Abstract
Background
Human papillomavirus (HPV) is thought to be the primary cause of cervical intraepithelial neoplasia and cervical cancer. We determined the age-specific prevalence of HPV infection and its risk factors in Ontario women.
Methods
We obtained 2 cervical specimens from randomly selected women (in 5-year age categories, from 15 to 49 years) who were being seen in 32 family practices for cytologic screening. The specimens were tested for carcinogenic HPV by the hybrid capture II assay (Digene Corp., Silver Spring, Md.) and by polymerase chain reaction (PCR) and genotyping.
Results
Of 1004 women eligible to participate, samples were obtained from 955 (95.1%). The prevalence of HPV (as determined by the hybrid capture II method) was highest, at 24.0% (95% confidence interval [CI] 16.5% to 31.5%), among women 20 to 24 years of age and was progressively lower in older age groups, reaching 3.4% (95% CI 0.1% to 6.7%) in women 45 to 49 years old. The prevalence of HPV (any type) as determined by PCR showed a similar pattern but was significantly higher (p = 0.01) among women 45 to 49 years old than among those 40 to 44 years old (13.0% [95% CI 6.4% to 19.6%] v. 3.3% [95% CI 0.1% to 6.5%]). Risk factors for positivity with the hybrid capture II method were never-married status, divorced or separated status, more than 3 lifetime partners, more than 1 partner in the preceding year, cigarette smoking and current use of oral contraceptives. The presence of squamous intraepithelial lesions on cytologic examination was strongly associated with positivity with the hybrid capture II assay (odds ratio 96.0, 95% CI 22.3 to 413.4; p < 0.01).
Interpretation
The highest prevalence of HPV was 24.0%, in women 20 to 24 years old. Risk factors supported a sexual mode of transmission, and there was a strong association between HPV and abnormal cervical cytologic result
There is consistent evidence of a causal association between certain types of human papillomavirus (HPV) and cervical intraepithelial neoplasia, a precursor of cervical cancer.1,2 The attributable fraction of cervical cancers due to HPV (any type) has been estimated at 82% in developed countries and 91% in developing countries.3 HPV is transmitted sexually, and natural history studies have shown that the probability of young women acquiring it is high and that they usually remain positive for a period of less than a year.4,5 What is less well understood worldwide is the burden of infection in men and women and the natural history of HPV infection. This information is important for a better understanding of which prevention strategies, such as screening programs, public health education and vaccines, are likely to be effective.
A large population-based survey of women in a rural province of Costa Rica recently determined the prevalence of cervical infection with HPV.6 The age-specific prevalences of HPV infection were highest in young women. Because HPV infection is the strongest risk factor for cervical cancer,7 surveys to determine HPV prevalences in different age groups are key to our understanding of the wide variations in the incidence of cervical cancer in populations worldwide.8 To obtain a clear picture of HPV infection as a correlate of the incidence of cervical cancer, the International Agency for Research on Cancer has fostered several surveys of HPV in women around the world.9
Consistent with this global effort to generate better epidemiologic data on HPV infection, we surveyed women in Ontario, Canada, using the most sensitive tests available. Women were randomly selected, proportionate to the regional populations, to determine the age-specific prevalences of cervical HPV infection and any associations with cytologic abnormalities and risk factors.
Methods
From May 1998 to January 1999, women 15 to 49 years of age who were seen for cervical cytologic screening by 32 participating family physicians were randomly selected for recruitment. The family practices were chosen from the 6 health planning regions of Ontario, proportionate to the regional populations. A maximum of 6 randomly selected, consenting women in each of the seven 5-year age categories from 15 to 49 years could be enrolled from each practice, in the following manner. The names of women visiting the physician for cytologic screening were entered consecutively on log sheets for the appropriate age category. These precoded forms (based on a random number table and a 2:1 sampling ratio) indicated which women should be approached for participation. Enrolment was completed after a woman had given written informed consent. Those who refused to participate were asked to complete a brief questionnaire. The protocol was approved by the Research Ethics Committee of St. Joseph's Hospital, Hamilton.
Each participant completed a self-administered questionnaire, in English or French, on demographic characteristics, reproductive history and sexual behaviour. The participant's physician then examined her by inserting a vaginal speculum and using an Ayre spatula and endocervical brush, as appropriate, to obtain a cervical smear, which was immediately fixed for cytologic examination. Two specimens, one for the hybrid capture II HPV assay (Digene Corp., Silver Spring, Md.) and the other for polymerase chain reaction (PCR), were obtained from the transformation zone with modified soft, cone-shaped cervical brushes (Cervical Sampler, Digene Corp.) and placed in transport tubes containing appropriate medium (specimen transport medium for the hybrid capture II assay and sterile phosphate-buffered saline for PCR). To test for any effect of sampling order on assay sensitivity, half of the practices obtained the PCR specimen first and the others obtained the hybrid capture II specimen first. Physicians completed a brief questionnaire about each woman with respect to history of cytologic abnormalities, colposcopy results, treatment of cervical lesions and visualization of anogenital warts. The usual pathology service providers reported the results of examination of the cervical cytologic specimens.
The 2 cervical brush specimens from each woman were stored at 4∘C and shipped within 2 weeks to the McMaster University Regional Virology and Chlamydiology Laboratory for HPV testing, one by the hybrid capture II method and the other by PCR. The tests were conducted without knowledge of the other data for each subject.
The hybrid capture II assay is a second-generation DNA probe test based on signal amplification, which uses a chemiluminescent readout to indicate the presence of one or more carcinogenic HPV types as a group (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59 and 68). The assay procedure has been described previously.10 The test was considered positive if the light emitted by a specimen was greater than the light emitted by the positive control.
For PCR, 0.2 mL of the brush specimen was digested with proteinase K, and then DNA was extracted with phenol-chloroform. Subsequently, all processed specimens were amplified using the consensus HPV L1 primers (highly conserved region o fthe viral genome), as well as human β-globin primers to assess specimen integrity. Specimens that were negative for β-globin were further processed and retested by PCR, as previously described.11 The 131 specimens that remained negative for β-globin, even after dilution to reduce the effect of any inhibitors, were excluded from the analysis of PCR results, as they apparently did not contain intact DNA. Detection and genotyping of the PCR specimens that were positive for 13 carcinogenic HPV types (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59 and 68) and 4 noncarcinogenic types (6, 11, 42 and 53) were processed according to protocols described by Bauer and colleagues.12
The data were analysed by means of the continuity-corrected χ2 method or Fisher's exact test, as required, and the probability of a type I error (α) was set at 0.05 (two-tailed). Gart's exact test13 for order effect was used to detect any association between order of specimen collection and positive test results. For risk factor analysis, the results of the hybrid capture II test were used to define the presence of carcinogenic HPV, and a reference category was designated for each variable. The effect of age was estimated using indicator variables for the different age categories using the oldest age group as the reference category. Age, geographic region and any variables that were significantly associated in univariate analyses with the presence of carcinogenic HPV (p < 0.10) were entered in a logistic regression model. Stepwise logistic regression, based on the likelihood ratio method, was used to determine the final multivariate model.
Results
Of the 1004 eligible women who were randomly selected and approached, 49 (4.9%) refused to participate. Our analysis is based on the 955 consenting women for whom questionnaires and 2 cervical specimens were obtained. The numbers of women enrolled in the 5-year age categories (15 to 49 years) and their responses to the items on the self-completed questionnaire are summarized in Table 1. The 49 women who refused to participate were not significantly different from the 955 participants with respect to age distribution (p = 0.77) or marital status (p = 0.48) (data not shown), but among those who refused there was a higher proportion of current smokers (52.2% v. 30.1%; p < 0.01) and a lower proportion who had had more than 3 lifetime sexual partners (6.1% v. 47.2%; p < 0.01).
Table 1
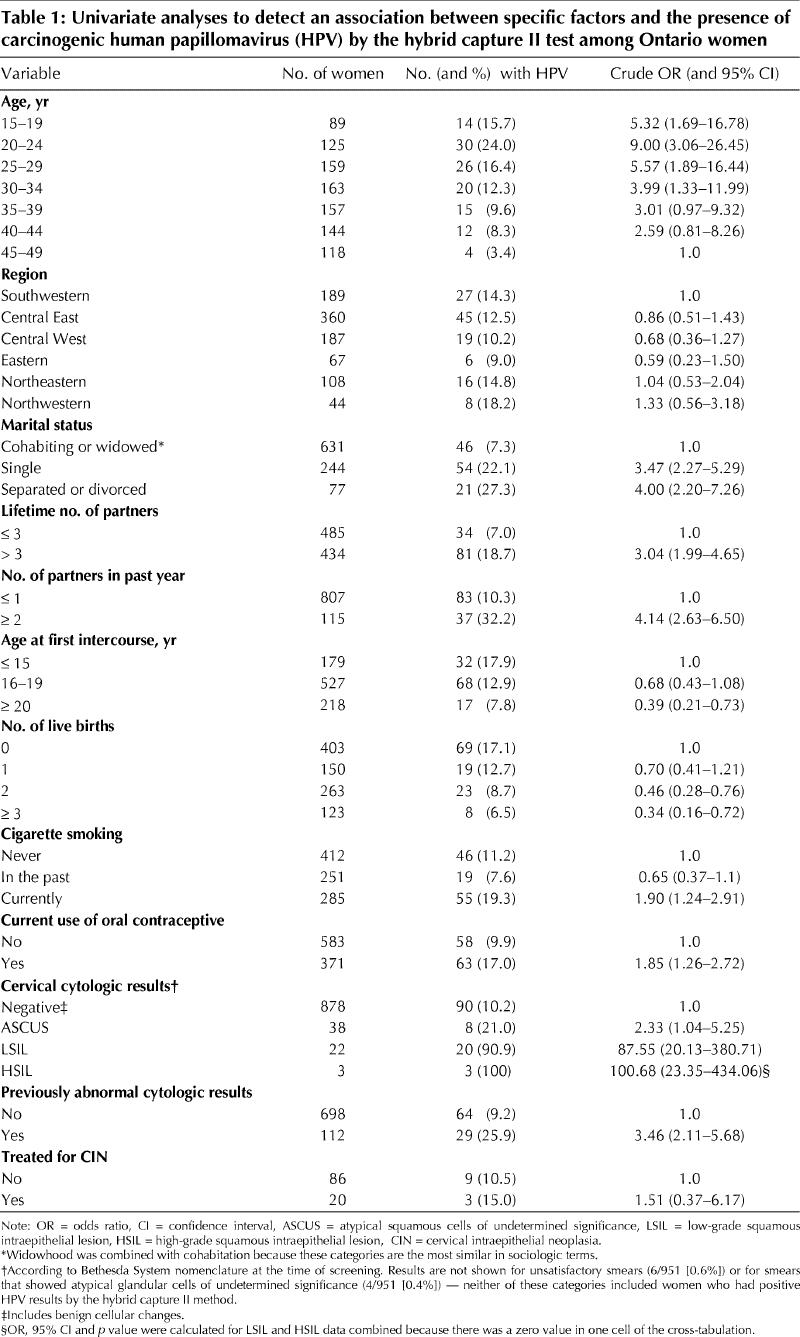
According to the results of the questionnaires completed by physicians, warts were visible in 10 (1.1%) of 909 women for whom a response was obtained. Physicians reported that 118 (13.1%) of 901 women had been referred at least once for colposcopic examination. The results of cervical cytologic examination of the specimens obtained at the visit are shown in Table 1.
The cervical specimens of 131 women were negative for β-globin and therefore not suitable for PCR (see Methods). The age-specific HPV prevalences by PCR (for any type including carcinogenic types) and by the hybrid capture II assay (for carcinogenic types only), based on the results for the 824 women with specimens positive for β-globin, are compared in Fig. 1. In the 7 age groups (Fig. 1), hybrid capture II was positive in 14 (17.5%), 25 (23.1%), 25 (17.5%), 18 (13.2%), 14 (10.3%), 10 (8.3%) and 4 (4.0%); PCR (for carcinogenic types only) was positive in 11 (13.8%), 21 (19.4%), 17 (11.9%), 11 (8.1%), 8 (5.9%), 3 (2.5%) and 8 (8.0%); and PCR (for any type) was positive in 16 (20.0%), 25 (23.1%), 21 (14.7%), 16 (11.8%), 15 (11.0%), 4 (3.3%) and 13 (13.0%) respectively.
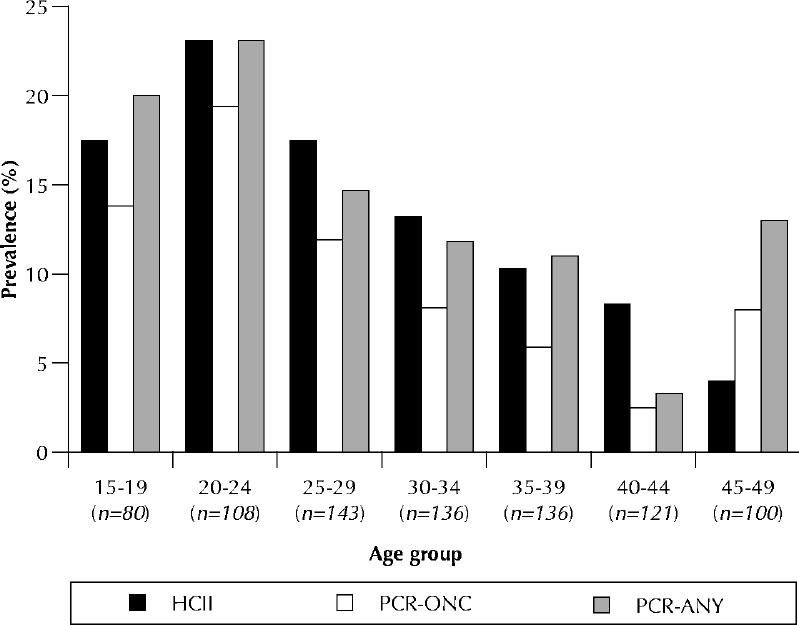
Fig. 1: Age-specific prevalence of HPV according to different tests. HCII = hybrid capture II test, used only for carcinogenic HPV types; PCR-ONC = polymerase chain reaction test for carcinogenic HPV types; PCR-ANY = polymerase chain reaction test for any HPV type. The data are based on results for 824 women who tested positive for β-globin (i.e., specimens contained intact DNA).
HPV of any type was detected in 110 (13.3%, 95% CI 11.0% to 15.6%) of the 824 women who tested positive for β-globin; 79 (9.6%, 95% CI 7.6% to 11.6%) of the 824 women tested positive for carcinogenic types of HPV by PCR and hybridization with type-specific probes. The order in which specimens were obtained was not significantly associated with the rate of positivity by PCR (for any HPV type) according to Gart's test (z score 1.33; p = 0.91). Analysis of the results of PCR (for carcinogenic types) and hybrid capture II, based on the paired specimens from the 824 women for whom PCR was positive for β-globin, showed that 66 (8.0%, 95% CI 6.3% to 10.1%) were positive by both tests, 13 (1.6%, 95% CI 0.8% to 2.7%) were negative by the hybrid capture II test and positive by PCR, 44 (5.3%, 95% CI 3.9% to 7.1%) were positive by the hybrid capture II test and negative by PCR, and 701 (85.1%, 95% CI 82.5% to 87.5%) were negative by both tests. The age-specific prevalences of carcinogenic types of HPV as determined by the hybrid capture II assay are shown for all 955 women in Table 1. Overall, 121 (12.7%, 95% CI 10.6% to 14.8%) of these women tested positive for carcinogenic types of HPV by this method.
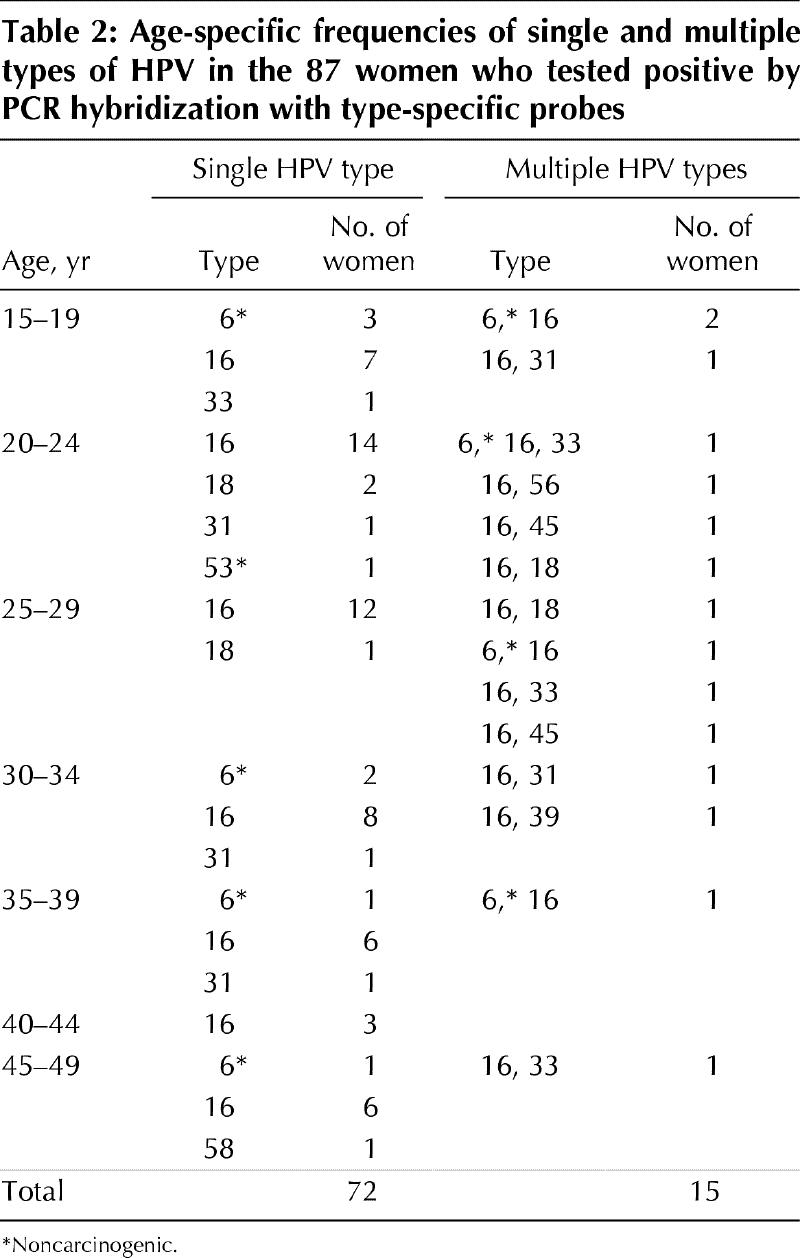
Logistic regression was used to compare the prevalences of HPV among age categories. The prevalence of HPV as determined by PCR was significantly higher for any type of HPV (p = 0.01), but not for carcinogenic types only (p = 0.07), among the oldest women (45 to 49 years of age) than among women 40 to 44 years of age. The prevalence of carcinogenic types of HPV as determined by PCR was significantly higher among women 20 to 24 years of age than among those in the oldest age category (p = 0.02). No other comparisons of data from the PCR assay were significant. The age-specific prevalence of HPV as determined by the hybrid capture II method was significantly lower among women in the oldest age category than among those in the categories less than 35 years of age (Table 1). Table 2 shows the distribution of HPV types by age.
Table 2
Univariate analyses of selected putative risk factors and the presence of carcinogenic HPV as determined by hybrid capture II are shown in Table 1. The presence of squamous intraepithelial lesions on cytologic examination was strongly associated with positivity by hybrid capture II (odds ratio 96.0, 95% CI 22.3 to 413.4; p < 0.01).
Lower educational level, inconsistent condom use, current pregnancy and previous history of a sexually transmitted infection were not significantly associated with HPV infection. No significant associations were found between the presence of HPV and a history of genital warts, genital herpes or chlamydial infection, and visible genital warts did not predict the presence of cervical HPV (data not shown).
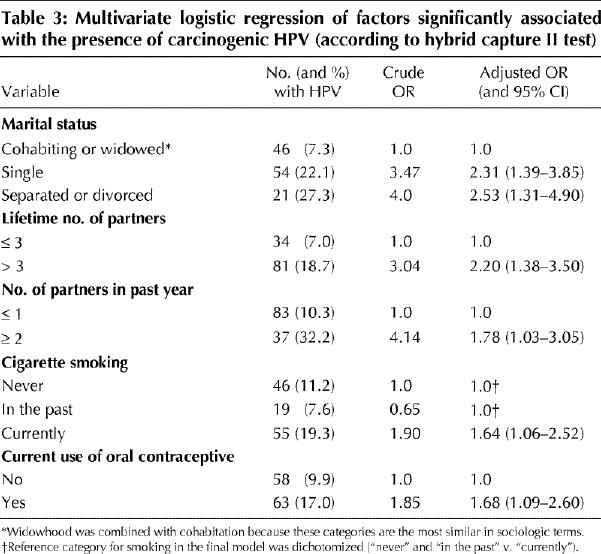
Logistic regression was performed with the following variables: age category, geographic region, marital status, lifetime number of sexual partners, number of sexual partners in the past year, age at first intercourse, parity, current smoking status and current use of oral contraceptives. The resulting model had the following factors: never-married status, divorced or separated status, more than 3 lifetime partners, more than 1 partner in the past year, current cigarette smoking and current use of oral contraceptives (Table 3). There was no significant association between the presence of HPV and geographic region, with or without adjustment for age.
Table 3
To explain the higher prevalence of any type of HPV as determined by PCR analysis in the oldest age category, we compared the distribution of risk factors between women 40 to 44 years of age and those 45 to 49 years of age (Table 1). Use of oral contraceptives by 15 (10.4%) of the 144 younger women (40 to 44 years of age) and 2 (1.7%) of the 117 older women (45 to 49 years) was the only significant association (p < 0.01), but this was in the opposite direction. There were no significant differences for marital status (p = 0.50), lifetime number of partners (p = 0.39), number of partners in the past year (p = 0.37), age at first intercourse (p = 0.97), parity (p = 0.83) or smoking status (p = 0.10).
Interpretation
In this sample of Ontario women, the burden of infection with carcinogenic types of HPV was high relative to other sexually transmitted infections, especially in young women.14 The rate of infection with carcinogenic HPV types was highest among women 20 to 24 years of age — 24.0% by the hybrid capture II method and 19.4% by PCR — and became progressively lower with age (Table 1 and Fig. 1). Although our survey was not strictly population based, it was possible to use proportionate sampling methods to randomly select women over the whole province when they accessed the universal-coverage primary health care system. Lower HPV prevalence with age has been observed in surveys of women in a rural province of Costa Rica6 and a suburb of Amsterdam15,16 and in controls selected for case-control studies of cervical cancer in Colombia, Brazil and Spain.17 The Dutch report of age-specific prevalences (of any HPV type, as determined by PCR) that changed with age, from a peak of 24% in women 20 to 24 years of age to less than 6% in the older age categories (35 to 54 years), closely resembled the findings in our survey.16
With respect to the higher HPV prevalence (any type) determined by PCR for our oldest age category, Herrero and colleagues,6 using a less sensitive version of the hybrid capture test, described a similar pattern in Costa Rican women 60 years of age or older. They questioned whether this was a cohort effect or re-emergence of latent infection. Given that PCR should detect a lower number of DNA copies than the hybrid capture II test,10 the discrepancy between the 2 tests in our study might have occurred because of the generally low levels of HPV DNA in the oldest women (45 to 49 years; data not shown). There was no obvious cohort effect that could explain the higher prevalence of HPV in our oldest subjects, so perhaps HPV infection becomes latent, and waning immune surveillance with age plays a role in its re-emergence; this pattern is well known for herpes zoster, which causes varicella primarily in children and shingles in older patients. The common occurrence of florid diseases caused by HPV (e.g., genital warts, cervical intraepithelial neoplasia) in immunosuppressed women after organ transplantation or HIV infection18 supports the hypothesis that HPV infection becomes latent in many people. From a recent natural history study in which college women were tested regularly for HPV, the cumulative 36-month incidence of infection was 43%. This result suggests that most sexually active women will become infected at some time in their lives, usually asymptomatically.5 Whether HPV infection is usually cleared or whether it becomes latent remains to be determined.
Risk factors for cervical HPV infection in control women participating in case-control studies in Brazil, Colombia and Spain were reported as higher number of lifetime sexual partners, lower level of family income and younger age at first sexual intercourse.17 In Spanish and Colombian but not Brazilian women, positive serologic results for Chlamydia trachomatis were associated with HPV positivity.17 Although we did not perform serologic tests, there was no association between history of chlamydial infection and HPV in our subjects. As others have reported,1,2 there was a strong association between abnormal cervical cytologic results and the presence of carcinogenic types of HPV. Hankins and associates18 found that HPV infection was present in two-thirds of HIV-positive Canadian women and that it was associated with a low CD4 cell count, nonwhite race, inconsistent condom use and lower age (less than 30 years).
To increase our understanding of the natural history of HPV infection it is important that HPV surveys be done in countries with various rates of cervical cancer incidence, so that we can assess risk factors, clearance rates and acquisition of different types over time. This information will increase our understanding of the natural history of HPV infection and assist in the development of effective HPV vaccines and screening programs to prevent cervical cancer.
Footnotes
This article has been peer reviewed.
We express our appreciation for the assistance provided to the project by Dr. Margaret Fearon, Laboratory Services Branch, Ministry of Health, Toronto, Ont.
Funding for this study was provided as a research grant by the Division of STD Prevention and Control, Bureau of HIV/AIDS, STD and TB, Laboratory Centre for Disease Control, Health Canada, Ottawa, and as an unrestricted research grant by the Pharmaceuticals Division, 3M Corp., London, Ont. The HC II kits were provided at no charge by Digene Corporation, Silver Spring, Md.
Reprint requests to: Dr. John W. Sellors, Department of Family Medicine, HSC-2V10, McMaster University, 1200 Main St. W, Hamilton ON L8N 3Z5; fax 905 521-5594; sellors@fhs.mcmaster.ca
References
- 1.Franco EL. Cancer causes revisited: human papillomavirus and cervical neoplasia. J Natl Cancer Inst 1995;87:779-80. [DOI] [PubMed]
- 2.Schiffman MH. New epidemiology of human papillomavirus infection and cervical neoplasia. J Natl Cancer Inst 1995;87:1345-7. [DOI] [PubMed]
- 3.Pisani P, Parkin M, Muñoz N, Ferlay J. Cancer and infection: estimates of the attributable fraction in 1990. Cancer Epidemiol Biomarkers Prev 1997;6: 387-400. [PubMed]
- 4.Koutsky LA, Holmes KK, Critchlow CW, Stevens CE, Paavonen J, Beckmann AM, et al. A cohort study of the risk of intraepithelial neoplasia grade 2 or 3 in relation to papillomavirus infection. N Engl J Med 1992;327:1272-8. [DOI] [PubMed]
- 5.Ho GYF, Bierman R, Beardsley L, Chang CJ, Burk RD. Natural history of cervicovaginal papillomavirus infection in young women. N Engl J Med 1998;338:423-8. [DOI] [PubMed]
- 6.Herrero R, Schiffman MH, Bratti C, Hildesheim A, Balmaceda I, Sherman ME, et al. Design and methods of a population-based natural history study of cervical neoplasia in a rural province of Costa Rica: the Guanacaste Project. Pan Am J Public Health 1997;1:362-75. [DOI] [PubMed]
- 7.Bosch FX, Manos M, Muñoz N, Sherman M, Jansen AM, Peto J, et al. Prevalence of human papillomavirus in cervical cancer: a worldwide perspective. International Biological Study on Cervical Cancer (IBSCC) Study Group. J Natl Cancer Inst 1995;87:796-802. [DOI] [PubMed]
- 8.Parkin DM, Pisani P, Ferlay J. Global cancer statistics. CA Cancer J Clin 1999;49:33-64. [DOI] [PubMed]
- 9.Herrero R. Prevalence surveys of HPV infection in high- and low-incidence areas for cervical cancer. In: International Agency for Research on Cancer. Biennial report 1996/1997. Lyon, France: IARC Press; 1997. pp. 68-9.
- 10.Lorincz A. Molecular methods for the detection of human papillomavirus infection. Obstet Gynecol Clin North Am 1996;23:707-30. [PubMed]
- 11.Coutlee F, Hankins C, Lapointe N, and the Canadian Women's HIV Study Group. Comparison between vaginal tampon and cervicovaginal lavage specimen collection for detection of human papillomavirus DNA with the polymerase chain reaction. J Med Virol 1997;51:42-7. [DOI] [PubMed]
- 12.Bauer HM, Greer CE, Manos MM. Determination of genital human papillomavirus infection using consensus PCR. In: Herrington CS, McGee JOD, editors. Diagnostic molecular pathology: a practical approach. Oxford: Oxford University Press; 1992. pp. 132-52.
- 13.Gart JJ. An exact test for comparing matched proportions in crossover designs. Biometrika 1969;56:75-9.
- 14.Sellors JW, Pickard L, Gafni A, Goldsmith CH, Jang D, Mahony JB, et al. Effectiveness and efficiency of selective versus universal screening for chlamydial infection in sexually active young women. Arch Intern Med 1992;152:1837-44. [PubMed]
- 15.Van den Brule AJC, Walboomers JMM, du Maine M, Kenemans P, Meijer CJL. Difference in prevalence of human papillomavirus genotypes in cytomorphologically normal cervical smears is associated with a history of cervical intraepithelial neoplasia. Int J Cancer 1991;48:404-8. [DOI] [PubMed]
- 16.Melkert PWJ, Hopman E, van den Brule AJC, Risse EKJ, van Diest PJ, Bleker OP, et al. Prevalence of HPV in cytomorphologically normal cervical smears, as determined by the polymerase chain reaction, is age-dependent. Int J Cancer 1993;53:919-23. [DOI] [PubMed]
- 17.Muñoz N, Kato I, Bosch FX, Eluf-Neto J, de Sanjose S, Ascunce N, et al. Risk factors for HPV detection in middle-aged women. Sex Transm Dis 1996;23:504-10. [DOI] [PubMed]
- 18.Hankins C, Coutlee F, Lapointe N, Simard P, Tran T, Samson J, et al. Prevalence of risk factors associated with human papillomavirus infection in women living with HIV. Canadian Women's HIV Study Group. CMAJ 1999;160:185-91. Abstract available: www.cma.ca/cmaj/vol-160/issue-2/0185.htm [PMC free article] [PubMed]


