Abstract
Objectives
To identify the best experimental approach to detect a SARS-CoV-2-specific T cell response using a whole-blood platform.
Methods
Whole-blood from 56 COVID-19 and 23 “NO-COVID-19” individuals were stimulated overnight with different concentrations (0.1 or 1 μg/mL) of SARS-CoV-2 PepTivator® Peptide Pools, including spike (pool S), nucleocapsid (pool N), membrane (pool M), and a MegaPool (MP) of these three peptide pools. ELISA was used to analyse interferon (IFN)-γ levels.
Results
The IFN-γ-response to every SARS-CoV-2 peptide pool was significantly increased in COVID-19 patients compared with NO-COVID-19 individuals. Pool S and MegaPool were the most potent immunogenic stimuli (median: 0.51, IQR: 0.14–2.17; and median: 1.18, IQR: 0.27–4.72, respectively) compared with pools N and M (median: 0.22, IQR: 0.032–1.26; and median: 0.22, IQR: 0.01−0.71, respectively). The whole-blood test based on pool S and MegaPool showed a good sensitivity of 77% and a high specificity of 96%. The IFN-γ-response was mediated by both CD4+ and CD8+ T cells, and independently detected of clinical parameters in both hospitalized and recovered patients.
Conclusions
This easy-to-use assay for detecting SARS-CoV-2-specific T cell responses may be implemented in clinical laboratories as a powerful diagnostic tool.
Keywords: COVID-19, SARS-CoV-2, T cell response, Whole-blood, IFN-γ-release assay (IGRA), Spike protein, Nucleocapsid protein, Membrane protein
Introduction
The COVID-19 pandemic caused by Severe Acute Respiratory Syndrome CoronaVirus 2 (SARS-CoV-2) has recently emerged as a new human-to-human transmissible disease with a serious global health impact (Braun et al., 2020). SARS-CoV-2 is an enveloped virus with a positive stranded RNA genome and four structural proteins, including spike glycoprotein (S), envelope protein (E), membrane protein (M), and nucleocapsid protein (N) (Koblischke et al., 2020, Le Bert et al., 2020).
Most infected patients present mild-to-moderate symptoms and approximately 15–20% develop severe disease (Wu and McGoogan, 2020). The majority of patients infected with COVID-19 have normal or reduced white cell counts and lymphocytopenia, and those with severe disease show significantly elevated levels of neutrophils, with a continuing decrease in lymphocytes (Costela-Ruiz et al., 2020). SARS-CoV-2 infection activates innate and adaptive immune responses (Shah et al., 2020). Recent studies have highlighted the role of the adaptive immune response in viral control and immunopathogenesis during acute SARS-CoV-2 infection, and particularly the role of T cells (CD4+ and CD8+) in establishing durable protective immunity against reinfection (Vabret et al., 2020, Shrotri et al., 2021).
Currently, the evaluation of population immunity is based on seroprevalence studies; however, in the context of evidence for cellular responses in seronegative exposed individuals (Gallais et al., 2021, Freeman et al., 2004, Heller et al., 2013, Mizukoshi et al., 2008) and the potential waning of antibody responses over time (Shrotri et al., 2021, Ojeda et al., 2021), current surveillance methods are likely to be underestimating both exposure and immunity. On the other hand, recent studies have demonstrated the presence of SARS-CoV-2-reactive T cells in a large number of patients with COVID-19 and also in unexposed individuals (Mateus et al., 2020, Ni et al., 2020, Grifoni et al., 2020, Echeverría et al., 2021). Moreover, CD4+ and CD8+ T cells targeting structural viral proteins appear to confer broad and long-lasting protection against SARS-CoV (Liu et al., 2017). Thus, a better understanding of the role of T cells in the long-term protection from COVID-19 is crucial in estimating population-level immunity, vaccine development, and long-term surveillance of vaccine efficacy (Dan et al., 2021).
Cytokine-release-based tests in whole-blood have been employed for several infectious diseases (Kim, 2020, Goletti et al., 2018a, Goletti et al., 2018b, Mahmoudi et al., 2017, Petrone et al., 2017, Petrone et al., 2021a, Dammermann et al., 2015). Recently, a whole-blood approach was scouted by the current (Petrone et al., 2021a, Petrone et al., 2021b, Petrone et al., 2021c) and other groups (Murugesan et al., 2020, Echeverría et al., 2021) to evaluate the specific immune response in COVID-19 patients. So far, different experimental approaches and clinical settings have been adopted, leading to different results. In particular, regarding the experimental procedures, peptides corresponding to different viral genome regions (spike, membrane, nuclear proteins or others), concentration of peptides, and read-out have been employed. Moreover, different clinical settings involving acute (Petrone et al., 2021a) or convalescent (Murugesan et al., 2020, Echeverría et al., 2021) COVID-19 subjects have been involved. This study used a whole-blood interferon (IFN)-γ release assay (IGRA) to characterize the IFN-γ response to different SARS-CoV-2 peptides in acute hospitalized and post-acute non-hospitalized COVID-19 patients, and to identify the best experimental approach to detect a SARS-CoV-2-specific T cell response.
Materials and methods
Study design
The prospective study was approved by the Ethical Committee of Lazzaro Spallanzani National Institute of Infectious Diseases (INMI) (approval number 59/2020) and was conducted between 10 December 2020 and 05 February 2021. Informed, written consent was required to consecutively enroll patients and controls by physicians. Demographic and clinical information were collected at enrollment. Inclusion criteria for COVID-19 patients were a diagnosis based on a positive nasopharyngeal swab for SARS-CoV-2 and a disease with the clinical characteristics already described [Lazzaro Spallanzani National Institute of Infectious Diseases (INMI) Recommendations for COVID-19 management] (Nicastri et al., 2020). Exclusion criteria were: HIV infection, inability to sign an informed consent and age <18 years.
The hospitalized COVID-19-patients were classified as mild, moderate, severe, and critical, according to WHO (WHO, 2020). This study reported the highest severity score of the disease occurring during hospitalization. Briefly, mild COVID-19 patients had symptoms but did not have viral pneumonia or hypoxia; moderate COVID-19 patients had pneumonia and SpO2 ≥ 90% on room air; severe COVID-19 patients had pneumonia and a respiratory rate >30 breaths/min or severe respiratory distress or SpO2 < 90% on room air; critical COVID-19 patients had acute respiratory distress syndrome. The “NO-COVID-19” group with 23 participants included 16 healthy blood donor (HD) volunteers from UOC Transfusion Medicine and Stem Cell Unit, San Camillo Forlanini Hospital (Rome, Italy), and seven patients with active tuberculosis. Inclusion criteria for the NO COVID-19 group were negative SARS-CoV-2 serology and no symptoms of COVID-19. A portion of them also had a negative swab for the molecular identification of SARS-CoV-2.
Peptide pools
SARS-CoV-2 PepTivator® Peptide Pools (Miltenyi Biotec, Germany) were used, including: the spike protein (PepTivator® SARS-CoV-2 Prot_S1, Prot_S, and Prot_S+) (pool S), the nucleocapsid phosphoprotein (PepTivator® SARS-CoV-2 Prot_N) (pool N), and the membrane glycoprotein (PepTivator® SARS-CoV-2 Prot_M) (pool M). The PepTivator® Peptide Pools comprise peptides of 15 amino acid length with 11 amino acid overlap. The peptides were grouped into different pools, including: pool S (equal amounts of Prot_S1, Prot_S, and Prot_S+), pool N, pool M, and a MegaPool (MP) of equal amounts of all of these peptides.
IFN-γ whole-blood assay
Whole-blood (600 μL) was stimulated or not with two different concentrations (0.1 μg/mL and 1 μg/mL) of different peptide pools. Staphylococcal enterotoxin B (SEB) (Sigma-Aldrich, Milan, Italy) (200 ng/mL) was used as a positive control. Plasma was harvested after overnight (20–24 h) stimulation in a 48-well flat-bottom plate at 37 °C (5% CO2) and stored at −80 °C. IFN-γ levels were evaluated by enzyme-linked immunosorbent assay (ELISA), according to manufacturer’s instructions (www.quantiFERON.com). IFN-γ values were subtracted from the unstimulated control. The lower and upper detection limits of the test were 0.065 and 10 IU/mL, respectively.
PBMCs culture conditions and stimulations
Peripheral blood mononuclear cells (PBMCs) were isolated using Ficoll density gradient centrifugation (Pancoll human, PAN Biotech, Germany) and resuspended in complete RPMI-1640 medium, with 10% fetal bovine serum, 1% L-glutamine, and 1% penicillin/streptomycin (Euroclone S.p.A, Italy). To characterize the specific T cell response by flow cytometry, fresh 1 × 106 PBMCs were resuspended in 1 mL of complete medium and stimulated with pools S, N, M at 0.1 μg/mL, and SEB (200 ng/mL) as a positive control. Anti-CD28 and anti-CD49d monoclonal antibodies (mAbs) at 2 μg/mL each were added to co-stimulate cells. After 1 h, 1 μL/1 × 106 of Golgi plug (BD Biosciences San Jose, USA) was added to each sample to inhibit cytokine secretion, according to manufacturer’s instructions. Following an incubation period of 16−24 h, cells were stained as described below.
T cell phenotyping and intracellular staining
PBMCs were stained with fluorochrome conjugated antibodies according to the standard operating procedure. T cells were stained with the following antibodies: CD3 phycoerythrin-cyanine 7 (PE-Cy7), CD4 Brilliant Violet (BV)711 and CD8 allophycocyanin-H7 (APC-H7) (all from BD Biosciences). The Cytofix/Cytoperm kit (BD Biosciences) was used for sub-sequential intracellular staining of IFN-γ allophycocyanin (APC). Dead cells were first excluded from analysis by side/forward scatter gating and then by Fixable Viability stain 700 (BD Biosciences). At least 100,000 gated events on living cells were analysed for each sample, whenever possible. Samples were acquired on a BD Lyric (BD Biosciences) cytometer. Data were analysed with FlowJo software, version 10 (Tree Star).
SARS-CoV-2 serology
SARS-CoV-2-specific IgM and IgG levels were measured by ELISA, according to manufacturer’s instructions (DIESSE Diagnostica Senese S.p.a., Monteriggioni, Italy). The ratio between the optical density (OD) of the sample and that of the cut-off reagent (index) was calculated. The samples were scored positive (index >1.1), doubtful (index between 1.1 and 0.9), and negative (index <0.9).
Statistical analysis
Data were analyzed using Graph Pad (GraphPad Prism 8 XML ProjecT) and Stata (Stata 15, StataCorp. 2017. Stata Statistical Software: Release 15. College Station, TX: StataCorp LLC). Medians and interquartile ranges (IQRs) were calculated. The following tests were used: Kruskal–Wallis test for comparisons among groups; Wilcoxon test for paired groups; Mann–Whitney U test with Bonferroni correction for pairwise comparisons; Friedman test to compare multiple paired groups; Chi-squared test for categorical variables; receiver operator characteristic (ROC) analysis for evaluating diagnostic performance; roccomp command in STATA for testing the equality of ROC areas; Spearman’s rank correlation for correlations: rs >0.7 high correlation, 0.7< rs >0.5 moderate correlation and rs <0.5 low correlation.
Results
Description of the studied population
Fifty-six (47 acute hospitalized and nine recovered non-hospitalized) COVID-19 patients and 23 NO-COVID-19 subjects were prospectively enrolled. COVID-19 patients were classified based on days from symptom onset and disease severity, as reported in Table 1 . A higher proportion of acute-hospitalized patients had a severe or critical illness compared with recovered patients (p < 0.0001). Demographic and clinical information are detailed in Table 1.
Table 1.
Demographical and clinical characteristics of the enrolled subjects.
| Characteristics | COVID-19 |
NO-COVID-19 | P value | ||
|---|---|---|---|---|---|
| Hospitalized | Non-Hospitalized | ||||
| N (%) | 47 (59.5) | 9 (11.4) | 23 (29.1) | ||
| Age median (IQR) | 61 (55−75) | 57 (31−61) | 45 (38−54) | <0.0001* | |
| Male N (%) | 31 (65.9) | 2 (22.2) | 18 (78.2) | 0.0112§ | |
| Origin N (%) | West Europe | 46 (97.9) | 7 (77.8) | 18 (78.3) | 0.0658§ |
| East Europe | 0 (0) | 1 (11.1) | 2 (8.7) | ||
| Asia | 1 (2.1) | 1 (11.1) | 1 (4.3) | ||
| Africa | 0 (0) | 0 (0) | 2 (8.7) | ||
| Swab positive results at the time of enrolment N (%) | 37 (72.5) | 3 (33.3) | 0 (0) | ||
| Days from symptom onset N (%) | Available | 41 (87.2) | 5 (55.6) | – | |
| 1−7 | 13 (31.7) | – | – | ||
| 8−14 | 12 (29.3) | – | – | ||
| 15−30 | 11 (26.8) | – | – | ||
| >30 | 5 (12.2) | 5 (100) | – | ||
| Lymphocyte count N (%) | Available | 47 (100) | – | – | |
| Lymphocyte count N (%) median (IQR) | <1 × 103/μL | 15 (31.9) 0.75 (0.58−0.92) |
– | ||
| ≤1 × 103/μL <2 × 103/μL | 23 (49) 1.53 (1.19−1.70) |
– | – | ||
| ≥2 × 103/μL | 9 (19.1) 2.80 (2.51−4.51) |
– | – | ||
| Serology results IgM N (%) | IgM+ | 24 (51) | 1 (11.1) | 0 (0) | 0.0871§§ |
| IgM− | 20 (42.6) | 7 (77.8) | 23 (100) | ||
| IgM doubtful | 3 (6.4) | 1 (11.1) | 0 (0) | ||
| Serology results IgG N (%) | IgG+ | 37 (78.7) | 7 (77.8) | 0 (0) | 0.892§§ |
| IgG− | 9 (19.2) | 2 (22.2) | 23 (100) | ||
| IgG doubtful | 1 (2.1) | 0 (0) | 0 (0) | ||
| Severity N (%)# | Available | 46 (97.9) | 7 (77.8) | – | <0.0001§§ |
| Mild | 1 (2.2) | 6 (85.7) | – | ||
| Moderate | 8 (17.4) | 0 (0) | – | ||
| Severe | 23 (50) | 1 (14.3) | – | ||
| Critical | 14 (30.4) | 0 (0) | – | ||
| Cortisone therapy N (%) | Available | 33 (70.2) | 1 (11.1) | – | |
| Severity of patients taking cortisone at the time of enrolment N (%) | Mild | 0 (0) | 1 (100) | – | |
| Moderate | 4 (12.1) | – | – | ||
| Severe | 19 (57.6) | – | – | ||
| Critical | 10 (30.3) | – | – | ||
COVID-19: coronavirus disease 19; N: number.
Kruskal–Wallis statistic test.
Chi-square test.
WHO criteria (ref WHO).
Chi-square test performed only on COVID-19 cohorts.
The IFN-γ response to SARS-CoV-2 peptides was increased in COVID-19 compared with NO-COVID-19 individuals
First, the study aimed to evaluate the optimum concentration of viral peptides to use in the whole-blood platform. It performed a dose concentration-response analysis of 0.1 μg/mL and 1 μg/mL concentrations of pools S, N, M, and MegaPool (MP) on a cohort of 23 COVID-19 and 22 NO-COVID-19 subjects (Figure S1 A–D). A significant difference was found in response to all SARS CoV-2 peptide pools between the concentrations tested both in COVID-19 (pool N p = 0.0005; pool M p < 0.0001; pool S p = 0.0003; and MP p = 0.0007) and NO-COVID-19 individuals (pool N p = 0.0047; pool M p = 0.0013; pool S p = 0.0003; and MP p < 0.0001). After stimulation with SEB, employed as a non-specific stimulation to evaluate the immune ability to respond, no significant differences were found within the COVID-19 and NO-COVID-19 groups (median: 11.31, IQR 0.82–16.69 vs median: 12.77, IQR 12.45–12.93; p = 0.051, respectively) (Figure S2 A).
A ROC analysis was then performed to define the best concentration of each peptide pool for discriminating COVID-19 patients from NO-COVID-19 individuals. Similar and significant area under the curves (AUC) were obtained based on both concentrations tested for pools N (AUC = 0.73, p = 0.006 vs AUC = 0.76, p = 0.003, respectively), M (AUC = 0.68, p = 0.036 vs AUC = 0.72, p = 0.011, respectively), S (AUC = 0.85, p < 0.0001 vs AUC = 0.85, p < 0.0001, respectively), and MP (AUC = 0.87, p < 0.0001 vs AUC = 0.86, p < 0.0001, respectively) (Figure S1 F–I). Comparison of the ROC curves showed no significant differences in terms of accuracy between the whole-blood test based on peptide pools used at 0.1 μg/mL and at 1 μg/mL (Table 2 ). Therefore, the following experiments were performed using the 0.1 μg/mL concentration for pools S, M, and MP. For pool N, a concentration of 1 μg/mL was chosen due to a higher accuracy found at 1 μg/mL compared with the 0.1 μg/mL concentration.
Table 2.
Comparison of the ROC curves generated from COVID-19 and NO-COVID-19 groups stimulated with SARS-CoV-2 peptide pools used at 0.1 μg/mL and 1 μg/mL.
| Peptide pools | AUC | 95% CI | P value | ROC comparison p value | |
|---|---|---|---|---|---|
| Pool N | 0.1 μg/mL | 0.7372 | 0.5860−0.8883 | 0.0064 | 0.5487 |
| 1 μg/mL | 0.7579 | 0.6104−0.9054 | 0.0030 | ||
| Pool M | 0.1 μg/mL | 0.6828 | 0.5251−0.8405 | 0.0357 | 0.4439 |
| 1 μg/mL | 0.7213 | 0.5692−0.8735 | 0.0110 | ||
| Pool S | 0.1 μg/mL | 0.8518 | 0.7354−0.9681 | <0.0001 | 0.8177 |
| 1 μg/mL | 0.8587 | 0.7449−0.9725 | <0.0001 | ||
| MegaPool | 0.1 μg/mL | 0.8775 | 0.7686−0.9863 | <0.0001 | 0.5221 |
| 1 μg/mL | 0.8597 | 0.7503−0.9691 | <0.0001 | ||
ROC: receiver-operator characteristic; AUC: area under the curve; CI: confidence interval.
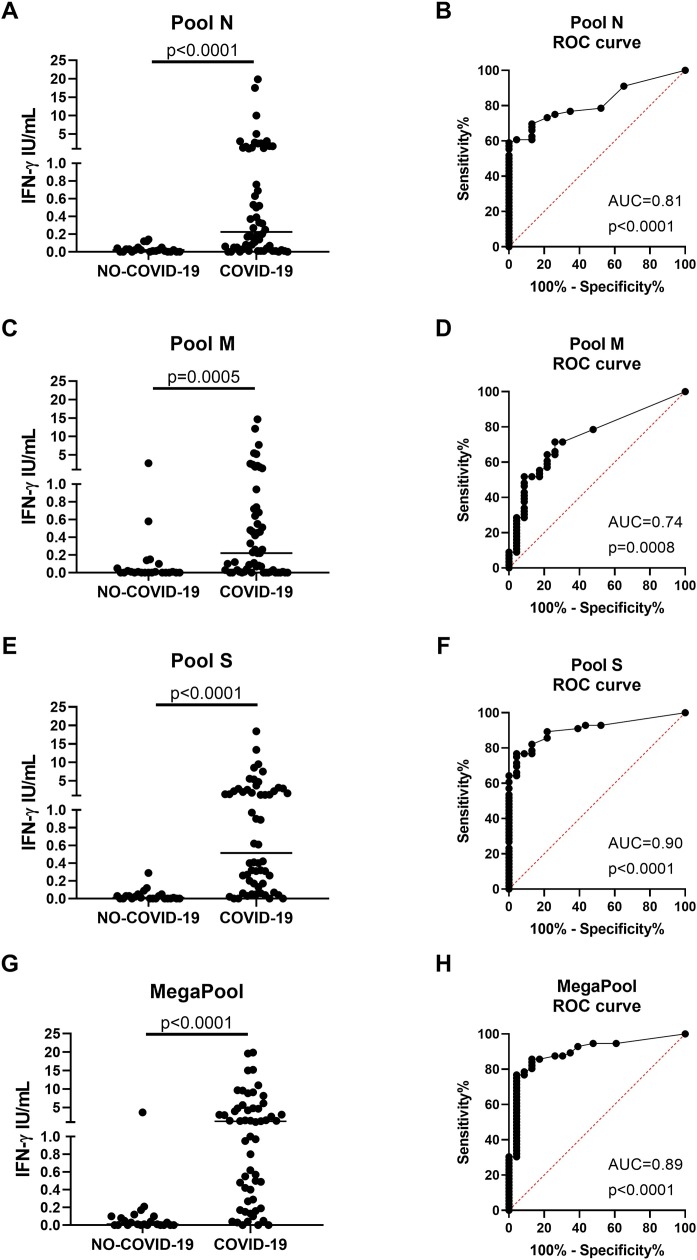
Once the best concentration for each peptide pool had been identified, the initial cohort of subjects reaching the number of 56 COVID-19 and 23 NO-COVID-19 individuals was formed. As expected, it was found that the IFN-γ levels in response to pools N, M, S, and MP stimulations were significantly higher in COVID-19 compared with NO-COVID-19 individuals (p < 0.0001, p = 0.0005, p < 0.0001, and p < 0.0001, respectively) (Figure 1A, C, E, G). In particular, pool S and MP were the most potent immunogenic stimuli (IFN-γ response to pool S, median: 0.51, IQR: 0.14–2.17; to MP, median: 1.18, IQR 0.27–4.72) compared with pools N and M that showed a similar lower response (pool N, median: 0.22, IQR: 0.032–1.26; pool M, median: 0.22, IQR 0.01−0.71, respectively). The ROC analyses performed on the larger cohort confirmed the accuracy of the test for the diagnosis of COVID-19, showing significant and even high AUC for pools S (AUC = 0.90, p < 0.0001) and MP (AUC = 0.89, p < 0.0001), followed by pools N (AUC = 0.81, p < 0.0001) and M (AUC = 0.74, p = 0.0008) (Figure 1B, D, F, H). Based on the likelihood ratio, the cut-off for scoring purposes was defined (0.13 IU/mL for the IFN-γ response to pool S and 0.24 IU/mL for MP) identifying a good sensitivity of 77% and a high specificity of 96% for both pool S and MP. For pools N and M, a cut-off of 0.13 IU/mL and 0.19 IU/mL was defined, respectively, which showed a sensitivity of 61% for pool N and 52% for pool M, and a specificity of 96% for pool N and 91% for pool M, respectively.
Figure 1.
Spike and MegaPool peptides were the most immunogenic SARS-CoV-2 antigens in the whole-blood platform. The IFN-γ level in response to SARS-CoV-2 peptides was significantly increased in COVID-19 patients compared with NO-COVID-19 individuals after stimulating whole-blood with 1 μg/mL of pool N (A) and 0.1 μg/mL of pool M (C), pool S (E) and MegaPool (G) antigens. The ROC analysis shows significant AUC results for pool N (AUC = 0.81, p < 0.0001, B), pool M (AUC = 0.74, p = 0.0008, D), but mainly for pool S (AUC = 0.90, p < 0.0001, F) and MegaPool (AUC = 0.89, p < 0.0001, H) antigens. For scoring purposes, the cut-off of 0.13 IU/mL and of 0.19 IU/mL were chosen for pools N and M antigens, respectively, that led to 61% sensitivity/96% specificity for pool N and 52% sensitivity/91% specificity for pool M. For scoring purposes, the cut-off of 0.13 IU/mL and of 0.24 IU/mL were chosen for pool S and MegaPool antigens, respectively, that led to 77% sensitivity/96% specificity for both antigens. IFN-γ was measured by ELISA in stimulated plasma. The horizontal lines represent the median; statistical analysis was performed using the Mann–Whitney test and ROC analysis, and p-value was considered significant if ≤0.05.
IFN, interferon; COVID-19: coronavirus disease 19; N: nucleocapsid; M: membrane; S: spike; ROC: receiver operating curve.
The IFN-γ production in response to SARS-CoV-2 specific stimulations was mediated by CD4+ and CD8+ T cells
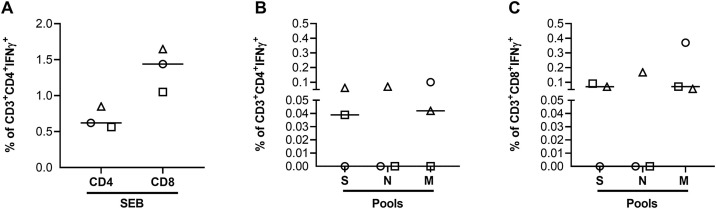
To define the T cell subsets responsible for the SARS-CoV-2 immune response, the ability of T cells to produce IFN-γ in response to stimulation with pools S, N, and M was evaluated by flow cytometry. The analysis was performed on fresh PBMCs isolated from a cohort of three hospitalized COVID-19 patients. It was demonstrated that all COVID-19 patients responded to SEB stimulation (Figure 2A) and that SARS-CoV-2 response was mediated by both CD4+ and CD8+ T cells (Figure 2B and C).
Figure 2.
The Sars-CoV-2-specific response was mediated by both CD4+ and CD8+ T cells. Freshly isolated PBMCs were stimulated overnight with different stimuli to evaluate by flow cytometry the CD4+ or CD8+ T cell-specific IFN-γ response.
A) Percentage of CD4+ or CD8+ T cells producing IFN-γ in response to the positive control SEB.
B) Percentage of IFN-γ+ CD4+ T cells in response to pools S, N and M.
C) Percentage of IFN-γ+ CD8+ T cells in response to pools S, N and M.
In all graphs the IFN-γ values were subtracted from the unstimulated control. The horizontal lines represent the median and statistical analysis was performed using the Friedman test and p ≤ 0.05 was considered significant.
Footnotes: IFN, interferon; N, nucleocapsid; M, membrane; S, spike.
The IFN-γ response to SARS-CoV-2 peptides was detected in COVID-19 patients independently of disease severity, symptom onset and lymphocyte count
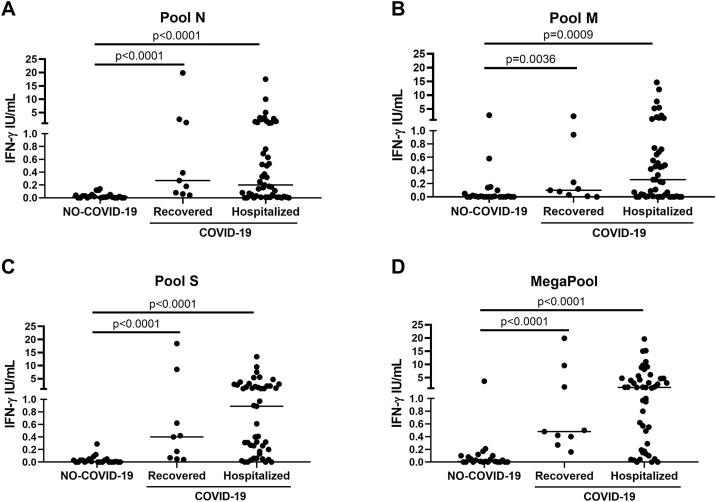
To evaluate whether the IFN-γ response was associated with the acute phase of the disease, the COVID-19 patients were stratified according to their hospitalization status. No significant differences were observed in the IFN-γ levels in response to all stimuli between acute hospitalized and post-acute non-hospitalized (recovered) COVID-19 patients (Figure 3). Within acute hospitalized COVID-19 patients, the characteristics of patients were evaluated as potential factors impacting the IFN-γ response to SARS-CoV-2 peptides (Table 3 ). Patients were stratified based on disease severity (mild/moderate, severe or critical), COVID-19 symptom onset within 1−7 days, 8−14 days, 15−30 days or >30 days in respect to whole-blood stimulation, serology or cortisone therapy. The IFN-γ response to all SARS-CoV-2 peptides was independent of the characteristics of patients. A positive and significant low correlation between IFN-γ levels and the number of lymphocytes (rs = 0.30, p = 0.048; rs = 0.31, p = 0.037, respectively) or IgG index (rs = 0.29, p = 0.043; rs = 0.29, p = 0.052, respectively) were found only for pool M and SEB.
Figure 3.
The IFN-γ response to pools N, M, S and MegaPool was detectable in both recovered and hospitalized COVID-19 patients. Analysis of the IFN-γ response to pools N (A), M (B), S (C) and MegaPool (D) antigens in COVID-19 patients either hospitalized (n = 47) or recovered (n = 9). No significant differences were observed in response to all stimuli between the two groups of COVID-19 patients. IFN-γ was measured by ELISA in stimulated plasma. Statistical analysis was performed using the Mann-Whitney test and p ≤ 0.05 was considered significant.
IFN, interferon; COVID-19: coronavirus disease 19; N, nucleocapsid; M, membrane; S, spike.
Table 3.
Impact of the characteristics of hospitalized COVID-19 patients on IFN-γ response induced by pools N, M, S and MegaPool.
| Characteristics | SEB |
Pool S |
MegaPool |
Pool N |
Pool M |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| median (IQR) | rs | p | median (IQR) | rs | p | median (IQR) | rs | p | median (IQR) | rs | p | median (IQR) | rs | p | ||
| Gender | Male | 10.7 (1.1–12.0) | na | 0.849 | 1.8 (0.2–2.8) | na | 0.329 | 1.4 (0.6–5.6) | na | 0.369 | 0.3 (0.0–1.1) | na | 0.551 | 0.3 (0.0 – 0.7) | na | 0.457 |
| Female | 10.8 (2.1–12.1) | 0.4 (0.1–2.1) | 0.7 (0.2–4.4) | 0.1 (0.0–1.7) | 0.1 (0.0 – 0.9) | |||||||||||
| Age | na | −0.14 | 0.349 | na | 0.0 | 0.996 | na | 0.01 | 0.947 | na | −0.01 | 0.637 | na | −0.04 | 0.790 | |
| Cortisone | No | 10.4 (2.7–12.0) | na | 0.710 | 1.0 (0.3–2.2) | na | 0.515 | 1.0 (0.2–4.6) | na | 0.954 | 0.2 (0.0–1.7) | na | 0.953 | 1.1 (0.0 – 0.5) | na | 0.086 |
| Yes | 10.9 (1.1–12.1) | 0.6 (0.2–2.2) | 1.4 (0.3–4.7) | 0.3 (0.0 – 0.8) | 0.3 (0.0–1.4) | |||||||||||
| Days from symptom onset* | na | 0.12 | 0.467 | na | 0.07 | 0.670 | na | 0.1 | 0.655 | na | 0.01 | 0.958 | na | 0.19 | 0.232 | |
| Severity of diseasea | Mild/Moderate | 10.9 (10.8–13.4) | na | 0.598 | 1.2 (0.3–2.9) | na | 0.478 | 4.0 (1.3–5.6) | na | 0.195 | 0.8 (0.1–1.6) | na | 0.242 | 0.4 (0.2 – 0.5) | na | 0.260 |
| Severe | 10.6 (1.5–12.1) | 0.4 (0.1–3.1) | 1.4 (0.2–6.1) | 0.2 (0.0–1.1) | 0.5 (0.0–1.8) | |||||||||||
| Critical | 6.8 (0.8–11.9) | 0.9 (0.2–2.0) | 0.9 (0.0–2.9) | 0.2 (0.0 – 0.3) | 0.0 (0.0 – 0.3) | |||||||||||
| Lymphocytes (x103) | Number | na | 0.31 | 0.037 | na | 0.02 | 0.872 | na | 0.16 | 0.286 | na | 0.17 | 0.260 | na | 0.30 | 0.048 |
| Serology | IgG index | na | 0.29 | 0.052 | na | 0.10 | 0.503 | na | 0.18 | 0.219 | na | 0.21 | 0.150 | na | 0.29 | 0.043 |
| IgG score: | ||||||||||||||||
| Negative | 10.1 (3.4–10.8) | na | 0.474 | 1.2 (0.2–3.7) | na | 0.490 | 1.0 (0.6–4.8) | na | 0.979 | 0.1 (0.0 – 0.5) | na | 0.343 | 0.2 (0.0 – 0.5) | na | 0.166 | |
| Positive | 10.9 (1.1–12.1) | 0.5 (0.2–2.2) | 1.4 (0.2–4.6) | 0.3 (0.0–1.2) | 0.3 (0.0–1.4) | |||||||||||
| IgM index | na | 0.14 | 0.360 | na | 0.05 | 0.738 | na | 0.06 | 0.711 | na | 0.15 | 0.306 | na | 0.11 | 0.461 | |
| IgM score: | ||||||||||||||||
| Negative | 10.3 (0.8–12.0) | na | 0.445 | 1.0 (0.2–3.3) | na | 0.533 | 1.2 (0.2–7.5) | na | 0.855 | 0.2 (0.0–1.1) | na | 0.763 | 0.3 (0.0 – 0.6) | na | 0.714 | |
| Positive | 10.9 (1.5–12.1) | 0.4 (0.1–2.2) | 1.3 (0.3–4.0) | 0.2 (0.0–1.2) | 0.2 (0.0–1.4) | |||||||||||
| IgG+/IgM+ | 10.9 (1.5–12.1) | na | 0.781 | 0.5 (0.3–2.2) | na | 0.339 | 1.4 (0.5–4.0) | na | 0.626 | 0.3 (0.0–1.2) | na | 0.435 | 0.2 (0.0–1.4) | na | 0.395 | |
| IgG+/IgM− | 9.1 (0.8–12.0) | 0.6 (0.1–2.1) | 1.3 (0.1–7.5) | 0.3 (0.0–1.7) | 0.4 (0.0–1.3) | |||||||||||
| IgG−/IgM+b | 3.8 | 0.0 | 0.0 | 0 | 0 | |||||||||||
| IgG−/IgM− | 10.3 (1.7–11.5) | 1.6 (0.7–4.6) | 1.2 (0.7–7.2) | 0.2 (0.0–1.1) | 0.2 (0.0 – 0.5) | |||||||||||
Mann–Whitney or Kruskal–Wallis test for categorical variables; Spearman’s correlation for continuous variables; rs: Spearman’s correlation coefficient; na: not applicable.
+ and − are related to the IgG and/or IgM scores.
Missing values: days from symptom onset, 4; severity of disease, 1.
IgG−/IgM+ there was one patient.
The IFN-γ response to SARS-CoV-2 peptides was detected in COVID-19 patients with both positive and negative SARS-CoV-2 serology
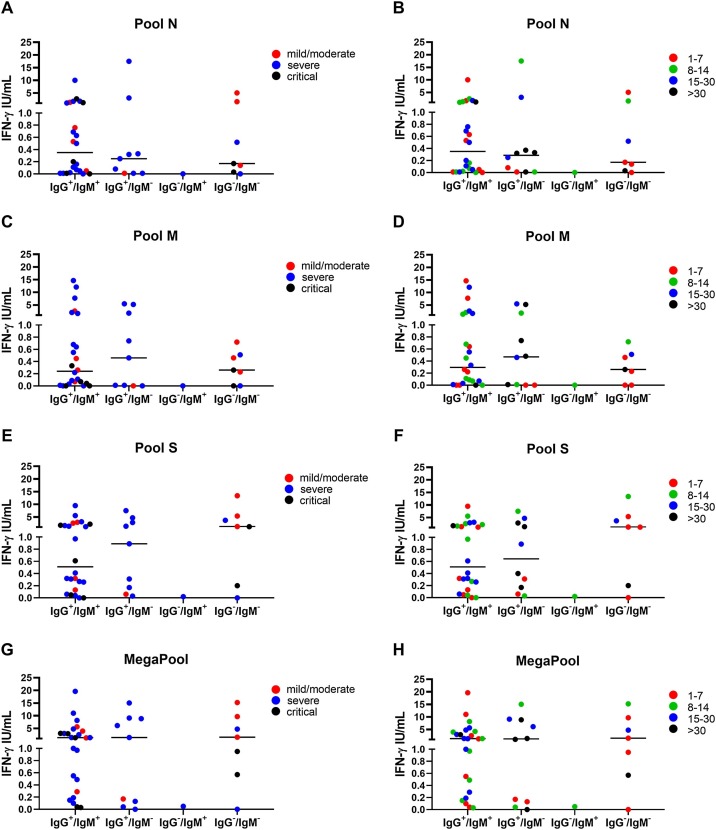
Acute hospitalized COVID-19 patients were stratified based on serology results. Patients who had doubtful serology only for IgG (n = 1) or IgM (n = 3) were included in the IgG+/IgM+ group. No significant differences in the IFN-γ levels were found in response to pools N (p = 0.43), M (p = 0.39), S (p = 0.63), and MP (p = 0.34) (Figure 4 ). Interestingly, the IFN-γ response was detected in seven patients who scored IgG-negative and IgM-negative that showed a higher IFN-γ response to pool S and MP (median: 1.22, IQR 0.20–5.41; median: 1.54, IQR 0.57–9.65, respectively) compared with pools N and M (median: 0.17, IQR 0.03–1.60; median: 0.26, IQR 0−0.51, respectively). Surprisingly, among the patients who scored negative to IgG/IgM there was one patient whose symptom onset dated back to 6 days before the time of blood stimulation and became IgG-positive after 5 days from the IFN-γ blood test.
Figure 4.
The IFN-γ response to pool S and MegaPool peptides can be detected in COVID-19 patients with a negative IgG/IgM serology. Evaluation of the IFN-γ response to pools N (A–B), M (C–D), S (E–F) and MegaPool (G–H) antigens in hospitalized COVID-19 patients (n = 41) stratified according to the IgG and IgM serology score and disease severity (A, C, E, G) or symptom onset (B, D, F, H). The IFN-γ response to pool S and MegaPool antigens can be detected in COVID-19 patients with negative IgG and IgM serology. IFN-γ was measured by ELISA in stimulated plasma. The horizontal lines represent the median; statistical analysis was performed using the Mann–Whitney test, and p-value was considered significant if ≤0.008.
IFN, interferon; N, nucleocapsid; M, membrane; S, spike.
Discussion
This study demonstrated in a cohort of hospitalized COVID-19 patients, COVID-19-recovered individuals and COVID-19-unexposed subjects that an IFN-γ test based on whole-blood stimulated with SARS-CoV-2-specific peptide pools corresponding to spike, nucleoprotein, membrane or a mix of them has good accuracy to discriminate COVID-19-hospitalized or -recovered patients from healthy unexposed individuals. Among the stimuli that were used, the best was pool S, followed by MP, pool N, and pool M. Interestingly, the T cell response was also found in individuals who scored negative to SARS-CoV-2 serology. Moreover, flow cytometry showed that the specific response to pools S, N and M was mediated by both CD4+ and CD8+ T cells. This T cell-based test may be a good approach with which to study the specific response in COVID-19 patients during the acute phase, at recovery and likely in SARS-CoV-2-vaccinated individuals (Manisty et al., 2021).
The assay described in the present study had a higher accuracy compared to whole-blood tests that have been previously described (Petrone et al., 2021a, Petrone et al., 2021b, Petrone et al., 2021c, Murugesan et al., 2020, Echeverría et al., 2021). The specificity of this test to detect SARS-CoV-2 infection was high. Based on the peptide concentration used, SARS-CoV-2-specific T cells were rarely found in NO-COVID-19 individuals, as differently reported in previous reports (Grifoni et al., 2020, Weiskopf et al., 2020, Sette and Crotty, 2020, Echeverría et al., 2021).
Different from an earlier study (Petrone et al., 2021a), this study analysed different peptide pools covering the whole spike region; additionally, it included other peptides from other viral proteins, as the nucleocapsid and membrane proteins. Based on this peptide selection, the IFN-γ-based test in response to spike was shown to be more accurate for SARS-CoV-2 detection of infection, with 77% sensitivity and 96% specificity compared with 60% sensitivity and 86.2% specificity reported in previous work (Petrone et al., 2021a). Different from other studies using a whole-blood platform (Murugesan et al., 2020, Echeverría et al., 2021), beside the different peptide used (Echeverría et al., 2021) or the different protocol performed, the current study enrolled both hospitalized and recovered patients, and evaluated the immune response based on the clinical stage, time of symptom onset, and, being an immune-based test, on lymphocyte counts or cortisone therapy. It showed that the IFN-γ response was independently detected of these clinical parameters. Therefore, the test seems robust and this is important for future clinical applications.
The kinetic of SARS-CoV-2 serology is not fully defined in terms of appearance of IgG and IgM (Sun et al., 2020, Fu et al., 2021, Long et al., 2020). The median seroconversion time of specific IgM and IgG against SARS-CoV-2 varies, ranging from 5 to 13 days and 11–14 days, respectively, after symptom onset (Fu et al., 2021). It is well documented that both humoral and cellular immune responses are crucial for SARS-CoV-2 infection and containment (Ni et al., 2020) and it has been found that cellular responses can also be detected in seronegative COVID-19 patients (Gallais et al., 2021, Freeman et al., 2004, Heller et al., 2013, Mizukoshi et al., 2008). Interestingly, the present study found T cell responses in seven hospitalized COVID-19 patients who scored negative to both IgM and IgG SARS-CoV-2 serology. Within this group there were five patients whose symptom onset dated back to 1–8 days before blood stimulation. Particularly, one patient had IgG seroconversion 5 days after the test. These data suggest that the IFN-γ T cell response might anticipate, in some cases, the B cell response as detected by antibody. This result is interesting and may offer clinical diagnostic applications. Considering that SARS-CoV-2-IgG or IgM levels are not constant over time (Sethuraman et al., 2020, Xiao et al., 2020), beside serology, the IFN-γ release assay (IGRA) may be a potential additional immune tool for further diagnostic and more in-depth clinical evaluations. The test may be used as an easy-to-use assay to better understand the prevalence of T cell-specific immunity in the population, access to pre-existing T cell immunity in seronegative individuals, vaccine-induced immunity, and also to pre-evaluate the SARS-CoV-2 vaccine candidates in clinical trials.
This study had some limitations. The sample size was relatively small (79 subjects) and not representative of the whole COVID-19 or NO-COVID-19 population. Moreover, it did not perform any longitudinal analyses of specific T-cell responses and antibodies to evaluate the levels of specific immunity over time. These issues may be assessed in future studies.
In conclusion, this study provided a simple IGRA platform for SARS-CoV-2-specific immune response detection, which can easily be scaled up for population-based studies. This test can be implemented in clinical laboratories as a powerful diagnostic tool and for understanding vaccine efficacy and potentially for surveillance strategies.
Authors’ contributions
Study conception and design: DG; acquisition of data: AA, SNF, CF, VV, GC, GG, SM, EN, LP, DG; analysis and interpretation of data: AA, SNF, CF, AN, EP, LP, DG.
Drafting the article: AA, SNF, CF, LP, DG; critically revising the article for important intellectual content: AA, SNF, EP, LP, VV, CF, GC, AN, GG, SM, LP, EN, DG; final approval of the version of the article to be published: AA, SNF, EP, LP, VV, CF, GC, AN, GG, SM, LP, EN, DG.
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Funding
This study was funded by Line one-Ricerca Corrente “Infezioni Emergenti e Riemergenti”, by Line four- Ricerca Corrente, by the projects COVID 2020-12371675 and COVID-2020-12371735, all funded by Italian Ministry of Health and by the funding from “Progetto 18 - Coinfezione di SARS-CoV-2 con HIV or M. tuberculosis” sponsored by “Valentino Spa”.
Ethical approval
The Ethical Committee of National Institute of Infectious Diseases (INMI) Lazzaro Spallanzani-IRCCS approved the study (approval number 59/2020).
Acknowledgements
The authors are grateful to all the patients and nurses who helped to conduct this study. The authors gratefully acknowledge the Collaborators Members of the National Institute for Infectious Diseases (INMI) COVID-19 study group: Maria Alessandra Abbonizio, Amina Abdeddaim, Chiara Agrati, Fabrizio Albarello, Gioia Amadei, Alessandra Amendola, Andrea Antinori, Mario Antonini, Tommaso Ascoli Bartoli, Francesco Baldini, Raffaella Barbaro, Barbara Bartolini, Rita Bellagamba, Martina Benigni, Nazario Bevilacqua, Gianlugi Biava, Michele Bibas, Licia Bordi, Veronica Bordoni, Evangelo Boumis, Marta Branca, Donatella Busso, Marta Camici, Paolo Campioni, Maria Rosaria Capobianchi, Alessandro Capone, Cinzia Caporale, Emanuela Caraffa, Ilaria Caravella, Fabrizio Carletti, Concetta Castilletti, Adriana Cataldo, Stefano Cerilli, Carlotta Cerva, Roberta Chiappini, Pierangelo Chinello, Carmine Ciaralli, Stefania Cicalini, Francesca Colavita, Angela Corpolongo, Massimo Cristofaro, Salvatore Curiale, Alessandra D'Abramo, Cristina Dantimi, Alessia De Angelis, Giada De Angelis, Maria Grazia De Palo, Federico De Zottis, Virginia Di Bari, Rachele Di Lorenzo, Federica Di Stefano, Gianpiero D'Offizi, Davide Donno, Francesca Faraglia, Federica Ferraro, Lorena Fiorentini, Andrea Frustaci, Matteo Fusetti, Vincenzo Galati, Roberta Gagliardini, Paola Gallì, Gabriele Garotto, Saba Gebremeskel Tekle, Maria Letizia Giancola, Filippo Giansante, Emanuela Giombini, Guido Granata, Maria Cristina Greci, Elisabetta Grilli, Susanna Grisetti, Gina Gualano, Fabio Iacomi, Giuseppina Iannicelli, Giuseppe Ippolito, Eleonora Lalle, Simone Lanini, Daniele Lapa, Luciana Lepore, Raffaella Libertone, Raffaella Lionetti, Giuseppina Liuzzi, Laura Loiacono, Andrea Lucia, Franco Lufrani, Manuela Macchione, Gaetano Maffongelli, Alessandra Marani, Luisa Marchioni, Andrea Mariano, Maria Cristina Marini, Micaela Maritti, Alessandra Mastrobattista, Giulia Matusali, Valentina Mazzotta, Paola Mencarini, Silvia Meschi, Francesco Messina, Annalisa Mondi, Marzia Montalbano, Chiara Montaldo, Silvia Mosti, Silvia Murachelli, Maria Musso, Emanuele Nicastri, Pasquale Noto, Roberto Noto, Alessandra Oliva, Sandrine Ottou, Claudia Palazzolo, Emanuele Pallini, Fabrizio Palmieri, Carlo Pareo, Virgilio Passeri, Federico Pelliccioni, Antonella Petrecchia, Ada Petrone, Nicola Petrosillo, Elisa Pianura, Carmela Pinnetti, Maria Pisciotta, Silvia Pittalis, Agostina Pontarelli, Costanza Proietti, Vincenzo Puro, Paolo Migliorisi Ramazzini, Alessia Rianda, Gabriele Rinonapoli, Silvia Rosati, Martina Rueca, Alessandra Sacchi, Alessandro Sampaolesi, Francesco Sanasi, Carmen Santagata, Alessandra Scarabello, Silvana Scarcia, Vincenzo Schinina, Paola Scognamiglio, Laura Scorzolini, Giulia Stazi, Fabrizio Taglietti, Chiara Taibi, Roberto Tonnarini, Simone Topino, Francesco Vaia, Francesco Vairo, Maria Beatrice Valli, Alessandra Vergori, Laura Vincenzi, Ubaldo Visco-Comandini, Serena Vita, Pietro Vittozzi, and Mauro Zaccarelli.
Delia Goletti is a professor of Pathology of Unicamillus, International Medical University of Rome.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.ijid.2021.04.034.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- Braun J., Loyal L., Frentsch M., Wendisch D., Georg P., Kurth F. SARS-CoV-2-reactive T cells in healthy donors and patients with COVID-19. Nature. 2020;587:270–274. doi: 10.1038/s41586-020-2598-9. [DOI] [PubMed] [Google Scholar]
- Costela-Ruiz V.J., Illescas-Montes R., Puerta-Puerta J.M., Ruiz C., Melguizo-Rodríguez L. SARS-CoV-2 infection: the role of cytokines in COVID-19 disease. Cytokine Growth Factor Rev. 2020;54:62–75. doi: 10.1016/j.cytogfr.2020.06.001. S1359-6101(20)30109-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dammermann W., Bentzien F., Stiel E.M., Kühne C., Ullrich S., Schulze Zur Wiesch J. Development of a novel IGRA assay to test T cell responsiveness to HBV antigens in whole blood of chronic Hepatitis B patients. J Transl Med. 2015;13:157. doi: 10.1186/s12967-015-0513-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dan J.M., Mateus J., Kato Y., Hastie K.M., Yu E.D., Faliti C.E. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science. 2021;371 doi: 10.1126/science.abf4063. Epub 2021 January 6. eabf4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echeverría G., Guevara Á, Coloma J., Ruiz A.M., Vasquez M.M., Tejera E. Pre-existing T-cell immunity to SARS-CoV-2 in unexposed healthy controls in Ecuador, as detected with a COVID-19 interferon-gamma release assay. Int J Infect Dis. 2021;105:21–25. doi: 10.1016/j.ijid.2021.02.034. S1201-9712(21)00120-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman A.J., Ffrench R.A., Post J.J., Harvey C.E., Gilmour S.J., White P.A. Prevalence of production of virus-specific interferon-gamma among seronegative hepatitis C-resistant subjects reporting injection drug use. J Infect Dis. 2004;190:1093–1097. doi: 10.1086/422605. JID32009. [DOI] [PubMed] [Google Scholar]
- Fu Y., Pan Y., Li Z., Li Y. The utility of specific antibodies against SARS-CoV-2 in laboratory diagnosis. Front Microbiol. 2021;11 doi: 10.3389/fmicb.2020.603058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallais F., Velay A., Nazon C., Wendling M.J., Partisani M., Sibilia J. Intrafamilial exposure to SARS-CoV-2 associated with cellular immune response without seroconversion, France. Emerg Infect Dis. 2021;27:113–121. doi: 10.3201/eid2701.203611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goletti D., Lee M.R., Wang J.Y., Walter N., Ottenhoff T.H.M. Update on tuberculosis biomarkers: from correlates of risk, to correlates of active disease and of cure from disease. Respirology. 2018;23:455–466. doi: 10.1111/resp.13272. [DOI] [PubMed] [Google Scholar]
- Goletti D., Lindestam Arlehamn C.S., Scriba T.J., Anthony R., Cirillo D.M., Alonzi T. Can we predict tuberculosis cure? What tools are available? Eur Respir J. 2018;52 doi: 10.1183/13993003.01089-2018. [DOI] [PubMed] [Google Scholar]
- Grifoni A., Weiskopf D., Ramirez S.I., Mateus J., Dan J.M., Moderbacher C.R. Targets of T cell responses to SARS-CoV-2 coronavirus in humans with COVID-19 disease and unexposed individuals. Cell. 2020;181:1489–1501.e15. doi: 10.1016/j.cell.2020.05.015. S0092-8674(20)30610-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller T., Werner J.M., Rahman F., Mizukoshi E., Sobao Y., Gordon A.M. Occupational exposure to hepatitis C virus: early T-cell responses in the absence of seroconversion in a longitudinal cohort study. J Infect Dis. 2013;208:1020–1025. doi: 10.1093/infdis/jit270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S.H. Interferon-γ release assay for cytomegalovirus (IGRA-CMV) for risk stratification of posttransplant CMV infection: is it time to apply IGRA-CMV in routine clinical practice? Clin Infect Dis. 2020;71:2386–2388. doi: 10.1093/cid/ciz1211. [DOI] [PubMed] [Google Scholar]
- Koblischke M., Traugott M.T., Medits I., Spitzer F.S., Zoufaly A., Weseslindtner L. Dynamics of CD4 T cell and antibody responses in COVID-19 patients with different disease severity. Front Med (Lausanne) 2020;7 doi: 10.3389/fmed.2020.592629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bert N., Tan A.T., Kunasegaran K., Tham C.Y.L., Hafezi M., Chia A. SARS-CoV-2- specific T cell immunity in cases of COVID-19 and SARS, and uninfected controls. Nature. 2020;584(7821):457–462. doi: 10.1038/s41586-020-2550-z. [DOI] [PubMed] [Google Scholar]
- Liu W.J., Zhao M., Liu K., Xu K., Wong G., Tan W. T-cell immunity of SARS-CoV: implications for vaccine development against MERS-CoV. Antiviral Res. 2017;137:82–92. doi: 10.1016/j.antiviral.2016.11.006. S0166-3542(16)30401-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long Q.X., Liu B.Z., Deng H.J., Wu G.C., Deng K., Chen Y.K. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat Med. 2020;26:845–848. doi: 10.1038/s41591-020-0897-1. [DOI] [PubMed] [Google Scholar]
- Mahmoudi S., Mamishi S., Suo X., Keshavarz H. Early detection of Toxoplasma gondii infection by using a interferon gamma release assay: a review. Exp Parasitol. 2017;172:39–43. doi: 10.1016/j.exppara.2016.12.008. [DOI] [PubMed] [Google Scholar]
- Manisty C., Otter A.D., Treibel T.A., McKnight Á, Altmann D.M., Brooks T. Antibody response to first BNT162b2 dose in previously SARS-CoV-2-infected individuals. Lancet. 2021 doi: 10.1016/S0140-6736(21)00501-8. S0140-6736(21)00501-00508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateus J., Grifoni A., Tarke A., Sidney J., Ramirez S.I., Dan J.M. Selective and cross-reactive SARS-CoV-2 T cell epitopes in unexposed humans. Science. 2020;370:89–94. doi: 10.1126/science.abd3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizukoshi E., Eisenbach C., Edlin B.R., Newton K.P., Raghuraman S., Weiler-Normann C. Hepatitis C virus (HCV)-specific immune responses of long-term injection drug users frequently exposed to HCV. J Infect Dis. 2008;198:203–212. doi: 10.1086/589510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murugesan K., Jagannathan P., Pham T.D., Pandey S., Bonilla H.F., Jacobson K. Interferon-gamma release assay for accurate detection of SARS-CoV-2 T cell response. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicastri E., Petrosillo N., Ascoli Bartoli T., Lepore L., Mondi A., Palmieri F. National Institute for the Infectious Diseases “L. Spallanzani”, IRCCS. Recommendations for COVID-19 clinical management. Infect Dis Rep. 2020;12:8543. doi: 10.4081/idr.2020.8543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni L., Ye F., Cheng M.L., Feng Y., Deng Y.Q., Zhao H. Detection of SARS-CoV-2-specific humoral and cellular immunity in COVID-19 convalescent individuals. Immunity. 2020;52:971–977.e3. doi: 10.1016/j.immuni.2020.04.023. S1074-7613(20)30181-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojeda D.S., Gonzalez Lopez Ledesma M.M., Pallarés H.M., Costa Navarro G.S., Sanchez L., Perazzi B. Emergency response for evaluating SARS-CoV-2 immune status, seroprevalence and convalescent plasma in Argentina. PLoS Pathog. 2021;17 doi: 10.1371/journal.ppat.1009161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrone L., Vanini V., Amicosante M., Corpolongo A., Gomez-Morales M.A., Ludovisi A. A T-cell diagnostic test for cystic echinococcosis based on antigen B peptides. Parasite Immunol. 2017;39(12):e12499. doi: 10.1111/pim.12499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrone L., Petruccioli E., Vanini V., Cuzzi G., Najafi Fard S., Alonzi T. A whole blood test to measure SARS-CoV-2-specific response in COVID-19 patients. Clin Microbiol Infect. 2021;27:286.e7–286.e13. doi: 10.1016/j.cmi.2020.09.051. S1198-743X(20)30605-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrone L., Petruccioli E., Alonzi T., Vanini V., Cuzzi G., Najafi Fard S. In-vitro evaluation of the immunomodulatory effects of Baricitinib: Implication for COVID-19 therapy. J Infect. 2021;82(4):58–66. doi: 10.1016/j.jinf.2021.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrone L., Petruccioli E., Vanini V., Cuzzi G., Gualano G., Vittozzi P. Coinfection of tuberculosis and COVID-19 limits the ability to in vitro respond to SARS-CoV-2. Int J Infect Dis. 2021 doi: 10.1016/j.ijid.2021.02.090. S1201-9712(21)00176-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethuraman N., Jeremiah S.S., Ryo A. Interpreting diagnostic tests for SARS-CoV-2. JAMA. 2020;323(22):2249–2251. doi: 10.1001/jama.2020.8259. [DOI] [PubMed] [Google Scholar]
- Sette A., Crotty S. Pre-existing immunity to SARS-CoV-2: the knowns and unknowns. Nat Rev Immunol. 2020;20:457–458. doi: 10.1038/s41577-020-0389-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah V.K., Firmal P., Alam A., Ganguly D., Chattopadhyay S. Overview of immune response during SARS-CoV-2 infection: lessons from the past. Front Immunol. 2020;11:1949. doi: 10.3389/fimmu.2020.01949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrotri M., van Schalkwyk M.C.I., Post N., Eddy D., Huntley C., Leeman D. T cell response to SARS-CoV-2 infection in humans: a systematic review. PLoS One. 2021;16 doi: 10.1371/journal.pone.0245532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun B., Feng Y., Mo X., Zheng P., Wang Q., Li P. Kinetics of SARS-CoV-2 specific IgM and IgG responses in COVID-19 patients. Emerg Microbes Infect. 2020;9:940–948. doi: 10.1080/22221751.2020.1762515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vabret N., Britton G.J., Gruber C., Hegde S., Kim J., Kuksin M. Immunology of COVID-19: current state of the science. Immunity. 2020;52:910–941. doi: 10.1016/j.immuni.2020.05.002. S1074-7613(20)30183-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiskopf D., Schmitz K.S., Raadsen M.P., Grifoni A., Okba N.M.A., Endeman H. Phenotype and kinetics of SARS-CoV-2-specific T cells in COVID-19 patients with acute respiratory distress syndrome. Sci Immunol. 2020;5 doi: 10.1126/sciimmunol.abd2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . 2020. Clinical management of COVID-19. Interim guidance, 27 May 2020. [Google Scholar]
- Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- Xiao K., Zhai J., Feng Y., Zhou N., Zhang X., Zou J.J. Isolation of SARS-CoV-2-related coronavirus from Malayan pangolins. Nature. 2020;583:286–289. doi: 10.1038/s41586-020-2313-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.