Abstract
Purpose
Natural Killer (NK) cells play a critical role in tumor immunosurveillance. Multiple activating and inhibitory receptors regulate NK cell-mediated tumor control. The inhibitory receptor TIGIT and its counter-receptor CD226 exert opposite effects on NK cell-mediated tumor reactivity.
Experimental design
We evaluated the frequency, phenotype and functions of NK cells freshly isolated from healthy donors and melanoma patients with multiparameter flow cytometry. We assessed TIGIT and CD226 cell surface expression and internalization upon binding to CD155. We evaluated the role of Interleukin (IL)-15 and TIGIT blockade in increasing NK cell-mediated cytotoxicity in vitro and in two mouse models.
Results
NK cells are present at low frequencies in metastatic melanoma, are dysfunctional and downregulate both TIGIT and CD226 expression. As compared with TIGIT− NK cells, TIGIT+ NK cells exhibit higher cytotoxic capacity and maturation but paradoxically lower cytotoxicity against CD155+ MHC class I-deficient melanoma cells. Membrane-bound CD155 triggers CD226 internalization and degradation, resulting in decreased NK cell-mediated tumor reactivity. IL-15 increases TIGIT and CD226 gene expression by tumor-infiltrating NK cells (TiNKs) and, together with TIGIT blockade, increases NK cell-mediated melanoma cytotoxicity in vitro and decreases tumor metastasis in two mouse melanoma models. Specific deletion of TIGIT on transferred NK cells enhances the anti-metastatic activity of IL-15, while CD226 blockade decreases the effects of IL-15 and TIGIT blockade.
Conclusion
Our findings support the development of novel combinatorial immunotherapy with IL-15 and TIGIT blockade to promote NK cell-mediated destruction of MHC class I-deficient melanoma, which are refractory to CD8+ T cell-mediated immunity.
Keywords: Melanoma, Immunotherapy, TIGIT, IL-15, NK cells
Introduction
There is ample evidence that NK cells exhibit tumor-killing capacity and play a critical role in mediating tumor immunosurveillance of primary tumors and controlling metastases (1). NK cells express multiple activating receptors (ARs) and inhibitory receptors (IRs) that regulate their function. Hence, therapeutic strategies to engage ARs and/or counteract NK cell inhibition have the potential to promote NK cell-mediated tumor reactivity (2). Solid tumors are usually poorly infiltrated by NK cells (3), thus the phenotypic and functional studies of TiNKs remain very challenging in humans. TiNKs in ovarian, breast, lung and prostate tumors downregulate multiple ARs, including DNAM-1/CD226, CD16, NKG2D, NKp30, NKp46, and 2B4, and are dysfunctional (4–9). NK cells upregulate multiple IRs that are also expressed by activated T cells, including CD94/NKG2A (10) and the T cell immunoglobulin and ITIM domain (TIGIT) (11). TIGIT binds with high and low affinity to CD155 (PVR) and CD112 (Nectin-2), respectively which are expressed on monocytes, dendritic cells, and tumor cells, including melanoma, and can also bind to the adhesion protein CD113 (Nectin-3) (12). TIGIT competes with its costimulatory counter-receptor CD226 (DNAM-1), which binds to CD155 with lower affinity (11,12). CD226 competes with CD112R for binding to CD112 (Nectin-2) (13,14). In mouse-bearing tumors and in humans, dual PD-1/TIGIT blockade potently augmented tumor-antigen CD8+ T cell functions and promoted tumor rejection (15,16). TIGIT acts in regulatory T cells (Tregs) to promote tumor growth (17), and CD226 opposes TIGIT to disrupt Treg stability in melanoma (18). Several lines of evidence support the critical role of the TIGIT/CD226 axis in regulating NK cell-mediated antitumor activity. First, TIGIT is highly expressed by circulating human NK cells (cNKs) and impedes NK cell-mediated killing of tumor cells (11). Upon CD155 binding to TIGIT, the ITT-like motif is phosphorylated and binds to Grb2 to recruit the SH domain-containing inositol-5-phosphatase (SHIP1), impeding PI3 and MAP kinase pathways, and NFKB signaling (19,20). Second, CD226 associates with LFA-1 and is recruited to the immunological synapse to promote NK cell-mediated tumor cytotoxicity (21,22). In vivo, CD226 is involved in NK cell-mediated tumor surveillance and control of melanoma metastases and NK cell-mediated lysis of melanoma (23–26). Third, one recent study in mouse tumor models has suggested that TIGIT acted primarily in NK cells to regulate CD8+ T cell-mediated tumor reactivity (27). Among the various cytokines that expand and activate NK cells, IL-15 potently enhances NK cell-mediated tumor killing and is being actively investigated in many clinical trials (1). NK cell-based therapies represent a powerful approach to kill MHC class I-deficient tumors that may arise upon CD8+ T cell-mediated immune destruction of MHC class I-presenting tumor cells, and may therefore counteract some of the mechanisms of resistance to PD-1 blockade (28,29). However, therapeutic strategies to potently reinvigorate NK cells in human tumors remain to be developed. Here, we show that membrane-bound (mb) CD155 triggers CD226 internalization and degradation, resulting in decreased NK cell-mediated tumor reactivity. We also show that IL-15 with TIGIT blockade augments the functional capacities of TiNKs in vitro and decreases tumor metastasis in mouse melanoma models. Altogether, our findings provide the rationale for combining IL-15 with TIGIT blockade to counteract melanoma-induced NK cell dysfunction and promote NK cell mediated lysis of MHC class I-deficient melanoma, which may prove useful in anti-PD-1 refractory patients (28,29).
Materials and Methods
Study subjects and cell lines
PBMCs from 30 HDs, and PBMCs and tumor samples from 30 stage IV MPs were obtained under the Internal Review Board (IRB)–approved protocols UPCI 05–140 and UPCI 96–099. Biopsies were obtained from 29 stage IV melanoma patients, including 22 males and 7 females, ranging from age 31 to 82. Metastatic sites included skin or soft tissue (20%), nodes (45%), lung (20%) and other visceral locations (20%). Ten out of the 29 patients had received prior interferon-α adjuvant therapy. The samples were collected before therapy for stage IV melanoma and more than three years after the end of interferon-α adjuvant therapy. The melanoma cell lines were derived from metastatic lesions of melanoma patients at the University of Pittsburgh. FO-I is a β2m-deficient human melanoma cell line recognized by NK cells (30). K562 and L cells were purchased (ATCC). Human CD155/PVR transcript variant 1 Gene cDNA Clone (full-length ORF Clone, Sino Biological Inc.) was transfected into L cells using lipofectamine 3000 (Invitrogen) according to manufacturer’s instructions. L-CD155 transfectants were selected upon exposure to Hygromycin (400μg/ml), transfectants and L-CD155+ clonal cell lines were obtained with limiting dilution.
Phenotypic analysis and cell sorting
Peripheral blood mononuclear cells (PBMCs) were used for ex-vivo flow cytometry analysis. We performed CD3 positive beads magnetic separation (Miltenyi biotec) from the single cell suspensions obtained from metastatic melanoma and used the CD3 negative fraction for ex-vivo flow cytometry. CD45+CD3−CD56+ TIGIT+ and TIGIT− NK cells were sorted under sterile conditions (FACSAria cytometer, BD). The following conjugated mAbs were used in flow cytometry experiments: TIGIT-PerCPeFluor710, TIGIT-PE, CD14-PacificBlue and CD19-PacificBlue (ThermoFisher Scientific), NKG2D-PE-Cy7, PD-1-PE-Cy7 and CD226-PE-Dazzle594 (Biolegend), CD96-APC and Tim-3-Alexa700 (R&D Systems), CD45-BV510, CD56-BUV395, CD8-BUV737, CD3-APC-Cy7, and CD3-FITC (BD), HLA-DR-ECD and CD16-PE-Cy7 (Beckman Coulter), CD226-PE or CD226-biotin (DX11, Abcam) coupled with streptavidin-PE-TexasRed (ThermoFisher Scientific) and/or IgG control mAbs. Viability was assessed using LIVE/DEAD violet, aqua kits (ThermoFisher Scientific) or Zombie-NIR (Biolegend). Intracellular staining was performed as previously reported (18). NK cells were labeled for Granzyme-A-PacificBlue (Biolegend), Granzyme-B-APC (ThermoFisher Scientific), Perforin-FITC (BD). Samples were acquired on a FACS LSR-II machine (BD) and analyzed using FlowJo software v9 (Tree star).
NK cell stimulation and functional assays
NK cells were obtained from PBMCs and MM single cell suspensions by a two-step cell separation. CD3 negative cells obtained after CD3 positive selection with magnetic beads (Miltenyi Biotec), were used for CD56 positive selection (magnetic beads, Miltenyi Biotec) to isolate CD56+ NK cells. NK cells were stimulated for 16 h with 100 U/mL IL-2 and/or 10 ng/mL IL-15 (Peprotech) prior to incubation for 4 h with with FO-I (1:1 ratio) +/− blocking aTIGIT 10D7.G8 (IgG4, BMS) (15), aCD226 (DX11, Abcam) or aCD155 (D171, ThermoFisher Scientific) mAbs. In some experiments, NK cells were stimulated (16 h, 48 h, 4 d or 6 d) with IL-2 (100U/mL), IL-15 (10 ng/mL), IL-7 (10ng/mL) or IL-21 (10ng/mL) (Peprotech) prior to 4 h-co-culture with FO-I, or in presence or absence of STAT5 inhibitor (CAS 285986–31-4, Sigma-Aldrich) or STAT3 inhibitor VI, S3I-201 (CAS 501919–59-1, Sigma-Aldrich) prior to receptor expression analysis. In other experiments, NK cells were incubated 48 h with IgG- or CD155-Fc beads +/− blocking antibodies. Cells were washed in PBS 4 μM EDTA and beads were removed with magnetic separation prior to the functional assays. NK cell degranulation capacity and intracellular IFN-γ expression were evaluated with flow cytometry using CD107a-PerCPcy5.5 and IFN-γ-PE/Cy7 (Biolegend), as previously reported (15). NK cell-mediated cytotoxicity (specific lysis) was evaluated using standard 51Chromium-release assay as previously reported (31). Briefly, 51Cr-(GE HealthCare) labeled FO-I cells were incubated 4 h at 37oC either alone (spontaneous release), with 2.5% Triton-X-100 (maximum release), or with NK (E:T=30:1) +/− aTIGIT and/or aCD226 blocking mAbs in triplicate wells.
Downregulation of CD226 and TIGIT expression by NK cells
1 × 105 purified NK cells were cocultured 48 h with L cells, L-CD155, FO-I, K562, HLA-I+ melanoma cell lines, immature dendritic cells (ratio 1:1) or CD155-Fc-coated beads (ratio 1:4). In some additional wells, L-CD155 and CD155-Fc beads were incubated 30 min with either blocking anti-CD155 mAbs (D171, ThermoFisher Scientific), 20 μM of the inhibitor of metalloproteases and ADAM17, TAPI-2 (Sigma-Aldrich), or 9 μM of the specific inhibitor of ADAM10, GI254023X (Tocris) prior to coculture with NK cells and flow cytometry using the following antibodies: CD56-PeCy7, CD226-PE-Dazzle594 (Biolegend) and TIGIT-PerCPefluor710 (ThermoFisher Scientific). Immature dendritic cells were obtained from CD14+ monocytes isolated from PBMCs of normal donors with magnetic separation (Miltenyi Biotec) and cultured 6 d with 1000 U/mL IL-4 and GM-CSF (Peprotech). CD155-Fc beads were obtained by coating a chimeric CD155-Fc (BMS) on M-450 Tosylactivated beads (Dynal) following the manufacturer’s protocol. Cell viability was assessed with LIVE/DEAD violet.
RT-PCR
5 × 105 NK cells were incubated 48 h either with L cells or L-CD155 +/− 10 ng/mL IL-15. RNA was extracted with RNeasy mini Kits (Qiagen) and cDNA was prepared by reverse transcription using M-MLV Reverse Transcriptase (ThermoFisher Scientific). RT-PCR was performed with StepOne System (ThermoFisher Scientific). All samples were analyzed and normalized to the expression of β-glucuronidase (β-Gus). CD226 and TIGIT expression were detected using previously described primers (18).
ELISA
sCD155 levels in sera were evaluated by sandwich ELISA. High-binding 96-well plates were coated with 2 μg/mL aCD155 mAb (D171, Abcam) for capture antibody for 30 min at 37oC in sodium phosphate buffer (pH 7.6) and blocked for 1 h at room temperature with blocking buffer (PBS 3% BSA and 0.05% Tween). Plates were washed three times (PBS 0.05% Tween). Human CD155-Fc and 1:10 diluted samples were plated at 100 μL for 2 h at room temperature and washed three times. Plates were incubated with 100 μL of secondary rabbit polyclonal aCD155 antibody (2 μg/mL in blocking buffer, Lifespan) for detection, washed, then incubated for 30 min at room temperature with 100 μL HRP-conjugated goat anti-rabbit (1:1000 in blocking buffer, ThermoFisher Scientific), washed, and reacted with 100 μL of substrate solution (1:1 tetramethylbenzidine and hydrogen peroxide, BD). The reaction was stopped after 5 min with 50 μL of 2 N sulfuric acid and absorbance was read at 450 nm. All values were determined in triplicates.
Imagestream flow cytometry
1 × 105 NK cells isolated from PBMCs were incubated 1 h at 37oC either with medium alone, L cells, L-CD155, or FO-I (1:1 ratio). Cells were washed in cold PBS and in refrigerated centrifuge to prevent further receptor internalization. Viability was assessed with the Zombie-NIR kit (Biolegend). NK cells were stained with CD45-BV510 (BD), CD56-PeCy7 (Biolegend), CD226-PE (DX11, Abcam) or TIGIT PerCPefluor710 (MBSA43, ThermoFisher Scientific), then permebealized and stained with CD226-biotin (DX11, Abcam) and streptavidin PE-TexasRed, or with TIGIT-PE (MBSA43, ThermoFisher Scientific) prior to analysis on ImagestreamX MARKII Imaging flow cytometer with INSPIRE software (Amnis, EMD Millipore). The flow rate was set at minimum and the objective magnification was set at 60x for all samples. A multifluorophore-labeled sample was used to determine accurate laser settings and avoid oversaturation. Gradient RMS and aspect ratio versus area on the brightfield channel were used during acquisition to ensure collection of focused single cells. At least 5 × 103 live CD45+CD56+ NK cells were acquired per sample. Data analysis was performed using IDEAS software (Amnis, EMD Millipore). CD226 and TIGIT internalization ratios were calculated using CD45 membrane expression as a mask (a region of interest) to determine the membrane and intracellular sections of the cells.
Intracellular receptor degradation
1 × 105 NK cells were incubated 30 min either with or without 0.5 μM bafilomycin A1 (Sigma-Aldrich), 25 ng/mL concanamycin-A (Sigma-Aldrich), or 1% DMSO as control, prior to 16 h or 48 h coculture with L cells, L-CD155 or FO-I (1:1 ratio) +/− IL-2 (100 U/mL), IL-15, IL-7 or IL-21 (10 ng/mL). Cells were washed with PBS and fixed with 1.3% paraformaldehyde. All cells were stained for surface CD45-BV510 (BD) and CD56-PeCy7 (Biolegend). Cells were either stained for surface CD226 and TIGIT expression, or permeabilized and stained for total CD226 and TIGIT expression prior to flow cytometry. Experiments were repeated and performed in triplicates.
Mice and experimental metastasis models
C57BL/6 wild-type (WT) and C57BL/6 Rag2−/−γc−/− mice were bred in-house. C57BL/6 Tigit−/− mice were kindly provided by Bristol Myers Squibb. All mice were bred and maintained at the QIMR Berghofer Medical Research Institute and used when more than 6 weeks of age. No mice were excluded based on pre-established criteria in all studies and no active randomization was applied to any experimental group. The investigators were not blinded to the group allocation during the experiment and/or when assessing the outcome. All experiments were approved by the QIMR Berghofer Medical Research Institute Animal Ethics Committee.
Mouse B16F10 melanoma cells were grown in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% Fetal Calf Serum (Bovogen), 1% Glutamine (Gibco), 1% HEPES (Gibco) and 1% Penicillin/Streptomycin (Gibco). LWT1 melanoma cells were cultured in RPMI 1640, supplemented with 10% Fetal Calf Serum (Bovogen), 1% Glutamine (Gibco), and 1% Penicillin-Streptomycin (Gibco). All cell lines were maintained at 37°C, 5% CO2. Cell injection and monitoring procedures were described in previous studies (32–34). All cell lines were routinely tested negative for Mycoplasma, but cell line authentication was not routinely performed. B16F10 melanoma (5 × 105) or LWT1 melanoma (7.5 × 105) cells were injected intravenously into the tail vein of WT or Tigit−/− mice. On days 0 and 3 after tumor inoculation, some mice were treated intraperitoneally (i.p.) with PBS or IL-15/IL-15Ra complexes (R&D Sytems) or cIg or anti-mouse TIGIT (4B1) at the indicated doses. Some groups of mice were additionally treated with an aCD226 mAb (480.1) to block CD226. In some experiments, CD3− NK1.1+ NK cells were sorted by flow cytometry from spleens of WT or Tigit−/− mice to 95% purity and WT or Tigit−/− NK cells (2 × 105) were injected i.v. into Rag2−/−γc−/− mice. After 6 d, blood was collected to check the equivalent reconstitution of NK cells by flow cytometry and B16F10 (5 × 105 or 1 × 104) melanoma cells were injected i.v. into Rag2−/−γc−/− mice. Lungs were harvested on day 14 and metastatic colonies on the surface of the lungs were counted using a dissecting microscope.
Statistical analysis
Statistical analyses were performed in Prism software (Graphpad). The normality of each variable was evaluated using the Shapiro-Wilk test. In case of normally distributed data, the comparison was performed using unpaired or paired two-tailed t-tests, one-way ordinary or repeated-measures ANOVA tests followed by Tukey’s multiple comparisons test to compare all data together or Dunnett’s multiple comparisons test to compare all data with control. Data that were not normally distributed were compared with Wilcoxon matched-pairs signed rank tests (two paired groups) or Kruskal-Wallis test followed by Dunn’s multiple tests (more than two groups, unpaired). Linear regressions were evaluated with Pearson correlation tests. Significant differences were indicated for each figure and defined as ns (non-significant), P>0.05; *, P<0.05; **, P<0.01; ***, P<0.001 and ****, P<0.0001.
Results
NK cells downregulate TIGIT and CD226 in metastatic melanoma
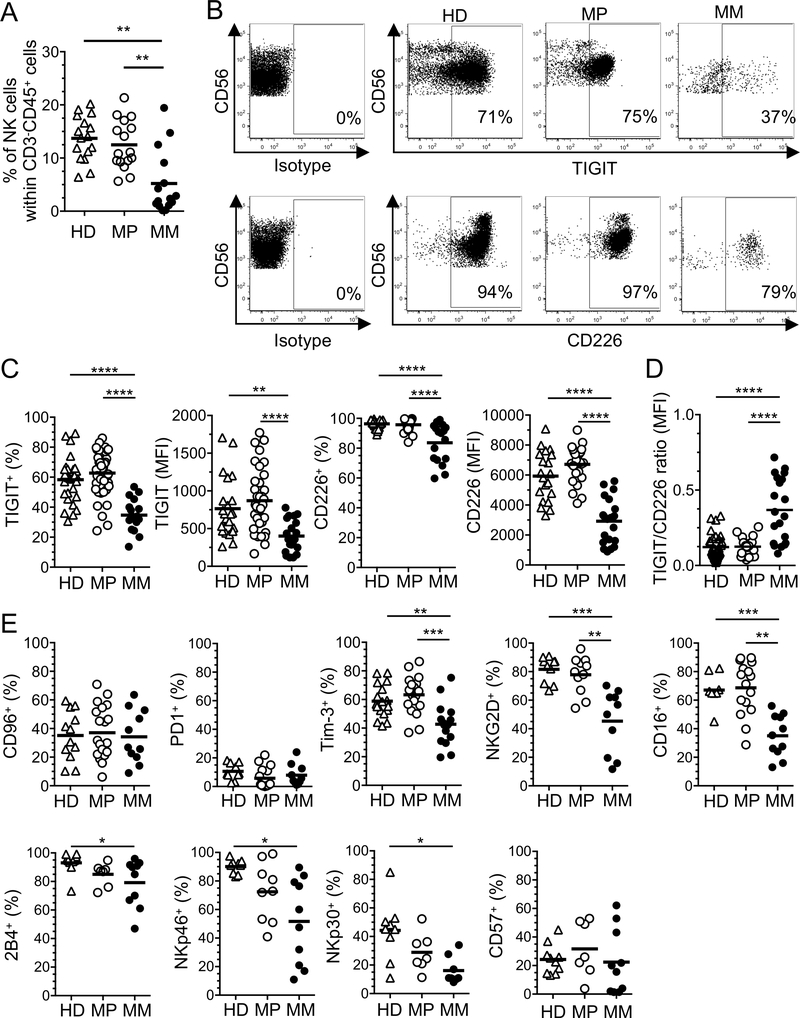
We first evaluated the expression of TIGIT and CD226 by NK cells (CD45+CD3−CD56+ cells, Supplemental Figure 1A) in the periphery and at tumor sites in melanoma patients (MPs). We measured lower NK cell frequencies in metastatic melanoma (MM) than in PBMCs of MPs (Figure 1A). In sharp contrast with CD8+ T cells (15), TiNKs exhibited lower TIGIT expression (frequency and MFI) than cNKs isolated from MPs and HDs (Figure 1, B and C). TiNKs exhibited lower CD226 expression (frequency and MFI) than cNKs, resulting in an increased TIGIT to CD226 expression ratio (Figure 1D). We observed a positive correlation between the percentages and MFI of TIGIT expression in cNKs and TiNKs for individual donors. We also observed a positive correlation between the percentages and MFI of CD226 expression in TiNKs, which downregulate CD226 expression, but not in cNKs. (Supplemental Figure 1B). CD56dim NK cells were enriched in MM and downregulated both CD226 and TIGIT expression as compared with the periphery (Supplemental Figure 1C). CD56bright NK cells, which represent a minority of NK cells in the periphery and at tumor sites, expressed low TIGIT level (Supplemental Figure 1C). TiNKs and cNKs exhibited similar levels of CD96 and CD57 expression and both expressed low-level PD-1 (Figure 1E). In addition, TiNKs displayed lower frequencies of activation/maturation markers such as Tim-3, NKG2D, CD16 (Figure 1E). Notably, TiNKs exhibited lower 2B4, NKp46, NKp30 expression (frequencies) as compared with HD cNKs but not MPs cNKs (Figure 1E).
Figure 1. Tumor-infiltrating NK cells downregulate TIGIT and CD226.
CD3−CD45+CD56+ NK cells were evaluated side by side in PBMCs of healthy donors (HD) and melanoma patients (MP) and in metastatic melanoma (MM) using flow cytometry. (A) Pooled data showing the frequencies of NK cells within CD3−CD45+ cells (n=16 per group). (B) Representative dot plots showing the percentages of TIGIT and CD226 expression by NK cells. (C) Pooled data of TIGIT and CD226 expression (% and MFI) by NK cells in PBMCs (HD: n=30 and n=25, respectively; MP: n=39 and n=32, respectively) and MM (n=17 and n=14, respectively). (D) TIGIT/CD226 expression ratio (MFI) by NK cells (from panel C). (E) Percentages of CD96, PD-1, Tim-3, NKG2D, CD16, 2B4, NKp46, NK30 and CD57 expression by cNKs isolated from HDs (n=9–12) and MPs (n=7–20), and TiNKs (n=10–11). Horizontal bars depict means and P values were obtained by one-way ANOVA tests followed by Tukey’s multiple comparisons test with *, P<0.05; **, P<0.01; ***, P<0.001 and ****, P<0.0001. Data are representative of ten independent experiments.
Collectively, our findings show that TiNKs are present at low frequencies in MM and downregulate both TIGIT and CD226 but not CD96 as compared with cNKs.
TIGIT+ NK cells exhibit higher lytic potential but lower degranulation capacity than TIGIT− NK cells in melanoma
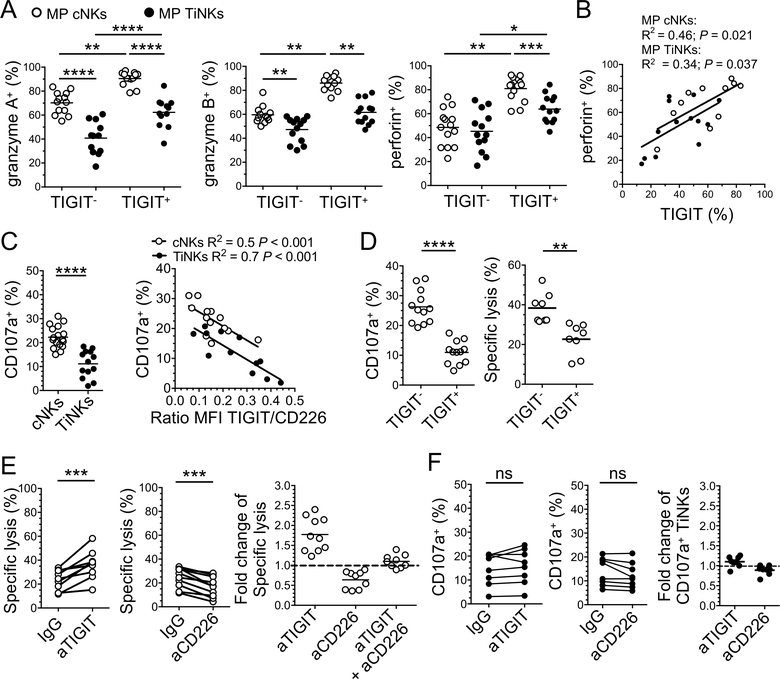
We next investigated the functional capacities of TIGIT+ and TIGIT− NK cells isolated from the periphery and tumor sites of MPs (Figure 2A). The frequencies of granzyme A+TIGIT− NK cells were significantly lower than granzyme A+TIGIT+ NK cells, both for cNKs and TiNKs (mean frequency 70% ± 9% vs. 90% ± SD 6%, respectively, for cNKs, P<0.01, and 41 ±14% vs. 62 ± 12%, respectively, for TiNKs, P <0.0001). The frequencies of granzyme B+ cells were also significantly lower in TIGIT+ TiNKs as compared with TIGIT+ cNKs (mean frequency 62 ± 10% vs. 86 ± 6.3%, P<0.01, respectively). Finally, the frequencies of perforin+ cells were also significantly lower in TIGIT+ TiNKs as compared with TIGIT+ cNKs (mean frequency 64 ± 11 vs. 81 ± 10%, P<0.001). Perforin+ NK cell frequencies positively correlated with TIGIT+ NK cell frequencies both in the periphery and at tumor site (Figure 2B). Also, TIGIT+ TiNKs displayed lower expression of granzymes and perforin than TIGIT+ cNKs. To evaluate the implication of these findings, cNKs and TiNKs were isolated from PBMCs and tumors of MPs, respectively (Supplemental Figure 1A), before degranulation assay in the presence of the MHC class I-deficient melanoma cell line FO-I or K562, which express high-level CD155 and CD112 but low-level MICA/B and ULBPs (Supplemental Figure 1D). TiNKs exhibited lower degranulation capacity than cNKs and CD107a+ TiNK frequencies inversely correlated with the TIGIT/CD226 expression ratio, supporting the role of the TIGIT/CD226 axis in modulating NK cell functions (Figure 2C and Supplemental Figure 1E). We then evaluated the lysis of FO-I by TIGIT− and TIGIT+ NK cells sorted from PBMCs of MPs. TIGIT+ NK cells exhibited lower lytic activity and CD107a expression than TIGIT− NK cells (Figure 2D). Altogether, our findings show that TiNKs are more dysfunctional than cNKs and that TIGIT+ NK cells display higher lytic potential but paradoxically lower lytic activity than TIGIT− NK cells, against CD155+ MHC-class I deficient melanoma cells.
Figure 2. TIGIT blockade reinvigorates NK cell-mediated tumor reactivity in the periphery but not at tumor sites.
NK cells were isolated from PBMCs (cNKs) and tumors (TiNKs) of MPs. (A) Frequency of granzyme A-, granzyme B- and perforin-expressing NKs according to TIGIT expression (n=14). (B) Pearson correlation between the frequencies of perforin+ and TIGIT+ NKs. (C) NK degranulation capacity against FO-I (CD107a %) and Pearson correlation between degranulation capacity and TIGIT/CD226 ratio by NK (n=13). (D) Degranulation against FO-I (CD107a, n=12) and specific lysis of FO-I lysis (Chromium 51 release assay, n=8) by TIGIT+ and TIGIT− cNKs. (E) Specific lysis of FO-I by cNKs +/− aTIGIT, aCD226 blocking mAbs, or IgG control mAbs (frequency and fold change, n=10) (F). CD107a expression by TiNKs (n=8) in response to FO-I +/− aTIGIT +/− aCD226, or IgG control mAbs. Horizontal bars depict means and P values were obtained by unpaired t-tests (A and C) or paired t-tests (A, C-F), with ns (non-significant), P>0.05; *, P<0.05; **, P<0.01; ***, P<0.001 and ****, P<0.0001. Data are representative of at least three independent experiments.
TIGIT blockade alone failed to reverse NK cell dysfunction in melanoma
We next evaluated the role of TIGIT and CD226 in regulating NK cell-mediated reactivity against MHC class I-deficient melanoma (Figure 2). NK cells isolated from PBMCs and tumor of MPs were stimulated with IL-2 and IL-15 prior to coculture with FO-I in the presence of blocking anti-(a)TIGIT and/or aCD226 as compared with IgG control mAbs. In line with previous published findings (11,27,35), TIGIT blockade increased the frequencies of lytic cNKs, while CD226 blockade impeded NK cell-mediated cytotoxicity and degranulation in the presence of FO-I (Figure 2E and Supplemental Figure 1F). The effects of TIGIT blockade on NK cell-mediated cytotoxicity and degranulation capacity in the presence of FO-I were abrogated by CD226 blockade (Figure 2E and Supplemental Figure 1F). CD155 blockade had no significant impact on the degranulation capacity of cNKs against FO-I (Supplemental Figure 1F). However, and in sharp contrast with the periphery, neither TIGIT blockade nor CD226 blockade significantly changed the low TiNK lytic activity against FO-I (Figure 2F). TIGIT blockade modestly increased IFN-γ production by cNK cells but had no significant effect on TiNKs (Supplemental Figure 1G).
Altogether, our findings show that, in sharp contrast with cNKs, TiNKs exhibit poor lytic function against MHC-deficient melanoma cells, which cannot be rescued upon TIGIT blockade.
Membrane-bound CD155 induces CD226 internalization/degradation and NK cell dysfunction
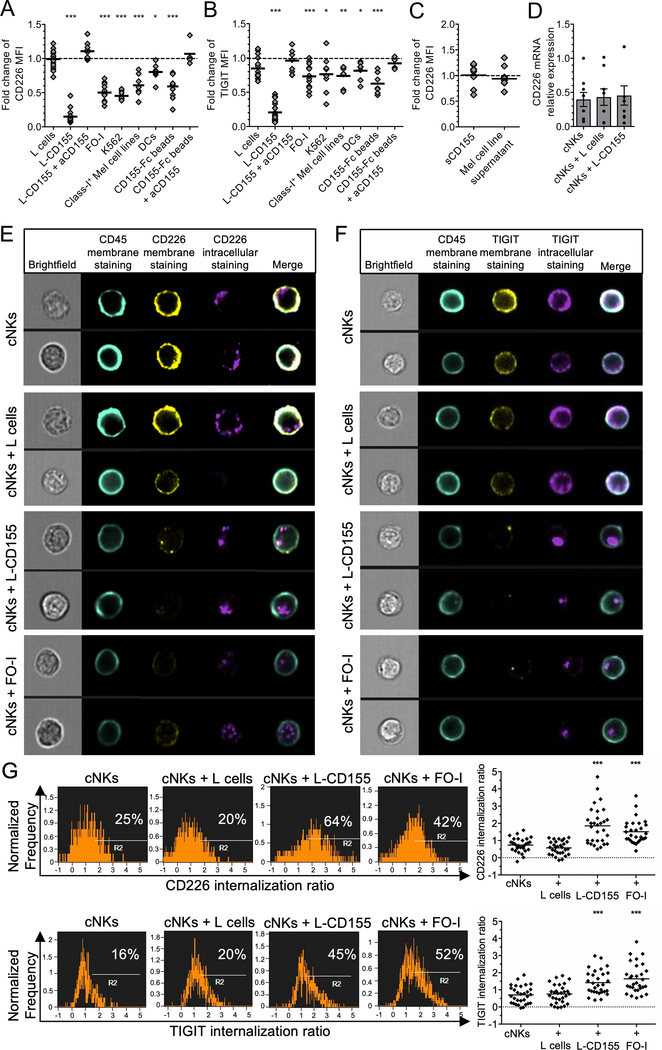
Because NK cells downregulate CD226 cell surface expression upon binding to membrane-bound (mb) CD155 (7), we next investigated the role of mb and soluble (s) CD155 in mediating the downregulation of CD226 and TIGIT by NK cells. To this end, cNKs were incubated in the presence of either CD155-Fc-coated beads, CD155-expressing cells including L-cells transfected or not with human CD155 (L-CD155), K562, FOI and dendritic cells, or sCD155 (Figure 3). cNKs strongly downregulated CD226 expression and to a lesser extent TIGIT expression in presence of L-CD155, FO-I, K562, immature DCs and CD155-Fc-coated beads, but not L-cells, resulting in an increased TIGIT/CD226 expression ratio (Figure 3, A and B). CD226 and TIGIT downregulation were abrogated in the presence of blocking aCD155 mAbs (Figure 3, A and B and Supplemental Figure 2A), supporting that CD226 and TIGIT downregulation occurred in a CD155-mediated fashion. In contrast, NK cells did not significantly downregulate CD96 nor Tim-3 expression as control (Supplemental Figure 2B). CD226 and TIGIT downregulation correlated with the level of CD155 expression (Supplemental Figure 2, C and D). Similarly, TiNKs showed decreased CD226 and increased TIGIT/CD226 expression ratio in correlation with the level of CD155 expression by the tumor cells in the TME (Supplemental Figure 2E). Notably, sCD155 or supernatants of melanoma cell lines containing high-level sCD155 (Supplemental Figure 2C), did not induce CD226 downregulation by NK cells (Figure 3C). CD155-induced CD226 downregulation by NK cells occurred at a post-transcriptional level since CD226 mRNA relative expression did not significantly change after 48-h co-culture with L-CD155 (Figure 3D). CD226 downregulaton by NK cells was not abrogated in the presence of protease inhibitors (TAPI-2 and GI254023X), suggesting that it did not occur upon ectodomain shedding (Supplemental Figure 2F). Notably, NK cells expressed similar Annexin-V expression in the presence of L-CD155 cells or CD155-Fc-coated beads, respectively, and compared with L cells or IgG beads, respectively (Supplemental Figure 2G).
Figure 3. Membrane-bound CD155 induces TIGIT and CD226 internalization in NK cells.
(A and B) CD226 (A) and TIGIT (B) expression by cNKs (fold change of MFI) after 48 h incubation with indicated targets +/− aCD155 blocking mAb, as compared with control (no target, n=15, or IgG-coated beads, n=8). (C) Fold change of CD226 expression in MFI by cNKs incubated 48 h with sCD155 or the supernatants of melanoma cell lines (n=7). (D) CD226 mRNA relative expression by cNKs either alone, co-cultured for 48 h with L-cells, or with L-CD155 cells. Dots are means of triplicates (n=7). (E and F) Representative pictures from ImageStream analysis of cNKs alone or with indicated cell lines (1 h) depicting CD226 (E) and TIGIT (F) membrane (yellow) and intracellular (violet) expression, as well as surface CD45 expression (turquoise). (G) Representative histograms gated on total live cNKs (left panels) and statistical analysis from a pool of 30 randomly selected cNKs (right panels) showing the ratio of CD226 and TIGIT internalization by cNKs in the presence of L-CD155 and FO-I as compared with L cells or cNKs alone. Cell surface CD45 expression was used to calculate the internalization ratio of CD226 and TIGIT intracellular staining using IDEAS software. P values were obtained from one-way ANOVA tests (A and B) or repeated-measure ANOVA tests (D and G) followed by Dunnett’s multiple comparisons test with *, P<0.05; **, P<0.01 and ***, P<0.001. Horizontal bars depict means (A-C and G) or means ± SEM (D). Data are representative of at least three independent experiments.
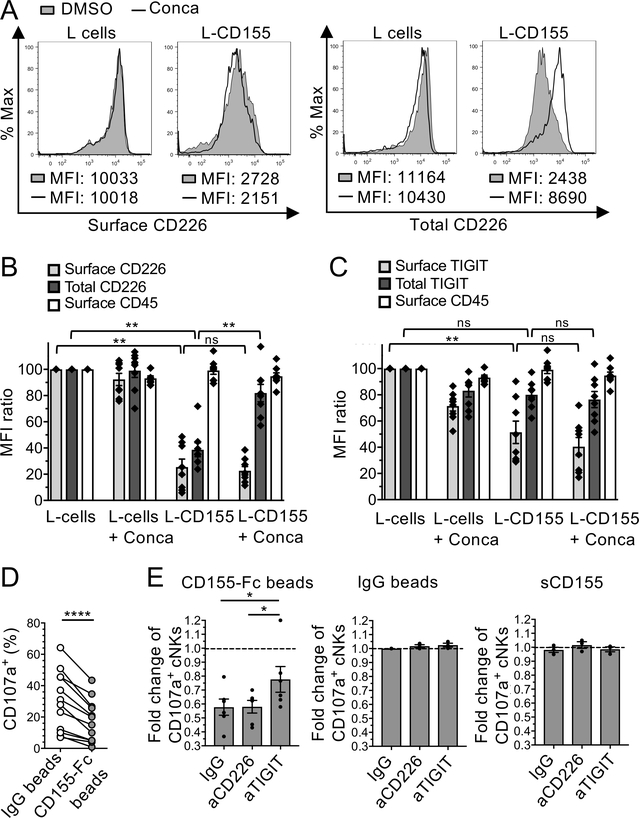
To investigate the fate of TIGIT and CD226 upon binding to mbCD155, including internalization, degradation and recycling, cNKs were co-incubated (1 h) with FO-I, L-CD155, L-cells or no cells prior to intracellular and extracellular staining of TIGIT, CD226, and CD45 followed by Imagestream flow cytometry. In presence of L-CD155 and FO-I but not L cells, the cell-surface expression of CD226 and TIGIT strongly decreased together with an increased intracellular expression of both molecules (Figure 3, E and F), resulting in increased internalization ratios (Figure 3G). To investigate whether CD226 and TIGIT undergo ligand-induced endocytosis followed by recycling or degradation (36), we next evaluated their surface and total cellular expression in cNKs incubated with L-cells or L-CD155 with or without V-ATPases inhibitors (16 h, Figure 4). In the presence of L-CD155 but not L cells, total CD226 but not TIGIT expression in NK cells (MFI) sharply decreased as compared with L cells (Figure 4, A, B and C). V-ATPase inhibitors, which inhibit protein degradation by acidified lysosomes (37), abrogated the decrease in total cellular CD226 expression (Figure 4B and Supplemental Figure 3, A and B).
Figure 4. Membrane-bound CD155 induces CD226 degradation and NK cell dysfunction.
(A-C). cNKs isolated from MP were incubated with L cells or L-CD155 for 16 h +/− concanamycin-A (Conca). (A) Representative histograms of flow cytometry showing the effects of Conca on cell surface and total CD226 expression (MFI) by NK cells in the presence of L-CD155 cells or L cells as compared with DMSO control. (B and C) Cell surface and total CD226 (B), TIGIT (C), and surface CD45 (as control) expressions by cNKs (normalized MFI expression as compared to cNKs + L cells). (D and E) cNKs were incubated 48 h with either sCD155, IgG- or CD155-Fc-beads +/− blocking antibodies and washed before functional assay in presence of FO-I. (D) CD107a expression (%) by cNKs pre-incubated or not with CD155-Fc beads (n=11) (E) CD107a expression (fold change) by cNKs pre-incubated with either CD155-Fc-beads (n=6), IgG-beads (n=3), or sCD155 (n=3) +/− aCD226 or aTIGIT blocking mAbs as compared with IgG-beads (dotted line). Horizontal bars depict means ± SEM (B, C, and E) and P values were obtained by one-way ANOVA tests followed by Tukey’s (B and C) or Dunnett’s (E) multiple comparisons test or by paired t-tests (D) with ns (non-significant), P>0.05; *, P<0.05; **, P<0.01 and ****, P<0.0001. Data are representative of at least three independent experiments done in duplicates.
We next assessed the functional implication of CD155-mediated CD226 degradation on NK cell functions. To this end, cNKs isolated from MPs were incubated in the presence of CD155-Fc- or IgG-coated beads and/or aTIGIT and/or aCD226 mAbs prior to stimulation with IL-2 and IL-15, and incubation with FO-I. CD155-Fc-treated cNKs exhibited lower CD107a expression as compared with IgG-treated cNKs (Figure 4D). TIGIT blockade partially reversed the effect of CD155-Fc on cNKs while CD226 blockade had no significant effect (Figure 4E and supplemental Figure 3C). As controls, IgG-coated beads and sCD155 had no significant impact on cNK cytolytic activity.
Altogether, our data show that mbCD155 but not sCD155 induced both TIGIT and CD226 internalization, and CD226 but not TIGIT degradation. They also show that mbCD155-mediated CD226 degradation promoted NK cell dysfunction that was only partially reversed upon TIGIT blockade.
IL-15 together with TIGIT blockade reverses CD155-mediated NK cell exhaustion and impedes experimental melanoma metastasis in vivo
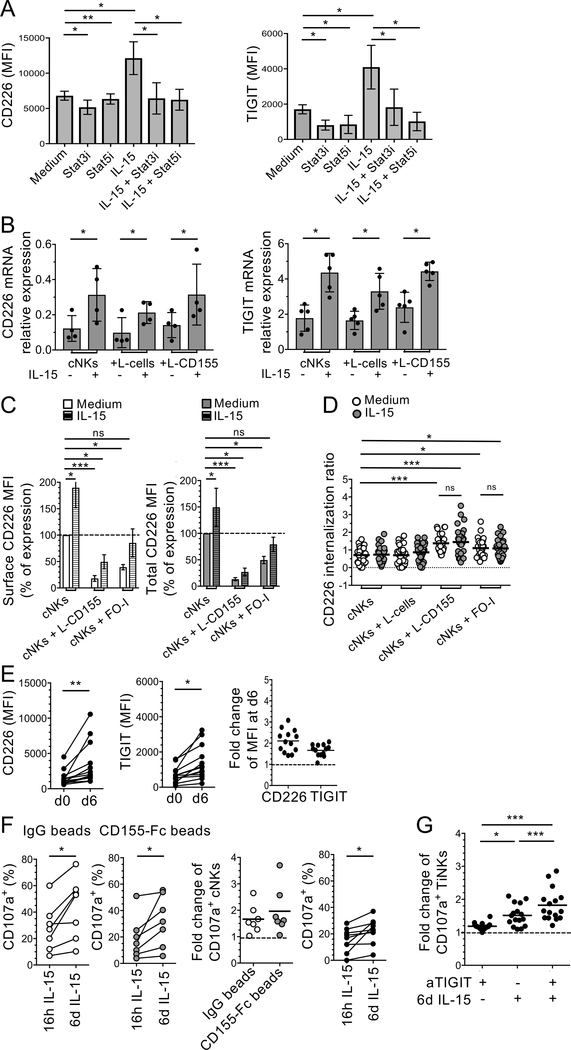
IL-15 upregulates CD226 expression by NK cells (38,39). In addition, IL-15 together with IL-12 also upregulates TIGIT expression by cNKs isolated from HIV-infected patients (40). We next investigated whether IL-15, which increases NK cell-mediated antitumor activity, regulates CD226 and TIGIT expression by cNKs and TiNKs as compared with other γ-chain cytokines including IL-2, IL-15, IL-7 and IL-21. IL-15 induced higher CD226 and TIGIT expression after 6 d stimulation than IL-2, IL-7, or IL-21 (Supplemental Figure 4A). We then incubated cNKs in the presence of IL-15 and L cells or L-CD155 prior to flow cytometry and RT-PCR for CD226 and TIGIT expression. IL-15 increased CD226 and TIGIT cell surface expression that was abrogated in the presence of STAT3 or STAT5 inhibitors (Figure 5A). IL-15 also increased Tim-3 and NKG2D expression by NK cells (Supplemental Figure 4A) as previously reported (41–43). IL-15 increased CD226 and TIGIT gene expression with or without L-CD155 (Figure 5B). To evaluate whether IL-15 reversed the downregulation of CD226 cell surface expression upon mbCD155, cNKs were incubated in the presence of IL-15 and L cells, L-CD155 or FOI prior to flow cytometry and Imagestream. In the presence of L-CD155 or FO-I, IL-15 significantly increased both cell surface and total CD226 expression by cNKs (Figure 5C) in contrast with the other γ-chain cytokines (Supplemental Figure 4B) but did not significantly impede CD226 internalization (Figure 5D). Notably, IL-15 also increased the expression of both CD226 and TIGIT by TiNKs (Figure 5E).
Figure 5. IL-15 together with TIGIT blockade reverses CD155-induced NK cell dysfunction.
(A) Pooled data showing the effect of STAT3 and STAT5 inhibitors (Stat3i and Stat5i, respectively) on CD226 and TIGIT expression (MFI) by cNKs after 6 d incubation +/− IL-15 (n=6). (B) Pooled data of from three independent experiments showing CD226 and TIGIT mRNA relative expression by cNKs from MPs after 48 h incubation with indicated cell lines +/− IL-15 (n=4 and n=5, respectively). (C) Surface and total CD226 expression by cNKs after 48 h incubation with L-CD155 or FO-I +/− IL-15 (normalized MFI ratio as compared with cNKs in medium alone). (D) Pool of 30 randomly selected cNKs from one representative ImageStream analysis (n=3), showing CD226 internalization ratio after 1 h incubation with indicated cell lines. (E) CD226 and TIGIT expression (MFI and fold change) by TiNKs, ex-vivo and after 6 d incubation with IL-15 (n=13). (F) Pooled data comparing CD107a expression by cNKs treated 48 h with indicated beads (frequency and fold change), and by TiNKs (frequency) after 16 h or 6 d IL-15 stimulation (n=7 and n=10, respectively), in response to FO-I. (G) Fold change of CD107a+ TiNKs after 16 h or 6 d IL-15 stimulation prior to incubation with FO-I +/− aTIGIT mAbs (n=16) as compared with 16 h IL-15 + IgG control mAb (dotted line). Horizontal bars depict means ± SD (A and B) or ± SEM (C). P values were obtained by paired t-tests (E and F), ordinary (D) or repeated-measures one-way ANOVA tests followed by Tukey’s (B, and G) or Dunnett’s (C) multiple comparisons test, with *, P<0.05; ***, P<0.01 and ***, P<0.001. Data are representative of at least three independent experiments.
We next assessed whether IL-15 alone or together with TIGIT blockade increased TiNK-mediated antitumor activity and counteracted CD155-mediated NK cell dysfunction. To this end, cNKs pre-incubated with CD155-Fc beads or IgG beads, or TiNKs were treated with IL-15 (16 h or 6 d) prior to evaluating their level of granzyme and perforin expression as well as CD107a expression in the presence of FO-I with or without aTIGIT mAbs. Prolonged IL-15 (6 d) increased cNK and TiNK degranulation in the presence of FO-I (Figure 5F) as well as perforin, granzyme A and granzyme B expression (Supplemental Figure 4, B and C) as compared with overnight stimulation with IL-15. Prolonged IL-15 stimulation alone (mean fold change: 1.49 ± SD 0.32), but not TIGIT blockade (1.17 ± 0.15), significantly increased TiNK degranulation in the presence of FO-I as compared with overnight stimulation with IL-15. Prolonged IL-15 together with TIGIT blockade further increased the degranulation of TiNK in the presence of FO-I (1.83 ± 0.48) (Figure 5G and Supplemental Figure 4E).
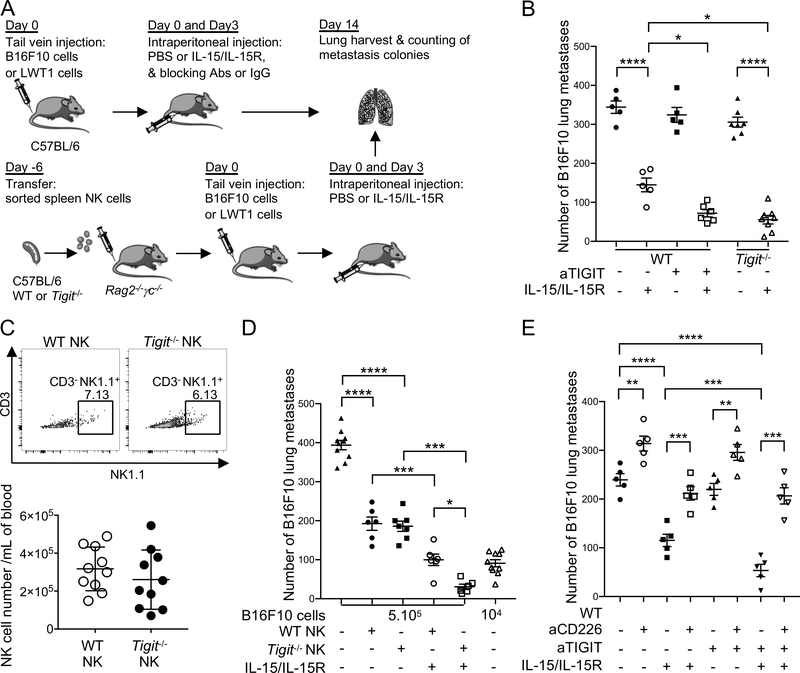
To support the relevance of our findings in vivo, we investigated whether IL-15 and TIGIT blockade promoted NK cell-mediated control of metastatic tumors in two mouse melanoma models (Figure 6A). Therapy with aTIGIT mAbs induced anti-metastatic activity against established B16F10 and LWT1 lung metastases only in mice treated with IL-15/IL-15Ra complexes (mean B16F10 metastasis numbers: 72 ± SD: 23, 144 ± 40, 324 ± 43 and 344 ± 36, respectively, for IL-15/IL-15R + aTIGIT mAbs, IL-15/IL-15R, aTIGITmAbs, and IgG + PBS, respectively) (Figure 6B and Supplemental Figure 5A and B). IL-15 was more effective in Tigit−/− mice compared with WT mice, despite Tigit−/− mice having no significant reduction in metastasis compared with WT mice as previously published (Figure 6B and Supplemental Figure 5A) (32,33). When NK cells from Tigit−/− and WT mice were transferred into immunodeficient Rag2−/−γc−/− mice (Figure 6A, C, D), they reduced B16F10 lung metastases by half as compared with no NK cell transfer (393 ± 38 vs. 186 ± 35 and 192 ± 42, respectively), but IL-15/IL-15Rα complexes were more effective in reducing metastases in mice transferred with Tigit−/− as compared with WT NK cells (31 ± 17 and 100 ± 36, respectively) (Figure 6D). Similar findings were observed in mice with the LWT1 lung metastases (Supplemental Figure 5C). In line with previous findings, CD226 blockade significantly increased the number of lung metastases in mice. Interestingly, CD226 blockade abrogated the effects of IL-15 alone or in combination with TIGIT blockade (Figure 6E and Supplemental Figure 5B). Additionally, NK cell but not CD8+ T cell depletion abrogated the antitumor effects of combined IL-15/IL-15R and TIGIT blockade in both B16F10 and LWT1 models. These findings support the role IL-15/IL-15R and TIGIT blockade in promoting direct NK cell-mediated tumor reactivity against lung metastases in vivo (Supplemental Figure 5D).
Figure 6. IL-15 and TIGIT blockade/deletion combine to suppress experimental lung metastasis of melanoma.
(A) Schematic of the mice tumor immunotherapy experiments. (B and E) Groups of C57BL/6 WT and Tigit−/− mice (n=5 mice/group) were injected i.v. with (B) 5 × 105 B16F10 or (E) 2 × 105 B16F10 on day 0. Some groups of mice then received PBS or IL-15 (0.5 μg)/IL-15Ra (3.0 μg) i.p., cIg or anti-TIGIT (200 μg) on days 0 and 3 and/or anti-CD226 (250 μg) on days −1, 0 and 7 (E). (C) Freshly sorted NK cells (TCRβ−NK1.1+) from spleens of wild-type (WT) or TIGIT−/− male mice were injected intravenously into Rag2−/−γc−/− recipient male mice (2 × 105 cells per mouse). At day 6, peripheral blood was collected from Rag2−/−γc−/− recipient mice to check NK cell reconstitution. Data are shown as representative flow and quantitative results of NK cell reconstitution. (D) Groups of C57BL/6 Rag2−/−γc−/− (n=6–10 mice/group) were i.v. reconstituted with 2 × 105 purified WT or Tigit−/− NK cells. Six days later, reconstituted mice were injected i.v. with 5 × 105 or 1 × 104 B16F10 (day 0). Some groups of mice then received PBS or IL-15 (0.5 μg)/IL-15Rα (3.0 μg) i.p. on d 0 and 3. In B-E on day 14, lungs were harvested, and the metastatic burden was quantified by counting colonies on the lung surface. Data are presented as mean ± SEM. Each experiment was performed once. Legends indicates the group of mice and the treatment per condition. Statistical significance was determined by one-way ANOVA with Tukey’s multiple comparisons test with *, P<0.05; **, P<0.01; ***P<0.001 and ****, P<0.0001.
Altogether, our findings show that IL-15 increased CD226 and TIGIT expression by NK cells in a STAT3/5-dependent fashion. Prolonged IL-15 stimulation together with TIGIT blockade increased the TiNK degranulation capacity and lysis of MHC class I-deficient melanoma. TIGIT blockade and TIGIT loss in NK cells were effective against tumor metastasis only in the presence of IL-15. The antitumor activity of IL-15 and TIGIT blockade were abrogated by CD226 blockade, supporting that TIGIT and CD226 act antagonistically to regulate the antitumor effector function of NK cells in vivo. Interestingly the antitumor effects of Tigit−/− NK cells in Rag2−/−γc−/− mice with melanoma suggest that TIGIT depletion in NK cells enhanced NK cell-mediated antitumor reactivity, independently of CD8+ T cells.
Discussion
In the present study, our findings support the development of combinatorial immunotherapy with IL-15 and TIGIT blockade to reinvigorate TiNKs against MHC class I-deficient melanoma. We observed that NK cells were present at low frequencies in human MM, were more dysfunctional and downregulated both TIGIT and CD226 as compared with cNKs. CD226 expression is downregulated in TiNKs as compared with cNKs and correlates with the levels of CD155 expression by melanoma cells in the TME. Noteworthy, CD226 appears to regulate T cell responses to PD-1 blockade and combinatorial therapy in two mouse tumor models (44). Upon PD-1 blockade, T cells upregulate CD226, which is the substrate for dephosphorylation by SHP2 upon PD-1 engagement (44). These findings in mouse T cells may not be relevant to human NK cells for several reasons. First, these data were obtained in mouse tumor models that are responsive to PD-1 blockade with no significant CD226 downregulation by T cells in the TME, unlike solid human tumors (15). Second, and most importantly, the reported CD226 effects are mediated by PD-1 signaling in T cells (SHP2), which upregulate PD-1 in the TME. In sharp contrast with these findings, we show that human NK cells, unlike mouse NK cells, do not upregulate PD-1 expression in the periphery nor at tumor sites. TiNKs displayed decreased expression of multiple activation/maturation markers including CD16 and NKG2D. Our findings are reminiscent of previous studies supporting that the downregulation of multiple ARs by TiNKs is associated with NK cell dysfunction and decreased tumor lysis capacity in many human tumors, including breast, ovarian, lung and prostate cancers (4–7,9). Furthermore, TiNKs exhibit decreased degranulation capacity that inversely correlated with the percentages of invading melanoma tumor cells (26), supporting the role of tumor cells in driving NK cell dysfunction. The phenotypic features of TiNKs strongly contrasts with those of human CD8+ tumor-infiltrating lymphocytes, which upregulate TIGIT as well as others IRs like PD-1 and Tim-3 through Prdm1 and c-Maf activation downstream of TCR activation (45) while they downregulate CD226 expression in the TME, resulting in an imbalance of TIGIT/CD226 expression. Strikingly, our findings in melanoma sharply contrast with those recently published in colon cancers, suggesting increased TIGIT expression by intratumor NK cells as compared with peritumor NKs (27). In this study including 19 colon tumors, it is unclear how the investigators precisely isolated low-frequency intratumor and peritumor NKs for flow cytometry. One may also wonder whether the pathogens in the gut microbiome, which act on innate and adative immunity (46), may critically influence the activation and phenotype of NK cells in colon tumors. In addition, and in contrast to humans, mouse cNKs express very low level TIGIT (19,47), which makes comparative studies of TIGIT expression between mice and humans very difficult.
Several lines of evidence support the role of the TIGIT/CD226 axis in regulating NK cell-mediated tumor killing capacity. As compared with TIGIT− NK cells, TIGIT+ NK cells, both in the periphery and at tumor site, upregulated multiple activation/effector markers including granzymes and perforin, supporting that TIGIT is a marker of NK cell activation (48). Paradoxically, TIGIT+ NK cells exhibited lower killing activity against CD155+ MHC class-I deficient melanoma FO-I as compared with TIGIT− NK cells. Such findings are in line with previous published findings (48,49). They are also reminiscent of exhausted CD8+ T cells, which upregulate multiple IRs, are dysfunctional, and display increased levels of perforin and granzymes despite lower degranulation capacities in the presence of target cells as compared to non-exhausted CD8+ T cells (50,51). The degranulation capacity of cNKs and TiNKs against CD155+ MHC class-I deficient melanoma FO-I inversely correlated with cell surface TIGIT/CD226 ratio, supporting the role of the TIGIT/CD226 axis in regulating NK cell effector functions. Our findings showed that mbCD155, but not sCD155, induced both CD226 and TIGIT internalization with CD226 but not TIGIT degradation. Multiple ARs and IRs, which regulate NK cell activation, are downregulated upon binding to their respective ligands, including NKG2D and 2B4 (52,53). Receptor downregulation upon ligand binding contributes to the regulation of receptor signaling in NK cells (54). In sharp contrast with NKG2D and soluble MIC-A/MIC-B (52), sCD155, which is present in the serum of patients with advanced cancers (55), did not induce CD226 endocytosis. In addition, CD226 downregulation correlated with the levels of mbCD155 expression, supporting that CD226 downregulation electively occurs in the TME. Although CD226 shedding by metalloproteases has been previously reported in vitro (56), metalloprotease inhibitors failed to prevent CD155-mediated CD226 downregulation by cNKs. Notably, CD96, which also binds to CD155, was not significantly downregulated by NKs in presence of mbCD155 in vitro. Collectively, our findings support that mbCD155 acts as a master regulator of the TIGIT/CD226 axis to limit NK cell tumor killing capacities in the TME. This observation adds to the previous findings supporting that the downregulation of ARs, such as NKG2D, NKp46 and NKp30, impedes the tumor-killing capacity of TiNKs (4–7). The nature of the intracellular motifs that differentially drive the internalization, recycling or degradation of TIGIT, CD226 and CD96 remain to be identified.
TIGIT blockade alone increased cNK cell killing of CD155+ MHC class I-deficient melanoma, which was abrogated upon CD226 blockade, but had no significant effect on dysfunctional TiNKs, which downregulate both TIGIT and CD226 and exhibit lower killing capacities. While IL-15 increased NK cell-mediated killing capacity, it also increased CD226 and TIGIT expression by NKs in a STAT3/5-dependent fashion. Prolonged IL-15 stimulation together with TIGIT blockade increased the human TiNK-mediated lysis of MHC class I-deficient melanoma. TIGIT blockade or TIGIT loss in NK cells only decreased tumor metastasis in two lung metastasis mouse models in the presence of IL-15. Interestingly the effects of IL-15 and TIGIT blockade on NK cells in vivo were abrogated upon CD226 blockade. These findings support that TIGIT and CD226 exert antagonistic effects to regulate the antitumor effector function of NK cells. They also suggest that combinatorial therapy with IL-15 and TIGIT blockade promotes CD226 engagement of CD155 on NK cells to augment their effector functions that may occur through phosphorylation-mediated inactivation of transcription factor FOXO1 (57). We cannot exclude that the upregulation of other activating receptors like NKG2D by NK cells upon IL-15 plays a role in NK cell-mediated tumor reactivity. This will need to be further investigated. Interestingly, sustained IL-15 stimulation of NK cells in vitro and in vivo appears to promote initial proliferation and maturation, followed with NK cell exhaustion with impaired activation, cytotoxicity and proliferative capacity (58,59). Therefore, IL-15 dosage and administration schedule will need to be carefully designed to avoid NK cell exhaustion.
In a recent study in mouse-tumor bearing models with lung metastases (27), the therapeutic effects of PD-1, TIGIT or dual PD-1/TIGIT blockade were reported to act primarily on NK cells to enhance antitumor activity mediated by CD8+ T cells. The mechanisms used by NK cells to regulate adaptive immunity upon TIGIT blockade have not yet been elucidated. Our findings in mice and in humans do not support these conclusions for several reasons. First, TIGIT blockade and TIGIT loss in NK cells were not effective against lung melanoma metastasis in the absence of IL-15. Second, the antitumor effects of Tigit−/− NK cells in Rag2−/−γc−/− mice with melanoma showed that TIGIT depletion in NK cells enhanced NK cell-mediated antitumor reactivity, but only in the presence of exogenous IL-15, and independently of CD8+ T cells. Whether NK cells participate in the environmental signals guiding CD8+ T cell priming, development of CD8+ effector T cells into CD8+ memory T cells or CD8+ memory T-cell maintenance remains to be evaluated. Additional mechanistic studies are needed to thoroughly investigate these questions.
In summary, the present study shows that mbCD155 triggers CD226 internalization and degradation by NK cells, resulting in increased cell surface TIGIT/CD226 expression ratio and decreased NK cell-mediated tumor reactivity. IL-15 together with TIGIT blockade reinvigorates TiNK-mediated killing of melanoma cells in vitro and in vivo in a CD226-dependent fashion. Altogether, our findings may support the development of novel combinational immunotherapy with IL-15 and TIGIT blockade to promote NK cell-mediated killing of MHC deficient tumors that are refractory to CD8+ T cell-mediated immunity.
Supplementary Material
Translational relevance.
Here, we show that membrane-bound poliovirus receptor (PVR)/CD155 triggers CD226 internalization and degradation, resulting in decreased NK cell-mediated tumor reactivity. We also show that IL-15 increases TIGIT and CD226 gene expression by tumor-infiltrating NK cells, and together with TIGIT blockade, increases NK cell-mediated melanoma cytotoxicity in vitro and decreases tumor metastasis in vivo. Collectively, our findings support the novel combinatorial therapy with IL-15 together with TIGIT blockade to promote NK cell-mediated destruction of MHC class I-deficient melanoma, which are refractory to CD8+ T cell-mediated immunity and PD-1 blockade.
Acknowledgments
This work was supported by NIH/NCI grants R01CA228181 and R01CA222203 (to HMZ), a research grant by Bristol-Myers Squibb BMS (to HMZ), a cancer vaccine collaborative clinical strategy team grant (to HMZ), and NCI grant P50CA121973 (to JMK). MJS was supported by a National Health and Medical Research Council of Australia (NH&MRC) Senior Principal Research Fellowship (1078671), a NH&MRC Program grant (1132519), a NH&MRC Project grant (1124784), a CLIP award from the Cancer Research Institute (New-York, NY), and a Project Grant from the Cancer Council of Queensland (1140251). This work benefited from ImageStreamX MARKII grant NIH 1S10OD019942-01.
Footnotes
MJS has a scientific research agreement with Bristol Myers Squibb and Tizona Therapeutics and is on the scientific advisory board of Tizona Therapeutics and Compass Therapeutics.
References
- 1.Guillerey C, Huntington ND, Smyth MJ. Targeting natural killer cells in cancer immunotherapy. Nat Immunol 2016;17(9):1025–36 doi 10.1038/ni.3518. [DOI] [PubMed] [Google Scholar]
- 2.Muntasell A, Ochoa MC, Cordeiro L, Berraondo P, Lopez-Diaz de Cerio A, Cabo M, et al. Targeting NK-cell checkpoints for cancer immunotherapy. Curr Opin Immunol 2017;45:73–81 doi 10.1016/j.coi.2017.01.003. [DOI] [PubMed] [Google Scholar]
- 3.Sconocchia G, Arriga R, Tornillo L, Terracciano L, Ferrone S, Spagnoli GC. Melanoma cells inhibit NK cell functions. Cancer Res 2012;72(20):5428–9; author reply 30 doi 10.1158/0008-5472.CAN-12-1181. [DOI] [PubMed] [Google Scholar]
- 4.Mamessier E, Sylvain A, Bertucci F, Castellano R, Finetti P, Houvenaeghel G, et al. Human Breast Tumor Cells Induce Self-Tolerance Mechanisms to Avoid NKG2D-Mediated and DNAM-Mediated NK Cell Recognition. Cancer Research 2011;71(21):6621–32 doi 10.1158/0008-5472.Can-11-0792. [DOI] [PubMed] [Google Scholar]
- 5.Mamessier E, Sylvain A, Thibult ML, Houvenaeghel G, Jacquemier J, Castellano R, et al. Human breast cancer cells enhance self tolerance by promoting evasion from NK cell antitumor immunity. J Clin Invest 2011;121(9):3609–22 doi 10.1172/Jci45816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Platonova S, Cherfils-Vicini J, Damotte D, Crozet L, Vieillard V, Validire P, et al. Profound coordinated alterations of intratumoral NK cell phenotype and function in lung carcinoma. Cancer Res 2011;71(16):5412–22 doi 10.1158/0008-5472.CAN-10-4179. [DOI] [PubMed] [Google Scholar]
- 7.Carlsten M, Norell H, Bryceson YT, Poschke I, Schedvins K, Ljunggren HG, et al. Primary human tumor cells expressing CD155 impair tumor targeting by down-regulating DNAM-1 on NK cells. J Immunol 2009;183(8):4921–30 doi 10.4049/jimmunol.0901226. [DOI] [PubMed] [Google Scholar]
- 8.Fregni G, Messaoudene M, Fourmentraux-Neves E, Mazouz-Dorval S, Chanal J, Maubec E, et al. Phenotypic and functional characteristics of blood natural killer cells from melanoma patients at different clinical stages. Plos One 2013;8(10):e76928 doi 10.1371/journal.pone.0076928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pasero C, Gravis G, Guerin M, Granjeaud S, Thomassin-Piana J, Rocchi P, et al. Inherent and Tumor-Driven Immune Tolerance in the Prostate Microenvironment Impairs Natural Killer Cell Antitumor Activity. Cancer Res 2016;76(8):2153–65 doi 10.1158/0008-5472.CAN-15-1965. [DOI] [PubMed] [Google Scholar]
- 10.Vivier E, Ugolini S, Blaise D, Chabannon C, Brossay L. Targeting natural killer cells and natural killer T cells in cancer. Nat Rev Immunol 2012;12(4):239–52 doi 10.1038/nri3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stanietsky N, Simic H, Arapovic J, Toporik A, Levy O, Novik A, et al. The interaction of TIGIT with PVR and PVRL2 inhibits human NK cell cytotoxicity. P Natl Acad Sci USA 2009;106(42):17858–63 doi 10.1073/pnas.0903474106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu X, Harden K, Gonzalez LC, Francesco M, Chiang E, Irving B, et al. The surface protein TIGIT suppresses T cell activation by promoting the generation of mature immunoregulatory dendritic cells. Nat Immunol 2009;10(1):48–57 doi 10.1038/ni.1674. [DOI] [PubMed] [Google Scholar]
- 13.Zhu Y, Paniccia A, Schulick AC, Chen W, Koenig MR, Byers JT, et al. Identification of CD112R as a novel checkpoint for human T cells. J Exp Med 2016;213(2):167–76 doi 10.1084/jem.20150785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murter B, Pan X, Ophir E, Alteber Z, Azulay M, Sen R, et al. Mouse PVRIG Has CD8(+) T Cell-Specific Coinhibitory Functions and Dampens Antitumor Immunity. Cancer Immunol Res 2019;7(2):244–56 doi 10.1158/2326-6066.CIR-18-0460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chauvin JM, Pagliano O, Fourcade J, Sun ZJ, Wang H, Sander C, et al. TIGIT and PD-1 impair tumor antigen-specific CD8(+) T cells in melanoma patients. J Clin Invest 2015;125(5):2046–58 doi 10.1172/Jci80445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnston RJ, Comps-Agrar L, Hackney J, Yu X, Huseni M, Yang Y, et al. The immunoreceptor TIGIT regulates antitumor and antiviral CD8(+) T cell effector function. Cancer Cell 2014;26(6):923–37 doi 10.1016/j.ccell.2014.10.018. [DOI] [PubMed] [Google Scholar]
- 17.Kurtulus S, Sakuishi K, Ngiow SF, Joller N, Tan DJ, Teng MW, et al. TIGIT predominantly regulates the immune response via regulatory T cells. J Clin Invest 2015;125(11):4053–62 doi 10.1172/JCI81187. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18.Fourcade J, Sun Z, Chauvin JM, Ka M, Davar D, Pagliano O, et al. CD226 opposes TIGIT to disrupt Tregs in melanoma. JCI Insight 2018;3(14) doi 10.1172/jci.insight.121157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li M, Xia P, Du Y, Liu S, Huang G, Chen J, et al. T-cell immunoglobulin and ITIM domain (TIGIT) receptor/poliovirus receptor (PVR) ligand engagement suppresses interferon-gamma production of natural killer cells via beta-arrestin 2-mediated negative signaling. J Biol Chem 2014;289(25):17647–57 doi 10.1074/jbc.M114.572420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu S, Zhang H, Li M, Hu D, Li C, Ge B, et al. Recruitment of Grb2 and SHIP1 by the ITT-like motif of TIGIT suppresses granule polarization and cytotoxicity of NK cells. Cell Death Differ 2013;20(3):456–64 doi 10.1038/cdd.2012.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ramsbottom KM, Hawkins ED, Shimoni R, McGrath M, Chan CJ, Russell SM, et al. Cutting edge: DNAX accessory molecule 1-deficient CD8+ T cells display immunological synapse defects that impair antitumor immunity. J Immunol 2014;192(2):553–7 doi 10.4049/jimmunol.1302197. [DOI] [PubMed] [Google Scholar]
- 22.Shibuya K, Lanier LL, Phillips JH, Ochs HD, Shimizu K, Nakayama E, et al. Physical and functional association of LFA-1 with DNAM-1 adhesion molecule. Immunity 1999;11(5):615–23 doi 10.1016/s1074-7613(00)80136-3. [DOI] [PubMed] [Google Scholar]
- 23.Gilfillan S, Chan CJ, Cella M, Haynes NM, Rapaport AS, Boles KS, et al. DNAM-1 promotes activation of cytotoxic lymphocytes by nonprofessional antigen-presenting cells and tumors. J Exp Med 2008;205(13):2965–73 doi 10.1084/jem.20081752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iguchi-Manaka A, Kai H, Yamashita Y, Shibata K, Tahara-Hanaoka S, Honda S, et al. Accelerated tumor growth in mice deficient in DNAM-1 receptor. J Exp Med 2008;205(13):2959–64 doi 10.1084/jem.20081611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lakshmikanth T, Burke S, Ali TH, Kimpfler S, Ursini F, Ruggeri L, et al. NCRs and DNAM-1 mediate NK cell recognition and lysis of human and mouse melanoma cell lines in vitro and in vivo. J Clin Invest 2009;119(5):1251–63 doi 10.1172/JCI36022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Messaoudene M, Fregni G, Fourmentraux-Neves E, Chanal J, Maubec E, Mazouz-Dorval S, et al. Mature cytotoxic CD56(bright)/CD16(+) natural killer cells can infiltrate lymph nodes adjacent to metastatic melanoma. Cancer Res 2014;74(1):81–92 doi 10.1158/0008-5472.CAN-13-1303. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Q, Bi J, Zheng X, Chen Y, Wang H, Wu W, et al. Blockade of the checkpoint receptor TIGIT prevents NK cell exhaustion and elicits potent anti-tumor immunity. Nat Immunol 2018;19(7):723–32 doi 10.1038/s41590-018-0132-0. [DOI] [PubMed] [Google Scholar]
- 28.Zaretsky JM, Garcia-Diaz A, Shin DS, Escuin-Ordinas H, Hugo W, Hu-Lieskovan S, et al. Mutations Associated with Acquired Resistance to PD-1 Blockade in Melanoma. N Engl J Med 2016;375(9):819–29 doi 10.1056/NEJMoa1604958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gettinger S, Choi J, Hastings K, Truini A, Datar I, Sowell R, et al. Impaired HLA Class I Antigen Processing and Presentation as a Mechanism of Acquired Resistance to Immune Checkpoint Inhibitors in Lung Cancer. Cancer Discov 2017;7(12):1420–35 doi 10.1158/2159-8290.CD-17-0593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.D’Urso CM, Wang ZG, Cao Y, Tatake R, Zeff RA, Ferrone S. Lack of HLA class I antigen expression by cultured melanoma cells FO-1 due to a defect in B2m gene expression. J Clin Invest 1991;87(1):284–92 doi 10.1172/JCI114984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zarour HM, Storkus WJ, Brusic V, Williams E, Kirkwood JM. NY-ESO-1 encodes DRB1*0401-restricted epitopes recognized by melanoma-reactive CD4+ T cells. Cancer Res 2000;60(17):4946–52. [PubMed] [Google Scholar]
- 32.Blake SJ, Stannard K, Liu J, Allen S, Yong MC, Mittal D, et al. Suppression of Metastases Using a New Lymphocyte Checkpoint Target for Cancer Immunotherapy. Cancer Discov 2016;6(4):446–59 doi 10.1158/2159-8290.CD-15-0944. [DOI] [PubMed] [Google Scholar]
- 33.Chan CJ, Martinet L, Gilfillan S, Souza-Fonseca-Guimaraes F, Chow MT, Town L, et al. The receptors CD96 and CD226 oppose each other in the regulation of natural killer cell functions. Nat Immunol 2014;15(5):431–8 doi 10.1038/ni.2850. [DOI] [PubMed] [Google Scholar]
- 34.Gao Y, Souza-Fonseca-Guimaraes F, Bald T, Ng SS, Young A, Ngiow SF, et al. Tumor immunoevasion by the conversion of effector NK cells into type 1 innate lymphoid cells. Nat Immunol 2017;18(9):1004–15 doi 10.1038/ni.3800. [DOI] [PubMed] [Google Scholar]
- 35.Xu F, Sunderland A, Zhou Y, Schulick RD, Edil BH, Zhu Y. Blockade of CD112R and TIGIT signaling sensitizes human natural killer cell functions. Cancer Immunol Immunother 2017;66(10):1367–75 doi 10.1007/s00262-017-2031-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cullen PJ, Steinberg F. To degrade or not to degrade: mechanisms and significance of endocytic recycling. Nat Rev Mol Cell Biol 2018;19(11):679–96 doi 10.1038/s41580-018-0053-7. [DOI] [PubMed] [Google Scholar]
- 37.Huss M, Wieczorek H. Inhibitors of V-ATPases: old and new players. J Exp Biol 2009;212(Pt 3):341–6 doi 10.1242/jeb.024067. [DOI] [PubMed] [Google Scholar]
- 38.Wilson EB, El-Jawhari JJ, Neilson AL, Hall GD, Melcher AA, Meade JL, et al. Human tumour immune evasion via TGF-beta blocks NK cell activation but not survival allowing therapeutic restoration of anti-tumour activity. Plos One 2011;6(9):e22842 doi 10.1371/journal.pone.0022842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Levanon D, Negreanu V, Lotem J, Bone KR, Brenner O, Leshkowitz D, et al. Transcription factor Runx3 regulates interleukin-15-dependent natural killer cell activation. Mol Cell Biol 2014;34(6):1158–69 doi 10.1128/MCB.01202-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yin X, Liu T, Wang Z, Ma M, Lei J, Zhang Z, et al. Expression of the Inhibitory Receptor TIGIT Is Up-Regulated Specifically on NK Cells With CD226 Activating Receptor From HIV-Infected Individuals. Front Immunol 2018;9:2341 doi 10.3389/fimmu.2018.02341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Easom NJW, Stegmann KA, Swadling L, Pallett LJ, Burton AR, Odera D, et al. IL-15 Overcomes Hepatocellular Carcinoma-Induced NK Cell Dysfunction. Front Immunol 2018;9:1009 doi 10.3389/fimmu.2018.01009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vuletic A, Jovanic I, Jurisic V, Milovanovic Z, Nikolic S, Spurnic I, et al. IL-2 And IL-15 Induced NKG2D, CD158a and CD158b Expression on T, NKT- like and NK Cell Lymphocyte Subsets from Regional Lymph Nodes of Melanoma Patients. Pathol Oncol Res 2020;26(1):223–31 doi 10.1007/s12253-018-0444-2. [DOI] [PubMed] [Google Scholar]
- 43.Ndhlovu LC, Lopez-Verges S, Barbour JD, Jones RB, Jha AR, Long BR, et al. Tim-3 marks human natural killer cell maturation and suppresses cell-mediated cytotoxicity. Blood 2012;119(16):3734–43 doi 10.1182/blood-2011-11-392951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang B, Zhang W, Jankovic V, Golubov J, Poon P, Oswald EM, et al. Combination cancer immunotherapy targeting PD-1 and GITR can rescue CD8(+) T cell dysfunction and maintain memory phenotype. Sci Immunol 2018;3(29) doi 10.1126/sciimmunol.aat7061. [DOI] [PubMed] [Google Scholar]
- 45.Chihara N, Madi A, Kondo T, Zhang H, Acharya N, Singer M, et al. Induction and transcriptional regulation of the co-inhibitory gene module in T cells. Nature 2018;558(7710):454–9 doi 10.1038/s41586-018-0206-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Belkaid Y, Hand TW. Role of the microbiota in immunity and inflammation. Cell 2014;157(1):121–41 doi 10.1016/j.cell.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stanietsky N, Rovis TL, Glasner A, Seidel E, Tsukerman P, Yamin R, et al. Mouse TIGIT inhibits NK-cell cytotoxicity upon interaction with PVR. Eur J Immunol 2013;43(8):2138–50 doi 10.1002/eji.201243072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang F, Hou HY, Wu SJ, Tang Q, Liu WY, Huang M, et al. TIGIT expression levels on human NK cells correlate with functional heterogeneity among healthy individuals. European Journal of Immunology 2015;45(10):2886–97 doi 10.1002/eji.201545480. [DOI] [PubMed] [Google Scholar]
- 49.Vendrame E, Seiler C, Ranganath T, Zhao NQ, Vergara R, Alary M, et al. TIGIT is upregulated by HIV-1 infection and marks a highly functional adaptive and mature subset of natural killer cells. AIDS 2020;34(6):801–13 doi 10.1097/QAD.0000000000002488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Paley MA, Kroy DC, Odorizzi PM, Johnnidis JB, Dolfi DV, Barnett BE, et al. Progenitor and terminal subsets of CD8+ T cells cooperate to contain chronic viral infection. Science 2012;338(6111):1220–5 doi 10.1126/science.1229620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sakhdari A, Mujib S, Vali B, Yue FY, MacParland S, Clayton K, et al. Tim-3 negatively regulates cytotoxicity in exhausted CD8+ T cells in HIV infection. Plos One 2012;7(7):e40146 doi 10.1371/journal.pone.0040146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Groh V, Wu J, Yee C, Spies T. Tumour-derived soluble MIC ligands impair expression of NKG2D and T-cell activation. Nature 2002;419(6908):734–8 doi 10.1038/nature01112. [DOI] [PubMed] [Google Scholar]
- 53.Sandusky MM, Messmer B, Watzl C. Regulation of 2B4 (CD244)-mediated NK cell activation by ligand-induced receptor modulation. Eur J Immunol 2006;36(12):3268–76 doi 10.1002/eji.200636146. [DOI] [PubMed] [Google Scholar]
- 54.Masilamani M, Peruzzi G, Borrego F, Coligan JE. Endocytosis and intracellular trafficking of human natural killer cell receptors. Traffic 2009;10(12):1735–44 doi 10.1111/j.1600-0854.2009.00973.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Iguchi-Manaka A, Okumura G, Kojima H, Cho Y, Hirochika R, Bando H, et al. Increased Soluble CD155 in the Serum of Cancer Patients. Plos One 2016;11(4):e0152982 doi 10.1371/journal.pone.0152982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xu Z, Zhang T, Zhuang R, Zhang Y, Jia W, Song C, et al. Increased levels of soluble CD226 in sera accompanied by decreased membrane CD226 expression on peripheral blood mononuclear cells from cancer patients. BMC Immunol 2009;10:34 doi 10.1186/1471-2172-10-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Du X, de Almeida P, Manieri N, de Almeida Nagata D, Wu TD, Harden Bowles K, et al. CD226 regulates natural killer cell antitumor responses via phosphorylation-mediated inactivation of transcription factor FOXO1. Proc Natl Acad Sci U S A 2018;115(50):E11731–E40 doi 10.1073/pnas.1814052115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Elpek KG, Rubinstein MP, Bellemare-Pelletier A, Goldrath AW, Turley SJ. Mature natural killer cells with phenotypic and functional alterations accumulate upon sustained stimulation with IL-15/IL-15Ralpha complexes. Proc Natl Acad Sci U S A 2010;107(50):21647–52 doi 10.1073/pnas.1012128107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Felices M, Lenvik AJ, McElmurry R, Chu S, Hinderlie P, Bendzick L, et al. Continuous treatment with IL-15 exhausts human NK cells via a metabolic defect. JCI Insight 2018;3(3) doi 10.1172/jci.insight.96219. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.