and Fisheries (MAAIF) undertook studies to determine the RVF sero-prevalence in risk prone areas.
Three datasets from cattle sheep and goats were obtained; one from retrospective samples collected in 2010–2011 from the northern region; the second from the western region in 2013 while the third was from a cross-sectional survey done in 2016 in the south-western region. Laboratory analysis involved the use of the Enzyme Linked Immunosorbent Assays (ELISA). Data were subjected to descriptive statistical analyses, including non-parametric chi-square tests for comparisons between districts and species in the regions.
Results
During the Yellow Fever outbreak investigation of 2010–2011 in the northern region, a total sero-prevalence of 6.7% was obtained for anti RVFV reacting antibodies (IgG and IgM) among the domestic ruminant population. The 2013 sero-survey in the western region showed a prevalence of 18.6% in cattle and 2.3% in small ruminants. The 2016 sero-survey in the districts of Kabale, Kanungu, Kasese, Kisoro and Rubirizi, in the south-western region, had the respective district RVF sero-prevalence of 16.0, 2.1, 0.8, 15.1and 2.7% among the domestic ruminants combined for this region; bovines exhibited the highest cumulative sero-prevalence of 15.2%, compared to 5.3 and 4.0% respectively for sheep and goats per species for the region.
Conclusions
The absence of apparent outbreaks in Uganda, despite neighboring enzootic areas, having minimal restrictions to the exchange of livestock and their products across borders, suggest an unexpected RVF activity in the study areas that needs to be unraveled. Therefore, more in-depth studies are planned to mitigate the risk of an overt RVF outbreak in humans and animals as has occurred in neighboring countries.
Keywords: Rift Valley fever virus, Sero-surveillance, ELISA, Epidemics
Background
Rift Valley fever (RVF) is a sub-acute to acute arthropod borne viral zoonotic disease, whose etiological agent is a ribonucleic acid (RNA) virus of the order Bunyavirales, family Phenuiviridae (the previous taxonomic division is Bunyaviridae) and of the genus Phlebovirus [1].
RVF was first described in 1910 and 1912, among exotic lambs in the Kenyan Rift Valley (between Lake Naivasha and Lake Elementaita). The virus was isolated and recognized as the etiological agent of the disease in 1931 [2, 3]. Later on, in 1944, K.C. Smithburn, working at the laboratories of the then Yellow Fever Research Institute in Entebbe, Uganda, obtained two virus isolates from mosquitoes of Aedes tarsalis and the Eretmapodites spp.collected in uninhabited forest in Western Uganda [4] from which isolates the Smithburn modified live virus vaccine (SMLVV) was derived [5, 6].
Before the disease emerged in Egypt in 1977, with severe epizootics in humans and animals [7], RVF was known to occur only in Eastern and Southern Africa. In 1987, RVF occurred for the first time in West Africa (in Mauritania) as a large and severe epizootic [8]. Further, in 1979, the disease emerged on the island of Madagascar [9] as well on the Arabian Peninsula in 2001 [10]. This dramatic extension was hypothesized to be associated with the trade routes and movements of viraemic animals [11].
In humans, RVF presents as an influenza-like illness that can evolve to severe hemorrhagic or neurological syndromes, [12]; more than 80% of infected people appear asymptomatic or present an influenza-like disease. In animals, RVF may occur in an epizootic form, over large areas following heavy rains with sustained flooding and is characterized by abortion storms, neonatal mortality and hepatitis, primarily in sheep, goats and cattle [13].
In Uganda, before the first recorded outbreak of RVF in March 2016, studies have indicated past serologic evidence of RVFV circulation [14–18] without any recorded overt outbreaks in humans or animals.
Neighboring countries in the Eastern African region, periodically experience large and severe RVF outbreaks such as the one of 1997–1998 that occurred in Kenya, Somalia, and Tanzania affecting over 100,000 people with over 450 deaths in Kenya alone [19, 20]. The RVF outbreaks spanning the period of December 2006 to June 2007, in the same Eastern African region, occurred in the neighboring countries of Kenya and Tanzania as well as Somalia and Sudan. Although, the outbreak was characterized by seven sequential outbreak foci; three in Kenya, two in Tanzania and two in Somalia, no RVF activity was reported, at the time, in Uganda despite its regional proximity to the affected countries and in spite of the early warning system developed by the USA National Aeronautics and Space Administration (NASA) that accurately predicted the latter outbreak as well as the outbreak areas. This same prediction indicated that Uganda was vulnerable and equally prone to RVF outbreaks, particularly so in the north eastern region [21, 22].
This paper examines three different sets of data with the aim of illuminating the serological status of domestic ruminant populations (cattle, goats and sheep) due to possible exposure to the RVF Phlevovirus, in the absence of overt outbreaks, in high-risk areas of northern, western and south-western regions of Uganda.
Results
The first dataset of 75 sera specimens collected from cattle and small ruminants obtained during the investigation of a Yellow Fever outbreak of 2010/2011 in Agago and Kitgum districts (northern Uganda) showed an overall RVF antibody sero-prevalence of 6.7%; species sero-prevalences in this region were 4.7% in cattle and 9.4% in small ruminants for the two districts combined; both IgG and IgM antibodies to the RVF virus were detected at the time. However, only one sample had anti RVFV IgM antibodies (Table 1).
Table 1.
Rift Valley fever sero-prevalence (on IgM, IgG ELISA tests) in cattle and small ruminants observed during a Yellow Fever outbreak investigation in Agago and Kitgum Districts in the northern region of Uganda (November 2010 to early 2011); aOne goat from Kitgum district tested positive for anti RVFV IgM antibodies
| Species | District | positive / total tested (%) | 95% CI |
|---|---|---|---|
| Cattle | Agago | 1/37 (2.7) | 0.0–14.2 |
| Kitgum | 1/6 (16.7) | 0.4–64.1 | |
| Sub Total | 2/43 (4.7) | 0.6–15.8 | |
| Small ruminants | Agago | 1/3 (33.3) | 0.8–90.6 |
| Kitgum | 2/29 (6.9)a | 0.8–22.8 | |
| Sub Total | 3/32 (9.40) | 2.0–25.0 | |
| Grand total | Agago | 2/40 (5.0) | 0.6–16.9 |
| Kitgum | 3/35 (8.6) | 1.8–23.1 | |
| Total | 5/75 (6.7) | 2.2–14.9 |
Caption: The chi-square test with Yates correction is not significant at p > 0.05 when comparing the two districts as well as comparison between cattle and small ruminants
The 2013 sero-survey specimens were collected from the domestic ruminant population in western Uganda from the districts of Hoima, Kibaale and Masindi; they were analyzed using an RVF inhibition ELISA test and showed a study sero-prevalence of 18.6% in the cattle population and 2.3% in the small ruminant population. District study sero-prevalences in cattle were respectively, 12.1, 10.0, and 25.0%, for the districts of Hoima, Kibaale and Masindi. In the small ruminant population, the district study sero-prevalence in Masindi was 3.1% while no antibodies were detected in Hoima district (Table 2).
Table 2.
Rift Valley fever sero-prevalence (on IgG ELISA test) among cattle and small ruminants from the three Districts of Hoima, Kibaale and Masindi in Western Uganda (2013)
| Species | District | positive / total tested (%) | 95% CI |
|---|---|---|---|
| Cattle | Hoima | 4/33 (12.1) | 3.4–28.2 |
| Kibaale | 2/20 (10.0) | 1.2–31.7 | |
| Masindi | 15/60 (25.0) | 14.7–37.9 | |
| Total cattle | 3 districts | 21/113 (18.6) | 11.9–27.0 |
| Small ruminants | Hoima | 0/11 (0.0) | – |
| Masindi | 1/32 (3.1) | 0.1–16.2 | |
| Total small ruminants | 2 districts | 1/43 (2.3) | 0.1–12.3 |
| Grand Total | 2 districts + 2 species | 23/156 (14.7) | 9.6–21.3 |
Caption. The chi-square statistic test on cattle vs small ruminants is 4.0687, with a p-value of 0.043684 showing a significant (p < 0.05) difference between the two population
After the March 2016 RVF outbreak in Kabale, a planned multi-sectoral bio-surveillance pilot study (sero survey) was conducted in south-western Uganda including the outbreak district of Kabale and the four surrounding ones of Kanungu, Kasese, Kisoro and Rubirizi which showed the respective percentage of RVF reacting IgG antibodies of 16.0, 2.1, 0.8, 15.1 and 2.7% (Table 3).
Table 3.
Rift Valley Fever sero-prevalence (on IgG ELISA test. Showing ‘positive / total tested (%)’) among cattle and small ruminants from five districts (Kabale, Kanungu, Kasese, Kisoro and Rubirizi) in south-western Uganda (2016)
| District | Cattle | Goats | Sheep | Total | ||||
|---|---|---|---|---|---|---|---|---|
| n/N (%) | 95% CI | n/N (%) | 95% CI | n/N (%) | 95% CI | n/N (%) | 95% CI | |
| Kabale | 66/280 (23.6)a | 18.7–29.0 | 13/190 (6.8) | 3.7–11.4 | 4/50 (8.0) | 2.2–19.2 | 83/520 (16.0) | 12.9–19.4 |
| Kanungu | 3/112 (2.7) | 0.6–7.6 | 1/81 (1.2) | 0.0–6.7 | 0/0 (0.0) | – | 4/193 (2.1) | 0.6–5.2 |
| Kasese | 1/69 (1.4) | 0.0–7.8 | 0/60 (0/0) | – | 0/1 (0.0) | – | 1/130 (0.8) | 0.0–4.2 |
| Kisoro | 17/67 (25.4) | 15.5–37.5 | 4/52 (7.7) | 2.1–18.5 | 0/20 (0.0) | – | 21/139 (15.1) | 9.6–22.2 |
| Rubirizi | 4/74 (5.4) | 1.5–13.3 | 0/71 (0.0) | – | 0/5 (0/0) | – | 4/148 (2.7) | 0.7–6.8 |
| Total | 91/600 (15.2) | 12.4–18.3 | 18/454 (4.0) | 2.4–6.2 | 4/76 (5.3) | 1.5–12.9 | 113/1130 (10.0) | 8.3–11.9 |
Legend: apositive / Total tested (percent sero-prevalence)
Caption. The chi-square with Yates correction, cattle to goat: Chi-square = 34.9763. p < 0.00001. Highly significant at p < 0.01; Cattle to sheep: Chi-square = 5.4776. p-value = 0.019262. Significant at p < 0.05.; Cattle to goat + sheep: Chi-square = 37.943. p < 0.00001. Highly significant at p < 0.01; Goat to sheep: not significant p > 0.6; Comparison of districts close to Rwanda andthose further away (in this case Kabale, versus Rubirizi) in the south western region: Chi-square = 27.5106. p < .00001. Highly Significant at p < 0.01.)
In south-western Uganda, the districts of Kabale and Kisoro registered the highest district sero-prevalence above 15% in the domestic ruminant populations while Rubirizi, Kanungu and Kasese districts had a sero-prevalence lower than 3%. Of the three species investigated, bovines exhibited the highest cumulative sero-prevalence reaching 15.2%, followed by ovines and caprines with 5.3 and 4.0% respectively.
Discussion
The three datasets provide recent serological evidence of possible RVFV circulation in selected areas of Uganda where anti RVFV antibodies were found in both cattle and small ruminant populations.
The pilot study of 2016 constitutes an initial multi-sectoral attempt to unveil the RVF sero-prevalence in a high-risk regions in Uganda, among domestic ruminant populations.
On a Chi-Square test comparison (with a Yates correction) among species, cattle from western and south-western regions had significantly higher anti-RVFV antibodies compared to goats and / or sheep. This can partly be explained by the longer life span of cattle compared to small ruminants with shorter life span. Additionally, the disproportionate sampling between the two species, particularly in the outbreak district of Kabale may have contributed to higher sero-prevalence in cattle. There was no significant difference between the goat and sheep populations sampled.
On the other hand, in the northern region, cattle sampled had lower anti-RVFV antibody sero-prevalence compared to small ruminants, but this was found not to be significant using the Chi-Square test.
The majority of the general sampling, from the northern region, gave negative results of IgM detection; only one test was found positive in a goat sample from the Kitgum district showing a recent infection and indicates a recent active transmission of RVF 6 to 8 weeks before our sampling, in the area or bordering areas.
The datasets from the three regions clearly reveal RVF virus reacting antibodies in susceptible hosts, it can be inferred that there has been previous, as well as ‘currently’ active, circulation of the virus within the domestic ruminant populations in the northern region of the country. The latter, however, cannot be inferred with certainty for datasets 2 and 3 obtained from the western and south western regions as anti RVFV IgM antibody data was not readily available.
It is clear that the domestic ruminant populations studied are not necessarily naïve to RVFV infection and it is, therefore, plausible to hypothesize that there is an unexplained transmission of the RVFV, probably mimicking disease occurrence during an ‘inter-epizootic’ period as is the case in the semi-arid regions which periodically experience large and severe outbreaks.
In the south-western region, the districts neighboring Rwanda had significantly higher anti-RVFV antibody sero-prevalence (p < 0.01) in the domestic ruminant population compared to the others; considering the case of Kabale vs Rubirizi, this can be partly explained by the prevailing eco-climatic condition as well as the socio-economic activities in the most southern districts (Kabale and Kisoro) which topographically, are characterized by steep hills and valleys; climatically Kabale district has a higher annual rainfall with a tendency of flooding; socioeconomically, there is trade in livestock and livestock products between Uganda and Rwanda. Whereas the more northerly districts (in the south-western region including Rubirizi) tend to be warmer, have lower annual rainfall and livestock trade is skewed in such a way that movement (of livestock and livestock products) tends to be more outwards from these areas to the eastern Democratic Republic of Congo (DRC).
The above trend in livestock trade notwithstanding, similar circumstances of serological evidence of RVF virus circulation in neighboring countries have been reported in the eastern Democratic Republic of Congo (DRC) in cattle using the ELISA assay [23]. The study areas included the provinces of North Kivu, South Kivu, and Ituri. The North Kivu province which borders much of western Uganda had the respective combined prevalence of anti-RVF IgG and IgM of 12.67 and 2%. In Rwanda, comparatively higher combined sero-prevalence of 16.8% in cattle were reported [24] although the Nyagatare district neighboring Uganda had a comparable seroprevalence of 7.9% in cattle.
Varying Rift Valley Fever sero-prevalences, in small ruminants, were reported in the DRC [25]; the study covered 15 territories within 7 provinces with the respective combined sero-prevalences of 0 to 23.81%, and 0 to 37.11% for goats and sheep. 12 out of 15 territories had seroprevalences of < 10% and some of these territories had comparable sero-prevalences reported in this study.
It is worth noting that in the above 3 studies (2 in DRC and 1 in Rwanda), there were no reported large and severe outbreaks, as was the case before the first outbreak in Uganda in March 2016.
It is also important to note that Uganda, Rwanda and Eastern DRC are largely part of the same African Tropical Pluvial-seasonal bioclimate with a number of limited areas of Tropical Xeric climate. The area is covered by deciduous forests, covering appreciable areas; bush-grass savannah in the northern region of Uganda and Mountain forest are shared by the 3 countries (mostly for the south-western part of Uganda). Altogether this shows an ecosystem unity, there are increasing changes in land cover and land use.
Consequently, this ecosystem unity can only partly explain the similarity of sero-prevalence as well as the reported circulation of RVFV; this is in view of the notion that transmissibility and spread of the disease are thought to be multi-factorial involving risk factors related not only to ecology but also climate, the vector and mechanical transmitters as well as socio-economic factors such as trade and cultural practices [21, 26, 27].
Depending on risk analysis studies, the cyclic nature of the disease and in consideration of the severity of outbreaks elsewhere there is need for preparedness plans to be setup.
Besides, it is also documented that despite the ‘quiescence’ of the RVFV without overt outbreaks, there is a possibility of large and severe outbreaks occurring; this is corroborated through a study in Malagasy, where severe outbreaks occurred in 2008 and 2009 in livestock and humans following two successive rainy seasons [9]. It would, therefore, be prudent for the Veterinary Services in Uganda to prepare contingency plans if ever the ‘right’ conditions should trigger an overt and large outbreak.
The studies in this paper also point out the need to characterize the RVFV potential variants which would possibly circulate at low noise without being detected in Uganda. Although the RVFV isolates belong to a particular serotype they are known to differ in virulence [28]; therefore, in this regard and given the non-overt manifestation of the RVFV in the study areas; a better understanding of the molecular epidemiology of the Ugandan isolates is highly warranted and should be determined along with the associated phenotype. Moreover, a report on the laboratory findings after the first reported RVF outbreak in Uganda [29], indicates that in 3 clinical specimens for which whole genome sequencing was done, the phylogenetic analysis inferred that the RVF Phlebovirus that was involved in the Kabale 2016 outbreak shows relatedness to the 2006–2007 RVF outbreak in Kenya.
Conclusion
The presence of anti-RVFV IgG, and a singular case of IgM, antibodies in susceptible hosts, indicate a possible but often hidden circulation (i.e. limited IgM response) of the RVFV within the domestic ruminant populations with possible undetected (false negative diagnoses) or unreported outbreaks. Therefore, there is a need for improved capacity to detect disease outbreaks in livestock at very early stages by the National Veterinary Services as well as to improve bio-surveillance and reporting and to extend such bio-surveillance to epizootic and zoonotic diseases in the country. This should also be coupled with predictive modeling as indicated by the most recent dataset obtained from a bio-surveillance cross sectional study and prompted by the first two studies. More in-depth studies are already being designed aiming to mitigate the risk of RVFV transmission to both humans and animals, as well as to characterize the origin (phylogeography), genotype (possible reassortment) and phenotypes (pathogenicity) of the RVFV strains potentially present in Uganda.
Methods
Method and study design
Study sites
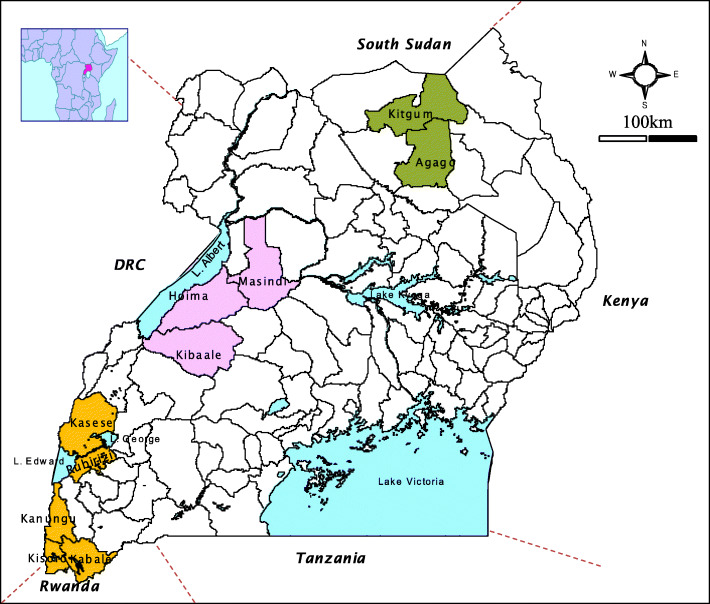
The districts in the 3 different regions in the country from which domestic ruminant samples were collected are shown in Fig. 1.
Fig. 1.
Uganda Map showing districts with suspected RVF endemic areas in domestic ruminants. Legend: Color coded Blue = lakes; Green = 2010 / 2011 RVFV reacting antibodies detected; Orange = 2016 RVFV reacting antibodies detected; Purple = 2013 RVFV reacting antibodies detected; white = non-tested districts. Source; this map was obtained from the Uganda Ministry of Agriculture, Animal Industry and Fisheries (MAAIF) and drawn using the ArcView GIS version 3.2 software
Retrospective and prospective sample analysis for RVFV reacting antibodies were carried out on the following sample sets, including: Set 1/ Samples were obtained during a yellow fever outbreak investigation based on syndromes during December 2010 to early 2011 in Agago and Kitgum districts; northern region. Sera were collected from cattle and small ruminants. Set 2/ Sampling from a cattle, sheep and goats sero-surveillance done in October 2013, in the districts of Hoima, Kibaale and Masindi; western region. Set 3/ A multi-sectoral bio-surveillance pilot study undertaken in the months of August to October 2016 in the RVF outbreak district of Kabale as well as in the surrounding districts of Kanungu, Kisoro and Rubirizi; south-western region.
Description of study sites
The south-western districts of Kabale, Kisoro, Kanungu and Rubirizi have a montane climate with bimodal rainfall pattern; with peaks during the months of March to May and September to November. The mean annual rainfall ranges from 1000 mm to 1480 mm with temperature range of 100 C to 270 C. However, Kanungu and Rubirizi tend to be warmer and have lower rainfall compared to Kabale and Kisoro.
These districts are located in the Western Rift Valley system; the whole of Rubirizi and part of Kanungu districts are on the Western Rift Valley escarpment; the districts have a relief of between 1200 and 3000 m above sea level. The districts can be described as mainly green with interlocking hills and stretches of valleys. The vegetation is characterized by tropical forests including Bwindi Impenetrable and Echuya forests; there are coniferous plantations of Cyprus and pines trees in forest reserves, Eucalyptus tree plantations and woodland are a common feature. The region has water bodies and vast wetlands (swamps and marshland) associated with Lake Bunyonyi. Like the rest of the country, agriculture is the major form of livelihood. It relies on the naturally fertile volcanic and peat soils. This region is well known for production of horticultural crops. The hilltops and valleys are also used for livestock production; other agricultural ventures include the growing of cash crops (coffee, tea, pyrethrum). The region is also well known for diary production, agro-forestry and apiary.
The district of Kasese is also within the Western Rift Valley, it is much drier with a wooded savannah vegetation on gentle rolling hills. The foothills of Mt. Rwenzori are appreciably forested.
The two northern Uganda districts have a bush grassland savannah, several water bodies and swamps with a bimodal rainfall pattern of between 800 mm and 1200 mm annually. The Western region districts of Kibaale, Hoima and Masindi are characterized by tropical rain forests.
The mean annual rainfall ranges from 1000 mm to 1300 mm with a temperature range of 17 °C to 28 °C.
In the 3 regions there are increasing changes in land use and therefore, land cover.
Selection of animals for the three study datasets
For the retrospective study in northern Uganda (dataset 1), syndromic surveillance was applied. Animals were selected by way of a non-probabilistic risk-based approach. Human medical samples for pathogen detection led to confirmed human cases of Yellow Fever. The samples (serum) from the domestic ruminant population (cattle, sheep and goats) had antibodies to RVFV.
Dataset 2 was part of the routine Ministry of Agriculture Animal Industry and Fisheries (MAAIF) sero-surveillance for RVF, animals in this dataset were part of a preselected sample based on multistage sampling; while dataset 3 was derived from a planned cross-sectional study where animals were selected also based on a multistage sampling design;
The approach used to select the total sample size involved selection of a region in a high risk area, namely, south-western Uganda; farms were selected from villages in the outbreak district and the surrounding ones. The minimum number of animals, irrespective of species, were randomly selected basing on the formula indicated here below [30].
nmin = minimum sample size of domestic ruminants (cattle, sheep and goats) (1153); DE = Design Effect (3); d = acceptable error margin (0.05); Z = Z score (for a 95% confidence interval); p = Assumed prevalence of the disease in the population (0.5).
Sample collection
Sera samples were collected from domestic ruminants (cattle, goats and sheep), preserved and transported along a cold chain following the standard operating procedures (SOPs) approved at the National Veterinary Laboratory of the MAAIF (National Veterinary Referral Laboratory).
A total of 1361 samples were tested, including 75 sera samples from the 2010 / 2011 Yellow Fever outbreak investigation, 156 sera samples from a routine RVF Sero-survey (2013) and 1130 sera samples from the 2016 RVF Pilot Study supported by the Defense Threat Reduction Agency (DTRA), Cooperative Biological Engagement Program CBEP [31].
All sera specimens from all districts sampled were tested using the ELISA and showed RVFV reacting antibodies among the domestic ruminant populations. Data were analyzed and the threshold of statistical significance was stated at p-value < 0.05.
Laboratory sample analyses
Sample set 1
Laboratory tests were performed in the Centers for Disease Control and Prevention (CDC) laboratory at Kenya Medical Research Institute (KEMR) Laboratory, Kenya, using an in-house direct immunoglobulin G (IgG) detection by Enzyme Linked Immunosorbent Assay (ELISA) as well as the immunoglobulin M (IgM) capture ELISA. Both IgG and IgM antibodies were detected. Only one sample exhibited anti RVFV IgM antibodies.
Sample set 2
Samples were analyzed using RVF inhibition ELISA at the National Veterinary Laboratory of the National Animal Disease Diagnostic and Epidemiology Centre; no tests were done for anti RVFV IgM antibodies.
Sample set 3
The sera specimens were analyzed using an in-house direct IgG antibody ELISA at the CDC Uganda Virus Research Institute (UVRI) laboratories. All sera specimens were tested in the lab using Enzyme Linked Immunosorbent Assay (ELISA) for anti-RVFV IgG antibodies only, no tests were done for anti RVFV IgM antibodies.
The laboratory test results of sample sets 1 and 3 were obtained using the CDC serological test protocols that classify a specimen as positive meeting 2 pre-established and conservative criteria; namely OD value at 1/400 titer must be > 0.2; and its OD Sum must be > 0.95. These criteria have been established over time and have high reproducibility of the results. Thereby, taking care of the lower limit of detection with respect to titter values that are set at 1/400.
The laboratory test results of datasets 2 were obtained using an inhibition Enzyme-linked immunsorbent oassay (ELISA) for the detection of antibodies to Rift Valley fever virus in humans, domestic and wild ruminants developed at the National Institute for Communicable diseases [32].
The lower control limits for net OD for the positive control and negative control were respectively minus 0.05 and 0.65; whereas the same values for the upper control limits were 0.07 and 1.34; the test in terms of Percent Inhibition (PI) for the positive control and negative control were, respectively, 94.26 and 4.26% for the lower control limits; whereas for the upper control limits corresponding values were 102.8 and 4.33%; while the diagnostic sensitivity and specificity were respectively 99.47% PI and 99.66% PI.
Data analysis
The data presented from the 3 datasets were principally observational, consequently unadjusted descriptive statistical analyses were employed including the computation of proportions to derive study sero-prevalences;
The data were captured, collated, analyzed using MS Excel® software spreadsheet in Microsoft office for dataset 3 and then annotated. The descriptive statistics and confidence intervals were calculated in R Statistical Software using the package binom [33]..
For each dataset a non-parametric chi-square test with Yates correction was used to do paired comparison between districts and species (cattle, sheep and goats) within each region for the 3 regions. The district comparisons were done for only datasets 1 and 3.
Acknowledgements
The Authors are greatly indebted to the DTRA-CBEP for providing the funding for the pilot study and enabling a preliminary presentation to be given at the American Society of Microbiology (ASM) Microbe 2018 conference, in particular the authors pay special tribute to Dr. Mary Lancaster, Dr. Jean Richards and Dr. Jarrad Marles and their teams for supporting the cross-sectional survey and initial presentation of results at the ASM, 2018. We are grateful to the Government of Uganda for the support both at the Central and Local district levels to obtain, store and analyze the specimens. Our thanks also go to the Central Veterinary Laboratory (CVL) of Kenya and the Kenya Medical Research Institute (KEMRI) for the initial analyses. We thank both the CDC – Kenya and CDC – Uganda for the laboratory analyses; the authors are particularly indebted to Trevor Shoemaker for supporting kick off of the multisectoral bio-surveillance pilot study and the laboratory analyses at the CDC-UVRI laboratory. We thank the European project PANDORA for supporting FV and Centaurus Biotech for supporting JPG for their participation to the completion of the study.
Abbreviations
- ASM
American Society of Microbiology
- CBEP
Collaborative Biological Engagement Program of DTRA
- CDC
Centers for Disease Control and Prevention
- CTP
Commonwealth Trading Partners
- CVL
Central Veterinary Laboratory (of Kenya)
- DTRA
Defense Threat Reduction Agency
- ELISA
Enzyme Linked Immunosorbent Assay
- GIS
Geographic Information System
- IgG
Immunoglobulin type G
- IgM
Immunoglobulin type M
- IRD
French Research Institute for Development
- KEMRI
Kenya Medical Research Institute
- LIPMC
Molecular Comparative Immuno-Physiopathology Lab
- MAAIF
Ministry of Agriculture, Animal Industry and Fisheries
- NASA
National Aeronautics and Space Administration of the USA
- OD
Optical Density
- OIE
Office Internationale des Epizootes (World Organization for Animal Health)
- PI
Percent Inhibition
- REC
Research Ethics Committee
- RVF
Rift Valley fever
- RVFV
Rift valley fever virus
- SMLVV
Smithburn modified live virus vaccine
- SOP
Standard Operating Procedure
- UMR-MD
Joint Research Unit-Ministry of Defense
- UNHRO
Uganda National Health Research Organization
- USA
United States of America
- UVRI
Uganda Virus Research Institute
- WHO
World Health Organization of the United Nations
Authors’ contributions
All authors have read and approved the present manuscript. ND, ME, BB and JPG worked on the concept and coordinated the study. JPG, ME, ND and NK designed and directed the study; ND, NJ, DR, BS, MF, TD, AE, KE, NM and KJ contributed to collection and analysis of the samples. ND, JPG, FV, MN and DR participated in the data analysis and writing of the manuscript. AR and RC contributed to the acquisition and interpretation of datasets 1 and 2 as well as providing administrative support. FV and JPG equally gave guidance for the data analysis and interpretation and actively participated the in writing of the manuscript.
Funding
DTRA-CBEP (US Department of Defense) provided the funding for the pilot study and supported preliminary presentation given at the American Society of Microbiology (ASM) 2018.
Availability of data and materials
All data and material of the present study are available upon request to the corresponding authors including all field and laboratory original data anonymized in an excel format.
Declarations
Ethics approval and consent to participate
Was required only for the pilot study (dataset 3) and was formally sought from and approved by the Uganda Virus Research Institute (UVRI) Research Ethics Committee (REC). The reviewed and approved documents included the following i) the UVRI REC Application Form ii) the study protocol, iii) informed consent documents, iv) Standard Operating Procedures (SOPs) and v) investigators’ Bio-sketches.
Additionally, for dataset 3; written consent was obtained from all livestock owners.
The ethical approval for datasets 1 and 2 was not deemed necessary as this was part of the MAAIF activities where the Veterinary Service of Uganda is mandated by the Animal Disease Act Cap. 38 Part II to undertake disease surveillance and investigations. This is also aligned to the OIE guidelines on surveillance and reporting; the latter are appropriately reflected by the Animal Health Master Plan for Uganda, at a procedural level, where the elements of information dissemination are described in schedule 2 on Communication.
Consent for publication
Not applicable.
Competing interests
All authors declare no financial and/or non-financial competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Deo B. Ndumu, Email: ndumudb@gmail.com
Barnabas Bakamutumaho, Email: bbarnabas2001@yahoo.com.
Edward Miller, Email: jaymiller.v@gmail.com.
Jesca Nakayima, Email: jescanl2001@yahoo.co.uk.
Robert Downing, Email: downingrg@gmail.com.
Stephen Balinandi, Email: balinandi@yahoo.com.
Fred Monje, Email: fredmonje230@gmail.com.
Dan Tumusiime, Email: drdantumusiime@gmail.com.
Mary Nanfuka, Email: nanfukamryl@yahoo.com.
Natascha Meunier, Email: nmeunier@rvc.ac.uk.
Eugene Arinaitwe, Email: earinaitwee@gmail.com.
Chris Rutebarika, Email: crutebarika@gmail.com.
Eugene Kidega, Email: eugenekidega@gmail.com.
Jackson Kyondo, Email: jacksonkyondo@gmail.com.
Rose Ademun, Email: ademunrose@yahoo.co.uk.
Kariuki M. Njenga, Email: mkariukinjenga@gmail.com
Francisco Veas, Email: francisco.veas@ird.fr.
Jean-Paul Gonzalez, Email: Jean.Paul.Gonzalez@georgetown.edu.
References
- 1.Adams MJ, Lefkowitz EJ, King AMQ, Harrach B, Harrison RL, Knowles NJ, Kropinski AM, Krupovic M, Kuhn JH, Mushegian AR, Nibert M, Sabanadzovic S, Sanfacon H, Siddell SG, Simmonds P, Varsani A, Zerbini FM, Gorbalenya AE, Davison AJ. Changes to taxonomy and the international code of virus classification and nomenclature ratified by the international committee on taxonomy of viruses. Arch Virol. 2017;162(8):2505–2538. doi: 10.1007/s00705-017-3358-5. [DOI] [PubMed] [Google Scholar]
- 2.Daubney R, Hudson JR, Granham PC. Enzootic hepatitis or Rift Valley fever: an undescribed virus disease of sheep, cattle and man from East Africa. J Pathol Bacteriol. 1931;34(4):545–579. doi: 10.1002/path.1700340418. [DOI] [Google Scholar]
- 3.Bishop DH, Calisher CH, Casals J, Chumakov MP, Gaidamovich SY, Hannoun C, Lvov DK, Marshall LD, Oker-Blom N, Pettersson RF, Porterfield JS, Russell PK, Shope RE, Westaway EG. Bunyaviridae. Intervirology. 1980;14(3-4):125–143. doi: 10.1159/000149174. [DOI] [PubMed] [Google Scholar]
- 4.Smithburn K.C. (1948); Haddow A.J, Gillett JD, Rift Valley fever; isolation of the virus from wild mosquitoes. Br J Exp Pathol. 1948, 29, 107–121. [PMC free article] [PubMed]
- 5.Smithburn KC. Rift Valley fever; the neurotropic adaptation of the virus and the experimental use of this modified virus as a vaccine. Br J Exp Pathol. 1949;1949:30. [PMC free article] [PubMed] [Google Scholar]
- 6.Faburay B., LaBeaud A.D., Scott McVey D., Wilson W..C and Richt J.A. (2017); Current status of Rift Valley fever vaccine development. Vaccines, 5, 29; doi:10.3390/vaccines5030029. www.mdpi.com/journal/vaccines. [DOI] [PMC free article] [PubMed]
- 7.Meegan JM, Hoogstraal H, Moussa MI. An epizootic of Rift Valley fever in Egypt in 1977. Vet Rec. 1979;105(6):124–125. doi: 10.1136/vr.105.6.124. [DOI] [PubMed] [Google Scholar]
- 8.Jouan A, Leguenno B, Digoutte JP, Philippe B, Riou O, Adam F. An RVF epidemic in southern Mauritania. Ann Inst Pasteur Virol. 1988;139:307–308. doi: 10.1016/S0769-2617(88)80046-7. [DOI] [PubMed] [Google Scholar]
- 9.Carroll S.A., Reynes. J.M.,. Khristova M.L, Andriamandimby F.S., Rollin P.E., and Nichol S.T. (2011); Genetic evidence for Rift Valley fever outbreaks in Madagascar resulting from virus introductions from the east African mainland rather than enzootic maintenance. J Virol, Vol. 85, No. 13p. 6162–6167, doi: 10.1128/JVI.00335-11. [DOI] [PMC free article] [PubMed]
- 10.Shoemaker TR., Boulianne C., Vincent MJ., Pezzanite L., Al-Qahtani MM., Al-Mazrou Y., Khan AS., Rollin PE., Swanepoel R, Ksiazek TG, and. Nichol ST. (2002);Genetic Analysis of Viruses Associated with Emergence of Rift Valley Fever in Saudi Arabia and Yemen, 2000–01. Emerg Infect Dis; 8, 12. [DOI] [PMC free article] [PubMed]
- 11.FAO and WHO . Rift Valley Fever distribution, outbreaks and Spread. 2010. [Google Scholar]
- 12.WHO . Rift Valley Fever Key Facts. 2017. [Google Scholar]
- 13.OIE . Rift Valley Fever, Information Note. 2009. [Google Scholar]
- 14.Digoutte JP, Cordellier R, Robin Y, Pajot FX. Le virus Zinga (Arb 1976) nouveau prototype d’arbovirus isole en Republique Centrafricaine. Ann Microbiol. 1974;125B(1):107–118. [PubMed] [Google Scholar]
- 15.Peters CJ, Anderson GW. Pathogenesis of Rift Valley fever. 1981. [DOI] [PubMed] [Google Scholar]
- 16.Meegan JM, Digoutte JP, Peters CJ, Shope RE. Monoclonal antibodies to identify Zinga virus as Rift Valley fever virus. Lancet. 1983;321(8325):641. [DOI] [PubMed]
- 17.Rodhain F, Gonzalez JP, Mercier E, Helynck B, Larouze B, Hannoun C. Arbovirus infections and viral haemorrhagic fevers in Uganda: a serological survey in Karamoja district, 1984. Trans R Soc Trop Med Hyg. 1989;83(6):851–854. doi: 10.1016/0035-9203(89)90352-0. [DOI] [PubMed] [Google Scholar]
- 18.Battles JK, Dalrymple JM. Genetic variation among geographic isolates of Rift Valley fever virus. American. J Trop Med Hygiene. 1988;39(6):617–631. doi: 10.4269/ajtmh.1988.39.617. [DOI] [PubMed] [Google Scholar]
- 19.Munywa P, Murithi RM, Wainwright S, Githinji J, Hightower A, Mutonga D, Macharia J, Ithondeka PM, Musaa J, Breiman RF, Bloland P, Njenga MK. Rift Valley Fever Outbreak in Livestock in Kenya, 2006–2007. Am J Trop Med Hyg. 2010;83(Suppl 2):58–64. doi: 10.4269/ajtmh.2010.09-0292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nanyingi MO, Munyua P, Kiama SG, Muchemi GM, Thumbi SM, Bitek AO, Bett B, Muriithi RM, Njenga MK. A systematic review of Rift Valley Fever epidemiology 1931 to 2014. Infect Ecol Epidemiol. 2015;5;28024. [DOI] [PMC free article] [PubMed]
- 21.Anyamba A., Linthicum K. J. and Tucker CJ, (2001) Climate-disease connections: Rift Valley Fever in Kenya https://pubmed.ncbi.nlm.nih.gov/11426274/Cad Saude Publica 17 Suppl:133–40. [DOI] [PubMed]
- 22.Anyamba A, Linthicum JK, Small J, Britch CS SC, Pak E, Rocque S, Formenty P, Hightower WA, FR CJP, Tucker JC, Schnabel D, Sang R, Haagsma K, Latham M, Lewandowski BH, Magdi M, Mohamed AM, Nguku MP, Reynes JM, Swanepoel R. Prediction, Assessment of the Rift Valley Fever Activity in East and Southern Africa 2006–2008 and Possible Vector Control Strategies. Am J Trop Med Hyg. 2010;83(Suppl 2):43–51. doi: 10.4269/ajtmh.2010.09-0289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tshilenge MG, Masumu J, Mbao V, Kayembe JM, Rweyemamu M, Mulumba MKL. Sero-prevalence and virus activity of Rift Valley fever in cattle in eastern region of Democratic Republic of the Congo; journal of veterinary medicine; volume 2018. Article ID. 2018;4956378:1–8. doi: 10.1155/2018/4956378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Umuhoza T., Berkvens D., Gafarasi I., Rukelibuga J., Mushonga B. and Biryomumaisho S. (2017) Seroprevalence of Rift Valley fever in cattle along the Akagera–Nyabarongo rivers, Rwanda; journal of the south African veterinary association ISSN: (online) 2224-9435, (print) 1019-9128. [DOI] [PMC free article] [PubMed]
- 25.Tshilenge GM, Mulumba MLK, Misinzo G, Noad R, Dundon WG. Rift Valley fever virus in small ruminants in the Democratic Republic of the Congo. Onderstepoort J Vet Res. 2019;86(1):e1–e5. doi: 10.4102/ojvr.v86i1.1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.WHO. Rift Valley fever outbreaks forecasting models. Joint FAO - WHO experts Consultation; Rome, Italy 29 September–1 October 2008. 2009. https://www.who.int/csr/resources/publications/WHO_HSE_GAR_BDP_2009_2/en/.
- 27.Lancelot R., Beral M.,. Rakotoharinomee V.M., Andriamandimby S.F., Heraud J.M., Costea C., Apolloni A., Squarzoni-Diawg C., Rocquea S. Formenty P.B.H., Bouyera J., Wint G.R.W.,and Cardinale E. (2017).Drivers of Rift Valley fever epidemics in Madagascar; in Proc Natl Acad Sci U S A. 2017; 114(5): 938–943.Online doi: 10.1073/pnas.1607948114 Applied biological sciences [DOI] [PMC free article] [PubMed]
- 28.Metwally S.; (2008); Rift Valley Fever. In; USA Animal Health Association, (ed). Foreign Animal Diseases. 7th Edition. 369–375. Boca Raton, Florida: Boca Publications Group, Inc.
- 29.ShoemakerT. R., Nyakarahuka L, Balinandi S, Ojwang J, Tumusiime A, Mulei S, Kyondo J, Lubwama B, Sekamatte M, Namutebi A, Tusiime P, Monje F, Mayanja M, Ssendagire S, Dahlke M, Kyazze S, Wetaka M, Makumbi I, J Borchert, Zufan S, Patel K, Whitmer S, Brown S, Davis WG, Klena JD, Nichol ST, Rollin PE and Lutwama J. (2019) First laboratory-confirmed outbreak of human and animal Rift Valley fever virus in Uganda in 48 years; am. J. Trop. Med. Hyg., 100(3), 2019, pp. 659–671;doi:10.4269/ajtmh.18-0732. [DOI] [PMC free article] [PubMed]
- 30.Thrusfield M. Veterinary epidemiology. 2. Oxford: Blackwell Science; 2007. [Google Scholar]
- 31.Ndumu D., Bakamutumaho B., Miller E., Balinandi S., Tumusiime D., Meunier N., Arinaitwe E., Rutebarika C., Downing R., Kidega E., Kyondo J., Ademun R. and Kariuki N. (2018); Covert Rift Valley fever in the domestic ruminant populations in Uganda; in Proc, American Society for Microbiology (ASM) microbe 2018, conference, Atlanta, USA; June 07 to 11, 2018.
- 32.Paweska JT, Mortimer E, Leman PA, Swanepoel R. An inhibition enzyme linked immunosorbent assay for the detection of antibody to Rift Valley fever in humans, domestic and wild ruminants. J Virol Meth. 2005;127(1):10–1E. doi: 10.1016/j.jviromet.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 33.R Core Team . R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2016. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data and material of the present study are available upon request to the corresponding authors including all field and laboratory original data anonymized in an excel format.