Abstract
T cells play a critical role in promoting tumor regression in both experimental models and humans. Yet, T cells that are chronically exposed to tumor antigen during cancer progression can become dysfunctional/exhausted and fail to induce tumor destruction. Such tumor-induced T cell dysfunction may occur via multiple mechanisms. In particular, immune checkpoint inhibitory receptors that are upregulated by tumor-infiltrating lymphocytes in many cancers limit T cell survival and function. Overcoming this inhibitory receptor-mediated T cell dysfunction has been a central focus of recent developments in cancer immunotherapy. Immunotherapies targeting inhibitory receptor pathways such as programmed cell death 1 (PD-1)/programmed death ligand 1 and cytotoxic T lymphocyte-associated antigen 4 (CTLA-4), alone or in combination, confer significant clinical benefits in multiple tumor types. However, many patients with cancer do not respond to immune checkpoint blockade, and dual PD-1/CTLA-4 blockade may cause serious adverse events, which limits its indications. Targeting novel non-redundant inhibitory receptor pathways contributing to tumor-induced T cell dysfunction in the tumor microenvironment may prove efficacious and non-toxic. This review presents preclinical and clinical findings supporting the roles of two key pathways—T-cell immunoglobulin and mucin-domain containing-3 (TIM-3) and T cell immunoreceptor with Ig and ITIM domain (TIGIT)/CD226/CD96/CD112R—in cancer immunotherapy.
Keywords: Cancer immunotherapy, inhibitory receptors, TIM-3, TIGIT, DNAM-1/CD226, CD96, CD112R
1. Introduction
Inhibitory receptors (IRs) on T cells regulate innate and adaptive immunity in chronic viral infections and cancer [1–4]. T cells exposed to chronic antigen stimulation can become dysfunctional/exhausted and upregulate many IRs, including programmed cell death receptor 1 (PD-1), T cell immunoglobulin mucin-3 (TIM-3), and T cell immunoreceptor with immunoglobulin (Ig) and ITIM domain (TIGIT) [2, 5–7]. At the same time, IR ligands are expressed by tumor cells and antigen-presenting cells (APCs) in the tumor microenvironment (TME). Targeting IRs with monoclonal antibodies (mAbs) has proven beneficial in mouse tumor models and humans, and immune checkpoint blockade (ICB) with anti-cytotoxic T lymphocyte-associated antigen 4 (CTLA-4), anti-PD-1, or both mAbs are standard treatments for many solid tumors [8–11]. However, most patients with cancer do not respond to current ICB, and dual PD-1/CTLA-4 often causes serious grade adverse events that often result in treatment discontinuation [12–14]. These results prompt a need to identify additional IR pathways for therapeutic targeting. Multiple lines of evidence indicate that the TIM-3 and TIGIT/CD226/CD96/CD112R pathways limit adaptive and innate immunity against tumors [15–20]. Further, preclinical evidence in experimental mouse tumor models suggests that dual PD-1/TIM-3 and PD-1/TIGIT blockade can confer clinical benefit. Here, we review the preclinical and clinical findings supporting the promising role of TIM-3 and TIGIT/CD96/CD112R pathways in cancer immunology.
2. TIM-3
TIM-3 (CD366, HAVCR2) was originally identified as a transmembrane protein expressed by interferon gamma (IFN-γ)-producing CD4+ T helper type 1 (Th1) and CD8+ cytotoxic T cells [21]. TIM-3 is a member of the TIM gene family, which includes eight members (Tim 18) on mouse chromosome band 11B1.1, and three members (TIM-1/Havcr1, TIM-3/Havcr2, and TIM-4) on human chromosome 5q33.2 [22]. In addition to CD8+ and CD4+ T cells, TIM-3 is also expressed by regulatory T cells (Tregs) [23], Th17 cells [24, 25], dendritic cells (DCs) [16, 26], myeloid cells [26], natural killer (NK) cells [27], macrophages [28, 29], mast cells [30], and cancer cell subsets (e.g., melanoma, glioma, osteosarcoma, cervical cancer and clear cell renal cell carcinoma) [31–35].
2.1. TIM-3 ligands
Four ligands bind TIM-3 to regulate anti-tumor immunity: Galectin-9 (Gal-9) [36], carcinoembryonic antigen-related cell adhesion molecule 1 (CEACAM1) [37], phosphatidylserine (PtdSer) [38], and high-mobility group box 1 (HMGB1) [16]. Gal-9 is a type C lectin, and was the first identified TIM-3 ligand [39]. This lectin is expressed by myeloid cells, T cells, B cells, mast cells, endothelial cells, tumor cells, and stromal cells and is secreted into the extracellular space [40]. Gal-9 binds to carbohydrates on the immunoglobulin V domain of TIM-3 in mice to induce apoptosis of TIM-3+ T cells [36]. The specific binding site of Gal-9 to TIM-3 remains unknown. Gal-9 binding induces the release of HLA-B-associated transcript 3 (BAT3) from the TIM-3 tail, resulting in T cell inhibitory signals and T cell dysfunction [15].
Multiple lines of evidence support the inhibitory effects of the TIM-3/Gal-9 pathway in cancer immunology. CT26 mouse colon tumor cells secrete Gal-9, which causes apoptosis of tumor-infiltrating TIM-3+CD8+ T cells; in contrast, TIM-3/Gal-9 blockade reduces apoptosis and inhibits tumor growth in mice [41]. In patients with hepatitis B virus (HBV)-related hepatocellular carcinoma (HCC), high numbers of HCC-infiltrating Gal-9+ Kupffer cells and TIM-3+ T cells appear to correlate with poor prognosis [42]. Transgenic overexpression of either TIM-3 or Gal-9 implicates the TIM-3/Gal-9 pathway in regulating Th1-type immunity by directly triggering Th1 cell death and expanding the suppressive CD11b+Ly-6G+ cells, which exhibit a phenotype consistent with granulocytic myeloid-derived suppressor cells (MDSCs) [43]. Notably, in human acute myeloid leukemia (AML), a TIM-3/Gal-9 autocrine stimulatory loop drives self-renewal of myeloid leukemia stem cells and leukemic progression through nuclear factor kappa B (NF-κB) and β-catenin signaling. Accordingly, applying anti-human Gal-9 antibodies in mice inhibits xenogeneic reconstitution of human AML [44]. Gal-9 also exerts TIM-3–independent immunological effects. Gal-9 induces pro-inflammatory cytokines from Th1/2 cells and suppresses Th17 cell development in the absence of TIM-3 [45, 46]. In addition, Gal-9 interacts with CD44 to promote expression and stability of forkhead box protein 3 (Foxp3) in induced Treg (iTreg) cells, enhancing their suppressive function [47].
PtdSer is a phospholipid normally localized in the inner leaflet of the plasma membrane that is exposed to the outer leaflet when a cell undergoes apoptosis, stress, or cell activation. PtdSer binds TIM-3 expressed on DCs or macrophages, resulting in phagocytosis of apoptotic cells and cross-presentation [38]. The binding site for PtdSer is located between the FG’ and CC’ loops of the TIM-3 IgV domain, distinct from the Gal-9-binding site on the opposite side of the IgV domain [48]. PtdSer binds to TIM-1 and TIM-4 with higher affinity than TIM-3 [48].
HMGB1 is an alarmin that interacts with multiple ligands such as receptor for advanced glycation endproducts (RAGE), toll-like receptor 4 (TLR4), and interleukin 1 (IL-1) receptor [49]. HMGB1 acts as a universal sentinel for nucleic acids and is required for nucleic acid-induction of innate responses mediated by pattern recognition receptors (PRRs) [50]. TIM-3 expressed by tumor-associated DCs binds HMGB1, which impairs the recruitment of nucleic acids into endosomes and disrupts PRR-mediated innate immune responses [16]. In mouse tumor models, TIM-3 blockade increases the therapeutic efficacy of DNA vaccination and chemotherapy in part by augmenting HMGB1-mediated nucleic acid-sensing systems [16]. These observations suggest that TIM-3 blockade promotes the capability of tumor-derived nucleic acids together with danger signals to activate innate immune response through activation of the PRR-mediated pathway.
CEACAM1 is co-expressed with TIM-3 on activated T cells. CEACAM1 interacts with TIM3 in cis and trans to facilitate cell-surface expression of TIM-3 and TIM-3 inhibitory function [37, 51]. CEACAM1 shares similar structures of IgV domains with TIM-3 and interacts with TIM-3 along the CC’ and FG cleft [37]. CEACAM1 is also expressed by DCs [52], monocytes [53], macrophages [54], and tumor cells (e.g., melanoma cells) [31]. Both CEACAM1 and Gal-9 induce phosphorylation of Tyr256 and Tyr263 in the TIM-3 cytoplasmic tail to promote the inhibition of T-cell receptor (TCR) signaling, although CEACAM1 and Gal-9 bind to different regions of the IgV domain of TIM-3 [15, 37].
Notably, blocking anti-TIM-3 antibodies evaluated to date appear to disrupt the interaction of TIM-3 with PtdSer or CEACAM1 but not HMGB1 [55]. Further investigations of TIM-3–ligand interactions and intracellular downstream signaling are required to obtain a deeper understanding of the regulatory functions of TIM-3.
2.2. Structure and signaling of TIM-3
TIM-3 belongs to the immunoglobulin superfamily and consists of a membrane-distal N-terminal immunoglobulin variable (IgV) domain, a membrane-proximal mucin domain, a transmembrane region, and a cytoplasmic tail [22]. The mouse TIM-3 IgV domain contains two N-linked glycosylation sites, which bind to Gal-9 [36]. The crystal structure of TIM-3 shows that the IgV domain possesses two disulfide bonds that bring the CC’ loop close to the FG loop, resulting in a unique Gal-9-independent ligand-binding cleft that binds CEACAM1 and PtdSer [38, 51]. TIM-3 does not contain classical inhibitory immunoreceptor tyrosine-based inhibition motifs (ITIMs) or immunoreceptor tyrosine-based switch signaling motifs found in other IRs (e.g., PD-1 and TIGIT). The intracellular mechanisms of TIM-3 signaling are not fully understood. Mouse and human TIM-3 cytoplasmic tails contain five conserved tyrosine residues, which can be phosphorylated by src kinases [56] or inducible T cell kinase [57]. Tyr256 and Tyr263 interact with BAT3 and tyrosine kinase FYN [15, 56, 57].
In addition to its ability to induce apoptosis of Th1 cells [36] and act as a coinhibitory receptor, TIM-3 may act as a positive regulator of T cell function [26, 56, 58]. Ectopic expression of TIM-3 in Jurkat T cells leads to the enhancement of TCR-dependent signaling pathways [56]. In addition, CD8+ T cells from Tim-3−/− mice have impaired recall responses upon infection with the acute armstrong strain of lymphocytic choriomeningitis virus, and enforced expression of TIM-3 in vivo drives the generation of short-lived effector CD8+ T cells in an AKT/mammalian target of rapamycin (mTOR)-dependent manner [58]. In this experimental model, sustained positive signals via TIM-3 eventually promote the exhaustion of TIM-3+ T cells by enhancing initial T cell activation and the generation of short-lived effector cells at the expense of memory T cell generation [58]. To explain these conflicting findings, Kuchroo and colleagues suggested that BAT3 acts as a master regulator of TIM-3 signaling. BAT3, which is a repressor of TIM-3 inhibition, binds to the TIM-3 cytoplasmic tail, recruiting the catalytically active form of LCK to permit TCR stimulation. However, when TIM-3 binds to its ligands CEACAM1 [37] and Gal-9 [57], Tyr256 and Tyr263 are phosphorylated, and BAT3 is released from the TIM-3 tail, allowing TIM-3 to exert inhibitory functions [15]. In the absence of BAT3, T cells lose the ability to produce large amounts of IFN-γ and IL-2 [15]. Notably, FYN and BAT3 bind to the same region of TIM-3, and FYN can promote T cell anergy through the activation of glycosphingolipid-enriched microdomains 1 [59]. Therefore, the potential competition between FYN and BAT3 for binding to TIM-3 may contribute to the inhibition of TCR signaling [60] (Fig. 1).
Figure 1. Structure and signaling of TIM-3.
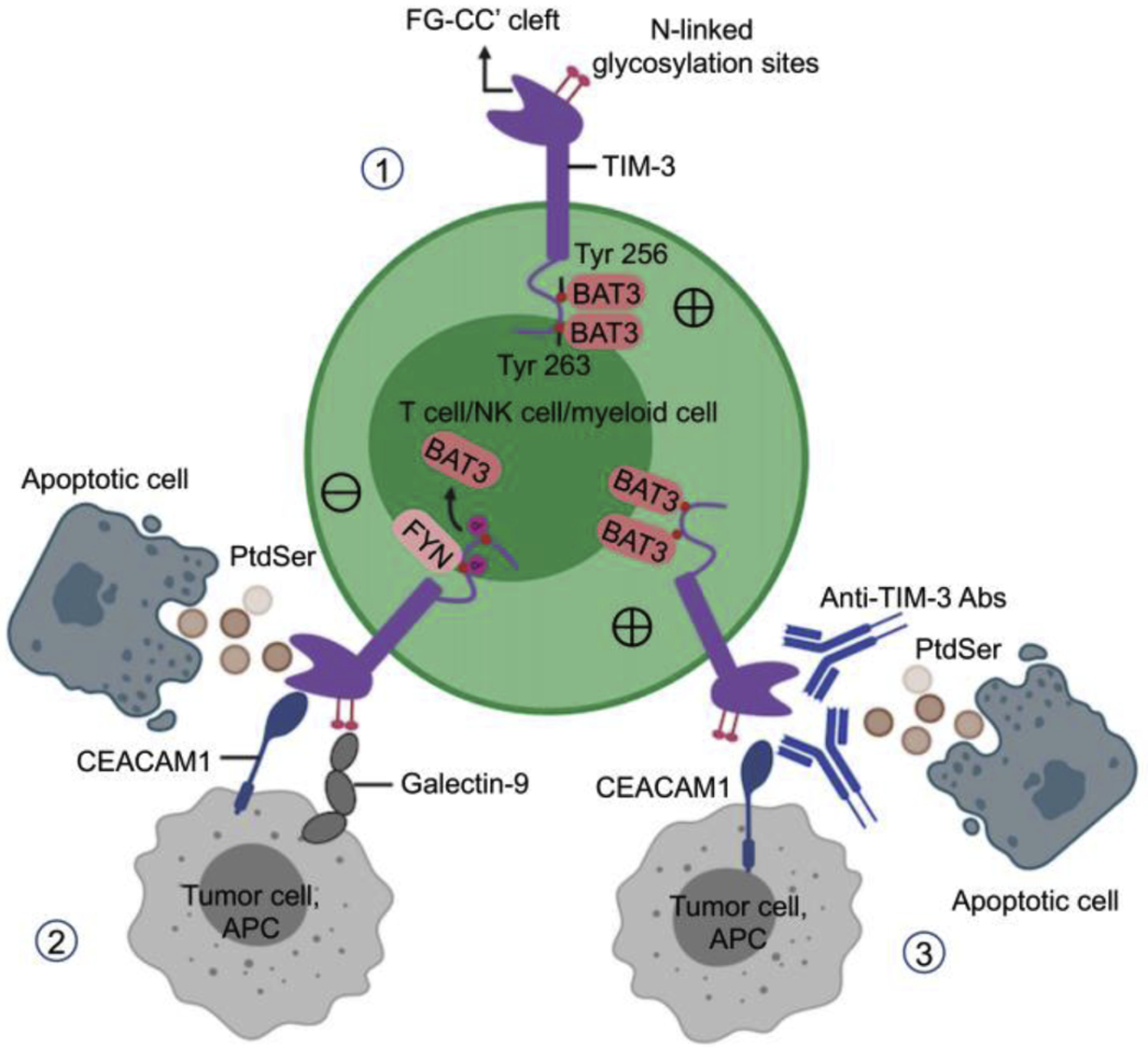
1) In the absence of TIM-3 ligands, BAT3 binds the TIM-3 cytoplasmic tail and recruits the active form of LCK to the TCR complex to activate TCR signaling. 2) In the presence of ligand, BAT3 is released from the TIM-3 cytoplasmic tail, resulting in the recruitment of phosphatases, dephosphorylation of LCK, and inhibition of TCR signaling. Tyrosine kinase FYN can be recruited to the TIM-3 tail after the release of BAT3. 3) TIM-3 blockade may promote TIM-3-BAT3 interaction and TCR signaling.
2.3. TIM-3 in cancer immunology
TIM-3 was initially associated with negative regulation of T cell functions in autoimmune diseases (experimental autoimmune encephalomyelitis, type 1 diabetes and multiple sclerosis) [21, 36, 61, 62]. High levels of TIM-3 expression by PD-1+ T cells correlate with T cell dysfunction and dampen T cell response during chronic viral infection and cancer [6, 63–69]. In patients with advanced melanoma, TIM-3 is co-expressed by a fraction of PD-1+ tumor antigen-specific CD8+ T cells in the periphery and at tumor sites, which are more dysfunctional than PD-1+TIM-3− and PD-1−TIM-3− CD8+ T cells [6]. Dual PD-1/TIM-3 blockade enhances cytokine production and proliferation of human antigen-specific CD8+ T cells [6]. There have been similar findings in mouse tumor-bearing models, and targeting TIM-3 and PD-1 pathways with monoclonal antibodies impede tumor growth and prolong survival in vivo [69, 70]. In addition, TIM-3 overexpression in T cells exacerbates tumor growth in the EL4 mouse model of lymphoma [43]. Increased TIM-3 expression on CD8+ tumor-infiltrating lymphocytes (TILs) correlates with poor prognosis in many solid tumors, including non-small cell lung cancer (NSCLC), renal cancer, HBV-associated HCC, and prostate cancer [42, 71–73]. TIM-3 upregulation by CD8+ TILs occurs upon adaptive resistance to PD-1 inhibitors in mice and humans with lung cancer, and TIM-3 blockade confers clinical benefits to PD-1 refractory mice [74]. Interestingly, TIM-3 and PD-1 are not only upregulated by tumor-antigen specific dysfunctional CD8+ T cells, but also activated vaccine-induced T cells in patients with advanced melanoma. Dual blockade of PD-1 and TIM-3 enhances the expansion and cytokine production of vaccine-induced CD8+ T cells upon stimulation with cognate antigen in vitro [68].
TIM-3 is upregulated on Tregs, which are potent suppressors of antitumor immune responses in the TME [75]. TIM-3+ Tregs appear to represent a subset of activated Tregs [75, 76] present in many solid tumors, including NSCLC [23], which correlate with tumor burden and progression. In healthy donors and patients with cancer, most peripheral NK cells express TIM-3. TIM-3 is expressed by all mature CD56dimCD16+ NK cells and is expressed heterogeneously in the immature CD56brightCD16− NK-cell subset in blood from healthy adults [27]. TIM-3 blockade reinvigorates peripheral NK cell function in vitro in the presence of cognate ligands [77]. In contrast to T cells, circulating TIM-3+ NK cells in healthy donors are not dysfunctional [27]. Compared with healthy donors, peripheral NK cells in patients with melanoma express significantly higher levels of surface TIM-3, although this increase is modest [77]. Higher TIM-3 expression on NK cells is associated with advanced stages of melanoma and poor prognostic clinical parameters. Blocking TIM-3 partially increases cytotoxicity and IFN-γ production by circulating NK cells in melanoma patients [77]. Notably and in sharp contrast with CD8+ TILs, tumor-infiltrating NKs are dysfunctional and do not upregulate TIM-3 compared to circulating NK cells in advanced melanoma [78]. In addition, dysfunctional NK cells in metastatic tumors downregulate many inhibitory and costimulatory receptors [79–84].
TIM-3 is constitutively expressed by APCs and is further upregulated by DCs in the TME [16]. In a mouse model of breast cancer, intratumoral CD103+ DCs upregulate TIM-3 expression and anti-TIM-3 antibody promotes chemokine (C-X-C motif) ligand 9 expression by cross-presenting BATF3+CD103+ DCs (type 1 conventional DCs) to enhance the CD8+ T cell-dependent response to the chemotherapeutic paclitaxel [85]. In this model, the effects of anti-TIM-3 antibody appear to be Gal-9-dependent. The precise mechanism used by anti-TIM-3 antibody to reprogram DCs in the TME and relevance of these data to humans remain unknown.
Transgenic overexpression of TIM-3 on T cells suppresses adaptive immunity in a murine model of EL4 thymoma by promoting the generation of MDSCs in a TIM-3/Gal-9–dependent manner [43]. Interestingly, one study recently reported the accumulation of lymphoid cells and monocytic MDSCs in PD-1 refractory patients with NSCLC [86]. Gal-9 but not PD-L1 expression is negatively correlated with IFN-γ production by peripheral blood mononuclear cells (PBMCs) upon stimulation with anti-CD3 antibodies. Dual PD-1/TIM-3 blockade restores cytokine production by PBMCs in vitro. Together, these findings suggest that TIM-3 and Gal-9 may be implicated in resistance to PD-1 blockade both in patients with primary or acquired secondary resistance to anti-PD-1.
Soluble TIM-3 is released upon shedding by sheddases (ADAM10 and ADAM17) [87] and may impair antitumor immunity. Highly levels of elevated soluble TIM-3 correlate with high risk of HCC and poor survival of patients with HCC [88], and soluble TIM-3 enhances B16F1 tumor growth in mice by inhibiting T cell-mediated antitumor immunity and antigen-specific stimulation [89]. TIM-3 is also expressed by tumor cells in melanoma, glioma, osteosarcoma, cervical cancer, and clear cell renal cell carcinoma and appears to facilitate their migration, proliferation, and invasion [31–35]. TIM-3 is highly expressed by leukemic stem cells (LSCs) in AML but not in normal hematopoietic stem cells [90–94], and represents a potential therapeutic target. In a mouse xenograft model, anti-TIM-3 blockade with Fc-binding capacity eradicates human AML LSCs without affecting normal human hematopoiesis [90, 94]. The mechanisms supporting the role of tumor-expressed TIM-3 in the regulation of antitumor immune responses remain unknown. Finally, a meta-analysis of published studies totaling 869 patients with advanced solid tumors suggests that high TIM-3 expression in the TME is associated with advanced tumor stage and shorter overall survival, supporting the role of TIM-3 as an inhibitory receptor in antitumor immune responses [95].
2.4. Genetic and epigenetic regulation of TIM-3 in cancer
Human TIM-3 contains a large number of single nucleotide polymorphisms (SNPs) [96]. These SNPs are associated with TIM-3 expression and risk of cancer including gastrointestinal tract cancer [97], breast cancer [98], renal cell carcinoma [99], HBV-related HCC [73], pancreatic cancer [100], NSCLC, and non-Hodgkin lymphoma [101]. In addition, germline mutations c.245A>G (p.Tyr82Cys) and c.291A>G (p.Ile97Met) in the TIM-3 IgV domain result in misfolding and post-translational modifications of TIM-3, abrogating TIM-3 cell surface expression by T cells and myeloid cells. These mutations were found in 16 of 27 patients with a rare form of non-Hodgkin T cell lymphoma, called subcutaneous panniculitis-like T cell lymphoma (SPTCL), which is often associated with hemophagocytic lymphohistiocytosis (HLH). Misfolding of TIM-3 promotes persistent immune activation and increases the production of inflammatory cytokines, including tumor necrosis factor-α (TNF-α) and IL-1β, promoting HLH and SPTCL. Such findings support the inhibitory function of TIM-3 [102–104].
Tumor-secreted molecules such as PGE2, which activate protein kinase A (PKA), upregulate transcriptomic TIM-3 expression in T cells [105]. CpG islands in the promoter regions of TIM-3 appear to be significantly hypomethylated in breast tumor tissues compared with normal tissues [106]. Also, bindings of repressive histones H3K9me3 and H3K27me3 in the TIM-3 promoter are significantly reduced in tumor tissues compared with normal tissues [106, 107].
2.5. Targeting TIM-3 in cancer immunotherapy
Ample evidence in preclinical models supports dual PD-1/TIM-3 blockade to augment the tumor antigen-specific T cell response and promote tumor regression. Based on these findings, several TIM-3-targeting antibodies are being tested in clinical trials (Table 1). TSR-022 is a humanized anti-TIM-3 IgG4 antibody developed by Tesaro/GlaxoSmithKline that is being evaluated alone and in combination with anti-PD-1 (TSR-042) and anti-lymphocyte activation gene-3 (LAG-3) (TSR-033) in an ongoing phase I/II study for PD-1 refractory melanoma and NSCLC patients. Anti-TIM-3 antibody alone or in combination with anti-PD-1 is safe, and the toxicity profile is similar to PD-1 inhibitors. Clinical benefits have been observed in the high dose of TSR-022 combination group with a 15% overall response rate (ORR) (3/20) and 40% stable disease (8/20) [108]. In a phase Ib clinical trial with anti-TIM-3 (MGB453) as monotherapy and in combination with anti-PD-1 (PDR001) in AML and high-risk myelodysplastic syndrome, 8 of 16 (50%) patients with HR-MDR and 4 of 14 (29%) patients with newly diagnosed AML exhibited clinical responses [109].
Table 1:
Active clinical trials targeting TIM-3, TIGIT, or CD112R
| Target | Agents (manufacturer) | Protocol (tumor types) | Combination agents |
|---|---|---|---|
| TIM-3 | TSR-022, humanized IgG4 (Tesaro) | NCT02817633, phase I (advanced solid tumors) | TSR-023 (anti-PD-1), TSR-033 (anti-LAG-3) |
| NCT03580508, phase II (advanced, localized/unresectable HCC) | TSR-023 (anti-PD-1) | ||
| NCT03307785, phase I (advanced solid tumors) | Niraparib, TSR-042 (anti-PD-1), Bevacizumab (anti-VEGF), platinum-based chemotherapy | ||
| MBG453, humanized IgG4 (Novartis) | NCT02608268, phase I/II (advanced malignancies) | PDR001 (anti-TJ-1) | |
| NCT03066648, phase I (AML or high risk MDS) | Decitabine, PDR001 (anti- PD-1) | ||
| NCT03940352, phase I (AML or high risk MDS) | HDM201, Venetoclax | ||
| NCT03961971, phase I (GBM) | Spartalizumab (anti-PD-1) | ||
| Sym023, human IgG1 (Symphogen) | NCT03489343, phase I (advanced malignancies) | None | |
| NCT03311412, phase I (advanced malignancies) | Sym021 (anti-PD-1), Sym022 (anti-LAG-3) | ||
| LY3321367, human IgG1 (Eli Lilly and Company) | NCT03099109, phase I (advanced relapsed/refractory solid tumors) | LY33000054 (anti-PD-L1) | |
| BGB-A425, humanized IgG1 (BeiGene) | NCT03744468, phase I/II (locally advance solid tumors) | Tislelizumab (anti-PD-1) | |
| RO7121661, bispecific anti-PD-1/TIM-3 (Hoffmann-La Roche) | NCT03099109, phase I (advanced solid tumors) | None | |
| INCAGN02390, IgG1k (Incyte Corporation) | NCT03652077, phase I (advanced malignances) | None | |
| NCT04370704, phase I/II (advanced malignances) | INCMGA00012 (anti-PD-1), INCAGN02385 (anti-LAG-3) | ||
| BMS-986258, (Bristol-Myers Squibb) | NCT03446040, phase I (advanced cancer) | Nivolumab (anti-PD-1), rHuPH20 | |
| SHR-1702 (Jiangsu HengRui) | NCT03708328, phase I (advanced malignances) | Camrelizumab (anti-PD-1) | |
| TIGIT | BMS-986207, human IgG1 (Bristol Myers Squibb) | NCT04150965, phase I/II (multiple myeloma with relapse) | None |
| BGB-A1217, humanized IgG1 (BeiGene) | NCT04047862, phase I/Ib (metastatic solid tumors) | Tislelizumab | |
| Tiragolumab, MTIG7192A, human IgG1 (Genentech) | NCT02794571, phase Ia/b (advanced tumors) | Atezolizumab (anti-PD-L1), carboplatin, cisplatin, pemetrexed, paclitaxel | |
| NCT04045028, phase I (myeloma and lymphoma) | Rituxomab (anti-CD20), Daratumumab/rHuPH20 (anti- CD38/hyaluronidase PH20) | ||
| NCT04045028, phase Ib/II (urothelial carcinoma) | Atezolizumab (anti-PD-L1) | ||
| NCT03281369, phase Ib/II (gastroesophageal cancers) | Atezolizumab (anti-PD-L1) | ||
| NCT03193190, phase Ib/II (pancreatic adenocarcinoma) | Atezolizumab (anti-PD-L1) | ||
| NCT03563716, phase II (lung cancer), | Atezolizumab (anti-PD-L1) | ||
| NCT04294810, phase III (PD-L1 positive non-small lung cancer) | Atezolizumab (anti-PD-L1) | ||
| NCT04300647, phase II (metastatic or recurrent PD-L1 positive cervical cancer) | Atezolizumab (anti-PD-L1) | ||
| NCT04256421, phase III (small cell lung cancer) | Atezolizumab (anti-PD-L1), carboplatin, etoposide | ||
| AB154, humanized IgG1 (Arcus Biosciences) | NCT03628677, phase I (advanced solid malignancies), | Zimberelimab (anti-PD-1) | |
| NCT04262856, phase II (PD-L1 positive non-small lung cancer) | Zimberelimab (anti-PD-1), AB928 (anti-A2a/bR antagonist) | ||
| ASP8374, human IgG4 (Astella Pharma Global Development) | NCT03260322, phase Ib (advanced tumors) | Pembrolizumab (anti-PD-1) | |
| MK-7684, humanied IgG1 (Merck Sharp & Dohme) | NCT4305041, phase I/II (melanoma) | Pembrolizumab (anti-PD-1), MK-1308 (anti-CTLA-4) | |
| NCT04303169, phase I/II (PD-1 refractory melanoma) | Pembrolizumab (anti-PD-1) | ||
| M6223, TIGIT inhibitor (Merck Sharp & Dohme) | NCT04457778, phase I (metastatic solid tumors) | Bintrafusp alfa (anti-PD-L1/TGFβ) | |
| C0M902, TIGIT inhibitor (Compugen) | NCT04354246, phase I (advanced malignancies) | None | |
| CD112R | COM701, inhibitor (Compugen) | NCT03667716, phase I (advanced solid tumors) | Nivolumab (anti-PD-1) |
Antibodies targeting TIM-3 and TIGIT and drugs targeting CD112R found on ClinicalTrials.gov (as of August 2020) that are active in clinical trials for the indicated tumor types and with the combinatorial treatment listed. AML, acute myeloid leukemia; GBM, glioblastoma multiforme; HCC, hepatocellular carcinoma; Ig, immunoglobulin; LAG-3, lymphocyte-activation gene 3 protein; mAb, monoclonal antibody; MDS: myelodysplastic syndrome; PD-1, programmed cell death 1; PD-L1, programmed death ligand 1
3. TIGIT
TIGIT (also called WUCAM, Vstm3, VSIG9) is an IR of the Ig superfamily expressed by multiple immune cells. TIGIT is part of a complex regulatory network involving multiple ligands [e.g., CD155 (PVR/NECL-5), CD112 (nectin-2/PVRL2)], two competitive IRs, CD96/TACTILE and CD112R/PVRIG, and one known competing co-stimulatory receptor, DNAM-1/CD226 [17, 18, 20, 110, 111]. This pathway is reminiscent of the CD28/CTLA-4/CD80/CD86 pathway, for which IRs and costimulatory receptors compete for binding to the same ligands [112]. Tigit−/− mice do not develop autoimmunity, in contrast with Ctla4−/− mice [113]. However, Tigit−/− mice develop more severe experimental autoimmune encephalitis than wild-type (WT) mice when immunized with myelin oligodendrocyte glycoprotein [113]. In humans, TIGIT is expressed on activated T cells, follicular helper T cells (Tfh), Tregs and NK cells in PBMCs [17, 114–116]. In the TME, TIGIT is upregulated by Tregs and TILs [118, 119]. DNA hypomethylation and binding of repressive histones (H3K9me3 and H3K27me3) in the TIGIT promoter appear to be significantly reduced in tumor tissues and compared with normal tissues, contributing to TIGIT upregulation [106]. In both mice and humans, TIGIT is co-expressed with PD-1 on CD8+ TILs and on tumor antigen-specific CD8+ T cells [120, 121]. TIGIT is also co-expressed with IRs such as TIM-3 and LAG-3 on exhausted CD8+ T cell subsets in the TME [120, 121]. TIGIT expression delineates Tregs from activated effector CD4+ T cells and its upregulation is associated with hypomethylation and Foxp3 binding at the Tigit locus [122]. Unlike mouse NK cells, TIGIT is highly expressed on human NK cells and regulates their antitumor activity [123]. Although TIGIT+ NK cells are more lytic and more mature than TIGIT− NK cells, they display lower cytotoxicity against CD155+ MHC class I-deficient melanoma [124]. In metastatic tumors, NK cells are found at a low frequency and they downregulate both TIGIT and CD226 expression in contrast with CD8+ T cells [124]. Membrane-bound CD155 promotes CD226 internalization and degradation, impeding NK cell-mediated tumor reactivity [124].
3.1. TIGIT ligands
TIGIT binds CD155 and CD112, which are members of the nectin protein family. TIGIT binds CD155 with high affinity as compared with its competitors and binds CD112 with low affinity [18] (Table 2). CD155 and CD112 are expressed by circulating and APCs including DCs, as well as many non-hematopoietic cells including tumor cells of different histological types [18, 120, 125–127]. CD155 and CD112 bind other nectin-like proteins including CD113 [18]. CD155 expression is upregulated upon reactive oxygen species-dependent activation of the DNA damage response, which regulates interactions among MDSCs, T cells, and NK cells [128, 129]. Fap2 protein from Fusobacterium nucleatum, an anaerobic Gram− commensal bacteria associated with colorectal carcinoma, binds TIGIT to inhibit NK- and T-cell mediated tumor reactivity. Such findings support the role of the gut microbiome in regulating antitumor immune responses via TIGIT [130].
Table 2:
Ligand binding affinities for human CD226, TIGIT, CD96, and CD112R
| Ligand/receptor affinity | CD226 | TIGIT | CD96 | CD112R |
|---|---|---|---|---|
| CD155 | 114–199 nM | 1–3 nM | 37.6 nM | - |
| CD112 | 0.31–8.97 μM | Not measurable | - | 88 nM |
3.2. Structure and signaling of TIGIT
TIGIT contains one extracellular Ig variable domain, a transmembrane domain, and a cytoplasmic tail containing two inhibitory motifs conserved in mice and humans: an ITIM and an Ig tail-tyrosine (ITT)-like motif [17, 18, 113, 114] (Fig. 2). Upon TIGIT/CD155 interaction, phosphorylation of the ITT-like motif (Tyr225) triggers TIGIT inhibitory signals in humans, whereas both the ITT and the ITIM motifs induce inhibitory signals in mice [17, 131]. In NK cells, TIGIT phosphorylation recruits the cytosolic adapters Grb2 [131] and β-arrestin 2 [132] to bind SH2-containing inositol phosphatase-1 (SHIP1). SHIP1 inhibits phosphoinositide 3 kinase and mitogen-activated protein kinase signaling [131]. SHIP1 also impedes the activation of TNF receptor-associated factor 6 (TRAF6) and NF-κB and disrupts IFN-γ production by NK cells [132].
Figure 2: TIGIT/CD96/CD112R inhibitory pathways.
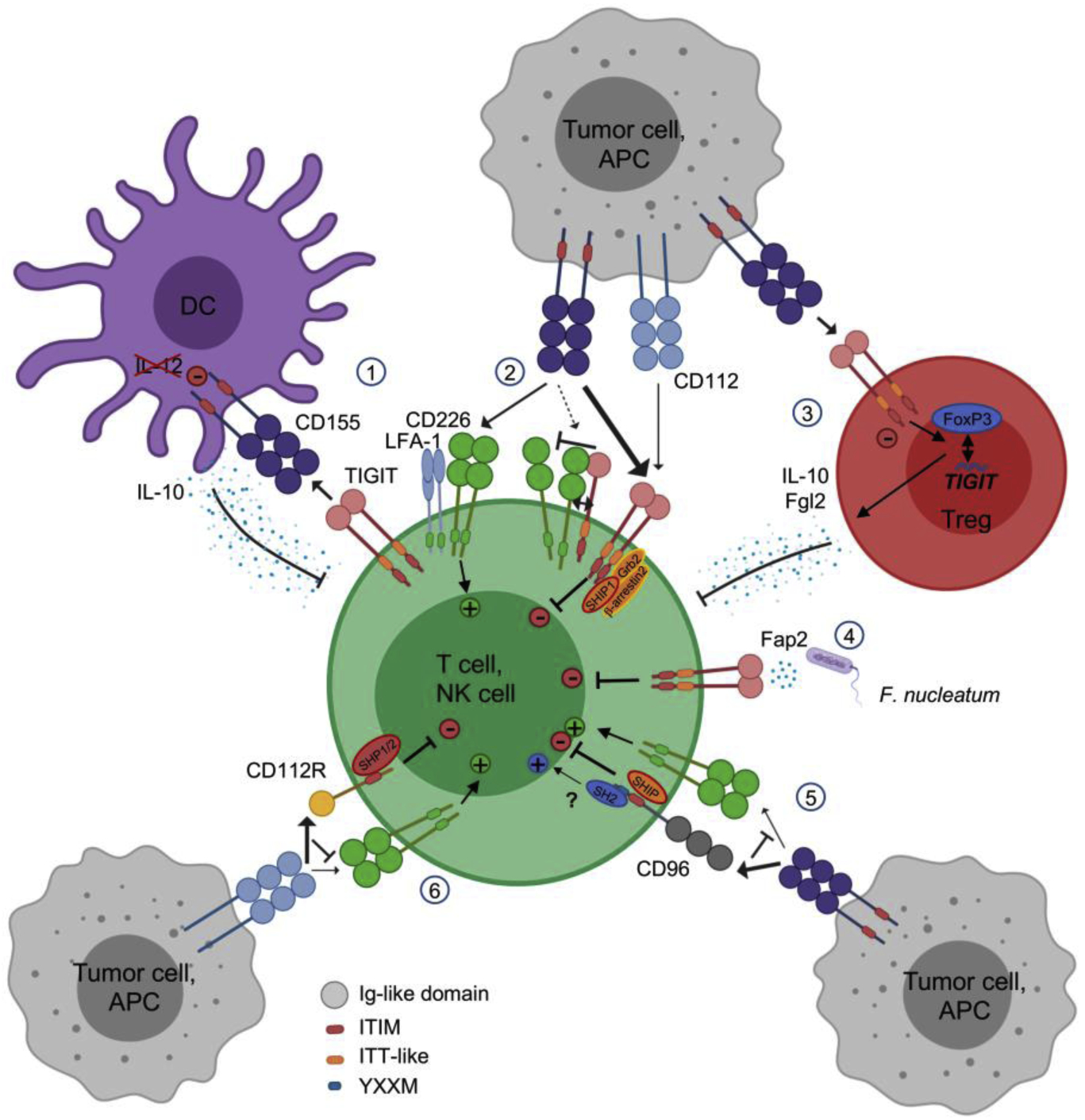
1) CD155 engagement with TIGIT drives DCs into producing IL-10 instead of IL-12. 2) TIGIT competes with CD226, binds CD226 in cis to reduce its binding stability to CD155, and induces direct intrinsic inhibitory signal via Grb2, SHIP1, and β-arrestin recruitment at the ITT-like motif. 3) TIGIT engagement promotes Treg stability, Foxp3 binding to the TIGIT locus, and suppressive functions with the production of Fgl2 and IL-10. 4) Fap2 protein from F. nucleatum can bind TIGIT, triggering direct inhibitory signals in the TME. 5) CD96 competition with CD226 for CD155 dampens stimulatory signals and its ITIM binds SHIP1/2 adaptors to trigger inhibitory signals while its YXXM might bind SH2 to promote stimulatory signals in T cells. 6) CD112R competition with CD226 for CD112 dampens stimulatory signals and its ITIM binds SHP1/2 adaptors to trigger inhibitory signals.
3.3. TIGIT in cancer immunology
TIGIT inhibits adaptive and innate immunity through multiple mechanisms [10, 11, 17, 114] (Fig. 2). First, in a mouse model, TIGIT binds CD155 on DCs to impair T cell functions [18]. TIGIT induces CD155 phosphorylation and triggers a signaling cascade promoting tolerogenic DCs that produce less IL-12 and more IL-10 [18]. Second, TIGIT exerts direct T cell-intrinsic inhibitory effects by dampening TCR signaling, leading to decreased T cell proliferation and function [113, 115, 126]. TIGIT also inhibits mouse and human NK cell degranulation, cytokine production, and NK cell-mediated cytotoxicity of CD155-expressing tumor cells [17, 131–133]. Notably, CD155+ MDSCs act on TIGIT+ NK cells to decrease phosphorylation of ZAP70/Syk and ERK1/2 and reduce their cytolytic capacity [129].
Third, TIGIT disrupts CD155-mediated DNAM-1/CD226 activation. CD226 is a costimulatory receptor widely expressed by immune cells including T cells, NK cells, monocytes, and platelets [134, 135]. This receptor is directly involved in tumor recognition by T cells and NK cells in mice and humans [134, 136]. CD226-deficient mouse CD8+ T cells and NK cells display immunological synapse defects impairing antitumor immunity [137, 138]. CD226 also associates with lymphocyte function-associated antigen 1 (LFA-1) to enhance TCR signaling [139]. TIGIT impedes CD226-mediated activation because it binds CD155 with higher affinity than CD226 [17, 18, 113], and directly binds CD226 in cis, impairing its homodimerization and binding capacity to CD155 [121].
Fourth, multiple lines of evidence suggest that the balance of TIGIT/CD226 expression plays a central role in regulating the effector function of T cells and NK cells. TIGIT knockdown with shRNA in TCR-activated CD4+ T cells increases T-bet expression and IFN-γ production, which are abolished upon CD226 or CD155 blockade. In contrast, CD226 silencing decreases T-bet expression and IFN-γ production [126]. Blocking anti-CD226 antibodies abrogates the immunological effects of dual PD-1 and TIGIT blockade on CD8+ T cell-mediated tumor in vitro and in CT26 tumor-bearing mice [120, 121]. Interestingly, anti-PD-1 blocking mAb and agonistic anti-GITR mAbs increase overall survival of mice bearing MC38 colon adenocarcinoma tumors. Notably, in MC38 tumor-bearing mice treated with anti-PD-1 blocking mAbs and agonistic anti-GITR mAbs, PD-1 blockade counteracts CD8+ T cell dysfunction by inhibiting Scr homology domain 2-containing tyrosine phosphatase (SHP2)-mediated CD226 dephosphorylation, while anti-GITR mAbs decrease TIGIT expression [140]. Such findings suggest that in addition to PD-1 and TIGIT blockades, other ICBs can exert beneficial clinical effects by favorably tipping the balance between CD226 and TIGIT in CD8+ T cells.
Fifth, TIGIT acts in Tregs to augment immunosuppressive function and stability. TIGIT is highly expressed by a subset of natural Tregs in mice [118] and the majority of Tregs in humans [118, 119, 141]. In Tregs, the TIGIT locus is hypomethylated and includes Foxp3 binding sites [122]. TIGIT+ Tregs are more suppressive than TIGIT− Tregs in healthy donors and patients with melanoma [119, 141]. Further, TIGIT+ Tregs in the periphery and at tumor sites upregulate many Treg gene signature markers as compared with TIGIT− Tregs [118] including FOXP3, IKZF2, NRP-1, CTLA-4, PD-1, and LAG-3 [118, 119]. TIGIT+ Tregs also suppress proinflammatory Th1 and Th17 but not Th2-type T cell responses [118, 142]. Upon TIGIT ligation, TIGIT+ Tregs produce IL-10 and fibrinogen-like protein 2 (FGL2), which mediate T cell suppression [118]. Compared to the periphery, Tregs exhibit lower CD226 expression in metastatic melanoma, resulting in an increased TIGIT/CD226 ratio [119]. TIGIT and CD226 oppose each other to augment or disrupt Treg suppression and stability, respectively [119]. A high TIGIT/CD226 ratio in Tregs appears to correlate with increased Treg frequencies in tumors and poor clinical outcome upon ICB. Whether the TIGIT/CD226 ratio in Tregs represent a biomarker of clinical response to ICB in patients with solid tumors remains to be determined.
3.4. Targeting TIGIT in cancer immunotherapy
Accumulating preclinical data suggest that dual PD-1/TIGIT blockade is a promising combinatorial cancer immunotherapy. While either single blockade does not significantly impede growth of CT26 tumors in mice, dual TIGIT and PD-1/PD-L1 blockade synergizes to augment CD8+ T cell-mediated tumor reactivity, resulting in protective memory T cells, complete tumor rejection, and prolonged overall survival [121, 143, 144]. Dual PD-1/TIGIT blockade in patients with melanoma also augments functions of isolated tumor-antigen-specific CD8+ T cells and TILs compared with effects of single blockade [120, 145]. Further, dual PD-L1/TIGIT blockade (atezolizumab [atezo]/tiragolumab [tira]) provides superior clinical benefits with improved ORR and progression-fee survival compared with PD-L1 blockade alone as a first-line therapy for patients with PD-L1-positive NSCLC, despite similar toxicity profiles [146]. This study included 135 NSCLC patients with an ORR of 37% for atezo/tira compared with 21% for atezo and placebo. In PD-L1+ tumors (tumor proportion score > 50%), ORR was 66% for atezo/tira vs 24% for atezo and placebo. Combinatorial therapy with anti-PD1 and anti-TIGIT antibodies is being evaluated in an ongoing phase III study.
The effects of dual PD-1/TIGIT blockade in mouse tumor models and in vitro are abrogated upon CD226 blockade, suggesting that TIGIT blockade acts primarily by tipping CD155-mediated signaling toward CD226 activation [120, 121]. In addition, PD-1 induces SHP2-mediated CD226 dephosphorylation, supporting the additive effects of dual PD-1/TIGIT blockade in enhancing CD226 signaling [140]. Membrane-bound CD155 plays a critical role in mediating CD226 downregulation by immune cells in the TME via CD226 internalization and degradation, supporting the role of CD155-mediated immune dysfunction [124]. CD226 downregulation by CD8+ TILs, which has been observed in many solid tumors, including melanoma, may limit the effects of dual PD-1/TIGIT blockade in patients with cancer [120, 147].
TIGIT blockade or TIGIT deletion augments NK cell-mediated antitumor reactivity in vitro and in vivo [17, 129, 131, 133, 148]. One study of B16 melanoma and CT26 lung metastasis mouse models reported that TIGIT blockade alone or in combination with PD-1 blockade acted primarily on NK cells to augment CD8+ T cell-mediated antitumor responses and impede tumor growth. In the same study, NK cell-specific TIGIT deficiency and NK cell depletion abrogated the effects of TIGIT blockade [143]. These findings do not fit well with the many observations supporting that TIGIT blockade alone fails to significantly augment CD8+ T cell immunity and promote tumor rejection in WT mice transplanted with solid tumors [121, 149] and in expanding tumor antigen-specific CD8+ T cells [120] compared with dual PD-1/TIGIT blockade. Mechanisms supporting potential helper effects of NK cells on CD8+ TILs as well as the relevance of these findings to cancer patients remain unclear. Whether and how NK cells contribute to environmental cues guiding CD8+ T cell priming, maturation, and memory differentiation needs to be determined. Interestingly, IL-15 together with TIGIT blockade increases NK cell-mediated melanoma cytotoxicity in vitro and decreases tumor metastasis in mouse melanoma models [124]. These findings support the development of novel combinatorial immunotherapy with IL-15 and TIGIT blockade to promote NK cell-mediated destruction of MHC class I-deficient melanoma, which is refractory to CD8+ T cell-mediated immunity.
One study in mouse tumor models showed that ICB with Fc variants of anti-TIGIT mAbs with selective FcγR co-engagement on APCs enhances antigen-specific T cell responses and tumor reactivity without evidence of Treg depletion [150]. Whether the antitumor effects of anti-TIGIT antibodies in cancer patients are Fc-dependent will need to be further investigated in ongoing clinical trials with different Fc-engineered anti-TIGIT mAbs (Table 1).
4. CD96
CD96 (also known as TACTILE) is a receptor of the Ig superfamily, first described as an activation receptor [151]. In humans, CD96 is expressed on T cells, NK cells, and gammadelta T cells [151, 152]. CD96 is a marker of T cell activation, which is co-expressed with CD226, TIGIT, and PD-1 on T cells upon TCR activation [152]. In multiple solid tumors, increased CD96 mRNA expression appears to correlate with increased expression of T cell markers, and the expression of CD96 and CD8A are higher in metastatic tumors than in primary tumors [152]. CD96 is also expressed by acute lymphoblastic leukemia, in AML and is a leukemic stem cell-specific marker in human acute myeloid leukemia [153].
4.1. CD96 ligands
CD96 competes with CD226 and TIGIT for binding to CD155 [17, 18, 110, 111]. The binding affinity of CD96 to CD155 is between that of TIGIT (high affinity) and DNAM-1 (low affinity) (Table 2). Notably, mouse CD96 also binds CD111 [18, 154].
4.2. Structure and signaling of CD96
CD96 contains three extracellular Ig-like domains, a transmembrane sequence, and a short intracellular domain. The first Ig domain of CD96 is necessary and sufficient for nectin-like protein 5 (NECL-5) binding via the canonical “lock-and-key” docking mode, although the second and third Ig domains of CD96 can influence the interaction [155]. In humans, alternative splicing of exon 4 generates two variants containing different second external Ig domains with either an I-like (variant 1) or a V-like (variant 2) Ig domain [156]. Variant 2 is more abundant than variant 1, and exhibits higher binding capacity to CD155, which promotes cell-to-cell adhesion [125, 156]. Both mouse and human cytoplasmic tails of CD96 contain a short basic/proline rich motif and a single ITIM motif, suggesting possible inhibitory signaling [156]. However, the human CD96 cytoplasmic tail also contains an YXXM motif, which is found in inducible T cell costimulator (ICOS) protein and CD28 and binds to the SH-2 domain [156], suggesting potential inhibitory or stimulatory function.
4.3. CD96 in cancer immunology and immunotherapy.
Whether CD96 acts as a costimulatory or an inhibitory receptor (Fig. 2) is under debate. CD96 was first described as an activating receptor [125]. In that study, the investigators tested the cytotoxicity of human polyclonal NK cell lines and NK92 against P815 cells in the presence of Abs that bound to the Fc receptor on P815 and engaged CD96. Anti-CD96 antibody increased the lysis of P815 by human polyclonal NK cell lines, although not as efficiently as the engagement of DNAM-1 [125]. The same anti-CD96 antibody failed to promote tumor lysis in the presence of primary NK cells, and did not influence the killing of CD155-expressing ovarian carcinoma or myeloma cells by NK cells, whereas anti-DNAM-1 significantly blocked NK cell degranulation and cytotoxicity [157, 158]. On one hand, CD96 appears to impede antitumor immune responses in mice. In an experimental mouse lung metastasis model with CD155-expressing tumors, cd96−/− mice developed less metastases via increased IFN-γ production by NK cells compared to WT mice [159]. CD96 blockade also inhibited experimental tumor metastasis in three different tumor models in an IFN-γ-, CD226- and NK cell-dependent fashion [160]. Blocking CD96 impedes primary tumor growth in many mouse tumor models, in a CD8+ T cell and CD226-dependent fashion. CD96 blockade is more effective in dual combination with anti-PD-1, anti-CTLA-4, or anti-TIGIT blockade [161]. Such studies fit well with the observation that PD-1 and CTLA-4 blockade are more effective in the setting in which CD155 is limiting [162]. Anti-CD96 antibody also enhances the clinical effects of dual PD-1/TIGIT blockade.
On the other hand, CD96 appears to exhibit costimulatory function both in mouse and human CD8+ T cells [163]. Anti-CD96 mAb co-crosslinking with anti-CD3 mAb promotes T cell proliferation, phosphorylation of the MEK-ERK pathway, expression of markers and transcription factors associated with antigen-specific activation like T-bet and Eomes, and production of proinflammatory cytokines such as IFN-γ and TNF-α. Using OT-I T cell transfer and a syngeneic tumor model, blockade or genetic ablation of CD96 results in functionally antigen-specific CD8+ T cells with no increased CD8+ T cell infiltrates or controlled tumor growth. The paradoxical observation of CD8+ T cell activation without clinical activity in mouse-bearing tumors treated with CD96 blockade remains to be explained. Additional studies are needed to determine whether CD96 blockade regulates CD8+ T cell priming, maturation, and memory differentiation in cancer immunotherapy. Also to be determined is whether PD-1 blockade may help enhance the immunological and clinical activity of the anti-CD96 antibody used in this study. Because of the conflicting findings on CD96 blockade reported in different mouse experimental tumor models with different anti-CD96 antibodies, it will be important to compare these antibodies in vitro and in vivo to further understand their mechanisms of action in CD8+ T cells and NK cells.
5. CD112R
CD112R is a recently described IR and a member of the Ig superfamily [20]. CD112R is expressed by T cells and NK cells in human PBMCs as well as in numerous solid tumors [164]. CD112R is also a member of the Ig superfamily [20]. This IR competes with CD226 for binding to CD112, which is expressed by DCs and the majority of human tumor cell lines [20]. In mouse, CD112R also binds CD155 with low affinity [165].
5.1. Structure and signaling of CD112R
CD112R contains an extracellular Ig-like domain, a transmembrane sequence, and a cytoplasmic tail that contains two tyrosine phosphorylation sites including an ITIM-like motif [20]. The binding of CD112R to its ligand recruits SHP1 and SHP2 that trigger the inhibition of the TCR/CD28 signaling in T cells [20] (Fig. 2).
5.2. CD112R in cancer immunology and immunotherapy
Several lines of evidence suggest that CD112R blockade combined with TIGIT blockade increases antitumor immune responses in mice and in vitro. In mice transplanted with MC38 tumors, CD112R-deficient mouse CD8+ T cells develop a stronger antigen-specific effector response compared with WT CD8+ T cells, resulting in reduced tumor growth compared to WT mice, and PD-L1 blockade further enhances the inhibition of tumor growth [165]. Dual CD112R/PD-L1 blockade also reduces tumor growth and increases overall survival [165]. CD112R blockade alone or together with either TIGIT blockade or PD-1 blockade increases cytokine production by TILs from ovarian, endometrial, and lung tumors in the presence of an allogeneic melanoma cell line expressing surface-bound anti-CD3 antibody [164]. The relevance of these findings to human cancer and autologous CD8+ T cell responses against well-defined tumor antigens remains to be demonstrated. Finally, CD112R blockade synergizes with TIGIT blockade to enhance human NK cell-triggered antibody-dependent cellular cytotoxicity against breast tumor cell lines in vitro [148]. CD112R blockade is being evaluated in an ongoing clinical trial (NCT03667716) with CD112R inhibitor COM701 (Compugen) alone or in combination with PD-1 blockade (nivolumab) (Table 1).
6. Concluding remarks
TIM-3 is expressed by multiple innate and adaptive immune cells and acts as a negative regulator of antitumor immunity. Ongoing clinical trials with anti-TIM-3 antibodies provide a unique opportunity to determine the immunogenicity and clinical activity of anti-TIM-3 antibodies in patients with cancer. Although TIM-3 binds many ligands, anti-TIM-3 antibodies with proven blocking capacity in vitro appear to bind PtdSer and CEACAM1 but not Gal-9–9. Whether PtdSer, CEACAM1, or TIM-3 expression in the TME may serve as potential biomarkers of response to TIM-3 blockade remains to be determined. Because of the superiority of dual PD-1/TIM-3 blockade in vitro and in experimental tumor models compared with single blockade, it is likely that the PD-1 and TIM-3 pathways are non-redundant. However, the mechanisms of action supporting the activity of dual PD-1/TIM-3 blockade remain to be defined. It will also be essential to identify the TIM-3+ cell subsets targeted by TIM-3 blockade that are responsible for the potential immunological and clinical effects in vivo. There is strong evidence in mice and humans that targeting TIGIT together with PD-1 with blocking antibodies augments antitumor immune responses and confers clinical benefits in cancer patients. However, such findings need to be confirmed in large randomized clinical trials. Whether CD112R blockade together with PD-1 blockade or TIGIT blockade enhances expansion and function human tumor antigen-specific T cell responses in vitro and reduces tumor growth in cancer patients remains to be demonstrated. Although CD96 blockade together with other IR blockade are efficacious in multiple mouse tumor models, more data are needed to determine whether CD96 blockade alone or in combination significantly augment human tumor antigen-specific T cell responses in vitro. CD226 acts as a master regulator of dual PD-1/TIGIT blockade in cancer immunotherapy. Therefore, additional strategies are needed to prevent CD226 downregulation or augment its expression by TILs. To this end, combinatorial therapy with IL-15 and dual PD-1/TIGIT blockade may prove useful. Whether the antitumor effects of anti-TIGIT antibodies in cancer patients are Fc-dependent will need to be thoroughly investigated. Many ongoing clinical trials (Table 1) are testing Fc-engineered anti-TIGIT mAbs: IgG1 (MTIG7192/Genentech, MK-7684/Merck, and OP-313M32/Oncomed), inert-Fc IgG1 (BMS-986207/Bristol-Myers Squibb; AB-154/Arcus), and IgG4 (ASP8374/Potenza/Astellas).
Highlights.
We discuss the role of TIM-3 as well as several members of the TIGIT pathway, including TIGIT, CD96 and CD112R as inhibitory receptors of both innate and adaptive immunity to cancer.
We review the preclinical data as well as early-phase clinical trials supporting that targeting Tim-3, TIGIT, CD96 or CD112R together with PD-1 increase antitumor immunity and clinical responses.
Tim-3, TIGIT, CD96 and CD112R represent attractive targets for cancer immunotherapy in combination with PD-1 blockade.
Acknowledgements
Funding
This work was supported by NIH/NCI grants R01CA228181 and R01CA222203 (to HMZ), a research grant by Bristol-Myers Squibb (to HMZ), a cancer vaccine collaborative clinical strategy team grant (to HMZ), and the James W. and Frances G. McGlothlin Chair in Melanoma Immunotherapy (to HMZ).
Abbreviations
- AML
acute myeloid leukemia
- ADAM
a disintegrin and metalloprotease
- atezo
atezolizumab
- BAT3
HLA-B associated transcript 3
- CTLA-4
cytotoxic T lymphocyte-associated antigen 4
- CEACAM1
carcinoembryonic antigen-related cell-adhesion molecule 1
- DCs
dendritic cells
- Gal-9
Galectin-9
- HCC
hepatocellular carcinoma
- HMGB1
high-mobility group box 1
- HLH
lymphohistiocytosis
- ICB
immune checkpoint blockade
- IFN
interferon
- Ig
immunoglobulin
- ITIM
immunoreceptor tyrosine-based inhibitory motif
- ITT
Ig tail-tyrosine
- IL
interleukin
- IR
inhibitory receptor
- LAG-3
lymphocyte activation gene-3
- LSC
leukemia stem cell
- mAbs
monoclonal antibodies
- MDSCs
myeloid-derived suppressive cells
- NK
natural killer
- NSCLC
non-small cell lung cancer
- ORR
overall response rate
- PBMCs
peripheral blood mononuclear cells
- PD-1
programmed cell death 1
- PD-L1
programmed death ligand 1
- PtdSer
phosphatidylserine
- SHIP
SH2-containing inositol phosphatase
- SHP
Scr homology domain 2-containing tyrosine phosphatase
- SNPs
single nucleotide polymorphisms
- SPTCL
subcutaneous panniculitis-like T cell lymphoma
- TCR
T-cell receptor
- TIGIT
T cell immunoreceptor with immunoglobulin and ITIM domain
- TIL
tumor-infiltrating lymphocyte
- TIM-3
T-cell immunoglobulin and mucin domain-containing molecule-3
- tira
tiragolumab
- TME
tumor microenvironment
- TNF-α
tumor necrosis factor-α
- Tregs
regulatory T cells
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Zajac AJ, Blattman JN, Murali-Krishna K, Sourdive DJ, Suresh M, Altman JD, Ahmed R, Viral immune evasion due to persistence of activated T cells without effector function, J Exp Med 188(12) (1998) 2205–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Blackburn SD, Shin H, Haining WN, Zou T, Workman CJ, Polley A, Betts MR, Freeman GJ, Vignali DA, Wherry EJ, Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection, Nat Immunol 10(1) (2009) 29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Ahmadzadeh M, Johnson LA, Heemskerk B, Wunderlich JR, Dudley ME, White DE, Rosenberg SA, Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired, Blood 114(8) (2009) 1537–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Fourcade J, Kudela P, Sun Z, Shen H, Land SR, Lenzner D, Guillaume P, Luescher IF, Sander C, Ferrone S, Kirkwood JM, Zarour HM, PD-1 is a regulator of NY-ESO-1-specific CD8+ T cell expansion in melanoma patients, J Immunol 182(9) (2009) 5240–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Wherry EJ, Ha SJ, Kaech SM, Haining WN, Sarkar S, Kalia V, Subramaniam S, Blattman JN, Barber DL, Ahmed R, Molecular signature of CD8+ T cell exhaustion during chronic viral infection, Immunity 27(4) (2007) 670–84. [DOI] [PubMed] [Google Scholar]
- [6].Fourcade J, Sun Z, Benallaoua M, Guillaume P, Luescher IF, Sander C, Kirkwood JM, Kuchroo V, Zarour HM, Upregulation of Tim-3 and PD-1 expression is associated with tumor antigen-specific CD8+ T cell dysfunction in melanoma patients, J Exp Med 207(10) (2010) 2175–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Chauvin JM, Pagliano O, Fourcade J, Sun Z, Wang H, Sander C, Kirkwood JM, Chen TH, Maurer M, Korman AJ, Zarour HM, TIGIT and PD-1 impair tumor antigen-specific CD8(+) T cells in melanoma patients, J Clin Invest 125(5) (2015) 2046–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, Segal NH, Ariyan CE, Gordon RA, Reed K, Burke MM, Caldwell A, Kronenberg SA, Agunwamba BU, Zhang X, Lowy I, Inzunza HD, Feely W, Horak CE, Hong Q, Korman AJ, Wigginton JM, Gupta A, Sznol M, Nivolumab plus ipilimumab in advanced melanoma, N Engl J Med 369(2) (2013) 122–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Zarour HM, Reversing T-cell Dysfunction and Exhaustion in Cancer, Clin Cancer Res 22(8) (2016) 1856–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Sosman JA, Atkins MB, Leming PD, Spigel DR, Antonia SJ, Drilon A, Wolchok JD, Carvajal RD, McHenry MB, Hosein F, Harbison CT, Grosso JF, Sznol M, Five-Year Survival and Correlates Among Patients With Advanced Melanoma, Renal Cell Carcinoma, or Non-Small Cell Lung Cancer Treated With Nivolumab, JAMA Oncol (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, Wolchok JD, Hersey P, Joseph R, Weber JS, Dronca R, Mitchell TC, Patnaik A, Zarour HM, Joshua AM, Zhao Q, Jensen E, Ahsan S, Ibrahim N, Ribas A, Five-year survival outcomes for patients with advanced melanoma treated with pembrolizumab in KEYNOTE-001, Ann Oncol 30(4) (2019) 582–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Amaria RN, Reddy SM, Tawbi HA, Davies MA, Ross MI, Glitza IC, Cormier JN, Lewis C, Hwu WJ, Hanna E, Diab A, Wong MK, Royal R, Gross N, Weber R, Lai SY, Ehlers R, Blando J, Milton DR, Woodman S, Kageyama R, Wells DK, Hwu P, Patel SP, Lucci A, Hessel A, Lee JE, Gershenwald J, Simpson L, Burton EM, Posada L, Haydu L, Wang L, Zhang S, Lazar AJ, Hudgens CW, Gopalakrishnan V, Reuben A, Andrews MC, Spencer CN, Prieto V, Sharma P, Allison J, Tetzlaff MT, Wargo JA, Neoadjuvant immune checkpoint blockade in high-risk resectable melanoma, Nat Med 24(11) (2018) 1649–1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Blank CU, Rozeman EA, Fanchi LF, Sikorska K, van de Wiel B, Kvistborg P, Krijgsman O, van den Braber M, Philips D, Broeks A, van Thienen JV, Mallo HA, Adriaansz S, Ter Meulen S, Pronk LM, Grijpink-Ongering LG, Bruining A, Gittelman RM, Warren S, van Tinteren H, Peeper DS, Haanen J, van Akkooi ACJ, Schumacher TN, Neoadjuvant versus adjuvant ipilimumab plus nivolumab in macroscopic stage III melanoma, Nat Med 24(11) (2018) 1655–1661. [DOI] [PubMed] [Google Scholar]
- [14].Rozeman EA, Menzies AM, van Akkooi ACJ, Adhikari C, Bierman C, van de Wiel BA, Scolyer RA, Krijgsman O, Sikorska K, Eriksson H, Broeks A, van Thienen JV, Guminski AD, Acosta AT, Ter Meulen S, Koenen AM, Bosch LJW, Shannon K, Pronk LM, Gonzalez M, Ch’ng S, Grijpink-Ongering LG, Stretch J, Heijmink S, van Tinteren H, Haanen J, Nieweg OE, Klop WMC, Zuur CL, Saw RPM, van Houdt WJ, Peeper DS, Spillane AJ, Hansson J, Schumacher TN, Long GV, Blank CU, Identification of the optimal combination dosing schedule of neoadjuvant ipilimumab plus nivolumab in macroscopic stage III melanoma (OpACIN-neo): a multicentre, phase 2, randomised, controlled trial, Lancet Oncol 20(7) (2019) 948–960. [DOI] [PubMed] [Google Scholar]
- [15].Rangachari M, Zhu C, Sakuishi K, Xiao S, Karman J, Chen A, Angin M, Wakeham A, Greenfield EA, Sobel RA, Okada H, McKinnon PJ, Mak TW, Addo MM, Anderson AC, Kuchroo VK, Bat3 promotes T cell responses and autoimmunity by repressing Tim-3-mediated cell death and exhaustion, Nat Med 18(9) (2012) 1394–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Chiba S, Baghdadi M, Akiba H, Yoshiyama H, Kinoshita I, Dosaka-Akita H, Fujioka Y, Ohba Y, Gorman JV, Colgan JD, Hirashima M, Uede T, Takaoka A, Yagita H, Jinushi M, Tumor-infiltrating DCs suppress nucleic acid-mediated innate immune responses through interactions between the receptor TIM-3 and the alarmin HMGB1, Nat Immunol 13(9) (2012) 832–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Stanietsky N, Simic H, Arapovic J, Toporik A, Levy O, Novik A, Levine Z, Beiman M, Dassa L, Achdout H, Stern-Ginossar N, Tsukerman P, Jonjic S, Mandelboim O, The interaction of TIGIT with PVR and PVRL2 inhibits human NK cell cytotoxicity, Proc Natl Acad Sci U S A 106(42) (2009) 17858–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Yu X, Harden K, Gonzalez LC, Francesco M, Chiang E, Irving B, Tom I, Ivelja S, Refino CJ, Clark H, Eaton D, Grogan JL, The surface protein TIGIT suppresses T cell activation by promoting the generation of mature immunoregulatory dendritic cells, Nat Immunol 10(1) (2009) 48–57. [DOI] [PubMed] [Google Scholar]
- [19].Dougall WC, Kurtulus S, Smyth MJ, Anderson AC, TIGIT and CD96: new checkpoint receptor targets for cancer immunotherapy, Immunol Rev 276(1) (2017) 112–120. [DOI] [PubMed] [Google Scholar]
- [20].Zhu Y, Paniccia A, Schulick AC, Chen W, Koenig MR, Byers JT, Yao S, Bevers S, Edil BH, Identification of CD112R as a novel checkpoint for human T cells, J Exp Med 213(2) (2016) 167–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Monney L, Sabatos CA, Gaglia JL, Ryu A, Waldner H, Chernova T, Manning S, Greenfield EA, Coyle AJ, Sobel RA, Freeman GJ, Kuchroo VK, Th1-specific cell surface protein Tim-3 regulates macrophage activation and severity of an autoimmune disease, Nature 415(6871) (2002) 536–41. [DOI] [PubMed] [Google Scholar]
- [22].Meyers JH, Sabatos CA, Chakravarti S, Kuchroo VK, The TIM gene family regulates autoimmune and allergic diseases, Trends Mol Med 11(8) (2005) 362–9. [DOI] [PubMed] [Google Scholar]
- [23].Gao X, Zhu Y, Li G, Huang H, Zhang G, Wang F, Sun J, Yang Q, Zhang X, Lu B, TIM-3 expression characterizes regulatory T cells in tumor tissues and is associated with lung cancer progression, PLoS One 7(2) (2012) e30676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Hastings WD, Anderson DE, Kassam N, Koguchi K, Greenfield EA, Kent SC, Zheng XX, Strom TB, Hafler DA, Kuchroo VK, TIM-3 is expressed on activated human CD4+ T cells and regulates Th1 and Th17 cytokines, Eur J Immunol 39(9) (2009) 2492–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Boenisch O, D’Addio F, Watanabe T, Elyaman W, Magee CN, Yeung MY, Padera RF, Rodig SJ, Murayama T, Tanaka K, Yuan X, Ueno T, Jurisch A, Mfarrej B, Akiba H, Yagita H, Najafian N, TIM-3: a novel regulatory molecule of alloimmune activation, J Immunol 185(10) (2010) 5806–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Anderson AC, Anderson DE, Bregoli L, Hastings WD, Kassam N, Lei C, Chandwaskar R, Karman J, Su EW, Hirashima M, Bruce JN, Kane LP, Kuchroo VK, Hafler DA, Promotion of tissue inflammation by the immune receptor Tim-3 expressed on innate immune cells, Science 318(5853) (2007) 1141–3. [DOI] [PubMed] [Google Scholar]
- [27].Ndhlovu LC, Lopez-Verges S, Barbour JD, Jones RB, Jha AR, Long BR, Schoeffler EC, Fujita T, Nixon DF, Lanier LL, Tim-3 marks human natural killer cell maturation and suppresses cell-mediated cytotoxicity, Blood 119(16) (2012) 3734–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Yan W, Liu X, Ma H, Zhang H, Song X, Gao L, Liang X, Ma C, Tim-3 fosters HCC development by enhancing TGF-beta-mediated alternative activation of macrophages, Gut 64(10) (2015) 1593–604. [DOI] [PubMed] [Google Scholar]
- [29].Jiang X, Yu J, Shi Q, Xiao Y, Wang W, Chen G, Zhao Z, Wang R, Xiao H, Hou C, Feng J, Ma Y, Shen B, Wang L, Li Y, Han G, Tim-3 promotes intestinal homeostasis in DSS colitis by inhibiting M1 polarization of macrophages, Clin Immunol 160(2) (2015) 328–35. [DOI] [PubMed] [Google Scholar]
- [30].Phong BL, Avery L, Sumpter TL, Gorman JV, Watkins SC, Colgan JD, Kane LP, Tim-3 enhances FcepsilonRI-proximal signaling to modulate mast cell activation, J Exp Med 212(13) (2015) 2289–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Wiener Z, Kohalmi B, Pocza P, Jeager J, Tolgyesi G, Toth S, Gorbe E, Papp Z, Falus A, TIM-3 is expressed in melanoma cells and is upregulated in TGF-beta stimulated mast cells, J Invest Dermatol 127(4) (2007) 906–14. [DOI] [PubMed] [Google Scholar]
- [32].Zhang J, Zhu ZQ, Li YX, Zhuang QF, Lai Y, Li SF, Xu XB, Liu JM, Tim-3 expression in glioma cells is associated with drug resistance, J Cancer Res Ther 15(4) (2019) 882–888. [DOI] [PubMed] [Google Scholar]
- [33].Shang Y, Li Z, Li H, Xia H, Lin Z, TIM-3 expression in human osteosarcoma: Correlation with the expression of epithelial-mesenchymal transition-specific biomarkers, Oncol Lett 6(2) (2013) 490–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Cao Y, Zhou X, Huang X, Li Q, Gao L, Jiang L, Huang M, Zhou J, Tim-3 expression in cervical cancer promotes tumor metastasis, PLoS One 8(1) (2013) e53834. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [35].Komohara Y, Morita T, Annan DA, Horlad H, Ohnishi K, Yamada S, Nakayama T, Kitada S, Suzu S, Kinoshita I, Dosaka-Akita H, Akashi K, Takeya M, Jinushi M, The Coordinated Actions of TIM-3 on Cancer and Myeloid Cells in the Regulation of Tumorigenicity and Clinical Prognosis in Clear Cell Renal Cell Carcinomas, Cancer Immunol Res 3(9) (2015) 999–1007. [DOI] [PubMed] [Google Scholar]
- [36].Zhu C, Anderson AC, Schubart A, Xiong H, Imitola J, Khoury SJ, Zheng XX, Strom TB, Kuchroo VK, The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity, Nat Immunol 6(12) (2005) 1245–52. [DOI] [PubMed] [Google Scholar]
- [37].Huang YH, Zhu C, Kondo Y, Anderson AC, Gandhi A, Russell A, Dougan SK, Petersen BS, Melum E, Pertel T, Clayton KL, Raab M, Chen Q, Beauchemin N, Yazaki PJ, Pyzik M, Ostrowski MA, Glickman JN, Rudd CE, Ploegh HL, Franke A, Petsko GA, Kuchroo VK, Blumberg RS, CEACAM1 regulates TIM-3-mediated tolerance and exhaustion, Nature 517(7534) (2015) 386–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].DeKruyff RH, Bu X, Ballesteros A, Santiago C, Chim YL, Lee HH, Karisola P, Pichavant M, Kaplan GG, Umetsu DT, Freeman GJ, Casasnovas JM, T cell/transmembrane, Ig, and mucin-3 allelic variants differentially recognize phosphatidylserine and mediate phagocytosis of apoptotic cells, J Immunol 184(4) (2010) 1918–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Wada J, Kanwar YS, Identification and characterization of galectin-9, a novel beta-galactoside-binding mammalian lectin, J Biol Chem 272(9) (1997) 6078–86. [DOI] [PubMed] [Google Scholar]
- [40].Tang R, Rangachari M, Kuchroo VK, Tim-3: A co-receptor with diverse roles in T cell exhaustion and tolerance, Semin Immunol 42 (2019) 101302. [DOI] [PubMed] [Google Scholar]
- [41].Kang CW, Dutta A, Chang LY, Mahalingam J, Lin YC, Chiang JM, Hsu CY, Huang CT, Su WT, Chu YY, Lin CY, Apoptosis of tumor infiltrating effector TIM-3+CD8+ T cells in colon cancer, Sci Rep 5 (2015) 15659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Li H, Wu K, Tao K, Chen L, Zheng Q, Lu X, Liu J, Shi L, Liu C, Wang G, Zou W, Tim-3/galectin-9 signaling pathway mediates T-cell dysfunction and predicts poor prognosis in patients with hepatitis B virus-associated hepatocellular carcinoma, Hepatology 56(4) (2012) 1342–51. [DOI] [PubMed] [Google Scholar]
- [43].Dardalhon V, Anderson AC, Karman J, Apetoh L, Chandwaskar R, Lee DH, Cornejo M, Nishi N, Yamauchi A, Quintana FJ, Sobel RA, Hirashima M, Kuchroo VK, Tim-3/galectin-9 pathway: regulation of Th1 immunity through promotion of CD11b+Ly-6G+ myeloid cells, J Immunol 185(3) (2010) 1383–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Kikushige Y, Miyamoto T, Yuda J, Jabbarzadeh-Tabrizi S, Shima T, Takayanagi S, Niiro H, Yurino A, Miyawaki K, Takenaka K, Iwasaki H, Akashi K, A TIM-3/Gal-9 Autocrine Stimulatory Loop Drives Self-Renewal of Human Myeloid Leukemia Stem Cells and Leukemic Progression, Cell Stem Cell 17(3) (2015) 341–52. [DOI] [PubMed] [Google Scholar]
- [45].Su EW, Bi S, Kane LP, Galectin-9 regulates T helper cell function independently of Tim-3, Glycobiology 21(10) (2011) 1258–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Oomizu S, Arikawa T, Niki T, Kadowaki T, Ueno M, Nishi N, Yamauchi A, Hirashima M, Galectin-9 suppresses Th17 cell development in an IL-2-dependent but Tim-3-independent manner, Clin Immunol 143(1) (2012) 51–8. [DOI] [PubMed] [Google Scholar]
- [47].Wu C, Thalhamer T, Franca RF, Xiao S, Wang C, Hotta C, Zhu C, Hirashima M, Anderson AC, Kuchroo VK, Galectin-9-CD44 interaction enhances stability and function of adaptive regulatory T cells, Immunity 41(2) (2014) 270–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Freeman GJ, Casasnovas JM, Umetsu DT, DeKruyff RH, TIM genes: a family of cell surface phosphatidylserine receptors that regulate innate and adaptive immunity, Immunol Rev 235(1) (2010) 172–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Sims GP, Rowe DC, Rietdijk ST, Herbst R, Coyle AJ, HMGB1 and RAGE in inflammation and cancer, Annu Rev Immunol 28 (2010) 367–88. [DOI] [PubMed] [Google Scholar]
- [50].Yanai H, Ban T, Wang Z, Choi MK, Kawamura T, Negishi H, Nakasato M, Lu Y, Hangai S, Koshiba R, Savitsky D, Ronfani L, Akira S, Bianchi ME, Honda K, Tamura T, Kodama T, Taniguchi T, HMGB proteins function as universal sentinels for nucleic-acid-mediated innate immune responses, Nature 462(7269) (2009) 99–103. [DOI] [PubMed] [Google Scholar]
- [51].Cao E, Zang X, Ramagopal UA, Mukhopadhaya A, Fedorov A, Fedorov E, Zencheck WD, Lary JW, Cole JL, Deng H, Xiao H, Dilorenzo TP, Allison JP, Nathenson SG, Almo SC, T cell immunoglobulin mucin-3 crystal structure reveals a galectin-9-independent ligand-binding surface, Immunity 26(3) (2007) 311–21. [DOI] [PubMed] [Google Scholar]
- [52].Kammerer R, Stober D, Singer BB, Obrink B, Reimann J, Carcinoembryonic antigen-related cell adhesion molecule 1 on murine dendritic cells is a potent regulator of T cell stimulation, J Immunol 166(11) (2001) 6537–44. [DOI] [PubMed] [Google Scholar]
- [53].Horst AK, Bickert T, Brewig N, Ludewig P, van Rooijen N, Schumacher U, Beauchemin N, Ito WD, Fleischer B, Wagener C, Ritter U, CEACAM1+ myeloid cells control angiogenesis in inflammation, Blood 113(26) (2009) 6726–36. [DOI] [PubMed] [Google Scholar]
- [54].Coutelier JP, Godfraind C, Dveksler GS, Wysocka M, Cardellichio CB, Noel H, Holmes KV, B lymphocyte and macrophage expression of carcinoembryonic antigen-related adhesion molecules that serve as receptors for murine coronavirus, Eur J Immunol 24(6) (1994) 1383–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Sabatos-Peyton CA, Nevin J, Brock A, Venable JD, Tan DJ, Kassam N, Xu F, Taraszka J, Wesemann L, Pertel T, Acharya N, Klapholz M, Etminan Y, Jiang X, Huang YH, Blumberg RS, Kuchroo VK, Anderson AC, Blockade of Tim-3 binding to phosphatidylserine and CEACAM1 is a shared feature of anti-Tim-3 antibodies that have functional efficacy, Oncoimmunology 7(2) (2018) e1385690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Lee J, Su EW, Zhu C, Hainline S, Phuah J, Moroco JA, Smithgall TE, Kuchroo VK, Kane LP, Phosphotyrosine-dependent coupling of Tim-3 to T-cell receptor signaling pathways, Mol Cell Biol 31(19) (2011) 3963–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].van de Weyer PS, Muehlfeit M, Klose C, Bonventre JV, Walz G, Kuehn EW, A highly conserved tyrosine of Tim-3 is phosphorylated upon stimulation by its ligand galectin-9, Biochem Biophys Res Commun 351(2) (2006) 571–6. [DOI] [PubMed] [Google Scholar]
- [58].Avery L, Filderman J, Szymczak-Workman AL, Kane LP, Tim-3 co-stimulation promotes short-lived effector T cells, restricts memory precursors, and is dispensable for T cell exhaustion, Proc Natl Acad Sci U S A 115(10) (2018) 2455–2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Davidson D, Schraven B, Veillette A, PAG-associated FynT regulates calcium signaling and promotes anergy in T lymphocytes, Mol Cell Biol 27(5) (2007) 1960–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Das M, Zhu C, Kuchroo VK, Tim-3 and its role in regulating anti-tumor immunity, Immunol Rev 276(1) (2017) 97–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Kanzaki M, Wada J, Sugiyama K, Nakatsuka A, Teshigawara S, Murakami K, Inoue K, Terami T, Katayama A, Eguchi J, Akiba H, Yagita H, Makino H, Galectin-9 and T cell immunoglobulin mucin-3 pathway is a therapeutic target for type 1 diabetes, Endocrinology 153(2) (2012) 612–20. [DOI] [PubMed] [Google Scholar]
- [62].Koguchi K, Anderson DE, Yang L, O’Connor KC, Kuchroo VK, Hafler DA, Dysregulated T cell expression of TIM3 in multiple sclerosis, J Exp Med 203(6) (2006) 1413–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].McMahan RH, Golden-Mason L, Nishimura MI, McMahon BJ, Kemper M, Allen TM, Gretch DR, Rosen HR, Tim-3 expression on PD-1+ HCV-specific human CTLs is associated with viral persistence, and its blockade restores hepatocyte-directed in vitro cytotoxicity, J Clin Invest 120(12) (2010) 4546–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Jin HT, Anderson AC, Tan WG, West EE, Ha SJ, Araki K, Freeman GJ, Kuchroo VK, Ahmed R, Cooperation of Tim-3 and PD-1 in CD8 T-cell exhaustion during chronic viral infection, Proc Natl Acad Sci U S A 107(33) (2010) 14733–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Ju Y, Hou N, Zhang XN, Zhao D, Liu Y, Wang JJ, Luan F, Shi W, Zhu FL, Sun WS, Zhang LN, Gao CJ, Gao LF, Liang XH, Ma CH, Blockade of Tim-3 pathway ameliorates interferon-gamma production from hepatic CD8+ T cells in a mouse model of hepatitis B virus infection, Cell Mol Immunol 6(1) (2009) 35–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Takamura S, Tsuji-Kawahara S, Yagita H, Akiba H, Sakamoto M, Chikaishi T, Kato M, Miyazawa M, Premature terminal exhaustion of Friend virus-specific effector CD8+ T cells by rapid induction of multiple inhibitory receptors, J Immunol 184(9) (2010) 4696–707. [DOI] [PubMed] [Google Scholar]
- [67].Wu W, Shi Y, Li J, Chen F, Chen Z, Zheng M, Tim-3 expression on peripheral T cell subsets correlates with disease progression in hepatitis B infection, Virol J 8 (2011) 113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Fourcade J, Sun Z, Pagliano O, Chauvin JM, Sander C, Janjic B, Tarhini AA, Tawbi HA, Kirkwood JM, Moschos S, Wang H, Guillaume P, Luescher IF, Krieg A, Anderson AC, Kuchroo VK, Zarour HM, PD-1 and Tim-3 regulate the expansion of tumor antigen-specific CD8(+) T cells induced by melanoma vaccines, Cancer Res 74(4) (2014) 1045–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Sakuishi K, Apetoh L, Sullivan JM, Blazar BR, Kuchroo VK, Anderson AC, Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity, J Exp Med 207(10) (2010) 2187–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Zhou Q, Munger ME, Veenstra RG, Weigel BJ, Hirashima M, Munn DH, Murphy WJ, Azuma M, Anderson AC, Kuchroo VK, Blazar BR, Coexpression of Tim-3 and PD-1 identifies a CD8+ T-cell exhaustion phenotype in mice with disseminated acute myelogenous leukemia, Blood 117(17) (2011) 4501–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Thommen DS, Schreiner J, Muller P, Herzig P, Roller A, Belousov A, Umana P, Pisa P, Klein C, Bacac M, Fischer OS, Moersig W, Savic Prince S, Levitsky V, Karanikas V, Lardinois D, Zippelius A, Progression of Lung Cancer Is Associated with Increased Dysfunction of T Cells Defined by Coexpression of Multiple Inhibitory Receptors, Cancer Immunol Res 3(12) (2015) 1344–55. [DOI] [PubMed] [Google Scholar]
- [72].Granier C, Dariane C, Combe P, Verkarre V, Urien S, Badoual C, Roussel H, Mandavit M, Ravel P, Sibony M, Biard L, Radulescu C, Vinatier E, Benhamouda N, Peyromaure M, Oudard S, Mejean A, Timsit MO, Gey A, Tartour E, Tim-3 Expression on Tumor-Infiltrating PD-1(+)CD8(+) T Cells Correlates with Poor Clinical Outcome in Renal Cell Carcinoma, Cancer Res 77(5) (2017) 1075–1082. [DOI] [PubMed] [Google Scholar]
- [73].Li Z, Li N, Li F, Zhou Z, Sang J, Chen Y, Han Q, Lv Y, Liu Z, Immune checkpoint proteins PD-1 and TIM-3 are both highly expressed in liver tissues and correlate with their gene polymorphisms in patients with HBV-related hepatocellular carcinoma, Medicine (Baltimore) 95(52) (2016) e5749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Koyama S, Akbay EA, Li YY, Herter-Sprie GS, Buczkowski KA, Richards WG, Gandhi L, Redig AJ, Rodig SJ, Asahina H, Jones RE, Kulkarni MM, Kuraguchi M, Palakurthi S, Fecci PE, Johnson BE, Janne PA, Engelman JA, Gangadharan SP, Costa DB, Freeman GJ, Bueno R, Hodi FS, Dranoff G, Wong KK, Hammerman PS, Adaptive resistance to therapeutic PD-1 blockade is associated with upregulation of alternative immune checkpoints, Nat Commun 7 (2016) 10501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Sakuishi K, Ngiow SF, Sullivan JM, Teng MW, Kuchroo VK, Smyth MJ, Anderson AC, TIM3(+)FOXP3(+) regulatory T cells are tissue-specific promoters of T-cell dysfunction in cancer, Oncoimmunology 2(4) (2013) e23849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Yan J, Zhang Y, Zhang JP, Liang J, Li L, Zheng L, Tim-3 expression defines regulatory T cells in human tumors, PLoS One 8(3) (2013) e58006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].da Silva IP, Gallois A, Jimenez-Baranda S, Khan S, Anderson AC, Kuchroo VK, Osman I, Bhardwaj N, Reversal of NK-cell exhaustion in advanced melanoma by Tim-3 blockade, Cancer Immunol Res 2(5) (2014) 410–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Chauvin JM, Ka M, Pagliano O, Menna C, Ding Q, DeBlasio R, Sanders C, Hou J, Li XY, Ferrone S, Davar D, Kirkwood JM, Johnston RJ, Korman AJ, Smyth MJ, Zarour HM, IL15 Stimulation with TIGIT Blockade Reverses CD155-mediated NK-Cell Dysfunction in Melanoma, Clin Cancer Res (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Mamessier E, Sylvain A, Bertucci F, Castellano R, Finetti P, Houvenaeghel G, Charaffe-Jaufret E, Birnbaum D, Moretta A, Olive D, Human Breast Tumor Cells Induce Self-Tolerance Mechanisms to Avoid NKG2D-Mediated and DNAM-Mediated NK Cell Recognition, Cancer Research 71(21) (2011) 6621–6632. [DOI] [PubMed] [Google Scholar]
- [80].Mamessier E, Sylvain A, Thibult ML, Houvenaeghel G, Jacquemier J, Castellano R, Goncalves A, Andre P, Romagne F, Thibault G, Viens P, Birnbaum D, Bertucci F, Moretta A, Olive D, Human breast cancer cells enhance self tolerance by promoting evasion from NK cell antitumor immunity, J Clin Invest 121(9) (2011) 3609–3622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Platonova S, Cherfils-Vicini J, Damotte D, Crozet L, Vieillard V, Validire P, Andre P, Dieu-Nosjean MC, Alifano M, Regnard JF, Fridman WH, Sautes-Fridman C, Cremer I, Profound coordinated alterations of intratumoral NK cell phenotype and function in lung carcinoma, Cancer Res 71(16) (2011) 5412–22. [DOI] [PubMed] [Google Scholar]
- [82].Carlsten M, Norell H, Bryceson YT, Poschke I, Schedvins K, Ljunggren HG, Kiessling R, Malmberg KJ, Primary human tumor cells expressing CD155 impair tumor targeting by down-regulating DNAM-1 on NK cells, J Immunol 183(8) (2009) 4921–30. [DOI] [PubMed] [Google Scholar]
- [83].Fregni G, Messaoudene M, Fourmentraux-Neves E, Mazouz-Dorval S, Chanal J, Maubec E, Marinho E, Scheer-Senyarich I, Cremer I, Avril MF, Caignard A, Phenotypic and functional characteristics of blood natural killer cells from melanoma patients at different clinical stages, Plos One 8(10) (2013) e76928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Pasero C, Gravis G, Guerin M, Granjeaud S, Thomassin-Piana J, Rocchi P, Paciencia-Gros M, Poizat F, Bentobji M, Azario-Cheillan F, Walz J, Salem N, Brunelle S, Moretta A, Olive D, Inherent and Tumor-Driven Immune Tolerance in the Prostate Microenvironment Impairs Natural Killer Cell Antitumor Activity, Cancer Res 76(8) (2016) 2153–65. [DOI] [PubMed] [Google Scholar]
- [85].de Mingo Pulido A, Gardner A, Hiebler S, Soliman H, Rugo HS, Krummel MF, Coussens LM, Ruffell B, TIM-3 Regulates CD103(+) Dendritic Cell Function and Response to Chemotherapy in Breast Cancer, Cancer Cell 33(1) (2018) 60–74 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Limagne E, Richard C, Thibaudin M, Fumet JD, Truntzer C, Lagrange A, Favier L, Coudert B, Ghiringhelli F, Tim-3/galectin-9 pathway and mMDSC control primary and secondary resistances to PD-1 blockade in lung cancer patients, Oncoimmunology 8(4) (2019) e1564505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Moller-Hackbarth K, Dewitz C, Schweigert O, Trad A, Garbers C, Rose-John S, Scheller J, A disintegrin and metalloprotease (ADAM) 10 and ADAM17 are major sheddases of T cell immunoglobulin and mucin domain 3 (Tim-3), J Biol Chem 288(48) (2013) 34529–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Li F, Li N, Sang J, Fan X, Deng H, Zhang X, Han Q, Lv Y, Liu Z, Highly elevated soluble Tim-3 levels correlate with increased hepatocellular carcinoma risk and poor survival of hepatocellular carcinoma patients in chronic hepatitis B virus infection, Cancer Manag Res 10 (2018) 941–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Geng H, Zhang GM, Li D, Zhang H, Yuan Y, Zhu HG, Xiao H, Han LF, Feng ZH, Soluble form of T cell Ig mucin 3 is an inhibitory molecule in T cell-mediated immune response, J Immunol 176(3) (2006) 1411–20. [DOI] [PubMed] [Google Scholar]
- [90].Akashi K, TIM-3 Is a Novel Therapeutic Target for Eradicating Acute Myelogenous Leukemia Stem Cells, in: Nakao K, Minato N, Uemoto S (Eds.), Innovative Medicine: Basic Research and Development, Tokyo, 2015, pp. 307–315. [PubMed] [Google Scholar]
- [91].Kikushige Y, Miyamoto T, Identification of TIM-3 as a Leukemic Stem Cell Surface Molecule in Primary Acute Myeloid Leukemia, Oncology 89 Suppl 1 (2015) 28–32. [DOI] [PubMed] [Google Scholar]
- [92].Jan M, Chao MP, Cha AC, Alizadeh AA, Gentles AJ, Weissman IL, Majeti R, Prospective separation of normal and leukemic stem cells based on differential expression of TIM3, a human acute myeloid leukemia stem cell marker, Proc Natl Acad Sci U S A 108(12) (2011) 5009–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Haubner S, Perna F, Kohnke T, Schmidt C, Berman S, Augsberger C, Schnorfeil FM, Krupka C, Lichtenegger FS, Liu X, Kerbs P, Schneider S, Metzeler KH, Spiekermann K, Hiddemann W, Greif PA, Herold T, Sadelain M, Subklewe M, Coexpression profile of leukemic stem cell markers for combinatorial targeted therapy in AML, Leukemia 33(1) (2019) 64–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Kikushige Y, Shima T, Takayanagi S, Urata S, Miyamoto T, Iwasaki H, Takenaka K, Teshima T, Tanaka T, Inagaki Y, Akashi K, TIM-3 is a promising target to selectively kill acute myeloid leukemia stem cells, Cell Stem Cell 7(6) (2010) 708–17. [DOI] [PubMed] [Google Scholar]
- [95].Zhang Y, Cai P, Liang T, Wang L, Hu L, TIM-3 is a potential prognostic marker for patients with solid tumors: A systematic review and meta-analysis, Oncotarget 8(19) (2017) 31705–31713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Lee J, Phong B, Egloff AM, Kane LP, TIM polymorphisms--genetics and function, Genes Immun 12(8) (2011) 595–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Gao X, Yang J, He Y, Zhang J, Quantitative assessment of TIM-3 polymorphisms and cancer risk in Chinese Han population, Oncotarget 7(24) (2016) 35768–35775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Wang Z, Liu X, Wang X, Chong T, Lin S, Wang M, Ma X, Liu K, Xu P, Feng Y, Dai Z, Polymorphisms in TIM-3 and breast cancer susceptibility in Chinese women: A case-control study, Oncotarget 7(28) (2016) 43703–43712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Cai C, Wang L, Wu Z, Li M, Chen W, Sun Y, T-cell immunoglobulin- and mucin-domain-containing molecule 3 gene polymorphisms and renal cell carcinoma, DNA Cell Biol 31(7) (2012) 1285–9. [DOI] [PubMed] [Google Scholar]
- [100].Tong D, Zhou Y, Chen W, Deng Y, Li L, Jia Z, Qi D, T cell immunoglobulinand mucin-domain-containing molecule 3 gene polymorphisms and susceptibility to pancreatic cancer, Mol Biol Rep 39(11) (2012) 9941–6. [DOI] [PubMed] [Google Scholar]
- [101].Bai J, Li X, Tong D, Shi W, Song H, Li Q, T-cell immunoglobulin- and mucin-domain-containing molecule 3 gene polymorphisms and prognosis of non-small-cell lung cancer, Tumour Biol 34(2) (2013) 805–9. [DOI] [PubMed] [Google Scholar]
- [102].Gayden T, Sepulveda FE, Khuong-Quang DA, Pratt J, Valera ET, Garrigue A, Kelso S, Sicheri F, Mikael LG, Hamel N, Bajic A, Dali R, Deshmukh S, Dervovic D, Schramek D, Guerin F, Taipale M, Nikbakht H, Majewski J, Moshous D, Charlebois J, Abish S, Bole-Feysot C, Nitschke P, Bader-Meunier B, Mitchell D, Thieblemont C, Battistella M, Gravel S, Nguyen VH, Conyers R, Diana JS, McCormack C, Prince HM, Besnard M, Blanche S, Ekert PG, Fraitag S, Foulkes WD, Fischer A, Neven B, Michonneau D, de Saint Basile G, Jabado N, Germline HAVCR2 mutations altering TIM-3 characterize subcutaneous panniculitis-like T cell lymphomas with hemophagocytic lymphohistiocytic syndrome, Nat Genet 50(12) (2018) 1650–1657. [DOI] [PubMed] [Google Scholar]
- [103].Dixon KO, Das M, Kuchroo VK, Human disease mutations highlight the inhibitory function of TIM-3, Nat Genet 50(12) (2018) 1640–1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Wolf Y, Anderson AC, Kuchroo VK, TIM3 comes of age as an inhibitory receptor, Nat Rev Immunol 20(3) (2020) 173–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Yun SJ, Lee B, Komori K, Lee MJ, Lee BG, Kim K, Park S, Regulation of TIM-3 expression in a human T cell line by tumor-conditioned media and cyclic AMP-dependent signaling, Mol Immunol 105 (2019) 224–232. [DOI] [PubMed] [Google Scholar]
- [106].Sasidharan Nair V, El Salhat H, Taha RZ, John A, Ali BR, Elkord E, DNA methylation and repressive H3K9 and H3K27 trimethylation in the promoter regions of PD-1, CTLA-4, TIM-3, LAG-3, TIGIT, and PD-L1 genes in human primary breast cancer, Clin Epigenetics 10 (2018) 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Sasidharan Nair V, Toor SM, Taha RZ, Shaath H, Elkord E, DNA methylation and repressive histones in the promoters of PD-1, CTLA-4, TIM-3, LAG-3, TIGIT, PD-L1, and galectin-9 genes in human colorectal cancer, Clin Epigenetics 10(1) (2018) 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Davar D, Boasberg P, Eroglu Z, Falchook G, Gainor J, Hamilton E, A phase 1 study of TSR-022, an anti-TIM-3 monoclonal antibody, in combination with TSR-042 (anti-PD-1) in patients with colorectal cancer and post-PD-1 NSCLC and melanoma, J Immunother Cancer 6(Suppl 1) (2018) 115O21.30400822 [Google Scholar]
- [109].Ahn M, MS28. 02 Combination IO+ IO, Journal of Thoracic Oncology 13(10) (2018) S299–S300. [Google Scholar]
- [110].Bottino C, Castriconi R, Pende D, Rivera P, Nanni M, Carnemolla B, Cantoni C, Grassi J, Marcenaro S, Reymond N, Vitale M, Moretta L, Lopez M, Moretta A, Identification of PVR (CD155) and Nectin-2 (CD112) as cell surface ligands for the human DNAM-1 (CD226) activating molecule, J Exp Med 198(4) (2003) 557–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Seth S, Maier MK, Qiu Q, Ravens I, Kremmer E, Forster R, Bernhardt G, The murine pan T cell marker CD96 is an adhesion receptor for CD155 and nectin-1, Biochem Biophys Res Commun 364(4) (2007) 959–65. [DOI] [PubMed] [Google Scholar]
- [112].Alegre ML, Frauwirth KA, Thompson CB, T-cell regulation by CD28 and CTLA-4, Nat Rev Immunol 1(3) (2001) 220–8. [DOI] [PubMed] [Google Scholar]
- [113].Levin SD, Taft DW, Brandt CS, Bucher C, Howard ED, Chadwick EM, Johnston J, Hammond A, Bontadelli K, Ardourel D, Hebb L, Wolf A, Bukowski TR, Rixon MW, Kuijper JL, Ostrander CD, West JW, Bilsborough J, Fox B, Gao Z, Xu W, Ramsdell F, Blazar BR, Lewis KE, Vstm3 is a member of the CD28 family and an important modulator of T-cell function, Eur J Immunol 41(4) (2011) 902–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Boles KS, Vermi W, Facchetti F, Fuchs A, Wilson TJ, Diacovo TG, Cella M, Colonna M, A novel molecular interaction for the adhesion of follicular CD4 T cells to follicular DC, Eur J Immunol 39(3) (2009) 695–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Joller N, Hafler JP, Brynedal B, Kassam N, Spoerl S, Levin SD, Sharpe AH, Kuchroo VK, Cutting Edge: TIGIT Has T Cell-Intrinsic Inhibitory Functions, Journal of Immunology 186(3) (2011) 1338–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Wu H, Chen Y, Liu H, Xu LL, Teuscher P, Wang S, Lu S, Dent AL, Follicular regulatory T cells repress cytokine production by follicular helper T cells and optimize IgG responses in mice, Eur J Immunol 46(5) (2016) 1152–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Bevington SL, Ng STH, Britton GJ, Keane P, Wraith DC, Cockerill PN, Chromatin Priming Renders T Cell Tolerance-Associated Genes Sensitive to Activation below the Signaling Threshold for Immune Response Genes, Cell Rep 31(10) (2020) 107748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Joller N, Lozano E, Burkett PR, Patel B, Xiao S, Zhu C, Xia J, Tan TG, Sefik E, Yajnik V, Sharpe AH, Quintana FJ, Mathis D, Benoist C, Hafler DA, Kuchroo VK, Treg cells expressing the coinhibitory molecule TIGIT selectively inhibit proinflammatory Th1 and Th17 cell responses, Immunity 40(4) (2014) 569–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Fourcade J, Sun Z, Chauvin JM, Ka M, Davar D, Pagliano O, Wang H, Saada S, Menna C, Amin R, Sander C, Kirkwood JM, Korman AJ, Zarour HM, CD226 opposes TIGIT to disrupt Tregs in melanoma, JCI Insight 3(14) (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Chauvin JM, Pagliano O, Fourcade J, Sun ZJ, Wang H, Sander C, Kirkwood JM, Chen THT, Maurer M, Korman AJ, Zarour HM, TIGIT and PD-1 impair tumor antigen-specific CD8(+) T cells in melanoma patients, Journal of Clinical Investigation 125(5) (2015) 2046–2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Johnston RJ, Comps-Agrar L, Hackney J, Yu X, Huseni M, Yang Y, Park S, Javinal V, Chiu H, Irving B, Eaton DL, Grogan JL, The immunoreceptor TIGIT regulates antitumor and antiviral CD8(+) T cell effector function, Cancer Cell 26(6) (2014) 923–937. [DOI] [PubMed] [Google Scholar]
- [122].Zhang Y, Maksimovic J, Naselli G, Qian J, Chopin M, Blewitt ME, Oshlack A, Harrison LC, Genome-wide DNA methylation analysis identifies hypomethylated genes regulated by FOXP3 in human regulatory T cells, Blood 122(16) (2013) 2823–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Wang F, Hou H, Wu S, Tang Q, Liu W, Huang M, Yin B, Huang J, Mao L, Lu Y, Sun Z, TIGIT expression levels on human NK cells correlate with functional heterogeneity among healthy individuals, Eur J Immunol 45(10) (2015) 2886–97. [DOI] [PubMed] [Google Scholar]
- [124].JM C, Ka M, P. O, M. C, D. Q, D. R, S. C, H. J, L. XY, Ferrone S, D. D, K. JM, J. R, K. AJ, S. MJ, Z. HM, IL-15 together with TIGIT blockade reverses PVR-mediated NK cell dysfunction in melanoma., Clin Cancer Res (In Press) (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Fuchs A, Cella M, Giurisato E, Shaw AS, Colonna M, Cutting edge: CD96 (tactile) promotes NK cell-target cell adhesion by interacting with the poliovirus receptor (CD155), J Immunol 172(7) (2004) 3994–8. [DOI] [PubMed] [Google Scholar]
- [126].Lozano E, Dominguez-Villar M, Kuchroo V, Hafler DA, The TIGIT/CD226 Axis Regulates Human T Cell Function, Journal of Immunology 188(8) (2012) 3869–3875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Pende D, Bottino C, Castriconi R, Cantoni C, Marcenaro S, Rivera P, Spaggiari GM, Dondero A, Carnemolla B, Reymond N, Mingari MC, Lopez M, Moretta L, Moretta A, PVR (CD155) and Nectin-2 (CD112) as ligands of the human DNAM-1 (CD226) activating receptor: involvement in tumor cell lysis, Mol Immunol 42(4) (2005) 463–9. [DOI] [PubMed] [Google Scholar]
- [128].Ardolino M, Zingoni A, Cerboni C, Cecere F, Soriani A, Iannitto ML, Santoni A, DNAM-1 ligand expression on Ag-stimulated T lymphocytes is mediated by ROS-dependent activation of DNA-damage response: relevance for NK-T cell interaction, Blood 117(18) (2011) 4778–86. [DOI] [PubMed] [Google Scholar]
- [129].Sarhan D, Cichocki F, Zhang B, Yingst A, Spellman SR, Cooley S, Verneris MR, Blazar BR, Miller JS, Adaptive NK Cells with Low TIGIT Expression Are Inherently Resistant to Myeloid-Derived Suppressor Cells, Cancer Research 76(19) (2016) 5696–5706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Gur C, Ibrahim Y, Isaacson B, Yamin R, Abed J, Gamliel M, Enk J, Bar-On Y, Stanietsky-Kaynan N, Coppenhagen-Glazer S, Shussman N, Almogy G, Cuapio A, Hofer E, Mevorach D, Tabib A, Ortenberg R, Markel G, Miklic K, Jonjic S, Brennan CA, Garrett WS, Bachrach G, Mandelboim O, Binding of the Fap2 protein of Fusobacterium nucleatum to human inhibitory receptor TIGIT protects tumors from immune cell attack, Immunity 42(2) (2015) 344–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Liu S, Zhang H, Li M, Hu D, Li C, Ge B, Jin B, Fan Z, Recruitment of Grb2 and SHIP1 by the ITT-like motif of TIGIT suppresses granule polarization and cytotoxicity of NK cells, Cell Death Differ 20(3) (2013) 456–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Li M, Xia P, Du Y, Liu S, Huang G, Chen J, Zhang H, Hou N, Cheng X, Zhou L, Li P, Yang X, Fan Z, T-cell immunoglobulin and ITIM domain (TIGIT) receptor/poliovirus receptor (PVR) ligand engagement suppresses interferon-gamma production of natural killer cells via beta-arrestin 2-mediated negative signaling, J Biol Chem 289(25) (2014) 17647–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Stanietsky N, Rovis TL, Glasner A, Seidel E, Tsukerman P, Yamin R, Enk J, Jonjic S, Mandelboim O, Mouse TIGIT inhibits NK-cell cytotoxicity upon interaction with PVR, European Journal of Immunology 43(8) (2013) 2138–2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Shibuya A, Campbell D, Hannum C, Yssel H, Franz-Bacon K, McClanahan T, Kitamura T, Nicholl J, Sutherland GR, Lanier LL, Phillips JH, DNAM-1, a novel adhesion molecule involved in the cytolytic function of T lymphocytes, Immunity 4(6) (1996) 573–81. [DOI] [PubMed] [Google Scholar]
- [135].Kojima H, Kanada H, Shimizu S, Kasama E, Shibuya K, Nakauchi H, Nagasawa T, Shibuya A, CD226 mediates platelet and megakaryocytic cell adhesion to vascular endothelial cells, J Biol Chem 278(38) (2003) 36748–53. [DOI] [PubMed] [Google Scholar]
- [136].Lakshmikanth T, Burke S, Ali TH, Kimpfler S, Ursini F, Ruggeri L, Capanni M, Umansky V, Paschen A, Sucker A, Pende D, Groh V, Biassoni R, Hoglund P, Kato M, Shibuya K, Schadendorf D, Anichini A, Ferrone S, Velardi A, Karre K, Shibuya A, Carbone E, Colucci F, NCRs and DNAM-1 mediate NK cell recognition and lysis of human and mouse melanoma cell lines in vitro and in vivo, J Clin Invest 119(5) (2009) 1251–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Ramsbottom KM, Hawkins ED, Shimoni R, McGrath M, Chan CJ, Russell SM, Smyth MJ, Oliaro J, Cutting edge: DNAX accessory molecule 1-deficient CD8+ T cells display immunological synapse defects that impair antitumor immunity, J Immunol 192(2) (2014) 553–7. [DOI] [PubMed] [Google Scholar]
- [138].Kim JS, Shin BR, Lee HK, Lee JH, Kim KH, Choi JE, Ji AY, Hong JT, Kim Y, Han SB, Cd226(−/−) natural killer cells fail to establish stable contacts with cancer cells and show impaired control of tumor metastasis in vivo, Oncoimmunology 6(8) (2017) e1338994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Shibuya K, Lanier LL, Phillips JH, Ochs HD, Shimizu K, Nakayama E, Nakauchi H, Shibuya A, Physical and functional association of LFA-1 with DNAM-1 adhesion molecule, Immunity 11(5) (1999) 615–23. [DOI] [PubMed] [Google Scholar]
- [140].Wang B, Zhang W, Jankovic V, Golubov J, Poon P, Oswald EM, Gurer C, Wei J, Ramos I, Wu Q, Waite J, Ni M, Adler C, Wei Y, Macdonald L, Rowlands T, Brydges S, Siao J, Poueymirou W, MacDonald D, Yancopoulos GD, Sleeman MA, Murphy AJ, Skokos D, Combination cancer immunotherapy targeting PD-1 and GITR can rescue CD8(+) T cell dysfunction and maintain memory phenotype, Sci Immunol 3(29) (2018). [DOI] [PubMed] [Google Scholar]
- [141].Fuhrman CA, Yeh WI, Seay HR, Saikumar Lakshmi P, Chopra G, Zhang L, Perry DJ, McClymont SA, Yadav M, Lopez MC, Baker HV, Zhang Y, Li Y, Whitley M, von Schack D, Atkinson MA, Bluestone JA, Brusko TM, Divergent Phenotypes of Human Regulatory T Cells Expressing the Receptors TIGIT and CD226, J Immunol 195(1) (2015) 145–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Kurtulus S, Sakuishi K, Ngiow SF, Joller N, Tan DJ, Teng MW, Smyth MJ, Kuchroo VK, Anderson AC, TIGIT predominantly regulates the immune response via regulatory T cells, J Clin Invest 125(11) (2015) 4053–62. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [143].Zhang Q, Bi J, Zheng X, Chen Y, Wang H, Wu W, Wang Z, Wu Q, Peng H, Wei H, Sun R, Tian Z, Blockade of the checkpoint receptor TIGIT prevents NK cell exhaustion and elicits potent anti-tumor immunity, Nat Immunol 19(7) (2018) 723–732. [DOI] [PubMed] [Google Scholar]
- [144].He W, Zhang H, Han F, Chen X, Lin R, Wang W, Qiu H, Zhuang Z, Liao Q, Zhang W, Cai Q, Cui Y, Jiang W, Wang H, Ke Z, CD155T/TIGIT Signaling Regulates CD8(+) T-cell Metabolism and Promotes Tumor Progression in Human Gastric Cancer, Cancer Res 77(22) (2017) 6375–6388. [DOI] [PubMed] [Google Scholar]
- [145].Inozume T, Yaguchi T, Furuta J, Harada K, Kawakami Y, Shimada S, Melanoma Cells Control Antimelanoma CTL Responses via Interaction between TIGIT and CD155 in the Effector Phase, J Invest Dermatol 136(1) (2016) 255–63. [DOI] [PubMed] [Google Scholar]
- [146].Rodriguez-Abreu D, Primary analysis of a randomized, double-blind, phase II study of the anti-TIGIT antibody tiragolumab (tira) plus atezolizumab (atezo) versus placebo plus atezo as first-line (1L) treatment in patients with PD-L1-selected NSCLC (CITYSCAPE). (2020).
- [147].Kong Y, Zhu L, Schell TD, Zhang J, Claxton DF, Ehmann WC, Rybka WB, George MR, Zeng H, Zheng H, T-Cell Immunoglobulin and ITIM Domain (TIGIT) Associates with CD8+ T-Cell Exhaustion and Poor Clinical Outcome in AML Patients, Clin Cancer Res 22(12) (2016) 3057–66. [DOI] [PubMed] [Google Scholar]
- [148].Xu F, Sunderland A, Zhou Y, Schulick RD, Edil BH, Zhu Y, Blockade of CD112R and TIGIT signaling sensitizes human natural killer cell functions, Cancer Immunol Immunother 66(10) (2017) 1367–1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Dixon KO, Schorer M, Nevin J, Etminan Y, Amoozgar Z, Kondo T, Kurtulus S, Kassam N, Sobel RA, Fukumura D, Jain RK, Anderson AC, Kuchroo VK, Joller N, Functional Anti-TIGIT Antibodies Regulate Development of Autoimmunity and Antitumor Immunity, J Immunol 200(8) (2018) 3000–3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Waight JD, Chand D, Dietrich S, Gombos R, Horn T, Gonzalez AM, Manrique M, Swiech L, Morin B, Brittsan C, Tanne A, Akpeng B, Croker BA, Buell JS, Stein R, Savitsky DA, Wilson NS, Selective FcgammaR Co-engagement on APCs Modulates the Activity of Therapeutic Antibodies Targeting T Cell Antigens, Cancer Cell 33(6) (2018) 1033–1047 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Wang PL, O’Farrell S, Clayberger C, Krensky AM, Identification and molecular cloning of tactile. A novel human T cell activation antigen that is a member of the Ig gene superfamily, J Immunol 148(8) (1992) 2600–8. [PubMed] [Google Scholar]
- [152].Lepletier A, Lutzky VP, Mittal D, Stannard K, Watkins TS, Ratnatunga CN, Smith C, McGuire HM, Kemp RA, Mukhopadhyay P, Waddell N, Smyth MJ, Dougall WC, Miles JJ, The immune checkpoint CD96 defines a distinct lymphocyte phenotype and is highly expressed on tumor-infiltrating T cells, Immunol Cell Biol (2018). [DOI] [PubMed] [Google Scholar]
- [153].Hosen N, Park CY, Tatsumi N, Oji Y, Sugiyama H, Gramatzki M, Krensky AM, Weissman IL, CD96 is a leukemic stem cell-specific marker in human acute myeloid leukemia, Proc Natl Acad Sci U S A 104(26) (2007) 11008–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Holmes VM, Maluquer de Motes C, Richards PT, Roldan J, Bhargava AK, Orange JS, Krummenacher C, Interaction between nectin-1 and the human natural killer cell receptor CD96, PLoS One 14(2) (2019) e0212443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Deuss FA, Watson GM, Fu Z, Rossjohn J, Berry R, Structural Basis for CD96 Immune Receptor Recognition of Nectin-like Protein-5, CD155, Structure 27(2) (2019) 219–228 e3. [DOI] [PubMed] [Google Scholar]
- [156].Meyer D, Seth S, Albrecht J, Maier MK, du Pasquier L, Ravens I, Dreyer L, Burger R, Gramatzki M, Schwinzer R, Kremmer E, Foerster R, Bernhardt G, CD96 interaction with CD155 via its first Ig-like domain is modulated by alternative splicing or mutations in distal Ig-like domains, J Biol Chem 284(4) (2009) 2235–44. [DOI] [PubMed] [Google Scholar]
- [157].Carlsten M, Bjorkstrom NK, Norell H, Bryceson Y, van Hall T, Baumann BC, Hanson M, Schedvins K, Kiessling R, Ljunggren HG, Malmberg KJ, DNAX accessory molecule-1 mediated recognition of freshly isolated ovarian carcinoma by resting natural killer cells, Cancer Res 67(3) (2007) 1317–25. [DOI] [PubMed] [Google Scholar]
- [158].El-Sherbiny YM, Meade JL, Holmes TD, McGonagle D, Mackie SL, Morgan AW, Cook G, Feyler S, Richards SJ, Davies FE, Morgan GJ, Cook GP, The requirement for DNAM-1, NKG2D, and NKp46 in the natural killer cell-mediated killing of myeloma cells, Cancer Res 67(18) (2007) 8444–9. [DOI] [PubMed] [Google Scholar]
- [159].Chan CJ, Martinet L, Gilfillan S, Souza-Fonseca-Guimaraes F, Chow MT, Town L, Ritchie DS, Colonna M, Andrews DM, Smyth MJ, The receptors CD96 and CD226 oppose each other in the regulation of natural killer cell functions, Nat Immunol 15(5) (2014) 431–8. [DOI] [PubMed] [Google Scholar]
- [160].Blake SJ, Stannard K, Liu J, Allen S, Yong MC, Mittal D, Aguilera AR, Miles JJ, Lutzky VP, de Andrade LF, Martinet L, Colonna M, Takeda K, Kuhnel F, Gurlevik E, Bernhardt G, Teng MW, Smyth MJ, Suppression of Metastases Using a New Lymphocyte Checkpoint Target for Cancer Immunotherapy, Cancer Discov 6(4) (2016) 446–59. [DOI] [PubMed] [Google Scholar]
- [161].Blake SJ, Dougall WC, Miles JJ, Teng MW, Smyth MJ, Molecular Pathways: Targeting CD96 and TIGIT for Cancer Immunotherapy, Clin Cancer Res 22(21) (2016) 5183–5188. [DOI] [PubMed] [Google Scholar]
- [162].Li XY, Das I, Lepletier A, Addala V, Bald T, Stannard K, Barkauskas D, Liu J, Aguilera AR, Takeda K, Braun M, Nakamura K, Jacquelin S, Lane SW, Teng MW, Dougall WC, Smyth MJ, CD155 loss enhances tumor suppression via combined host and tumor-intrinsic mechanisms, J Clin Invest 128(6) (2018) 2613–2625. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [163].Chiang EY, de Almeida PE, de Almeida Nagata DE, Bowles KH, Du X, Chitre AS, Banta KL, Kwon Y, McKenzie B, Mittman S, Cubas R, Anderson KR, Warming S, Grogan JL, CD96 functions as a co-stimulatory receptor to enhance CD8(+) T cell activation and effector responses, Eur J Immunol (2020). [DOI] [PubMed] [Google Scholar]
- [164].Whelan S, Ophir E, Kotturi MF, Levy O, Ganguly S, Leung L, Vaknin I, Kumar S, Dassa L, Hansen K, Bernados D, Murter B, Soni A, Taube JM, Fader AN, Wang TL, Shih IM, White M, Pardoll DM, Liang SC, PVRIG and PVRL2 Are Induced in Cancer and Inhibit CD8(+) T-cell Function, Cancer Immunol Res 7(2) (2019) 257–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Murter B, Pan X, Ophir E, Alteber Z, Azulay M, Sen R, Levy O, Dassa L, Vaknin I, Fridman-Kfir T, Salomon R, Ravet A, Tam A, Levin D, Vaknin Y, Tatirovsky E, Machlenkin A, Pardoll D, Ganguly S, Mouse PVRIG Has CD8(+) T Cell-Specific Coinhibitory Functions and Dampens Antitumor Immunity, Cancer Immunol Res 7(2) (2019) 244–256. [DOI] [PMC free article] [PubMed] [Google Scholar]


