Abstract
Since the outbreak of the novel severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), the control of virus spread has remained challenging given the pitfalls of the current diagnostic tests. Nevertheless, RNA amplification techniques have been the gold standard among other diagnostic methods for monitoring clinical samples for the presence of the virus. In the current paper, we review the shortcomings and strengths of RT-PCR (real-time polymerase chain reaction) techniques for diagnosis of coronavirus disease (COVID)-19. We address the repercussions of false-negative and false-positive rates encountered in the test, summarize approaches to improve the overall sensitivity of this method. We discuss the barriers to the widespread use of the RT-PCR test, and some technical advances, such as RT-LAMP (reverse-transcriptase-loop mediated isothermal amplification). We also address how other molecular techniques, such as immunodiagnostic tests can be used to avoid incorrect interpretation of RT-PCR tests.
Abbreviations: cDNA, complementary DNA; CLIA, chemiluminescence immunoassay; COVID-19, Coronavirus Disease 2019; CRISPR, clustered regularly interspaced short palindromic repeats; CT, computed tomography scan; DNA, deoxyribonucleic acid; EDTA, ethylene-diamine-tetra-acetic acid; ELISA, enzyme-linked immunosorbent assay; IgG, immunoglobulin G; iLACO, isothermal LAMP-based method for COVID-19; NP, nasopharyngeal; NPV, negative predictive value; OP, oropharyngeal; RBD, receptor binding domain of the virus; RNA, ribonucleic acid; RT-LAMP, reverse transcription loop-mediated isothermal amplification; RT–PCR, reverse transcription of polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus; SRT, sampling-to-result time; TCEP, tris (2-carboxyethyl) phosphine
Keywords: SARS-CoV-2, COVID-19, Reverse transcription polymerase chain reaction, Diagnostic tests, Reverse transcription loop-mediated isothermal amplification, Serologic tests
1. Introduction
In early December 2019, the first case of a severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was reported in Wuhan, China, and ever since, there has been a growing spread of the coronavirus disease 19 (COVID-19) all over the world [1]. While the disease is widely known to be a deadly disease, some patients are asymptomatic but can still transmit the virus [2]. This has made tracing the disease difficult solely based on clinical symptoms, and undetected SARS-CoV-2 infection has posed serious challenges regarding control of the disease spread. Currently, the virus outbreak has reached pandemic proportions with over 3 million deaths across the world [2], underlining the rapid spread and the urgent need for control of disease transmission. Therefore, because widespread vaccination against SARS-CoV-2 will take some considerable time, keeping the disease transmission under control is a high priority, and there is a need to drastically improve the efficiency of the present diagnostic tests.
Currently, diagnostic techniques based on viral RNA amplification, specifically qRT-PCR (quantitative real-time polymerase chain reaction), are the gold standard diagnostic methods for COVID-19 [3,4]. Unlike other molecular tests that do not have perfect diagnostic specificity, qRT-PCR is highly specific with a specificity of almost 100 % [5]. This has led to RT-PCR becoming the gold standard molecular diagnostic test.
However, the RT-PCR test for SARS-CoV-2 virus does have some pitfalls that necessitate improvements in the way the method is used. As with immunodiagnostic tests, the RT-PCR test can have difficulties in distinguishing between true positive and true negative COVID-19 infected individuals [6]. The test fails in a considerable proportion of suspected and confirmed patients with clinical implications; as a result, it is a wise precaution not to rely on PCR test results alone, and to consider other clinical and molecular evidence [5]. This means that one always should take into account a combination of clinical and molecular evidence before sending a suspected patient home as disease-free. Furthermore, given the challenges with RT-PCR test results, repetition of the test over time and on multiple samples enhances the overall sensitivity of the test.
Moreover, it is necessary to improve the RT-PCR methodology to tackle the problem of less than perfect sensitivity. This could be achieved by designing more simple versions of the test. Simple tests provide opportunities for more wide-spread application among different components of the health-care system. A simple test requires less training and could enable other health-care staff to use the test correctly. It also minimizes the risk of disease transmission to the staff, and test failure due to improper manipulation of the clinical samples. Furthermore, simplification of the test can shorten the gap between sampling and results, allowing the repetition of the test over time or on multiple samples if needed. Finally, the simpler the test is, the more likely it can be offered at a lower cost per test [7].
In the present paper, we will review publications discussing the diagnostic ability of the RT-PCR test as well as the implications of its failure, and some ways of maximizing the current molecular diagnosis for COVID-19 will be addressed. We cover the clinical evidence for RT-PCR results in COVID-19 patients, approaches adopted to enhance the test efficacy, and recent technological developments in the design of the test. The false-negative and false-positive results of the RT-PCR test are discussed, along with the advantages of the combined use of immunodiagnostic tests together with RT-PCR.
2. False-negative RT-PCR results in infected cases
False-negative results in a screening test can have serious implications during a pandemic, such as COVID-19 because a proportion of true infected cases are categorized as disease-free and can unintentionally transmit the disease. Unfortunately, there is no single molecular test that can guarantee the infection free status for a suspected case; therefore, the clinical history and social contacts of the individual should be always taken into account in the assessment of the infection probability. Repetition of the molecular tests over time also helps to increase the selectivity.
Reports have described RT-PCR on various specimens obtained from the respiratory tract; however, there are accumulating reports indicating the lack of adequate sensitivity for the test. RT-PCR examination of nasal and oropharyngeal swabs, nasopharyngeal washing or aspirate is recommended as the gold standard for the diagnosis of COVID-19 [[8], [9], [10]], However, the overall sensitivity of the RT-PCR test is reportedly between 45–60% in nasopharyngeal aspirate and swab samples [11]. For instance, Yang et al. reported a false negative rate of 11 % for sputum, 27 % for nasal, and 40 % for throat swabs within the first seven days from onset of illness in 213 patients hospitalized with COVID-19 [12]. Similarly, Zhao et al. reported a false negative rate of RT-PCR tests within the same range. These researchers reported that 33 % of respiratory samples gave false-negative results in 173 hospitalized patients with COVID-19 as diagnosed with typical chest CT scans and acute respiratory symptoms [12]. The false-negative rate ranged from 2% to 29 % in a systematic review of five other studies containing a total of 957 patients suspected or confirmed with COVID-19 [13].
One of the main reasons for such a high false-negative rate in RT-PCR results, is the time of sampling after the onset of symptoms. The time of sampling is important because it was shown that the false-negative rate of the test varies over time [14]. The false-negative rate of RT-PCR testing on nasopharyngeal (NP) and oropharyngeal samples was described as "shockingly high" in a study of 1330 confirmed cases. In their investigation, the authors pooled the data on the confirmed COVID-19 cases from seven previously published studies. They analyzed these data using a Bayesian hierarchical model to estimate the false-negative rate from 5 days before the onset of symptoms up to 21-days post-emergence of symptoms. They found that the median false-negative rate reduced gradually from 100 % to 20 % during days 3 and 4 post-onset of symptoms. The false-negative rate was 67 % one day before the onset of symptoms and 38 % on the day of symptom manifestation, and returned gradually to 66 % on day 21 post-onset of symptoms. Consequently, the false-negative rate of the test changes over time depending on when the samples were obtained from the onset of symptoms, and even at best, the RT-PCR fails to detect a considerable fraction (one out of five) of the infected cases [14].
Another explanation for the high negative rate of RT-PCR tests for COVID-19 could be related to the viral load present in the sampling site from each patient. This could vary among different specimens and patients. The highest viral loads are found in the lower respiratory tracts of COVID-19 patients compared to the upper respiratory tract [15]. However, sampling from the lower respiratory tract is difficult in patients with severe respiratory symptoms who are receiving oxygenation intervention [16]. In the upper tract, nasopharyngeal and oropharyngeal swaps or aspirates are recommended for early diagnosis of the infection. NP samples exhibited much higher viral loads compared to OP samples, giving a better chance detecting SARS-CoV-2 infection and lowering the risk of missing the infection [17]. Moreover, false-negative results occurred in some patients with gastrointestinal symptoms. In these patients, sampling from fecal samples revealed the presence of SARS-CoV-2 [16]. Therefore, some false-negative results are inevitable depending on the specimen chosen and the patient clinical symptoms.
Given the imperfect selectivity of the RT-PCR test, other diagnostic information should be taken into account to achieve the desirable sensitivity for true-positives or true-negatives for COVID-19. These factors include the clinical symptoms, immunodiagnostic test results, and prevalence of the disease within the community. These factors can help clinicians to better estimate how likely any particular case is to have disease. For instance, whether or not a case demonstrates the typical clinical symptoms of COVID-19 can give a primary estimate of the probability of the case being infected, and successive addition of the molecular test results (e.g. RT-PCR and serologic tests) will increase the confidence to distinguish between disease-free or infected. Furthermore, RT-PCR in combination with an immunodiagnostic test will improve the overall selectivity [18]. For example, in a retrospective study of 375 patients, the combined selectivity of RT-PCR and antibody testing was significantly higher compared to each test alone. The diagnostic sensitivity of the RT-PCR and antibody test were 95.7 % and 92.2 % respectively, which rose significantly to 98.6 % when these tests were combined [17]. Lastly, the prevalence of the disease should be taken into account in deciding whether or not a particular result is enough to send a person home as disease-free. The low prevalence of disease implies a low probability of having the disease, and a post-test negative result with a given sensitivity (say 95 %) could suggest a person is highly likely to be disease-free. However, when the prevalence of disease increases throughout a community, that level of sensitivity is less valuable to ensure a suspected patient is disease-free. In technical terms, the negative predictive value (NPV) of the test decreases with an increase in the prevalence of the disease [19].
To sum up, the false-negative rate of RT-PCR is significant and varies across different specimens and time periods. However, the false-negative rate can be minimized when immunodiagnostic tests and clinical symptoms are considered along with the RT-PCR test result. Moreover, it is importannt to stick to social distancing and recommended hygiene protocols to keep the prevalence of the disease as low as possible, in order to maintain the NPV of the tests at a high level. Otherwise, the negative results of PCR tests will no longer give us enough confidence that the suspected case is disease-free.
3. How to use the RT-PCR test more effectively given its false-negative results?
Due to the high rate of false-negative results in RT-PCR of COVID-19 patients, and the epidemiological prevalence, some improvements can be suggested.
The false-negative rate of the RT-PCR test can occur due to several reasons. Firstly, the viral load can be low or absent within the samples [19]. The viral load governs the amount of RNA in the samples. The higher the viral load in the sample, the more RNA with a better chance for a test to get a truly positive result. Secondly, the viral RNA might be subjected to denaturation or degradation in the samples due to improper manipulation or storage, which lowers the final amount of intact RNA for the test [20]. Thirdly, a sufficient viral load is limited to specific time periods when the virus rapidly replicates itself and is shed from the cells. Fourthly, the viral load has also shown to vary in terms of the anatomical site from which the specimen is obtained Lastly, the virus is present at low numbers or is absent in some specimens from some patients, while other specimens might have a higher viral load in the same patients [21]. Therefore, the variability of the false-negative rate depends on the viral load, which in turn, fluctuates over the course of the disease, and between specimens from patients with different clinical characteristics.
The above-mentioned problems can be solved with optimized RT-PCR diagnostic protocols. Given the mentioned viral load variability over time, specimen, and patients, an improved RT-PCR test should be more simple, rapid, and cost-effective to allow frequent repettition [22]. This will increase the chance of detecting the infection if the test is repeated over time and on different samples. If the test can be made rapid and less labor-intensive, the sampling-to-PCR gap time will be shortened, which will reduce the loss of viral RNA due to denaturation during this period. Moreover, a simplified test will require less sophisticated laboratory equipment. These simplified tests could enable rapid point-of-care sample manipulation and analysis, with a higher throughput.
The current RT-PCR test could be used more effectively by conducting the test in pooled samples. Pooling different samples from either the same patient or the patient's family members can reduce the number of tests and lower the costs positive rate of the test. Because in some patients the viral infection is limited to the lower respiratory tract, combining sputum, nasal and pharyngeal swabs coulsd be useful. In other patients with gastrointestinal involvement, the virus was only found in fecal material, while RT-PCR of the NP swabs and sputum were negative. Therefore conducting the test on pooled samples from different specimens can improve the probability of getting a sample with sufficient viral load to increase the accuracy of RT-PCR. The other benefit of pooling samples is to allow better at-home quarantine decisions amongst communities. For instance, pooled samples from the whole family of a suspected case can provide guidance on strict quarantine for the entire family, to reduce disease transmission in the community [23].
Therefore, the repetition of the RT-PCR test in pooled samples might offset the high false-negative rate of the test. Also, the conduction of the test in pooled samples appears to increase the utility of the test for screening purposes. To this end, recent cutting-edge technology has attempted to provide simple point-of-care or at home RT-PCR kits.
4. The preparation of point-of-care RT-PCR tests
The preparation of a simple, rapid, and inexpensive RT-PCR test requires understanding the technical difficulties that have restricted the use of the RT-PCR method. By overcoming these obstacles, the laboratory RT-PCR test can be turned into a convenient, rapid, and budget-friendly kit that can be used more widely in clinics.
5. The problems of the RT-PCR test for extensive use
Technically, the RT-PCR procedure for SARS-CoV-2-infected samples consists of several steps, and needs laboratory equipment that makes the process tedious and difficult to be conducted outside the laboratory setting. First of all, the RNA material must be extracted from the cells and the virions (viral particles) and preserved from destruction by RNase enzymes. This step needs laboratory equipment such as a centrifuge and a laminar flow cabinet, and might lose some of the RNA materials due to denaturation. Secondly, the process of PCR requires thermal-cycling equipment for creating a cyclic temperature change during the process of RNA amplification. The third difficulty is the readout method used, which in most cases required expensive sophisticated spectrofluorometric equipment [24].
Before the RT-PCR step, the viral RNA has to be extracted from the samples. During this process, certain laboratory chemicals and equipment are used for specific purposes. Firstly, the infected cells and the virions are disintegrated by the addition of lysis buffer typically containing detergents (Tween 20 or Triton X100). The lysis of the cells and virions causes all the biomolecules, including viral RNAs to be released into the medium and be readily available for the test. The lysis buffer also contains salts such as sodium iodide (NaI) or guanidinium thiocyanate (GuSCN) that facilitate the separation of the viral RNA from other biomolecules (e.g. cell and viral proteins). Centrifugation of samples containing these salts assists in the separation of these proteins from the viral RNA fraction. Besides, cellular RNase enzymes are inactivated by the addition of detergents and thermal treatment. Some detergents such as TCEP or EDTA are added to the samples, which are incubated at 95 °C for 5 min. The RNase inactivation inhibits the process of RNA denaturation and causes the RNA to be preserved for the RT-PCR test [25].
Subsequently, the RNA samples are amplified during the RT-PCR process using a thermal cycling program. In each cycle, the temperature of the samples is alternatively decreased to 70 °C and increased to 95 °C for a certain time, and at each cycle the number of RNA replicates is doubled. At 70 °C, the single stranded C-DNA replicates are used as the template for the synthesis of new stands of C-DNA. During this step, Taq DNA polymerase synthesizes a new strand of C-DNA from the template which gives rise to a double-strand DNA composed of the old and new strands of C-DNA. At 95 °C, these strands must be detached from each other to give twice the number of single strand templates for the next round of C-DNA replication. This cycle is repeated several times (e.g. for 30 cycles) until the number of C-DNA replicates reaches an amount capable of being detected by spectrofluorimetric techniques. The thermocycler apparatus that provides this accurate cycle of temperature changes is expensive equipment that is often confined to a laboratory [25].
Finally, the increasing number of C-DNA replicates is monitored using a real-time spectrofluorimetric technique that is also expensive and not always available. This technique offers a readout of the C-DNA amplification on a computer screen based on the fluorescent signal that changes increases in line with C-DNA numbers. Different fluorescent probes are used for the quantification of C-DNA replicates in the PCR. For instance, in the SYBR green assay, the fluorescent probe intercalates into the C-DNA double strands that quenches the probe fluorescence. This fluorescent probe de-quenches upon the separation of the C-DNA strands from each other. In the TaqMan assay, the fluorescent probe is de-quenched by the binding of the TaqMan primer to the C-DNA templates. In both techniques, a spectrofluorimetric apparatus coupled to a computer is required for the final readout of the RNA amount in the samples. These pieces of equipment are expensive and may not be available everywhere in large numbers [25].
6. Changing RT-PCR towards a point-of-care test for COVID-19
Given the aforementioned difficulties of the RT-PCR test, enormous efforts have been made to produce an easier, faster, and more convenient test capable of being used outside the laboratory environment. A simple and rapid test can reduce the sampling-to-result time (SRT) and encourage its wider application. The test procedure should require fewer steps and laboratory tools. A shorter SRT and easier manipulation of the sample will have some other benefits, including an increase in the test sensitivity.
One important simplification in the nucleic acid amplification procedure was the invention of an isothermal PCR method that eliminated the need for a thermal cycling apparatus. This allowed the amplification of RNA or DNA using a widely available kitchen oven maintained at a specific constant temperature. In this method there is no need to increase the temperature up to 90 °C to unwind the double-stranded DNA. Instead, the DNA polymerase itself displaces one of the strands of the DNA as it acts on the other strand and synthesizes a new copy. During this process, primers fold back on themselves and create loops of DNA that provide 3′ starting points for the new round of DNA replication by DNA polymerases. Therefore, the technique is called the loop-mediated isothermal amplification (LAMP) technique, described in reference [26]. LAMP only needs an optimum temperature of 65 °C for DNA polymerase activity. The provision of a constant temperature is technically much easier than a temperature cycling program that is required for conventional PCR [19].
Another invention which has made the test more convenient and quicker is where the pre-PCR RNA extraction step is combined with the PCR step itself. This reduction in the number of steps of the test offers some advantages. Firstly, a single step preparation of RNA reduces the SRT and increases the potential of the test for wider application. A shorter SRT decreases the probability of disease transfer by individuals whose test results have yet to be determined. Secondly, a one-step preparation of the RNA samples is much easier for potential users to learn how to use the test correctly. Thirdly, during the extraction of RNA from the sample, there is a risk of viral transmission from the samples to the laboratory staff, and cross-sample contamination due to unintentionally errors in sample manipulation. A shorter and easier process of RNA preparation can minimize the mentioned risks. Fourthly, the use of an RNA purification protocol increased the sensitivity of the RT-LAMP test shown by a reduction in the detection limit of the test from 100 copies/μL to 1 copy/μL. Lastly, the combination of the steps has been shown to eliminate the need for apparatus that limits the test to a lab environment [19].
Furthermore, further simplification of RT-PCR can be achieved by providing RT-LAMP kits with simpler result readouts. In the case of COVID-19 infection, it is only necessary to know whether or not viral RNA is present in the samples; therefore, there is no need for expensive quantification methods like spectrofluorimetry. Instead of quantitation, qualitative readouts such as a color change are much easier to achieve, and are more appropriate for diagnosis of SARS-CoV-2 infection. By using these kinds of readout, one can simply observe the results with the naked eye [19]. For instance, Yu et al. [27]. recently developed an iLACO assay (isothermal LAMP-based method for COVID-19) with a fluorescent readout. Using this kit, they could achieve the results within 15−40 min and observe the results without any need for expensive spectrofluorimetric equipment. In this test, the positive samples with Genefinder dye turned bright white, while the negative samples remained blue under blue light. Another example was the Penn-LAMP technique that produced a color change upon the amplification of the viral RNA. In this technique, the sample color changes from white to blue if the samples contained the amplified RNA material. Also, the entire RT-LAMP process can be done in a single vial without any need for specific equipment.
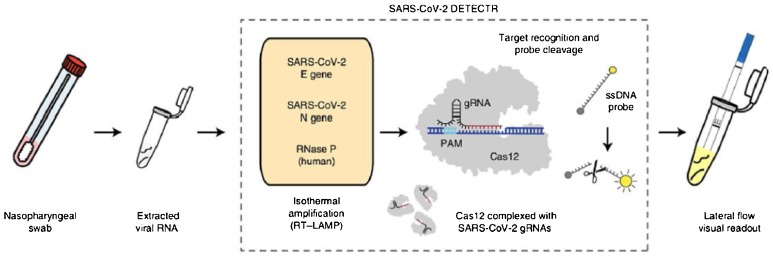
Previous studies have suggested using CRISPR/Cas systems in the diagnosis and treatment of COVID-19 [28,29]. Perhaps the best solution to produce a "fast and simple test" may be the "integrated RT-LAMP and CRISPR-Cas-12 method' (Fig. 1 ), that literally can be used anywhere by anyone. The method contains a kit with a lateral flow visual readout using a strip of paper. In this test, one just needs to dip the correct end of the designed strip in the vial of the final RT-LAMP product and wait to observe either a positive or negative result. These results appear in the form of a band at specific distances from the starting point. In the designed strip, the detection method relies on the CRISPR Cas-12 endonuclease enzyme and a trans-reporter molecule. The CRISPR Cas-12 detects a specific E gene and N gene sequence in the amplified viral DNA and acts as a “shredder” on other irrelevant RNA sequences. Therefore, all other RNA sequences are eliminated except for the target sequence that remains intact in the RT-LAMP product. The FAM-biotin trans-reporter is already placed and affixed to the strip. As the sample flows laterally across the strip, the remaining target sequence interacts with the FAM-biotin trans-reporter molecules on the strip producing the band. Using this DNA Endonuclease-Targeted CRISPR Trans Reporter (DETECTOR) technique, a fluorescently-labeled band can be observed in less than one minute [8].
Fig. 1.
Integrated RT-LAMP and CRISPR-Cas-12 for SARS-CoV-2 detection. Reprinted from ref. 10.1038/s41587-020-0513-4.
Taken together, the RT-LAMP methodology has provided a new alternative for rapid, simple, and home-use molecular diagnostic tests. Being rapid and simple has enabled wider and more frequent use of these tests for COVID-19 detection bymembers of the public, therefore, overcoming the high negative rate of RNA-based tests. On the other hand, the false-positive rate of these tests poses some issues regarding the management of the COVID-19 pandemic that will be discussed in the following section.
7. False-positive RT-PCR rates in COVID-19 cases
Another question that needs to be addressed is to be certain that a positive PCR test result for COVID-19 truly reflects the infected status of the patient. To this end, a positive PCR test result can be confirmed when the sample is examined by the gold standard viral culture test. Although data on viral culture results are sparse, there is some evidence that can help us to evaluate the predictive value of the PCR test as a screening method under different conditions. To what extent a positive PCR result predicts the chance of someone being infectious may be governed by different factors. These factors include the time after symptom onset, symptom severity, and the specimen used when the PCR test is carried out [30].
First of all, we should consider the time of symptom onset when interpreting the probability of being infectious according to RT-PCR results. It has been reported that the viral load is maximum by the 3rd day from the onset of symptoms in samples from the upper respiratory tract, and that live virus can still be detected at 8 days after the onset of the disease symptoms by the viral culture test. However, beyond this period the virus might no longer be infectious, although RT-PCR results continue to detect the presence of viral RNA material [31]. In one study [32] conducted on 129 hospitalized patients with COVID-19, RT-PCR testing showed that the duration of virus shedding was longer, and ranged from 0 to 20 days post-onset of symptoms. Moreover, it was reported that SARS-CoV-2 RT-PCR continued to detect the virus until the 63rd day from the onset of symptoms in one case report study. However, there is some evidence from serum samples suggesting that the RT-PCR could give positive results by detecting viral RNA remnants long after infectious virus had disappeared. Therefore, it is possible that the RT-PCR result was positive even after the infectious virus had been neutralized by the immune system.
The source of the specimen can also reflect the disease progression. Viral shedding can be detected only during a specific period that varies according to the sampling site. For example, within 5–6 days from the onset of symptoms, high viral loads were reportedly found in the upper and lower respiratory tracts in COVID-19 patients. As a result, nasopharyngeal (NP) and oropharyngeal (OP) swabs are recommended for early diagnosis of the infection. In this regard, NP samples were found to be better than OP samples in terms of the chance of detecting SARS-CoV-2 infection (63 % in NP swabs versus 32 % in OP swabs) showing there were higher viral loads in the nasal cavity. However, upper tract respiratory samples might fail to give sufficient viral load for detection purposes in a given time point of the infection [16]. For instance, one case report showed that the virus was only detected within the first 18 days from the onset of respiratory specimens [33], while the presence of the virus in fecal samples was detected for a longer period after respiratory samples became negative [34]. Some patients with COVID-19 pneumonia exhibited a longer-lasting shedding of the virus in the respiratory tract, whereas there had been high loads of SARS-CoV-2 in their fecal samples from the beginning of the symptoms [[35], [36], [37], [38]]. The fecal shedding of the viral RNA continued between days 1–33, while at least 3-days post-onset of symptom was identified as the optimum timepoint for a high positive rate of RT-PCR test in upper respiratory tract samples [34]., The amount of virus in the serum correlates with infectious SARS-CoV-2, and once neutralizing antibodies are detected in the serum, the virus is no longer infectious [32]. Consequently, RT-PCR positive results in fecal and upper respiratory tract samples will continue for a specific period of time (probably longer for fecal samples), but the infectious status of the patient might be limited to the period when active virus can be detected in serum samples.
As with other RNA viruses, SARS-CoV-2 RNA can be detected long after the infectious virus has been neutralized by the immune system and disappeared. This is because that the RNA of the inactivated virus degrades slowly over time, and during this period, RT-PCR will still give a false positive result based on the RNA remnants. In this regard, the application of other molecular techniques, such as those that detect viral protein targets, or patient antibodies against these proteins is required to confirm the results of RT-PCR as "truly infectious” [39].
Lastly, the initial viral RNA load in the specimen can influence the likelihood of getting a positive PCR result and can result in the test being oversensitive. A lower initial viral RNA load can be detected by an increase in the sensitivity of the RT-PCR test, but at the same time, this can prolong the period of getting RT-PCR positive results [3]. The conventional RT-PCR fails to detect the infection in samples containing <106 copies/mL (or copies per sample) [30]. This detection limit can be improved (lowered) by making modifications in the test, such as improving the viral RNA extraction method and the fluorescent probes. However, reducing the detection limit of the test might also increase the false positive rate of the test in the later stages of the infection, because lower amounts of remnant RNA from the inactivated virus would be sufficient to give a positive result. Therefore, other molecular and clinical evidence in combination with RT-PCR results should be used to confirm the status of the infection [39,40].
Taken together, the PCR results for COVID-19 should be carefully considered to confirm the infection, and special attention should be paid to the stage of disease development and the type of specimens collected for the test.
8. The public health implications of false-positive rates
The false-positive rate of the diagnostic tests might at first glimpse, seem not to be as important as the false-negative rate, given the current global prevalence of the disease. However, erroneous positive results are indeed important, and can have serious implications for public health services [3].
Currently, the global health policy is to maintain COVID-19 transmission as low as possible within communities. Therefore, COVID-19 hygienic protocols contain strict quarantine guidelines based on positive results of RT-PCR tests. This occurs despite knowing that the RT-PCR test suffers a low positive predictive value [3]. When the PCR test remains positive over time, the positive results will be taken seriously, and the suspect patient is recommended for stay-at-home quarantine as long as the repetition of the test gives positive results. For instance, as of September 19, 2020, the false positive rate of the swab tests was estimated to be between 0.8 % and 4.0 % in the UK population, which suggested a significant proportion of false-positive results were due to the low prevalence of the disease globally (the global prevalence of the disease was 0.50 % at that time) [3].
Despite the low positive predictive value for the test, patients are still recommended to follow a strict quarantine which will not cause a serious social problem.
However, the low positive predictive value of RT-PCR tests causes problems for health and social services. The prevalence of the disease is likely to be much higher in the health-care environment, and the high false-positive rate of PCR tests will lead to the quarantine of significant numbers of social health-care workers and health-care personnel, that might have been avoided. This could cause a serious shortage of health-care workers especially at the peak of waves of disease transmission [3].
Therefore, the high false-positive rate of the RT-PCR test is indeed a problem among health-care personnel and the results of the test should be confirmed based on other clinical evidence.
9. Combination of RT-PCR and serologic testing during infection
Although the RT-PCR test is the frontline diagnostic test in the early stages, the test still suffers from a high rate of failure in the detection of true COVID-19 infections. However, when the RT-PCR test is accompanied by other diagnostic molecular tests, the sensitivity of COVID-19 testing is enhanced. Moreover, the RT-PCR and serologic tests display opposite trends in sensitivity during the infection, in which one test can cover the failure of the other as the disease progresses [18].
In the early phase of the infection, the rate of viral detection by the RT-PCR method is low; however, RT-PCR results are improved when ELISA molecular tests are used in combination. The combination of these techniques has already been shown to improve the sensitivity in the early stages. Within 5–6 days of the onset of the symptoms, RT-PCR has a low chance of viral detection in nasopharyngeal samples; however, when RT-PCR tests and ELISA techniques were used in combination, the sensitivity improved dramatically [18]. Guo et al. [41] reported a set of 58 suspected cases with negative RT-PCR results, and showed that 93 % were positive when nasopharyngeal (NP) samples were tested by ELISA for the presence of IgM antibodies. Out of 82 RT-PCR confirmed cases, 76 % were also found to be positive by the IgM-ELISA test. The overall sensitivity of the RT-PCR alone was 52 %, failing to detect almost half the cases; however, the combined IgM-ELISA/RT-PCR sensitivity was much higher at 99 %. Besides, the rate of success in positive cases was found to be increased even further when the combined detection of IgG and IgM were combined with RT-PCR. In another study, over 70 % of suspected cases with negative RT-PCR results were identified as positive when IgG/IgM ELISA was used [42].
As the disease progresses, the sensitivity of the RT-PCR and serological tests both change. While the RT-PCR was highly sensitive during the first week after symptoms emerge, the serological tests had higher sensitivity in the second week, underlining the advantage of the combination [17]. During the course of the infection, Zhao et al. [12] followed the sensitivity using an ELISA test measuring total antibodies to the RBD viral antigen. In addition to serological testing, RT-PCR results were obtained as well. In the early (1–7) days after onset of symptoms, the sensitivity of RT-PCR was 67 %, but decreased to 54 % between days 8–14, and to 46 % between days 15–39 post-onset of symptoms. In comparison, the sensitivity of the total antibody ELISA significantly increased as the disease progressed, and reached a maximum of 100 % during the last 15–39 days. Interestingly, the combined sensitivity of both tests was much higher at 79 % during the first 1–8 days of the onset of symptoms, and grew to 97 % from the 8th day onward.
Therefore, the use of RT-PCR combined withg ELISA tests can enhance the overall sensitivity and reduce the false-negative rate. Besides, these tests follow opposite trends in the sensitivity during the infection period; therefore, the use of both tests can improve the
10. Relation of serological response with progression and severity of COVID-19
The results of serologic tests depends on the amount of antibodies produced, which may vary according to the severity of the disease. Some studies proposed the monitoring of antibody titers as a prognostic indicator for early aggressive treatment of the disease. Other studies observed higher antibody titers in elderly patients compared to other age groups regardless of the disease severity. Moreover, it is traditionally believed that seroconversion from IgM to IgG takes place during the development of the humoral immune response; however, some reports found that the expression of IgG and IgM could occur simultaneously, and the median time of appearance from onset of symptoms varied in different patients [18].
The median time of seroconversion of antibody isoforms may vary according to the type of immunodiagnostic test and the choice of the target antigen. In a large multicenter study conducted by Long et al. [43], the median time of seroconversion for both IgM and IgG was observed on day 13 by an NP and SP-targeted direct chemiluminescence immunoassay (CLIA). Zhao et al. [12] found a median seroconversion of IgM and IgG against RBD antigen, at 12th and 14th days after onset of symptoms, respectively. The median seroconversion time varied more widely in other studies. Guo et al. [41] used an ELISA kit for detection of antibodies to the NP antigen, and found that the IgM and IgG response appeared on day 5 and 14 after the onset of symptoms, respectively. In that study, IgA to the NP antigen was also found on day 5. Xiang et al. [44] also used an NP-targeted ELISA and found the median appearance of IgM and IgG at day 4 post-onset of symptoms. Overall, while IgM appearance preceded IgG in some studies appearing during the first week, in other studies both isoforms appeared simultaneously by two weeks from the onset of symptoms. In this regard, the detection time of antibody appearance may have been affected by the target antigens chosen and different immunodiagnostic assays used.
Few studies have examined the relationship between antibody titer and the disease aggressiveness. These studies investigated the relationship between specific antibody profiles and disease severity. There was generally a positive correlation between the level of antibodies and the severity of the disease. For example, Tan et al. [45] reported that they found an earlier presence of IgG, as well as higher IgG titers in severe patients compared to less severe patients. Wang et al. [46] reported significantly higher titers of IgM only in deceased patients (n = 15) compared to those with mild-to-moderate symptoms (n = 115, p = 0.19). No correlation was observed between IgG titer and disease severity in this study. This trend towards higher IgM and lower IgG levels was also observed in a large study of 338 confirmed COVID-19 patients. Also, the level of IgA was significantly increased in a cohort of 216 patients that was correlated with the severity of the disease [47].
Whether or not there is a relationship between IgG titer and disease severity may have depended on the choice of antigen used in the test. While some studies failed to show a relationship, other studies did find a relationship between IgG and disease severity. For instance, Sun et al. [46] reported that IgG titers against the SP antigen were significantly higher in non-ICU patients, while IgG titers to NP antigen were higher in ICU patients. This was in contrast with the results of To et al. [42] who failed to observe a relationship of any kind between IgG titers and disease severity when IgG titers to NP and RBD antigens were measured by an ELISA test.
Although there is a positive correlation between age and severity of the disease and poor outcomes, it was found that increasing age was associated with high antibody titers to both non-SARS-CoV-2 and SARS-CoV-2 infections. Given that an elevated antibody response to non-SARS-CoV-2 infection appears to have little effect on health, the high antibody titers seem not to be the cause of the disease severity in older age groups. Using neutralizing antibody assays, participants over sixty years of age exhibited a higher overall antibody titer compared to a healthy young adult with non–SARS-CoV-2 human coronavirus infection. This implies that the antibody response is stronger in the elderly than young adults in the case of non-SARS-CoV-2 infection, where no serious clinical outcomes are reported. Consequently, although the elderly and middle-aged patients who recovered from COVID-19 demonstrated higher titers of SP-reactive antibodies in their samples than young adults, this elevated antibody titer may not be related to the poor outcome of treatment in elderly COVID-19 patients [47,48].
11. Concluding remarks
Fig. 2 illustrates the benefits and shortcomings of the different screening tests discussed in this paper. We addressed the shortcomings and strengths of RT-PCR tests for the screening and diagnosis of COVID-19 patients. Up to now, the RNA PCR amplification tests are accepted as the gold standard method for the detection of SARS-CoV-2 infection in clinical samples. The test is highly specific for SARS-CoV-2 virus detection, and it can give a result within a short period. However, the test is too difficult to be widely used and it requires expensive laboratory equipment and highly-trained laboratory staff. The current RT-PCR test also suffers from a level of insensitivity. This implies that other clinical and immunodiagnostic tests should be also taken into account when interpreting the results of RT-PCR tests. PCR tests suffer from an alarming level of false-negative and false-positive results, so it is necessary to look for further improvements to the RT-PCR test.
Fig. 2.
An overview of the advantages and disadvantages of the SARS_CoV-2 molecular diagnostic test.
As with other screening tests, the RT-PCR method has some drawbacks when applied to clinical samples. There are a few factors that might interfere with the test reliability. Firstly, there is a narrow time window to obtain high sensitivity depending on the viral load in the samples. The false-negative rate of the test varies over the time from the onset of clinical symptoms. Within the first 3 days from the onset of symptoms, the RT-PCR test could offer the fewest false-negative results, however, the test failure was shockingly high for detection of the virus. Secondly, there is also variability in the test sensitivity in different clinical samples and patients with varying clinical symptoms. Lastly, the improper manipulation of clinical samples might result in test failure due to the viral RNA degradation and loss during the handling of the samples.
Therefore, the repetition of the RT-PCR test is recommended to enhance the likelihood of virus detection, which requires the test to be easier and more rapid for frequent repetition. It has been shown that repeating the test over time and on different clinical samples can enhance the overall positive rates of the test. The repetition of RT-PCR testing is currently recommended in standard protocols to allow clinicians to confirm the results in suspected cases, as it is more reliable than a single test result. Another point is that pooling the different clinical samples or samples from the patient's family could reduce the number of test repetitions required, but still reap the benefits of enhanced sensitivity. As a result, RT-PCR test repetition over time or on different samples can increase the overall sensitivity of the test. Therefore alternative RT-PCR tests which are simpler, less expensive, and easier to conduct are under investigation.
There are some obstacles regarding the widespread application of RT-PCR tests. Firstly, the test necessitates the use of sophisticated equipment that confines the test to the laboratory setting where everything is at hand. Secondly, the test takes many steps to be completed, and requires the staff to be well trained to follow every step correctly. Thirdly, the time between sampling and the result is lengthy, and should be shortened. Therefore, for the test to be applied more widely, it requires simplification and to be easily learned and completed.
Up to now, great strides have been made in the improvement of RT-PCR testing. Alternative methods of viral RNA amplification have already been implemented with a simpler technical procedure compared to the conventional RT-PCR method. With the invention of the RT-LAMP method, there is now no need for a thermal cycler apparatus for viral RNA amplification, and the viral RNA sequence can be amplified to a detectable level by incubation in an ordinary oven that provides a constant temperature. Steps have also been taken to enhance both the overall sensitivity of the test and allow the test to be carried out outside the laboratory setting. For instance, the sensitivity of the test has been improved by reducing the detection limit so that it will give positive results at a lower viral load in the sample. In the Penn-LAMP and DETECTOR techniques, the final result can be observed with the naked eye, and the need for the expensive spectrofluorimetric readout methods is eliminated.
Furthermore, a more reliable diagnosis can be achieved when the RT-PCR test and an immunodiagnostic test are applied together. It has been shown that the overall sensitivity is increased when these two test results are combined. Moreover, the sensitivity of the test can be improved over the course of the infection by combining both RT-PCR and serologic ELISA tests. The serologic monitoring of the patient's antibody response might give a picture of the disease progression and severity. However, the positive correlation of disease severity with antibody titer is uncertain, because confounding factors like the choice of the antigen and antibody can affect the results.
Taken together, the RT-PCR test is still the best choice for diagnosis of SARS-CoV-2 infection in patients; however, due to its above-mentioned drawbacks, the RT-PCR test should be used sensibly and the RT-PCR technical features should be improved.
Declaration of Competing Interest
The authors declare that they have no conflict of interest.
References
- 1.Tan W., Aboulhosn J. Coronavirus disease 2019 (COVID-19) and cardiovascular burden including congenital heart disease. Int. J. Cardiol. 2020 doi: 10.1016/j.ijcard.2020.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Long B., Brady W., Koyfman A., Gottlieb M. Cardiovascular complications in COVID-19. Am. J. Emerg. Med. 2020 doi: 10.1016/j.ajem.2020.04.048. [published online ahead of print April 18, 2020] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Surkova E., Nikolayevskyy V., Drobniewski F. False-positive COVID-19 results: hidden problems and costs. Lancet Respir. Med. 2020;8:1167–1168. doi: 10.1016/S2213-2600(20)30453-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hong K.H., Lee S.W., Kim T.S., Huh H.J., Lee J., Kim S.Y., Park J.-S., Kim G.J., Sung H., Roh K.H. Guidelines for laboratory diagnosis of coronavirus disease 2019 (COVID-19) in Korea. Ann. Lab. Med. 2020;40:351–360. doi: 10.3343/alm.2020.40.5.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tahamtan A., Ardebili A. Taylor & Francis; 2020. Real-time RT-PCR in COVID-19 Detection: Issues Affecting the Results. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.D’Cruz R.J., Currier A.W., Sampson V.B. Laboratory testing methods for novel severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) Front. Cell Dev. Biol. 2020;8 doi: 10.3389/fcell.2020.00468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Won J., Lee S., Park M., Kim T.Y., Park M.G., Choi B.Y., Kim D., Chang H., Kim V.N., Lee C.J. Development of a laboratory-safe and low-cost detection protocol for SARS-CoV-2 of the Coronavirus disease 2019 (COVID-19) Exp. Neurobiol. 2020;29:107. doi: 10.5607/en20009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Broughton J.P., Deng X., Yu G., Fasching C.L., Servellita V., Singh J., Miao X., Streithorst J.A., Granados A., Sotomayor-Gonzalez A. CRISPR–Cas12-based detection of SARS-CoV-2. Nat. Biotechnol. 2020:1–5. doi: 10.1038/s41587-020-0513-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Esbin M.N., Whitney O.N., Chong S., Maurer A., Darzacq X., Tjian R. Overcoming the bottleneck to widespread testing: a rapid review of nucleic acid testing approaches for COVID-19 detection, RNA. RNA. 2020 doi: 10.1261/rna.076232.120. 076120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang Y., Yang M., Shen C., Wang F., Yuan J., Li J., Zhang M., Wang Z., Xing L., Wei J. Laboratory diagnosis and monitoring the viral shedding of 2019-nCoV infections. MedRxiv. 2020 doi: 10.1016/j.xinn.2020.100061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dong Y.M., Sun J., Li Y.X., Chen Q., Liu Q.Q., Sun Z., et al. Development and Validation of a Nomogram for Assessing Survival in Patients With COVID-19 Pneumonia. Clin. Infect. Dis.: Off. Publ. Infect. Dis. Soc. Am. 2021;72(4):652–660. doi: 10.1093/cid/ciaa963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Linton N.M., Kobayashi T., Yang Y., Hayashi K., Akhmetzhanov A.R., Jung S.-m., Yuan B., Kinoshita R., Nishiura H. Incubation period and other epidemiological characteristics of 2019 novel coronavirus infections with right truncation: a statistical analysis of publicly available case data. J. Clin. Med. 2020;9:538. doi: 10.3390/jcm9020538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arevalo-Rodriguez I., Buitrago-Garcia D., Simancas-Racines D., Zambrano-Achig P., del Campo R., Ciapponi A., Sued O., Martinez-Garcia L., Rutjes A., Low N. False-negative results of initial RT-PCR assays for COVID-19: a systematic review. medRxiv. 2020 doi: 10.1371/journal.pone.0242958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kucirka L.M., Lauer S.A., Laeyendecker O. Variation in false-negative rate of reverse transcriptase polymerase chain reaction-based SARS-CoV-2 tests by time since exposure. Ann. Intern. Med. 2020 doi: 10.7326/M20-1495. [published online ahead of print May 13, 2020] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abbasi-Oshaghi E., Mirzaei F., Farahani F., Khodadadi I., Tayebinia H. Diagnosis and treatment of coronavirus disease 2019 (COVID-19): laboratory, PCR, and chest CT imaging findings. Int. J. Surg. 2020 doi: 10.1016/j.ijsu.2020.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tang Y.W., Schmitz J.E., Persing D.H., Stratton C.W. The laboratory diagnosis of COVID‐19 infection: current issues and challenges. J. Clin. Microbiol. 2020;10:00512–00520. doi: 10.1128/JCM.00512-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang P. Combination of serological total antibody and RT-PCR test for detection of SARS-COV-2 infections. J. Virol. Methods. 2020;283 doi: 10.1016/j.jviromet.2020.113919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Espejo A.P., Akgun Y., Al Mana A.F., Tjendra Y., Millan N.C., Gomez-Fernandez C., Cray C. Review of current advances in serologic testing for COVID-19. Am. J. Clin. Pathol. 2020;154:293–304. doi: 10.1093/ajcp/aqaa112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thompson D., Lei Y. Mini review: recent progress in RT-LAMP enabled COVID-19 detection. Sens. Actuators Rep. 2020 doi: 10.1016/j.snr.2020.100017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karthik K., Babu R.P.A., Dhama K., Chitra M.A., Kalaiselvi G., Senthilkumar T.M.A., Raj G.D. Biosafety concerns during the collection, transportation, and processing of COVID-19 samples for diagnosis. Arch. Med. Res. 2020 doi: 10.1016/j.arcmed.2020.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mallett S., Allen A.J., Graziadio S., Taylor S.A., Sakai N.S., Green K., Suklan J., Hyde C., Shinkins B., Zhelev Z. At what times during infection is SARS-CoV-2 detectable and no longer detectable using RT-PCR-based tests? A systematic review of individual participant data. BMC Med. 2020;18 doi: 10.1186/s12916-020-01810-8. 1-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wee S.K., Sivalingam S.P., Yap E.P.H. Rapid direct nucleic acid amplification test without RNA extraction for SARS-CoV-2 using a portable PCR thermocycler. bioRxiv. 2020 doi: 10.3390/genes11060664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deka S., Kalita D. Effectiveness of sample pooling strategies for SARS-CoV-2 mass screening by RT-PCR: a scoping review. J. Lab. Physicians. 2020;12:212. doi: 10.1055/s-0040-1721159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Premraj A., Aleyas A.G., Nautiyal B., Rasool T.J. Nucleic acid and immunological diagnostics for SARS-CoV-2: processes, platforms and pitfalls. Diagnostics. 2020;10:866. doi: 10.3390/diagnostics10110866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Espy M., Uhl J., Sloan L., Buckwalter S., Jones M., Vetter E., Yao J., Wengenack N., Rosenblatt J., Cockerill F. Real-time PCR in clinical microbiology: applications for routine laboratory testing. Clin. Microbiol. Rev. 2006;19:165–256. doi: 10.1128/CMR.19.1.165-256.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang W.E., Lim B., Hsu C.C., Xiong D., Wu W., Yu Y., Jia H., Wang Y., Zeng Y., Ji M. RT‐LAMP for rapid diagnosis of coronavirus SARS‐CoV‐2. Microb. Biotechnol. 2020;13:950–961. doi: 10.1111/1751-7915.13586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yu L., Wu S., Hao X., Li X., Liu X., Ye S., Han H., Dong X., Li X., Li J. Rapid colorimetric detection of COVID-19 coronavirus using a reverse tran-scriptional loop-mediated isothermal amplification (RT-LAMP) diagnostic plat-form: iLACO. medRxiv. 2020 doi: 10.1093/clinchem/hvaa102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goudarzi K.A., Nematollahi M.H., Khanbabaei H., Nave H.H., Mirzaei H.R., Pourghadamyari H., Sahebkar A. Targeted delivery of CRISPR/Cas13 as a promising therapeutic approach to treat SARS-CoV-2. Curr. Pharm. Biotechnol. 2020 doi: 10.2174/1389201021666201009154517. [DOI] [PubMed] [Google Scholar]
- 29.Palaz F., Kalkan A.K., Tozluyurt A., Ozsoz M. CRISPR-based tools: alternative methods for the diagnosis of COVID-19. Clin. Biochem. 2021;89:1–13. doi: 10.1016/j.clinbiochem.2020.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alvarez-Moreno C.A., Rodríguez-Morales A.J. Testing dilemmas: post negative, positive SARS-CoV-2 RT-PCR–is it a reinfection? Travel Med. Infect. Dis. 2020 doi: 10.1016/j.tmaid.2020.101743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bernacki E., Tsourmas N. 2020. COVID-19 Workplace Issues: Retail Industry. [Google Scholar]
- 32.van Kampen Jeroen J., van de Vijver A.David, Fraaij Pieter L. Shedding of infectious virus in hospitalized patients with coronavirus disease-2019 (COVID-19): duration and key determinants. medRxiv. 2020 doi: 10.1038/s41467-020-20568-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu W.-D., Chang S.-Y., Wang J.-T., Tsai M.-J., Hung C.-C., Hsu C.-L., Chang S.-C. Prolonged virus shedding even after seroconversion in a patient with COVID-19. J. Infect. 2020 doi: 10.1016/j.jinf.2020.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gupta S., Parker J., Smits S., Underwood J., Dolwani S. Persistent viral shedding of SARS‐CoV‐2 in faeces‐a rapid review. Colorectal Dis. 2020 doi: 10.1111/codi.15138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cheng P.K., Wong D.A., Tong L.K., Ip S.-M., Lo A.C., Lau C.-S., Yeung E.Y., Lim W.W. Viral shedding patterns of coronavirus in patients with probable severe acute respiratory syndrome. Lancet. 2004;363:1699–1700. doi: 10.1016/S0140-6736(04)16255-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu S., Hou X., Chen J. Therapeutic potential of duodenal electrical stimulation for obesity: acute effects on gastric emptying and water intake. Am. J. Gastroenterol. 2005;100:792–796. doi: 10.1111/j.1572-0241.2005.40511.x. [DOI] [PubMed] [Google Scholar]
- 37.Xu D., Zhang Z., Jin L., Chu F., Mao Y., Wang H., Liu M., Wang M., Zhang L., Gao G. Persistent shedding of viable SARS-CoV in urine and stool of SARS patients during the convalescent phase. Eur. J. Clin. Microbiol. Infect. Dis. 2005;24:165–171. doi: 10.1007/s10096-005-1299-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yeo C., Kaushal S., Yeo D. Enteric involvement of coronaviruses: is faecal–oral transmission of SARS-CoV-2 possible? Lancet Gastroenterol. Hepatol. 2020;5:335–337. doi: 10.1016/S2468-1253(20)30048-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Feng W., Newbigging A.M., Le C., Pang B., Peng H., Cao Y., Wu J., Abbas G., Song J., Wang D.-B. Molecular diagnosis of COVID-19: challenges and research needs. Anal. Chem. 2020;92:10196–10209. doi: 10.1021/acs.analchem.0c02060. [DOI] [PubMed] [Google Scholar]
- 40.Jayamohan H., Lambert C.J., Sant H.J., Jafek A., Patel D., Feng H., Beeman M., Mahmood T., Nze U., Gale B.K. SARS-CoV-2 pandemic: a review of molecular diagnostic tools including sample collection and commercial response with associated advantages and limitations. Anal. Bioanal. Chem. 2020:1–23. doi: 10.1007/s00216-020-02958-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li G., Lili R., Siyuan Y., Meng X., De C., Fan Y., et al. Profiling early humoral response to diagnose novel coronavirus disease (COVID-19) Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.To K.K.-W., Tsang O.T.-Y., Leung W.-S., Tam A.R., Wu T.-C., Lung D.C., Yip C.C.-Y., Cai J.-P., Chan J.M.-C., Chik T.S.-H. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect. Dis. 2020 doi: 10.1016/S1473-3099(20)30196-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee Y.-H., Hong C.M., Kim D.H., Lee T.H., Lee J. Clinical course of asymptomatic and mildly symptomatic patients with coronavirus disease admitted to community treatment centers, South Korea. Emerging Infect. Dis. 2020;26:2346. doi: 10.3201/eid2610.201620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xiang F., Wang X., He X., Peng Z., Yang B., Zhang J., Zhou Q., Ye H., Ma Y., Li H. Antibody detection and dynamic characteristics in patients with coronavirus disease 2019. Clin. Infect. Dis. 2020;71 doi: 10.1093/cid/ciaa461. 1930-1. 934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tan W., Lu Y., Zhang J., Wang J., Dan Y., Tan Z., He X., Qian C., Sun Q., Hu Q. Viral kinetics and antibody responses in patients with COVID-19. MedRxiv. 2020 [Google Scholar]
- 46.Sun B., Feng Y., Mo X., Zheng P., Wang Q., Li P., Peng P., Liu X., Chen Z., Huang H. Kinetics of SARS-CoV-2 specific IgM and IgG responses in COVID-19 patients. Emerg. Microbes Infect. 2020:1–36. doi: 10.1080/22221751.2020.1762515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gorse G.J., Donovan M.M., Patel G.B. Antibodies to coronaviruses are higher in older compared with younger adults and binding antibodies are more sensitive than neutralizing antibodies in identifying coronavirus‐associated illnesses. J. Med. Virol. 2020;92:512–517. doi: 10.1002/jmv.25715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Crawford K.H., Dingens A.S., Eguia R., Wolf C.R., Wilcox N., Logue J.K., Shuey K., Casto A.M., Fiala B., Wrenn S. Dynamics of neutralizing antibody titers in the months after severe acute respiratory syndrome Coronavirus 2 infection. J. Infect. Dis. 2020 doi: 10.1093/infdis/jiaa618. [DOI] [PMC free article] [PubMed] [Google Scholar]