Abstract
Background
Staphylococcus aureus has been associated with the exacerbation and severity of atopic dermatitis (AD). Studies have not investigated the colonisation dynamics of S. aureus lineages in African toddlers with AD. We determined the prevalence and population structure of S. aureus in toddlers with and without AD from rural and urban South African settings.
Methods
We conducted a study of AD-affected and non-atopic AmaXhosa toddlers from rural Umtata and urban Cape Town, South Africa. S. aureus was screened from skin and nasal specimens using established microbiological methods and clonal lineages were determined by spa typing. Logistic regression analyses were employed to assess risk factors associated with S. aureus colonisation.
Results
S. aureus colonisation was higher in cases compared to controls independent of geographic location (54% vs. 13%, p < 0.001 and 70% vs. 35%, p = 0.005 in Umtata [rural] and Cape Town [urban], respectively). Severe AD was associated with higher colonisation compared with moderate AD (86% vs. 52%, p = 0.015) among urban cases. Having AD was associated with colonisation in both rural (odds ratio [OR] 7.54, 95% CI 2.92–19.47) and urban (OR 4.2, 95% CI 1.57–11.2) toddlers. In rural toddlers, living in an electrified house that uses gas (OR 4.08, 95% CI 1.59–10.44) or utilises kerosene and paraffin (OR 2.88, 95% CI 1.22–6.77) for heating and cooking were associated with increased S. aureus colonisation. However, exposure to farm animals (OR 0.3, 95% CI 0.11–0.83) as well as living in a house that uses wood and coal (OR 0.14, 95% CI 0.04–0.49) or outdoor fire (OR 0.31, 95% CI 0.13–0.73) were protective. Spa types t174 and t1476, and t272 and t1476 were dominant among urban and rural cases, respectively, but no main spa type was observed among controls, independent of geographic location. In urban cases, spa type t002 and t442 isolates were only identified in severe AD, t174 was more frequent in moderate AD, and t1476 in severe AD.
Conclusion
The strain genotype of S. aureus differed by AD phenotypes and rural-urban settings. Continued surveillance of colonising S. aureus lineages is key in understanding alterations in skin microbial composition associated with AD pathogenesis and exacerbation.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12879-021-06044-4.
Introduction
Atopic dermatitis (AD) is a common childhood inflammatory skin disease that frequently presents in early childhood [1]. The prevalence of AD is high in developed countries where it affects 10–20% of children [2]. However, recent epidemiological data indicate an increase in the prevalence of AD among children in developing countries, including South Africa [3–5]. The increasing prevalence of AD and allergy is also associated with urbanisation with a lower prevalence and microbial-related protective environmental factors noted in rural areas [3, 6]. Patients with AD usually suffer from persistent or relapsing itchy and dry eczematous skin lesions with inflammation and increased susceptibility to cutaneous Staphylococcus aureus (S. aureus) colonisation associated with perturbation of the skin microbial community [7, 8]. In addition to skin colonisation, S. aureus has also been reported to colonise the nasal cavity as a primary reservoir for extra-nasal auto-transmission [9]. Skin and nasal S. aureus colonisation have been demonstrated in both AD patients and healthy individuals; however, a higher colonisation density and prevalence have been described in AD patients [9]. S. aureus colonisation has also been associated with AD pathogenesis [10], with colonisation preceding the clinical onset of AD in early childhood [11]. S. aureus produces a variety of virulence factors, including superantigens, proteases, as well as dermolytic and cytolytic toxins which contribute to the progression of AD [12]. Nonetheless, other staphylococcal species, including S. epidermidis and S. haemolyticus have been implicated in the pathophysiology of AD by the degradation of epidermal structural proteins [13, 14]. Molecular epidemiological studies have shown that while colonisation occurs in both AD patients and healthy individuals, the genetic background of colonising S. aureus strains differ across AD disease phenotypes and may influence disease pathogenesis and severity [1, 15]. We hypothesised that geographic location affects S. aureus colonisation in AD and health through distinct environmental exposures. Here, we report the prevalence and genotypes of S. aureus from skin and nasal samples of AmaXhosa AD and non-AD toddlers in rural and urban South African settings. In addition, we evaluated the risk factors for S. aureus colonisation in each geographic location.
Materials and methods
Study design, setting and population
Participant recruitment
We conducted a cross-sectional study of 220 toddlers with and without AD aged 12–36 months (overall mean age 22.4 months; standard deviation 0.54 months) from February 2015 to May 2016 (Fig. 1) [6, 16]. Urban control subjects (n = 50) were recruited as a sub-study from non-allergic, non-food-sensitised subjects participating in the South African Food Allergy (SAFFA) study at randomly selected creches in the Cape Town metropole. As creches are rarely found in the rural district, rural controls (n = 54) were recruited from toddlers of eligible age from the areas surrounding 10 district community health clinics in the rural Mqanduli district of Umtata. Patients with moderate to severe AD (n = 56) were recruited from the Department of Paediatric Dermatology of the Red Cross War Memorial Children’s Hospital in Cape Town and rural cases (n = 60) from the Department of Dermatology, Nelson Mandela Academic Hospital, in Umtata. AD was clinically diagnosed by a dermatologist using the validated UK Working Party diagnosis criteria for AD [17]. Disease severity was determined using the objective SCORAD (SCORing of Atopic Dermatitis) index into moderate (15–40) and severe (> 40) [18]. Guardians completed a questionnaire aimed at determining environmental exposures as previously described [19].
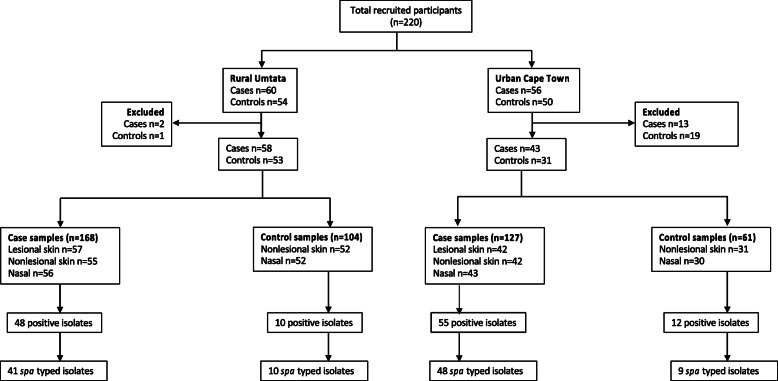
Fig. 1.
Flow chart of participants’ sample processing. Eleven participants (eight cases and three controls) had one unavailable specimen for either the lesional skin, nonlesional skin or anterior nares
Specimen collection and processing
Sterile Copan nylon-tipped flocked swabs (Cat. no. 516C; Copan Italia, Brescia, Italy) were used to collect samples from lesional (i.e., most active area of eczematous skin with acute and/or chronic changes) and non-lesional skin (i.e., area with the most normal-appearing skin – usually the back). The swab was pre-moistened with sterile distilled water and a 4 cm2 area of the skin lesion was swabbed for at least 1 min in a non-overlapping manner. In addition, nasal swabs were collected from all participants to determine the S. aureus carriage status according to previously described methodology [20]. The collected swabs were immediately placed into 1 ml skim milk-tryptone-glucose-glycerol (STGG), transported at 4 °C to the laboratory within two hours of collection and frozen at − 80 °C for subsequent batch processing. All lesional, non-lesional, and nasal swabs stored in STGG were allowed to thaw at room temperature, vortexed for 30 s and 100 μl was inoculated onto Mannitol Salt Agar (MSA) (National Health Laboratory Services [NHLS], Green Point Media Laboratory Cape Town, South Africa), and aerobically incubated at 37 °C for 48 h. Isolates that were positive for both mannitol fermentation and DNase production were presumptively identified as S. aureus [21].
Nucleic acid extraction
Recovered S. aureus isolates were aerobically subcultured onto 2% sheep blood agar at 37 °C overnight. Genomic DNA extraction was completed using a modified heat lysis method [22]. Briefly, colonies were re-suspended in AVE buffer (Qiagen, Hilden, Germany) instead of phosphate-buffered saline and centrifuged at 13,000 g for two minutes. The supernatant containing genomic DNA was diluted in AVE buffer depending on the initial DNA concentration to a final concentration range of 20-70 ng/μl.
Molecular identification of the S. aureus isolates
Isolates presumptively identified as S. aureus were screened for the thermonuclease (nuc) gene using species-specific primers as previously described [23].
Molecular characterisation
S. aureus isolates were characterised by staphylococcus protein A (spa) typing targeting the variable X-region of the gene using the conventional primers spa-1113F/spa-1514R [24, 25]. Isolates that failed to yield a spa amplicon or had poor sequence quality were re-analysed using alternative spa primers T3F/1517R or 1095F/1517R [26, 27]. Clustering was based on their genetic relatedness to spa-clonal complexes (spa-CCs) using the Based Upon Repeat Pattern (BURP) clustering algorithm of the Ridom Staph Type software (Ridom GmbH, Münster, Germany) [28]. PCR detection of the nuc gene was performed to rule out misidentification of isolates that failed to yield a spa amplicon [23].
Statistical analysis
All data analyses were performed using Stata version SE16.0 (1985–2019 StataCorp LP, Texas, USA). The significance threshold for all analyses was 0.05. Univariate and multivariate analyses to assess risk factors for S. aureus colonisation were performed using logistic regression and presented as odd ratios (OR) and adjusted ORs (aOR) reported with a 95% confidence interval (CI). The level of statistical significance in the logistic regression analysis was determined by a Chi-square test. Variables that were significant determinants for colonisation were included in the multivariate logistic regression model. Comparison of categorical data was performed by Chi-squared test unless stated otherwise. Comparison of means was performed using the t-test for two independent samples reported with a standard deviation (SD). Participants with missing data were excluded from the analyses relating to that variable.
Results
S. aureus colonisation in cases and controls
A total of 185 (84 controls and 101 cases) toddlers were assessed for S. aureus colonisation (Table 1). Thirty-five participants were excluded (missing specimen) from the study analysis (Fig. 1). Of these, 79 (43%) were colonised with S. aureus in at least one of the sampled body sites. There was an overall higher prevalence of colonisation among urban participants compared to rural participants (55% [41/74] vs. 34% [38/111], p = 0.006). S. aureus was commonly detected from cases compared to controls in both rural (54% [31/58] vs. 13% [7/53], p < 0.001 and urban settings (70% [30/43] vs. 35% [11/31], p = 0.005). Furthermore, cases were more frequently colonised on non-lesional skin compared to controls, and this was independent on geographic location (Additional file 1: Table S1). Among cases, colonisation was more common on lesional skin compared to non-lesional skin (rural: p = 0.035 and urban: p = 0.021) and the anterior nares (rural: p = 0.008). The prevalence of colonisation was common among urban cases with severe disease (86% [19/22] vs. 52% [11/21], p = 0.015), however, this was not associated with the site of colonisation (Additional file 2: Table S2). Overall, these findings show that geographic location influences the dynamics of S. aureus colonisation on skin and nares in AD and non-AD, and this is dependent on the site of colonisation and disease severity.
Table 1.
Participant characteristics of atopic dermatitis cases and healthy controls
| Explanatory variable | Umtata | Cape Town | ||||||
|---|---|---|---|---|---|---|---|---|
| Total, n (%) | Case, n (%) | Control, n (%) | p-value | Total, n (%) | Case, n (%) | Control, n (%) | p-value | |
| Total | 111 (100) | 58 (52) | 53 (48) | 0.502a | 74 (100) | 43 (58) | 31 (42) | 0.049a |
| Age (months) | ||||||||
| Mean [standard deviation] | 21.27 [7.15] | 21.03 [7.41] | 21.53 [6.90] | 0.718 | 24.19 [7.37] | 23.98 [7.44] | 24.48 [7.38] | 0.773 |
| Sex | ||||||||
| Female | 42 (39) | 24 (43) | 18 (34) | 0.431 | 36 (49) | 19 (44) | 17 (55) | 0.480 |
| Male | 67 (61) | 32 (57) | 35 (66) | 38 (51) | 24 (56) | 14 (45) | ||
| AD severity | ||||||||
| Moderate | 23 (40) | 23 (40) | – | 21 (49) | 21 (49) | – | ||
| Severe | 35 (60) | 35 (60) | – | 22 (51) | 22 (51) | – | ||
| Atopic disease | ||||||||
| Food allergy | 11 (10) | 10 (17) | 1 (2) | 0.009 | 9 (12) | 9 (21) | 0 (0) | 0.008 |
| Asthma | 0 (0) | 0 (0) | 0 (0) | 1 (1) | 1 (3) | 0 (0) | 1.000 | |
| Allergic rhinitis | 7 (8) | 1 (2) | 6 (11) | 0.242 | 1 (1) | 1 (3) | 0 (0) | 1.000 |
| Mode of birth | ||||||||
| Caesarean section | 25 (23) | 14 (24) | 11 (21) | 0.821 | 33 (46) | 20 (49) | 13 (42) | 0.637 |
| Vaginal | 86 (77) | 44 (76) | 42 (79) | 39 (54) | 21 (51) | 18 (58) | ||
| Breastfeeding | 35 (32) | 10 (17) | 25 (47) | 0.001 | 9 (12) | 7 (16) | 2 (6) | 0.288 |
| Antibiotic exposure | 92 (82) | 49 (83) | 43 (81) | 0.810 | 54 (72) | 30 (70) | 24 (77) | 0.598 |
| Immunisation status | ||||||||
| Complete | 107 (96) | 56 (95) | 52 (98) | 0.620 | 64 (86) | 33 (77) | 31 (100) | 0.004 |
| Incomplete | 4 (4) | 3 (5) | 1 (2) | 10 (14) | 10 (23) | 0 (0) | ||
| Large family a | 62 (55) | 30 (52) | 32 (60) | 0.445 | 24 (32) | 14 (33) | 10 (32) | 1.000 |
| Animal exposure | 93 (84) | 39 (67) | 53 (100) | 0.001 | 2 (3) | 2 (6) | 0 (0) | 0.495 |
| Parental education | ||||||||
| Primary | 8 (7) | 2 (3) | 6 (11) | 0.001 | 1 (1) | 1 (2) | 0 (0) | 0.025 |
| Secondary | 70 (63) | 31 (53) | 39 (74) | 33 (45) | 14 (33) | 19 (61) | ||
| Tertiary | 31 (28) | 25 (43) | 6 (11) | 40 (54) | 28 (65) | 12 (39) | ||
| Other | 2 (2) | 0 (0) | 2 (4) | 0 (0) | 0 (0) | 0 (0) | ||
| Maternal factors | ||||||||
| Animal exposure | 96 (86) | 44 (76) | 52 (98) | 0.001 | 4 (60) | 4 (11) | 0 (0) | 0.120 |
| Pregnant smoking | 1 (1) | 0 (0) | 1 (2) | 0.482 | 3 (45) | 0 (0) | 3 (10) | 0.094 |
| Smoking | 1 (1) | 0 (0) | 1 (2) | 0.477 | 4 (6) | 1 (3) | 3 (10) | 0.324 |
| Asthma | 2 (2) | 2 (3) | 0 (0) | 0.496 | 6 (8) | 4 (10) | 2 (6) | 1.000 |
| Allergic rhinitis | 4 (4) | 4 (7) | 0 (0) | 0.120 | 5 (68) | 4 (10) | 1 (3) | 0.387 |
| Atopic dermatitis | 2 (2) | 2 (3) | 0 (0) | 0.496 | 3 (4) | 2 (5) | 1 (3) | 1.000 |
| Food allergy | 3 (3) | 2 (3) | 1 (2) | 1.000 | 1 (1) | 1 (2) | 0 (0) | 1.000 |
| Paternal factors | ||||||||
| Smoking | 15 (14) | 9 (16) | 6 (12) | 0.589 | 20 (31) | 11 (31) | 9 (31) | 1.000 |
| Asthma | 3 (3) | 3 (5) | 0 (0) | 0.245 | 0 (0) | 0 (0) | 0 (0) | |
| Allergic rhinitis | 3 (3) | 3 (5) | 0 (0) | 0.245 | 7 (10) | 7 (17) | 0 (0) | 0.018 |
| Atopic dermatitis | 1 (1) | 1 (2) | 0 (0) | 1.000 | 2 (3) | 2 (5) | 0 (0) | 0.505 |
| Food allergy | 1 (1) | 1 (2) | 0 (0) | 1.000 | 1 (1) | 1 (2) | 0 (0) | 1.000 |
| Household factors | ||||||||
| Electricity + gas | 69 (62) | 56 (97) | 13 (25) | 0.001 | 66 (99) | 35 (97) | 31 (100) | 1.000 |
| Kerosene + paraffin | 64 (58) | 44 (76) | 20 (38) | 0.001 | 43 (64) | 21 (58) | 22 (71) | 0.317 |
| Paraffin | 38 (34) | 6 (10) | 32 (60) | 0.001 | 0 (0) | 0 (0) | 0 (0) | |
| Indoor fire | 4 (4) | 2 (3) | 2 (4) | 1.000 | 0 (0) | 0 (0) | 0 (0) | |
| Outdoor fire | 49 (44) | 12 (21) | 37 (70) | 0.001 | 0 (0) | 0 (0) | 0 (0) | |
| Wood + fire | 31 (28) | 4 (7) | 27 (51) | 0.001 | 0 (0) | 0 (0) | 0 (0) | |
Bold text indicates statistical significance. AD atopic dermatitis, CI confidence interval, IQR interquartile range; a Large family is arbitrarily defined as 7 or more members living within one household
Risk factors associated with S. aureus colonisation across the locations
The effect of various risk factors on colonisation with S. aureus in toddlers from both locations using logistic regression are shown in Tables 2 and 3, for rural and urban toddlers, respectively. The univariate analysis models showed that having AD was associated with colonisation in both rural (OR 7.54, 95% CI 22.92–19.47) and urban (OR 4.2, 95% CI 1.57–11.2) toddlers. Also, living in an electrified house that utilises gas (OR 4.08, 95% CI 1.59–10.44) and kerosene and paraffin (OR 2.88, 95% CI 1.22–6.77) for heating and cooking were associated with an increased risk of S. aureus among the rural toddlers. Surprisingly, exposure to farm animals (OR 0.3, 95% CI 0.11–0.83) as well as living in a house that uses wood and coal (OR 0.14, 95% CI 0.04–0.49) and outdoor fire (OR 0.31, 95% CI 0.13–0.73) were associated with lower odds of colonisation. In the multivariate model of rural toddlers, having AD (aOR 8.02, 95% CI 1.28–50.37) was retained as a risk factor for S. aureus colonisation, while living in a house that uses wood and coal for cooking and heating (aOR 0.02, 95% CI 0.02–0.99) remained protective against S. aureus colonisation. No regression analysis was performed for urban toddlers because only AD showed an association with S. aureus. In summary, the findings highlight the importance of the immediate environment, or exposome, in S. aureus colonisation.
Table 2.
Unconditional logistic regression analysis of child, parental, domestic and environmental characteristics associated with S. aureus colonisation in Umtata participants
| Explanatory variable | Colonised a, n (%) | Not colonised, n (%) | OR [95% CI] | p-value | aOR [95% CI] | p-value |
|---|---|---|---|---|---|---|
| AD: case | 31 (28) | 27 (24) | 7.54 [2.92–19.47] | 0.000 | 8.02 [1.28–50.37] | 0.026 |
| Sex: male | 21 (19) | 46 (42) | 0.74 [0.33–1.67] | 0.469 | 0.83 [0.32–2.16] | 0.696 |
| Child characteristics | ||||||
| Breastfeeding | 10 (9) | 25 (23) | 0.69 [0.29–1.63] | 0.395 | 1.46 [0.48–4.47] | 0.503 |
| Allergic rhinitis | 1 (1) | 6 (7) | 0.43 [0.05–3.79] | 0.449 | Excluded | |
| Asthma § | 0 (0) | 0 (0) | Omitted d | Excluded | ||
| Food allergy | 5 (5) | 6 (5) | 1.69 [0.48–5.95] | 0.413 | Excluded | |
| Mode of delivery: vaginal | 29 (26) | 57 (51) | 0.9 [0.36–2.29] | 0.833 | Excluded | |
| Incomplete immunisation status | 2 (2) | 2 (2) | 1.97 [0.27–14.58] | 0.506 | Excluded | |
| Antibiotic exposure | 33 (30) | 58 (52) | 1.71 [0.57–5.12] | 0.34 | 1.54 [0.39–6] | 0.536 |
| Large family size b | 15 (14) | 35 (32) | 0.71 [0.32–1.57] | 0.395 | 0.94 [0.36–2.44] | 0.903 |
| Animal exposure c | 27 (24) | 65 (59) | 0.3 [0.11–0.83] | 0.021 | 0.53 [0.11–2.54] | 0.429 |
| Fossil fuel exposure | ||||||
| Electricity + gas | 31 (28) | 38 (34) | 4.08 [1.59–10.44] | 0.003 | 0.35 [0.05–2.47] | 0.295 |
| Kerosene + paraffin | 28 (25) | 36 (32) | 2.88 [1.22–6.77] | 0.015 | 0.69 [0.19–2.49] | 0.571 |
| Indoor fire | 1 (1) | 3 (3) | 0.63 [0.06–6.27] | 0.694 | Excluded | |
| Outdoor fire | 10 (9) | 39 (35) | 0.31 [0.13–0.73] | 0.008 | 0.54 [0.17–1.67] | 0.283 |
| Wood + coal | 3 (3) | 28 (25) | 0.14 [0.04–0.49] | 0.002 | 0.14 [0.02–0.99] | 0.048 |
| Maternal factors | ||||||
| Allergic rhinitis | 0 (0) | 4 (4) | Omitted d | Excluded | ||
| Asthma | 1 (1) | 1 (1) | 1.95 [0.12–32] | 0.641 | Excluded | |
| Atopic dermatitis | 1 (1) | 1 (1) | 1.95 [0.12–32] | 0.641 | Excluded | |
| Food allergy | 1 (1) | 2 (2) | 0.96 [0.08–10.93] | 0.973 | Excluded | |
| Smoking | 0 (0) | 1 (1) | Omitted | Excluded | ||
| Pregnant smoker | 0 (0) | 1 (1) | Omitted | Excluded | ||
| Animal exposure c | 31 (28) | 65 (59) | 0.55 [0.18–1.64] | 0.28 | 1.93 [0.37–10.16] | 0.438 |
| Paternal factors | ||||||
| Allergic rhinitis § | 0 (0) | 3 (3) | Omitted | Excluded | ||
| Asthma § | 1 (1) | 2 (2) | 0.96 [0.08–10.93] | 0.973 | Excluded | |
| Atopic dermatitis § | 0 (0) | 1 (1) | Omitted | Excluded | ||
| Food allergy § | 0 (0) | 1 (1) | Omitted | Excluded | ||
| Smoking | 5 (5) | 10 (9) | 0.94 [0.3–2.98] | 0.267 | Excluded | |
AD atopic dermatitis, OR odds ratio, aOR adjusted odds ratio, CI confidence interval; § No within group variance; a Colonisation with Staphylococcus aureus; b Large family size is arbitrarily defined as 7 or more members within a household; c Animal exposure refers to farm animals; d Independent variables omitted due to dependency in the regression model
Table 3.
Unconditional logistic regression analysis of child, parental, domestic and environmental characteristics associated with S. aureus colonisation in Cape Town participants
| Explanatory variable | Colonised a, n (%) | Not colonised, n (%) | OR [95% CI] | p-value |
|---|---|---|---|---|
| AD: case | 30 (41) | 13 (18) | 4.2 [1.57–11.2] | 0.004 |
| Sex: male | 19 (26) | 19 (25) | 0.74 [0.33–1.67] | 0.469 |
| Child characteristics | ||||
| Breastfeeding | 6 (8) | 3 (4) | 1.71 [0.39–7.45] | 0.472 |
| Atopic dermatitis | ||||
| Allergic rhinitis | 1 (1) | 0 (0) | Omitted d | |
| Asthma § | 1 (1) | 0 (0) | Omitted d | |
| Food allergy | 6 (8) | 3 (4) | 1.71 [0.39–7.45] | 0.472 |
| Mode of delivery: vaginal | 22 (31) | 17 (24) | 0.95 [0.37–2.43] | 0.921 |
| Incomplete immunisation status | 8 (11) | 2 (3) | 3.76 [0.74–19.09] | 0.11 |
| Antibiotic exposure | 31 (42) | 23 (31) | 1.35 [0.48–3.77] | 0.57 |
| Large family size b | 9 (12) | 8 (11) | 0.88 [0.3–2.61] | 0.816 |
| Animal exposure c | 1 (1) | 1 (1) | 0.86 [0.05–14.3] | 0.915 |
| Fossil fuel exposure | ||||
| Electricity + gas | 36 (54) | 30 (45) | Omitted d | |
| Kerosene + paraffin | 20 (30) | 23 (34) | 0.43 [0.15–1.23] | 0.116 |
| Indoor fire | 0 (0) | 0 (0) | Omitted d | |
| Outdoor fire | 0 (0) | 0 (0) | Omitted d | |
| Wood + coal | 0 (0) | 0 (0) | Omitted d | |
| Maternal factors | ||||
| Allergic rhinitis | 0 (0) | 5 (7) | Omitted d | |
| Asthma | 3 (4) | 3 (4) | 0.76 [0.14–4.06] | 0.751 |
| Atopic dermatitis | 1 (1) | 2 (3) | 0.38 [0.03–4.33] | 0.432 |
| Food allergy | 1/73 | 0 (0) | Omitted d | |
| Smoking | 3 (4) | 1 (1) | 2.65 [0.26–26.82] | 0.41 |
| Pregnant smoker | 2 (3) | 1 (1) | 1.76 [0.15–20.45] | 0.65 |
| Animal exposure c | 3 (5) | 1 (2) | 2.64 [0.26–26.76] | 0.412 |
| Paternal factors | ||||
| Allergic rhinitis § | 5 (7) | 2 (3) | 2.08 [0.38–11.52] | 0.4 |
| Asthma § | 0 (0) | 0 (0) | Omitted d | |
| Atopic dermatitis § | 2 (3) | 0 (0) | Omitted d | |
| Food allergy § | 1 (1) | 0 (0) | Omitted d | |
| Smoking | 13 (20) | 7 (11) | 1.86 [0.62–5.54] | 0.267 |
AD atopic dermatitis, OR odds ratio, CI confidence interval; § No within group variance; a Colonisation with Staphylococcus aureus. b Large family size is arbitrarily defined by more than 6 members within a household; c Animal exposure refers to exposure to farm animals; d Independent variables omitted due to dependency in the regression model
Clonal lineages of recovered S. aureus isolates
A total of 125 skin and nasal S. aureus isolates were recovered from cases and controls, however, only 108 isolates were characterised by spa typing (Fig. 1). Seventeen isolates were excluded from molecular analysis due to their failure to amplify the spa gene using the described primers or poor sequence quality for spa type assignment despite repeated sequencing. BURP analysis grouped 19 spa types into 6 spa-clonal complexes (spa-CCs) and 15 spa types were singletons. Among toddlers with spa typed isolates, 25% (19/76) were colonised with one spa type, while 7% (5/76) were colonised with different spa types on at least two of the sampled sites which were positive for S. aureus. One rural case toddler was colonised with spa type t062 on lesional skin and anterior nares, and with spa type t1399 on non-lesional skin which belongs to the same spa-CC. The most frequent spa types were spa-CC002/t002 (spa-CC/spa type; 8%), spa cluster 4/t272 (9%), spa cluster 6/t174 (14%) and spa cluster 5/t1476 (18%). Furthermore, we identified four new (t15783, t18354, t18750 and t19774) and one unassigned spa types (i.e., txAC).
Distribution of S. aureus spa clonal lineages across locations by AD disease and severity
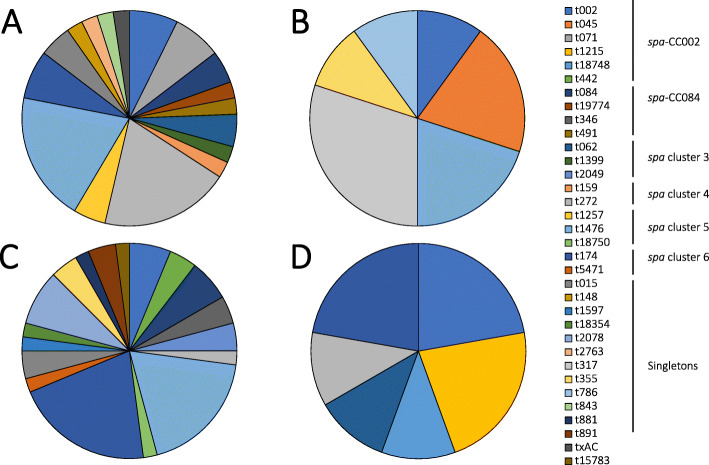
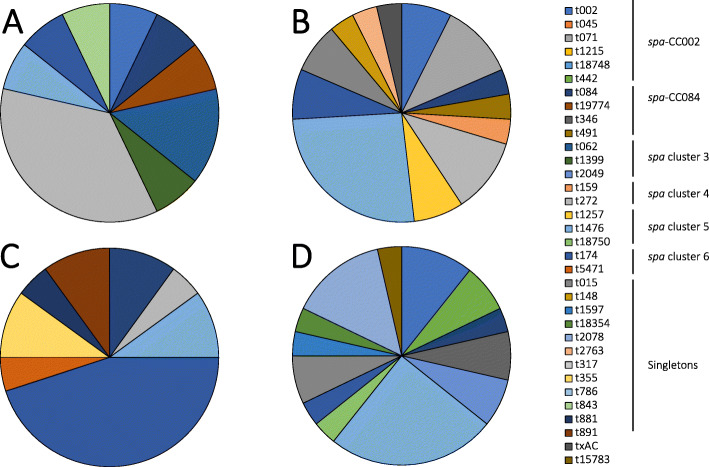
The rural and urban toddlers were colonised by different S. aureus spa clonal lineages. The spa cluster 4 was frequently identified among rural toddlers (18% [9/51] vs. 4% [2/57], p = 0.015) and spa cluster 6 in urban toddlers (23% [13/57] vs. 6% [3/51], p = 0.013) compared to their respective counterparts based on all sampled sites (Table 4). The diversity of spa types among cases was higher compared to controls in both locations (Fig. 2). Moreover, comparative analysis revealed that there was an overall significant difference in the distribution of spa clonal lineages between urban cases and controls (p = 0.009), with spa cluster 5/t1476 and spa cluster 6/t174 identified as predominant among cases. There was no overall difference between rural cases and controls (p = 0.224), albeit, spa cluster 4/t272 and spa cluster 5/t1476 were the dominant spa clonal lineages among cases with no single most dominant spa clonal lineage among controls (Fig. 2). We also noted a significant difference in the distribution of spa clonal lineages among urban cases based on AD severity (p = 0.001). In these cases, spa-CC002 (t002 and t442) isolates were only identified in severe AD, spa cluster 6/t174 was more frequent in moderate AD, and spa cluster 5/t1476 in severe AD. Although no significant difference was observed between AD severity and the identified spa types in rural cases (p = 0.126), spa cluster 3 (t062 and t1399) isolates were only detected in moderate cases while spa cluster 5 (t1476 and t1257) isolates predominated in severe cases (Fig. 3).
Table 4.
Distribution of clonal lineages of S. aureus isolates among Umtata and Cape Town participants
| spa-CC | Umtata | Cape Town | ||||
|---|---|---|---|---|---|---|
| No. of isolates (%) | No. of spa types (%) | spa types (no. of isolates) | No. of isolates (%) | No. of spa types (%) | spa types (no. of isolates) | |
| spa-CC002 | 9 | 3 (14) | t002 (4); t045 (2); t071 (3) | 10 | 4 (19) | t002 (5); t1215 (2); t18748 (1); t442 (2) |
| spa-CC084 | 3 | 2 (10) | t084 (2); t491 (1); t19774 (1) | 5 | 2 (10) | t084 (3); t346 (2) |
| spa cluster 3 | 3 | 2 (10) | t062 (2); t1399 (1) | 3 | 2 (10) | t062 (1); t2049 (2) |
| spa cluster 4 | 9 | 2 (10) | t159 (1); t272 (8) | 2 | 1 (5) | t272 (2) |
| spa cluster 5 | 12 | 2 (10) | t1476 (10); t1257 (2) | 10 | 2 (10) | t1476 (9); t18750 (1) |
| spa cluster 6 | 3 | 1 (5) | t174 (3) | 13 | 2 (10) | t174 (12); t5471 (1) |
| Singletons | 10 | 7 (33) | t015 (2); t148 (1); t2763 (1); t317 (3); t355 (1); t786 (1); t843 (1) | 13 | 7 (33) | t015 (2); t18354 (1); t1597 (1); t2078 (4); t335 (2); t881 (1); t891 (2) |
| Unaligned/spa types with unknown repeat succession | 2 | 2 (10) | txAC (1) | 1 | 1 (5) | t15783 (1) |
| Total | 51 | 21 | 57 | 21 | ||
Bold text indicates spa types that were identified in only one location
Fig. 2.
Distribution of spa types by disease phenotype stratified by location. a rural case, (b) rural control, (c) urban case, and (d) urban control. Percentages were calculated by the number of isolates for a spa type divided by the total number of spa types in each group
Fig. 3.
Distribution of spa types by disease severity. a rural moderate, (b) rural severe, (c) urban moderate, and (d) urban severe. Percentages were calculated by the number of isolates for a spa type divided by the total number of spa types in each group
Discussion
We conducted a cross-sectional, case-control study to determine the molecular epidemiology of S. aureus colonising the skin and nasal cavity of AD-affected and healthy South African AmaXhosa toddlers. We observed a higher prevalence of colonisation in cases compared to controls, regardless of geographic location. The distribution of S. aureus spa clonal lineages differed between rural-urban settings and differentially associated with AD disease and severity. Moreover, determinants of S. aureus colonisation varied across the rural-urban settings.
The pathogenesis of AD is characterised by epidermal barrier defects and activation of inflammatory responses leading to impaired clearance of skin pathogens and a decrease in skin microbial diversity [10]. S. aureus dominance is consistently linked with acute AD flares and severe forms of the disease [29, 30]. We noted a higher prevalence of S. aureus colonisation among cases compared to controls which was independent of geographic location (55% vs. 13 and 70% vs. 35% in rural and urban locations, respectively). These findings are consistent with a similar study in Italy that reported a prevalence of 57% vs. 20% in cases compared to controls [31]. Therefore, these findings support the relationship between S. aureus predominance and AD, regardless of population and location [9, 31].
AD-lesional skin has been shown to be more susceptible to S. aureus colonisation compared to AD-uninvolved, non-lesional skin, with a reported prevalence of colonisation of 23–70% vs. 6–39% [9, 32, 33]. Similarly, we noted a higher frequency of colonisation on lesional skin compared to non-lesional skin among urban and rural cases. Furthermore, similar colonisation rates on lesional skin and anterior nares have been reported in AD, with S. aureus nasal colonisation suggested as the main source of the increased skin colonisation in AD [9, 34]. However, we observed that lesional skin was more frequently colonised compared to the anterior nares among rural cases, suggesting a non-nasal source of S. aureus for the increased colonisation on lesional skin in rural AD or transient nasal colonisation [31, 35].
Skin barrier dysfunction in AD lesions, particularly in severe AD, has been correlated with increased S. aureus colonisation [36, 37]. In agreement with recent studies [9, 33, 37], we noted a higher prevalence of colonisation based on all sampled sites in cases with severe AD, however, this was limited to urban cases and not rural cases. Geographical location has been postulated to influence microbial colonisation and may explain the varied susceptibility of geographical populations to skin pathologies [38]. In this regard, the study communities each represent a geographic population that is uniquely affected by S. aureus colonisation in the pathophysiology of AD. Moreover, the rural and urban populations, regardless of disease, are generally different populations with distinct sensitisation patterns to environmental exposures [16] and inflammatory immune responses [39]. These may in turn affect microbial colonisation and the contribution thereof to disease pathogenesis and pathophysiology.
Risk factors for bacterial colonisation on the skin and nasal cavity differ with rural-urban living [40, 41]. The association between S. aureus colonisation and AD is well studied, with some studies reporting colonisation preceding the onset of clinically appreciable AD in toddlers and further associated with disease severity [11]. Consistent with previous reports [30], having AD in both communities was associated with S. aureus colonisation. Exposure to air pollutants has also been associated with increased skin barrier damage [42] which increases the propensity to S. aureus colonisation [43]. In rural toddlers, we observed that living in a house that uses kerosene and paraffin which release fine air particulates [44] was associated with increased S. aureus colonisation. However, exposure to the burning of wood/coal or outdoor fire, which also release fine air pollutants that may induce cutaneous irritation was associated with reduced S. aureus colonisation in rural toddlers. The effect of environmental air pollutants in children is a function of exposure time [45]. Although the toddlers are living in homes that use wood/coal or an outdoor fire for cooking and heating, they might have limited exposure to the produced particulates which restrict the possible effect on skin irritation and susceptibility to S. aureus. Electricity and biogas are relatively “clean fuels” with minimal air pollution emission at the household level [46]. In contrast, we found that rural living in an electrified house that also utilises gas increased the risk of S. aureus colonisation. Animals are reservoirs for human S. aureus colonisation [47], however, we found that rural toddlers living in a house with farm animals were associated with a reduced risk of S. aureus colonisation. Similarly, this finding could be due to the absence of direct interaction between toddlers and animals hence there are no animal-to-human S. aureus transmission events. Nonetheless, AD remained a risk factor while living in a house that uses wood and coal was protective against S. aureus colonisation in rural toddlers in the multivariate regression model. These findings highlight the importance of the immediate environment in shaping bacterial colonisation dynamics and the potential implication thereof in AD pathogenesis.
In addition to microbial colonisation, geographic location also determined the genotype of the colonising bacteria [48]. We noted heterogeneity in the distribution of the colonising spa clonal lineages based on geographic location, with rural toddlers mostly colonised by spa types belonging to the spa cluster 4 (previously associated with MLST CC121, Table S1) [49] while urban toddlers were predominantly colonised with spa cluster 6 (CC1) isolates [50]. This is similar to studies that suggest that location may play a role in the colonisation dynamics of childhood skin and nares [1, 15, 51, 52]. Furthermore, urban cases and controls exhibited distinct S. aureus spa clonal lineages, however, there was no difference in the distribution of S. aureus lineages between rural cases and controls. These findings suggest that the rural-urban locations provide a specific niche for the selection of certain S. aureus clonal lineages which sequentially influence the population structure in these settings, and associated colonisation dynamics. Future studies are essential to investigate site-specific features in this cohort that contribute to the observed S. aureus population structures and their association with disease phenotype.
The relationship between disease severity and the clonal lineages of the colonising S. aureus isolates is unclear with some studies reporting an association between specific clonal lineages and AD severity [15, 51] and others demonstrating none [34, 53]. In spite of this, we noted different distributions of S. aureus clonal lineages depending on AD severity among urban cases. Here, spa clonal lineages spa cluster 5 (CC5) [54] and spa cluster 6 (CC1) [50] were the most common in severe and moderate AD, respectively. The spa-CC002 (CC5) [15] isolates were only detected in severe AD cases. These findings are in agreement with a study in Spanish children which reported a predominance of CC5 isolates in severe AD [51] and another on the predominance of CC1 isolates in moderate AD [34], but in contrast to a report of the predominance of CC5 in moderate disease among Canadian children with AD [15]. There was a difference in the distribution of spa clonal lineages among rural cases based on disease severity. Albeit, spa cluster 3 (CC5) [49, 55] isolates were only identified in rural cases with moderate AD and spa cluster 6 (CC1) [50] isolates were frequent in rural cases with severe AD. The predominance of spa cluster 3 (CC5) isolates is similar to that noted in moderate AD elsewhere [15] while that of spa cluster 6 (CC1) isolates in severe AD is in contrast to previous reports of the high prevalence of CC1 isolates in children with moderate AD [34]. The contrasting predominance of S. aureus clonal lineages based on disease severity across the rural-urban communities emphasises the importance of the environment in the contribution of bacterial clonality in disease. Therefore, more investigations are needed to determine if certain S. aureus clonal lineages are associated with differential AD disease severity and the concomitant contribution to AD and disease severity.
Our data are subject to a few limitations. BURP analyses are limited to spa types with a cut-off ≤ 5 repeats, which excludes spa types with the number of repeats below the set parameter [28]. Therefore, spa type t15783 was excluded from BURP clustering analyses. Secondly, we predicted the corresponding MLST sequence types (STs) and CCs of the S. aureus spa types identified in this study by extrapolating data from previous studies (Additional file 3: Table S3). Furthermore, 14% (17/125) of the isolates were untypeable which highlights the need for whole-genome sequencing (WGS) to provide both spa and MLST data for detailed characterisation [1].
Conclusion
Our study shows that toddlers with AD are more frequently colonised with S. aureus compared to non-AD controls. The genetic background of colonising S. aureus is a unique signature of AD and disease severity, however, this is largely dependent on rural-urban living. These findings highlight the importance of geographic location on the colonisation epidemiology and population structure of S. aureus as well as the associated colonisation determinants in childhood health and AD disease in South Africa. Future studies are planned to examine the mechanisms within the rural-urban environments that contribute to S. aureus colonisation dynamics and the association thereof with AD and disease severity. This information will provide insights into population-specific therapeutic strategies that may be harnessed in the restoration of microbial diversity in AD-affected toddlers.
Supplementary Information
Additional file 1: Table S1. Participant colonisation among all, rural and urban cases and controls. This table is showing the distribution of S. aureus colonisation in AD and non-AD toddlers in the rural and urban locations.
Additional file 2: Table S2. Colonisation in cases stratified by disease severity among all, rural and urban cases. This table is showing the distribution of S. aureus colonisation based on disease severity in AD toddlers in the rural and urban locations.
Additional file 3: Table S3. Extrapolated MLST sequence types and clonal complexes for spa types identified in the present study. This table is correlating the spa types identified in this study to MLST clonal complexes and sequence types reported in previous studies.
Acknowledgements
We would like to acknowledge the SOSALL study participants and families and research team. We would also like to thank Division of Medical Microbiology staff, particularly Charmaine Barthus and members of Dube Lab for their help and technical assistance. We thank Anne von Gottberg, Linda de Gouveia and the staff of the Centre for Respiratory Diseases and Meningitis (CRDM), National Institute for Communicable Diseases (NICD) of the National Health Laboratory Service for training, sharing of standard operating procedures, supplying control isolates. The abstract of this study was published as part of the International Congress on Infectious Diseases 2020 conference and is available online at 10.1016/j.ijid.2020.09.1083.
Supporting information
Supplementary results on S. aureus colonisation based on AD and health, S. aureus colonisation based on disease severity, study spa types and MLST sequence types are available as additional files on the Journal’s website.
Abbreviations
- AD
Atopic dermatitis
- BURP
Based Upon Repeat Pattern
- CC
Clonal complex
- CI
Confidence interval
- MLST
Multi-locus sequence typing
- nuc
Thermonuclease
- OR
Odds ratio
- aOR
Adjusted odds ratio
- STGG
Skim milk-tryptone-glucose-glycerol
- SD
Standard deviation
- SAFFA
South African Food Allergy study
- SCORAD
Scoring of atopic dermatitis
- Spa
Staphylococcus protein A
- Spa-CC
Spa clonal cluster
- ST
Sequence type
Authors’ contributions
FSD, MEL, CH, NL, MPN, SMA, REA, CLD and AOS conceptualised and supervised this study. FSD, NL, CH and MEL obtained funding. NL and AM collected all clinical specimens. GONN, FSD, SMA and REA performed the experiments, data collection and analysis with support from DJ and AOS. GONN and FSD prepared the first draft manuscript. All authors contributed to manuscript review. The author(s) read and approved the final manuscript.
Funding
We acknowledge the Medical Research Council of South Africa, Nestlé Foundation, Mylan, Thermo Fisher Scientific funding of the parent study, and the Allergy Society of South Africa (ALLSA) for funding of this study. GONN acknowledges the National Research Foundation and the University of Cape Town Vice Chancellor’s Research Scholarship for their financial assistance towards her MSc degree. REA is currently an Organisation of Women in Science for the Developing World (OWSD) and L’OREAL-UNESCO Women in Science PhD Fellow. She also acknowledges the financial support of the Swedish International Development Cooperation Agency (Sida) and Margaret McNamara Education Grants. SMA holds the Claude Leon Postdoctoral Research Fellowship. AOS is currently an awardee of the Georg Forster Research Fellowship (for Experienced Researchers) of the Alexander von Humboldt Foundation. FSD is supported by the National Research Foundation of South Africa (112160), Future Leaders – African Independent Research (FLAIR) Fellowship, NIHR-MPRU, the University of Cape Town and the Allergy Society of South Africa (ALLSA).
Availability of data and materials
The datasets used and analysed during the current study are available from the corresponding author on reasonable request and ethical approval.
Declarations
Ethics approval and consent to participate
The study was approved by the Human Research and Ethics Committee of the Faculty of Health Science, University of Cape Town (HREC/REF: 451/2014) and the Western Cape Provincial Child Health Research Committee. No additional data was collected other than that approved in the parent study. Written informed consent and assent were given by guardians and participants, respectively. All data obtained and generated during the study were kept confidential. This research was conducted in accordance with the Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
Adebayo Shittu and Mark Nicol are members of the editorial board for the BMC Infectious Diseases journal.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Harkins CP, Pettigrew KA, Oravcova K, et al. The microevolution and epidemiology of Staphylococcus aureus colonization during atopic eczema disease flare. J Invest Dermatol. 2018;138(2):336–343. doi: 10.1016/j.jid.2017.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gur Cetinkaya P, Sahiner UM. Childhood atopic dermatitis: current developments, treatment approaches, and future expectations. Turk J Med Sci. 2019;49(4):963–984. doi: 10.3906/sag-1810-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nutten S. Atopic dermatitis: global epidemiology and risk factors. Ann Nutr Metab. 2015;66(Suppl 1):8–16. doi: 10.1159/000370220. [DOI] [PubMed] [Google Scholar]
- 4.Williams H, Stewart A, von Mutius E, Cookson W, Anderson HR. Is eczema really on the increase worldwide? J Allergy Clin Immunol. 2008;121(4):947–954. doi: 10.1016/j.jaci.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 5.Civelek E, Sahiner UM, Yuksel H, et al. Prevalence, burden, and risk factors of atopic eczema in schoolchildren aged 10-11 years: a national multicenter study. J Investig Allergol Clin Immunol. 2011;21(4):270–277. [PubMed] [Google Scholar]
- 6.Levin ME, Botha M, Basera W, Facey-Thomas HE, Gaunt B, Gray CL, Kiragu W, Ramjith J, Watkins A, Genuneit J. Environmental factors associated with allergy in urban and rural children from the south African food allergy (SAFFA) cohort. J Allergy Clin Immunol. 2020;145(1):415–426. doi: 10.1016/j.jaci.2019.07.048. [DOI] [PubMed] [Google Scholar]
- 7.Eyerich K, Eyerich S, Biedermann T. The multi-modal immune pathogenesis of atopic eczema. Trends Immunol. 2015;36(12):788–801. doi: 10.1016/j.it.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 8.Mernelius S, Carlsson E, Henricson J, Löfgren S, Lindgren PE, Ehricht R, Monecke S, Matussek A, Anderson CD. Staphylococcus aureus colonization related to severity of hand eczema. Eur J Clin Microbiol Infect Dis. 2016;35(8):1355–1361. doi: 10.1007/s10096-016-2672-2. [DOI] [PubMed] [Google Scholar]
- 9.Totte JE, van der Feltz WT, Hennekam M, van Belkum A, van Zuuren EJ, Pasmans SG. Prevalence and odds of Staphylococcus aureus carriage in atopic dermatitis: a systematic review and meta-analysis. Br J Dermatol. 2016;175(4):687–695. doi: 10.1111/bjd.14566. [DOI] [PubMed] [Google Scholar]
- 10.Magnifico I, Petronio Petronio G, Venditti N, et al. Atopic Dermatitis as a Multifactorial Skin Disorder. Can the Analysis of Pathophysiological Targets Represent the Winning Therapeutic Strategy? Pharmaceuticals (Basel). 2020;13(11). [DOI] [PMC free article] [PubMed]
- 11.Meylan P, Lang C, Mermoud S, Johannsen A, Norrenberg S, Hohl D, Vial Y, Prod’hom G, Greub G, Kypriotou M, Christen-Zaech S. Skin colonization by Staphylococcus aureus precedes the clinical diagnosis of atopic dermatitis in infancy. J Invest Dermatol. 2017;137(12):2497–2504. doi: 10.1016/j.jid.2017.07.834. [DOI] [PubMed] [Google Scholar]
- 12.Geoghegan JA, Irvine AD, Foster TJ. Staphylococcus aureus and atopic dermatitis: a complex and evolving relationship. Trends Microbiol. 2018;26(6):484–497. doi: 10.1016/j.tim.2017.11.008. [DOI] [PubMed] [Google Scholar]
- 13.Bjerre RD, Bandier J, Skov L, Engstrand L, Johansen JD. The role of the skin microbiome in atopic dermatitis: a systematic review. Br J Dermatol. 2017;177(5):1272–1278. doi: 10.1111/bjd.15390. [DOI] [PubMed] [Google Scholar]
- 14.Cau L, Williams MR, Butcher AM, et al. Staphylococcus epidermidis protease EcpA can be a deleterious component of the skin microbiome in atopic dermatitis. J Allergy Clin Immunol. 2020. [DOI] [PMC free article] [PubMed]
- 15.Yeung M, Balma-Mena A, Shear N, Simor A, Pope E, Walsh S, McGavin MJ. Identification of major clonal complexes and toxin producing strains among Staphylococcus aureus associated with atopic dermatitis. Microbes Infect. 2011;13(2):189–197. doi: 10.1016/j.micinf.2010.10.023. [DOI] [PubMed] [Google Scholar]
- 16.Botha M, Basera W, Facey-Thomas HE, Gaunt B, Gray CL, Ramjith J, Watkins A, Levin ME. Rural and urban food allergy prevalence from the south African food allergy (SAFFA) study. J Allergy Clin Immunol. 2019;143(2):662–668. doi: 10.1016/j.jaci.2018.07.023. [DOI] [PubMed] [Google Scholar]
- 17.Williams HC, Burney PG, Pembroke AC, Hay RJ. The U.K. working Party's diagnostic criteria for atopic dermatitis. III. Independent hospital validation. Br J Dermatol. 1994;131(3):406–416. doi: 10.1111/j.1365-2133.1994.tb08532.x. [DOI] [PubMed] [Google Scholar]
- 18.Oranje AP. Practical issues on interpretation of scoring atopic dermatitis: SCORAD index, objective SCORAD, patient-oriented SCORAD and three-item severity score. Curr Probl Dermatol. 2011;41:149–155. doi: 10.1159/000323308. [DOI] [PubMed] [Google Scholar]
- 19.Basera W, Botha M, Gray CL, Lunjani N, Watkins ASM, Venter C, Allen KJ, Hlela C, Zar HJ, Levin ME. The south African food sensitisation and food allergy population-based study of IgE-mediated food allergy: validity, safety, and acceptability. Ann Allergy Asthma Immunol. 2015;115(2):113–119. doi: 10.1016/j.anai.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 20.Dube FS, Kaba M, Whittaker E, Zar HJ, Nicol MP. Detection of Streptococcus pneumoniae from different types of nasopharyngeal swabs in children. PLoS One. 2013;8(6):e68097. doi: 10.1371/journal.pone.0068097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kateete DP, Kimani CN, Katabazi FA, Okeng A, Okee MS, Nanteza A, Joloba ML, Najjuka FC. Identification of Staphylococcus aureus: DNase and Mannitol salt agar improve the efficiency of the tube coagulase test. Ann Clin Microbiol Antimicrob. 2010;9(1):23. doi: 10.1186/1476-0711-9-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leung MH, Oriyo NM, Gillespie SH, Charalambous BM. The adaptive potential during nasopharyngeal colonisation of Streptococcus pneumoniae. Infect Genet Evol. 2011;11(8):1989–1995. doi: 10.1016/j.meegid.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 23.Brakstad OG, Aasbakk K, Maeland JA. Detection of Staphylococcus aureus by polymerase chain reaction amplification of the nuc gene. J Clin Microbiol. 1992;30(7):1654–1660. doi: 10.1128/JCM.30.7.1654-1660.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koreen L, Ramaswamy SV, Graviss EA, Naidich S, Musser JM, Kreiswirth BN. Spa typing method for discriminating among Staphylococcus aureus isolates: implications for use of a single marker to detect genetic micro- and macrovariation. J Clin Microbiol. 2004;42(2):792–799. doi: 10.1128/JCM.42.2.792-799.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stegger M, Andersen PS, Kearns A, Pichon B, Holmes MA, Edwards G, Laurent F, Teale C, Skov R, Larsen AR. Rapid detection, differentiation and typing of methicillin-resistant Staphylococcus aureus harbouring either mecA or the new mecA homologue mecA (LGA251) Clin Microbiol Infect. 2012;18(4):395–400. doi: 10.1111/j.1469-0691.2011.03715.x. [DOI] [PubMed] [Google Scholar]
- 26.Votintseva AA, Fung R, Miller RR, Knox K, Godwin H, Wyllie DH, Bowden R, Crook DW, Walker AS. Prevalence of Staphylococcus aureus protein a (spa) mutants in the community and hospitals in Oxfordshire. BMC Microbiol. 2014;14(1):63. doi: 10.1186/1471-2180-14-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shopsin B, Gomez M, Montgomery SO, Smith DH, Waddington M, Dodge DE, Bost DA, Riehman M, Naidich S, Kreiswirth BN. Evaluation of protein a gene polymorphic region DNA sequencing for typing of Staphylococcus aureus strains. J Clin Microbiol. 1999;37(11):3556–3563. doi: 10.1128/JCM.37.11.3556-3563.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mellmann A, Weniger T, Berssenbrugge C, et al. Based upon repeat pattern (BURP): an algorithm to characterize the long-term evolution of Staphylococcus aureus populations based on spa polymorphisms. BMC Microbiol. 2007;7(1):98. doi: 10.1186/1471-2180-7-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Di Domenico EG, Cavallo I, Bordignon V, et al. Inflammatory cytokines and biofilm production sustain Staphylococcus aureus outgrowth and persistence: a pivotal interplay in the pathogenesis of atopic dermatitis. Sci Rep. 2018;8(1):9573. doi: 10.1038/s41598-018-27421-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tauber M, Balica S, Hsu CY, Jean-Decoster C, Lauze C, Redoules D, Viodé C, Schmitt AM, Serre G, Simon M, Paul CF. Staphylococcus aureus density on lesional and nonlesional skin is strongly associated with disease severity in atopic dermatitis. J Allergy Clin Immunol. 2016;137(4):1272–1274. doi: 10.1016/j.jaci.2015.07.052. [DOI] [PubMed] [Google Scholar]
- 31.Pascolini C, Sinagra J, Pecetta S, et al. Molecular and immunological characterization of Staphylococcus aureus in pediatric atopic dermatitis: implications for prophylaxis and clinical management. Clin Dev Immunol. 2011;2011:718708. doi: 10.1155/2011/718708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wrobel J, Tomczak H, Jenerowicz D, Czarnecka-Operacz M. Skin and nasal vestibule colonisation by Staphylococcus aureus and its susceptibility to drugs in atopic dermatitis patients. Ann Agric Environ Med. 2018;25(2):334–337. doi: 10.26444/aaem/85589. [DOI] [PubMed] [Google Scholar]
- 33.Bilal JA, Ahmad MI, Robaee AA, Alzolibani AA, Shobaili HA, Al-Khowailed MS. Pattern of bacterial colonization of atopic dermatitis in saudi children. J Clin Diagn Res. 2013;7(9):1968–1970. doi: 10.7860/JCDR/2013/5506.3371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Clausen ML, Edslev SM, Andersen PS, Clemmensen K, Krogfelt KA, Agner T. Staphylococcus aureus colonization in atopic eczema and its association with filaggrin gene mutations. Br J Dermatol. 2017;177(5):1394–1400. doi: 10.1111/bjd.15470. [DOI] [PubMed] [Google Scholar]
- 35.Fard-Mousavi N, Mosayebi G, Amouzandeh-Nobaveh A, Japouni-Nejad A, Ghaznavi-Rad E. The dynamic of Staphylococcus aureus nasal carriage in Central Iran. Jundishapur J Microbiol. 2015;8(7):e20760. doi: 10.5812/jjm.20760v2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Park HY, Kim CR, Huh IS, Jung MY, Seo EY, Park JH, Lee DY, Yang JM. Staphylococcus aureus colonization in acute and chronic skin lesions of patients with atopic dermatitis. Ann Dermatol. 2013;25(4):410–416. doi: 10.5021/ad.2013.25.4.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kong HH, Oh J, Deming C, Conlan S, Grice EA, Beatson MA, Nomicos E, Polley EC, Komarow HD, NISC Comparative Sequence Program. Murray PR, Turner ML, Segre JA. Temporal shifts in the skin microbiome associated with disease flares and treatment in children with atopic dermatitis. Genome Res. 2012;22(5):850–859. doi: 10.1101/gr.131029.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gupta VK, Paul S, Dutta C. Geography, Ethnicity or Subsistence-Specific Variations in Human Microbiome Composition and Diversity. Front Microbiol. 2017;8:1162. doi: 10.3389/fmicb.2017.01162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cooper PJ, Amorim LD, Figueiredo CA, Esquivel R, Tupiza F, Erazo S, Oviedo Y, Vaca M, Chico ME, Barreto ML. Effects of environment on human cytokine responses during childhood in the tropics: role of urban versus rural residence. World Allergy Organ J. 2015;8(1):22. doi: 10.1186/s40413-015-0071-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sollid JU, Furberg AS, Hanssen AM, Johannessen M. Staphylococcus aureus: determinants of human carriage. Infect Genet Evol. 2014;21:531–541. doi: 10.1016/j.meegid.2013.03.020. [DOI] [PubMed] [Google Scholar]
- 41.Andersen PS, Larsen LA, Fowler VG, Jr, Stegger M, Skov RL, Christensen K. Risk factors for Staphylococcus aureus nasal colonization in Danish middle-aged and elderly twins. Eur J Clin Microbiol Infect Dis. 2013;32(10):1321–1326. doi: 10.1007/s10096-013-1882-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Valacchi G, Sticozzi C, Pecorelli A, Cervellati F, Cervellati C, Maioli E. Cutaneous responses to environmental stressors. Ann N Y Acad Sci. 2012;1271(1):75–81. doi: 10.1111/j.1749-6632.2012.06724.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Addor FA, Takaoka R, Rivitti EA, Aoki V. Atopic dermatitis: correlation between non-damaged skin barrier function and disease activity. Int J Dermatol. 2012;51(6):672–676. doi: 10.1111/j.1365-4632.2011.05176.x. [DOI] [PubMed] [Google Scholar]
- 44.Lam NL, Smith KR, Gauthier A, Bates MN. Kerosene: a review of household uses and their hazards in low- and middle-income countries. J Toxicol Environ Health B Crit Rev. 2012;15(6):396–432. doi: 10.1080/10937404.2012.710134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wangchuk T, Mazaheri M, Clifford S, Dudzinska MR, He C, Buonanno G, Morawska L. Children's personal exposure to air pollution in rural villages in Bhutan. Environ Res. 2015;140:691–698. doi: 10.1016/j.envres.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 46.Puzzolo E, Zerriffi H, Carter E, Clemens H, Stokes H, Jagger P, Rosenthal J, Petach H. Supply considerations for scaling up clean cooking fuels for household energy in low- and middle-income countries. Geohealth. 2019;3(12):370–390. doi: 10.1029/2019GH000208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Verkade E, Kluytmans J. Livestock-associated Staphylococcus aureus CC398: animal reservoirs and human infections. Infect Genet Evol. 2014;21:523–530. doi: 10.1016/j.meegid.2013.02.013. [DOI] [PubMed] [Google Scholar]
- 48.Campbell SJ, Deshmukh HS, Nelson CL, Bae IG, Stryjewski ME, Federspiel JJ, Tonthat GT, Rude TH, Barriere SL, Corey R, Fowler VG. Genotypic characteristics of Staphylococcus aureus isolates from a multinational trial of complicated skin and skin structure infections. J Clin Microbiol. 2008;46(2):678–684. doi: 10.1128/JCM.01822-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yu F, Liu Y, Lv J, Qi X, Lu C, Ding Y, Li D, Liu H, Wang L. Antimicrobial susceptibility, virulence determinant carriage and molecular characteristics of Staphylococcus aureus isolates associated with skin and soft tissue infections. Braz J Infect Dis. 2015;19(6):614–622. doi: 10.1016/j.bjid.2015.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rijnders MI, Deurenberg RH, Boumans ML, et al. Population structure of Staphylococcus aureus strains isolated from intensive care unit patients in the Netherlands over an 11-year period (1996 to 2006) J Clin Microbiol. 2009;47(12):4090–4095. doi: 10.1128/JCM.00820-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Benito D, Aspiroz C, Gilaberte Y, Sanmartín R, Hernández-Martin Á, Alonso M, Gómez P, Lozano C, Torres C. Genetic lineages and antimicrobial resistance genotypes in Staphylococcus aureus from children with atopic dermatitis: detection of clonal complexes CC1, CC97 and CC398. J Chemother. 2016;28(5):359–366. doi: 10.1179/1973947815Y.0000000044. [DOI] [PubMed] [Google Scholar]
- 52.Egyir B, Guardabassi L, Esson J, Nielsen SS, Newman MJ, Addo KK, Larsen AR. Insights into nasal carriage of Staphylococcus aureus in an urban and a rural community in Ghana. PLoS One. 2014;9(4):e96119. doi: 10.1371/journal.pone.0096119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim DW, Park JY, Park KD, et al. Are there predominant strains and toxins of Staphylococcus aureus in atopic dermatitis patients? Genotypic characterization and toxin determination of S. aureus isolated in adolescent and adult patients with atopic dermatitis. J Dermatol. 2009;36(2):75–81. doi: 10.1111/j.1346-8138.2009.00592.x. [DOI] [PubMed] [Google Scholar]
- 54.Schaumburg F, Ngoa UA, Kosters K, et al. Virulence factors and genotypes of Staphylococcus aureus from infection and carriage in Gabon. Clin Microbiol Infect. 2011;17(10):1507–1513. doi: 10.1111/j.1469-0691.2011.03534.x. [DOI] [PubMed] [Google Scholar]
- 55.Li T, Lu H, Wang X, et al. Molecular characteristics of Staphylococcus aureus causing bovine mastitis between 2014 and 2015. Front Cell Infect Microbiol. 2017;7:127. doi: 10.3389/fcimb.2017.00127. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Participant colonisation among all, rural and urban cases and controls. This table is showing the distribution of S. aureus colonisation in AD and non-AD toddlers in the rural and urban locations.
Additional file 2: Table S2. Colonisation in cases stratified by disease severity among all, rural and urban cases. This table is showing the distribution of S. aureus colonisation based on disease severity in AD toddlers in the rural and urban locations.
Additional file 3: Table S3. Extrapolated MLST sequence types and clonal complexes for spa types identified in the present study. This table is correlating the spa types identified in this study to MLST clonal complexes and sequence types reported in previous studies.
Data Availability Statement
The datasets used and analysed during the current study are available from the corresponding author on reasonable request and ethical approval.