Abstract
Background
Radical resection is associated with good prognosis among patients with cT1/T2Nx rectal cancer. However, still some of the patients experienced cancer recurrence following radical resection. This study tried to identify the postoperative risk factors of local recurrence and distant metastasis separately.
Methods
This retrospective, single-center study comprised of 279 consecutive patients from Linkou branch of Chang Gung Memorial Hospital in 2005–2016 with rectal adenocarcinoma, pT1/T2N0M0 at distance from anal verge ≤ 8cm, who received curative radical resection.
Results
The study included 279 patients with pT1/pT2N0 mid-low rectal cancer with median follow-up of 73.5 months. Nineteen (6.8%) patients had disease recurrence in total. Nine (3.2%) of them had local recurrence, and fourteen (5.0%) of them had distant metastasis. Distal resection margin < 0.9 (cm) (hazard ratio = 4.9, p = 0.050) was the risk factor of local recurrence. Preoperative carcinoembryonic antigen (CEA) ≥ 5 ng/mL (hazard ratio = 9.3, p = 0.0003), lymph node yield (LNY) < 14 (hazard ratio = 5.0, p = 0.006), and distal resection margin < 1.4cm (hazard ratio = 4.0, p = 0.035) were the risk factors of distant metastasis.
Conclusion
For patients with pT1/pT2N0 mid-low rectal cancer, current multidisciplinary treatment brings acceptable survival outcome. Insufficient distal resection margin attracted the awareness of risk factors for local recurrence and distant metastasis as a foundation for future research.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12957-021-02223-4.
Keywords: Rectal cancer, Local recurrence, Distant metastasis, Radical resection, Total mesorectal excision
Background
Transabdominal radical resection without neoadjuvant therapy is recommended for patients with rectal cancer at clinical T1/T2 and negative N stage [1], and this sphincter-saving surgery with total mesorectal excision (TME) has been associated with high survival rates and low recurrence rate [2].
On the other hand, a growing number of patients with clinical T1/T2 tumors have undergone local excision (LE) which has improved their quality of life. However, concerns remain surrounding treatment, and though quality of life has improved, patients may still be at higher risk for disease recurrence [3, 4]. Radical resection generally guarantees disease-free survival at the expense of quality of life. Still, some patients with radical resection experience cancer recurrence which can be very frustrating and discouraging for both the patients and surgeons.
Previous studies reporting on rates of local recurrence (LR) and distant metastasis (DM) in patients with rectal cancer have not been consistent and owing to the limited data available, and the clear recommendations for preventing rectal cancer recurrence have not been established well. By knowing the risk factors, improvements in surgical planning and follow-up strategies may help improve cancer-free survival. Therefore, our study aimed to identify the risk factors for postoperative LR and DM in those with early-stage rectal cancer.
Methods
Data was retrieved from medical records of Chang Gung Memorial Hospital (CGMH) between 2005 and 2016, from 493 adult patients who had pT1/T2 rectal cancer; the data was finally collected from 279 patients with solitary, localized, resectable pT1/T2, N0 rectal adenocarcinomas with a distance from anal verge (DAV) ≤ 8 cm (Fig. 1). This study was approved by Institutional Review Board of CGMH with number 202000644B0. Rectal sonography, pelvic MRI, and PET-CT were performed for clinical staging at cT1/T2. All patients received chest to pelvis CT to assess for preoperative occult metastasis. If the patients received neoadjuvant therapy, only the patients who received short-course radiotherapy (RT) with 500 cGy × 5 days and underwent TME within 7 days were included. All patients received radical TME with curative intent. This procedure can be performed as an open method, laparoscopically assisted, or as a robotic surgery. All specimens were examined carefully by a well-trained pathologist with precise pT1/T2 (Table 1).
Fig. 1.
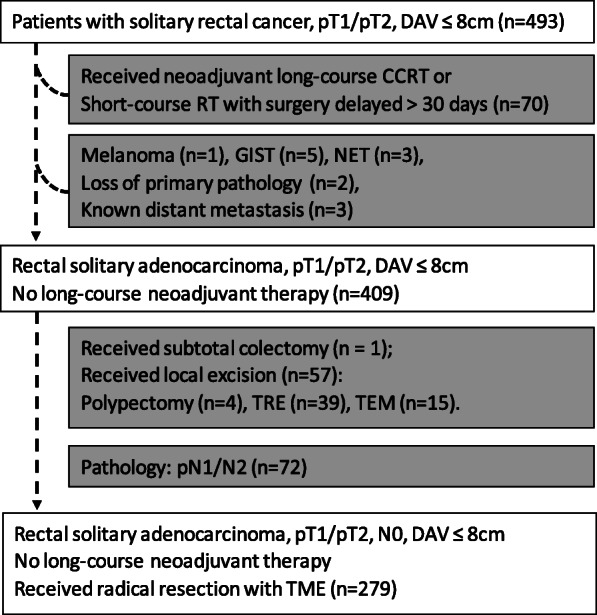
Patient selection of this study: patients with mid and low rectal cancer with lesions ≤8 cm away from the anal verge were included. Patients who were administered long-term (more than 30 days) neoadjuvant chemoradiotherapy or received radical resection after waiting for more than 30 days after receiving short-term (5 days) radiotherapy were excluded from this study due to the possibility of tumor regression. Other histologic types other than adenocarcinoma were listed and excluded. Patients who received subtotal colectomy due to familial polyposis, local excision including four who underwent transrectal polypectomies, 39 who had transanal excisions, and 15 who underwent transanal endoscopic microsurgeries were excluded. In the last step, we moved the patients who had positive N stage to supplement information
Table 1.
Patient characteristics for pT1/T2, N0
| Variable | 279 patients (% or [Q1 − Q3] †) | |
|---|---|---|
| No recurrence (n=260, %) | Recurrence (n=19, %) | |
| Age | 63.8± 12.4 | 58.4 ± 11.0 |
| BMI (kg/m2) | 24.1± 3.2 | 24.8 ± 3.4 |
| Male gender | 141 (54.2) | 12 (63.2) |
| Family cancer history | 79 (30.4) | 8 (42.1) |
| Preoperative CEA (ng/mL) | 1.8 [1.1–2.7] † | 2.3 [1.5–6.7] † |
| Preoperative CEA ≥ 5* | 30 (11.5) | 7 (36.8) |
| Preoperative hemoglobin (g/dL) | 12.8 ± 2.0 | 13.8 ± 1.8 |
| Preoperative albumin (g/dL) | 4.23 ± 0.40 | 4.32 ± 0.37 |
| Distance from anal verge (cm)* | 5.9 ± 1.7 | 4.9 ± 1.9 |
| Distance from anal verge ≤ 5 | 108 (41.5) | 12 (63.2) |
| Operation type | ||
| Low anterior resection | 241 (92.7) | 17 (89.5) |
| Abdomino-perineal resection | 17 (6.5) | 2 (10.5) |
| Hartmann’s procedure | 2 (0.8) | 0 |
| Neo-adjuvant radiotherapy ‡ | 41 (15.8) | 6 (31.6) |
| Adjuvant therapy | 4 (1.5) | 0 |
| Chemotherapy | 3 (1.2) | 0 |
| CRT | 1 (0.4) | 0 |
| PeriOP colostomy/ileostomy | 149 (57.3) | 15 (78.9) |
| PostOP complication/morbidity | 68 (26.2) | 3 (15.8) |
| Early | 43 (16.5) | 3 (15.8) |
| Late | 37 (14.2) | 1 (5.3) |
| Resection margin (cm) | 1.5 [0.8–2.2] † | 0.8 [0.5–1.7] † |
| Resection margin < 0.9 * | 78 (30.0) | 10 (52.6) |
| Resection margin < 1.4 * | 118 (45.4) | 14 (73.7) |
| Tumor diameter (cm) | 3.0 ± 1.4 | 3.3 ± 1.1 |
| Tumor diameter ≥ 2.7 | 137 (52.7) | 13 (72.2) |
| T stage | ||
| T1 | 98 (37.7) | 5 (26.3) |
| T2 | 162 (62.3) | 14 (73.7) |
| Lymph node yield | 20 [14–28] † | 16 [11–33] † |
| Lymph node yield ≥ 14* | 207 (79.6) | 11 (57.9) |
| Lymphovascular invasion | 12 (4.6) | 1 (5.3) |
| Perineural invasion | 13 (5.0) | 0 |
| Differentiation | ||
| Poor | 4 (1.5) | 1 (5.3) |
| Moderate | 195 (75.0) | 15 (78.9) |
| Well | 61 (23.5) | 3 (15.8) |
| Follow-up (month) | 73.5 [48–108] † | |
| Total follow-up length | 79.6 [51–109] † | 64.9 [54–102] † |
| Time to local recurrence | 25.6 [13.7–38.8] † | |
| Time to distant metastasis | 31.4 [12.9–59.2] † | |
BMI body mass index, CEA carcinoembryonic antigen, CRT chemoradiotherapy
*p value < 0.05
†Median [25 percentile–75 percentile]
‡Short-course radiotherapy 500cGy*5days
Following discharge, all patients returned to the clinic following a 7–10-day period for assessment. Patients were advised to return to the clinic for carcinoembryonic antigen (CEA) evaluations and chest x-rays every 3 months. As part of the follow-up evaluations, patients also underwent computed tomography (CT) and colonoscopy annually for the first 3 years following the surgery. LR was defined as intrapelvic recurrence to the area of anastomosis, presacral space, anterior side of the rectum, to organs with adhesions found in close proximity, internal iliac nodes, and lateral pelvic wall. DM was defined as recurrence outside the pelvic cavity detected after at least 6 months following curative resection.
We used receiver operating characteristic curve (ROC curve), which provided area under the curve (AUC), to determine the cutoff point for distance from anal verge (DAV), the lymph node yield (LNY), tumor diameter, and distal resection margin (DRM). After the cutoff points were identified, we examined the risk factors including family cancer history, sex, high CEA level (≥ 5 ng/mL), rate of postoperative morbidity (early and late), preoperative radiotherapy, T stage, lymphovascular invasion (LVI), perineural invasion (PNI), and tumor cell differentiation with Kaplan-Meier survival analysis. If the “p value < 0.1” was observed from Log rank test, then, we applied the risk factor into the COX regression model. A univariate COX regression model was applied followed by multivariate COX regression model in backward stepwise (Wald) that was used to provide an estimate of the hazard ratio (HR) and its confidence interval (CI) for investigating the association between the survival time of patients and one or more predictor variables/factors.
Results
Overall, 279 patients with pT1/pT2 mid-low rectal cancer were included in the analysis. The median follow-up period was 73.5 months. Overall, 19 (6.8%) patients had disease recurrence. Nine (3.2%) had LR, and 14 (5.0%) had DM. The median interval of time to recurrence was 25.6 months for LR and 31.4 months for DM. Three- and 5-year disease-free survival were 90% and 86%, respectively, while the 3- and 5-year cumulative recurrence rates were 4% and 6%, respectively.
After univariable COX regression, we selected these factors below for multivariable COX regression. CEA ≥ 5 with HR = 9.3 (95% CI 2.79–30.76, p = 0.0003), LNY < 14 with HR = 5.0 (95% CI 1.57–15.63, p = 0.006), DRM < 1.4 (cm) with HR = 4.0 (95% CI 1.10–14.41, p = 0.035), and preoperative radiotherapy with HR = 3.8 (95% CI 1.27–11.13, p = 0.035) were risk factors for DM. DRM < 0.9 (cm) with HR = 4.9 (95% CI 1.00–24.42, p = 0.050) and DAV ≤ 5 (cm) with HR = 7.1 (95% CI 0.86–59.19, p = 0.068) were risk factors for LR with borderline significance. All adjusted parameters, HR along with 95% CI and p value, are listed in Table 2 and Fig. 2.
Table 2.
Risk factors for local recurrence and distant metastasis in Cox regression model
| Variable | Univariable | Multivariable | ||
|---|---|---|---|---|
| Hazard ratio (95% CI for Exp(B)) | p value | Hazard ratio (95% CI for Exp(B)) | p value | |
| Local recurrence † | ||||
| Distal resection margin < 0.9 (cm) | 7.7 (1.60–37.17) | 0.011* | 4.9 (1.00–24.42) | 0.050 |
| Distance from anal verge ≤ 5 (cm) | 11.0 (1.37–87.89) | 0.024* | 7.1 (0.86–59.19) | 0.068 |
| Family cancer history | 2.9 (0.78–10.88) | 0.111 | ||
| Preoperative radiotherapy | 2.0 (0.24–15.93) | 0.533 | ||
| T stage | 1.1 (0.28–4.50) | 0.878 | ||
| Distant metastasis‡ | ||||
| CEA ≥ 5 (ng/mL) | 6.1 (2.06–18.26) | 0.001* | 9.3 (2.79–30.76) | 0.0003* |
| Lymph node yield < 14 | 3.5 (1.22–9.93) | 0.020* | 5.0 (1.57–15.63) | 0.006* |
| Distal resection margin < 1.4 (cm) | 4.4 (1.23–15.91) | 0.023* | 4.0 (1.10–14.41) | 0.035* |
| Tumor diameter ≥ 2.7 (cm) | 3.2 (0.89–11.50) | 0.074 | ||
| Preoperative radiotherapy | 3.4 (1.16–9.86) | 0.025* | 3.8 (1.27–11.13) | 0.016* |
| T stage | 1.4 (0.44–4.47) | 0.575 | ||
CEA carcinoembryonic antigen
*p value < 0.05
†Adjusted parameters (local recurrence): distal resection margin, distance from anal verge, family cancer history, preoperative radiotherapy, and T stage
‡Adjusted parameters (distant metastasis): CEA, distal resection margin, lymph node yield, tumor diameter, pre-operative radiotherapy, and T stage
Fig. 2.
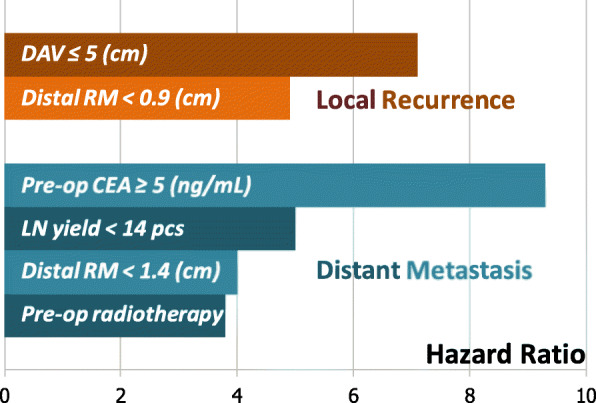
Hazard ratio for local recurrence and distant metastasis
Overall, 44% patients with LR were first evaluated by digital exam and subsequently diagnosed, while 71% of patients with DM were detected first by CEA elevation. Three (33%) patients with endoluminal LR and six (66%) with presacral or perirectal recurrence were identified in LR group. Eight (57%) patients with lung metastases and seven (50%) with liver metastases were identified in the DM group. There were 9 local recurrences and 14 distant metastases remaining from 19 patients. In these 4 patients who had both local recurrence and distant metastasis, two of them were detected at the same time. For the other two patients, one of them was detected local recurrence at postoperative 3 years, and then, CEA elevation came with the detection of lung metastasis 8 months later; the other was detected local recurrence at postoperative 15 months, and then, CEA elevation came with the detection of bone metastasis 2.5 years later.
Discussion
Currently, the published data on recurrence rates for patients with pT1/T2 mid-low rectal cancer is very limited. Pre-treatment CEA elevation, T2 stage, tumor distance from anal verge, close distal resection margin, lymphovascular invasion, perineural invasion, young age, male gender, ulcerative gross appearance (rather than polypoid appearance), and anastomotic leakage have been reported for risk factors of tumor recurrence, time to recurrence, and/or the recurrence patterns [5–10]. However, there was no consensus result, and sometimes, controversy existed. Some of the studies focused on transanal endoscopic surgery, which might have different results from those who received TME; some of the studies excluded patients who received any type of neoadjuvant therapy, which may generate another type of selection bias. For real-world data, some patients may receive neoadjuvant therapy due to clinically suspicious advanced T stage or possible N+ stage. After the specimen is examined, patients at pathological stage III were recommended to adjuvant chemotherapy and excluded from this research (Supplementary table 1).
Local recurrence
However, the existing reports suggest that once surgical treatment is performed and R0 resection is confirmed, good outcomes can be expected [11]. The most common sites for locoregional recurrence are generally the area around the anastomotic site, anterior side of the rectum, and the presacral site [12]. For those with mid-low rectal cancer, failure to achieve successful TME or receive preoperative RT may cause LR [13]. Lower DAV increases the difficulty of the surgery, and thus is thought to have a negative impact on survival. One recent prospective study reported a higher proportion of patients with positive resection margins in those with rectal cancer <5 cm DAV [6]. DAV has also been found to impact metastatic spread to the liver and lungs, a finding that was consistent with our data showing that those with mid-low rectal cancer had higher rates of lung metastasis [10].
In the US and European societies, perioperative RT is considered an acceptable adjuvant treatment for controlling LR. One Dutch TME trial reported lower 5-year LR rates in a TME + RT group than that for a TME only group (4.6% vs 11%) [14]. Another recent study reported no benefits associated with long-term neoadjuvant chemoradiotherapy (CRT) in terms of reduced early-stage rectal cancer recurrence [15]. In our study, one of the 47 patients who received short-course RT following TME had LR. The policy of preoperative radiotherapy in our hospital usually suggests for clinical T3 stage and above or positive N stage. However, it was flexible for mid-low rectal cancers in some circumstances. There was no significant difference for LR between RT and non-RT groups, but patients in the RT group tended to develop DM, and this might be related to patient selection policy. Patients in the RT group had higher perioperative colostomy/ileostomy rate; this group had higher proportion of T2 tumor and lower proportion of well-differentiated tumor (supplementary table 2).
Carcinoembryonic antigen (CEA)
CEA is a protein produced during prenatal development that decreases to very low or undetectable levels following delivery. In current practice, CEA is mostly utilized to complete preoperative evaluations and to assess patients for occult recurrence of colorectal cancer on follow-up. In recent studies, high pretreatment CEA was regarded as a poor prognostic factor for colorectal cancer after curative surgery [7, 16]. In a retrospective study that included 16,659 patients, elevated pre-operative CEA levels predicted poor prognosis much more accurately in pT1 patients who were considered to have a better prognosis according to the TNM system [17]. In our study, 37 patients had preoperative CEA elevation. Seven (18.9%) of them had two LRs, and six had DM (one of them had both LR and DM) when evaluated during the postoperative follow-up. Preoperative CEA elevation was considered to be a poor prognostic factor in our study.
Lymph node yield (LNY)
The presence of metastatic LNs identified by pathological examination indicates systemic tumor spread and is therefore the major determinant for adjuvant therapy. There is a current consensus that at least 12 lymph nodes (LNs) should be yielded when obtaining the surgical specimen in order to conduct an appropriate pathological examination; appropriate LNY can help to stage colorectal cancer more precisely. Inappropriate LNY may lead to underreporting, and thus result in higher recurrence rates and poorer survival [18].
LNY number is possibly affected by factors such as age, gender, tumor size, location, T stage, N stage, preoperative CRT, tumor regression grade, or the pathologic investigation [19, 20]. A few recent large-scale retrospective studies reported survival benefits with LNY ≥ 12 in those with colorectal cancer [20, 21]. However, rectal cancer is thought to be more difficult than colon cancer in achieving a LNY ≥ 12 [22]. In our study, 279 patients had a median LNY of 20. Overall, 244 of 279 (87.5%) patients had a LNY ≥ 12.
Recently, one large SEER database retrospective study based on 154,208 patients with colon cancer found that LNY did not have a unique, strong threshold for assessing survival (i.e., 12 lymph nodes) [23]. Interestingly, the study reported that patients without LN metastasis had a lower risk of death for each LN examined up to approximately 25 LNs. With a higher LNY, oversights made in staging due to false-negative N stages might decrease. Some studies reported survival benefits with a LNY ≥ 14 or more. The effect on an adequate LNY might bring survival benefits even for those at a pN0 stage [24]. This suggests that the survival benefits associated with increasing LNY may not be completely associated with N stage. A possible explanation is that an increased number of negative lymph nodes are associated with a higher immune response and longer survival [25]. In our study, we used ROC curve and identified that LNY = 13.5 had the largest AUC for 280 pN0 patients. For our analysis, those with LNY ≥ 14 had better outcomes in distant-metastasis-free survival (p = 0.013) and disease-free survival (p = 0.047).
Distal resection margin
In our study, DRM was found to be a significant risk factor for both LR (<0.9 cm) and DM (<1.4 cm). Retained intramucosal cancer cells can potentially increase the risk of resection site recurrence, and migration of cancer to the perirectal tissue may lead to locoregional recurrence in the pelvic cavity. In addition, insufficient DRM is associated with a higher risk of LR [26]. Though the 1-cm rule is still controversial in some studies, especially for patients who undergo preoperative RT [12, 27], a DRM 1 to 2 cm is acceptable according to the current NCCN guidelines [28]. Some studies regarding transanal TME revealed that with appropriate DRM, short-term and long-term oncological outcomes improved for those with mid-low rectal cancers [29–31], and so forth, transanal TME may provide better outcome from preventing DM in correlation to our findings.
Miscellaneous
Age, postoperative complication, LVI, and T stage may be risk factors for LR and/or DM. A meta-analysis that included five prospective cohort and six retrospective cohort studies reported that anastomotic leakage after radical resection of rectal cancer adversely impacted cancer-specific mortality and LR [5]. Age younger than 63 and DAV ≤ 5 cm were reported to have a higher chance of early DMs in a recent study [16]. In addition, LVI was reported to be a risk factor of DM in some studies [7]. However, this factor was not significant in our study. Those with advanced T stage tend to have poor prognoses and higher risk of disease recurrence; however, our study did not reveal the difference in impact between T1 and T2 stage on disease-free or recurrence-free survival. The possible explanation was the patient selection policy which may encourage pT2 for radiotherapy because of the inaccurate preoperative clinical staging.
Limitations
Our study was limited by its retrospective design, small case number, and experience from a single tertiary center. All patients were treated by the same colorectal team, including surgeons with similar training background and surgical concepts, and this may have led to similar preferences among the surgeons, which may have resulted in bias. The selection criteria for preoperative radiotherapy were a confounding factor. Even though the study had a long-term follow-up period, the evolution of surgical techniques could not be evaluated.
Conclusion
For patients with pT1/pT2N0 mid-low rectal cancer, multidisciplinary management that includes awareness of risk factors for local recurrence and distant metastasis is needed for treatment and to improve survival outcomes. Our study identified distal resection margin < 0.9 (cm) to be the main risk factor of local recurrence, while CEA ≥ 5 (ng/mL), lymph node yield < 14, and distal resection margin < 1.4 (cm) were risk factors for distant metastasis. For achieving more sufficient distal resection margin by the surgical planning and evolution of technique and devices, we hope that the current study can lay a foundation to improve survival outcomes in the future.
Supplementary Information
Additional file 1: Supplementary table 1 Patient characteristics
Additional file 2: Supplementary table 2 Patient characteristics for non-RT vs. RT
Acknowledgements
The authors appreciate all the colorectal surgeons, oncologists, radiologists, pathologists, and the nursing and medical staff of CGMH. The authors appreciate the Clinical Informatics and Medical Statistics Research Center (CIMS) of Chang Gung University for offering the consult of statistics.
Abbreviations
- CEA
Carcinoembryonic antigen
- CGMH
Chang Gung Memorial Hospital
- CI
Confidence interval
- CRT
Chemoradiotherapy
- CT
Computed tomography
- DAV
Distance from anal verge
- DM
Distant metastasis
- DRM
Distal resection margin
- HR
Hazard ratio
- LAR
Low anterior resection
- LE
Local excision
- LNY
Lymph node yield
- LR
Local recurrence
- LVI
Lymphovascular invasion
- RT
Radiotherapy
- TME
Total mesorectal excision
Authors’ contributions
IL and YH made the concept and design of this study. Collection and assembly of data was done by IL and YC. The data analysis and interpretation were done by IL. IL, YH, YC, JY, and WT wrote and edited the manuscript. JC reviewed and corrected this manuscript. JY, WT, PH, HH, and JC provided patients for this study. All authors read and approved the final manuscript.
Funding
This study was not funded by any grants or financial support.
Availability of data and materials
The detailed patients’ databases generated and analyzed during this study are not publicly available due to appropriate protection of patients’ personal information but are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The study protocol was approved by the institutional review board of Taoyuan branch of Chang Gung Memorial Hospital as 202000644B0. Due to the retrospective design of the study, the local ethics committee confirmed that informed consent was not necessary from the participants.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
I-Li Lai, Email: ryane92a5@gmail.com.
Jeng-Fu You, Email: jenodyssey@gmail.com.
Yih-Jong Chern, Email: ufo789.ufo789@gmail.com.
Wen-Sy Tsai, Email: wensyt@gmail.com.
Jy-Ming Chiang, Email: jmjiang@cgmh.org.tw.
Pao-Shiu Hsieh, Email: hsiehps@yahoo.com.
Hsin-Yuan Hung, Email: hsinyuan@adm.cgmh.org.tw.
Yu-Jen Hsu, Email: blueslun@gmail.com.
References
- 1.Benson AB, Venook AP, Al-Hawary MM, Cederquist L, Chen YJ, Ciombor KK, Cohen S, Cooper HS, Deming D, Engstrom PF, et al. Rectal cancer, version 2.2018, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2018;16(7):874–901. doi: 10.6004/jnccn.2018.0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kobayashi H, Mochizuki H, Morita T, Kotake K, Teramoto T, Kameoka S, Saito Y, Takahashi K, Hase K, Oya M, Maeda K, Hirai T, Kameyama M, Shirouzu K, Sugihara K. Characteristics of recurrence after curative resection for T1 colorectal cancer: Japanese multicenter study. J Gastroenterol. 2011;46(2):203–211. doi: 10.1007/s00535-010-0341-2. [DOI] [PubMed] [Google Scholar]
- 3.Halverson AL, Morris AM, Cleary RK, Chang GJ: For patients with early rectal cancer, does local excision have an impact on recurrence, survival, and quality of life relative to radical resection? Ann Surg Oncol 2019 [DOI] [PubMed]
- 4.Hwang Y, Yoon YS, Bong JW, Choi HY, Song IH, Lee JL, Kim CW, Park IJ, Lim SB, Yu CS, Kim JC. Long-term transanal excision outcomes in patients with T1 rectal cancer: comparative analysis of radical resection. Ann Coloproctol. 2019;35(4):194–201. doi: 10.3393/ac.2018.10.18.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lu ZR, Rajendran N, Lynch AC, Heriot AG, Warrier SK. Anastomotic leaks after restorative resections for rectal cancer compromise cancer outcomes and survival. Dis Colon Rectum. 2016;59(3):236–244. doi: 10.1097/DCR.0000000000000554. [DOI] [PubMed] [Google Scholar]
- 6.Khan MAS, Ang CW, Hakeem AR, Scott N, Saunders RN, Botterill I. The impact of tumour distance from the anal verge on clinical management and outcomes in patients having a curative resection for rectal cancer. J Gastrointest Surg. 2017;21(12):2056–2065. doi: 10.1007/s11605-017-3581-0. [DOI] [PubMed] [Google Scholar]
- 7.Lee JH, Lee JL, Park IJ, Lim SB, Yu CS, Kim JC. Identification of recurrence-predictive indicators in stage I colorectal cancer. World J Surg. 2017;41(4):1126–1133. doi: 10.1007/s00268-016-3833-2. [DOI] [PubMed] [Google Scholar]
- 8.Wang S, Liu J, Wang S, Zhao H, Ge S, Wang W. Adverse effects of anastomotic leakage on local recurrence and survival after curative anterior resection for rectal cancer: a systematic review and meta-analysis. World J Surg. 2017;41(1):277–284. doi: 10.1007/s00268-016-3761-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zeng WG, Liu MJ, Zhou ZX, Wang ZJ. A distal resection margin of </=1 mm and rectal cancer recurrence after sphincter-preserving surgery: the role of a positive distal margin in rectal cancer surgery. Dis Colon Rectum. 2017;60(11):1175–1183. doi: 10.1097/DCR.0000000000000900. [DOI] [PubMed] [Google Scholar]
- 10.Augestad KM, Keller DS, Bakaki PM, Rose J, Koroukian SM, Oresland T, Delaney CP. The impact of rectal cancer tumor height on recurrence rates and metastatic location: a competing risk analysis of a national database. Cancer Epidemiol. 2018;53:56–64. doi: 10.1016/j.canep.2018.01.009. [DOI] [PubMed] [Google Scholar]
- 11.Bhangu A, Ali SM, Darzi A, Brown G, Tekkis P. Meta-analysis of survival based on resection margin status following surgery for recurrent rectal cancer. Colorectal Dis. 2012;14(12):1457–1466. doi: 10.1111/j.1463-1318.2012.03005.x. [DOI] [PubMed] [Google Scholar]
- 12.Kusters M, Marijnen CA, van de Velde CJ, Rutten HJ, Lahaye MJ, Kim JH, Beets-Tan RG, Beets GL. Patterns of local recurrence in rectal cancer; a study of the Dutch TME trial. Eur J Surg Oncol. 2010;36(5):470–476. doi: 10.1016/j.ejso.2009.11.011. [DOI] [PubMed] [Google Scholar]
- 13.Bernstein TE, Endreseth BH, Romundstad P, Wibe A, Norwegian Colorectal Cancer R What is a safe distal resection margin in rectal cancer patients treated by low anterior resection without preoperative radiotherapy? Colorectal Dis. 2012;14(2):e48–e55. doi: 10.1111/j.1463-1318.2011.02759.x. [DOI] [PubMed] [Google Scholar]
- 14.Kapiteijn E, Marijnen CA, Nagtegaal ID, Putter H, Steup WH, Wiggers T, Rutten HJ, Pahlman L, Glimelius B, van Krieken JH, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med. 2001;345(9):638–646. doi: 10.1056/NEJMoa010580. [DOI] [PubMed] [Google Scholar]
- 15.Hayes IP, Milanzi E, Gibbs P, Reece JC: Neoadjuvant chemoradiotherapy and tumor recurrence in patients with early T-stage cancer of the lower rectum. Ann Surg Oncol 2019. [DOI] [PubMed]
- 16.Restivo A, Delrio P, Deidda S, Spolverato G, Rega D, Cerci M, et al. Predictors of early distant relapse in rectal cancer patients submitted to preoperative chemoradiotherapy. Oncol Res Treat. 2020:1–7. [DOI] [PubMed]
- 17.Shen F, Cui J, Hong X, Yu F, Bao X. Preoperative serum carcinoembryonic antigen elevation in stage I colon cancer: improved risk of mortality in stage T1 than in stage T2. Int J Colorectal Dis. 2019;34(6):1095–1104. doi: 10.1007/s00384-019-03298-y. [DOI] [PubMed] [Google Scholar]
- 18.Rullier A, Laurent C, Capdepont M, Vendrely V, Belleannee G, Bioulac-Sage P, Rullier E. Lymph nodes after preoperative chemoradiotherapy for rectal carcinoma: number, status, and impact on survival. Am J Surg Pathol. 2008;32(1):45–50. doi: 10.1097/PAS.0b013e3180dc92ab. [DOI] [PubMed] [Google Scholar]
- 19.Bustamante-Lopez LA, Nahas CSR, Nahas SC, Marques CFS, Pinto RA, Cotti GC, Imperiale AR, de Mello ES, Ribeiro UJ, Cecconello I. Pathologic complete response implies a fewer number of lymph nodes in specimen of rectal cancer patients treated by neoadjuvant therapy and total mesorectal excision. Int J Surg. 2018;56:283–287. doi: 10.1016/j.ijsu.2018.07.001. [DOI] [PubMed] [Google Scholar]
- 20.Orsenigo E, Gasparini G, Carlucci M. Clinicopathological factors influencing lymph node yield in colorectal cancer: a retrospective study. Gastroenterol Res Pract. 2019:5197914. [DOI] [PMC free article] [PubMed]
- 21.Budde CN, Tsikitis VL, Deveney KE, Diggs BS, Lu KC, Herzig DO. Increasing the number of lymph nodes examined after colectomy does not improve colon cancer staging. J Am Coll Surg. 2014;218(5):1004–1011. doi: 10.1016/j.jamcollsurg.2014.01.039. [DOI] [PubMed] [Google Scholar]
- 22.Vergara-Fernandez O, Navarro-Navarro A, Rangel-Rios HA, Salgado-Nesme N, Reyes-Monroy JA, Velazquez-Fernandez D. Oncological implications of lymph nodes retrieval and perineural invasion in colorectal cancer: outcomes from a referral center. Rev Invest Clin. 2018;70(6):291–300. doi: 10.24875/RIC.18002505. [DOI] [PubMed] [Google Scholar]
- 23.Gleisner AL, Mogal H, Dodson R, Efron J, Gearhart S, Wick E, Lidor A, Herman JM, Pawlik TM. Nodal status, number of lymph nodes examined, and lymph node ratio: what defines prognosis after resection of colon adenocarcinoma? J Am Coll Surg. 2013;217(6):1090–1100. doi: 10.1016/j.jamcollsurg.2013.07.404. [DOI] [PubMed] [Google Scholar]
- 24.Chandrasinghe PC, Ediriweera DS, Hewavisenthi J, Kumarage S, Deen KI. Total number of lymph nodes harvested is associated with better survival in stages II and III colorectal cancer. Indian J Gastroenterol. 2014;33(3):249–253. doi: 10.1007/s12664-013-0406-2. [DOI] [PubMed] [Google Scholar]
- 25.He WZ, Xie QK, Hu WM, Kong PF, Yang L, Yang YZ, Jiang C, Yin CX, Qiu HJ, Zhang HZ, Zhang B, Xia LP. An increased number of negative lymph nodes is associated with a higher immune response and longer survival in colon cancer patients. Cancer Manag Res. 2018;10:1597–1604. doi: 10.2147/CMAR.S160100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Junginger T, Goenner U, Hitzler M, Trinh TT, Heintz A, Roth W, Blettner M, Wollschlaeger D. Analysis of local recurrences after transanal endoscopic microsurgery for low risk rectal carcinoma. Int J Colorectal Dis. 2017;32(2):265–271. doi: 10.1007/s00384-016-2715-2. [DOI] [PubMed] [Google Scholar]
- 27.Manegold P, Taukert J, Neeff H, Fichtner-Feigl S, Thomusch O. The minimum distal resection margin in rectal cancer surgery and its impact on local recurrence - a retrospective cohort analysis. Int J Surg. 2019;69:77–83. doi: 10.1016/j.ijsu.2019.07.029. [DOI] [PubMed] [Google Scholar]
- 28.Leo E, Belli F, Miceli R, Mariani L, Gallino G, Battaglia L, Vannelli A, Andreola S. Distal clearance margin of 1 cm or less: a safe distance in lower rectum cancer surgery. Int J Colorectal Dis. 2009;24(3):317–322. doi: 10.1007/s00384-008-0604-z. [DOI] [PubMed] [Google Scholar]
- 29.de Lacy FB, van Laarhoven J, Pena R, Arroyave MC, Bravo R, Cuatrecasas M, Lacy AM. Transanal total mesorectal excision: pathological results of 186 patients with mid and low rectal cancer. Surg Endosc. 2018;32(5):2442–2447. doi: 10.1007/s00464-017-5944-8. [DOI] [PubMed] [Google Scholar]
- 30.Marks JH, Myers EA, Zeger EL, Denittis AS, Gummadi M, Marks GJ. Long-term outcomes by a transanal approach to total mesorectal excision for rectal cancer. Surg Endosc. 2017;31(12):5248–5257. doi: 10.1007/s00464-017-5597-7. [DOI] [PubMed] [Google Scholar]
- 31.Hol JC, van Oostendorp SE, Tuynman JB, Sietses C. Long-term oncological results after transanal total mesorectal excision for rectal carcinoma. Tech Coloproctol. 2019;23(9):903–911. doi: 10.1007/s10151-019-02094-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Supplementary table 1 Patient characteristics
Additional file 2: Supplementary table 2 Patient characteristics for non-RT vs. RT
Data Availability Statement
The detailed patients’ databases generated and analyzed during this study are not publicly available due to appropriate protection of patients’ personal information but are available from the corresponding author on reasonable request.


